Abstract
Recent reports indicate that cytotoxic T cells are critically involved in contact hypersensitivity reactions in animals. In this study we sought to investigate the in vivo expression of cytotoxic granule proteins in the elicitation phase of allergic contact dermatitis in humans. Skin biopsy specimens were obtained from patients with allergic contact dermatitis (n = 8) and psoriasis (n = 6) and from controls with normal skin (n = 6). Expression of perforin and granzyme B was investigated by in situ hybridization and immunohistochemistry. In contrast to normal skin and psoriasis, a significant enhancement of perforin and granzyme B gene expression and immunoreactivity was observed in the mononuclear cell infiltrate of allergic contact dermatitis. Immunoreactivity for perforin and granzyme B was mainly found in the cytoplasm of lymphocytic cells, which were located in the dense perivascular infiltrate as well as at sites of marked spongiosis in the epidermis. Double immunostaining revealed that both CD4+ and CD8+ T cells are capable of expressing perforin and granzyme B. In conclusion, our data suggest that T-cell-mediated mechanisms involving cytotoxic granule proteins may elicit epidermal cell injury in vivo and thereby strongly contribute to the development of allergic contact dermatitis in humans.
Allergic contact dermatitis (ACD) is a common skin disease occurring after epicutaneous exposure to haptens in sensitized individuals. 1,2 It is well established that delayed cell-mediated immune mechanisms involving hapten-specific T cells play an important part in the pathogenesis of this skin disease. 1,2 The histopathology of ACD is characterized by a varying degree of spongiosis and exocytosis of mononuclear cells into the epidermis. These epidermal changes are accompanied by a perivascular lymphohistiocytic infiltrate in the dermis, which is composed predominantly of CD4+ T cells. To date, the precise underlying mechanisms leading to spongiosis and epidermal cell injury in vivo are still poorly understood.
In previous years, increasing evidence indicated that cytotoxic T lymphocytes (CTL) are involved in eliciting contact hypersensitivity (CHS) reactions in animals. 2-7 In particular, Kehren et al have recently demonstrated that CHS in mice is mediated by cytotoxic mechanisms involving Fas/Fas ligand interactions and perforin. 7 To our knowledge, the precise localization and distribution of cytotoxic proteins like perforin and granzyme B in ACD in humans has not yet been elucidated. The aim of this study, therefore, was to investigate the in situ expression of these cytotoxic proteins in skin biopsy specimens from individuals with ACD by in situ hybridization and immunohistochemistry. To assess differences in other inflammatory skin conditions, the expression of perforin and granzyme B was also analyzed in psoriatic skin lesions.
Materials and Methods
Subjects and Skin Biopsy Specimens
The Ethical Committee of the Medical Faculty of the University of Bern approved the study. Eight Caucasian patients (5 females, 3 males; mean age 35 years; range, 21–45 years) with ACD were included in the study after giving their informed consent. The contact allergens involved were nickel sulfate (n = 5) and one each of potassium dichromate, 4-phenylenediamine, and mercapto mix. The diagnosis of ACD was based on the typical clinical presentation and histology of the lesions and was also confirmed by a positive allergic epicutaneous test, excluding an irritant contact reaction to these substances. Punch biopsy specimens 5 mm in diameter were taken from the eczematous skin lesions, all typically showing erythema, papulo-vesicles, and some excoriations. Normal skin from non-atopic controls (n = 6) as well as lesional psoriatic skin (n = 6) were obtained as control groups. Before the investigation, the patients did not receive any systemic corticosteroids or topical treatment with corticosteroids at the site of the punch biopsy. Biopsy specimens were snap-frozen in tissue embedding medium using isopentane precooled in liquid nitrogen and stored at −70°C until use.
Preparation of 35S-Labeled RNA Probes
A 700-bp fragment of the human granzyme B cDNA (generously provided by Dr. G. M. Griffiths, University of Oxford, Oxford, UK) and a 1953-bp cDNA fragment of the human perforin gene (kindly provided by J. Tschopp, University of Lausanne, Lausanne, Switzerland) were cloned into the expression vectors pGEM-1 and pBluescript SK, respectively, and used to prepare 35S-labeled sense and antisense RNA probes as described previously. 8
In Situ Hybridization
In situ hybridization was performed as described previously. 8 Hybridized slides were exposed for 28 days at 4°C, developed, and subsequently counterstained with nuclear fast red (0.05% in 5% aluminum sulfate) by standard techniques.
Immunohistochemistry
The monoclonal antibodies used in the study and their specificity are shown in Table 1 ▶ . Substitution of the primary antibody with isotype-matched IgG and omission of the primary antibody served as negative controls.
Table 1.
Monoclonal Antibodies Used in the Study
| Antibody against [clone] | Specificity | Concentration (μg/ml) | Supplier |
|---|---|---|---|
| CD3 [UCHT1] | T cells | 3 | DAKO, Glostrup, Denmark |
| CD4 [MT310] | T cell subset | 2.0 | DAKO, Glostrup, Denmark |
| CD4 [YNB46.1.8] | – | 10.0 | Serotec, Oxford, UK |
| CD8 [DK25] | T cell subset | 1.5 | DAKO, Glostrup, Denmark |
| CD8 [YTC 141.1HL] | – | 10.0 | Serotec, Oxford, UK |
| CD56 [MY31] | NK cells, subset of activated T cells | 1.0 | Becton Dickinson, Mountain View, CA |
| perforin [δG9] | cytotoxic protein | 33 | Ancell Corporation, Bayport, MN |
| granzyme B [GrB-11] | cytotoxic protein | 30 | Hölzel Diagnostik, Köln, Germany |
Immunostaining for granzyme B was performed using the alkaline phosphatase anti-alkaline phosphatase method. 9 Briefly, 6-μm cryostat tissue sections were air-dried, fixed in 2% formaldehyde for 8 minutes, and rehydrated in Tris-buffered saline with 0.1% saponin. Slides were then incubated for 3 hours with the primary mouse antibody, followed by a rabbit anti-mouse antibody (Z-259; DAKO, Glostrup, Denmark) and the alkaline phosphatase anti-alkaline phosphatase complexes (D-651; DAKO). To enhance the signal, incubation with the bridging antibody and the alkaline phosphatase anti-alkaline phosphatase complexes were repeated once.
Immunostaining for perforin, CD3, CD4, CD8, and CD56 was performed using the avidin-biotin-complex/alkaline phosphatase method. 9 Briefly, after fixation for 8 minutes with 2% formaldehyde (for perforin) or acetone (for the remaining antibodies) slides were incubated with the primary mouse antibody for 3 hours at room temperature, followed by a biotinylated rabbit-anti-mouse IgG (E0413; DAKO) and thereafter with avidin-biotin-complex/alkaline phosphatase (K0376; DAKO). Finally, all sections were developed in new fuchsin-naphtol (Sigma, St. Louis, MO) and counterstained with hematoxylin.
Double immunostaining was performed by combining two indirect staining methods with two unlabeled primary antibodies of mouse (anti-perforin or anti-granzyme B) and rat (anti-CD4 [YNB46.1.8] or anti-CD8 [YTC 141.1HL]) origin as described previously. 9 Briefly, slides were initially incubated with the primary antibodies, followed by alkaline phosphatase-conjugated goat anti-mouse (D0486, DAKO) and peroxidase-conjugated goat anti-rat immunoglobulins (Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were then developed using new fuchsin-naphtol and diaminobenzidine, which revealed red and brown staining color, respectively, and finally counterstained with hematoxylin. As a further control, sections were also developed using tetramethylbenzidine (TMB; Rockland, Gilbertsville, PA) instead of diaminobenzidine, which revealed a green staining color. The specificity of the reaction was confirmed by omitting the primary antibodies and using appropriate isotype-matched antibodies as negative controls.
Evaluation of Slides
The slides were coded with numbers and evaluated in a blinded fashion. After in situ hybridization with the 35S-labeled antisense RNA probe, cells were considered positive for gene expression when they had at least three times as many silver grains as cells hybridized with the corresponding sense RNA probe, which served as a negative control. With regard to perforin and granzyme B immunoreactivity, cells displaying a strong cytoplasmic staining were considered to be positive. Only positively stained cells with a nucleus were included in the counting. In each section, 10 to 15 fields were analyzed at 400× magnification with a Leitz Dialux 20EB microscope (Leica Microsystems AG, Wetzlar, Germany) by two independent investigators. The positive cells were counted using a 0.063 mm 2 grid and the number of positive cells/mm 2 (mean ± SEM) was calculated.
The degree of spongiosis was evaluated on five randomly selected fields using the following grading: 0 = no spongiosis; 1 = slight spongiosis; 2 = moderate spongiosis; 3 = strong spongiosis with microvesicles.
Statistical Analysis
Statistical analysis was performed by using the Kruskal-Wallis nonparametric analysis of variance test, and, if results were significant, the Mann-Whitney U test (with the Bonferroni correction) was applied to compare any two groups. The Spearman rank test was used to analyze the correlation between the degree of spongiosis and number of perforin- and granzyme B-positive cells per field. A P value <0.05 was considered statistically significant.
Results
Perforin and Granzyme B Gene Expression and Immunoreactivity Are Significantly Enhanced in ACD
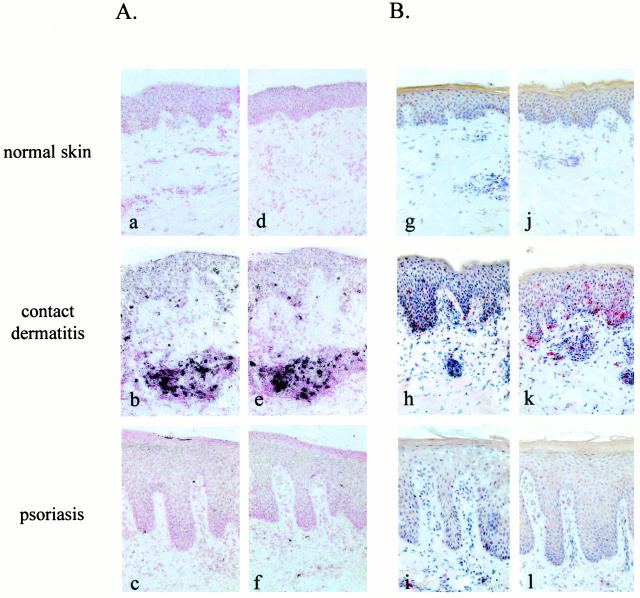
The distribution of perforin and granzyme B gene expression ascertained by in situ hybridization and the quantification of positive cells in normal skin, lesional skin from ACD, and psoriasis are shown in Figures 1A and 2A ▶ ▶ . In normal skin, expression of perforin and granzyme B was not detectable or was detected in only a few cells. In comparison to normal skin and psoriasis, a significant enhancement of perforin and granzyme B expression was observed in ACD. A significantly higher number of perforin- and granzyme B-positive cells was also observed in psoriasis as compared to normal skin.
Figure 1.
Expression of perforin and granzyme B is strongly enhanced in contact dermatitis. In situ hybridizations (A) and immunohistochemistry (B) of cryostat sections form normal skin, lesional contact dermatitis and psoriatic skin are shown. Perforin (a and g) and granzyme B (d and j) gene expression and immunoreactivity was barely detectable in normal skin. A marked enhancement of perforin (b and h) and granzyme B (e and k) expression was observed in the mononuclear cell infiltrate in contact dermatitis. Perforin (c and i) and granzyme B (f and l) expression was found in a few cells in psoriasis. Original magnification, ×250.
Figure 2.
Quantification of perforin- and granzyme B-positive cells in normal skin (n = 6), contact dermatitis (n = 8), and psoriatic skin lesions (n = 6). Mean values ± SEM.
Localization of perforin and granzyme B immunoreactivity and the quantification of positively stained cells are shown in Figures 1B and 2B ▶ ▶ . In correlation with the data on in situ hybridization, a significant enhancement of perforin and granzyme B immunoreactivity was also observed in the mononuclear cell infiltrate of ACD as compared to psoriasis and normal skin. No positive staining was detected when the primary antibody was substituted with an irrelevant isotype-matched IgG or when omitting the primary antibody (not shown).
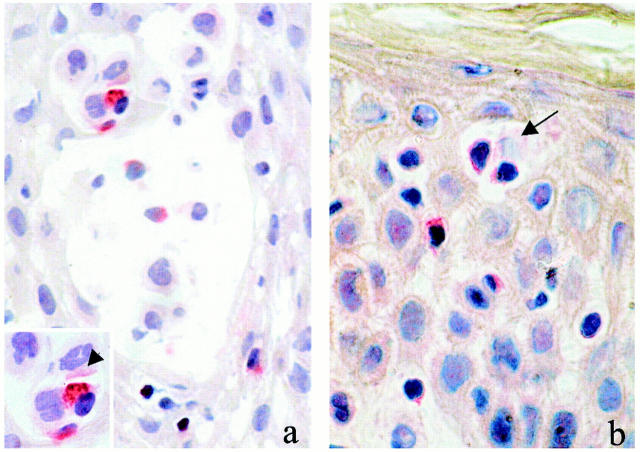
Perforin was expressed on up to 20 and 5% of the mononuclear cells in ACD and psoriasis, respectively; and granzyme B was expressed on up to 30 and 10% of the mononuclear cells in ACD and psoriasis, respectively. Immunoreactivity for these cytotoxic proteins was found mainly in the cytoplasm of the cells scattered throughout the dermis and the dermoepidermal junction zone. In ACD, increased numbers of perforin- and granzyme B-positive cells were also observed in the epidermis. Positively stained cells were particularly detected at sites of marked spongiosis and often in close proximity to keratinocytes, some of which showed signs of cell damage (Figure 3, a and b) ▶ . A weaker positive staining for perforin and granzyme B was also observed on some of these keratinocytes (Figure 3 ▶ , insert), suggesting that these cells may have been exposed and damaged by cytotoxic proteins released from the neighboring T cells. A significant correlation was found between the degree of spongiosis and the number of perforin-positive (rSpearman = 0.90, P < 0.05) and granzyme B-positive (rSpearman = 0.92, P < 0.05) cells infiltrating the epidermis.
Figure 3.
Perforin (a) and granzyme B (b) immunoreactivity was found particularly at sites of spongiosis or near spongiotic vesicles in the epidermis. Positively stained lymphocytic cells were observed in close relationship with damaged keratinocytes (arrow). Note that positive immunostaining was also observed on keratinocytes (arrowhead) next to lymphocytic cells expressing cytotoxic proteins (inset). Original magnification, ×400.
To analyze the phenotype of perforin- and granzyme B-positive cells, staining of serial sections and double-immunostaining experiments were performed. The morphology of the stained cells as well as staining of serial sections strongly suggest that CD3+ T lymphocytes (not shown) are the major source of these proteins. Furthermore, double-immunostaining experiments using new fuchsin and diaminobenzidine as chromogens confirmed that both CD4+ and CD8+ cells express perforin (Figure 4, a and b) ▶ and granzyme B (not shown). In addition, staining with new fuchsin and TMB as chromogens was also performed with similar results (shown for granzyme B in Figure 4, c and d ▶ ). Immunoreactivity for CD56 was not detectable, so that double-immunostaining experiments with CD56 and perforin and/or granzyme B were not feasible.
Figure 4.
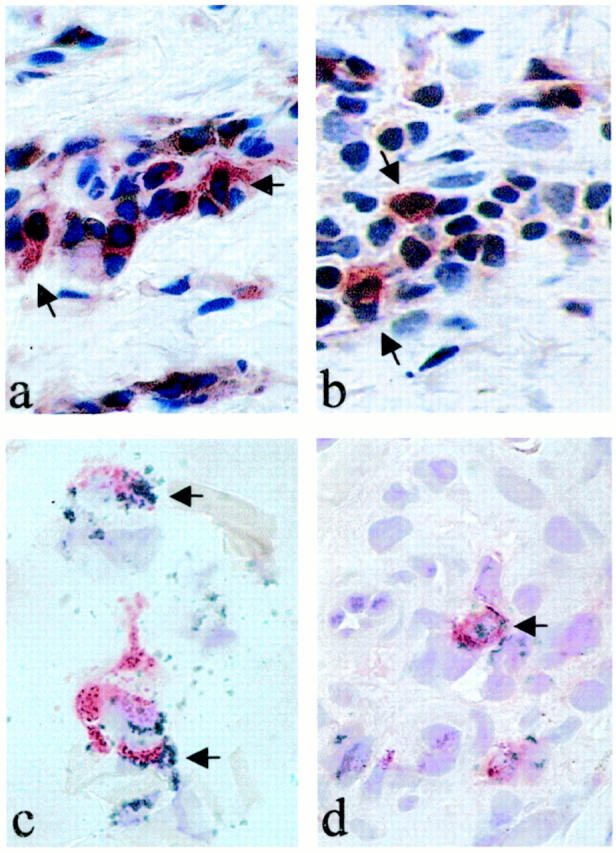
Perforin and granzyme B are expressed in CD4+ and CD8+ cells. Double immunostaining was performed as described in Materials and Methods. Representative stainings of perforin and granzyme B with different chromogens are shown. In the upper panel CD4+ (a) and CD8+ (b) cells were determined with diaminobenzidine (brown), perforin with new fuchsin-naphtol (red). In the lower panel CD4+ (c) and CD8+ (d) cells were determined with TMB (green), granzyme B with Fast Red (red). Arrows indicate double-positive cells. Original magnification, ×1000.
Discussion
In this study the in situ expression of perforin and granzyme B was investigated in normal skin as well as in two common inflammatory skin diseases, namely ACD and psoriasis. Our data demonstrate for the first time that the expression of these cytotoxic proteins is particularly enhanced in ACD and is often found in the epidermis at sites with signs of marked spongiosis.
Perforin and granzyme B are important mediators of cell-mediated cytotoxic reactions. 10-14 These cytotoxic granule proteins are stored in secretory lysosomes of natural killer cells and CTLs. On activation perforin and granzyme B are released through exocytosis and cooperatively trigger death of their target cells. Perforin is thought to cause cell death by forming pores on the target cell membrane and inducing osmotic cell lysis. 11 The formation of these pores also facilitates the entry of granzymes, which trigger cell death by inducing degradation of DNA to fragments. The enhanced expression of perforin and granzyme B in ACD, and to a lesser extent in psoriasis, suggests that such cell-mediated cytotoxic mechanisms may play an integral part in the pathogenesis of these inflammatory skin diseases. Interestingly, our data revealed that the number of T cells expressing these cytotoxic proteins is significantly increased in ACD as compared to psoriasis. These differences may account for the distinct clinical morphology and histological features of these two inflammatory skin diseases. ACD typically represents a spongiotic dermatitis with an infiltration of lymphocytic cells into the epidermis. In contrast, psoriatic skin lesions do not usually show signs of spongiosis, although T cells may also be present in the epidermis of these lesions. The precise mechanism leading to keratinocyte damage and spongiosis in ACD has so far remained unclear. The enhanced expression of perforin and granzyme B at sites of marked spongiosis suggest that T-cell-mediated cytotoxicity may play a decisive role in inducing epidermal cell damage in ACD. This assumption is particularly substantiated by the close cellular contact between CTLs and damaged keratinocytes as well as the presence of such cytotoxic proteins on these epidermal cells. Further evidence for a role of T-cell-mediated cytotoxic mechanisms is also obtained from recently published data, demonstrating that elicitation of CHS is partly mediated by CTLs containing perforin in mice. 7 Nevertheless, it will be of interest to determine in future studies whether such T-cell-mediated cytotoxic mechanisms are specific for ACD or may also contribute to the development of irritant contact reactions.
A further notable finding of this study was the detection of perforin and granzyme B immunoreactivity in both CD4+ and CD8+ T cells. In recent years increasing evidence indicates that CD8+ as well as CD4+ T cells are able to mediate cytotoxic reactions. 9,15-19 The data of this study confirm the presence of CD4+ CTLs in humans and provide further evidence for a role of these cells in inducing cutaneous inflammation in vivo. Although some previous reports have suggested that cytotoxic CD8+ T cells are the main effector cells in CHS, 2,20 very recent data from knockout mice deficient for CD4+ or CD8+ T cells indicate that both T cell subsets are important as effector cells in eliciting cutaneous inflammation. 21 Thus, it is conceivable that in ACD, activated keratinocytes present haptens to both CD8+ as well as CD4+ CTLs, which subsequently elicit tissue damage through perforin and granzyme B exocytosis.
It is well established that CTLs may also mediate cytotoxicity via molecules like Fas/Fas ligand. Interestingly, however, recent evidence indicates that the main pathway of cytotoxicity mediated by alloantigen-specific CD4+ and CD8+ CTLs in human is granule exocytosis, not the Fas/Fas ligand system. 22 These findings are in accordance with our observations showing that drug-specific T cell clones mainly kill keratinocytes in a perforin-dependent manner. 16 Nevertheless, it is noteworthy that Fas/Fas ligand-induced reactions have also been reported recently to be important in ACD. 23 This report, together with our present results, indicates that CTL-induced cytotoxicity against keratinocytes may not be limited to one pathway and may involve complex mechanisms. These might also include other molecules like tumor necrosis factor receptor type 1 (TNF-R1), TNF-related apoptosis-inducing ligand (TRAIL R1/R2), TNF-receptor-related apoptosis-mediated protein (TRAMP), and DR6. The contribution of these molecules in ACD is still unknown and remain to be determined in future studies.
In conclusion, our data provide evidence that CTLs containing perforin and granzyme B participate in inducing cutaneous inflammation and suggest that T-cell-mediated cytotoxic reactions particularly play an important part in the pathogenesis of ACD in humans.
Acknowledgments
We thank J. Mirkovitch for excellent technical help.
Footnotes
Address reprint requests to N. Yawalkar, Harvard Skin Disease Research Center, 77 Louis Pasteur Avenue, Room 671, Boston, MA 02115. E-mail: nyawalker@RICS.bwh.harvard.edu.
Supported by the Swiss National Science Foundation grant 32–48885.96, SCORE B (to N. Y.).
References
- 1.Krasteva M, Kehren J, Ducluzeau MT, Sayag M, Cacciapuoti M, Akiba H, Descotes J, Nicolas JF: Contact dermatitis. I. Pathophysiology of contact sensitivity. Eur J Dermatol 1999, 9:65-77 [PubMed] [Google Scholar]
- 2.Grabbe S, Schwarz T: Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today 1998, 19:37-44 [DOI] [PubMed] [Google Scholar]
- 3.Cavani A, Mei D, Guerra E, Corinti S, Giani M, Pirrotta L, Puddu P, Girolomoni G: Patients with allergic contact dermatitis to nickel and nonallergic individuals display different nickel-specific T cell responses: evidence for the presence of effector CD8+ and regulatory CD4+ T cells. J Invest Dermatol 1998, 111:621-628 [DOI] [PubMed] [Google Scholar]
- 4.Moulon C, Wild D, Dormoy A, Weltzien HU: MHC-dependent and -independent activation of human nickel-specific CD8+ cytotoxic T cells from allergic donors. J Invest Dermatol 1998, 111:360-366 [DOI] [PubMed] [Google Scholar]
- 5.Bouloc A, Cavani A, Katz SI: Contact hypersensitivity in MHC class II-deficient mice depends on CD8 T lymphocytes primed by immunostimulating Langerhans cells. J Invest Dermatol 1998, 111:44-49 [DOI] [PubMed] [Google Scholar]
- 6.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF: Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol 1995, 25:3006-3010 [DOI] [PubMed] [Google Scholar]
- 7.Kehren J, Desvignes C, Krasteva M, Ducluzeau MT, Assossou O, Horand F, Hahne M, Kagi D, Kaiserlian D, Nicolas JF: Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med 1999, 189:779-786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller C, Gershenfeld HK, Lobe CG, Okada CY, Bleackley RC, Weissman IL: A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J Exp Med 1988, 167:1124-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR, Pichler WJ: Infiltration of cytotoxic T cells in drug-induced cutaneous eruptions. Clin Exp Allergy 2000, 30:847-855 [DOI] [PubMed] [Google Scholar]
- 10.Griffiths GM: The cell biology of CTL killing. Curr Opin Immunol 1995, :343-348 [DOI] [PubMed] [Google Scholar]
- 11.Liu CC, Walsh CM, Young JD: Perforin: structure and function. Immunol Today 1995, 16:194-201 [DOI] [PubMed] [Google Scholar]
- 12.Masson D, Tschopp JA: family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987, 49:679-685 [DOI] [PubMed] [Google Scholar]
- 13.Squier MK, Cohen JJ: Cell-mediated cytotoxic mechanisms. Curr Opin Immunol 1994, 6:447-452 [DOI] [PubMed] [Google Scholar]
- 14.Trapani JA: Target cell apoptosis induced by cytotoxic T cells and natural killer cells involves synergy between the pore-forming protein, perforin, and the serine protease, granzyme B. Aust N Z J Med 1995, 25:793-799 [DOI] [PubMed] [Google Scholar]
- 15.Pichler WJ, Yawalkar N: Pathophysiology of drug-elicited exanthema. ACI Int 2000, 12:166-170 [Google Scholar]
- 16.Schnyder B, Frutig K, Yawalkar N, Limat A, Pichler WJ: T cell cytotoxicity in sulfamethoxazole induced skin reaction. Clin Exp Allergy 1998, 28:1412-1417 [DOI] [PubMed] [Google Scholar]
- 17.Liu CC, Rafii S, Granelli-Piperno A, Trapani JA, Young JD: Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med 1989, 170:2105-2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Susskind B, Shornick MD, Iannotti MR, Duffy B, Mehrotra PT, Siegel JP, Mohanakumar T: Cytolytic effector mechanisms of human CD4+ cytotoxic T lymphocytes. Hum Immunol 1996, 45:64-75 [DOI] [PubMed] [Google Scholar]
- 19.Vergelli M, Hemmer B, Muraro PA, Tranquill L, Biddison WE, Sarin A, McFarland HF, Martin R: Human autoreactive CD4+ T cell clones use perforin- or Fas/Fas ligand-mediated pathways for target cell lysis. J Immunol 1997, 158:2756-2761 [PubMed] [Google Scholar]
- 20.Xu H, Banerjee A, Dilulio NA, Fairchild RL: T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med 1996, 183:1001-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B, Fujisawa, Zhuang L, Freed I, Howell BG, Shahid S, Shivji GM, Mak TW, Sauder DN: CD4(+) Th1 and CD8(+) type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol 2000, 165:6783-6790 [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, Fujita S: Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood 2000, 95:2352-2355 [PubMed] [Google Scholar]
- 23.Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, Graeve T, Noll M, Bröcker EB, Blaser K, Akdis CA: T cell mediated FAS-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 2000, 106:25-35 [DOI] [PMC free article] [PubMed] [Google Scholar]