Abstract
Juvenile nasopharyngeal angiofibromas (JNAs) are locally aggressive vascular tumors occurring predominantly in adolescent males. The pathogenesis of JNAs is unknown. Recently, JNAs have been reported to occur at increased frequency among patients with familial adenomatous polyposis, suggesting that alterations of the adenomatous polyposis coli (APC)/β-catenin pathway might also be involved in the pathogenesis of sporadic JNAs. We analyzed somatic β-catenin and APC gene mutations in 16 sporadic JNAs from nonfamilial adenomatous polyposis patients using immunohistochemistry for β-catenin, and direct DNA sequencing for exon 3 of the β-catenin gene and the mutation cluster region of the APC gene. Nuclear accumulation of β-catenin was diffusely present in the stromal cells but not in the endothelial cells of all 16 JNAs. Activating β-catenin gene mutations were present in 75% (12 of 16) of JNAs. Six JNA patients also had recurrent tumors after surgery, and in all cases the β-catenin gene status of the recurrent JNA was identical to the initial tumor. No mutations in the mutation cluster region of the APC gene were detected in the four JNAs without β-catenin mutations. The high frequency of β-catenin mutations in sporadic JNAs and the presence of identical β-catenin gene mutations in recurrent tumors indicates that activating β-catenin gene mutations are important in the pathogenesis of JNAs. The immunohistochemical localization of β-catenin only to the nuclei of stromal cells further suggests that the stromal cells, rather than endothelial cells, are the neoplastic cells of JNAs.
Juvenile nasopharyngeal angiofibroma (JNA) is an uncommon vascular tumor occurring almost exclusively in adolescent males. JNAs constitute ∼0.5% of head and neck tumors. 1 Although histologically benign, they have been reported to recur after surgical therapy in 21 to 34% of patients and can be locally aggressive. 1,2 JNAs typically arise from the posterolateral wall of the nasal cavity and grow by erosion of bone and displacement of adjacent structures; eventually these lesions can involve the nasopharynx, paranasal sinuses, orbit, and skull base with intracranial extension. 1 Histopathologically, JNAs are characterized by proliferating, irregular vascular channels within a fibrous stroma. The stromal compartment consists of plump cells that can be spindled or stellate in shape and give rise to varying amounts of collagen fibers. 3
The pathogenesis of JNAs is unknown. However, a causal association between JNAs and familial adenomatous polyposis (FAP) has recently been suggested, with JNAs occurring 25 times more frequently in patients with FAP than in an age-matched population. 4,5 FAP is an autosomal-dominant condition characterized by upper and lower gastrointestinal tract adenomas, a high tendency for the development of colorectal and periampullary adenocarcinomas, and by various extraintestinal manifestations including benign epidermal cysts, osteomas, desmoid tumors, and malignant tumors of the thyroid, liver, biliary tree, and brain. 6-11
FAP results from germline mutations in the adenomatous polyposis coli (APC) gene on chromosome 5q. The APC gene product regulates the level of β-catenin protein, which acts both as a submembranous component in cadherin-mediated cell-cell adhesion and as a downstream transcriptional activator in the Wnt signaling pathway. 12,13 APC tumor suppressor protein, along with glycogen synthase kinase-3β (GSK-3β), promotes phosphorylation of serine/threonine residues encoded in exon 3 of the β-catenin gene. 12,14,15 Phosphorylation is then followed by ubiquitin-mediated degradation of β-catenin protein. 16,17 Loss of β-catenin regulatory activity resulting in accumulation of β-catenin protein can occur either by truncating APC gene mutations or by stabilizing β-catenin gene mutations at GSK-3β phosphorylation sites. 15,18,19 A majority of colorectal adenomas and carcinomas can be shown to contain either bi-allelic inactivation of the APC gene or activating β-catenin gene mutations. 20,21
The increased incidence of JNA in patients with FAP raised the possibility that either APC or β-catenin gene mutations affecting the APC/β-catenin pathway might also be involved in the pathogenesis of sporadic JNAs. We therefore analyzed a series of JNAs from patients without FAP for mutations in both the APC and β-catenin genes.
Materials and Methods
Case Selection
The study population consisted of 16 primary JNAs and six recurrent JNAs from 16 patients who underwent surgical resection at The Johns Hopkins Hospital between 1984 and 1999. Tumor recurrences were resected from 4 months to 5 years after the original surgery (mean, 24 months). All patients were male and ranged in age from 10 to 24 years (mean, 14 years) at the time of initial surgery. None of the patients was known to have FAP and none was enrolled in The Johns Hopkins Polyposis Registry.
Immunohistochemistry for β-Catenin
Immunoperoxidase stain using diaminobenzidine as the chromogen was performed on the Techmate 1000 automatic staining system (BioTek Solutions, Tucson, AZ). Deparaffinized sections of formalin-fixed tissue were stained with β-catenin antibody (mouse monoclonal antibody; Becton Dickinson Transduction Laboratories, Lexington, KY) at 1:500 dilution after heat-induced antigen retrieval. 22
DNA Extraction
Microdissection of JNAs from hematoxylin and eosin-stained slides for DNA extraction was performed from formalin-fixed, paraffin-embedded specimens. Genomic DNA was extracted as described previously. 23 Corresponding normal control DNA was extracted from nonneoplastic nasopharyngeal mucosa in 15 of 16 patients (normal tissue was not available in the remaining case).
Mutation Analysis of the β-Catenin Gene
Genomic DNA from each sample was amplified by polymerase chain reaction (PCR) using the primer pair: 5′-ATGGAACCAGACAGAGGGGC-3′ and 5′-GCTACTTGTTCTGAGTGAAG-3′. These amplified a 200-bp fragment of exon 3 of the β-catenin gene that encompasses the region for GSK-3β phosphorylation. PCR reaction was performed under standard conditions in a 50-μl volume using PCR Master [containing 1.25 U of Taq polymerase in Brij 35, 0.005% (v/v), dATP, dCTP, dGTP, and dTTP at 0.2 mmol/L, 10 mmol/L Tris-HCl, 50 mmol/L KCl, and 1.5 mmol/L MgCl2 (Boehringer Mannheim, Mannheim, Germany)] and 1 μmol/L of both 5′ and 3′ oligonucleotides with 40 cycles (94°C for 1 minute, 58°C for 1 minute, and 72°C for 2 minutes). PCR products were treated with shrimp alkaline phosphatase and exonuclease I (Amersham, Buckinghamshire, UK) before sequencing. Treated PCR products were sequenced directly with SequiTherm EXCEL II DNA sequencing kit (Epicentre, Madison, WI) using the internal primers: 5′-AAAGCGGCTGTTAGTCACTFF-3′ and 5′-GACTTGGGAGGTATCCACATCC-3′. Oligonucleotides were end-labeled with (γ-32P)-ATP (New England Nuclear-DuPont, Boston, MA) using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). All mutations were verified in both sense and antisense directions. Base substitutions in codons 32, 33, and the second position of codon 34 were further confirmed by HinfI restriction endonuclease (Promega, Madison, WI) assay. The 200-bp PCR product for β-catenin contains two HinfI restriction endonuclease sites, which yield DNA fragments of 7 bp, 55 bp, and 138 bp after digestion of the wild-type allele. β-catenin mutations in codons 32 and 33 yield only 62-bp and 138-bp fragments after digestion because of ablation of the first HinfI site. Mutations in the second position of codon 34 yield 55-bp and 145-bp fragments because of ablation of the other HinfI site.
Mutation Analysis of the APC Gene
Mutation analysis of the APC gene was performed only on cases that did not show detectable β-catenin mutations. Four sets of oligonucleotide primers (A1: 5′-CAGACTTATTGTGTAGAAGA-3′ and A2: 5′-CTCCTGAAGAAAATTCAACA-3′ for codons 1260 to 1359; B1: 5′-AGGGTTCTAGTTTATCTTCA-3′ and B2: 5′-TCTGCTTGGTGGCATGGTTT-3′ for codons 1339 to 1436; C1: 5′-GGCATTATAAGCCCCAGTGA-3′ and C2: 5′-AAATGGCTCATCGAGGCTCA-3′ for codons 1417 to 1516; D1: 5′-ACTCCAGATGGATTTTCTTG-3′ and D2: 5′-GGCTGGCTTTTTTGCTTTAC-3′ for codons 1497 to 1596) were used to amplify the mutation cluster region of the APC gene. 24 PCR reaction was performed under standard conditions in a 50-μl volume using PCR Master (Boehringer Mannheim) and 1 μmol/L of both 5′ and 3′ oligonucleotides with 40 cycles (94°C for 1 minute, 55°C for 1 minute, and 68°C for 2 minutes for APC-B, -C, and -D primer pairs and 94°C for 1 minute, 52°C for 1 minute, and 68°C for 2 minutes for APC-A). PCR products were purified using shrimp alkaline phosphatase and exonuclease I. Purified PCR products were sequenced directly with SequiTherm EXCEL II DNA sequencing kit using the same primers as for DNA amplification.
Results
Nuclear Accumulation of β-Catenin in JNAs
All 16 primary JNAs and the six recurrent tumors showed strong, diffuse nuclear staining for β-catenin (Figure 1) ▶ . In all cases the nuclear staining was present only within stromal cell nuclei. Endothelial cell nuclei and the nuclei of vascular smooth muscle cells showed faint cytoplasmic staining but no nuclear β-catenin accumulation. Immunohistochemical staining of normal nasopharyngeal tissue was also evaluated in a total of 14 cases that included this tissue on the slides stained for evaluation of the JNAs (comprising 10 cases with surface epithelium, eight cases with nonlesional fibrovascular stroma, seven cases with subepithelial lamina propria, and one case with cartilage). Strong membranous staining and light cytoplasmic staining for β-catenin was present within surface epithelium and seromucinous glands of the lamina propria in all cases that contained these elements. However, no nuclear accumulation of β-catenin was present in any nontumorous tissues.
Figure 1.

Histopathological appearance and immunohistochemical staining for β-catenin in JNAs. A: Tumors are comprised of spindled to stellate-shaped stromal cells and numerous vascular channels. B: Nuclear accumulation of β-catenin is diffusely present in stromal cells but not in endothelial cells or vascular smooth muscle cells, which show only faint cytoplasmic staining.
Somatic β-Catenin Gene Mutations in JNAs
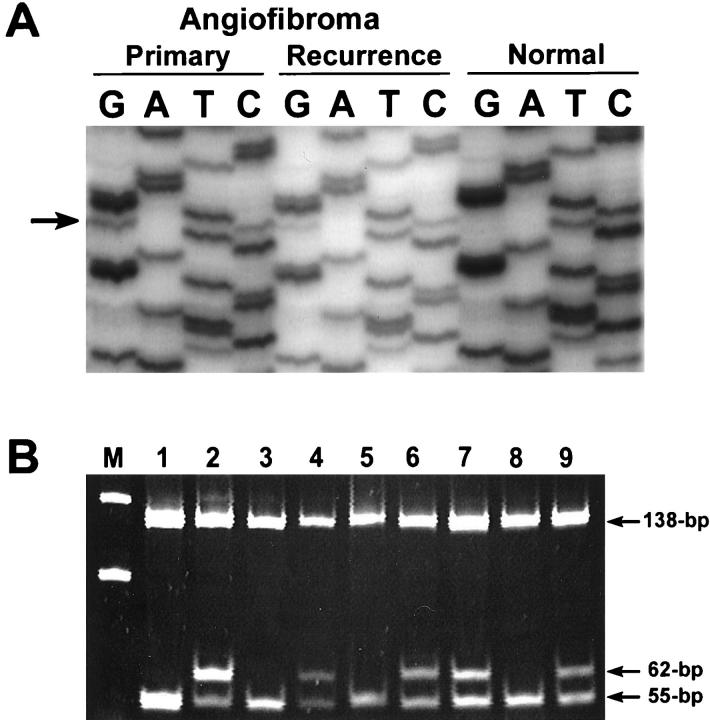
Mutations in exon 3 of the β-catenin gene were detected in 12 (75%) of 16 JNAs. The somatic nature of the mutations was confirmed by finding no β-catenin gene mutations in the corresponding normal nasopharyngeal tissue from these patients (available in 15 patients). In nine JNAs, mutations were 1-bp missense mutations, predominantly in one of the serine/threonine residues at GSK-3β phosphorylation sites: codon 33 (five cases), codon 34 (two cases), codon 35 (one case), and codon 37 (one case) (Figure 2) ▶ . Three additional JNAs contained deletions in exon 3 of β-catenin (an 18-bp deletion spanning codons 33 to 39, a 36-bp deletion spanning codons 28 to 40, and a 27-bp deletion spanning codons 39 to 48) (Figure 3) ▶ . In all tumors with β-catenin gene mutations, a mixture of wild-type and mutant bands was present, as would be expected because of the dominant-positive effect of β-catenin gene alterations. All five tumors with codon 33 mutations that could be evaluated with HinfI restriction endonuclease digestion showed the expected ablation of the HinfI recognition site.
Figure 2.
β-catenin gene mutations in JNAs. A: DNA sequencing autoradiograph of a TCT (serine)→TGT (cysteine) mutation (arrow) at codon 33, present in both the primary and recurrent JNA from patient 3. No β-catenin mutation is present in the corresponding normal nasopharyngeal mucosa from the same patient. B:HinfI restriction endonuclease assay to verify the presence of a point mutation in this case and in other representative cases with codon 33 mutations. DNA samples are from JNAs from patient 3 (lane 1, normal; lane 2, tumor), patient 6 (lane 3, normal; lane 4, tumor), patient 7 (lane 5, normal; lanes 6 and 7, primary and recurrent tumors), and patient 15 (lane 8, normal; lane 9, tumor). A molecular weight marker of 50-bp ladder is in lane M. The normal 200-bp PCR product for β-catenin contains two HinfI restriction endonuclease sites, yielding 7-bp, 55-bp, and 138-bp DNA fragments after digestion of the wild-type allele (the 7-bp fragment is too small to be visualized on the gel). β-catenin mutations in codon 33 yield 62-bp and 138-bp fragments after digestion because of ablation of the first HinfI site.
Figure 3.
Deletion mutations in exon 3 of β-catenin in JNAs. A: Three JNAs with deletion mutations show β-catenin PCR products that are 164 bp, 182 bp, and 173 bp in addition to the normal 200-bp product (arrow). Patient 2 (lane 1, normal; lanes 2 and 3, primary and recurrent tumors); patient 8 (lane 4, normal; lane 5, tumor); and patient 11 (lane 6, normal; lane 7, tumor). Lane M contains a molecular weight marker of 50-bp ladder. B: DNA sequencing autoradiograph from patient 2, showing a 36-bp deletion beginning at codon 28 (arrow) in the primary and recurrent JNAs, but not in the normal nasopharyngeal mucosa from the same patient. Sequencing is shown in the 3′ to 5′ direction because of use of the reverse primer for sequencing.
Somatic APC Gene Mutations in JNAs
The four JNAs that did not show β-catenin gene mutations were further evaluated for APC mutations by sequencing of the APC mutation cluster region. However, no APC gene mutations were detected.
β-Catenin/APC Genes Mutations in Recurrent JNAs
Analysis of the six recurrent JNAs demonstrated identical genetic status between the primary tumor and recurrence. In five cases, identical β-catenin gene mutations were present in both primary and recurrent tumor, and in the remaining case no alteration in either β-catenin or APC was detected in the primary or recurrent tumor. A summary of genetic alterations in primary and recurrent JNAs is given in Table 1 ▶ .
Table 1.
β-Catenin Gene Mutations in JNAs
| Patient | Age (years) | JNA type | β-Catenin mutation | Nuclear accumulation of β-catenin protein |
|---|---|---|---|---|
| 1 | 15 | Primary | Codon 33, TCT → GCT | + |
| 2 | 24 | Primary | Codons 28–40, 36-bp deletion | + |
| 29 | Recurrent | Codons 28–40, 36-bp deletion | + | |
| 3 | 14 | Primary | Codon 33, TCT → TGT | + |
| 14 | Recurrent | Codon 33, TCT → TGT | + | |
| 4 | 12 | Primary | − | + |
| 15 | Recurrent | − | + | |
| 5 | 19 | Primary | Codon 35, ATC → AGC | + |
| 20 | Recurrent | Codon 35, ATC → AGC | + | |
| 6 | 12 | Primary | Codon 33, TCT → TGT | + |
| 14 | Recurrent | Codon 33, TCT → TGT | + | |
| 7 | 12 | Primary | Codon 33, TCT → TGT | + |
| 13 | Recurrent | Codon 33, TCT → TGT | + | |
| 8 | 12 | Primary | Codons 33–39, 18-bp deletion | + |
| 9 | 14 | Primary | − | + |
| 10 | 15 | Primary | Codon 34, GGA → CGA | + |
| 11 | 10 | Primary | Codons 39–48, 27-bp deletion | + |
| 12 | 12 | Primary | Codon 37, TCT → TTT | + |
| 13 | 10 | Primary | − | + |
| 14 | 16 | Primary | − | + |
| 15 | 13 | Primary | Codon 33, TCT → TGT | + |
| 16 | 14 | Primary | Codon 34, GGA → CGA | + |
Discussion
The occurrence of nasopharyngeal angiofibromas in the nasal region of pubescent males, their histological similarity to erectile tissue, and their expression of multiple sex hormone receptors have remained tantalizing observations with possible implications about the origin of these neoplasms. 25-27 However, little information concerning the molecular pathogenesis and specific neoplastic cell of origin of JNAs has previously been elucidated. We hypothesized that the increased frequency of JNAs among patients with FAP and germline mutations in the APC gene suggests that these tumors might arise through alterations of the APC/β-catenin pathway.
We identified mutations in exon 3 of the β-catenin gene in 75% (12 of 16) sporadic JNAs from non-FAP patients. These mutations were predominantly 1-bp missense mutations in codons 33 and 37 (six cases total) leading to loss of serine/threonine sites for GSK-3β phosphorylation. Other exon 3 mutations in codons 34 and 35 (three cases total) did not involve loss of a phosphorylation site but may still interfere with degradation of the β-catenin gene product. 21 Three cases contained deletion mutations ranging from 18 bp to 36 bp and would be expected to interfere with β-catenin protein degradation. All six recurrent JNAs in our series showed β-catenin gene status that was identical to that of the corresponding primary tumors (β-catenin gene mutations in five cases and no β-catenin or APC mutation in the remaining case).
This high frequency of β-catenin gene mutations in sporadic JNAs, coupled with the increased frequency of JNAs among patients with FAP, suggests that these lesions arise through alterations of the APC/β-catenin pathway. The presence of identical β-catenin gene mutations in the primary and recurrent tumors further supports that these genetic alterations are important and are maintained through the temporal growth of the tumors.
Somatic alterations of the APC/β-catenin pathway have now been demonstrated in the sporadic counterparts to most tumors that occur frequently in FAP patients, including gastrointestinal adenomas, 20,21,28,29 desmoid tumors, 30-32 medulloblastomas, 33 childhood hepatoblastomas, 34-35 and gastric fundic gland polyps. 36 β-catenin mutations have also been reported in a variety of other tumors such as anaplastic thyroid carcinoma, 37 prostatic carcinoma, 38 endometrial carcinoma, 39-41 pilomatricoma, 42 Wilms’ tumor, 43 and hepatocellular carcinoma. 44-46 Among patients with FAP, intestinal and extra-intestinal neoplasms typically arise through bi-allelic (germline then somatic) inactivation of the APC gene, whereas the corresponding tumors in non-FAP patients occur either through somatic bi-allelic APC inactivation or somatic mutation of a single β-catenin allele. In contrast to the high frequency of β-catenin gene mutations detected in JNAs in this study, APC mutations in the mutation cluster region were not found. However, we did not sequence the entire APC gene in this study and we did not analyze tumors from patients with FAP, and therefore it remains possible that a specific subset of JNAs would contain bi-allelic inactivation of the APC gene.
The demonstration of nuclear β-catenin accumulation in all JNAs but β-catenin mutations in only 75% of the JNAs is interesting. Nuclear β-catenin accumulation can result from mutations in APC, β-catenin, or AXIN1 (axis inhibitor 1) genes. The AXIN1 gene encodes a key factor for the WNT signaling pathway, and interacts with APC, β-catenin, and GSK3β, playing an important role in the degradation of β-catenin. 47 Somatic mutations of AXIN1 have been reported in three of six hepatocellular carcinoma (HCC) cell lines and five of 87 primary HCCs. 48 In this study, we did not analyze for mutations in the AXIN1 gene. However, the role of AXIN1 in the pathogenesis of JNAs remains to be clarified.
All JNAs in our series showed strong immunohistochemical accumulation of β-catenin protein within the nuclei of stromal cells, whereas endothelial cells and vascular smooth muscle cells showed only faint cytoplasmic staining without nuclear β-catenin accumulation. JNAs are well known to be characterized histologically by proliferations of both vascular channels and spindled to stellate-shaped stromal cells that show ultrastructural features of fibroblasts, 3 although the specific neoplastic cell has been debated. Some investigators have regarded JNAs as predominantly vasoproliferative malformations because of their tendency to bleed, their striking vascularity, and the presence of angiogenic growth factors within lesional endothelial cells. 3,49 Schiff and colleagues 49 demonstrated intense immunohistochemical staining for the angiogenic protein basic fibroblast growth factor or FGF within all endothelial cells of JNAs, whereas only some stromal cells were stained. In contrast, Hwang and colleagues 27 found androgen receptor positivity by immunohistochemistry in both the endothelial cells and stromal cells of the majority of JNAs studied, although staining was typically more intense in the stromal cells. Our results indicate that JNAs are true neoplasms containing clonal alterations in the β-catenin oncogene, and suggest that the stromal component is the key neoplastic element of JNAs.
Footnotes
Address reprint requests to Tsung-Teh Wu, M.D., Ph.D., Division of Gastrointestinal/Liver Pathology, Department of Pathology, Ross Building, Room 632, The Johns Hopkins University School of Medicine, 720 Rutland Ave., Baltimore, MD 21205-2196. E-mail: ttwu@welch.jhu.edu.
References
- 1.Tewfik TL, Tan AKW, Al Noury K, Chowdhury K, Tampieri D, Raymond J, Vuong T: Juvenile nasopharyngeal angiofibroma. J Otolaryngol 1999, 28:145-151 [PubMed] [Google Scholar]
- 2.McCombe A, Lund VJ, Howard DJ: Recurrence in juvenile angiofibroma. Rhinology 1990, 28:97-102 [PubMed] [Google Scholar]
- 3.Beham A, Kainz J, Stammberger H, Aubock L, Beham-Schmid C: Immunohistochemical and electron microscopical characterization of stromal cells in nasopharyngeal angiofibromas. Eur Arch Otorhinolaryngol 1997, 254:196-199 [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Hamilton SR, Krush AJ, Offerhaus JA, Booker SV, Petersen GM: Nasopharyngeal angiofibroma in patients with familial adenomatous polyposis. Gastroenterology 1993, 105:1550-1552 [DOI] [PubMed] [Google Scholar]
- 5.Ferouz AS, Mohr RM, Paul P: Juvenile nasopharyngeal angiofibroma and familial adenomatous polyposis: an association? Otolaryngol Head Neck Surg 1995, 113:435-439 [DOI] [PubMed] [Google Scholar]
- 6.Gardner EJ: Follow-up study of a family group exhibiting dominant inheritance for a syndrome including intestinal polyps, osteomas, fibromas, and epidermal cysts. Am J Hum Genet 1962, 14:376-390 [PMC free article] [PubMed] [Google Scholar]
- 7.Klemmer S, Pascoe L, DeCosse J: Occurrence of desmoids in patients with familial adenomatous polyposis of the colon. Am J Med Genet 1987, 28:385-392 [DOI] [PubMed] [Google Scholar]
- 8.Krush AJ, Traboulsi EI, Offerhaus GJA, Maumenee IH, Yardley JH, Levin LS: Hepatoblastoma, pigmented ocular fundus lesions and jaw lesions in Gardner syndrome. Am J Med Genet 1988, 29:323-332 [DOI] [PubMed] [Google Scholar]
- 9.Bell B, Mazzaferri EL: Familial adenomatous polyposis (Gardner’s syndrome) and thyroid carcinoma: a case report and review of the literature. Dig Dis Sci 1993, 38:185-190 [DOI] [PubMed] [Google Scholar]
- 10.Walsh N, Qizilbash A, Banerjee R, Waugh GA: Biliary neoplasia in Gardner’s syndrome. Arch Path Lab Med 1987, 111:76-77 [PubMed] [Google Scholar]
- 11.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, Krush AJ, Berk T, Cohen Z, Tetu B, Berger PC, Wood PA, Taqi F, Brooker SV, Petersen GM, Offerhaus GJA, Tersmette AC, Giardiello FM, Vogelstein B, Kinzler KW: The molecular basis of Turcot’s syndrome. N Engl J Med 1995, 332:839-847 [DOI] [PubMed] [Google Scholar]
- 12.Barth AI, Nathke IS, Nelson WJ: Cadherins, catenins, and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol 1997, 9:683-690 [DOI] [PubMed] [Google Scholar]
- 13.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W: Functional interaction of β-catenin with the transcriptional factor LEF-1. Nature 1996, 382:638-642 [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P: Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 1996, 272:1023-1026 [DOI] [PubMed] [Google Scholar]
- 15.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P: Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA 1995, 92:3046-3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R: β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997, 16:3797-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW: Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J Biol Chem 1997, 272:24735-24738 [DOI] [PubMed] [Google Scholar]
- 18.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancers by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 19.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler W, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon cancer. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 20.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 21.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW: Mutational analysis of the APC/β-catenin/Tcf pathway in colorectal cancer. Cancer Res 1998, 58:1130-1134 [PubMed] [Google Scholar]
- 22.Bankfalvi A, Navabi H, Bier B, Bocker W, Jasani B, Schmid KW: Wet autoclave pretreatment for antigen retrieval in diagnostic immunohistochemistry. J Pathol 1994, 174:223-228 [DOI] [PubMed] [Google Scholar]
- 23.Moskaluk CA, Kern SE: Microdissection and polymerase chain reaction amplification of genomic DNA from histological tissue sections. Am J Pathol 1997, 150:1547-1552 [PMC free article] [PubMed] [Google Scholar]
- 24.Yashima K, Nakamori S, Murakami Y, Yamaguchi A, Hayashi K, Ishikawa O, Konishi Y, Sekiya T: Mutations of the adenomatous polyposis coli gene in the mutation cluster region: comparison of human pancreatic and colorectal cancers. Int J Cancer 1994, 59:43-47 [DOI] [PubMed] [Google Scholar]
- 25.Farag MM, Ghanimah SE, Ragaie A, Saleem TH: Hormonal receptors in juvenile nasopharyngeal angiofibroma. Laryngoscope 1987, 97:208-211 [DOI] [PubMed] [Google Scholar]
- 26.Brentani MM, Butugan O, Oshima CTF, Torloni H, Paiva LJ: Multiple steroid receptors in nasopharyngeal angiofibromas. Laryngoscope 1989, 99:398-401 [DOI] [PubMed] [Google Scholar]
- 27.Hwang HC, Mills SE, Patterson K, Gown AM: Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Mod Pathol 1998, 11:1122-1126 [PubMed] [Google Scholar]
- 28.Iwao K, Nakamori S, Kameyama M, Imaoka S, Kinoshita M, Fukui T, Ishiguro S, Nakamura Y, Miyoshi Y: Activation of the β-catenin gene by interstitial deletions involving exon 3 in primary colorectal carcinomas without adenomatous polyposis coli mutations. Cancer Res 1998, 58:1021-1026 [PubMed] [Google Scholar]
- 29.Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML: β-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res 1999, 59:1442-1444 [PubMed] [Google Scholar]
- 30.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ: Increased β-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997, 151:329-334 [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y: Frequent mutations in the β-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res 1998, 10:591-594 [PubMed] [Google Scholar]
- 32.Tejpar S, Nollet F, Li C, Wunder JS, Michilis G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman JJ, Alman BA: Predominance of β-catenin mutations and β-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999, 11:6615-6620 [DOI] [PubMed] [Google Scholar]
- 33.Zurawel RH, Chiappa SA, Allen C, Raffel C: Sporadic medulloblastomas contain oncogenic β-catenin mutations. Cancer Res 1998, 58:896-899 [PubMed] [Google Scholar]
- 34.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T: Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the β-catenin gene. Cancer Res 1999, 59:269-273 [PubMed] [Google Scholar]
- 35.Oda H, Imai Y, Makatsuru Y, Hata J, Ishikawa T: Somatic mutations of the APC gene in sporadic hepatoblastomas. Cancer Res 1996, 56:3320-3323 [PubMed] [Google Scholar]
- 36.Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT: Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 2000, 157:747-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL: Frequent mutation and nuclear localization of β-catenin in anaplastic thyroid carcinoma. Cancer Res 1999, 59:1811-1815 [PubMed] [Google Scholar]
- 38.Voeller HJ, Truica C, Gelmann EP: β-catenin mutations in human prostate cancer. Cancer Res 1998, 58:2520-2523 [PubMed] [Google Scholar]
- 39.Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S: β-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 1998, 58:3526-3528 [PubMed] [Google Scholar]
- 40.Palacios J, Gamallo C: Mutations in the β-catenin gene in endometrioid ovarian carcinomas. Cancer Res 1998, 58:1344-1347 [PubMed] [Google Scholar]
- 41.Mirabelli-Primdahl L, Gryfe R, Kim H, Millar A, Luceri C, Dale D, Holowaty E, Bapat B, Gallinger S, Redston M: β-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res 1999, 59:3346-3351 [PubMed] [Google Scholar]
- 42.Chan EF, Gat U, McNiff JM, Fuchs E: A common human skin tumour is caused by activating mutations in β-catenin (letter). Nat Genet 1999, 21:410-413 [DOI] [PubMed] [Google Scholar]
- 43.Koesters R, Ridder R, Kopp-Schneider A, Betts D, Adams V, Niggli F, Briner J, von Knebel Doeberitz M: Mutational activation of the β-catenin proto-oncogene is a common event in the development of Wilms’ tumors. Cancer Res 1999, 59:3880-3882 [PubMed] [Google Scholar]
- 44.Miyoshi Y, Iwao K, Nagasawa Y, Aihara T, Sasaki Y, Imaoka S, Murata M, Shimano T, Nakamura Y: Activation of the β-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 1998, 58:2424-2427 [PubMed] [Google Scholar]
- 45.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H: β-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 1999, 155:1795-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hus HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY: β-catenin mutations are associated with a subset of low-grade hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol 2000, 157:763-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W: Functional interaction of an axin homolog, conductin, with β-catenin, APC and GSK3β. Science 1998, 280:596-599 [DOI] [PubMed] [Google Scholar]
- 48.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y: AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 2000, 24:245-250 [DOI] [PubMed] [Google Scholar]
- 49.Schiff M, Gonzalez AM, Ong M, Baird A: Juvenile nasopharyngeal angiofibroma contain an angiogenic growth factor: basic FGF. Laryngoscope 1992, 102:940-945 [DOI] [PubMed] [Google Scholar]