Abstract
Carbonic anhydrases (CA) influence intra- and extracellular pH and ion transport in varied biological processes. We recently identified CA9 and CA12 as hypoxia-inducible genes. In this study we examined the expression of these tumor-associated CAs by immunohistochemistry in relation to necrosis and early breast tumor progression in 68 cases of ductal carcinoma in situ (DCIS) (39 pure DCIS and 29 DCIS associated with invasive carcinoma). CA IX expression was rare in normal epithelium and benign lesions, but was present focally in DCIS (50% of cases) and in associated invasive carcinomas (29%). In comparison, CA XII was frequently expressed in normal breast tissues (89%), in DCIS (84%), and in invasive breast lesions (71%). In DCIS, CA IX was associated with necrosis (P = 0.0053) and high grade (P = 0.012). In contrast, CA XII was associated with the absence of necrosis (P = 0.036) and low grade (P = 0.012). Despite this, augmented CA XII expression was occasionally observed adjacent to necrosis within high-grade lesions. Neither CA IX nor CA XII expression was associated with regional or overall proliferation as determined by MIB1 staining. Assessment of mammographic calcification showed that CA XII expression was associated with the absence of calcification (n = 43, P = 0.0083). Our results demonstrate that induction of CA IX and CA XII occurs in regions adjacent to necrosis in DCIS. Furthermore, these data suggest that proliferation status does not influence expression of either CA in breast tissues, that hypoxia may be a dominant factor in the regulation of CA IX, and that factors related to differentiation, as determined by tumor grade, dominate the regulation of CA XII. The existence of differential regulation and associations with an aggressive phenotype may be important in the development of selective inhibitors of CAs, because the latter have recently been shown to prevent tumor invasion.
The management of pre-invasive ductal carcinoma in situ (DCIS) of the breast has become an increasingly significant problem. This is due in part to both the increasing number of these lesions detected by mammography, 1,2 and the impetus provided by the demonstration that invasive breast cancer may be delayed or inhibited by tamoxifen therapy in women at high risk. 3,4 Assessment of DCIS and the risk of progression to invasive disease is complicated by the small size and focal nature of most breast lesions, and has traditionally been based primarily on morphological classification and grading of pre-invasive disease by the pattern of growth into comedo and noncomedo subtypes. Recently, radiological studies have suggested that abnormal patterns of calcification may be associated with high-grade DCIS, 5,6 whereas pathological studies have developed more reproducible and discriminating schema for classification of DCIS lesions. 7 Consequently, the presence of necrosis and nuclear grade 8,9 have emerged as important aspects to consider when assessing breast lesions, although consistent recognition and quantification of both parameters remains problematic. 10
Necrosis is believed to represent the extreme manifestation of hypoxia within tissues. 11 Interestingly, tumor hypoxia has been shown to be a prognostic indicator for many tumor types, being associated with aggressive growth, metastasis, and poor response to treatment not only in patients treated with radiotherapy, but also in those treated with surgery alone. 12-16 Of potential importance for understanding these effects is the role of hypoxia in regulating patterns of gene expression. Studies of gene expression have defined several classes of genes that are up-regulated by hypoxia and demonstrated that activation of the transcriptional complex hypoxia-inducible factor-1 is a key mediator of many of these effects. 17,18 Genes that are up-regulated by microenvironmental hypoxia through hypoxia-inducible factor-1 activation include glucose transporters, glycolytic enzymes, and angiogenic growth factors.
We recently identified the two tumor-associated transmembrane carbonic anhydrases (CA) CA9 19-21 and CA12 20,22 as being up-regulated by hypoxia in epithelial tumor cell lines. 23 Furthermore, we demonstrated focal perinecrotic expression of CA IX in invasive human tumors, co-localizing with vascular endothelial growth factor mRNA expression and pimonidazole activation. 23
CA9 and CA12 are members of a family of catalytically active CAs that share the capacity to catalyze the reversible hydration of carbon dioxide to carbonic acid. 24 CA IX 19,21,25 has been studied extensively in several tumor types including lung, kidney, colon, and cervix, where its expression has been established as a marker of cellular proliferation and early dysplasia. 26-30 CA XII was initially identified in renal carcinoma, 22 and subsequently shown to be associated with colon carcinoma where altered expression occurs in early stages of tumorigenesis. 31 However, the expression of these CAs in breast cancer has not been examined.
We investigated CA IX and CA XII expression in breast cancer in anticipation that their expression might serve as indicators of tissue hypoxia, altered pH, and tumor progression. Specifically we wished to assess the pattern of expression of these genes in DCIS, where the appearance of necrosis and abnormal calcification is associated with a high risk of progression to invasive disease.
Materials and Methods
Tissue Specimens
Sixty-eight pathological specimens containing DCIS of the breast were selected from review of surgical resections collected from 1997 to 1999 at the John Radcliffe Hospital and the Churchill Hospital, Oxford, UK. The cohort comprised 39 cases of pure DCIS (DCIS−) and 29 cases of DCIS associated with invasive carcinoma in the same biopsy (DCIS+), either independent from or directly associated with adjoining invasive carcinoma.
All DCIS lesions were classified into histological grades on the basis of the predominant grade present in the tissue section studied for gene expression according to the Van Nuys grading system. 9,32 The presence of intraductal necrosis within any component of DCIS within the tissue section was evaluated in hematoxylin and eosin-stained sections by light microscopy. The radiological appearance was classified according to the presence and pattern of calcification 5,6 in preoperative mammograms for a subset of cases in which films were available (n = 43). These classifications were performed by a single pathologist (PHW) and radiologist (RE), respectively, without reference to the cohort’s immunohistochemical data and outcome. Among the series of cases, the histological grades were as follows: 18 low grade (8 DCIS−, 10 DCIS+), 24 intermediate grade (15 DCIS−, 9 DCIS+), and 26 high grade (16 DCIS−, 10 DCIS+). Intraductal necrosis was present in 51 cases (75%), among which 29 were DCIS− and 22 DCIS+. Mammographic calcifications were present in 35 of 43 cases, among which 27 of 35 were DCIS− and eight of 35 DCIS+. The pattern of calcification was classified as linear type (14 cases) if the presence of any linear calcification was seen, nonlinear type (21 cases), or absent (eight cases).
Cell Lines and Immunoblotting
MDA-MB-231 and ZR-75.1 cell lines were from ATCC (Rockville, MD). Hypoxic conditions were generated in a Napco 7001 incubator (Precision Scientific, Winchester, VA) with 0.1% O2, 5% CO2, and balance N2 for 16 hours. Whole-cell protein extracts were prepared from tissue culture cells by 10 seconds of homogenization in denaturing conditions as described. 33 Whole-cell protein extracts were prepared from tumors by fine section of frozen tissue and 30 seconds of homogenization in denaturing conditions identical to tissue-culture extracts. For Western analysis, aliquots were separated under reducing conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes (Millipore, Hertfordshire, UK). CA IX was detected using the mouse monoclonal anti-human CA IX antibody M75 (1:50) as described. 34 CA XII was detected using a rabbit polyclonal anti-human CA XII antibody (1:2000) as described. 22 horseradish peroxidase-conjugated goat-anti-mouse and swine anti-rabbit immunoglobulins (DAKO, Cambridgeshire, UK) (1:2000), respectively, were applied for 1 hour at room temperature. ECL Plus (Amersham Pharmacia, Buckinghamshire, UK) was used for visualization.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tissue specimens collected by standard surgical oncology procedures were obtained from the Pathology Department, John Radcliffe Hospital, Oxford, UK. Immunostaining of paraffin sections was performed after dewaxing and rehydrating 5-μm sections. Staining for CA IX, CA XII, and MIB1 was performed on serial sections. Endogenous peroxidase was blocked with 0.5% hydrogen peroxide in water for 30 minutes. Ten percent normal human serum in Tris-buffered saline was applied for 15 minutes at room temperature to block nonspecific protein binding. Primary antibodies: anti-human CA IX murine monoclonal antibody M75 (1:50); 35 anti-human CA XII rabbit polyclonal antibody (1:2000); anti-human Ki67 murine monoclonal antibody MIB1 (1:50) (Immunotech). Primary antibodies were incubated for 30 minutes at room temperature in Tris-buffered saline with 5% normal human serum, followed by a 30-minute incubation with a peroxidase-conjugated secondary antibody. After each incubation, slides were washed twice with Tris-buffered saline for 5 minutes. Visualization of staining was by diaminobenzidine substrate for 8 minutes. Slides were counterstained with hematoxylin before mounting in Aquamount (BDH). Substitution of primary antibody with PBS was used as a negative control for each antibody. All staining was performed on an automated IHC stainer (MiniPrep 75; Tecan) at room temperature.
Assessment of CA IX, CA XII, and MIB1 Staining
Immunostaining for CA IX and CA XII was assessed by light microscopy and semiquantitative scoring was performed by a single pathologist (PHW), independently of the pathological assessment. Expression and intensity was scored (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining), together with the percentage of normal or neoplastic epithelial cells showing expression within the tissue section (0 to 100%). The product of the intensity and the percentage gave a final immunostaining score (0 to 300; IHC score). MIB1 expression was assessed using a Chalkley point array method adapted from methods used to assess vascular density in breast sections. 11 Briefly, MIB1-immunostained section was reviewed at low magnification and five areas showing the highest density of MIB1-positive tumor cells were selected. These hot spots were then assessed at higher magnification (×25 objective) and the number of grid points that coincided with positive and negative tumor cell nuclei was counted. The mean ratio of MIB1-positive/MIB1-negative cells was then calculated. Each hot spot contained between 200 and 1000 tumor cells depending on DCIS histology.
Results
CA IX and CA XII Antibody Specificity
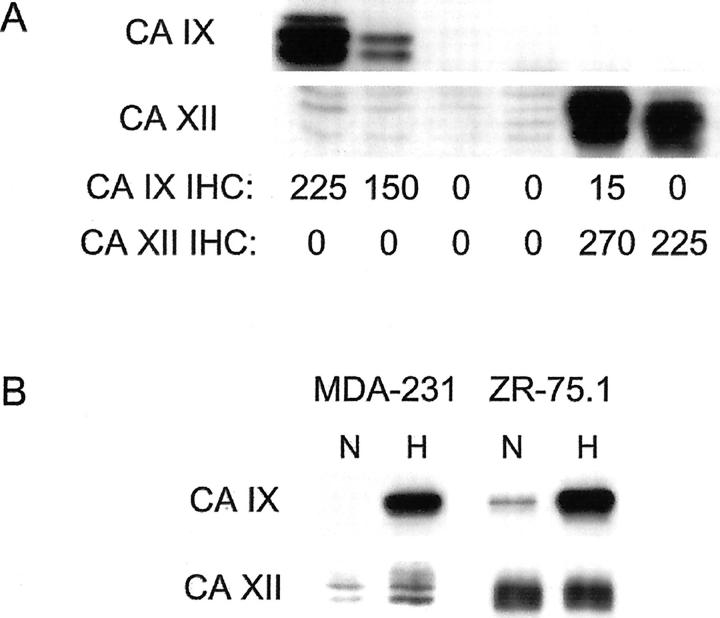
The anti-CA IX and anti-CA XII antibodies used in this study have been previously characterized for immunostaining in many human tissues. 22,31,34,36 However, neither antibody has been applied extensively to breast specimens. Therefore, initial experiments were performed to compare IHC profiles with immunoblotting signals from a set of six invasive breast ductal carcinomas. By IHC, two cases exhibited strong membranous staining for CA IX that was restricted to the invasive ductal carcinoma cells, one was weakly positive, and three were negative (data not shown). Two of the tumors that were either negative or weakly positive for CA IX by IHC exhibited strong membranous staining for CA XII, whereas four cases were negative (data not shown). Immunoblotting for CA IX and CA XII was performed in parallel on protein lysates obtained from the same tumor specimens. As shown in Figure 1A ▶ , immunoblotting for CA IX revealed a 54- to 58-kd doublet restricted to the two cases that were strongly positive by IHC. Similarly, immunoblotting for CA XII revealed a 46- to 48-kd doublet restricted to the two cases that were positive by IHC.
Figure 1.
Immunoblotting for CA IX and CA XII. A: CA IX and CA XII expression detected by Western blot correlates with the respective immunostaining (IHC) score in invasive breast carcinomas. CA IX and CA XII are detected in tumor extracts that are strongly positive for CA IX or CA XII by IHC in adjacent paraffin sections (IHC score for each CA is indicated). B: CA IX and CA XII expression and response to hypoxia in two breast tumor cell lines exposed to either normoxia (N; 20% O2) or hypoxia (H; 0.1% O2) for 16 hours.
CA IX and CA XII Expression in Breast Cell Lines
We have previously demonstrated wide-spread hypoxic induction of CA9 and CA12 mRNA in various tumor cell lines. 23 Here we compared hypoxic induction of CA IX and CA XII in two breast cell lines selected as representative of estrogen receptor (ER)-negative and poorly differentiated (MDA-MB-231), and ER-positive and well-differentiated (ZR-75.1) breast cancer (Figure 1B) ▶ . CA IX had an undetectable or low basal level of expression and was markedly induced by hypoxia. In comparison, CA XII had a higher level of normoxic expression and was further induced by hypoxia in one of the two cell lines.
CA IX Expression in Breast Tissues
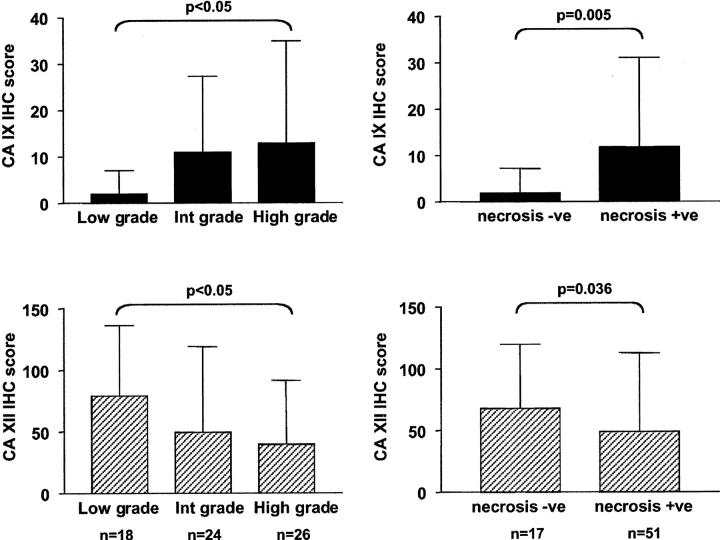
A series of 68 DCIS breast cases were studied for CA IX expression by IHC. Subsets of these cases also contained normal lobular and ductal components (n = 47), and invasive ductal carcinoma components (n = 29). CA IX expression was present in normal epithelium in one of 47 cases (2%), and in this case was limited to focal expression adjacent to the site of a recent biopsy. In many cases, benign breast lesions were present within the tissue section, including cystic changes, apocrine metaplasia, blunt duct, and sclerosis adenosis. No expression was observed in any benign breast lesion. In DCIS lesions, focal membranous CA IX staining, typically adjacent to areas of necrosis, was present in 34 of 68 (50%) cases, including 23 of 39 (59%) pure DCIS and 11 of 29 (38%) DCIS associated with invasive disease. In those cases in which invasive disease was present on the same tumor section, CA IX was expressed adjacent to regions of necrosis where this was present within the invasive component in four of 14 cases (29%). The presence of CA IX staining in both DCIS and invasive components was correlated (r = 0.55, P = 0.04). The focal perinecrotic nature of expression was reflected in the distribution of IHC scores with only 13 (19%) tumors scoring >10 (potential range of IHC score was 0 to 300, as described in Materials and Methods). The range of IHC scores was from 0 to 100 (median, 1; mean, 9; and SD, 17). Representative examples of low and high CA IX expression are illustrated in Figure 2, A and B ▶ . CA IX was significantly associated with high grade (grade low versus intermediate versus high; mean (SD), 2 (5), 11 (16), 13 (22), P = 0.012 analysis of variance) and the presence of necrosis (necrosis negative versus positive; mean (SD), 2 (5), 12 (19), P = 0.0053, Mann Whitney; Figure 3 ▶ and Table 1 ▶ ).
Figure 2.
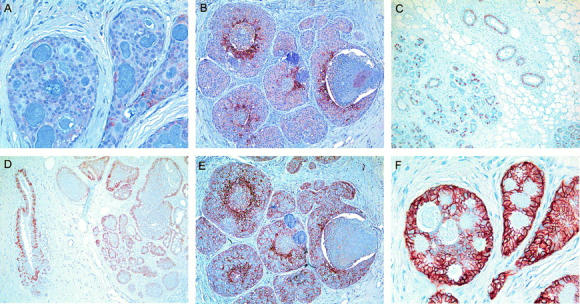
CA IX and CA XII have different expression profiles in non-neoplastic and neoplastic breast tissues. Low levels of CA IX expression are detected in low-grade DCIS (A) and more prominently in high-grade DCIS associated with central necrosis (B). CA XII expression in normal breast lobules and ducts (C), in ductal hyperplasia (D), in high-grade DCIS with accentuation adjacent to central luminal necrosis (E), and at higher levels in low-grade DCIS (F). Original magnifications, ×10 (B–E) and ×20 (A and F).
Figure 3.
CA IX and CA XII expression are inversely related to grade and the presence of necrosis in DCIS. CA IX expression shown relative to grade (upper left) and necrosis (upper right) and CA XII expression shown relative to grade (lower left) and necrosis (lower right). Columns (CA IX, black; CA XII, hatched) represent the mean IHC score with bars showing SD relative to DCIS grade (low, intermediate, high) and necrosis (absent, present).
Table 1.
Relationship between CA IX or CA XII Expression and Grade, Necrosis, and MIB1 Staining
| DCIS parameter | No. | CA IX | CA XII | ||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||||
| Grade | |||||||
| Low | 18 | 14 | 4 | P = 0.0091 P = 0.0023 (t) | 7 | 11 | ns (P = 0.072) P = 0.025 (t) |
| Intermediate | 24 | 12 | 12 | 15 | 9 | ||
| High | 26 | 8 | 18 | 19 | 7 | ||
| Necrosis | |||||||
| Negative | 17 | 14 | 3 | P = 0.002 | 7 | 10 | ns (P = 0.55) |
| Positive | 51 | 20 | 31 | 17 | 34 | ||
| MIB1 | |||||||
| Low | 13 | 6 | 7 | ns (P = 0.42) | 6 | 7 | ns (P = 0.69) |
| High | 13 | 4 | 9 | 5 | 8 | ||
Statistical significance estimated by χ2 test and χ2 test for trend (t).
CA XII Expression in Breast Tissues
The expression of CA XII was assessed by IHC in sections adjacent to CA IX-stained sections for all 68 DCIS cases. Membranous staining of the basal-lateral aspects of breast epithelial cells was present in normal lobular and normal ductal epithelium in 42 of 47 (89%) cases and in every benign breast lesion observed (Figure 2, C and D) ▶ . In ductal hyperplasia, CA XII expression was predominately limited to basal epithelial cells. In DCIS lesions, widespread membranous CA XII staining was present in 57 of 68 (84%) cases, including 31 of 39 (79%) pure DCIS and 26 of 29 (90%) DCIS associated with invasive disease. In those cases in which invasive disease was present on the same tumor section, CA XII was expressed in 10 of 14 cases (71%). The presence of CA XII staining in both DCIS and invasive components was highly correlated (r = 0.91, P < 0.0001). Although expression in DCIS was typically homogeneous throughout the tumor section, in intermediate- and high-grade DCIS where CA XII expression tended to be lower, expression was increased adjacent to areas of necrosis (Figure 2E) ▶ . The distribution of IHC scores was wider than for CA IX, with 17 (25%) tumors scoring 60 or more (potential range, 0 to 300). The range of IHC scores was from 0 to 270 (median, 40; mean, 54; and SD, 61). Representative examples of low and high CA XII expression are illustrated in Figure 2, E and F ▶ . CA XII was significantly associated with low grade (grade low versus intermediate versus high; mean (SD), 79(57), 50(69), 40(52), P = 0.012 analysis of variance) and the absence of necrosis (necrosis negative versus positive; mean (SD), 68(52), 49(64), P = 0.036, Mann Whitney; Figure 3 ▶ and Table 1 ▶ ).
CA IX and CA XII Expression Relative to the Proliferation Marker MIB1
A correlation between CA IX and proliferation has been suggested previously. 26 We therefore examined the relationship between proliferation and CA IX and CA XII in our breast tissue specimens (Figure 4) ▶ . Comparison of mitotic rates within positively stained ducts for CA IX and CA XII and within different zones in these ducts (adjacent to the stroma or the lumen), indicated that neither CA IX nor CA XII expression correlated regionally with mitosis. Whereas CA IX expression was typically localized to areas adjacent to necrosis, mitotic figures did not show a similar distribution, being most numerous in the cells adjacent to stroma and farthest removed from necrosis. Similarly, CA XII expression was not restricted to the areas of highest mitotic activity and was typically uniform throughout the intraductal epithelium, with occasional accentuation in luminal cells adjacent to necrosis. To confirm these morphological observations, a random subset of cases (n = 26) were immunostained for the proliferation marker MIB1 and the MIB1 score was determined by Chalkley counting. In agreement with previous observations, 37 we found MIB1 to be associated with both grade (low versus intermediate versus high; mean (SD), 0.16(0.07), 0.4(0.18), 0.56(0.33), P = 0.0026 analysis of variance) and the presence of necrosis (necrosis negative versus positive; mean (SD), 0.19(0.09), 0.51(0.28), P = 0.001 Mann Whitney; Figure 5 ▶ ). However, MIB1 was not significantly related to the expression of either CA IX or CA XII (CA IX negative versus positive; MIB1 mean (SD), 0.44(0.37), 0.37(0.21); CA XII negative versus positive; MIB1 mean (SD), 0.43(0.31), 0.38(0.26); Table 1 ▶ ). Similarly, when CA IX and CA XII expression were divided into low and high expression using the median IHC score of the series for each (CA IX positive, >1 and CA XII negative, >40), no association was detected between the expression of either CA and MIB1 staining.
Figure 4.
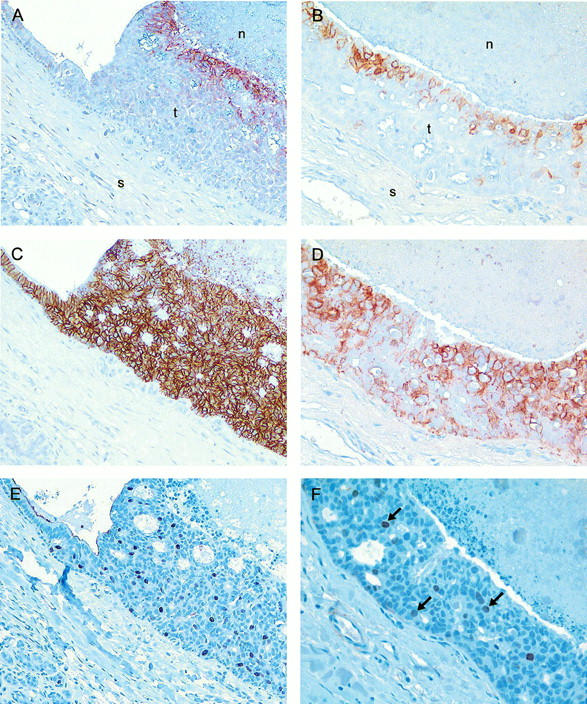
CA IX and CA XII expression is unrelated to MIB1 expression. The pattern of expression of CA IX (A and B), CA XII (C and D), and MIB1 (E and F), was assessed in serial sections from intermediate-grade DCIS (left column) and high-grade DCIS (right column). CA IX expression is restricted to the inner zone of luminal epithelium adjacent to central necrosis. CA XII expression is present throughout the duct wall, but accentuated adjacent to necrosis in high-grade DCIS, and also in the adjacent portion of non-neoplastic ductal epithelium (C, upper left). In contrast, MIB1-positive nuclei are distributed throughout the duct in both intermediate- and high-grade DCIS, and are absent from normal epithelium (E, upper left). Arrows within F indicate MIB1-positive nuclei. Original magnifications, ×20.
Figure 5.
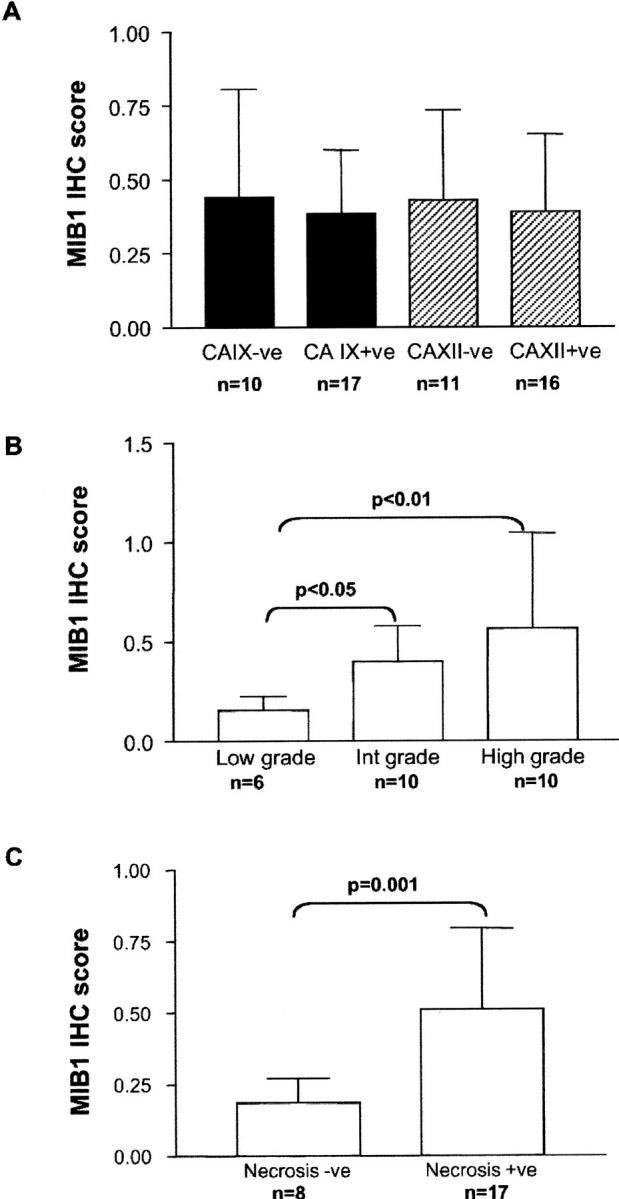
MIB1 expression relative to CA IX or CA XII expression, DCIS grade, and the presence of necrosis. Columns (CA IX, black; CA XII, hatched; MIB1, clear) represent the mean MIB1 IHC score with bars showing SD relative to CA IX or CA XII expression (negative, positive), DCIS grade (low, intermediate, high), and necrosis (absent, present).
CA IX and CA XII Expression Relative to Mammographic Calcification
CA IX and CA XII expression were assessed in relation to the presence and pattern of calcification detected in preoperative mammograms in a subset of cases in which films were available for review (n = 43). The presence of calcification was associated with the presence of necrosis (P = 0.0036, chi-square) and higher grade (P = 0.0057, chi-square) (Table 2) ▶ . When CA gene expression was classified as low or high on the basis of the median IHC score of the series (CA IX positive, >1 and CA XII positive, >40) a significant relationship was observed between lower CA XII expression and the presence of calcification (P = 0.0083, chi-square), as shown in Table 2 ▶ . The level of CA XII expression was also inversely associated with calcification (calcification absent versus present, mean (SD), 94(60), 42(60), P = 0.03, Mann Whitney). The pattern of calcification was not significantly different with respect to either CA gene expression. Despite this, comparison of cases with nonlinear versus cases with some component of linear calcification revealed a trend toward an increased proportion of CA IX positive cases (8 of 21 vs. 9 of 14 or 38% vs. 64%), whereas the proportion of CA XII positive cases was no different (6 of 21 vs. 3 of 14 or 29% vs. 21%). Additionally, cases with linear calcification tended to be associated with higher levels of CA IX expression than cases with nonlinear calcification (CA IX IHC score mean (SD), 9 (12) vs. 5 (10), P = n.s.) whereas there was no such trend for CA XII (CA XII IHC score, 41 (69) vs. 43 (55), P = n.s.).
Table 2.
Relationship in DCIS between the Presence of Calcification and CA IX or CA XII Expression, Necrosis, and Grade
| DCIS parameter | No. | Mammogram calcification | ||
|---|---|---|---|---|
| Negative | Positive | |||
| CA IX | ||||
| Negative | 21 | 3 | 18 | ns (P = 0.477) |
| Positive | 22 | 5 | 17 | |
| CA XII | ||||
| Negative | 28 | 2 | 26 | P = 0.0083 |
| Positive | 15 | 6 | 9 | |
| Necrosis | ||||
| Negative | 10 | 5 | 5 | P = 0.0036 |
| Positive | 33 | 3 | 30 | |
| Grade | ||||
| Low | 9 | 5 | 4 | P = 0.0057, P = 0.0137 (t) |
| Intermediate | 15 | 1 | 14 | |
| High | 19 | 2 | 17 | |
Statistical significance estimated by χ2 test and χ2 test for trend (t).
Discussion
We have shown that the tumor-associated CAs CA IX and CA XII are both expressed by malignant breast epithelium. CA IX expression was rare in normal ductal and lobular epithelium, and in benign breast lesions, occur- ring primarily in pre-invasive DCIS and invasive breast carcinomas. In DCIS, expression was focal and specifically associated with regions of necrosis and high-grade lesions. In contrast, CA XII was frequently expressed in normal breast tissue as well as in benign, pre-invasive, and invasive breast lesions. In DCIS, expression was typically homogeneous and associated with the absence of necrosis and low-grade lesions. However, focal induction of CA XII was observed in high-grade DCIS adjacent to necrosis. The finding that both CA IX and CA XII are induced in vivo in breast tumor cells adjacent to regions of necrosis suggests that these CAs may be important components of the breast epithelial cellular response to hypoxia. This observation is compatible with our recent findings in a variety of tissue-culture cell lines, 23 where both CA9 and CA12 are induced by hypoxia, and that at least for CA9 this induction is hypoxia-inducible factor-1-dependent. However, future studies to establish co-localization of CA expression with hypoxia-inducible factor-1 in tissue sections will be important to confirm in vivo.
CAs have been studied in a spectrum of tumor types in relation to their potential role as diagnostic and prognostic markers. Earlier studies focusing on CAs I, II, and IV revealed no clear relationships with tumorigenesis. 26 More recently, CA9 has been identified as overexpressed in multiple tumor cell lines and in several human tumor types. In various studies, CA9 has been found to be associated with aspects of early tumorigenesis, 20,26,30,35,38 and it has therefore been proposed to serve as a biomarker for dysplasia. In accordance with these findings, we have shown that whereas CA IX expression is rare in normal or benign breast lesions, CA IX expression occurs in pre-invasive DCIS of the breast where it is limited to malignant epithelium. In the breast specimens examined, CA IX expression was not related to proliferation and was strongly associated with necrosis, indicating that hypoxia may be an important pathway for induction of CA IX in breast tumors in vivo.
CA12 was initially identified as a renal carcinoma-associated gene 20,22 and has subsequently been found to be expressed in a range of normal tissues including endometrium, pancreas, and colon. 31,39,40 Interestingly, in the normal colon CA XII is expressed highly by the differentiated surface epithelium relative to the cells of the crypt base, and whereas no change in the surface expression occurs with tumorigenesis, increased basal/deep mucosal expression is associated with increasing dysplasia and invasive tumor stage. 31 Similarly, and in striking contrast to CA IX expression, we have observed constitutive expression of CA XII in normal breast epithelium and benign ductal hyperplasia. This suggests that CA XII may play a role in the control of pH in normal breast tissue. The function of this membrane-associated extracellular CA may be coupled to that of an intracellular CA such as CA II, as has been hypothesized for other secretory/excretory organs such as the salivary glands, pancreas, and kidney. 40 Clearly, a detailed examination of the interplay between the many CAs is warranted. We have also shown that CA XII expression persists in malignant pre-invasive DCIS. Although focal induction of CA XII was observed in areas adjacent to necrosis, the differentiation status of the DCIS lesion (as indicated by grade) had a more dominant role in determining CA XII expression, which was reflected in the pattern of expression observed in the ER-negative and ER-positive cell lines examined. Of note, differentiation has been proposed to play a role in the expression of other CAs, including CA I whose induction is association with differentiation in the colon, 41 and CA II whose expression is associated with differentiation in pancreatic cell lines under the influence of tumor necrosis factor-α. 42
An abnormal pattern of calcification in a breast mammogram is an important indicator of DCIS. 5 In particular, the presence of linear type calcification is associated with high-grade DCIS and may predict outcome of associated small invasive tumors. 5,6,43,44 Calcification is believed to reflect a disruption of the normal vascular architecture caused by abnormal proliferation within the intraductal epithelium. 45 This leads to a reduction in luminal pH, changes in the equilibria of many ions, and resulting calcification. 46 Inherited alteration and deficiency of CA II activity causes metabolic acidosis and ectopic tissue calcification. 47 Similarly, changes in extracellular pH influenced by CA IX and CA XII expression may affect the extent and pattern of calcification in DCIS of the breast. In the current series, increased mammographically detectable calcification was associated with reduced CA XII expression, as well as the presence of high-grade DCIS and necrosis, as previously reported. 48 Our inability to demonstrate a relationship between calcification and CA IX staining could relate to the fact that CA IX staining was only present very focally. Therefore, the tissue block assessed for CA IX expression may not correspond to the status of the area of the mammogram assessed for calcification, which encompassed the entire biopsy. Furthermore, our results suggest that whereas overall loss of CA XII expression is important in the development of calcification, local gain of CA IX expression adjacent to the ductal lumen may influence the pattern of calcification. However the significance of these observations awaits confirmation by larger studies as this subset of cases is small and includes a disproportionate number of cases with calcification present, reflecting the fact that calcification is a key factor in detection of tumors by mammography. 5
The effect on local pH and the significance for breast tumor progression of reciprocal changes in the expression of these CAs remains to be determined. However, there is additional evidence to suggest that a switch in pH regulatory pathways may occur in breast tumor progression. Although a decrease in the activity of the Na+/H+ exchanger was noted in response to serum deprivation in nontumor breast cells, stimulation of this exchanger and an increased capacity for extracellular acidification was observed in tumor cells. 49 In terms of tumor progression, maintenance of high levels of CA XII may be important for both the function and survival of the ductal epithelium in normal tissue. Loss of CA XII expression with progression to higher grade DCIS may reflect the acquisition of alternative cellular responses to ameliorate the effects of disruption of tissue architecture, altered pH, and hypoxia. 18,50 One facet of this adaptation may be provided by the induction of other CAs such as CA IX to modulate the effects of local hypoxia. This view predicts that overall loss of CA XII and/or gain of CA IX expression may be associated with a high risk of progression to invasive disease and therefore be of prognostic significance. Interestingly, inhibition of CA activity has recently been demonstrated to suppress invasion of some tumor cell lines. 51
In conclusion, we have shown that CA IX and CA XII are expressed in breast tissues, and that the profile of expression of these CAs in DCIS suggests that whereas hypoxia may be a dominant factor in the regulation of CA IX, the regulation of CA XII is dominated by other factors related to cellular differentiation.
Footnotes
Address reprint requests to Dr. Peter Watson, Dept. of Pathology, University of Manitoba, D212-770 Bannatyne Ave., Winnipeg, Manitoba, R3E 0W3, Canada. E-mail: pwatson@cc.umanitoba.ca.
Supported by the Imperial Cancer Research Fund and the Wellcome Trust. P. H. W. is supported by a Scientist Award from the Medical Research Council of Canada, an Academic Award from the U. S. Army Medical Research and Materiel Command, and a Research Travel Fellowship from Burroughs Wellcome. S. K. C. is supported by the Shane Fellowship and the Canadian Breast Cancer Foundation.
References
- 1.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C: Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA 1996, 275:913-918 [PubMed] [Google Scholar]
- 2.Ernster VL, Barclay J: Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. J Natl Cancer Inst Monogr 1997, 22:151-156 [DOI] [PubMed] [Google Scholar]
- 3.Fentiman IS: Trials of treatment for non-invasive breast cancer. Recent Results Cancer Res 1998, 152:135-142 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B: Highlights from recent National Surgical Adjuvant Breast and Bowel Project studies in the treatment and prevention of breast cancer. CA Cancer J Clin 1999, 49:159-177 [DOI] [PubMed] [Google Scholar]
- 5.Holland R, Hendriks JH: Microcalcifications associated with ductal carcinoma in situ: mammographic-pathologic correlation. Semin Diagn Pathol 1994, 11:181-192 [PubMed] [Google Scholar]
- 6.Tabar L, Chen HH, Duffy SW, Yen MF, Chiang CF, Dean PB, Smith RA: A novel method for prediction of long-term outcome of women with T1a, T1b, and 10–14 mm invasive breast cancers: a prospective study. Lancet 2000, 355:429-433 [DOI] [PubMed] [Google Scholar]
- 7.Shoker BS, Sloane JP: DCIS grading schemes and clinical implications. Histopathology 1999, 35:393-400 [DOI] [PubMed] [Google Scholar]
- 8.Fisher ER, Costantino J, Fisher B, Palekar AS, Redmond C, Mamounas E: Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) Protocol B-17. Intraductal carcinoma (ductal carcinoma in situ). The National Surgical Adjuvant Breast and Bowel Project Collaborating Investigators. Cancer 1995, 75:1310-1319 [DOI] [PubMed] [Google Scholar]
- 9.Silverstein MJ, Lagios MD, Craig PH, Waisman JR, Lewinsky BS, Colburn WJ, Poller DN: A prognostic index for ductal carcinoma in situ of the breast. Cancer 1996, 77:2267-2274 [DOI] [PubMed] [Google Scholar]
- 10.Schnitt SJ, Harris JR, Smith BL: Developing a prognostic index for ductal carcinoma in situ of the breast. Are we there yet? Cancer 1996, 77:2189-2192 [DOI] [PubMed] [Google Scholar]
- 11.Leek RD, Landers RJ, Harris AL, Lewis CE: Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999, 79:991-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wetzels RH, Kuijpers HJ, Lane EB, Leigh IM, Troyanovsky SM, Holland R, van Haelst UJ, Ramaekers FC: Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol 1991, 138:751-763 [PMC free article] [PubMed] [Google Scholar]
- 13.Vaupel P, Hoeckel M: Predictive power of the tumor oxygenation status. Adv Exp Med Biol 1999, 471:533-539 [DOI] [PubMed] [Google Scholar]
- 14.Dachs GU, Chaplin DJ: Microenvironmental control of gene expression: implications for tumor angiogenesis, progression, and metastasis. Semin Radiat Oncol 1998, 8:208-216 [DOI] [PubMed] [Google Scholar]
- 15.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW: Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 1997, 38:285-289 [DOI] [PubMed] [Google Scholar]
- 16.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W: High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000, 60:916-921 [PubMed] [Google Scholar]
- 17.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ: Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 1997, 94:8104-8109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW: Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999, 59:5830-5835 [PubMed] [Google Scholar]
- 19.Grabmaier K, Vissers JL, De Weijert MC, Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH, Noessner E, Mulders PA, Merkx G, Figdor CG, Adema GJ, Oosterwijk E: Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer 2000, 85:865-870 [DOI] [PubMed] [Google Scholar]
- 20.Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI: Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA 1998, 95:12596-12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opavsky R, Pastorekova S, Zelnik V, Gibadulinova A, Stanbridge EJ, Zavada J, Kettmann R, Pastorek J: Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics 1996, 33:480-487 [DOI] [PubMed] [Google Scholar]
- 22.Tureci O, Sahin U, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS: Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA 1998, 95:7608-7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wykoff C, Beasley NJP, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks K, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL: Hypoxia inducible expression of tumor associated carbonic anhydrases. Cancer Res 2000, 60:7075-7083 [PubMed] [Google Scholar]
- 24.Jiang W, Gupta D: Structure of the carbonic anhydrase VI (CA6) gene: evidence for two distinct groups within the alpha-CA gene family. Biochem J 1999, 344:385-390 [PMC free article] [PubMed] [Google Scholar]
- 25.Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavsky R, Zat’ovicova M, Liao S, Portetelle D, Stanbridge EJ: Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994, 9:2877-2888 [PubMed] [Google Scholar]
- 26.Nogradi A: The role of carbonic anhydrases in tumors. Am J Pathol 1998, 153:1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao SY, Stanbridge EJ: Expression of the MN antigen in cervical Papanicolaou smears is an early diagnostic biomarker of cervical dysplasia. Cancer Epidemiol Biomarkers Prev 1996, 5:549-557 [PubMed] [Google Scholar]
- 28.Liao SY, Aurelio ON, Jan K, Zavada J, Stanbridge EJ: Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res 1997, 57:2827-2831 [PubMed] [Google Scholar]
- 29.Liao SY, Stanbridge EJ: Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer 2000, 88:1108-1121 [DOI] [PubMed] [Google Scholar]
- 30.Vermylen P, Roufosse C, Burny A, Verhest A, Bosschaerts T, Pastorekova S, Ninane V, Sculier JP: Carbonic anhydrase IX antigen differentiates between preneoplastic malignant lesions in non-small cell lung carcinoma. Eur Respir J 1999, 14:806-811 [DOI] [PubMed] [Google Scholar]
- 31.Kivela A, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, Tureci O, Rajaniemi H: Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol 2000, 156:577-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverstein MJ, Poller DN, Waisman JR, Colburn WJ, Barth A, Gierson ED, Lewinsky B, Gamagami P, Slamon DJ: Prognostic classification of breast ductal carcinoma-in-situ. Lancet 1995, 345:1154-1157 [DOI] [PubMed] [Google Scholar]
- 33.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH: Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 1998, 92:2260-2268 [PubMed] [Google Scholar]
- 34.Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J: A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992, 187:620-626 [DOI] [PubMed] [Google Scholar]
- 35.Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastorekova S, Pastorek J, Kairaluoma MI, Karttunen TJ: Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol 1998, 153:279-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastorekova S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS: Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 1998, 46:497-504 [DOI] [PubMed] [Google Scholar]
- 37.Zafrani B, Leroyer A, Fourquet A, Laurent M, Trophilme D, Validire P, Sastre-Garau X: Mammographically-detected ductal in situ carcinoma of the breast analyzed with a new classification. A study of 127 cases: correlation with estrogen and progesterone receptors, p53 and c-erbB-2 proteins, and proliferative activity. Semin Diagn Pathol 1994, 11:208-214 [PubMed] [Google Scholar]
- 38.Turner JR, Odze RD, Crum CP, Resnick MB: MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol 1997, 28:740-744 [DOI] [PubMed] [Google Scholar]
- 39.Karhumaa P, Parkkila S, Tureci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS, Rajaniemi H: Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod 2000, 6:68-74 [DOI] [PubMed] [Google Scholar]
- 40.Nishimori I, Fujikawa Adachi K, Onishi S, Hollingsworth MA: Carbonic anhydrase in human pancreas: hypotheses for the pathophysiological roles of CA isozymes. Ann NY Acad Sci 1999, 880:5-16 [DOI] [PubMed] [Google Scholar]
- 41.Sowden J, Leigh S, Talbot I, Delhanty J, Edwards Y: Expression from the proximal promoter of the carbonic anhydrase 1 gene as a marker for differentiation in colon epithelia. Differentiation 1993, 53:67-74 [DOI] [PubMed] [Google Scholar]
- 42.Franz MG, Winkler BC, Norman JG, Fabri PJ, Gower WR, Jr: Tumor necrosis factor-alpha induces the expression of carbonic anhydrase II in pancreatic adenocarcinoma cells. Biochem Biophys Res Commun 1994, 205:1815-1821 [DOI] [PubMed] [Google Scholar]
- 43.Evans AJ, Pinder SE, Snead DR, Wilson AR, Ellis IO, Elston CW: The detection of ductal carcinoma in situ at mammographic screening enables the diagnosis of small, grade 3 invasive tumours. Br J Cancer 1997, 75:542-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holland R, Hendriks JH, Vebeek AL, Mravunac M, Schuurmans Stekhoven JH: Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet 1990, 335:519-522 [DOI] [PubMed] [Google Scholar]
- 45.Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL: Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol 1997, 181:207-212 [DOI] [PubMed] [Google Scholar]
- 46.Stubbs M, Rodrigues L, Howe FA, Wang J, Jeong KS, Veech RL, Griffiths JR: Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res 1994, 54:4011-4016 [PubMed] [Google Scholar]
- 47.Sly WS, Sato S, Zhu XL: Evaluation of carbonic anhydrase isozymes in disorders involving osteopetrosis and/or renal tubular acidosis. Clin Biochem 1991, 24:311-318 [DOI] [PubMed] [Google Scholar]
- 48.Evans AJ, Pinder S, Ellis IO, Sibbering M, Elston CW, Poller DN, Wilson R: Screening-detected and symptomatic ductal carcinoma in situ: mammographic features with pathologic correlation. Radiology 1994, 191:237-240 [DOI] [PubMed] [Google Scholar]
- 49.Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V: Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na(+)/H(+) exchange, motility, and invasion induced by serum deprivation. J Biol Chem 2000, 275:5361-5369 [DOI] [PubMed] [Google Scholar]
- 50.Tannock IF, Rotin D: Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 1989, 49:4373-4384 [PubMed] [Google Scholar]
- 51.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS: Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA 2000, 97:2220-2224 [DOI] [PMC free article] [PubMed] [Google Scholar]