Abstract
Type I and type III procollagen are reduced in photodamaged human skin. This reduction could result from increased degradation by metalloproteinases and/or from reduced procollagen synthesis. In the present study, we investigated type I procollagen production in photodamaged and sun-protected human skin. Skin samples from severely sun-damaged forearm skin and matched sun-protected hip skin from the same individuals were assessed for type I procollagen gene expression by in situ hybridization and for type I procollagen protein by immunostaining. Both mRNA and protein were reduced (∼65 and 57%, respectively) in photodamaged forearm skin compared to sun-protected hip skin. We next investigated whether reduced type I procollagen production was because of inherently reduced capacity of skin fibroblasts in severely photodamaged forearm skin to synthesize procollagen, or whether contextual influences within photodamaged skin act to down-regulate type I procollagen synthesis. For these studies, fibroblasts from photodamaged skin and matched sun-protected skin were established in culture. Equivalent numbers of fibroblasts were isolated from the two skin sites. Fibroblasts from the two sites had similar growth capacities and produced virtually identical amounts of type I procollagen protein. These findings indicate that the lack of type I procollagen synthesis in sun-damaged skin is not because of irreversible damage to fibroblast collagen-synthetic capacity. It follows, therefore, that factors within the severely photodamaged skin may act in some manner to inhibit procollagen production by cells that are inherently capable of doing so. Interactions between fibroblasts and the collagenous extracellular matrix regulate type I procollagen synthesis. In sun-protected skin, collagen fibrils exist as a highly organized matrix. Fibroblasts are found within the matrix, in close apposition with collagen fibers. In photodamaged skin, collagen fibrils are shortened, thinned, and disorganized. The level of partially degraded collagen is ∼3.6-fold greater in photodamaged skin than in sun-protected skin, and some fibroblasts are surrounded by debris. To model this situation, skin fibroblasts were cultured in vitro on intact collagen or on collagen that had been partially degraded by exposure to collagenolytic enzymes. Collagen that had been partially degraded by exposure to collagenolytic enzymes from either bacteria or human skin underwent contraction in the presence of dermal fibroblasts, whereas intact collagen did not. Fibroblasts cultured on collagen that had been exposed to either source of collagenolytic enzyme demonstrated reduced proliferative capacity (22 and 17% reduction on collagen degraded by bacterial collagenase or human skin collagenase, respectively) and synthesized less type I procollagen (36 and 88% reduction, respectively, on a per cell basis). Taken together, these findings indicate that 1) fibroblasts from photoaged and sun-protected skin are similar in their capacities for growth and type I procollagen production; and 2) the accumulation of partially degraded collagen observed in photodamaged skin may inhibit, by an as yet unidentified mechanism, type I procollagen synthesis.
Damage to the collagenous extracellular matrix that comprises the skin connective tissue is a characteristic feature of chronically sun-exposed human skin, and is thought to underlie the coarse, rough, wrinkled appearance that is the hallmark of photoaged skin. 1-6 Acute exposure of human skin in vivo to ultraviolet (UV) irradiation up-regulates synthesis of several matrix metalloproteinases (MMPs) including MMP-1 (interstitial collagenase), MMP-3 (stromelysin-1), and MMP-9 (92-kd gelatinase B) that degrade skin collagen. 7,8 Repeated induction of these matrix-degrading enzymes, after exposure to solar irradiation, throughout a period of years or decades likely initiates much of the damage to connective tissue that is seen in chronically sun-exposed skin. In addition, previous studies have demonstrated decreased levels of type I and type III collagen precursors (procollagens and pN collagens) in photodamaged human skin, relative to sun-protected skin. 9,10 This suggests that failure to replace damaged collagen through new synthesis also occurs.
Reduced procollagen levels in severely sun-damaged skin may result from irreversible, UV-induced damage to the cellular and molecular machinery governing collagen synthesis and breakdown in skin fibroblasts. Alternatively, damaged extracellular matrix may act in some manner to down-regulate procollagen synthesis by resident skin fibroblasts. It is known in this regard that interactions between collagen peptides and cell surface integrins induce intracellular signaling events, 11-14 and that procollagen synthesis in fibroblasts is regulated by these interactions. 15-18 In the presence of degraded collagen fibers, intracellular signaling is disrupted. 19,20 To distinguish between these two possibilities, we have assessed type I procollagen synthesis by human fibroblasts in photodamaged skin in vivo, and by fibroblasts from photodamaged skin cultured in vitro. In addition, we have determined the level of partially degraded collagen in photodamaged human skin relative to sun-protected skin, and have examined the effect of partially degraded collagen on type I procollagen synthesis. The data reported herein demonstrate that collagen fragmentation is increased, and that type I procollagen synthesis is reduced in sun-damaged human skin compared to sun-protected skin. Despite this, fibroblasts isolated from severely photodamaged skin possess similar capacity for in vitro procollagen synthesis as fibroblasts from sun-protected skin. Furthermore, in vitro type I procollagen synthesis is reduced in the presence of partially degraded collagen relative to intact collagen. Taken together, these data suggest that reduced collagen synthesis in photoaged skin occurs as a result of contextual influences, including the effects of damaged collagen on dermal fibroblast function.
Materials and Methods
Study Population and Skin Samples
A total of 42 individuals (22 males and 20 females) participated in this study. All of the individuals were characterized by the presence of severe photodamage on their forearms based on clinical criteria (eg, coarseness of the skin and degree of wrinkling). The age range was 46 to 83 years with the average age being 69 years. Replicate 4-mm full-thickness punch biopsies of forearm and sun-protected hip skin were obtained from each individual. All procedures involving human study subjects were approved by the University of Michigan Institutional Review Board, and all study participants provided written informed consent before their inclusion in the study. It should be noted that in 18 of the individuals who participated in this study, we were able to obtain biopsies of sun-protected underarm skin as well as skin from the other two sites. Overall, sun-protected skin from the underarm and sun-protected hip skin were very similar in regards to the parameters assessed (ie, collagen fragmentation, fibroblast isolation rates, proliferation, and collagen synthesis; see below).
Electron Microscopy
Skin biopsies from forearm and hip skin were fixed overnight in 4% electron-microscopic grade glutaraldehyde in 0.1 mol/L of cacodylate buffer (Sigma, St. Louis, MO) at pH 7.4. After fixation with 2% osmium tetroxide (EM Sciences, Fort Washington, PA) buffered in 0.1 mol/L of cacodylate buffer, sections were dehydrated with graded alcohol to 2× 100% alcohol and 2× propylene oxide (EM Sciences). The samples were embedded in pure epon resin. One-μm tissue sections were cut, stained with Toluidine blue, and examined at the light microscopic level. Ultrathin sections were cut from areas of interest, stained with lead citrate and uranyl acetate (all from EM Sciences), and observed in a Phillips 400 transmission electron microscope.
Assessment of Collagen Degradation in Human Skin
Skin biopsies from forearm and hip skin were homogenized in Tris buffer (20 mmol/L, pH 7.3) and centrifuged. The pellet, containing the collagenous extracellular matrix, was resuspended in 150 μl of Tris buffer containing 75 μg of α-chymotrypsin, and incubated for 8 hours at 37°C. The pellet from homogenized skin biopsies incubated in buffer alone served as control. At the end of the incubation period, the reaction tubes were centrifuged at 10,000 × g for 10 minutes. Supernatants were collected and assayed for hydroxyproline using automated amino acid analysis. Unlike intact fibrillar collagen, partially degraded collagen can be further broken down and the hydrolysis products liberated from tissue by α-chymotrypsin. 21 The amount of released collagen hydrolysis product can be determined by measurement of hydroxyproline, which is a modified amino acid present in collagen but rarely found in other proteins. 22
Assessment of Type I Procollagen Synthesis in Human Skin in Vivo
Assays for type I procollagen mRNA and protein were used to identify and quantify collagen-elaborating cells in skin samples. Type I procollagen (α1) gene expression was assessed by in situ hybridization. Fresh skin samples were immersed in OCT and frozen in liquid nitrogen. Frozen sections (6 μm) were hybridized with digoxigenin-labeled antisense and sense type I procollagen α1 cRNA probes, as described previously. 23 Cells expressing type I procollagen (α1) mRNA were quantified by counting under light microscopy. Type I procollagen protein was assessed by immunohistology. Frozen sections (6 μm) were stained with either one of two mouse monoclonal antibodies (SP1.D8, and M38) to human type I procollagen (α1 chain) and an immunoperoxidase-conjugated secondary antibody. 7,10 The SP1.D8 antibody was developed by Dr. Heinz Furthmayr and obtained from the Developmental Studies Hybridoma Bank under the auspices of the National Institutes of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA. This antibody predominantly stains extracellular type I procollagen. The M38 antibody was obtained from Takara Biomedicals, Shiga, Japan. This antibody stains cellular and extracellular type I procollagen. Stained sections were examined by light microscopy. The amount of staining (cellular and extracellular) with M38 was assessed visually and scored as 0, 0.25, 0.5, 0.75, or 1, where 0 indicates no staining and 1 indicates extensive extracellular staining as well as staining of most of the interstitial cells. The three other values (0.25, 0.5, and 0.75) represent intermediates between these two extremes.
Quantitative Fibroblast Outgrowth Assay
Skin samples were cut into small fragments (12 to 15 fragments per 4-mm biopsy) and each fragment placed in a separate well of a 96-well plate. Tissue fragments were incubated for up to 1 month in Dulbecco’s modified minimal essential medium of Eagle with nonessential amino acids and 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. The number of tissue fragments that yielded fibroblasts was determined at the end of the incubation period 24 and expressed as a percentage of the total number of tissue fragments incubated. Cells were defined as fibroblasts on the basis of spindle-shaped morphology, reactivity with antibodies to vimentin, and a lack of reactivity with antibodies to keratin. Fibroblasts isolated in this manner were used without subculture or passaged 1 to 2 times before use.
Assessment of Type I Procollagen Synthesis and Fibroblast Proliferation in Vitro
Fibroblasts cultured from photodamaged forearm and sun-protected hip skin were plated in Dulbecco’s modified minimal essential medium of Eagle with nonessential amino acids and 10% fetal bovine serum at 8 × 10 4 cells per well in a 24-well culture plate. After allowing the cells to attach and spread, cells were washed twice in MCDB-153 basal medium (Clonetics Inc., Walkersville, MD), supplemented with 1.4 mmol/L Ca2+ (final concentration) and incubated for 2 days at 37°C and 5% CO2. At the end of the 2-day incubation period, cells were washed twice in Ca2+-supplemented MCDB-153 and incubated for an additional 1 hour at 37°C and 5% CO2. The 1-hour culture fluid was collected and analyzed for type I procollagen protein by enzyme-linked immunoassay (Takara Biomedicals). Preliminary studies showed that the rate of accumulation of immunoreactive type I procollagen in medium conditioned by 8 × 10 4 dermal fibroblasts was linear through at least 2 hours. After collection of the culture medium, cells were harvested by brief exposure to trypsin/ethylenediaminetetraacetic acid (EDTA) and counted with the aid of a particle counter.
Preparation of Polymerized Collagen Gels
Rat tail collagen (4.7 mg/ml in 1 N HCl; Collaborative Biomedical Products, Bedford, MA) was diluted to 1 mg/ml with Ca2+-supplemented MCDB-153. The solution was made isotonic by addition of an appropriate amount of a 10× concentrated solution of Hanks’ balanced salt solution, and the pH brought to 7.2. The collagen solution was added to wells of a 24-well plate (0.5 ml/well) and incubated for 2 hours at 37°C. During this period, the collagen formed a polymerized gel. 25,26
Collagen-Degrading Enzyme Preparations
A collagenolytic enzyme preparation from Clostridium histolyticum (Collagenase type I; Worthington Biochemical Corp, Freehold, NJ) was used to produce fragmentation of the collagen. This enzyme preparation contains collagenolytic activities at 105 and 55 kd, 27 and the presence of these activities was confirmed by reactivity with gelatin and monomeric collagen, but not with β-casein in zymography. Reactivity was lost when 10 mmol/L of EDTA was included in the overnight incubation buffer. The bacterial enzyme preparation cleaves intact collagen at numerous sites to produce low molecular weight fragments. 27 Collagenolytic activity was quantified by exposing 1 mg of rat tail (monomeric) collagen to varying concentrations of enzyme preparation for 5 hours at 37°C. Intact collagen exposed to buffer alone served as control. At the end of the incubation period, the control collagen and enzyme-treated collagen were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue. Laser densitometry was used to quantify α1(I) and α2(I) bands in the intact and digested preparations. When 10 mmol/L of EDTA was included in the reaction mixture, no detectable collagen breakdown occurred.
Human basal cell carcinoma tissue was used as a source of collagen-degrading enzymes from human skin. Fresh tumor specimens obtained at surgery were cut into 2-mm pieces, and 6 to 8 tissue pieces incubated for 72 hours in 0.5 ml of Ca2+-supplemented MCDB-153. Incubation was at 37°C and 5% CO2. At the end of the incubation period, the culture fluid was obtained and used as the enzyme source. The conditioned medium from basal cell tumors contains large amounts of active MMP-1 as well as small amounts of MMP-8 (neutrophil collagenase) and MMP-13 (collagenase-3). 28 Active forms of gelatinolytic enzymes (eg, MMP-2 and MMP-9) are also present. 28 Zymography with gelatin, collagen, and β-casein was used in the present study to confirm the presence of these activities, and collagen-degrading activity was quantified using digestion of monomeric collagen followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis resolution as described above. As with the bacterial enzyme preparation, inclusion of 10 mmol/L of EDTA in the incubation buffer suppressed zymographic activities, and inclusion of 10 mmol/L of EDTA in the reaction mixture suppressed collagen degradation.
Polymerized collagen gels were treated for 5 hours at 37°C with varying amounts of either the bacterial enzyme or human-skin enzyme preparation. At the end of the incubation period, the collagenase solutions were decanted. The polymerized collagen gels were briefly exposed in sequence to 10 mmol/L of EDTA and 14 mmol/L of Ca2+, and then rinsed exhaustively with Ca2+-supplemented MCDB-153.
Assessment of Collagen Contraction and Type I Procollagen Synthesis by Fibroblasts on Polymerized Collagen Gels
Four isolates of adult fibroblasts (two from forearm and two from hip) and five isolates of neonatal foreskin fibroblasts at passage 1 to 2 were added to the collagen gels at a final concentration of 1 to 8 × 10 4 cells per culture. For this, Ca2+-containing MCDB-153 medium was further supplemented with 0.1 ng/ml of epidermal growth factor, 0.5 μg/ml of insulin, and 2% of pituitary extract. Cells were incubated for 4 days, with fresh culture medium provided on day 2. Contraction of the collagen gels occurred during a 2-day period. The diameter of the collagen gel was measured at day 2 using a microscope with a calibrated grid in the eyepiece. Collagen contraction in this assay depends on fibroblasts binding to the collagen fibers and pulling the fibers as the cells themselves undergo actin-mediated and myosin-sliding filament-mediated contraction. 29,30
At the end of the incubation period (day 4), the culture fluid was removed, and the collagen gels rinsed two times with Ca2+-supplemented MCDB-153 (without the added growth factors). Fresh culture medium (Ca2+-supplemented MCDB-153 without growth factors) was added to the wells and incubated for a further 1 hour. The 1-hour culture fluid was collected and assayed for type I procollagen by enzyme-linked immunosorbent assay as described above. The cells were then released from the collagen gels by sequential treatment with a high concentration of the bacterial collagenase preparation (100 μg for 2 hours) and trypsin (0.5% for 15 minutes) and counted.
Results
Collagen Destruction Is Increased in Photodamaged Forearm Skin Relative to Sun-Protected Hip Skin
Damage to the collagenous matrix of the dermis has been observed at both the light- and electron-microscopic levels in photoaged skin. 31-37 Reductions in both the number and size of the collagen fiber bundles as well as ultrastructural abnormalities in the collagen fibrils themselves have been noted. However, the presence of elastotic material often masks structural evidence of damage, and makes quantification of damage difficult. In the present study we used transmission electron microscopy in conjunction with a sensitive (albeit, indirect) biochemical assay to compare structural features of the collagen in severely photodamaged skin and in matched sun-protected skin from the same individuals. Consistent with past reports, 32-34,37 large bundles of collagenous fibers were present throughout the dermis of sun-protected skin. Healthy fibroblasts in intimate contact with the collagen bundles could be seen (Figure 1, A and C) ▶ . In contrast, severely photodamaged skin was characterized by the presence of fewer bundles of collagen, and many individual, disorganized fibers. The space between the collagen bundles, where not occupied with elastotic material, was filled with mostly acellular debris. Instead of being in contact with intact collagen, many of the fibroblasts in the damaged skin were surrounded by the debris. Some of the cells demonstrated a rounded rather than elongated morphology and, in some cases, there were aggregates of two or more cells. These features are shown in Figure 1, B and D ▶ . Thus, electron microscopy proved useful for identifying a reduction in the relative amount of intact collagen in the photodamaged skin, the presence of acellular debris, and contact/interaction of dermal fibroblasts with this debris rather than with intact collagen.
Figure 1.
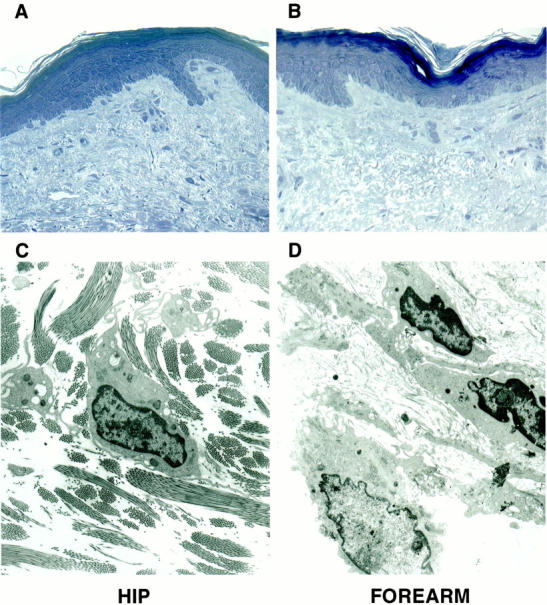
Histological and ultrastructural appearance of collagen fibers and dermal fibroblasts in severely photodamaged skin and sun-protected hip skin. Top: Light microscopy of Toluidine blue-stained thick sections. Bottom: Transmission electron microscopy. A and C: Sections of sun-protected hip skin. B and D: Sections of severely sun-damaged forearm skin. Original magnifications: ×160 (A and B), ×4,600 (C and D).
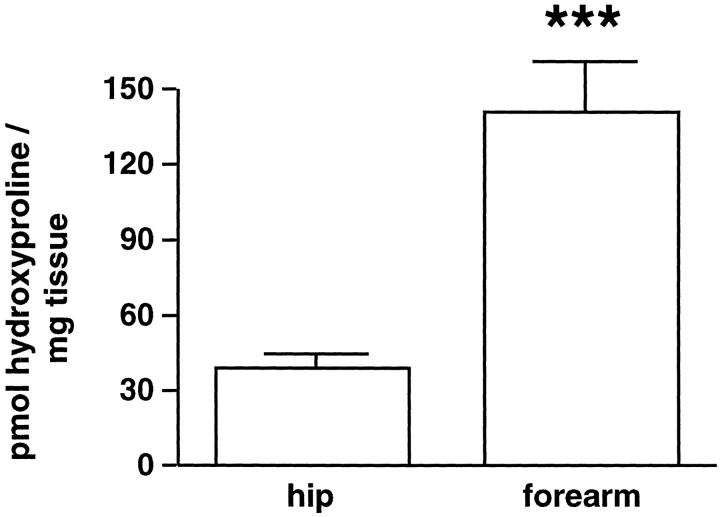
Ultrastructural analysis also provided evidence of damage to the collagen fibers themselves. Although some of the collagen fibers in photodamaged skin demonstrated the same overall width (∼1,500 A) and periodicity in photoaged skin as in sun-protected skin, others appeared shortened and thinned. To quantitatively assess collagen fragmentation, we took advantage of the fact that intact collagen is insensitive to in vitro hydrolysis by α-chymotrypsin, whereas collagen that has been partially degraded in vivo is susceptible to further hydrolysis by this enzyme in vitro. 21 Digestion of partially degraded collagen by α-chymotrypsin liberates collagen fragments from the tissue, and the liberated collagen fragments can be quantified by hydroxyproline measurement. 22 Hydroxyproline content after α-chymotrypsin digestion is, therefore, a measure of partially degraded collagen in the tissue. Figure 2 ▶ compares amounts of hydroxyproline released by α-chymotrypsin treatment of matched samples of severely photodamaged forearm skin and sun-protected hip skin from nine individuals. The amount released from photodamaged skin was 3.6-fold higher than the amount released from matched sun-protected skin.
Figure 2.
Degraded collagen is increased in severely photodamaged forearm skin compared to sun-protected hip skin. Values shown represent amounts of hydroxyproline released per mg of homogenized tissue after treatment with α-chymotrypsin ± standard errors (n = 9). Statistical significance was determined using the Student’s t-test. ***, P < 0.001.
Type I Procollagen Synthesis Is Reduced in Photodamaged Forearm Skin Compared to Sun-Protected Hip Skin
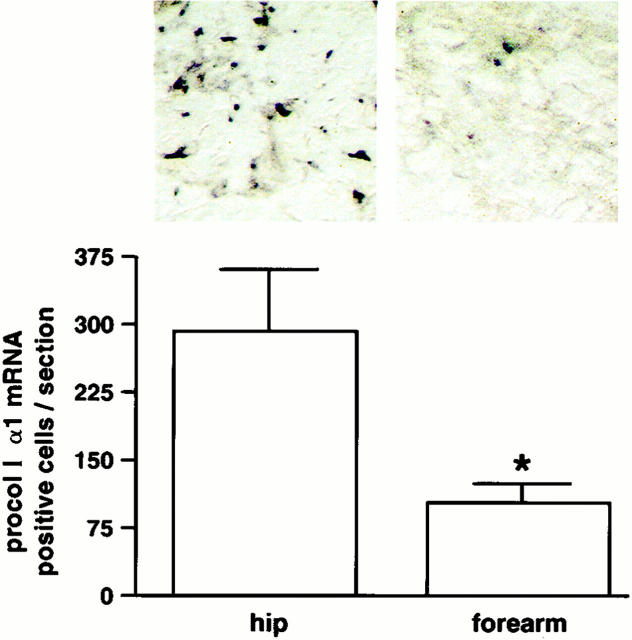
We next assessed levels of type I procollagen (α1) gene expression and type I procollagen (α1) protein expression in severely photodamaged forearm and sun-protected hip skin. As shown in Figure 3 ▶ , type I procollagen (α1) mRNA-expressing cells were readily detected in the dermal connective tissue of sun-protected hip skin. mRNA-expressing cells were observed throughout the entire dermis. In severely photodamaged forearm skin from the same individuals, cellular expression of type I procollagen (α1) mRNA was markedly reduced (Figure 3) ▶ . The number of cells expressing type I procollagen (α1) mRNA was reduced by ∼65% in severely photodamaged forearm skin, compared to sun-protected hip skin (n = 7).
Figure 3.
Type I procollagen (α1) mRNA expression is reduced in cells in severely photodamaged forearm skin compared to sun-protected hip skin. Values shown represent average numbers of type I procollagen (α1) mRNA-positive cells per section ± standard errors (n = 7). Statistical significance was determined using the Student’s t-test. *, P < 0.05. Inset: Examples of forearm and hip skin. Original magnification, ×160.
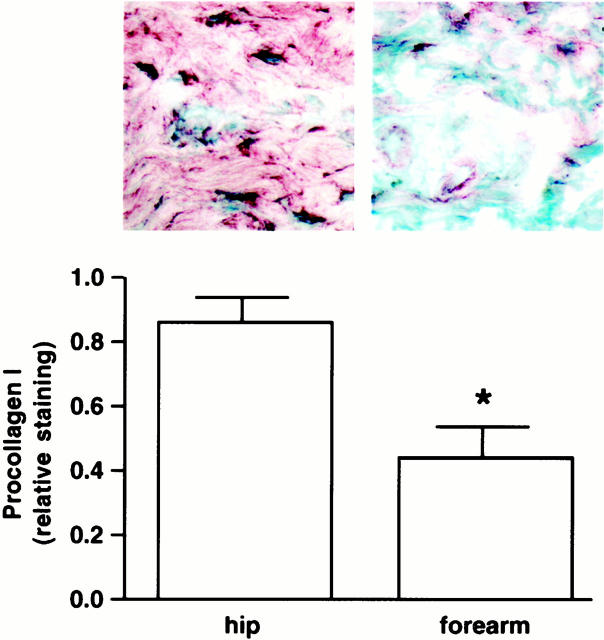
Immunohistology using monoclonal antibodies to the N-terminal and C-terminal propeptides of type I procollagen (α1) revealed a pattern of type I procollagen expression similar to that observed with in situ hybridization. Using antibody M38, protein expression was observed within cells and extracellularly throughout the dermis in sun-protected hip skin (Figure 4) ▶ . In contrast, cellular and extracellular expression of type I procollagen protein was nearly absent in severely damaged forearm skin (Figure 4) ▶ . Semiquantitative scoring for both cellular and extracellular staining (as described in Materials and Methods) revealed a decrease in staining of ∼57% in severely photodamaged skin, compared to sun-protected hip skin (n = 9). When a second antibody (SP1.D8) was used in place of M38, cellular bodies did not stain well but extracellular type I procollagen did. The extracellular staining pattern with this antibody was virtually identical to that seen with M38 (not shown). Together, the reduced level of type I (α1) procollagen gene expression and reduced level of cellular type I procollagen protein expression indicate that type I procollagen synthesis is decreased in photodamaged skin relative to sun-protected skin. The reduced procollagen synthesis in photodamaged skin is unlikely to reflect the presence of fewer interstitial fibroblasts in the sun-damaged skin. Past studies have reported no change or an increased number of interstitial cells present in photodamaged skin. 38 Likewise, the ability of topical retinoid treatment to induce type I procollagen synthesis in photodamaged skin implies the existence of cells with potential for collagen synthesis in the tissue. 9,10 Finally, the present studies (see below) show that equivalent numbers of fibroblasts can be isolated from severely sun-damaged forearm skin and matched sun-protected hip skin. Based on these data, we favor the interpretation that interstitial fibroblasts are present in photodamaged skin but are producing little type I procollagen.
Figure 4.
Type I procollagen (α1) protein expression is reduced in cells in severely photodamaged forearm skin compared to sun-protected hip skin. Values shown represent relative amount of cellular and extracellular staining ± standard errors (n = 9). Statistical significance was determined using the Student’s t-test. *, P < 0.05. Inset: Examples of forearm and hip skin. Original magnification, ×160.
Proliferation of Fibroblasts from Photodamaged Forearm Skin and Sun-Protected Hip Skin Is Similar
We next determined if reduced type I procollagen synthesis in vivo resulted from a permanent incapacitation of collagen synthetic activity in fibroblasts from photodamaged skin. To do this, we isolated dermal fibroblasts from fragments of severely photodamaged skin and from matched sun-protected skin. The frequency of fibroblast outgrowth was similar for the two skin sites. A total of 36 fibroblast isolates were obtained from 108 fragments of photodamaged skin (33%) and 43 isolates were obtained from 122 fragments of sun-protected hip skin (35%) (not statistically different based on chi-square test).
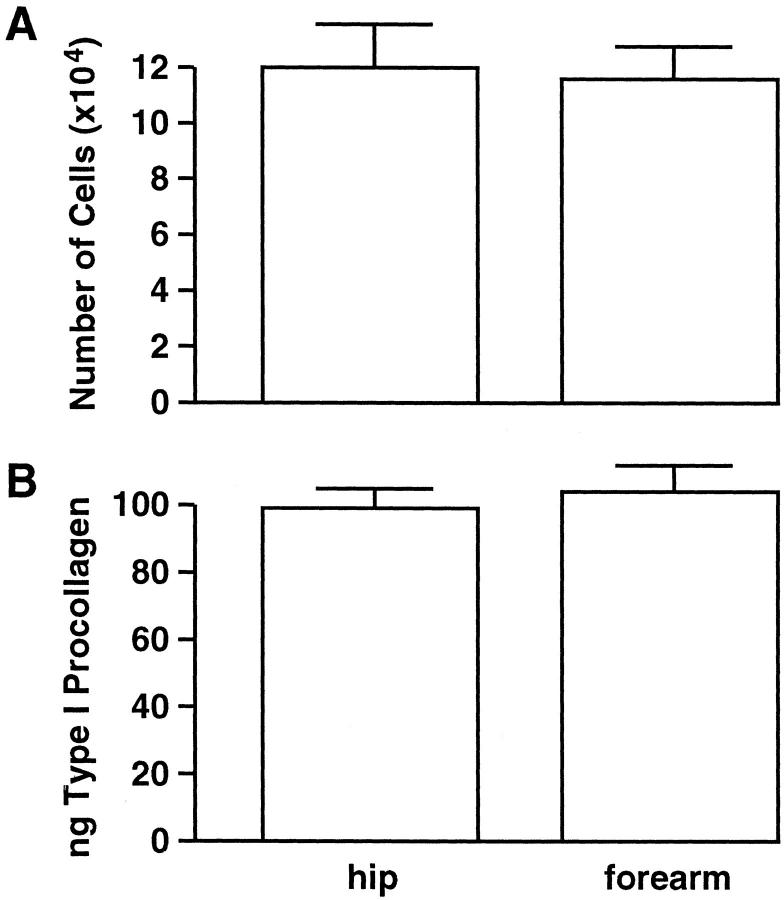
Fibroblasts isolated from severely photodamaged forearm skin and corresponding sun-protected hip skin were examined for proliferation in vitro in a 2-day assay. As shown in Figure 5A ▶ , cell growth was virtually indistinguishable between fibroblast isolates from the two skin sites.
Figure 5.
Proliferation and type I procollagen synthesis by fibroblasts from severely photodamaged forearm skin and sun-protected hip skin are similar. Cell number values (top) are averages ± standard errors, based on 22 and 29 isolates from forearm and hip, respectively. Collagen synthesis values (bottom) represent average amounts of type I procollagen secreted into 1 ml of culture medium (normalized to 8 × 10 4 cells) during a 1-hour incubation period ± standard errors, based on 12 and 23 isolates from forearm and hip, respectively. Statistical significance was determined by Student’s t-test. Values from the two groups were not different from one another at the P < 0.05 level.
Type I Procollagen Synthesis by Fibroblasts Cultured from Photodamaged Forearm Skin and Sun-Protected Hip Skin Is Similar
Having demonstrated that growth potential of fibroblasts from photodamaged and sun-protected skin was similar, we next determined the capacity of these cells to synthesize type I procollagen. As shown in Figure 5B ▶ , type I procollagen protein production was virtually indistinguishable between fibroblast isolates from photodamaged and sun-protected skin.
Type I Procollagen Production in Vitro Is Inhibited on Partially Degraded Collagen
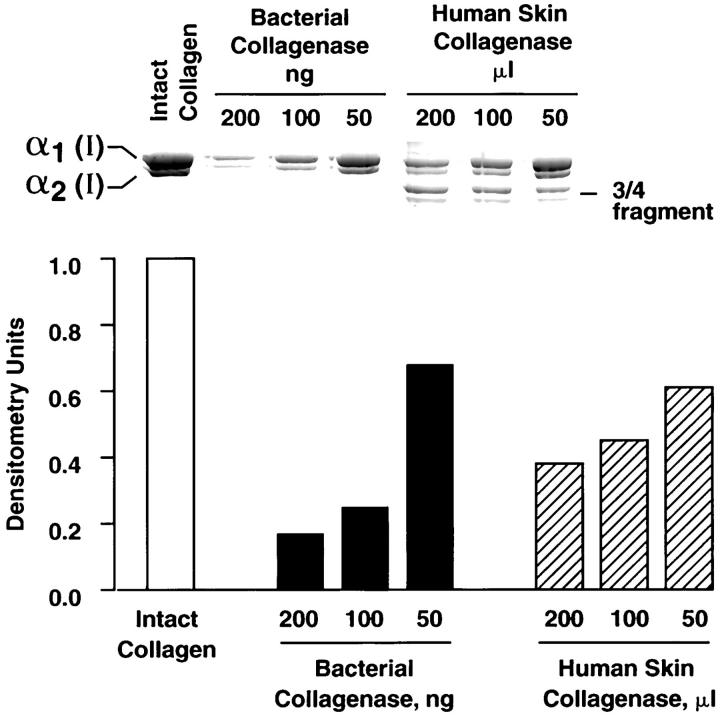
Polymerized collagen gels were prepared as described in Materials and Methods and treated with bacterial collagenase or with human skin collagenase from conditioned medium of basal cell carcinomas. Enzyme concentrations were standardized based on degradation of monomeric collagen, as shown in Figure 6 ▶ . Both enzyme preparations produced dose-dependent degradation of intact collagen. The major difference between the two enzymes was the direct formation of low molecular weight fragments by the bacterial enzyme and the initial formation of three-quarter-size and one-quarter-size fragments by the human skin collagenase, followed by subsequent degradation of these fragments (Figure 6) ▶ .
Figure 6.
Degradation of monomeric collagen by bacterial collagenase and human skin collagenase. Intact collagen and collagenase-treated collagen were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. α1(I) and α2(I) bands were quantified (together) by densitometry scanning after staining with Coomassie brilliant blue. Values for intact collagen were set at 1.0 and the others valued normalized to this. Inset: Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showing the appearance of intact and degraded collagen. Note: the major difference between the two enzyme preparations is the production of characteristic one-quarter-size and three-quarter-size fragments by the human skin collagenase but not the bacterial collagenase.
Collagen gels treated with high concentrations of either enzyme preparation (up to 200 ng of the bacterial enzyme per gel or 200 μl of the tumor culture fluid per gel) remained polymerized and appeared indistinguishable from untreated control collagen gels. However, after exposure of the collagen gels to the same concentrations of collagenolytic enzymes that produced fragmentation of monomeric collagen and subsequent addition of dermal fibroblasts (8 × 10 4 cells) to the gels, the behavior of the cells was considerably different from when the cells were added to intact collagen. Specifically, cells plated on intact collagen spread out diffusely and uniformly on the collagen surface (as they do on plastic or on a dried collagen film). Some cells also migrated into the collagen matrix as single cells. These features can be seen in Figure 7A ▶ , which shows a vertical section through the plane of the collagen surface. On partially degraded collagen, dermal fibroblasts initially spread on the surface. Throughout the subsequent 1 to 2 day period, extensive cell-cell contacts were formed. Cell-cell aggregation occurred, and as it did, the collagen contracted around the aggregated cells. This can be seen in Figure 7B ▶ , which is a section through the three-dimensional cell aggregate produced as the cells contracted the collagen around them.
Figure 7.
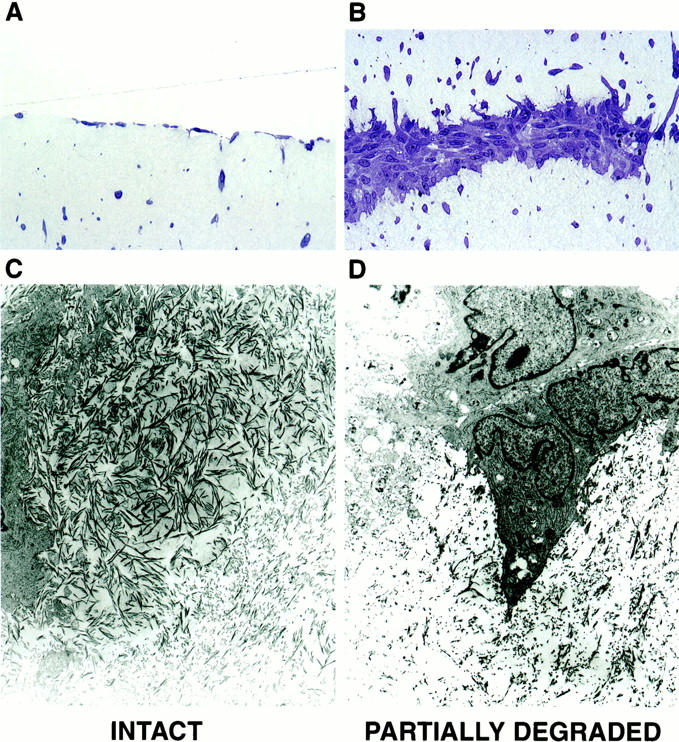
Histological and ultrastructural appearance of collagen fibers and dermal fibroblasts on polymerized intact collagen and polymerized collagen after partial degradation by collagenolytic enzymes. Top: Light microscopy of Toluidine blue-stained thick sections. Bottom: Transmission electron microscopy. A and C: Intact collagen. B and D: Partially degraded collagen. Original magnifications: ×260 (A and B), ×8,000 (C and D).
The appearance of the collagen fibers and the interaction of dermal fibroblasts with intact or partially degraded collagen were examined at the electron microscopic level (Figure 7, C and D) ▶ . In both cases, collagen fibers of similar length and width were seen. As compared to the collagen in whole skin, the reconstituted fibers were shorter; periodicity was evident. The density of fibers was high in the collagen gels not exposed to collagenolytic enzymes. Both vertical and horizontal orientation of fibers was evident, and fibroblasts with their numerous processes were in close and frequent contact with these fibers. In contrast, the density of intact collagen fibers was much lower in the gels that had been exposed to the collagenolytic enzymes. Many of the fibers appeared shortened and fragmented. There was no discernable orientation to the fibers in these gels, and a large amount of debris was evident. Fibroblasts were surrounded by the debris, and many cells showed decreased process formation. Overall, there were few cell contacts with intact collagen fibers. Thus, the interactions of dermal fibroblasts with reconstituted intact or partially degraded collagen in vitro paralleled what was observed in sun-protected versus severely sun-damaged skin in vivo.
Figure 8 ▶ demonstrates that collagen contraction requires both collagen digestion and fibroblast activity. Figure 8A ▶ shows the relationship between enzyme concentrations needed to produce collagen fragmentation (see Figure 6 ▶ ) and concentrations that facilitated collagen contraction. Inhibitor studies indicated that MMPs in the enzyme preparations are, in fact, responsible for collagen digestion. When collagen gels were exposed to either enzyme preparation in the presence of 10 mmol/L of EDTA, and then subsequently exposed to fibroblasts (after neutralization of the EDTA with Ca2+), no contraction of the collagen occurred (Figure 8B) ▶ . Other gels were treated with 10 μg of human recombinant tissue inhibitor of metalloproteinase-2 (TIMP-2) or 10 μg of aprotinin along with the human tumor enzyme preparation. Collagen contraction was inhibited by TIMP-2 but no inhibition was observed in the presence of aprotinin (Figure 8B) ▶ . Figure 8C ▶ demonstrates that contraction of partially digested collagen was dependent on fibroblast activity. Full contraction occurred in the presence of 4 to 8 × 10 4 cells and partial contraction was observed with as few as 2 × 10 4 cells. However, contraction did not occur when fewer cells (1 × 104) were used.
Figure 8.
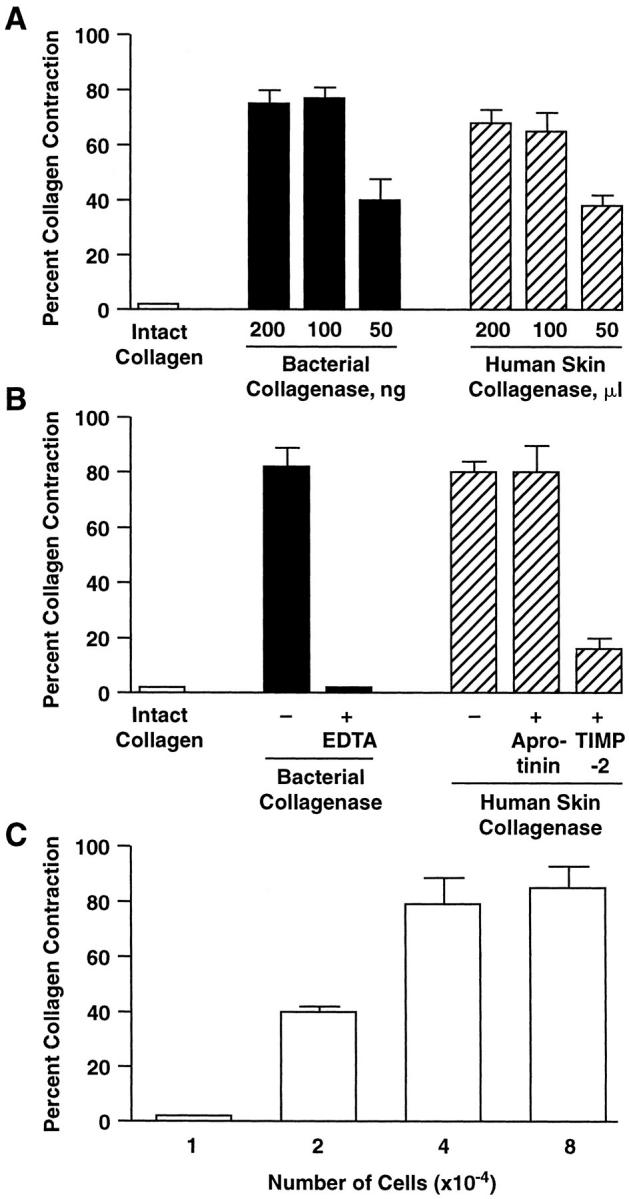
Contraction of intact and partially degraded collagen by human dermal fibroblasts. Collagen contraction was assessed at day 2 as described in the Materials and Methods. Values shown represent the average diameter of the contracted collagen ± the differences between duplicate samples and averages in a single experiment. A: Dose responses for the two enzyme preparations. B: Inhibitor sensitivity profile. C: Dermal fibroblast dose response.
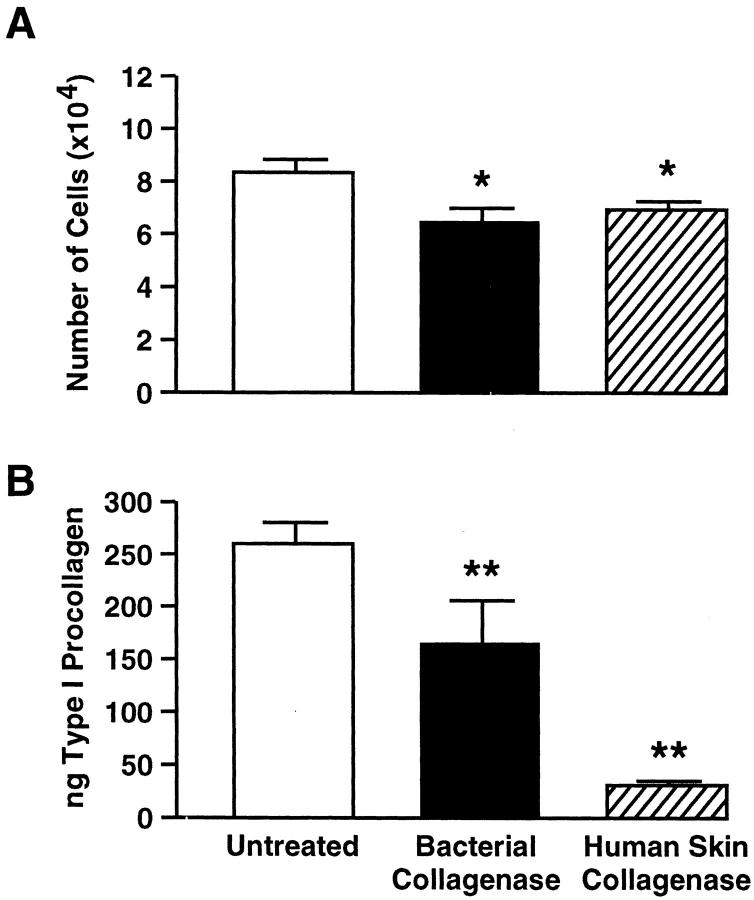
Cell growth and type I procollagen production by fibroblasts on intact collagen gels and gels partially degraded by either enzyme preparation were assessed. For these studies, four different adult isolates (two from forearm and two from hip) and five different isolates of neonatal (foreskin) fibroblasts were used. Both cell growth and the amount of type I procollagen produced were lower on partially degraded collagen than on intact collagen (Figure 9, A and B) ▶ . Reduced cell growth and type I procollagen elaboration were observed with all of the neonatal and adult dermal fibroblast isolates tested.
Figure 9.
Cell growth and type I procollagen synthesis by human dermal fibroblasts are reduced on partially degraded collagen gels compared to intact collagen gels. A: Cell growth. Values represent the mean number of cells present at day 4 ± standard errors (n = 5 foreskin isolates and 4 adult isolates). B: Type I procollagen synthesis. Values represent average ng of type I procollagen per ml ± standard errors (n = 5 foreskin isolates and 4 adult isolates). Before assay, culture media volume from the control and treated groups were adjusted to a common cell number (based on the cell counts presented in A). Statistical significance was determined using the Student’s t-test. *, P < 0.05; **, P < 0.01.
Discussion
Damage to the collagenous matrix is thought to underlie the coarse, rough, wrinkled appearance of photoaged skin. How collagen damage is brought about during photoaging is not fully understood. Exposure of skin to UV irradiation transiently up-regulates production of MMPs that are capable of degrading skin collagen. 7,8 Repeated MMP induction throughout years or decades could give rise to the damage seen in the matrix of chronically sun-exposed skin. Although proteolytic attack on structural collagen is clearly part of the overall process, failure to replace damaged collagen with newly synthesized collagen also contributes to the progressive degenerative changes that occur in the connective tissue of sun-exposed skin throughout time. 8-10
Mechanisms underlying decreased collagen synthesis by fibroblasts in severely photodamaged skin are not completely understood. Based on the results of the present study, we conclude that whereas fibroblast synthesis of type I procollagen is greatly diminished in photoaged human skin in vivo, growth capacity and synthesis of type I procollagen by fibroblasts from sun-damaged skin and age-matched sun-protected skin are indistinguishable when the cells are removed from the skin and examined in vitro. It should be noted that whereas the studies described here made use of sun-exposed forearm skin and sun-protected hip skin for most of the comparisons, we did, in fact, have the opportunity to assess a number of parameters (ie, collagen fragmentation in vivo and fibroblast isolation rates, growth rates and type I procollagen production in vitro) in underarm skin from 18 of the same volunteers. Sun-protected underarm skin and sun-protected hip skin from these individuals were similar in regard to all of the parameters assessed.
Because our data indicate that equivalent numbers of fibroblasts can be isolated from photodamaged skin and sun-protected skin, and because our data are based on results of multiple isolates from both tissue sites (from nine different individuals), it is unlikely that the in vitro data are skewed by a small subpopulation of cells in the photodamaged skin that grow out from the tissue and demonstrate the same phenotype as fibroblasts from sun-protected skin in vitro. Rather, these studies indicate that reduced procollagen production observed in vivo in severely sun-damaged skin is not because of reduced synthetic capacity of the fibroblasts per se. Consistent with these observations, it has been demonstrated previously that synthesis of collagen (as well as fibronectin) is low or undetectable in organ cultures of sun-exposed skin relative to organ cultures of healthy young skin. 24,39 Synthesis of both matrix components is normalized 24,39 when the organ cultures are treated with concentrations of all-trans retinoic acid that induce collagen expression in photoaged skin in vivo. 23 Taken together with these previous observations, the present finding that fibroblasts in severely photoaged skin are not intrinsically damaged (with respect to collagen production) provides a rationale for therapeutic intervention with agents such as all-trans retinoic acid to stimulate collagen synthesis to repair photodamaged skin. 40,41 In a like manner, any number of other agents may be found that have the capacity to restore collagen synthetic capacity to dermal fibroblasts that have been switched off in some manner by the presence of severely damaged connective tissue. It should be noted that the present data, particularly the mRNA data, strongly argue for reduced procollagen synthesis (independent of changes in collagen degradation). None-the-less, altered (ie, increased) elaboration of matrix-degrading MMPs could also lead to reduced procollagen deposition in the extracellular matrix. In fact, we assessed a number of forearm and hip skin samples for collagenase levels, but no significant differences were noted (J Varani and SC Datta, unpublished observation). This is in contrast to findings from studies with acute UV-irradiated skin, where increased collagenase was observed relative to nonirradiated controls. 7,8 This is also in contrast to findings in natural aging, where a higher level of collagenase was observed in sun-protected skin from >80-year-old individuals than in sun-protected skin of younger (18- to 29-year-old) individuals. 42
Because dermal fibroblasts do not seem to be intrinsically damaged in severely photoaged skin, it follows that inhibitory influences within the in vivo environment of severely photodamaged skin may act in some way to prevent cells that are inherently capable of elaborating collagen from doing so. In vitro studies performed with intact and partially degraded collagen gels support this suggestion. When skin fibroblasts (either neonatal or adult) were added to polymerized collagen, they rapidly attached and spread; they continued to proliferate and synthesize type I procollagen. In contrast, when fibroblasts were added to collagen gels that had been exposed to collagenase, cell growth and type I procollagen synthesis were reduced. Although extrapolating from in vitro experiments to what may occur in vivo is difficult, these data provide evidence that fibroblast functions that are important for maintenance of dermal connective tissue are inhibited in the presence of fragmented collagen. It should be noted that whereas both cell growth and type I procollagen production were reduced on the degraded collagen, the decrease in procollagen production was greater. Whether this reflects a specific inhibition of procollagen synthesis or whether procollagen elaboration is simply a more sensitive indicator of the overall functioning of the cells cannot be distinguished from the present data.
How damaged collagen exerts its influence on dermal fibroblast function is not known. A number of potential mechanisms exist. One possibility involves a change in cell shape that occurs during collagen contraction. Fibroblast interaction with structural collagen depends on the primary, secondary, and tertiary structure of the collagen fibrils. 11 Fibroblasts attach to collagen fibers and express the typical, elongated spindle-cell morphology. When enough breaks are introduced into the three-dimensional collagen scaffold, it is no longer capable of resisting the contractile force of the cells and collapses. As the collagen scaffold collapses, the cytoskeleton disassembles and cell shape changes from elongated to round. Previous studies have demonstrated that optimal fibroblast function (including growth and collagen production) depend on maintenance of the elongated cell shape. 43-45 Thus, it may be that loss of cell shape in the presence of degraded collagen directly underlies reduced growth and collagen production on the partially degraded collagen gels. Based on the in vivo ultrastructural findings presented here, we suggest that loss of cell shape could underlie reduced collagen synthesis in severely photodamaged skin.
Alternatively, it may not be loss of cell shape per se that underlies reduced growth and collagen production. Rather, it may be abnormal signaling, brought about by cellular interactions with degraded collagen rather than with the intact triple helical molecule, that directly causes abnormal cell function. Cellular interactions with collagen are mediated by multiple members of the β1 integrin family, including α1, α2, and α3. 12-16,46 Although a number of different α subunits mediate cell adhesion to collagen, other functions including collagen synthesis, elaboration of matrix-degrading enzymes, and collagen contraction are mediated more specifically by different α subunits. 12,13,15,16,46 When the collagen fibers are degraded, the contribution of different integrins to cell-matrix interactions changes, 20 leading to alterations in motility and, perhaps, alterations in proliferation/matrix production. In support of this, Gardner and colleagues 15 have argued that fibroblast interactions with collagen through specific integrins rather than contraction of the collagen is directly responsible for modulating collagen production because reduced collagen synthesis is seen in the dermis of mice with a specific integrin gene defect. It should be noted that although signaling through integrin receptors has been well studied, these molecules are probably not the only molecules through which cell-matrix interactions occur. Recent studies have shown, for example, that cell interactions with collagen can occur through discoidin domain receptors. 47 A role for these receptors in collagen metabolism has been suggested. Which cell surface receptors are ultimately responsible for the modulation of cell behavior in the presence of degraded collagen fibers will need to be addressed experimentally in future studies.
Ultimately, it may not be possible to completely distinguish between altered cell shape because of contraction of the collagen versus altered signaling because of cell interactions with collagen fragments as the reason for decreased cell growth and new collagen synthesis. It has recently been shown that collagen fragments actively promote disassembly of focal adhesion contacts, resulting in cleavage of cytoskeletal proteins and loss of cell shape. 19
Finally, it must be noted that although our studies have focused mainly on type I collagen (because it is by far the most abundant structural protein in the skin), the dermis contains a number of collagenous and noncollagenous extracellular matrix molecules. Virtually every one of these molecules can be degraded by members of the MMP family. 48 Fibroblast interactions with enzyme-degraded forms of any of these molecules could result in faulty signaling and subsequent alterations in fibroblast function. By focusing on collagenous components in the present study, we do not mean to rule out possible contributions of other components of the extracellular matrix. Regardless of the molecular mechanisms underlying reduced collagen synthesis in photodamaged skin, the beneficial effects of agents such as all-trans retinoic acid may derive not only from direct action on fibroblasts to stimulate collagen synthesis 9,10 and decrease collagenase expression, 7,8 but also from indirectly promoting (through the newly synthesized collagen) additional matrix-regenerative signals not present in untreated photodamaged skin.
A final question concerns the possible relationship between fibroblast-induced contraction of partially degraded collagen and the structural features seen in severely photodamaged skin. Until now, we have considered collagen contraction only as an indicator of collagen damage or as a modulator of new collagen synthesis. Could the presence of extensive amounts of contracted collagen contribute directly to the clinical appearance of severely photodamaged skin? Although there is no direct evidence to show that contraction of damaged collagen contributes to coarse wrinkling in photoaged skin, collagen contraction in the context of repeated cycles of damage and repair could distort and disrupt structural features of the tissue. 46 In support of such a possibility, it has been shown in a past study that skin wounds with minimal collagen damage (for example freeze wounds) heal without contraction of the collagen at the wound site and consequently, the healed skin at these sites is smooth. In contrast, where damage to the matrix is more extensive (in burn wounds or traumatic injury), collagen contraction occurs during wound closure. Healed skin at these sites is rough and wrinkled. 49 Studies to determine whether collagen contraction could play a similar role in photoaged skin are in progress.
Acknowledgments
We thank Suzan Rehbine, Robin Kunkel, and Lisa Riggs for technical assistance; Laura VanGoor for preparation of graphic material; and Ted Hamilton for statistical analysis.
Footnotes
Address reprint requests to James Varani, Ph.D., Department of Pathology, The University of Michigan Medical School, 1301 Catherine Rd./Box 0602, Ann Arbor, MI 48109. E-mail: varani@umich.edu.
Supported in part by a grant from Johnson and Johnson, and by grant CA 60958 from the United States Public Health Service.
References
- 1.Smith JG, Davidson EA, Sams WM, Clark RD: Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol 1962, 39:347-350 [DOI] [PubMed] [Google Scholar]
- 2.Kligman AM, Balin AK: Aging of human skin. Balin AK Kligman AM eds. Aging and the Human Skin. 1989, :pp 1-11 Raven Press, New York [Google Scholar]
- 3.Schwartz E, Cruickshank FA, Perlish JS, Fleischmajer R: Alterations in dermal collagen in ultraviolet irradiated hairless mice. J Invest Dermatol 1989, 93:142-146 [DOI] [PubMed] [Google Scholar]
- 4.Marks R (ed): Sun-Damaged Skin. London, Martin Dunitz, 1992
- 5.Maloney SJ, Edmonds SH, Giddens LD, Learn DB: The hairless mouse model of photoaging: evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles. Photochem Photobiol 1992, 56:505-511 [DOI] [PubMed] [Google Scholar]
- 6.Schwartz E, Cruickshank FA, Christensen CC, Perlish JS, Lebwohl M: Collagen alterations in chronically sun-damaged human skin. Photochem Photobiol 1993, 58:841-844 [DOI] [PubMed] [Google Scholar]
- 7.Fisher GJ, Datta SC, Talwar HS, Wang Z-Q, Varani J, Kang S, Voorhees JJ: The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1966, 379:335-338 [DOI] [PubMed] [Google Scholar]
- 8.Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ: Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 1977, 337:1419-1428 [DOI] [PubMed] [Google Scholar]
- 9.Griffiths CEM, Russman G, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ: Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med 1993, 329:530-534 [DOI] [PubMed] [Google Scholar]
- 10.Talwar HS, Griffiths CEM, Fisher GJ, Hamilton TA, Voorhees JJ: Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol 1995, 105:285-290 [DOI] [PubMed] [Google Scholar]
- 11.Humphries MJ: The molecular basis and specificity of integrin-ligand interactions. J Cell Sci 1990, 97:585-592 [DOI] [PubMed] [Google Scholar]
- 12.Schiro JA, Chan BMC, Poswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS: Integrin α2β1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell 1991, 67:403-410 [DOI] [PubMed] [Google Scholar]
- 13.Chan BMC, Kassner PD, Shiro JA, Byers HR, Kupper TS, Hemler ME: Distinct cellular functions mediated by different VLA integrin α subunit cytoplasmic domains. Cell 1992, 68:1051-1060 [DOI] [PubMed] [Google Scholar]
- 14.Boudreau NJ, Jones PL: Extracellular matrix and integrin signalling. Biochem J 1999, 339:481-488 [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner H, Broberg A, Pozzi A, Laato M, Heino J: Absence of integrin α1β1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 1999, 112:263-272 [DOI] [PubMed] [Google Scholar]
- 16.Gotwals PJ, Chi-Rosso G, Lindner V, Yang J, Ling L, Fawell SE, Koteliansky VE: The α1β1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J Clin Invest 1996, 97:2469-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B: Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by alpha 1 beta 1 and alpha 2 beta 1 integrins. J Cell Biol 1995, 131:1903-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, Heino J: Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem 1995, 270:13548-13552 [DOI] [PubMed] [Google Scholar]
- 19.Carragher NO, Levkau B, Ross R, Raines EW: Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125fak, paxillin and talin. J Cell Biol 1999, 147:619-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringa E, Knauper V, Murphy G, Gavrilovic J: Collagen degradation and platelet-derived growth factor stimulate the migration of vascular smooth muscle cells. J Cell Sci 2000, 113:2055-2064 [DOI] [PubMed] [Google Scholar]
- 21.Bank RA, Krikken M, Beckman B, Stoop R, Maroudas A, Lafeber F, Koppele J: A simplified measurement of degraded collagen in tissues: applications in healthy, fibrotic and osteoarthritic collagen. Matrix Biol 1997, 16:233-243 [DOI] [PubMed] [Google Scholar]
- 22.Miller EJ, Gay S: Collagen structure and function. Cohen IK Diegelmann RF Lindblad WJ eds. Wound Healing; Biochemical and Clinical Aspects. 1992, :pp 130-151 W. B. Saunders Company, Philadelphia [Google Scholar]
- 23.Kang S, Duell EA, Fisher GJ, Datta SC, Wang Z-Q, Reddy AP, Tavakkol A, Voorhees JJ: Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid-binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol 1995, 105:549-556 [DOI] [PubMed] [Google Scholar]
- 24.Varani J, Perone P, Griffiths CEM, Inman DR, Fligiel SEG, Voorhees JJ: All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. J Clin Invest 1994, 94:1747-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell E, Ivarson B, Merrill C: Production of a tissue-like structure by contraction of collagen lattice by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979, 76:1274-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravanti L, Heino J, Lopez-Otin C, Kahari V-M: Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts in three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem 1999, 274:2446-2455 [DOI] [PubMed] [Google Scholar]
- 27.Mandl L, MacLennan JD, Howes EL, DeBellis RH, Sohler A: Isolation and characterization of proteinase and collagenase from Cl. Histolyticum. J Clin Invest 1953, 32:1323-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varani J, Hattori Y, Chi Y, Schmidt T, Perone P, Zeigler ME, Fader DJ, Johnson TJ: Elaboration of collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors by basal cell carcinomas of skin: comparison with normal skin. Br J Cancer 2000, 82:657-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreiss TE, Birchmeier W: Stress fiber sarcomeres of fibroblasts are contractile. Cell 1980, 22:555-561 [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich HP, Rajaratnam JBM, Griswold TR: ATP-induced cell contraction in dermal fibroblasts: effects of cAMP and myosin light-chain kinase. J Cell Physiol 1986, 128:223-230 [DOI] [PubMed] [Google Scholar]
- 31.Oikrinen A, Kallioinen M: A biochemical and immunohistochemical study of collagen in sun-exposed and protected skin. Photodermatology 1989, 6:24-31 [PubMed] [Google Scholar]
- 32.Constantine VS, Hartley MW: Collagen and elastic fibers in normal dermis and severe actinic (senile) elastosis: a light and electron microscopic study. Ala J Med Sci 1966, 3:329-342 [PubMed] [Google Scholar]
- 33.Mitchell RE: Chronic solar dermatosis: a light and electron microscopic study of the dermis. J Invest Dermatol 1967, 48:203-220 [DOI] [PubMed] [Google Scholar]
- 34.Lavker RM: Structural alterations in exposed and unexposed aged skin. J Invest Dermatol 1979, 73:59-66 [DOI] [PubMed] [Google Scholar]
- 35.Bruce SA: Ultrastructure of dermal fibroblasts during development and aging: relationship to in vitro senescence of dermal fibroblasts. Exp Gerontol 1991, 26:3-16 [DOI] [PubMed] [Google Scholar]
- 36.Toyoda M, Bhawan J: Electron-microscopic observations of cutaneous photoaging versus intrinsic aging. J Geriatr Dermatol 1995, 3:131-143 [Google Scholar]
- 37.Moragas A, Garcia-Bonaf M, Sans M, Toran N, Huguet P, Martin-Plata C: Image analysis of dermal collagen changes during skin aging. Analyt Quant Cytol. Histol 1998, 20:493-499 [PubMed] [Google Scholar]
- 38.Kligman LH, Kligman AM: The nature of photoaging: its prevention and repair. Photodermatology 1986, 3:215-221 [PubMed] [Google Scholar]
- 39.Varani J, Fisher GJ, Kang S, Voorhees JJ: Molecular mechanisms of intrinsic skin aging and retinoid-induced repair and reversal. J Invest Dermatol 1988, 3:57-60 [PubMed] [Google Scholar]
- 40.Kligman LH: Effects of all-trans retinoic acid on the dermis of hairless mice. J Am Acad Dermatol 1986, 15:779-785 [DOI] [PubMed] [Google Scholar]
- 41.Kligman LH, Duo CH, Kligman AM: Topical retinoic acid enhances the repair of ultraviolet-damaged dermal connective tissue. Connect Tissue Res 1984, 12:139-150 [DOI] [PubMed] [Google Scholar]
- 42.Varani J, Warner RL, Mehrnaz G-K, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ: Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000, 114:480-486 [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa S, Pawelek P, Grinnell F: Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res 1989, 182:572-582 [DOI] [PubMed] [Google Scholar]
- 44.Fukamizu H, Grinnell F: Spatial organization of extracellular matrix and fibroblast activity: effects of serum, transforming growth factor b and fibronectin. Exp Cell Res 1990, 190:276-282 [DOI] [PubMed] [Google Scholar]
- 45.Lin Y-C, Grinnell F: Decreased level of PDGF-stimulated receptor autophosphorylation by fibroblasts in mechanically relaxed collagen matrices. J Cell Biol 1993, 122:663-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racine-Samson L, Rockey DC, Bissell DM: The role of α1β1 integrin in wound contraction: a quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem 1977, 272:30911-30917 [DOI] [PubMed] [Google Scholar]
- 47.Vogel W: Discoidin domain receptors: structural relations and functional implications. FASEB J 1999, 13(Suppl):S77-S82 [DOI] [PubMed] [Google Scholar]
- 48.Cawston TE: Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther 1996, 70:163-182 [DOI] [PubMed] [Google Scholar]
- 49.Li AK, Chir B, Ehrlich HP, Trelstad RL, Koroly MJ, Schattenkerk ME, Malt RA: Differences in healing of skin wounds caused by burn and freeze injuries. Ann Surgery 1980, 191:244-248 [DOI] [PMC free article] [PubMed] [Google Scholar]