Abstract
Transgenic mice (Tg2576) overexpressing human β-amyloid precursor protein with the Swedish mutation (APP695SWE) develop Alzheimer’s disease-like amyloid β protein (Aβ) deposits by 8 to 10 months of age. These mice show elevated levels of Aβ40 and Aβ42, as well as an age-related increase in diffuse and compact senile plaques in the brain. Senile plaque load was quantitated in the hippocampus and neocortex of 8- to 19-month-old male and female Tg2576 mice. In all mice, plaque burden increased markedly after the age of 12 months. At 15 and 19 months of age, senile plaque load was significantly greater in females than in males; in 91 mice studied at 15 months of age, the area occupied by plaques in female Tg2576 mice was nearly three times that of males. By enzyme-linked immunosorbent assay, female mice also had more Aβ40 and Aβ42 in the brain than did males, although this difference was less pronounced than the difference in histological plaque load. These data show that senescent female Tg2576 mice deposit more amyloid in the brain than do male mice, and may provide an animal model in which the influence of sex differences on cerebral amyloid pathology can be evaluated.
Alzheimer’s disease (AD), the most common cause of dementia, is characterized neuropathologically by senile plaques, neurofibrillary tangles, and neuronal degeneration. The cores of senile plaques consist mainly of the amyloidogenic peptide Aβ, which is derived from the β-amyloid precursor protein (βAPP). 1 Mutations in the genes for βAPP and presenilins 1 and 2 cause rare, familial forms of AD that are indistinguishable clinically and pathologically from sporadic AD. 1-4 Specific mutations in both βAPP and the presenilins alter the processing of βAPP, thereby increasing the generation of Aβ, especially Aβ42.
Women have been reported to be disproportionately susceptible to AD, even after accounting for their longer survival. 5,6 After 85 years of age, the preponderance of dementia in women increases even further. 7 The basis for this sex difference remains unknown, although the postmenopausal decline in circulating estrogen 8 has been implicated, in part because estrogen replacement therapy decreases the risk, or delays the onset, of AD in women. 9
Mice transgenic for mutated human βAPP exhibit age-related deposition of cerebral Aβ. 10-12 Tg2576 mice overexpressing human βAPP with the Swedish mutation begin to develop Aβ-positive senile plaques by 8 to 10 months of age, 11,13 and have a substantial plaque burden by ∼15 months. We report that senile plaques are significantly more abundant in female Tg2576 mice than in males, suggesting that sex and/or endocrine factors strongly modulate cerebral β-amyloidogenesis in βAPP-transgenic mice.
Materials and Methods
Animals
Tg2576 mice (n = 134) with the Swedish mutation (APP695SWE) were bred from lines described previously. 11 Male Tg2576 mice were backcrossed to (C57BL/6 × SJL)F1 female breeders. Mice were singly housed under a 12-hour light:12-hour dark schedule with food and water provided ad libitum. Animal care and surgical procedures were conducted in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Housing facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care. All experimental procedures were approved by an internal animal use committee.
Ninety-one mice (32 males, 59 females) were studied at 15 months of age. Additional mice were analyzed at the ages of 8 (five males, seven females), 12 (nine males, nine females), and 19 (six males, seven females) months. Mice were killed under deep pentobarbital anesthesia (200 mg/kg, intraperitoneally) and perfused for 2 minutes with cold phosphate-buffered saline. The cerebellum and caudal brainstem were removed and the forebrain bisected midsagittally. The left hemisphere of the brain was placed in fixative (4% paraformaldehyde) for histopathology, and the right hemisphere was quickly frozen for enzyme-linked immunosorbent assay (ELISA) measurement of Aβ40 and Aβ42 levels.
Quantitative Histopathological Analysis
Sagittal sections (30 μm) through the entire left hemisphere were stained with the Campbell-Switzer silver AD stain (Neuroscience Assoc., Knoxville, TN), a highly sensitive marker of Aβ deposits. 14 Four sections, starting at a random plane near the midline and evenly spaced from medial to lateral, were quantitated by point-counting techniques 15,16 using CAST-Grid software version 1.20 (Olympus, Albertslund, Denmark). The PC-based, color-video image analysis system was linked to an Olympus microscope. A motorized stage for movement in the x-y-z axes was interfaced between the microscope and the computer. To determine the combined area of the hippocampus and neocortex, the system software superimposed grid marks over a low-magnification video image of each tissue section, and the grid marks overlying the area of interest were counted. A calibration slide was used to calculate the area in μm 2 represented by each grid mark in the field. A similar calibration was performed at higher magnification (×20 objective) and used to estimate the percent area occupied by amyloid deposits in the hippocampus and neocortex. In addition, tissue sections from 15-month-old mice were stained immunohistochemically with monoclonal antibody 4G8 (Senetek, Maryland Heights, MO) to amino acids 17 to 24 of Aβ, or with Congo Red (n = 17 and 10, respectively).
ELISA Analysis
Aβ40 and Aβ42 were quantitated by sandwich ELISAs in the right forebrain of 32 15-month-old mice (11 males, 21 females). The tissues were maintained at −80°C until the day of assay and then brought up to 4°C by placing the samples on ice and covering each one with 1 ml of 0.5 mol/L Tris-buffered saline (TBS) that contained a protease inhibitor cocktail and 0.05 mol/L ethylenediaminetetraacetic acid. Tissues were Dounce-homogenized and then centrifuged at 100,000 × g for 45 minutes. The supernatants were drawn off and Tween-20 was added to bring the final concentration to 0.02%. The pellet was resuspended in 1 ml of 2% diethylamine (DEA) in 50 mmol/L NaCl with a probe-sonicator and centrifuged at 100,000 × g for 45 minutes at 4°C. The supernatant was drawn off and 0.5 ml of 2 mol/L Tris-HCl was added to each sample to bring the pH to 8.0. The remaining pellet was dissolved in 1 ml of 88% glass-distilled formic acid, probe-sonicated, and centrifuged at 100,000 × g for 45 minutes. The aqueous supernatant layer, between the small pellet at the bottom and the lipid layer on top, was carefully removed. These supernatant samples were completely dried overnight in a speed-vac (Savant Instruments, Holbrook, NY). The dried pellets were resolubilized in DEA buffer, sonicated, and centrifuged at 1,000 × g for 15 minutes to pellet the DEA-insoluble fraction. The resolubilized, acid-extracted supernatant samples were then neutralized to pH 8.0 by addition of 2 mol/L Tris-HCl. The supernatants from each of the above extraction steps were analyzed for Aβ40 and Aβ42 by ELISA using a biotinylated detection antibody as described previously. 17 In all assays the monoclonal antibody 4G8 was biotinylated and used as the detection antibody. Capture antibodies for coating the wells of the microtiter plates were the rabbit polyclonal antibodies R163 or R165 (5 μg/ml), which specifically recognize AβX-40 or AβX-42, respectively. 18 No cross-reactivity of R165 against Aβ1-40 or of R163 for Aβ1-42 was detected at peptide concentrations <100 ng/ml. The limit of sensitivity for both assays was 0.3 ng/ml Aβ1-40 or Aβ1-42. A protein assay was run on the Triton-TBS (TTBS) extracts and this value was used to express Aβ concentrations obtained from the ELISA assay in ng/mg protein. The amount of soluble Aβ in TTBS was obtained from the assay of the initial TTBS extracts. Insoluble Aβ was derived by summing the values obtained in the DEA and formic acid extraction assays.
Statistics
Differences between the groups were evaluated for statistical significance using analysis of variance followed by pairwise comparison of the means using a post hoc Newman-Keuls test. The analyses were performed using Sigmastat version 2.03 software (SPSS Inc., Chicago, IL).
Results
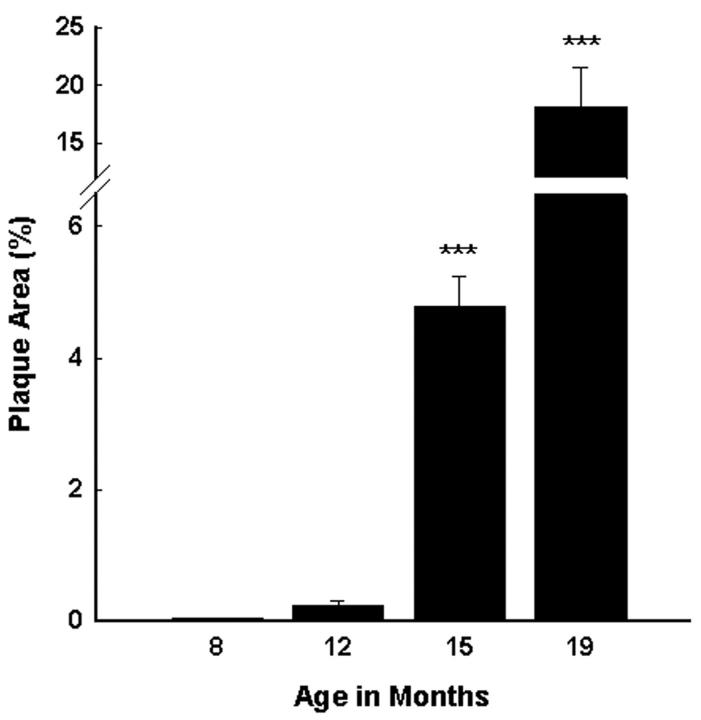
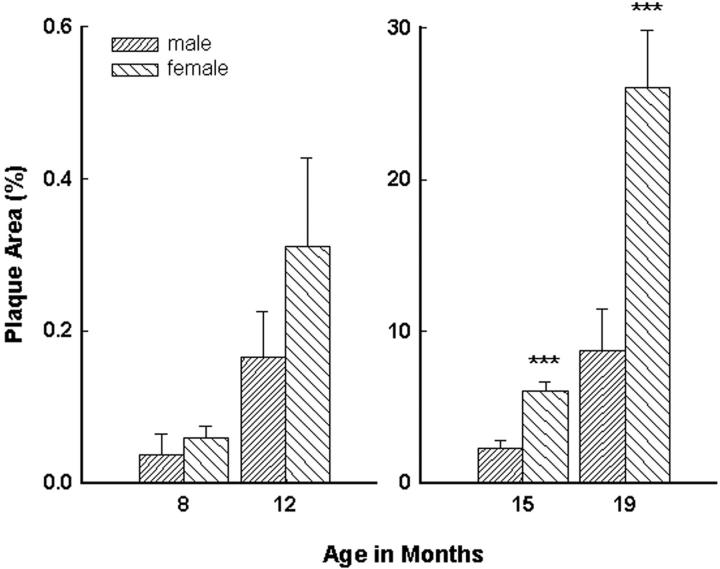
Tg2576 mice show a marked age-related increase in amyloid deposition in the hippocampus and neocortex [F(3,130) = 36.51, P < 0.001] (Figure 1) ▶ . Amyloid deposition in female Tg2576 mice was consistently greater than in males (Figure 2) ▶ . This difference between males and females was statistically significant in 15- and 19-month-old animals [F(7,126) = 34.42, P < 0.001], when amyloid burden is high (Figure 3) ▶ . Within each age group, the percent area of the hippocampus and neocortex occupied by amyloid deposits was quite variable. For example, in 15-month-old mice, the percent area occupied by amyloid in males ranged from 0.3% to 13.8%, and in females from 0.4% to 15.3%. However, in a given mouse, the calculated percent plaque area was usually consistent in all of the four tissue sections analyzed. Quantitation of Aβ deposits immunostained with antibody 4G8 showed a strong correlation with the areal density seen with the Campbell-Switzer method (r = 0.82, P < 0.0001). Congo Red staining revealed a number of congophilic deposits at 15 months of age, but the majority (98%) of the plaque area assessed was occupied by diffuse, noncongophilic plaques.
Figure 1.
Tg2576 mice have an age-related increase in the percent area of the hippocampus and neocortex occupied by Aβ deposits as quantitated using a point-counting technique. Data evaluated by analysis of variance followed by a post hoc Newman-Keuls test. ***, P < 0.001, compared to all other age groups.
Figure 2.
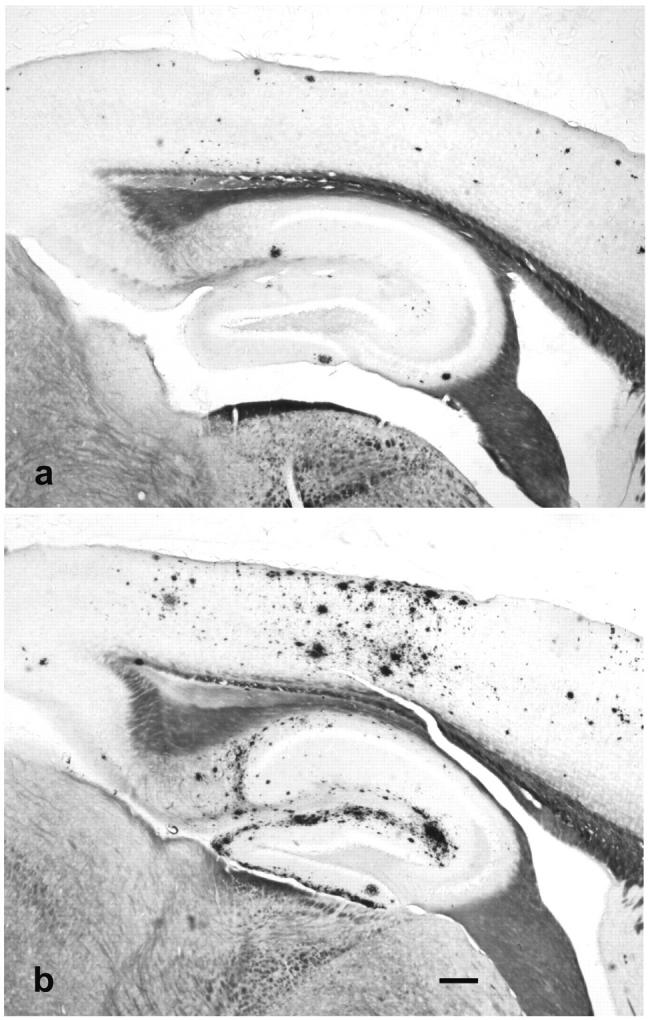
Campbell-Switzer silver AD-stained sagittal tissue sections under low (×4 objective) magnification from male (a) and female (b) Tg2576 mice representing mean percent plaque areas of 2.31% and 6.11%, respectively, at 15 months of age. The Aβ deposits are stained black or dark brown with this method. The silver stain normally turns myelinated pathways a golden brown color; these are easily distinguished from Aβ deposits under the microscope. Scale bar, 200 μm.
Figure 3.
At 15 months of age, female Tg2576 mice have a threefold greater percent area of the hippocampus and neocortex occupied by senile plaques. Data evaluated by analysis of variance followed by a post hoc Newman-Keuls test. ***, P < 0.001 compared to male mice.
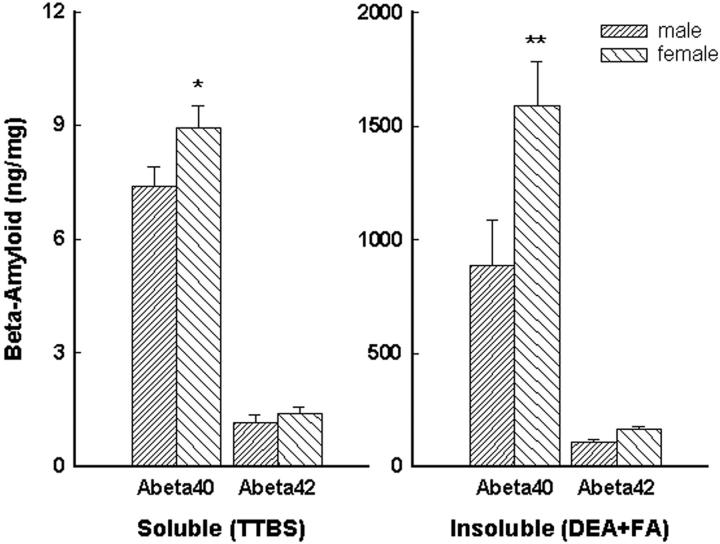
Aβ40 and Aβ42 levels in 15-month-old mice, measured by ELISA, also were higher in females than in males (Figure 4) ▶ . This difference was statistically significant for Aβ40 measured in the soluble TTBS extract [F(3,60) = 87.3, P < 0.001] and in the insoluble DEA and formic acid extract [F(3,60) = 18.5, P < 0.001]. Aβ42 levels, though slightly higher in females, did not differ significantly between males and females. The levels of Aβ measured by ELISA correlated with the calculated percent plaque area in the opposite hemisphere of the same mice. Statistically significant correlations were seen between amyloid plaques and soluble Aβ40 (r = 0.57, P < 0.001) and Aβ42 (r = 0.36, P < 0.05), and insoluble Aβ40 (r = 0.58, P < 0.001), and Aβ42 (r = 0.71, P < 0.0001).
Figure 4.
In 15-month-old Tg2576 mice, soluble and insoluble Aβ levels, measured by ELISA, were increased in females compared to males. This increase was significant for Aβ40 in both the soluble and insoluble extracts. Data evaluated by analysis of variance followed by post hoc Newman-Keuls test. *, P < 0.05; **, P < 0.01 compared to male mice.
Discussion
Aged female Tg2576 mice with the human βAPP695SWE transgene deposit significantly more Aβ in the brain than do aged male mice. This sex difference in amyloid load was apparent by 12 months of age, but was most pronounced at 15 and 19 months. At 15 months (the age at which our largest group of mice was studied) female transgenic mice had approximately three times the senile plaque load of males. By ELISA, Aβ levels also were higher in female mice than in males; this difference was particularly true for Aβ40, which is the most abundant Aβ-peptide species in Tg2576 mice. Although Aβ-peptide levels in these mice correlated significantly with senile plaque load, the correlation coefficients were not as high as might be expected (r = 0.36 to 0.57 for soluble Aβ, and 0.58 to 0.71 for insoluble Aβ). The reasons for this partial disconnection are unknown, but the results suggest the possible presence of a significant pool of Aβ in the aged brain that is not histologically detectable.
Our preliminary data suggest that 15-month-old female Tg2576 mice perform slightly, but not significantly, worse than males on the Morris Water Maze task (Lipinski WJ, Pack A, Callahan MJ and Walker LC, unpublished). However, we have seen no correlation between Aβ load and water maze performance, at least in mice up to 15 months of age. 19 More sensitive tests are needed to rule out an influence of β-amyloid deposition on behavior in male and female Tg2576 mice.
The higher age-specific rate of AD in women than in men 7,20 may be linked to changes in hormonal levels at menopause; 21 senescent men are thought to be slightly more resistant to AD because they secrete relatively constant levels of testosterone, which is partially converted to estradiol. Epidemiological studies indicate that estrogen replacement therapy in postmenopausal women decreases the risk of AD or delays its onset. 9 The interaction of estrogen with neurotrophins, 22 oxidative stress, 23,24 apolipoprotein E levels, 25 and Aβ processing 26-28 are among the mechanisms suggested to modulate the pathogenesis of AD. Although AD treatment trials with estrogen have been disappointing to date, 29-31 prevention trials may be necessary to demonstrate the beneficial effects of estrogen. 29
The appearance of senile plaques in Tg2576 mice coincides approximately with the onset of reproductive senescence. In mice, females display normal signs of estrus, including mating behavior, every 4 to 5 days. The estrous cycle is constantly repeated until ∼11 months of age and becomes irregular between 12 and 14 months, with cessation of the cycles and ultimately persistent anestrus thereafter. 32 These changes indicate that female mice, like humans and nonhuman primates, 33 experience pronounced age-related changes in reproductive physiology.
Various reports have suggested a role for gonadal hormones in Aβ processing. Physiological concentrations of 17β-estradiol reduce the release of Aβ40 and Aβ42 by primary neuronal cultures. 27 In vivo, ovariectomized guinea pigs demonstrate elevated levels of Aβ that are partially reversed by estrogen replacement. 28 However, preliminary studies in neuroblastoma cells bearing βAPPSWE suggest that estrogen actually increases the release of Aβ40 and Aβ42, in contrast to the reduction of Aβ in neuroblastoma cells expressing wild-type βAPP (H. Xu, P. Greengard, and S. Gandy, personal communication). Thus, it may be that the augmented plaque load in female Tg2576 mice is because of this paradoxical ability of estrogen to stimulate Aβ generation from βAPPSWE during development and young adulthood (stages associated with normal levels of circulating estrogen), rather than to hormonal changes that occur in later life.
βAPP-transgenic mice are now widely used in academic and industrial laboratories to decipher the mechanisms of β-amyloidogenesis and to test therapeutic compounds. The discovery that female Tg2576 mice are particularly disposed to deposit Aβ suggests that β-amyloid pathogenesis may differ in subtle but important ways in males and females. The experimental manipulation of hormonal (and other) parameters in βAPP-transgenic mice may yield clues to the factors regulating the age-related accumulation of cerebral Aβ and the pathogenesis of AD. Our results also caution that the gender of βAPP-transgenic mice should be considered in the design of studies on murine β-amyloid pathology.
Acknowledgments
We thank Drs. Sam Gandy (New York University) and Arnold Gerall (Tulane University) for their helpful comments on the manuscript; and Drs. Mark Emmerling and Harry LeVine (Pfizer) for many enlightening discussions.
Footnotes
Address reprint requests to Michael J. Callahan, Neuroscience Therapeutics, PGRD, Ann Arbor Laboratories, 2800 Plymouth Rd., Ann Arbor, MI 48105. E-mail: michael.callahan@pfizer.com.
Supported by Pfizer Global Research and Development.
References
- 1.Selkoe DJ: Biology of β-amyloid precursor protein and the mechanism of Alzheimer disease. ed 2 Terry RD Katzman R Bick KL Sisodia SS eds. Alzheimer Disease, 1999, :pp 293-310 Lippincott Williams and Wilkins, Philadelphia [Google Scholar]
- 2.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L: A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1992, 1:345-347 [DOI] [PubMed] [Google Scholar]
- 3.Hardy J: Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 1997, 20:154-159 [DOI] [PubMed] [Google Scholar]
- 4.Price DL, Sisodia SS: Mutant genes in familial Alzheimer’s disease and transgenic models. Ann Rev Neurosci 1998, 21:479-505 [DOI] [PubMed] [Google Scholar]
- 5.Molsa PK, Marttila RJ, Rinne UK: Epidemiology of dementia in a Finnish population. Acta Neurol Scand 1982, 65:541-552 [DOI] [PubMed] [Google Scholar]
- 6.Jorm AF, Korten AE, Henderson AS: The prevalence and incidence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987, 76:465-479 [DOI] [PubMed] [Google Scholar]
- 7.Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B: Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology 1997, 48:132-138 [DOI] [PubMed] [Google Scholar]
- 8.Sherman BM, West JH, Korenman SG: The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentration during menstrual cycles of older women. J Clin Endocrinol Metab 1976, 42:629-636 [DOI] [PubMed] [Google Scholar]
- 9.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R: Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 1996, 348:429-432 [DOI] [PubMed] [Google Scholar]
- 10.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guild T, Hagoplan S, Johnson-Wood K, Kahn K, Lee M, Leibowitz P, Lieberbug I, Little S, Masilah E, McConlogue L, Montoya-Zavals M, Mucke L, Paganni L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao B: Alzheimer-type neuropathology in transgenic mice overexpressing V717 β-amyloid precursor protein. Nature 1995, 373:523-527 [DOI] [PubMed] [Google Scholar]
- 11.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G: Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274:99-102 [DOI] [PubMed] [Google Scholar]
- 12.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold K-H, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B: Two amyloid precursor protein transgenic mouse models with Alzheimer’s disease-like pathology. Proc Natl Acad Sci USA 1997, 94:13287-13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao K, Pogemiller L, Clark HB: Age-related neuropathology in transgenic mice with human mutant APP. Soc Neurosci Abstr 1997, 23:1647 [Google Scholar]
- 14.Campbell SK, Switzer RC, III, Martin TL: Alzheimer’s plaques and tangles: a controlled and enhanced silver staining method. Soc Neurosci Abstr 1987, 13:687 [Google Scholar]
- 15.Gundersen HJ, Jensen EB: The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987, 147:229-263 [DOI] [PubMed] [Google Scholar]
- 16.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ: The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988, 96:875-881 [DOI] [PubMed] [Google Scholar]
- 17.Carroll RT, Lust MR, Kim KS, Doyle PD, Emmerling MR: An age-related correlation between levels of beta-amyloid precursor protein and beta-amyloid in human cerebrospinal fluid. Biochem Biophys Res Commun 1995, 210:345-349 [DOI] [PubMed] [Google Scholar]
- 18.Mehta PD, Dalton AJ, Mehta SP, Kim KS, Sersen EA, Wisniewski HM: Increased plasma amyloid beta protein 1-42 levels in Down syndrome. Neurosci Lett 1998, 241:13-16 [DOI] [PubMed] [Google Scholar]
- 19.Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D: Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 1999, 29:177-185 [DOI] [PubMed] [Google Scholar]
- 20.Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL: Development of dementing illnesses in a 80-year-old volunteer cohort. Ann Neurol 1989, 25:317-324 [DOI] [PubMed] [Google Scholar]
- 21.Seeman MV: Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997, 154:1641-1647 [DOI] [PubMed] [Google Scholar]
- 22.Miranda RC, Sohrabji F, Toran-Allerand CD: Presumptive estrogen target neurons express mRNAs for both the neurotrophins and neurotrophin receptors: a basis for potential developmental interactions of estrogen with neurotrophins. Mol Cell Neurosci 1993, 4:510-525 [DOI] [PubMed] [Google Scholar]
- 23.Niki E, Nakano M: Estrogens as antioxidants. Methods Enzymol 1990, 186:330-333 [DOI] [PubMed] [Google Scholar]
- 24.Goodman Y, Bruce AJ, Cheng B, Mattson MP: Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury and amyloid β-peptide toxicity in hippocampal neurons. J Neurochem 1996, 66:1836-1844 [DOI] [PubMed] [Google Scholar]
- 25.Applebaum-Bowden D, McLean P, Steinmetz A, Fontana D, Matthys C, Warnick GR, Cheung M, Albers JJ, Hazzard WR: Lipoprotein, apolipoprotein, and lipolytic enzyme changes following estrogen administration in postmenopausal women. J Lipid Res 1989, 30:1895-1906 [PubMed] [Google Scholar]
- 26.Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE: Estrogen regulates metabolism of Alzheimer amyloid β precursor protein. J Biol Chem 1994, 269:13065-13068 [PubMed] [Google Scholar]
- 27.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S: Estrogen reduces neuronal generation of Alzheimer β-amyloid peptides. Nat Med 1998, 4:447-451 [DOI] [PubMed] [Google Scholar]
- 28.Petanceska SS, Nagy V, Frail D, Gandy S: Ovariectomy and 17β-estradiol modulate the levels of Alzheimer’s amyloid β peptides in brain. Neurology 2000, 54:2212-2217 [DOI] [PubMed] [Google Scholar]
- 29.Sano M: Understanding the role of estrogen on cognition and dementia. J Neural Transm Suppl 2000, 59:223-229 [DOI] [PubMed] [Google Scholar]
- 30.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ: Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA 2000, 283:1007-1015 [DOI] [PubMed] [Google Scholar]
- 31.Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC: Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 2000, 54:2061-2066 [DOI] [PubMed] [Google Scholar]
- 32.Maekawa A, Maita K: Changes in the uterus and vagina. Mohr U Dungworth DL Capen CC Carlton WW Sundberg JP Ward JM eds. Pathobiology of the Aging Mouse, 1996, vol 1.:pp 469-493 International Life Sciences Institute Press, Washington, DC [Google Scholar]
- 33.Walker ML: Menopause in female rhesus monkeys. Am J Primatol 1995, 35:59-71 [DOI] [PMC free article] [PubMed] [Google Scholar]