Abstract
Hypertrophy of mesangial cells is one of the earliest morphological alterations in the kidney after the onset of diabetes mellitus. We have previously shown that cultured mesangial cells exposed to high ambient glucose arrest in the G1 phase of the cell cycle and that this is associated with an increased expression of inhibitors of the cyclin-dependent kinase (CDK)-inhibitors p21Cip and p27Kip1. To further investigate a potential role of p27Kip1 in the development of glucose-induced hypertrophy, mesangial cells from p27Kip1 wild-type (+/+) and knockout (−/−) mice were established. High glucose medium (450 mg/dl) increased p21Cip1 protein in p27Kip1+/+ and −/− mesangial cells, and increased p27Kip1 protein levels in p27Kip1+/+ cells. In contrast to high glucose increasing de novo protein synthesis in p27Kip1+/+ cells, high glucose did not increase protein synthesis in p27Kip1−/− cells. High glucose also reduced DNA synthesis and caused cell cycle arrest in p27Kip1+/+ cells. In contrast, despite an increase in transforming growth factor (TGF)-β mRNA and protein expression, DNA synthesis and cell cycle progression were increased by high glucose in p27Kip1−/− cells. Exogenous TGF-β comparably induced fibronectin mRNA in p27Kip1+/+ and −/− cells suggesting intact TGF-β receptor transduction. In addition, high glucose failed to increase the total protein/cell number ratio in p27Kip1−/− cells. However, in the presence of high glucose, reconstituting p27Kip1 expression by transient or stable transfection in p27Kip1−/− cells, using an inducible expression system, increased the de novo protein synthesis and restored G1-phase arrest. These results show that p27Kip1 is required for glucose-induced mesangial cell hypertrophy and cell cycle arrest.
Mesangial expansion, one of the earliest renal abnormalities observed after the onset of hyperglycemia in diabetes mellitus, is because of growth of mesangial cells, as well as an increase in glomerular extracellular matrix accumulation. 1-4 In vivo studies in different models of type I and II diabetes mellitus, and cell culture studies using mesangial cells exposed to high glucose, have shown a characterized biphasic growth response. First, there is an early and self-limited proliferation of mesangial cells, which is followed by cell cycle arrest in the G1 phase of the cell cycle. This is followed by glomerular cell persistent and progressive hypertrophy. 5-7
Cell proliferation is regulated at the level of the cell cycle by cell cycle proteins, where activation of cyclin-dependent kinases (CDK) is required for progression through the cell cycle. In contrast, CDK inhibitors inactivate CDKs, and cause cell cycle arrest. There is a growing body of literature showing that CDK inhibitors may also be critical regulators of cell hypertrophy. 8-10 The present study was undertaken to determine the role of the CDK inhibitor p27Kip1 in mediating glucose-induced mesangial cell hypertrophy. We show that in contrast to p27Kip1+/+ mesangial cells, p27Kip1−/− mesangial cells do not undergo glucose-induced hypertrophy. However, reconstituting p27 levels is necessary to induce hypertrophy in p27Kip1−/− cells. Our results provide evidence for a role of p27Kip1 in high glucose-induced hypertrophy of cultured mesangial cells.
Materials and Methods
Cell Culture
Mesangial cells from litter mate p27Kip1+/+ and p27Kip1−/− mice were isolated by differential sieving and characterized as previously described. 11 Cells were grown in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Eggenstein, Germany) containing 100 mg/dl d-glucose (G100) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/L glutamine. Mesangial cells were cultured at 37°C in 5% CO2, and passaged every 4 to 5 days. Experiments were done using cells of passages 10 to 20.
Inducible p27Kip1 Expression Construct and Transfections
A full-length mouse p27Kip1 cDNA was constructed using reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. Briefly, total RNA was isolated from murine mesangial cells 12 rested in serum-free medium. Ten μg of total RNA was reverse-transcribed using 0.7 μg of poly-d(T)primer (Pharmacia Diagnostics, Freiburg, Germany) in the presence of 500 U of Maloney murine leukemia virus reverse transcriptase diluted in 50 μl of a buffer containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L dithiothreitol, and 500 μmol/L dNTP. After incubation for 2 hours at 37°C, 5 μl of the cDNA preparation was directly used for the PCR amplification with 5 μl of 10× amplification buffer, 25 mmol/L MgCl2, 10 mmol/L dNTPs, and 1.5 μl of each primer (50 ng/μl), and 2.5 U Taq-polymerase (Promega, Madison, WI). The following primers specific for the murine p27Kip1 were used: 5′GGGCCACCATGCAAACGTGAGAGTG3′, 5′GCTGTTTACGTCTGGCGTCGAAGG3′. 13 The 5′end primer contained an optimized Kozak sequence for efficient mRNA translation initiation. 14 A total of 40 amplification cycles (denaturing for 30 seconds at 94°C, annealing for 90 seconds at 50°C, and extension for 90 seconds at 72°C) were performed. Fifteen μl of the reaction product were separated in a 1.8% agarose gel containing 0.5 μg/ml ethidium bromide and a single band of the predicted 607 bp was isolated using a DEAE membrane.
The full-length p27Kip1 cDNA was then cloned into the pIND TOPO vector (Invitrogen, Leek, The Netherlands) and the correct orientation was confirmed by sequencing. The expression plasmid pIND contains five modified ecdysone response elements upstream of a minimal heat-shock promoter allowing inducible expression. 15 For transient transfections, 10 5 cells were rested in serum-free medium for 24 hours and were co-transfected with 10 μg of pINDp27Kip1 and 10 μg of the plasmid pVgRXR (Invitrogen) encoding subunits of the ecdysone receptor using lipofectin (Gibco-BRL) as previously described. 8 After 12 hours, cells were split into 24- or 96-well plates (for measurement of leucine and thymidine incorporation) or left in cell culture flasks (for Western blots). Cells were then treated with 0 to 5 μg/ml muristone (Invitrogen) to induce p27Kip1 expression.
To establish stabile cell lines, p27Kip1−/− mesangial cells were first transfected with pVgRXR and stable clones were selected in medium with 500 μg/ml Zeocin (Invitrogen) by several rounds of limited dilution. A stable cell line expressing pVgRXR was subsequently transfected with pINDp27Kip1 and selection was then performed in medium with 500 μg/ml of Zeocin and 250 μg/ml of G418 (Sigma). Surviving cells were cloned by three rounds of limited dilution and several stable cell lines containing pVgRXR and pINDp27Kip1 were established. These cell lines were carried normally in medium containing Zeocin and G418 to prevent back mutations.
Measuring the Expression of CDK Inhibitors
A total of 10 6 cells from the various cell lines and clones were incubated in serum-free G100 or G450 (450 mg/dl d-glucose) medium for 48 hours. To restore p27Kip1 expression, p27Kip1−/− cells were transient-transfected with pINDp27Kip1 or permanent-transfected clones (clones 5.2, 6.2) were treated with 0 to 5 μg/ml of muristone for 24 hours. Cells were rinsed twice in ice-cold phosphate-buffered saline (PBS) at the end of the incubation period. After removing all PBS, monolayers were directly lysed in 150 μl of lysis buffer (2% sodium dodecyl sulfate, 60 mmol/L Tris-HCl, pH 6.8), and the protein content was measured in supernatants after centrifugation by a modification of the Lowry method that is insensitive to the used concentrations of sodium dodecyl sulfate. 8 Protein concentrations were adjusted to 80 μg/sample, and 100 mmol/L dithiothreitol, 5% glycerol, and 0.03% bromophenol blue were added and samples were boiled for 5 minutes. After centrifugation, supernatants were loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel. Low molecular rainbow markers (comprising 2,350 to 45,000 Daltons; Amersham, Braunschweig, Germany) served as the molecular weight standards.
After completion of electrophoresis, proteins were electroblotted onto a nitrocellulose membrane (Highbond-N, Amersham) in transfer buffer (50 mmol/L Tris-HCl, pH 7.0; 380 mmol/L glycine, 20% methanol). Filters were stained with Ponceau S to control for equal loading and transfer. Membranes were blocked for 1 hour at room temperature with 5% nonfat dry milk redissolved in PBS with 0.1% Tween 20. For the detection of p27Kip1 protein, a 1:1,000 dilution of a mouse monoclonal anti-p27Kip1 antibody (Transduction Laboratories, Lexington, MA) was used. This antibody reacts with murine p27Kip1. p21Cip1 protein expression was detected with a mouse monoclonal anti-human p21Cip1 antibody exhibiting cross-reactivity with the murine protein at a 1:500 dilution (DAKO, Glostrup, Denmark). After incubation for another hour, membranes were washed in PBS with 0.1% Tween for 3 × 10 minutes, and a horseradish peroxidase-conjugated rabbit polyclonal anti-mouse antibody (Transduction Laboratories) was added at a 1:1,000 dilution. The luminescence detection of peroxidase activity was performed with the ECL system (Amersham) according to the manufacturer’s recommendations. To control for small variations in protein loading and transfer, membranes were washed and re-incubated with a mouse monoclonal anti-β-actin antibody (Sigma). Incubation with secondary antibody and detection was performed as described above. Exposed films were scanned with Fluor-S multi-imager (Bio-Rad Laboratories, Hercules, CA), and data were analyzed with the computer program MultiAnalyst from Bio-Rad. Western blots were independently performed three to four times with qualitatively similar results.
Northern Blot Hybridization for TGF-β and Fibronectin
p27Kip1−/− or +/+ cells (10 7 cells) were made quiescent in serum-free G100 medium and were stimulated for 48 hours in either serum-free G100 or G450 medium. Some cells were also treated in G100 with 1 ng/ml of ultrapure human transforming growth factor (TGF)-β1 (Sigma). After washing in RNase-free PBS, cells were directly lysed with acid guanidinium thiocyanate, and total RNA was isolated. 5 Equal amounts of total RNA (25 μg per lane) were denatured in formamide-formaldehyde at 65°C and electrophoresed through a 1.2% agarose gel containing 2.2 mol/L formaldehyde. Blotting, hybridization, and washing conditions were exactly as previously described. 5 A 0.7-kb PvuII cDNA fragment encoding human fibronectin was used. For control hybridizations, a 2.0-kb cDNA insert of the plasmid pMCI encoding the murine ribosomal 18S band was used. Northern blots were repeated twice with qualitatively similar result.
Determination of TGF-β Protein in Culture Supernatants
To investigate whether p27Kip1−/− and +/+ mouse mesangial cells increased the synthesis of TGF-β, 2 × 10 6 cells were plated in small culture flasks. After incubation for 12 hours in serum-free medium with normal glucose, cells were incubated for another 48 hours in either normal or high glucose medium. The supernatant was harvested and dried using a speed vac. Cell layers were lysed in 0.5 mol/L of NaOH and protein was determined with a modification of the Lowry method. TGF-β1 protein measurements were performed with a commercially available enzyme-linked immunosorbent assay (Predicta, Genzyme, Cambridge, MA). In brief, dried supernatants were reconstituted in 200 μl of sample diluent, activated by addition of 20 μl of 1 mol/L HCl, and neutralized by 15 μl of 1 mol/L NaOH. Measurements of TGF-β1 were done according to the manufacturer’s recommendations. Concentrations were calculated as pg TGF-β1 per μg protein. TGF-β1 measurements were independently repeated five times with qualitatively similar results.
Measuring Protein and DNA Synthesis
The incorporation of [3H]leucine was used to assess de novo protein synthesis. 5,8,9 Cells were plated (10 5 per well) in 24-well plates, and were made quiescent for 12 hours in normal glucose-containing medium. After an additional 12 hours, the medium was changed to normal glucose or high glucose for another 48 hours. Five μCi of [3H]leucine (142 Ci/mmol, Amersham) were included per well for the last 12 hours. At the end of the incubation period, cells were washed twice in ice-cold PBS and proteins were subsequently precipitated with ice-cold 10% trichloroacetic acid. After redissolving the precipitates in 0.5 mol/L NaOH containing 0.1% Triton X-100, 5 ml of scintillation cocktail (Roth, Karlsruhe, Germany) was added, and vials were measured by liquid scintillation spectroscopy. [3H]leucine incorporation experiments were repeated five times with duplicate measurements for each experiment.
The incorporation of [3H]thymidine into DNA was used to measure proliferation. Cells (10 4 cells per well) were transferred to a 96-well micrometer plate. After incubation for 12 hours in normal glucose medium, they were subsequently incubated for another 48 hours in either normal or high glucose. They were pulsed with 1 μCi [3H]thymidine (5 Ci/mmol, Amersham) during the last 6 hours of culture. At the end of the incubation period, MMCs were washed in PBS, trypsinized for 10 minutes at 37°C, and finally collected on glass-fiber paper with an automatic cell harvester. Radioactivity of dry filters was measured by liquid scintillation spectroscopy. [3H]thymidine experiments were independently performed four times with triplicate measurements.
A ratio of total protein content to cell number was determined as another parameter of cellular hypertrophy. For this experiment, 10 5 cells were seeded into each well of a 6-well plate and were made quiescent for 12 hours in normal glucose-containing medium. After incubation for another 48 hours as appropriate, cells were shortly trypsinized, scraped off the plate with a rubber policeman, and were washed twice in PBS. A small aliquot of cells was counted in a Fuchs-Rosenthal chamber after resuspension of cells in PBS. The remaining cells were lysed in 0.5 mol/L NaOH and total protein content was measured by a modified Lowry method. Total protein content was expressed as μg protein per 10 3 cells. These experiments were independently performed five times.
Statistical Analysis
All values are presented as means ± SEM. Statistical significance among multiple groups was tested with nonparametric Kruskal-Wallis test. Individual groups were then tested using the Wilcoxon-Mann-Whitney test. A P value of < 0.05 was considered significant.
Results
High Glucose Increases the Levels of CDK Inhibitors p21Cip1 and p27Kip1 in p27Kip1+/+ Cells
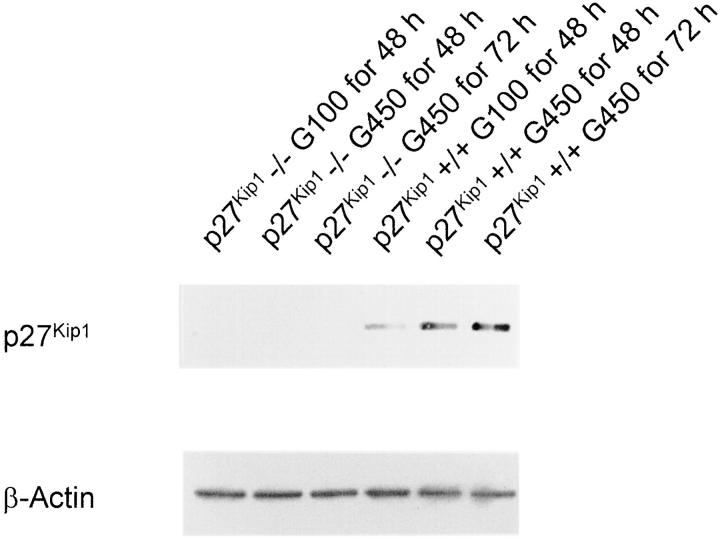
Figure 1 ▶ shows that quiescent p27Kip1+/+ mesangial cells express p27Kip1 protein. However, the incubation of p27Kip1+/+ mesangial cells in serum-free media with high glucose (450 mg/dl) for 48 to 72 hours increased the levels of p27Kip1 protein (Figure 1) ▶ (G100 for 48 hours:1.0 ± 0.0; G450 for 48 hours: 4.8 ± 0.6*; G450 for 72 hours: 6.2 ± 0.8* relative changes in p27Kip1 expression normalized to β-actin; *P < 0.05, n = 3.). As expected, p27Kip1 protein expression was totally absent in p27Kip1−/− mesangial cells and was not induced by high glucose (Figure 1) ▶ .
Figure 1.
Western blot of total cell lysates incubated with an antibody against p27Kip1. p27Kip1 expression was, as predicted, not induced in p27Kip1−/− mesangial cells incubated in serum-free medium with high glucose (G450; 450 mg/dl) for up to 72 hours. However, a strong high glucose-mediated induction of p27Kip1 was detectable in wild-type (p27Kip1+/+) cells. The membrane was re-incubated with an antibody against β-actin to demonstrate that these changes were not because of unequal loading or transfer of proteins. This blot is representative of three independent experiments with qualitatively similar results.
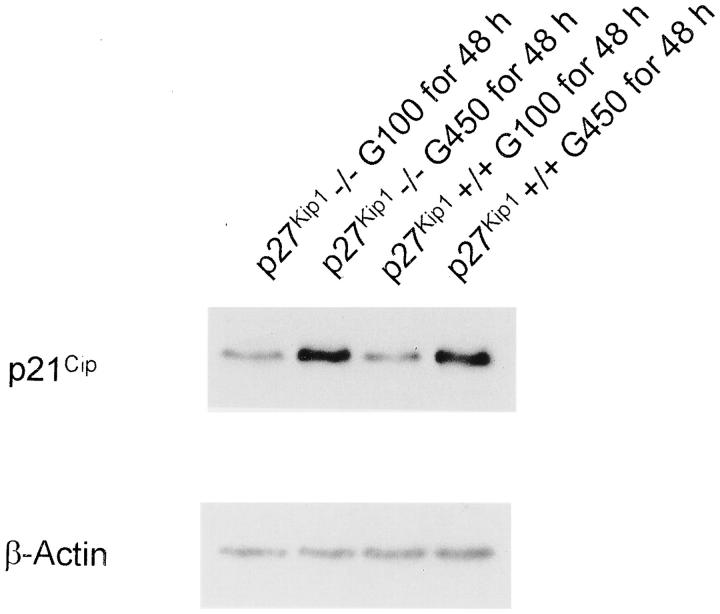
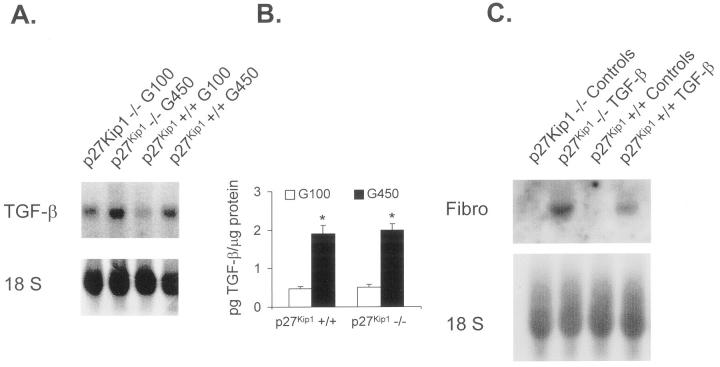
High glucose induced an increase in levels for the CDK inhibitor p21Cip1 in p27Kip1+/+ mesangial cells, and a similar increase in p21Cip1 expression was observed in p27Kip1−/− cells in response to high glucose (Figure 2) ▶ (p27Kip1−/− G100 for 48 hours: 1.0 ± 0.0; p27Kip1−/− G450 for 48 hours: 8.1 ± 1.1*; p27Kip1+/+ G100 for 48 hours: 1.0 ± 0.0, p27Kip1+/+ G450 for 48 hours: 8.6 ± 0.9*, relative changes in p21Cip expression normalized to β-actin; *P < 0.05, n = 3). To ensure that any differences in glucose-induced hypertrophy were not because of alterations in TGF-β expression, 5 TGF-β mRNA and protein were measured in p27Kip1+/+ and p27Kip1−/− mesangial cells grown in high glucose. Figure 3, A and B ▶ , shows that TGF-β mRNA as well as protein were increased similarly in both cell lines when incubated with high glucose medium (G450) for 48 hours. Moreover, exogenous TGF-β1 increased fibronectin mRNA expression in both p27Kip1−/− and +/+ cells (Figure 3C) ▶ . These results convincingly show that the response to high glucose is not different in p27Kip1+/+ and −/− mesangial cells, except for the expected lack of p27Kip1 response in the p27Kip1−/− cells. Furthermore, putative TGF-β1 receptors and signaling transduction pathways involved in fibronectin transcription are present in both cell lines.
Figure 2.
Western blot for p21Cip1 expression. In contrast to p27Kip1, high glucose for 48 hours increased p21Cip1 protein abundance in p27Kip1−/− and +/+ mesangial cells. This blot is representative of three independent experiments with qualitatively similar results.
Figure 3.
A: mRNA expression of TGF-β1. High glucose medium induces TGF-β transcripts independent of whether cells express p27Kip1 or not. This blot is representative of three independent experiments with qualitatively similar results. B: High glucose also leads to an equal amount of TGF-β1 protein synthesis in p27Kip1+/+ and −/− mesangial cells. *, P < 0.01, n = 5. C: Exogenous TGF-β1 induced fibronectin mRNA expression in p27Kip1+/+ and −/− cells indicating that both cell lines express TGF-β receptors and exhibit appropriate signal transduction systems downstream of the receptors. This blot is representative of three independent experiments with qualitatively similar results
High Glucose-Induced Hypertrophy Occurs in p27Kip1+/+ but Not p27Kip1−/− Cells
Proliferation was measured by [3H]thymidine incorporation. There was no significant difference in DNA synthesis in p27Kip1+/+ and −/− mesangial cells when grown in serum-free media with normal glucose (Table 1) ▶ . However, incubation of p27Kip1+/+ mesangial cells in serum-free medium with high glucose (G450) for 48 hours significantly reduced proliferation, consistent with glucose-induced cell cycle arrest in p27Kip1+/+ cells. In marked contrast, high glucose caused a significant increase in cell cycle progression and proliferation in p27Kip1−/− cells.
Table 1.
Effect of High Glucose Medium on Proliferation and Protein Synthesis of Cultured Mesangial Cells from Wild-Type and p27Kip1 Knockout Mice
| [3H]Thymidine (proliferation) | [3H]Leucine (de novo protein synthesis) | |
|---|---|---|
| p27Kip1+/+ in 100 mg/dl glucose | 4,646 ± 675 | 276 ± 16 |
| p27Kip1+/+ in 450 mg/dl glucose | 2,511 ± 277* | 369 ± 20* |
| p27Kip1−/− in 100 mg/dl glucose | 6,914 ± 1,126 | 878 ± 47* |
| p27Kip1−/− in 450 mg/dl glucose | 11,696 ± 1,720†‡ | 900 ± 26† |
cpm, incubation for 48 hours, n = 10–12.
*P < 0.05 versus p27Kip1+/+ in normal glucose (100 mg/dl).
†P < 0.05 versus p27Kip1−/− in normal glucose (100 mg/dl).
‡P < 0.05 versus p27Kip1+/+ in high glucose (450 mg/dl).
The de novo synthesis of protein in response to high glucose was measured by [3H]leucine incorporation and the results are shown in Table 1 ▶ . High glucose stimulated de novo protein synthesis in p27Kip1+/+ mesangial cells. In contrast, [3H]leucine incorporation did not increase in p27Kip1−/− mesangial cells in response to high glucose (Table 1) ▶ .
In addition, total protein content and cell number were determined and a hypertrophy index (protein content divided by cell number) was calculated. As shown in Table 2 ▶ , high glucose significantly increased protein content/cell number only in p27Kip1 cells. Taken together, these results show that high glucose induced hypertrophy in p27Kip1+/+ mesangial cells, but not in p27Kip1−/− cells.
Table 2.
Effect of High Glucose Medium on Hypertrophy Index (Total Protein Content/Cell Number) of Cultured Mesangial Cells from Wild-Type and p27Kip1 Knockout Mice
| Protein content/cell number (μg/103 cells) | |
|---|---|
| p27Kip1+/+ in 100 mg/dl glucose | 1.63 ± 0.49 |
| p27Kip1+/+ in 450 mg/dl glucose | 4.27 ± 1.00* |
| p27Kip1−/− in 100 mg/dl glucose | 1.71 ± 0.26 |
| p27Kip1−/− in 450 mg/dl glucose | 2.08 ± 0.31 |
Incubation for 48 hours, n = 5–6.
*P < 0.05 versus p27Kip1+/+ in normal glucose (100 mg/dl).
Transient Reconstituting p27Kip1 Rescues the Hypertrophic Phenotype in p27Kip1−/− Cells
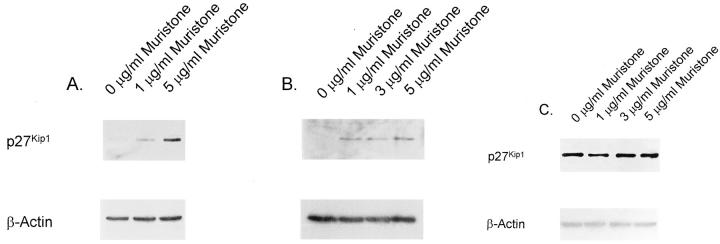
To confirm a role for p27Kip1 in glucose-induced hypertrophy, we used an ecdysone-inducible gene expression system to reconstitute p27Kip1 expression in p27Kip1−/− mesangial cells. This system offers the advantage of a lower basal activity compared to tetracycline-based expression vectors and the absence of any toxicity of the inducer muristone, a synthetic analog of the insect molting hormone ecdysone. 15 Figure 4A ▶ shows that p27Kip1−/− cells transiently transfected with pINDp27Kip1/pVgRXR grown in normal glucose medium (100 mg/dl) showed no detectable p27Kip1 protein expression in the absence of muristone. However, incubating cells with 1 and 5 μg/ml of muristone for 24 hours increased p27Kip1 expression (0 μg/ml muristone, 1.0 ± 0.0; 1 μg/ml muristone, 2.1 ± 0.3*; 5 μg/ml muristone, 4.7 ± 0.9* relative changes in p27Kip1 expression normalized to β-actin; P < 0.05, n = 3). A similar pattern was observed when cells were grown in high glucose medium (450 mg/dl; Figure 4B ▶ ; 0 μg/ml muristone: 1.0 ± 0.0, 1 μg/ml muristone: 3.1 ± 0.4*, 3 μg/ml muristone: 2.8 + 0.3*, 5 μg/ml muristone: 3.7 ± 0.5* relative changes in p27Kip1 expression normalized to β-actin; *P < 0.05, n = 3). However, the levels of p27Kip1 were not increased in p27Kip1+/+ cells transiently transfected with pINDp27Kip1/pVgRXR (Figure 4C) ▶ .
Figure 4.
A-C: Inducible p27Kip1 expression in transient transfected mesangial cells. A: p27Kip1−/− cells were transiently transfected with pINDp27Kip1/pVgRXR and grown in normal glucose medium without serum. One and 5 μg/ml of the synthetic ecdysone analog muristone for 24 hours strongly induced p27Kip1 expression as detected in this Western blot. B: However, no further induction of p27Kip1 was obtained when grown in high glucose indicating that the expression of the transgene is not under control of glucose. C: No additional induction of p27Kip1 was obtained with muristone in p27Kip1+/+ mesangial cells grown in high glucose medium that were transiently transfected with pINDp27Kip1/pVgRXR. These blots are representative of four independent experiments with qualitatively similar results.
The biological effect of transiently transfecting p27Kip1−/− cells is shown in Table 3 ▶ . Restoring p27Kip1 expression with muristone inhibited [3H]thymidine incorporation in p27Kip1−/− cells, and induced cell cycle arrest (Table 3) ▶ . Moreover, in the presence of high glucose, muristone induced cellular hypertrophy in p27Kip1−/− cells to levels comparable to p27Kip1+/+ cells (Table 4) ▶ . Thus, transiently reconstituting p27Kip1 was required to induced hypertrophy in p27Kip1−/− cells independent of the medium glucose content (Table 4) ▶ .
Table 3.
Effect of the Inducer Muristone on Proliferation and Protein Synthesis of p27Kip1−/− Transiently Transfected with pINDp27Kip1/pVgRXR
| [3H]Thymidine (proliferation) | [3H]Leucine (de novo protein synthesis) | |
|---|---|---|
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in mg/dl glucose without muristone | 1,061 ± 42 | 948 ± 73 |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 100 mg/dl glucose+ 2 μg/ml muristone | 846 ± 33* | 1,105 ± 198 |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 450 mg/dl glucose without muristone | 1,027 ± 35 | 1,272 ± 123 |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 450 mg/dl glucose+ 2 μg/ml muristone | 792 ± 36‡ | 1,787 ± 154† |
cpm, incubation for 24 hours, n = 10–12.
*P < 0.01 versus cells in 100 mg/dl glucose without muristone.
†P < 0.05 versus cells in 450 mg/dl glucose without muristone.
‡P < 0.01 versus cells in 450 mg/dl glucose without muristone.
Table 4.
Effect of the Inducer Muristone on Hypertrophy Index (Total Protein Content/Cell Number) of p27Kip1−/− Transiently Transfected with pINDp27Kip1/pVgRXR
| Protein content/cell number (μg/103 cells) | |
|---|---|
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 100 mg/dl glucose without muristone | 1.33 ± 0.22 |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 100 mg/dl glucose+ 2 μg/ml muristone | 2.04 ± 0.18* |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 450 mg/dl glucose without muristone | 1.93 ± 0.17 |
| p27Kip1−/− cotransfected with pINDp27Kip1/pVgRXR in 450 mg/dl glucose+ 2 μg/ml muristone | 2.50 ± 0.15† |
Incubation for 24 hours, n = 5.
*P < 0.05 versus cells in 100 mg/dl glucose without muristone.
†P < 0.05 versus cells in 450 mg/dl glucose without muristone.
p27Kip1−/− Cells Stably Transfected with Inducible p27Kip1 Undergo Glucose-Induced Hypertrophy
To circumvent some of the intrinsic problems using mass cultures of transiently transfected cells, stable cell lines were generated. Therefore, mesangial cells from p27Kip1−/− mice were first transfected with pVgRXR and selected in Zeocin to permanently express the ecdysone receptor. After several rounds of limited dilutions, a cell line was obtained that showed muristone-inducible expression of p27Kip1 after transient transfection with pINDp27Kip1 (data not shown). This cell line was subsequently transfected with pINDp27Kip1 and selection of surviving cells was performed in medium containing Zeocin and G418. Two cell lines (clones 5.2 and 6.2) were selected for further analysis.
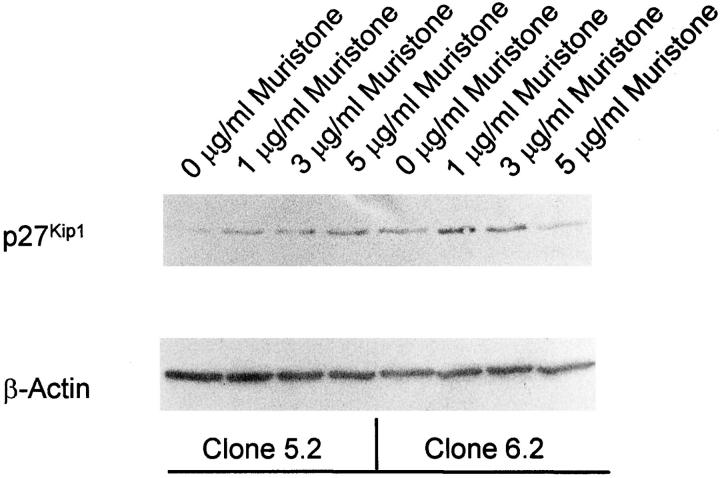
As shown in Figure 5 ▶ , muristone induced p27Kip1 expression in these two cell lines suggesting that the inducible expression systems works in permanent-transfected cell lines. However, in contrast to transient-transfected cells, clones 5.2 and 6.2 revealed some minimal basal p27Kip1 expression even in the absence of the inducer muristone indicating that the suppression is somewhat leaky (Figure 5) ▶ . Similar to the transiently transfected cells, muristone-induced p27Kip1 expression inhibited the proliferation of both cell clones in high glucose medium (Table 5) ▶ . In addition, muristone converted the proliferation into a hypertrophy phenotype only in the presence of high glucose (Table 5) ▶ .
Figure 5.
Western blot for p27Kip1 in clones 5.2 and 6.2, two stable transfected clones. Muristone induced p27Kip1 expression in both cell lines. However, in contrast to transient-transfected cells, both clones demonstrated a minimal basal expression in the absence of the inducer indicating that genomic integration had occurred distal to a minimal promoter. Cells were incubated in normal glucose medium. These blots are representative of four independent experiments with qualitatively similar results.
Table 5.
Effect of the Inducer Muristone on Proliferation and Protein Synthesis of Clones 5.2 and 6.2
| [3H]Thymidine (proliferation) | [3H]Leucine (de novo protein synthesis) | |
|---|---|---|
| Clone 5.2 in 100 mg/dl glucose without muristone | 1,970 ± 132 | 193 ± 18 |
| Clone 5.2 in 100 mg/dl glucose+ μg/ml muristone | 1,620 ± 153* | 186 ± 10 |
| Clone 5.2 in 450 mg/dl glucose without muristone | 1,435 ± 151 | 184 ± 11 |
| Clone 5.2 in 450 mg/dl glucose+ 2 μg/ml muristone | 1,138 ± 146† | 256 ± 30† |
| Clone 6.2 in 100 mg/dl glucose without muristone | 709 ± 61 | 259 ± 12 |
| Clone 6.2 in 100 mg/dl glucose+ μg/ml muristone | 708 ± 67 | 260 ± 15 |
| Clone 6.2 in 450 mg/dl glucose without muristone | 893 ± 72 | 225 ± 8 |
| Clone 6.2 in 450 mg/dl glucose+ 2 μg/ml muristone | 684 ± 43† | 316 ± 42† |
cpm, incubation for 24 hours, n = 10–12.
*P < 0.05 versus cells in 100 mg/dl glucose without muristone.
†P < 0.05 versus cells in 450 mg/dl glucose without muristone.
Discussion
Glomerular hypertrophy, defined as an increase in cell size is because of an increase in protein content without DNA replication and occurs very early after the onset of diabetes mellitus. 1,2-4 A better understanding of the potential molecular mechanisms causing renal hypertrophy in diabetes mellitus is necessary because these events precede the development of irreversible structural changes such as glomerulosclerosis and tubulointerstitial fibrosis. 1 Although hemodynamic changes such as glomerular hyperfiltration may contribute through mechanical alteration to growth of glomerular cells, 16 there is now evidence that the diabetic milieu itself is pivotal in the development of mesangial hypertrophy. 1 Yet, the nuclear mechanisms underlying this are not well established.
High glucose as well as advanced glycation end products induce in vitro and in vivo TGF-β in the kidney. 17-20 Furthermore, angiotensin II may additionally induce TGF-β synthesis, particularly in the setting of high ambient glucose. 21 Neutralization experiments have clearly demonstrated that TGF-β is a necessary prerequisite for the development of glomerular hypertrophy in streptozotocin-induced diabetic mice. 20
We and others have been interested in the role of specific cell cycle proteins in diabetic hypertrophy, because we have previously described that high glucose, in the absence of other factors, induces immediate early genes in mesangial cells and the early entry G0 to G1 phases. 5 However, after a very limited proliferation, mesangial cells exposed to high glucose are growth-arrested in the G1 phase, and do not progress into the S phase of the cell cycle. 5 Moreover, high glucose-mediated expression of TGF-β is pivotal for this G1-phase arrest because neutralizing anti-TGF-β antibodies convert the G1-phase arrest into a proliferative phenotype. 5 Young and colleagues 7 showed similar results in vivo where an early and limited glomerular proliferation occurred in the streptozotocin model which preceded glomerular hypertrophy.
CDK inhibitors cause cell cycle arrest by inactivating specific cyclin-CDK complexes required for cell cycle progression. In the current study we focused on specific CDK inhibitors because we have previously shown p21Cip1 and p27Kip1 are increased in vitro and in vivo in response to high glucose. 8-10 The major finding in the current study was that an induction of p27Kip1 is necessary for high glucose-induced mesangial cell hypertrophy. Our results show that in contrast to an increase in protein synthesis and decrease in DNA synthesis (measures of hypertrophy) in p27Kip1+/+ cells, high glucose did not stimulate the de novo synthesis of proteins in p27Kip1−/− cells. Moreover, in contrast to glucose-induced cell cycle arrest in p27Kip1+/+ cells, glucose increased cell cycle progression in p27Kip1−/− cells. Finally, we showed that in the presence of high glucose, reconstituting p27Kip1 levels in p27Kip1−/− mesangial cells by either a transient or stable transfection converted these cells from a proliferative to hypertrophic phenotype.
A second major finding in this study was that in accord with our previous in vitro and in vivo studies, 10 the CDK inhibitor p21Cip1 was increased in both p27Kip1+/+ and p27Kip1−/− mesangial cells in response to high glucose. 22,23 Because TGF-β can mediate p21Cip1 expression by p53-dependent and -independent pathways, 24-26 it is possible that high glucose-induced TGF-β is responsible for the increase in p21Cip1 in the diabetic environment. This expression of p21Cip1 is required for glomerular hypertrophy because p21Cip1−/− mice made diabetic by streptozotocin did not develop glomerular hypertrophy as measured by computer image analysis of glomerular tufts. 27 This absence of glomerular hypertrophy in p21Cip1−/− diabetic mice seemed to be protective of renal function because these animals did not develop proteinuria. 27
A role for specific CDK inhibitors in hypertrophy has been recently shown. We found that treatment of BBdp rats, a model of autoimmune diabetes mellitus type I, with angiotensin-converting enzyme inhibitors prevented glomerular expression of p16 and p27Kip1, but not of p21Cip1. 28 Increased kidney weight, a parameter of hypertrophy, was also abolished by angiotensin-converting enzyme inhibitor treatment. 28 Hence, a picture is emerging in which both CDK inhibitors, p27Kip1 and p21Cip1, are required for the development of high glucose-mediated hypertrophy, but interference with the expression of one of these proteins may attenuate hypertrophy.
Terada and co-workers 29 overexpressed p21Cip1 and p27Kip1 in tubular LLC-PK1 cells using adenovirus vectors. Although overexpression of each of the CDK inhibitors alone was sufficient to stimulate de novo protein synthesis of tubular cells in the presence of the mitogen epidermal growth factor, these overexpressions surprisingly failed to cause an inhibition of proliferation. 29 On the other hand, overexpression of p21Cip1 in vascular smooth muscle cells suppressed serum-induced proliferation and stimulated hypertrophy. 30 Although species differences and/or the particular cell type may all explain these somewhat inconsistent findings, a more likely explanation is the fact that different total amounts of CDK inhibitors may have been expressed in the various systems.
It has been suggested that CDK inhibitors, in particular those of the Cip/Kip family, are redundant in some of their cell-cycle regulatory functions. 31,32 The rationale for this assumption was the observation that mice with targeted disruption of the p27Kip1 gene, although exhibiting more cells in several organs, nevertheless revealed normal cell cycle arrest of lymphocytes treated with TGF-β or rapamycin. 33,34 Moreover, despite a high frequency of pituitary tumors in p27Kip1-deficient mice, these animals are not predisposed to a general increase in cancer frequency suggesting that p27Kip1 is not a genuine tumor suppressor gene. 33,34
p27Kip1 inhibits several cyclin/CDK complexes including cyclin E/CDK2, and cyclin D/CDK4,6. 31 However, these cyclin/CDK complexes may show different susceptibilities to inhibition by p27Kip1. 35 For example, binding of p27Kip1 does not necessarily inhibit cyclin D/CDK4 in proliferating cells, whereas it always inactivates cyclin E/CDK2 and cyclin A/CDK2. 31,35 Studies with inducible p27Kip1 expression have shown that the amount of p27Kip1 required for the inhibition of cyclin D/CDK4 is much larger than that required for the inhibition of cyclin A/CDK2. 35 TGF-β decreases the expression of CDK4 36 and also induces the CDK4-specific inhibitor p15INK4b. 37 These changes will disrupt binding of p27Kip1 to cyclin D/CDK4 complexes leading to a redistribution and binding of p27Kip1 to cyclin E/CDK2 and cyclin A/CDK2 complexes with their inhibition. 36,37 Thus, our observation that induced p27Kip1 expression leads to hypertrophy only in high glucose medium may be explained by the fact that TGF-β-mediated down-regulation of CDK4 expression and/or induction of p15INK4b, which occurs only in high glucose medium, is necessary for liberation of p27Kip1 from cyclin D/CDK4 complexes with consecutive inhibition of cyclin E/CDK2. In accordance with this theory, Huang and Preisig 38 have demonstrated that cyclin D/CDK2 is activated, but cyclin E/CDK2 kinase is inhibited during diabetic hypertrophy. 38 This effect is mediated by TGF-β. The switch from the initial hyperplastic to the hypertrophic response of tubular cells was mediated by a decrease in cyclin E activity and p21Cip1, p27Kip1, and p57 played a role in this inhibition 38 suggesting that all three CKI may be necessary in diabetic hypertrophy.
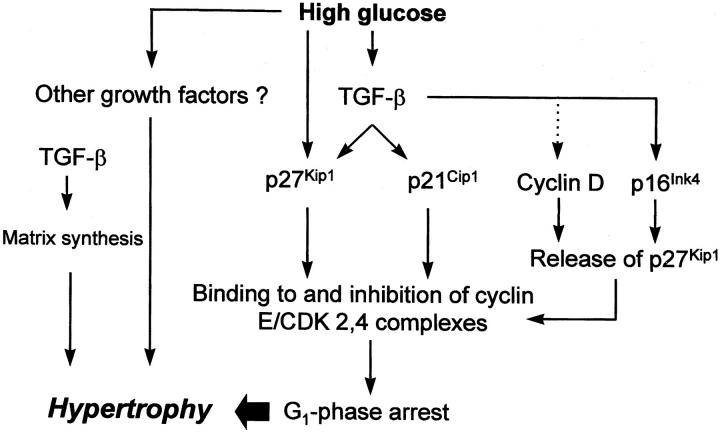
In summary, we would like to propose the following orderly molecular course of glucose-mediated hypertrophy of mesangial cells as shown in Figure 6 ▶ : high ambient glucose primarily induces dormant cells to re-enter the cell cycle. After completing one or two rounds, CDK inhibitors such as p21Cip1 and p27Kip1 are induced, 8-10 likely through TGF-β-dependent as well as -independent mechanisms. Direct phosphorylation of p27Kip1 by high glucose-activated MAP kinases could additionally increase the protein stability. 39-41 These CDK inhibitors interact with cyclin/CDK complexes, inhibit their activities, and arrest cells in the G1 phase. It seems that p27Kip1 is the major CDK inhibitor being necessary for this arrest. In addition, p21Cip1 and p27Kip1 may counteract apoptosis so that the overall number of G1-phase-arrested cells remain intact. 11,40 Arrested cells are undergoing cellular hypertrophy through stimulated protein synthesis, increases in extracellular matrix, reduction in protein and matrix turnover, and maybe cellular enlargement by additional osmotic changes. 42-44 It is likely that TGF-β plays a central role in several of these processes. 20,45 Hence, interference with any of these consecutive events would inevitably abolish mesangial hypertrophy. However, whether a prevention of glomerular hypertrophy would ultimately attenuate renal function and structure in diabetic nephropathy remains unclear. 46
Figure 6.
Proposed molecular events leading to high glucose-induced hypertrophy of mesangial cells. High glucose induces TGF-β. TGF-β in turn stimulates the expression of the CDK inhibitors p27Kip1 and p21Cip1. We have previously shown that the high glucose-mediated induction of p27Kip1 is to some extent independent of TGF-β and is mediated by protein kinase C. 8 Both CDK inhibitors, likely in concert, mediate G1-phase arrest by binding to and inhibiting G1-phase CDK 2, 4-cyclin E complexes. However, for the full development of cellular hypertrophy of G1-phase-arrested cells other high glucose-induced factors are necessary such as other hypertrophic growth factors and/or cell-cycle-independent effects of TGF-β including stimulated matrix synthesis. Moreover, it has been shown that TGF-β leads to a down-regulation of cyclin D and induces p16Ink4 with a potential release of p27Kip1 from cyclin D-containing complexes that could now bind to cyclin E and further reinforce the G1-phase arrest. —➤ = induction; · · · ·➤ = inhibition.
Acknowledgments
We thank James M. Roberts (Fred Hutchinson Cancer Center, Seattle, WA) for providing the p27Kip1+/+ and −/− mice used in this study.
Footnotes
Address reprint requests to Gunter Wolf, M.D., University of Hamburg, University Hospital Eppendorf, Department of Medicine, Division of Nephrology and Osteology, Pavilion 61, Martinistrasse 52, D-20246 Hamburg, Germany. E-mail: wolf@uke.uni-hamburg.de.
Supported by the Deutsche Forschungsgemeinschaft (Wo 460/2-4, and a Heisenberg scholarship (to G. W.), and by National Institutes of Health grants DK52121, DK51096, DK47659 (to S. J. S.).
Parts of this study were presented at the 32nd Annual Meeting of the American Society of Nephrology, November 1 to 8, 1999, Miami Beach, Florida, and are published in abstract form (J Am Soc Nephrol 10:692A0, 1999).
References
- 1.Wolf G, Ziyadeh FN: Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 1999, 56:393-405 [DOI] [PubMed] [Google Scholar]
- 2.Mogensen CE, Andersen MJ: Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes 1973, 22:706-712 [DOI] [PubMed] [Google Scholar]
- 3.Seyer-Hansen K: Renal hypertrophy in streptozotocin-diabetic rats. Clin Sci Mol 1976, 51:551-555 [DOI] [PubMed] [Google Scholar]
- 4.Osterby R, Gundersen HJG: Glomerular size and structure in diabetes mellitus. I. Early abnormalities. Diabetologica 1975, 11:225-229 [DOI] [PubMed] [Google Scholar]
- 5.Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN: High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int 1992, 42:647-656 [DOI] [PubMed] [Google Scholar]
- 6.Ziyadeh FN, Sharma K, Wolf G: Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest 1994, 93:536-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG: Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int 1995, 47:935-944 [DOI] [PubMed] [Google Scholar]
- 8.Wolf G, Schroeder R, Ziyadeh FN, Thaiss F, Zahner G, Stahl RAK: High glucose stimulates expression of p27Kip1 in cultured mouse mesangial cells: relationship to hypertrophy. Am J Physiol 1997, 273:348-356 [DOI] [PubMed] [Google Scholar]
- 9.Wolf G, Schroeder R, Thaiss F, Ziyadeh FN, Helmchen U, Stahl RAK: Glomerular expression of p27Kip1 in diabetic db/db mouse: role of hyperglycemia. Kidney Int 1998, 53:869-879 [DOI] [PubMed] [Google Scholar]
- 10.Kuan CJ, Al-Douahji M, Shankland SJ: The cyclin kinase inhibitor p21WAF1,CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J Am Soc Nephrol 1998, 9:986-993 [DOI] [PubMed] [Google Scholar]
- 11.Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ: Modulation of apoptosis by the cyclin-dependent kinase inhibitor p27Kip1. J Clin Invest 1999, 103:597-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf G, Haberstroh U, Neilson EG: Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol 1992, 140:95-107 [PMC free article] [PubMed] [Google Scholar]
- 13.Polyak K, Le MH, Erdjument-Bromag H, Koff A, Roberts JM, Tempel P, Massagué J: Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78:59-66 [DOI] [PubMed] [Google Scholar]
- 14.Kaufman RJ: Vectors used for expression in mammalian cells. Methods Enzymol 1990, 185:487-513 [DOI] [PubMed] [Google Scholar]
- 15.No D, Yao TP, Evans RM: Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci USA 1996, 93:3346-3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes P, Zhao X, Riser BL, Narins RG: Role of glomerular mechanical strain in the pathogenesis of diabetic nephropathy. Kidney Int 1997, 51:57-68 [DOI] [PubMed] [Google Scholar]
- 17.Shankland SJ, Scholey JW: Expression of transforming growth factor-β1 during diabetic renal hypertrophy. Kidney Int 1994, 46:430-442 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 1993, 90:1814-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziyadeh FN, Han DC, Cohen J, Guo J, Cohen MP: Glycated albumin stimulates fibronectin gene expression in glomerular mesangial cells: involvement of the TGF-β system. Kidney Int 1998, 53:631-638 [DOI] [PubMed] [Google Scholar]
- 20.Sharma K, Jin Y, Guo J, Ziyadeh FN: Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996, 45:522-530 [DOI] [PubMed] [Google Scholar]
- 21.Wolf G, Neilson EG, Goldfarb S, Ziyadeh FN: The influence of glucose concentration on angiotensin II-induced hypertrophy of proximal tubular cells in culture. Biochem Biophys Res Commun 1991, 176:902-909 [DOI] [PubMed] [Google Scholar]
- 22.Shankland SJ, Pippin J, Flanagan M, Coats SR, Nangaku M, Gordon KL, Roberts JM, Couser WG, Johnson RJ: Mesangial cell proliferation mediated by PDGF and bFGF is determined by levels of the cyclin kinase inhibitor p27Kip1. Kidney Int 1997, 51:1088-1099 [DOI] [PubMed] [Google Scholar]
- 23.Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ: The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med 1998, 4:575-580 [DOI] [PubMed] [Google Scholar]
- 24.Li CY, Suardett L, Little JB: Potential role of Waf1/Cip1/p21 as a mediator of TGF-β cytoinhibitory effect. J Biol Chem 1995, 270:4971-4974 [DOI] [PubMed] [Google Scholar]
- 25.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 26.Elbendary A, Berchuck A, Davis P, Havrilesky L, Bast RC, Iglehart JD, Marks JR: Transforming growth factor β1 can induce CIP1/WAF1 expression independent of the p53 pathway in ovarian cancer cells. Cell Growth Differ 1994, 5:1301-1307 [PubMed] [Google Scholar]
- 27.Al-Douahji M, Brugarolas J, Brown PAJ, Stehman-Breen CO, Alpers CE, Shankland SJ: The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic hypertrophy. Kidney Int 1999, 56:1691-1699 [DOI] [PubMed] [Google Scholar]
- 28.Wolf G, Wenzel U, Ziyadeh FN, Stahl RAK: ACE inhibitor treatment reduces glomerular p16INK4 and p27Kip1 expression in diabetic BBdp rats. Diabetologia 1999, 42:1425-1432 [DOI] [PubMed] [Google Scholar]
- 29.Terada Y, Inoshita S, Nakashima O, Tamamori M, Ito H, Kuwahara M, Sasaki S, Marumo F: Cell cycle inhibitors (p27Kip1 and p21CIP1) cause hypertrophy in LLC-PK1 cells. Kidney Int 1999, 56:494-501 [DOI] [PubMed] [Google Scholar]
- 30.Kato S, Yamaguchi M, Fujii T, Miyagi N, Terasaki M, Hamada T, Sugita Y, Morimatsu M: Overexpression of p21Waf-1 in vascular smooth muscle cells: regulation of proliferation, differentiation, and cell size. Exp Mol Pathol 1999, 66:39-52 [DOI] [PubMed] [Google Scholar]
- 31.Hengst L, Reed SI: Inhibitors of the Cip/Kip family. Curr Top Microbiol Immunol 1998, 227:25-41 [DOI] [PubMed] [Google Scholar]
- 32.Shankland SJ: Cell-cycle control and renal disease. Kidney Int 1997, 52:294-308 [DOI] [PubMed] [Google Scholar]
- 33.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K: Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85:707-720 [DOI] [PubMed] [Google Scholar]
- 34.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM: A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 1996, 85:733-744 [DOI] [PubMed] [Google Scholar]
- 35.Blain SW, Montalvo E, Massagué J: Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-cdk4. J Biol Chem 1997, 272:25863-25872 [DOI] [PubMed] [Google Scholar]
- 36.Ewen ME, Sluss HK, Whitehouse LL, Livingston DM: TGF beta inhibition of cdk4 synthesis is linked to cell cycle arrest. Cell 1993, 74:1009-1020 [DOI] [PubMed] [Google Scholar]
- 37.Reynisdottir I, Polyak K, Iavarone A, Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 1995, 9:1831-1845 [DOI] [PubMed] [Google Scholar]
- 38.Huang HC, Preisig PA: G1 kinases and transforming growth factor-β signaling are associated with a growth pattern switch in diabetes-induced renal growth. Kidney Int 2000, 58:162-172 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen H, Gitig DM, Koff A: Cell-free degradation of p27(Kip1), a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol Cell Biol 1999, 19:1190-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K, Roberts JM, Ross R: Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of cdk2: role of as caspase cascade. Mol Cell 1998, 1:553-563 [DOI] [PubMed] [Google Scholar]
- 41.Awazu M, Ishikura K, Hida M, Hoshiya M: Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J Am Soc Nephrol 1999, 10:738-745 [DOI] [PubMed] [Google Scholar]
- 42.Wolf G, Thaiss F: Hyperglycaemia—pathophysiological aspects at the cellular level. Nephrol Dial Transplant 1995, 10:1109-1112 [PubMed] [Google Scholar]
- 43.Olbricht CJ, Geissinger B: Renal hypertrophy in streptozotocin diabetic rats: role of proteolytic lysosomal enzymes. Kidney Int 1992, 11:966-972 [DOI] [PubMed] [Google Scholar]
- 44.Häussinger D: The role of cellular hydration in the regulation of cell function. Biochem J 1996, 313:697-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma K, Ziyadeh FN: Hyperglycemia and diabetic kidney disease: the case for transforming growth factor-β as a key mediator. Diabetes 1995, 44:1139-1146 [DOI] [PubMed] [Google Scholar]
- 46.Al-Awqati Q, Preisig PA: Size does matter: will knockout of p21WAF1/CIP1 save the kidney by limiting compensatory renal growth ? Proc Natl Acad Sci USA 1999, 96:10551-10553 [DOI] [PMC free article] [PubMed] [Google Scholar]