Abstract
Vascular endothelial growth factor (VEGF) is an important mediator of angiogenesis in both physiological and pathological processes. Hepatocyte growth factor (HGF) is a mesenchyme-derived mitogen that also stimulates cell migration, and branching and/or tubular morphogenesis of epithelial and endothelial cells. In the present study, we tested the hypothesis that simultaneous administration of HGF and VEGF would synergistically promote new blood vessel formation. HGF acted in concert with VEGF to promote human endothelial cell survival and tubulogenesis in 3-D type I collagen gels, a response that did not occur with either growth factor alone. The synergistic effects of VEGF and HGF on endothelial survival correlated with greatly augmented mRNA levels for the anti-apoptotic genes Bcl-2 and A1. Co-culture experiments with human neonatal dermal fibroblasts and human umbilical vein endothelial cells demonstrated that neonatal dermal fibroblasts, in combination with VEGF, stimulated human umbilical vein endothelial cells tubulogenesis through the paracrine secretion of HGF. Finally, in vivo experiments demonstrated that the combination of HGF and VEGF increased neovascularization in the rat corneal assay greater than either growth factor alone. We suggest that combination therapy using HGF and VEGF co-administration may provide a more effective strategy to achieve therapeutic angiogenesis.
Vascular endothelial cell growth factor (VEGF) is produced by a variety of cell types, including keratinocytes, pericytes, smooth muscle cells, macrophages, and mast cells. 1 Elevated expression of VEGF and its receptors has been reported in many different tumors, rheumatoid synovial fluid, and synovial tissue, and diabetic retina and vitreous fluid. 1-5 However, recent negative data from a placebo-controlled clinical study with VEGF has raised questions about the ability of VEGF to mount an effective angiogenic response, at least when administered to patients with extensive atherosclerosis. 6,7 This concept is supported by a number of in vitro studies. For example, VEGF is not sufficient to promote endothelial survival or tubulogenesis 8-10 when human endothelial cells are suspended in 3-D collagen gels.
Hepatocyte growth factor (HGF) is a potent mitogen for a number of cell types, including hepatocytes, myeloid precursor cells, and various epithelial and endothelial cells. 11 HGF also promotes epithelial and endothelial cell motility and branching morphogenesis and/or tubular morphogenesis. 12,13 HGF may also mediate mesenchymal-epithelial and endothelial interactions, which contribute to tissue repair and embryogenesis. 14-16 In vivo, intravenous administration of HGF has been reported to improve collateral formation in ischemic hind limb. 17,18 HGF and its receptor c-met, are up-regulated in a number of tumors including breast cancer, urothelial-bladder cancer and gliomas, and like VEGF, HGF levels have been shown to correlate with tumor vascularity. 11 HGF can also induce VEGF production by a variety of cells and tissues. 17,19,20 In the present study we investigated the possibility that HGF may augment VEGF driven endothelial angiogenesis, independent of the ability to increase VEGF production. To evaluate the in vitro interactions of HGF and VEGF in human umbilical vein endothelial cell (HUVEC) tubulogenesis, we used a modification of type I collagen gel model that incorporates endothelial cells into the collagen before gelation. 8 The in vivo activity of HGF and VEGF was evaluated using the rat cornea as previously described. 21 We report here that HGF acts as a co-activator (with VEGF) promoting both endothelial survival and tubulogenesis in 3-D collagen gels in vitro and angiogenesis in vivo in the rat cornea. These observations suggest that combination therapy using HGF and VEGF co-administration may be a more effective means to achieve therapeutic angiogenesis.
Materials and Methods
Endothelial Tube Assay
HUVECs were obtained from Clonetics (San Diego, CA) and grown in Clonetics Endothelial Growth Medium (EGM) medium supplemented with 10% fetal bovine serum and endothelial cell growth supplements provided by the manufacturer. Cells from passages 4 to 7 were used throughout the study. Collagen gels (3-D) containing HUVECs were prepared as described previously. 8,21 After gelation at 37°C for 30 minutes, the gels were overlaid with 1× basal medium (BM) 8 supplemented with phosphate-buffered saline (PBS) (control), HGF, VEGF or the combination of HGF and VEGF at the indicated concentrations. Tube formation was quantified by measuring total tube length (long axis) in three random fields/well at a fixed layer at low-power (×10) magnification using Openlab software (Improvision Inc, Coventry, UK). For each experiment, each group consisted of two to three replicate wells, and all experiments were repeated at least three times. Digital images were captured using Hoffman modulation optics and a Hamamatsu 9600 charge-coupled device camera.
Fibroblast and HUVEC Co-Culture
Neonatal dermal fibroblasts (NDFBs) and FGM2 culture media were purchased from Clonetics (San Diego, CA) and cells were grown in FGM2 supplemented with 10% fetal bovine serum. All cells were used at passages 4 to 6. To co-culture NDFBs with HUVECs, equal ratios of HUVECs and NDFBs (3 × 10 6 cells/ml each) were mixed and embedded in 3-D collagen gels in BM supplemented with different growth factors as indicated in Results. To distinguish the origin of the tube structures in co-culture experiments, confluent HUVECs grown in monolayer were labeled with Di-I-Ac-LDL (Biomedical Technologies Inc., Stoughton, MA) at final concentration of 10 μg/ml for 4 hours. Cells were then thoroughly washed with PBS, trypsinized, and used as the source of endothelial cells for the co-culture studies. To quantitate the effects of NDFBs on HUVEC tube structure formation, average tube length was measured as described above except that rhodamine fluorescent optics were used in conjunction with the Hoffman modulation optics to confirm that the tubes measured were comprised of endothelial cells. NDFB-conditioned media (CM), was harvested from subconfluent NDFB monolayers that had been incubated in BM for 48 hours. CM were spun at 800 rpm for 5 minutes to remove cell debris and used directly as the culture media for HUVECs in 3-D collagen gels. Controls used media that had not been previously conditioned by the fibroblasts. The HGF content in NDFBs and NDFB-HUVEC co-cultures CM was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Neutralizing antibodies to HGF were purchased from R&D Systems, and the HGF mutants, NK1 and NK2, were provided by Dr. Ralph Schwall (Genentech).
Cell Viability
The collagen gel matrix made conventional methods (eg, trypan blue dye exclusion, annexin V binding) to assess cell viability problematic. Therefore, we assessed cell viability based on nuclear morphology. Gels were fixed in 3.7% buffered formalin and cell nuclei stained with 10 μmol/L 4,6′-diamino-2-phenylindole dihydrochloride (DAPI) for 20 minutes. The cells/tubes were viewed under UV optics to evaluate nuclei. Cells with condensed nuclei or pyknotic nuclei were counted as dead, as were cell ghosts in which nuclei were no longer visibly stained with DAPI. The percentage of viable cells/high-power field was then determined. For each group, six random fields in nine replicate wells were evaluated. In other experiments nuclei were stained with acridine orange and images captured using a Leica confocal microscope.
Real Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) (Taqman) Assay
Total RNA was extracted from HUVECs cultured in 3-D type I collagen gels for various times in 1× BM supplemented with the indicated growth factors. A gene-specific PCR oligonucleotide primer pair and an oligonucleotide probe labeled with a reporter fluorescent dye at the 5′-end and a quencher fluorescent dye at the 3′-end were designed using the Oligo 4.0 software (National Bioscience, Plymouth, MN) following guidelines suggested in the Taqman Model 7700 Sequence Detection instrument manual (PE Applied Biosystems, Foster City, CA). The primers and probes used are provided in Table 1 ▶ , and mRNA was quantified as described previously. 21 Standard curves for the expression of each gene were generated by serial dilution of a standard preparation of total RNA isolated from quiescent HUVECs (ie, maintained 24 hours in EGM 100% confluency culture media with 10% fetal bovine serum without additional growth factors) grown in monolayer culture. The mRNA levels are expressed as the ratio to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase.
Table 1.
Taqman Primers and Probes
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Bcl-2 | CAAGCACCGCTTCGTCGTGTG | ATGGTGATCCGGCCAACA | TGTTCTGTGCCTGTAAACATAGATTCGCTTTCC |
| A-1 | CAGCTCAAGACTTTGCTCTCCA | AGTCCTGAGCCAGCCTGTAAAT | ATCCAAATTCACAGTCTGTCATCTTCTGCCTG |
Electron Microscopy
HUVECs grown in 3-D collagen gels for 48 hours were fixed in 1/2 Karnovsky’s solution (2% paraformaldehyde, 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer, pH 7.4) and then washed twice in 0.1 mol/L sodium cacodylate, pH 7.4, (15 minutes per wash) before being postfixed in 1% aqueous osmium tetroxide for 2 hours at room temperature. Following washing in ddH2O the samples were dehydrated through an ethanol series: 50%, 70%, 90%, for 15 minutes each, and then 100% for 2 × 15 minutes, followed by propylene oxide for 2 × 15 minutes. The samples were infiltrated with Eponate 12 (Ted Pella, Redding, CA) first with 1:1 propylene oxide:Eponate 12 overnight, followed by 100% Eponate 12 for 8 hours. The samples were transferred to fresh resin and polymerized in a 60°C oven overnight and ultrathin (80 nm) sections were cut. The sections were stained with uranyl acetate and lead citrate and were observed on a Philips CM12 transmission electron microscope. Images were captured with a GATAN Retractable MultiScan Camera using Digital Micrograph software.
In Vivo Angiogenesis Corneal Assay
In vivo angiogenic activity of the combination of HGF and VEGF was examined by using the rat corneal angiogenesis assay as described previously. 21 Hydron pellets containing excipient (control), HGF (50 or 200 ng), VEGF (50 or 200 ng), or the combination of HGF and VEGF (50 or 200 ng each) were implanted into the corneas of 250 to 300 g male Sprague-Dawley rats. All hydron pellets contained 100 ng of sucralfate. At day 6, the animals were euthanized and injected with fluorescein isothiocyanate-dextran to allow for visualization of the vasculature. Corneal whole mounts were made of the enucleated eyes and analyzed for neovascular area using a computer-assisted image analysis (Image Pro-Plus 2.0, Silver Spring, MD).
Statistics
Statistical analyses were performed using one-analysis of variance (INSTAT, GraphPad Software, Sorrento Valley, CA). Multiple comparisons against the control were first analyzed by one-way analysis of variance followed by Bonferroni’s modified Student’s t-test to determine differences between groups. A P value <0.05 was accepted as significant.
Results
HGF and VEGF, in Combination, Stimulate HUVEC Tubulogenesis
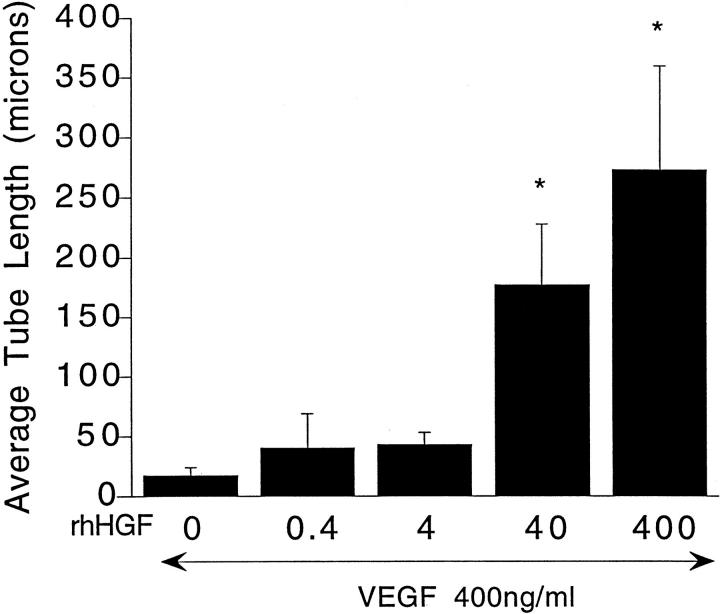
When grown in 3-D collagen gels in BM alone, HUVECs underwent rapid cell death, visible as early as 4 hours and at 24 hours. At 72 hours virtually all of the cells were dead (Figure 1A) ▶ in agreement with earlier reports. 8 Addition of VEGF to the BM, even at concentrations as high as 800 ng/ml, did not promote cell survival, nor induce tubulogenesis (Figure 1B) ▶ . HUVECs cultured in 3-D collagen gels in BM supplemented with 0.4 to 800 ng/ml HGF also underwent rapid cell death (data not shown except for 200 ng/ml, Figure 1C ▶ ). HGF-treated cells did start to form small vacuoles at 4 hours, but some of the cells exhibited pyknotic nuclei. At 24 hours, small sprouts were observed in some cells but numerous dead cells were also observed. Few viable HUVECs were observed at 72 hours. However, HGF (200 ng/ml), when combined with VEGF (200 ng/ml), supported cell viability and tubulogenesis (Figure 1D) ▶ . The sequential changes in cell morphology were similar to those described in our earlier report detailing endothelial tubulogenesis in 3-D collagen gels when incubated with phorbol myristate acetate (PMA) in combination with VEGF and bFGF. 8 Frequent small vacuole-like structures were observed at ∼6 to 8 hours, and an interconnected network of endothelial cells with lumens was observed by 48 to 72 hours (Figure 1D) ▶ . Figure 2; A, B, and C ▶ , documents the appearance of the endothelial cells at 4, 24, and 48 hours in 3-D collagen gels in BM supplemented with HGF and VEGF (200 ng/ml each), and Figure 2D ▶ shows a representative transmission electron micrograph of a representative tubular structure demonstrating a lumen-like structure formed by several adjoining endothelial cells. The combinatorial effects of HGF with VEGF were both HGF and VEGF dose-dependent. As shown in Figure 3 ▶ , when the VEGF concentration was fixed at 400 ng/ml, HGF stimulated a dose-dependent increase in tube formation, with significant effects observed at 40 and 400 ng/ml HGF. Similar data were obtained when the HGF concentration was fixed at 400 ng/ml and the VEGF concentration varied from 0.4 to 400 ng/ml (not shown). These results demonstrated a synergistic interaction between the two endothelial mitogens, HGF and VEGF, in the promotion of endothelial survival and tubulogenesis in vitro.
Figure 1.
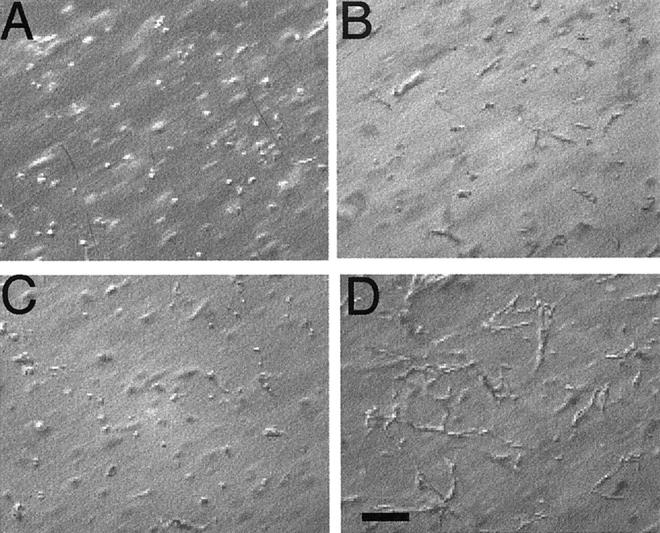
Synergistic induction of HUVEC tubulogenesis by the combination of HGF and VEGF. HUVECs were cultured in 3-D collagen gels in BM alone (A), or BM supplemented with VEGF (200 ng/ml) (B), HGF (200 ng/ml) (C), or a combination of VEGF and HGF (200 ng/ml each) (D). Photographs were taken at 72 hours. Size marker, 100 μm. Note the interconnected network of HUVECs in the combined HGF- and VEGF-treated group.
Figure 2.
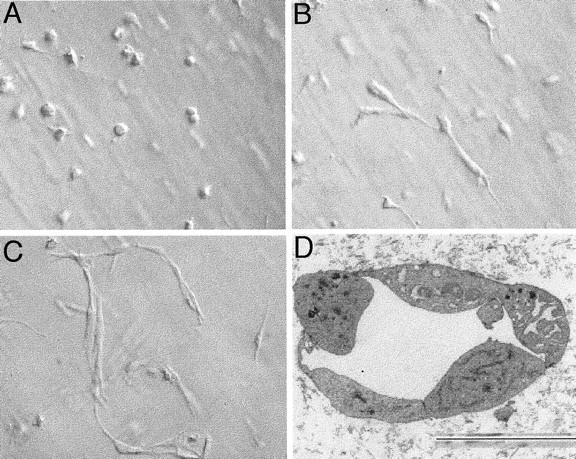
Morphology of HUVECs at 4, 24, and 48 hours in 3-D collagen gels when cultured in BM and VEGF (200 ng/ml) and HGF (200 ng/ml). A–C: Representative photomicrographs taken at 4 (A), 24 (B), and 48 (C) hours using Hoffman modulation optics. D: Representative transmission electron micrograph showing lumen-like structure (arrow). Size marker shown at bottom, 3 μm.
Figure 3.
HGF dose-dependently induced HUVEC tube formation in 3-D collagen gels in the presence of a fixed concentration of VEGF. HUVECs were cultured in 3-D collagen gels in BM supplemented with VEGF (400 ng/ml) in the presence of the indicated concentrations of HGF for 72 hours. Average tube length was obtained by measuring as described in Materials and Methods and is expressed as the mean ± SEM from three independent experiments. *, P < 0.05 versus VEGF treatment.
HGF and VEGF, in Combination, Promote Survival in 3-D Collagen Gels
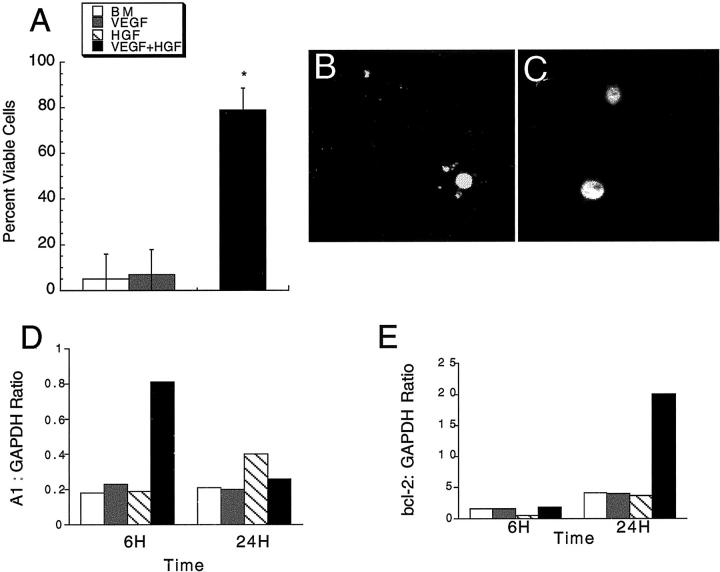
VEGF has been reported to be a survival factor for HUVECs when grown in monolayer in low or no serum. 22 Similarly, HGF is a survival factor for endothelial cells. 23 Endothelial cells were incubated in BM alone or BM supplemented with HGF (200 ng/ml), VEGF (200 ng/ml), or the combination of HGF and VEGF (both at 200 ng/ml), and cell viability (based on nuclear morphology) at 24 hours (confocal microscopy) and 48 hours (DAPI stained nuclei, examination of multiple high-powered fields) determined as described in Materials and Methods. As shown in Figure 4 ▶ , the majority of cells incubated in BM alone, VEGF alone, or HGF alone exhibited an apoptotic appearance or were dead. Representative photomicrographs of pyknotic nuclei and normal nuclei are shown in Figure 4, B and C ▶ , respectively. However, the combination of VEGF and HGF greatly improved cell survival, with ∼80% of the cells in the gel exhibiting normal nuclear morphology at 48 hours. In addition, the cells in the VEGF plus HGF-treated groups demonstrated interconnected branching networks, as shown above.
Figure 4.
The combination of HGF and VEGF inhibits HUVEC death (at 48 hours) in 3-D collagen gels. A: HUVECs were cultured in 3-D gels in BM alone or BM supplemented with HGF (200 ng/ml), VEGF (200 ng/ml), or the combination of HGF and VEGF (200 ng/ml each) for 24 hours. Cell viability was determined as described in Materials and Methods (means ± SD, n = 9). B and C: Photomicrographs of acridine orange-stained nuclei taken using a confocal microscope showing representative pyknotic (B) and normal (C) nuclei. D and E: Effects of HGF and VEGF on the expression of A1 (D) and bcl-2 (E). HUVECs were cultured in 3-D collagen gels in BM supplemented with HGF (200 ng/ml), VEGF (200 ng/ml), or the combination of HGF and VEGF (200 ng/ml each). Total RNA were extracted at 6 hours and 24 hours and duplicate samples from two independent experiments analyzed by quantitative RT-PCR (Taqman) as described in Materials and Methods. Results are expressed as the ratio of the indicated mRNA to the level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase in the same sample. The legend for A, D, and E, is shown in the inset to A.
Bcl-2 family members have been implicated in endothelial cell survival. 22,24 The mRNA expression of the apoptotic regulatory gene, Bcl-2 and a related Bcl-2 family member, A1, in endothelial cells cultured in 3-D gels under different conditions were determined at 6 hours and 24 hours. Both Bcl-2 and A1 mRNA were detected in HGF- and VEGF-treated groups, but the combination of HGF and VEGF resulted in a synergistic induction of mRNA levels for both genes (A1 at 6 hours, Bcl-2 at 24 hours) (Figure 4, D and E) ▶ . These results suggest that the survival activity of HGF in combination with VEGF in 3-D collagen gels might be due in part to the synergistic induction of the anti-apoptotic genes, A1 and Bcl-2.
HGF Is the Mediator of Endothelial Tubulogenesis in Fibroblast/HUVEC Co-Cultures
Fibroblast and fibroblast-derived factors have been previously reported to facilitate endothelial morphogenesis into capillary-like tubes 25-27 although the identity of the fibroblast-derived factor has not been conclusively determined. Because a major cellular source of HGF is the fibroblast 11 we examined the potential role of HGF as the mediator of fibroblast-supported endothelial morphogenesis in 3-D collagen gels. NDFBs were cultured alone and in combination with HUVECs as described. NDFBs, cultured in BM with VEGF (at concentrations ranging from 0 to 400 ng/ml) were viable and were observed as single, elongated cells (not shown except at 40 ng/ml VEGF, Figure 5b ▶ ). When NDFBs were co-cultured with HUVECs in BM alone, at least half of the cells were dead, and the viable cells exhibited a similar morphology to the NDFBs when cultured without HUVECs (not shown). To determine which cells were viable, the HUVECs were preloaded with Di-I-Ac-LDL (an endothelial cell-specific marker) and the co-cultures observed under rhodamine fluorescent optics. These studies demonstrated that the viable cells (which did not take up the Di-I-Ac-LDL) were NDFBs (not shown). However, when NDFBs were co-cultured with HUVECs in BM supplemented with VEGF (40 ng/ml) elongated network-like structures were observed (Figure 5c) ▶ . HUVECs preloaded with Di-I-Ac-LDL clearly demonstrated that the networks were endothelial in origin (Figure 5, d and e) ▶ . CMs were collected from NDFBs as described in Materials and Methods, supplemented with VEGF (200 ng/ml), and added to HUVECs in 3-D collagen gels immediately after gelation. Unconditioned media supplemented with VEGF (200 ng/ml) were used as the control. HUVECs cultured in NDFB-CM formed tube-like structures with a time course and morphology essentially identical to HUVECs cultured in BM supplemented with HGF and VEGF, although average tube length tended to be reduced (see Figure 6A ▶ ). NDFB-CM without VEGF and unconditioned media with VEGF did not support HUVEC survival or tube formation in 3-D collagen gels (not shown). The physicochemical properties of the factor in NDFB-CM indicated the factor was >30 kd, bound to heparan Sepharose, and was sensitive to heat-inactivation (90°C, 10 minutes), properties shared by many growth factors including HGF.
Figure 5.
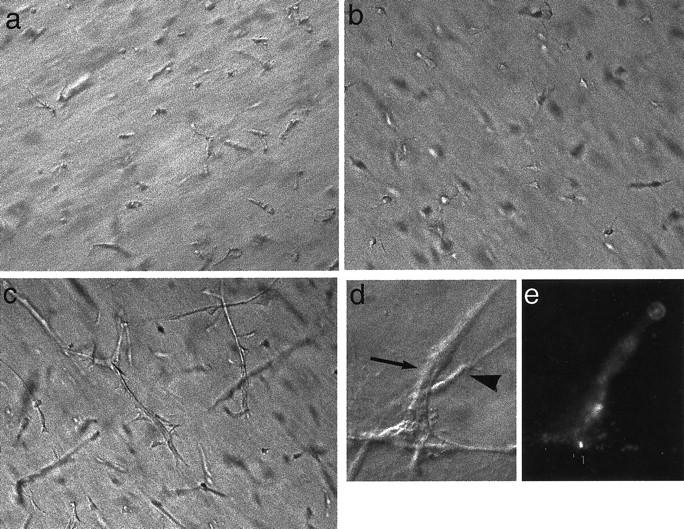
Induction of HUVEC tubulogenesis in NDFB/HUVEC co-cultures in the presence of VEGF. a: NDFBs alone. b: HUVECs alone. c: 1:1 mixture of NDFBs/HUVECs. d and e: Same field of a mixture of NDFB co-cultured with Di-I-Ac-LDL-prelabeled HUVECs under phase (d) and fluorescent (e) optics. All cells were cultured in 3-D collagen gels in BM supplemented with VEGF (200 ng/ml) for 72 hours. Note that NDFBs remained as intact, single cells (a) whereas the majority of the HUVECs appeared apoptotic or dead (b) in 3-D collagen gels. Tubular structures were formed in NDFB/HUVEC co-culture (c). Size marker, 100 μm. Corresponding Di-I-Ac-LDL-labeled HUVECs comprise the interconnected tube-like structures (d and e). Large arrow, endothelial cell labeled with Di-I-Ac-LDL; arrowhead, fibroblast, which is elongated but not fluorescent.
Figure 6.
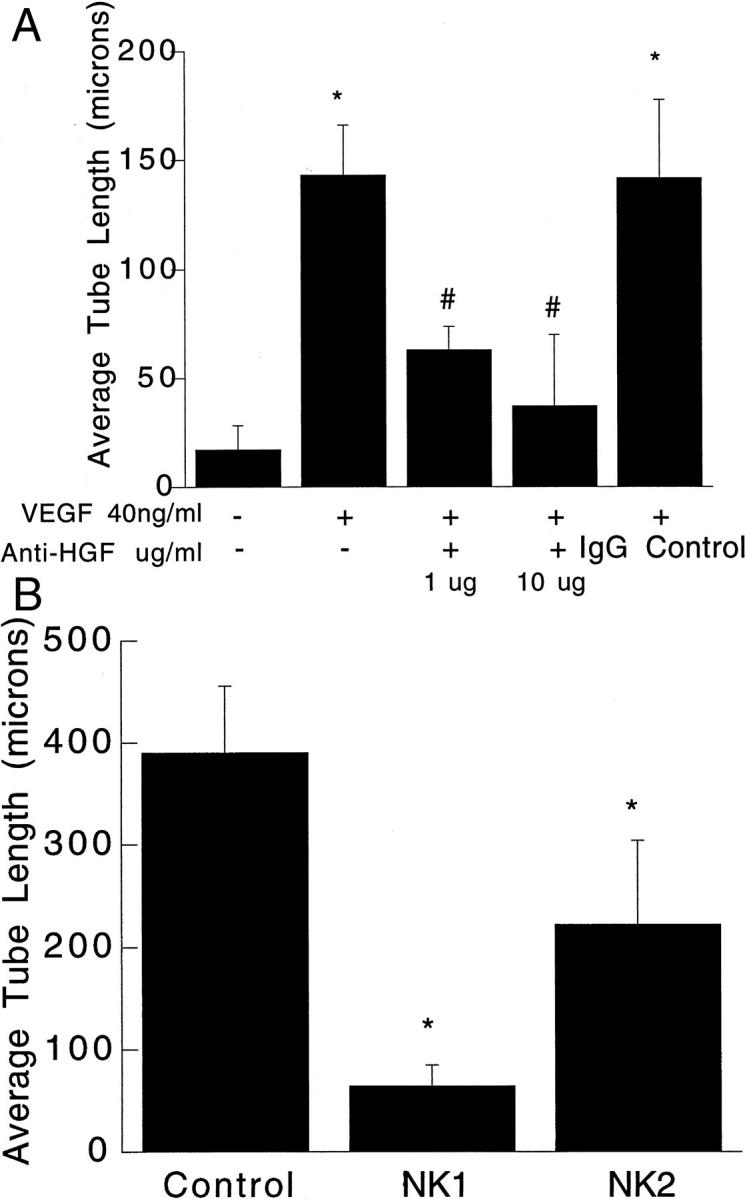
Neutralizing antibody against HGF and HGF mutants NK1 and NK2 blocked NDFB CM-induced HUVEC tube formation in 3-D collagen gels. A: CM from NDFBs were obtained and used as the culture medium for HUVEC in 3-D collagen gels supplemented with VEGF (200 ng/ml) as described in Materials and Methods. Anti-HGF antibodies were given at time 0 at concentrations of 1 μg/ml or 10 μg/ml. Results are expressed as mean ± SEM from three independent experiments. #, P < 0.05 versus CM groups with supplemental VEGF (200 ng/ml). *, P < 0.05 versus CM without VEGF. B: HUVECs were co-cultured with NDFBs in 3-D collagen gels for 72 hours in BM containing 200 ng/ml VEGF in the presence of PBS (control), NK1, or NK2 (1 μg/ml). Data are shown as the mean tube length ± SEM of three independent experiments. *, P < 0.05 compared to control (NDFBs: HUVEC co-cultures incubated with 200 ng/ml VEGF).
Tube formation elicited by NDFB-CM was completely blocked by 10 μg/ml anti-HGF (Figure 6A) ▶ . Similar data were obtained in NDFB/HUVEC co-culture experiments (not shown). Two naturally occurring HGF mutants and antagonists, NK1 and NK2, 28 also significantly inhibited HUVEC tubulogenesis in the co-culture model (Figure 6B) ▶ . We determined the levels of HGF in the media of the NDFB-HUVEC 3-D gel co-cultures. At 72 hours, there was ∼1 ng/ml HGF in the supernatants recovered from co-cultures in the absence of VEGF, and 750 pg/ml in the supernatants recovered from the co-cultures when 200 ng/ml VEGF was added to the BM at time 0. There was no detectable HGF in unconditioned media. Although the recoverable levels of HGF measured in CM were lower than those apparently required to promote tube formation, the amount recovered in the supernatant probably underestimates the local concentration of HGF in the gels because HGF is known to interact strongly with heparan-sulfate proteoglycans. Additionally, a significant amount of HGF may also be bound to cell surface receptors. Further, the addition of exogenous HGF to the gels at a concentration of 200 ng/ml may not result in a local concentration of 200 ng/ml. Based on the antibody neutralization data and antagonism with NK1 and NK2, the fibroblast facilitation of endothelial tube formation in co-cultures (with VEGF) is most likely mediated by fibroblast secretion of HGF.
HGF Amplifies VEGF-Driven Angiogenesis in Vivo
Hydron pellets containing excipient (control), HGF, VEGF, or the combination of HGF and VEGF were implanted into the corneas of Sprague-Dawley rats. Summary data from this experiment show that pellets containing the combination of VEGF and HGF demonstrated a significant increase in vessel area compared with either growth factor alone (Figure 7, A–E) ▶ . Results from this experiment were repeated in two separate experiments. These in vivo data strongly support our in vitro observations that HGF can augment VEGF-driven angiogenesis.
Figure 7.
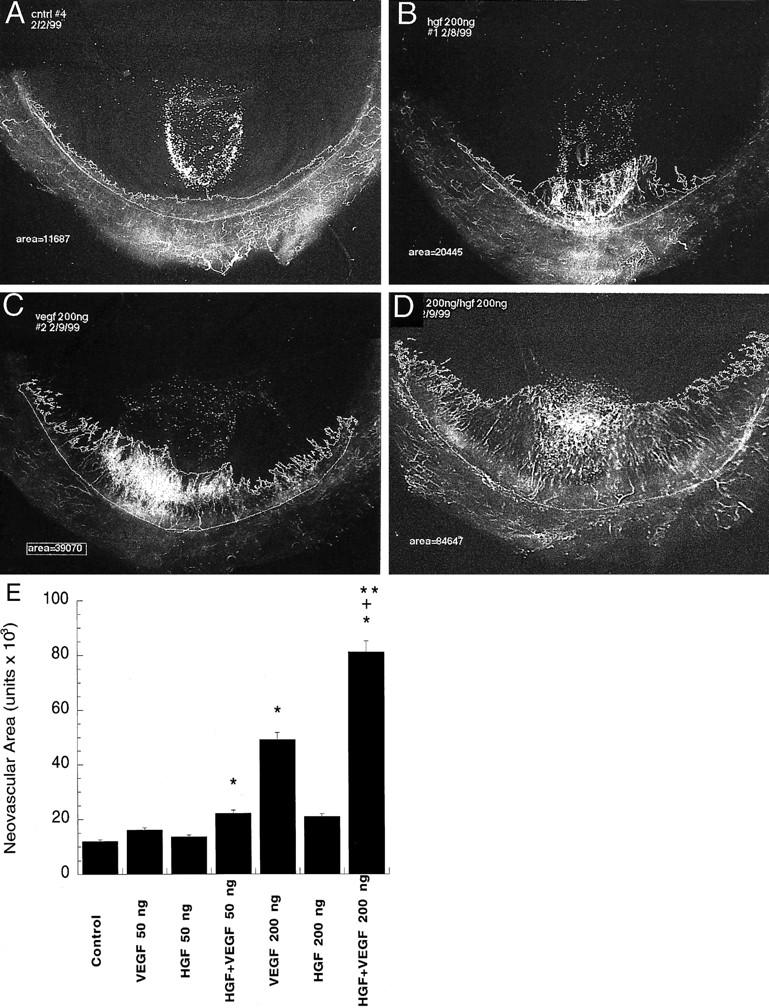
. Representative photomicrographs of the effects of VEGF and HGF alone and in combination on angiogenesis in vivo. A–D: Representative flat-mount photomicrographs of rat corneas 6 days after implantation of hydron pellets A: Control, excipient alone. B: HGF (200 ng/ml). VEGF (200 ng/ml) (C) and VEGF (200 ng/ml) and HGF (200 ng/ml) in combination (D). E: Summary data of the in vivo angiogenic response to control, VEGF-, HGF-, and VEGF and HGF-treated groups. Data are expressed as mean ± SE, n = 5 animals/group. *, Significantly different from control; **, significantly different from VEGF alone. +, Significantly different from HGF alone (Mann-Whitney test for nonparametric values).
Discussion
In vivo studies have suggested that either VEGF or bFGF, administered by repeat intramuscular or intrarterial injection, markedly improve collateral development in animal models of limb ischemia. 29,30 The combination of these angiogenic mitogens was also shown to produce a significantly greater and more rapid improvement in collateral circulation than administration of either mitogen alone. 31,32 Synergistic interactions between growth factors have also been demonstrated in a number of in vitro systems. Co-administration of VEGF and bFGF induced endothelial tube formation on the surface of collagen, a response that was greater than additive, and which occurred with greater rapidity than the response to either cytokine alone. 33 It has also been reported that transforming growth factor-β1 may further augment the in vitro angiogenic activities of bFGF and VEGF. 34
HGF shares many properties with bFGF and VEGF. It is a potent endothelial cell mitogen, and it stimulates endothelial migration and invasion through extracellular matrix proteins. However, in contrast to VEGF and bFGF, HGF is a potent morphogen for multiple cell types. 11 For example, in addition to promoting endothelial organization into tubule-like structures on Matrigel and on the surface of collagen gels, HGF will also induce Madin-Darby canine kidney epithelial cells to organize into a branching network of tubules with the proper apical-basolateral polarity, 35 and mammary epithelial cells to form duct-like structures. 28 HGF synergistically interacts with VEGF to induce endothelial cell proliferation and migration (in monolayer cultures). 17 We and others 8-10 have observed that HUVECs cultured in 3-D collagen gels do not proliferate (independent of the mixture of growth factors used), but undergo differentiation into tubule-like structures. In this 3-D model, we and others 8-10 have observed that the combination of bFGF and VEGF did not support cell survival or tubulogenesis. For example, Ilan and co-workers 9 demonstrated that HUVECs cultured in 3-D collagen gels (under conditions virtually identical to those used in the present study) in the presence of VEGF (20 ng/ml) underwent rapid apoptosis as indicated by terminal dUTP nick-end labeling staining, nuclear morphology, and poly(ADP-ribose) polymerase hydrolysis. Previous work by Ilan, and others 9,10 demonstrated that PMA, alone or in combination with bFGF and VEGF could support cell survival and branching morphogenesis. Because PMA is not a naturally occurring stimulus, in preliminary experiments we tested a number of different growth factors and cytokines in combination with either VEGF or bFGF. As shown in the present study, the combination of HGF and VEGF (or HGF and bFGF, data not shown) enabled endothelial cell survival and morphogenesis into a network of tubule-like structures. These observations suggest that the synergistic interactions between the HGF and VEGF signaling pathways differ from those of bFGF and VEGF co-administration. The synergy between VEGF and HGF was clearly demonstrated by the more than additive changes in the mRNA levels of bcl-2, A1, cell survival indices and tubule formation. Further, the inability of even very high concentrations of VEGF to support endothelial survival or tubulogenesis in the 3-D collagen gel model suggests that the synergistic actions of HGF are likely independent of further increases in VEGF production.
The receptor for HGF, c-met, is expressed on endothelial and smooth muscle cells as well as pericytes, 36 and HGF stimulates the proliferation of all of these vascular cell types in vitro. 11 Activation of c-met is known to stimulate phosphatidylinositol-3-kinase, phospholipase Cγ, pp60src, and Grb2/Sos1; signaling pathways also activated by VEGF. 37 However, the observed synergistic interactions between VEGF and HGF suggest that there may be differences in the signal transduction pathways. For example, induction of epithelial tubules by HGF is dependent on the STAT pathway. 38 A role of the STAT pathway in VEGF-mediated responses has not been reported. There are undoubtedly other differences in the nature of cellular responses to VEGF and HGF that have yet to be discovered.
Hypoxia is potent inducer of VEGF mRNA and protein, and the expression of the VEGF receptors kdr and flt-1 are also increased in response to ischemia. 1 However, HGF expression is reduced in diseased segments of blood vessels from patients with critical limb ischemia compared to disease-free segments. 18 HGF mRNA and protein can be down-regulated by hypoxia, 39 although this may be a time- and tissue-dependent phenomenon. 15,40 In patients as well as in a myocardial ischemia model, c-met is up-regulated in response to hypoxia. 40-42 Down-regulation of HGF in response to hypoxia may be an important contributor to the pathology associated with peripheral vascular disease, and the up-regulation of c-met in peripheral vascular disease may offer an opportunity for therapeutic intervention with HGF.
In summary, in the present study we have demonstrated that HGF and VEGF act in synergy to induce human endothelial morphogenesis into tube-like structures in 3-D collagen gels. These morphogenic changes are accompanied by synergistic induction of the anti-apoptotic genes (bcl-2 and A1). Our in vitro and in vivo results demonstrate that by acting in concert with VEGF, HGF may amplify an angiogenic response. These observations highlight the importance of biological context, because the activity of any angiogenesis-regulating cytokine will likely depend on the presence and concentration of other cytokines in the pericellular environment. Angiogenesis is a multistep process orchestrated by a complex mixture of different growth factors, cytokines, proteolytic enzymes, and matrix molecules and thus the optimal therapy to induce a functional angiogenic response may require a mixture of the appropriate factors.
Acknowledgments
We thank Ms. Holly Aaron of the Genentech confocal facility for technical assistance.
Footnotes
Address reprint requests to Dr. Mary E. Gerritsen, Department of Cardiovascular Research, Genentech Inc., MS 42, South San Francisco, CA 94080. E-mail: meg@gene.com.
References
- 1.Ferrara N: Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 1999, 56:794-814 [DOI] [PubMed] [Google Scholar]
- 2.Plate KH, Breier G, Farrell CL, Risau W: Platelet-derived growth factor receptor-beta is induced during tumor development and upregulated during tumor progression in endothelial cells in human gliomas. Lab Invest 1992, 67:529-534 [PubMed] [Google Scholar]
- 3.Duh E, Aiello LP: Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes 1999, 48:1899-1906 [DOI] [PubMed] [Google Scholar]
- 4.Maeno N, Takei S, Imanaka H, Takasaki I, Kitajima I, Maruyama I, Matsuo K, Miyata K: Increased circulating vascular endothelial growth factor is correlated with disease activity in polyarticular juvenile rheumatoid arthritis. J Rheumatol 1999, 26:2244-2248 [PubMed] [Google Scholar]
- 5.Harada M, Mitsuyama K, Yoshida H, Sakisaka S, Taniguchi E, Kawaguchi T, Ariyoshi M, Saiki T, Sakamoto M, Nagata K, Sata M, Matsuo K, Tanikawa K: Vascular endothelial growth factor in patients with rheumatoid arthritis. Scand J Rheumatol 1998, 27:377-380 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Alitalo K: Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999, 5:1359-1364 [DOI] [PubMed] [Google Scholar]
- 7.Henry T: Double blind placebo controlled, trial of recombinant human vascular endothelial growth factor: the VIVA trial. J Am Coll Cardiol 1999, 33:384A [Google Scholar]
- 8.Yang S, Graham J, Kahn J, Schwartz E, Gerritsen M: Differential roles for CD31 and VE-cadherin in formation of vascular tubes and lumens in three dimensional collagen gels. Am J Pathol 1999, 155:887-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilan N, Mahooti S, Madri JA: Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J Cell Sci 1998, 111:3621-3631 [DOI] [PubMed] [Google Scholar]
- 10.Davis GE, Camarillo CW: An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res 1996, 224:39-51 [DOI] [PubMed] [Google Scholar]
- 11.Rosen EM, Lamszus K, Laterra J, Polverini PJ, Rubin JS, Goldberg ID: HGF/SF in angiogenesis. Ciba Found Symp 1997, 212:215-229 [DOI] [PubMed] [Google Scholar]
- 12.Morimoto A, Okamura K, Hamanaka R, Sato Y, Shima N, Higashio K, Kuwano M: Hepatocyte growth factor modulates migration and proliferation of human microvascular endothelial cells in culture. Biochem Biophys Res Commun 1991, 179:1042-1049 [DOI] [PubMed] [Google Scholar]
- 13.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM: Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 1992, 119:629-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C: Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol 1993, 123:223-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennische E, Ekberg S, Matejka GL: Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am J Physiol 1993, 265:C122-C128 [DOI] [PubMed] [Google Scholar]
- 16.Igawa T, Matsumoto K, Kanda S, Saito Y, Nakamura T: Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol 1993, 265:F61-F69 [DOI] [PubMed] [Google Scholar]
- 17.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM: Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation 1998, 97:381-390 [DOI] [PubMed] [Google Scholar]
- 18.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 1999, 33:1379-1384 [DOI] [PubMed] [Google Scholar]
- 19.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, Binder BR: Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest 1999, 79:427-438 [PubMed] [Google Scholar]
- 20.Gille J, Khalik M, Konig V, Kaufmann R: Hepatocyte growth factor/scatter factor (HGF/SF) induces vascular permeability factor (VPF/VEGF) expression by cultured keratinocytes. J Invest Dermatol 1998, 111:1160-1165 [DOI] [PubMed] [Google Scholar]
- 21.Xin X, Yang S, Kowalski J, Gerritsen ME: Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 1999, 274:9116-9121 [DOI] [PubMed] [Google Scholar]
- 22.Gerber HP, Dixit V, Ferrara N: Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998, 273:13313-13316 [DOI] [PubMed] [Google Scholar]
- 23.Morishita R, Higaki J, Hayashi SI, Yo Y, Aoki M, Nakamura S, Moriguchi A, Matsushita H, Matsumoto K, Nakamura T, Ogihara T: Role of hepatocyte growth factor in endothelial regulation: prevention of high d-glucose-induced endothelial cell death by prostaglandins and phosphodiesterase type 3 inhibitor. Diabetologia 1997, 40:1053-1061 [DOI] [PubMed] [Google Scholar]
- 24.Kondo S, Yin D, Aoki T, Takahashi JA, Morimura T, Takeuchi J: bcl-2 gene prevents apoptosis of basic fibroblast growth factor-deprived murine aortic endothelial cells. Exp Cell Res 1994, 213:428-432 [DOI] [PubMed] [Google Scholar]
- 25.Montesano R, Pepper MS, Orci L: Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci 1993, 105:1013-1024 [DOI] [PubMed] [Google Scholar]
- 26.Martin T, Harding K, Jiang W: Regulation of angiogenesis and endothelial cell motility by matrix bound fibroblasts. Angiogenesis 1999, 3:69-76 [DOI] [PubMed] [Google Scholar]
- 27.Villaschi S, Nicosia RF: Paracrine interactions between fibroblasts and endothelial cells in a serum-free coculture model. Modulation of angiogenesis and collagen gel contraction. Lab Invest 1994, 71:291-299 [PubMed] [Google Scholar]
- 28.Montesano R, Soriano JV, Malinda KM, Ponce ML, Bafico A, Kleinman HK, Bottaro DP, Aaronson SA: Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ 1998, 9:355-365 [PubMed] [Google Scholar]
- 29.Chleboun JO, Martins RN, Mitchell CA, Chirila TV: bFGF enhances the development of the collateral circulation after acute arterial occlusion. Biochem Biophys Res Commun 1992, 185:510-516 [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner I, Isner JM: Stimulation of peripheral angiogenesis by vascular endothelial growth factor (VEGF). Vasa 1998, 27:201-206 [PubMed] [Google Scholar]
- 31.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM: Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 1995, 92:II365-II371 [DOI] [PubMed] [Google Scholar]
- 32.Rakue H, Nakajima H, Katoh T, Usui M, Amemiya T, Miyagi M, Hara T, Tamura K, Sasame A, Naito Y, Nagai Y, Ibukiyama C: Low-dose basic fibroblast growth factor and vascular endothelial growth factor for angiogenesis in canine acute hindlimb insufficiency. Jpn Circ J 1998, 62:933-939 [DOI] [PubMed] [Google Scholar]
- 33.Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K: Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998, 177:439-452 [DOI] [PubMed] [Google Scholar]
- 34.Pepper MS, Vassalli JD, Orci L, Montesano R: Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res 1993, 204:356-363 [DOI] [PubMed] [Google Scholar]
- 35.Kadono Y, Shibahara K, Namiki M, Watanabe Y, Seiki M, Sato H: Membrane type 1-matrix metalloproteinase is involved in the formation of hepatocyte growth factor/scatter factor-induced branching tubules in Madin-Darby canine kidney epithelial cells. Biochem Biophys Res Commun 1998, 251:681-687 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura Y, Morishita R, Higaki J, Kida I, Aoki M, Moriguchi AKY, Hayashi S, Yo Y, Matsumotot K, Nakamura T, Ogihara T: Expression of local hepatocyte growth factor system in vascular tissues. Biochem Biophys Res Commun 1995, 215:483-488 [DOI] [PubMed] [Google Scholar]
- 37.Trusolino L, Pugliese L, Comoglio PM: Interactions between scatter factors and their receptors: hints for therapeutic applications. FASEB J 1998, 12:1267-1280 [DOI] [PubMed] [Google Scholar]
- 38.Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM: Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature 1998, 391:285-288 [DOI] [PubMed] [Google Scholar]
- 39.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation 1999, 100:II301-II308 [DOI] [PubMed] [Google Scholar]
- 40.Ueda H, Sawa Y, Matsumoto K, Kitagawa-Sakakida S, Kawahira Y, Nakamura T, Kaneda Y, Matsuda H: Gene transfection of hepatocyte growth factor attenuates reperfusion injury in the heart. Ann Thorac Surg 1999, 67:1726-1731 [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Fujieda H, Murao S, Sato H, Takeuchi T, Ohtsuki Y: Sequential changes of hepatocyte growth factor in the serum and enhanced c-Met expression in the myocardium in acute myocardial infarction. Jpn Circ J 1999, 63:906-908 [DOI] [PubMed] [Google Scholar]
- 42.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S: Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reperfusion in a rat model. J Cardiol 1998, 31:184-185 [PubMed] [Google Scholar]