Abstract
Acute treatment with the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) or chronic environmental enrichment (EE) hasten behavioral recovery after experimental traumatic brain injury (TBI). The aim of this study was to determine if combining these interventions would confer additional benefit. Anesthetized adult male rats received either a cortical impact or sham injury followed 15 min later by a single intraperitoneal injection of 8-OH-DPAT (0.5 mg/kg) or saline vehicle (1.0 mL/kg) and then randomly assigned to either enriched or standard (STD) housing. Behavioral assessments were conducted utilizing established motor and cognitive tests on post-injury days 1-5 and 14-18, respectively. Hippocampal CA1/CA3 neurons were quantified at 3 weeks. Both 8-OH-DPAT and EE attenuated CA3 cell loss. 8-OH-DPAT enhanced spatial learning in a Morris water maze (MWM) as revealed by differences between the TBI+8-OH-DPAT+STD and TBI+VEHICLE+STD groups (P=0.0014). EE improved motor function as demonstrated by reduced time to traverse an elevated narrow beam in both the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups vs. the TBI+VEHICLE+STD group (P=0.0007 and P=0.0016, respectively). EE also facilitated MWM learning as evidenced by both the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups locating the escape platform quicker than the TBI+VEHICLE+STD group (P's<0.0001). MWM differences were also observed between the TBI+8-OH-DPAT+EE and TBI+8-OH-DPAT+STD groups (P=0.0004) suggesting that EE enhanced the effect of 8-OH-DPAT. However, there was no difference between the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups. These data replicate previous results from our laboratory showing that both a single systemic administration of 8-OH-DPAT and EE improve recovery after TBI and extend those findings by elucidating that the combination of treatments in this particular paradigm did not confer additional benefit. One explanation for the lack of an additive effect is that EE is a very effective treatment and thus there is very little room for 8-OH-DPAT to confer additional statistically significant improvement.
Keywords: beam-walking, controlled cortical impact, functional recovery, learning and memory, Morris water maze, neurobehavior, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) affects almost two million individuals in the United States each year [33] and renders approximately 100,000 of the severely injured with motor and cognitive disabilities [91] that require lengthy and costly medical and rehabilitative care [63]. Experimental and clinical efforts aimed at treating this significant health care issue have resulted in numerous therapeutic approaches such as, but not limited to, anti-inflammatory and anti-oxidative strategies [4,34,36,39,51,56,101], hypothermia [5,9,13,14,21,57], and the exogenous administration of neurotrophins [19,84,88]. Another line of research that has received significant attention as a potential treatment strategy is the administration of various pharmacologic agents targeting both specific and non-specific neurotransmitter systems in an attempt to restore neural activity that has been altered by TBI [2,25,44,51-55]. While the catecholaminergic and glutamatergic systems have received considerable interest [24,26-28,41,44,49,72-74,76,83], there is a paucity of studies describing the consequences of altering serotonergic (5-hydroxytryptamine; 5-HT) neurotransmission on functional outcome after TBI [6,7,11,100]. There are, however, numerous experiments in intact animals showing that the 5-HT system is an integral component of cognitive processing [3,59,67,68] and thus is an important target to investigate after TBI.
The 5-HT1A receptor is the most studied and best-characterized 5-HT receptor subtype to date [3,76]. 5-HT1A receptors are abundantly expressed in cortical and hippocampal (CA1/CA3 and dentate gyrus) regions that are critically involved in learning and memory and susceptible to neuronal damage induced by TBI or other CNS injury [15-17]. Studies from our laboratory have demonstrated that the selective and high affinity 5-HT1A receptor agonists repinotan HCL and 8-OH-DPAT enhance spatial learning and memory performance in a Morris water maze (MWM) task, reduce hippocampal neuron loss, and decrease cortical lesion size [50,54,55]. Beneficial effects of 5-HT1A receptor agonists have also been reported in other models of CNS injury, such as focal or global cerebral ischemia [15-17,62,78-80,87,94] and acute subdural hematoma [1].
Another intervention strategy that has been shown to improve function after experimental TBI is the exposure of rats to complex, stimulatory, and social housing (enriched environment (EE)). EE, which may be considered a rodent correlate of physiotherapeutic intervention, has been extensively studied in various experimental conditions and has been shown to produce a myriad of physiological and anatomical responses such as increased brain weight and dendritic arborization, cortical and hippocampal synaptogenesis [29,95], alterations in DA neurotransmission [96,98], increased 5-HT1A receptor mRNA expression [81], increased cortical thickness [32], and reduced lesion size after TBI [75]. In addition to the plasticity-associated adaptations, EE has also been shown to improve behavioral performance and sensory neglect after brain trauma in both adult [31,40,82] and developing rats [32]. EE also improves motor function and spatial learning and memory after TBI using several injury models. For example, exposing adult rats to a complex environment for 11 or 15 days following moderate FP injury or 18 days after controlled cortical impact has been reported to significantly improve water maze performance vs. standard-housing [38,42,75,97].
An approach that has received modest attention, despite having the potential to contribute significantly to functional outcome after TBI, is the combining of potentially efficacious therapies. The limited studies utilizing this experimental paradigm have generated mixed results with some treatment combinations being counter productive [23,35,46], neutral [50,89], or providing benefits that are increased beyond what is seen after single treatments [60,104]. Based on the positive studies showing significant benefit with the 5-HT1A receptor agonist 8-OH-DPAT and EE when provided singly after experimental TBI, we hypothesized that combining these interventions would confer greater benefit than either intervention alone.
2. Materials and methods
2.1. Subjects
Fifty-eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were initially housed in standard steel-wire mesh cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with food and water available ad libitum. After one week of acclimatization the rats underwent beam walk training and then were randomly assigned to EE or standard (STD) housing conditions with either 8-OH-DPAT or saline vehicle as treatments after traumatic brain injury (TBI) or sham surgery. The following group conditions were evaluated: TBI+8-OH-DPAT+EE (n=10), TBI+VEHICLE+EE (n=10), Sham+8-OH-DPAT+EE (n=5), Sham+VEHICLE+EE (n=5), TBI+8-OH-DPAT+STD (n=10), TBI+VEHICLE+STD (n=10), Sham+8-OH-DPAT+STD (n=4), Sham+VEHICLE+STD (n=4).
2.2. Surgery
Surgical anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane (IsoFlo®, Abbott Laboratories, North Chicago, IL), respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. After endotracheal intubation the rats were secured in a stereotaxic frame and ventilated mechanically (Harvard Rodent Ventilator, Model 683, Harvard Apparatus, Inc., Holliston, MA) during surgery. Utilizing aseptic techniques a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6-mm in diameter) was made in the right hemisphere (encompassing bregma and lambda and between the sagittal suture and the coronal ridge) with a hand held Michele trephine (Miltex Instrument Company, Inc., Bethpage, NY). The resulting bone flap was removed and the craniectomy was enlarged with cranial rongeurs. Controlled cortical impact (CCI) injury was produced as previously described [18,48]. Briefly, the impacting shaft was extended and the impact tip was centered and lowered over the craniectomy until it contacted the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Immediately after the CCI, anesthesia was discontinued and the incision was promptly closed with nylon sutures. The rats were subsequently extubated and assessed for acute neurological outcome before being placed in a temporary cage where core temperature was monitored. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
All experimental procedures were approved by the Animal Care and Use Committee at the University of Pittsburgh and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). Every attempt was made to limit the number of subjects used and to minimize suffering.
2.3. Acute neurological evaluation
Hind limb reflexive ability was assessed by gently squeezing the rats paw every 5 sec following the cessation of anesthesia and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required for a rat to turn from the supine to prone position.
2.4. Drug administration
8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) was purchased from Sigma (Sigma-Aldrich, Inc., St. Louis, MO) and was prepared daily by dissolving in sterile saline, which also served as the vehicle. 8-OH-DPAT (0.5 mg/kg) or a comparable volume of vehicle (1 mL/kg) was administered intraperitoneally 15 min after cortical impact or sham injury. The dose of 8-OH-DPAT and route of administration were selected based on an earlier dose-response study from our laboratory [55] and a more recent publication showing this regimen to be neuroprotective and to promote behavioral recovery after TBI [50].
2.5. Housing conditions: (environmental manipulation)
Following surgery and acute drug administration, the rats were returned to the colony where those designated for enrichment were immediately placed in an EE, which consisted of a 36×30×20 inch steel-wire cage with three levels (and ladders to ambulate from one level to another) containing various toys (e.g., balls, blocks, tubes for tunneling, swing ropes etc) and materials (e.g., cloth and paper towels) for nesting (Fig. 1). In addition to ad libitum food and water, rats in the EE were also given palatable foods such as dry cereal (e.g., fruit loops). To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was approximately every 3 days. Ten to twelve rats, which included both 8-OH-DPAT and vehicle-treated TBI and sham controls, were housed in the EE at any given time. Rats in the STD conditions were placed in standard steel-wire mesh cages (2 rats per cage) with only food and water.
Fig. 1.
Photograph of the enrichment cage. Note the multiple levels, wide array of sensory stimuli in the form of balls, tunnels, ramps, ropes, etc. Ten to twelve rats are housed at any given time and are removed only briefly for behavioral assessments.
2.6. Motor performance
Established beam-balance (BB) and beam-walk (BW) tasks were utilized to assess motor function. The BB task consists of placing the rat on an elevated (90 cm) narrow wooden beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. The BW task, originally devised by Feeney and colleagues (1982), consists of training/assessing rats using a negative-reinforcement paradigm to escape ambient light and white noise by traversing an elevated narrow wooden beam (2.5 × 100 cm) and entering a darkened goal box situated at the opposite end. When the rat enters the goal box the adverse stimuli (light and noise) are terminated thus serving as reinforcement (reward) for completing the task. Performance was assessed by recording the elapsed time to traverse the beam. Rats were tested for BB and BW performance on post-operative days 1–5 and were provided three trials (60 sec allotted time) per day on each task. The average daily scores for each subject were used in the statistical analyses.
2.7. Cognitive function
2.7.1. Spatial learning acquisition
Spatial learning was assessed in a Morris water maze (MWM) task demonstrated to be sensitive to cognitive function/dysfunction after TBI [37,52,86,90]. Briefly, the maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning acquisition began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14–18) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). An additional day (postoperative day 19) was provided to locate the platform when it was raised 2 cm above the water surface (i.e., visible to the rat). While the visible platform task has been used to test for non-hippocampal damage [8], its use in the present study was as a control procedure to determine the contributions of non-spatial factors (e.g., sensory-motor performance, motivation, and visual acuity) on MWM performance. For each daily block of trials the rats were placed in the pool facing the wall at each of the four possible start locations (north, east, south, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the goal within the allotted time were manually guided to it. All rats remained on the platform for 30 sec before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses. The data were obtained using a spontaneous motor activity recording & tracking (SMART) system (San Diego Instruments, San Diego, CA).
2.7.2. Probe trial assessment
One day after the final acquisition training session (Day 19), all rats were given a probe trial to measure retention. Briefly, the platform was removed from the pool and the rats were placed in the MWM from the location point most distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 60 sec. Typically, rats that have learned the specific location of the escape platform exhibit a spatial bias and spend significantly more time in the target quadrant.
2.8. Histology
Quantification of hippocampal neurons
Three weeks after CCI or sham injury, the rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and then perfused transcardially with 200 mL heparinized 0.1 M phosphate buffered saline (pH 7.4) followed by 300 mL 10% buffered formalin. The brains were extracted, post-fixed in 10% buffered formalin for one week, dehydrated with alcohols, and embedded in paraffin. Seven-micrometer thick coronal sections were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on gelatin-coated glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with Cresyl violet. An observer blinded to experimental conditions analyzed one coronal section underlying the area of contusion (∼ 3.5 mm posterior to bregma) from all rats in each group for determination of treatment efficacy on selectively vulnerable hippocampal CA1 and CA3 neurons. To reduce counting errors associated with false positive identification of dying neurons, the total number of CA1 and CA3 morphologically intact neurons (i.e., those with a clearly defined cell body and nucleus) were counted using a Nikon Eclipse E600 microscope (Nikon Corporation, Tokyo, Japan) with a 40x objective. The data are reported as the percent of total neurons in the ipsilateral (injured) CA1 and CA3 regions relative to the contralateral hippocampus.
2.9. Data analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statview 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological, probe trial, histological, and swim speed data were analyzed by one-factor ANOVAs. When the overall ANOVA revealed a significant effect, the data were further analyzed with the Bonferroni/Dunn post-hoc test to determine specific group differences. The data are presented as the mean ± standard error (SE) and are considered significant when corresponding P values are < 0.05 or as determined by the Bonferroni/Dunn statistic after adjusting for multiple comparisons.
3. Results
All rats survived the surgical procedures and thus the statistical analyses for motor, cognitive, and histological outcome are based on the initial fifty-eight rats. However, because there were no significant differences in behavioral outcomes among the sham-injured groups [P's > 0.05], the data were pooled and analyzed as one group (designated as SHAM).
3.1. Acute neurological function
No significant differences were observed among the TBI groups in latency to recover the hind limb withdrawal reflex in response to a brief paw pinch [range 177.3 ± 4.4 sec to 185.5 ± 4.2 sec, P > 0.05] or for return of righting ability [range 350.9 ± 9.1 sec to 389.4 ± 10.7 sec, P > 0.05] following the cessation of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced an equivalent level of injury and anesthesia.
3.2. Motor function
All rats were capable of balancing on the beam and as such no pre-surgical differences were observed among groups. However, significant impairments were detected in all injured groups vs. SHAMS for the first 2 days after surgery, regardless of treatment. There were trends for faster BB improvement on post-operative days 3–5 for the TBI+8-OH-DPAT+EE group vs. TBI+VEHICLE+EE [P = 0.07], TBI+VEHICLE+STD [P = 0.08], and TBI+8-OH-DPAT+STD [P = 0.009]. According to the Bonferroni/Dunn post-hoc test an alpha level of 0.005 was required for significance. Similar to BB, there were no differences [P > 0.05] in pre-surgical BW times among groups as all rats were proficient and traversed the beam in approximately 5 sec. However, after the traumatic insult all groups demonstrated a marked increase in BW time vs. SHAM. EE facilitated BW recovery as revealed by quicker traversal times in both the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups vs. the TBI+VEHICLE+STD group [P = 0.0007 and P = 0.0016, respectively]. There was no difference between the STD housed groups [P = 0.29]. Lastly, although not statistically significant, there was a trend for the TBI+8-OHDPAT+EE group to perform better than the TBI+8-OH-DPAT+STD group [P = 0.018] suggesting some additive benefit with the combined treatments (Fig. 2).
Fig. 2.
Mean (± SE) walking ability as measured by time (sec) to traverse an elevated wooden beam prior to, and after, TBI or SHAM injury. All TBI groups exhibited significant initial impairment vs. SHAM controls, but both EE groups displayed facilitated recovery. *P < 0.05 vs. TBI+VEHICLE+STD.
3.3. Cognitive function
3.3.1. Spatial learning acquisition
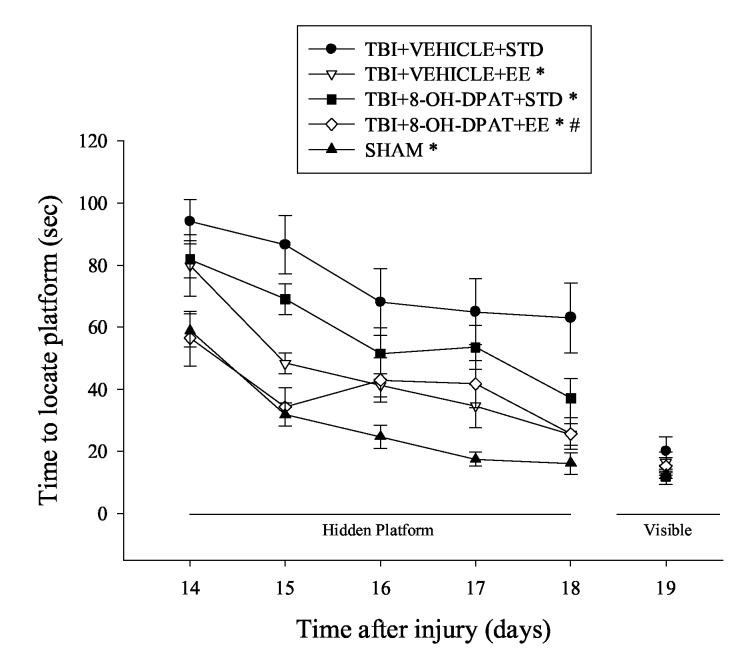
Analysis of spatial learning acquisition revealed significant Group [F4,53 = 25.536, P < 0.0001] and Day [F 4, 212 = 31.694, P < 0.0001] effects. All injured groups with the exception of TBI+8-OH-DPAT+EE were significantly impaired relative to SHAMs. EE facilitated spatial learning and memory as evidenced by shorter times to locate the target platform in the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups vs. the TBI+VEHICLE+STD group [P < 0.0001 and 0.0001, respectively]. 8-OH-DPAT also improved water maze performance as evidenced by enhanced performance in the TBI+8-OH-DPAT+STD vs. TBI+VEHICLE+STD group [P = 0.0014]. Moreover, as depicted in Fig. 3, a significant difference in spatial acquisition was observed between the TBI+8-OH-DPAT+EE and TBI+8-OH-DPAT+STD groups [P = 0.0004], suggesting that EE can enhance the effects of 8-OH-DPAT. However, the data do not support an additive effect with combined treatments as there was no difference between the TBI+8-OHDPAT+EE and TBI+VEHICLE+EE groups.
Fig. 3.
Mean (± SE) time (sec) to locate either a hidden (submerged) or visible (raised) platform in a water maze. All TBI groups, except the TBI+8-OH-DPAT+EE group, had significant difficulty with the cognitive task vs. SHAM controls. However, over the subsequent 5 days of training, all groups, except the TBI+VEHICLE+STD group, became proficient at locating the escape platform. *P < 0.05 vs. TBI+VEHICLE+STD. #P < 0.05 vs. TBI+8-OH-DPAT+STD. No differences were observed in locating the visible platform.
3.3.2. Probe trial assessment
Analysis of probe assessment revealed a significant Group [F4,53 = 3.727, P < 0.0096] effect, which was attributed to the SHAM group exhibiting a significant bias for the target quadrant vs. all TBI groups. There were no significant differences among the TBI groups, regardless of condition. The amount of time spent in the target quadrant for each group was: SHAM = 45.43 ± 2.5 %, TBI+VEHICLE+EE = 35.2 ± 2.8 %, TBI+VEHICLE+STD = 28.8 ± 3.1 %, TBI+8-OH-DPAT+EE = 34.2 ± 3.2 %, and TBI+8-OH-DPAT+STD = 35.0 ± 5.7 %.
3.3.3. Swim speed and visible platform performance
No significant differences in swim speed (range = 28.2 ± 2.1 cm/sec to 34.3 ± 1.9 cm/sec) or visible platform acquisition (Fig. 3) were observed among the TBI groups vs. SHAM, suggesting that neither motor impairments nor visual disparities influenced the assessment of place learning.
3.4. Histology
Quantification of hippocampal neurons
CCI produced significant reductions in normal appearing (i.e., morphologically intact) CA1 and CA3 neurons in the hippocampus ipsilateral to the impact. All TBI groups differed from the SHAM group, but not from one other in the percentage of normal appearing CA1 neurons. The mean ratio of morphologically intact CA3 neurons was significantly reduced in all TBI groups, regardless of condition, vs. SHAM [P < 0.05]. Furthermore, as depicted in Fig. 4 and elaborated on in Table 1, EE conferred neuroprotection as revealed by greater CA3 intact cells in both the TBI+8-OH-DPAT+EE and TBI+VEHICLE+EE groups vs. the TBI+VEHICLE+STD group [P = 0.0012 and 0.0015, respectively]. In addition, 8-OH-DPAT also provided protection as revealed by differences between the TBI+8-OH-DPAT+STD vs. TBI+VEHICLE+STD groups [P = 0.0001]. However, no additive effect of treatments was observed [P = 0.44, TBI+8-OHDPAT+EE vs. TBI+8-OH-DPAT+STD].
Fig. 4.
Mean (± SE) morphologically intact CA3 neurons (% of contralateral hippocampus) at three weeks after a controlled cortical impact or sham injury. All injured groups expressed significantly fewer normal appearing neurons compared to the SHAM controls. **P < 0.05 vs. all TBI groups. *P < 0.05 vs. TBI+VEHICLE+STD.
Table 1.
Acute 8-OH-DPAT treatment, chronic EE, and their combination on hippocampal cell survival quantified 3 weeks after TBI or sham injury.
| Groups | CA1 | CA3 |
|---|---|---|
| TBI+8-OH-DPAT+EE | 51.8 ± 7.5 | 60.3 ± 3.0* |
| TBI+8-OH-DPAT+STD | 46.5 ± 6.0 | 65.5 ± 10.3* |
| TBI+VEHICLE+EE | 47.9 ± 5.4 | 59.6 ± 4.8* |
| TBI+VEHICLE+STD | 35.6 ± 4.6 | 36.3 ± 1.5 |
| SHAM | 99.5 ± 0.5** | 98.9 ± 1.6** |
Mean (± SE) normal appearing neurons expressed as a percentage of the contralateral hippocampus.
P < 0.05 vs. TBI+VEHICLE+STD.
P < 0.05 vs. all TBI groups.
4. Discussion
Previous studies have shown that both acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and EE confer neurobehavioral benefit when provided singly after TBI. Given the established effectiveness of these therapies, the current study sought to determine if combining these treatments would yield additional benefit. A single and early administration of 8-OH-DPAT improved performance in the water maze and attenuated the loss of hippocampal CA3 neurons vs. vehicle-treated controls when both groups were housed in standard (STD) conditions, thus replicating prior work from our laboratory [50,54,55]. EE improved both cognitive and motor (i.e., beam walk) performance, which is also consistent with data from our laboratory [97]. In addition, EE attenuated hippocampal CA3 cell loss relative to STD housed vehicle-treated controls. Although decreased lesion volume has been shown after fluid percussion (FP) TBI [75], this is the first report of EE-induced histological protection after controlled cortical impact injury. However, the combination of 8-OH-DPAT and EE was not significantly more efficacious than either of the two therapeutic approaches alone. Thus, our hypothesis that combined treatments would confer greater neurobehavioral benefits than either intervention alone was not supported. A possible explanation for the lack of an additive effect is that EE is an effective treatment and thus there was very little room for an additional treatment like 8-OH-DPAT to confer additional statistically significant improvement.
No studies have been reported regarding an 8-OH-DPAT plus EE treatment paradigm and thus our study investigating the effect of these two therapies on functional improvement after TBI is novel. However, the notion that two separate neuroprotective strategies may be able to enhance recovery beyond the limits of either alone is not new as other studies, albeit few, have shown enhanced improvement with combined treatments. For example, the combination of scopolamine and morphine after moderate FP brain injury enhanced motor function (i.e., beam-walking) significantly more than either treatment individually [60]. Beneficial effects have also been observed when two separate therapies work in synergy, such as that reported by Yan et al (2000) who showed that the combination of fibroblast growth factor-2 (FGF-2) and hypothermia resulted in enhanced motor performance as indicated by shorter beam-walking latencies vs. FGF-2 and hypothermia that on their own did not differ from controls [104]. In addition to the aforementioned studies showing added improvement with joint therapies, there are an equal number of reports describing neutral or counter productive effects with multiple treatment or “cocktail” paradigms. Recent studies from Kline and colleagues have shown that 8-OH-DPAT induces a rapid, mild, and transient hypothermic response [55] that does not augment the cognitive improvement observed after drug treatment [50]. Smith et al (1993) evaluated the effect of two NMDA receptor antagonists, magnesium and ketamine, on memory function after parasagittal FP TBI and found that while both treatments were beneficial when provided alone, there was no additive effect when combined [89]. These studies suggest a neutral effect of combined treatments. Paradoxically, the administration of the anti-inflammatory agent interleukin-10 (IL-10) in combination with moderate hypothermia after controlled cortical impact injury suppressed the effects of hypothermia, which on its own enhanced both motor and cognitive outcome [46]. Similar findings were observed in a study showing that magnesium benefited outcome when provided alone after FP TBI, but not when combined with FGF-2 [35]. Lastly, combined treatment with the thyrotropin-releasing hormone analog YM14673 and the NMDA antagonist dextrorphan after FP brain trauma resulted in significantly less behavioral recovery than either treatment alone [23]. These observations indicate that combining neuroprotective pharmacotherapies that exert benefit when used alone does not always equate to enhanced functional outcome and, in fact, may actually be suboptimal or deleterious signifying the need for further empirical investigation of drug-drug interactions in the treatment of TBI.
Potential mechanisms for the observed beneficial effects may be varied as acute administration of 8-OH-DPAT and chronic EE are acting, presumably, on distinct mechanisms. For example, unpublished observations from our laboratory have shown that the beneficial effect on cognitive performance seen after an early and single systemic administration of 8-OH-DPAT (same treatment paradigm as in the current study) is restricted to a narrow therapeutic time window as evidenced by a lack of effectiveness when treatment is delayed by even 1 hr post-injury. This observation suggests that early 8-OH-DPAT treatment is producing its beneficial effects by acting on acute pathophysiological responses of TBI such as the attenuation of glutamate-induced excitotoxicity, which is known to occur early after TBI. 5-HT1A receptor agonists reduce Na+ influx [66], decrease glutamate release in experimental models of brain insult [15-17,62,79] and Parkinsons disease [69], and induce neuronal hyperpolarization [78]. Taken together, these data provide support for neuroprotection mediated by neuronal membrane hyperpolarization and increased resistance to glutamate-induced excitotoxicity. 5-HT1A receptor agonists also increase dopamine (DA) levels [3,85], which may confer benefit by restoring DA neurotransmission in a system that is known to be altered after TBI [22,43,49,61,65,98,102,103]. Evidence to support this assertion comes from studies showing that the DA receptor agonists amantadine, bromocriptine, and methylphenidate improve functional outcome and/or preserve hippocampal CA3 cell survival after TBI [20,47,51-53,64,77,99]. A potential mechanism for the EE-induced benefits may include neural-adaptive changes such as increased hippocampal neurogenesis [10,45,71]. Although not examined in the current study, it is possible that new hippocampal neurons participated in learning and memory improvement. EE-induced neurogenesis may have enhanced the effect on learning and memory that was observed after 8-OH-DPAT. Increased expression of the synaptic proteins synaptophysin and PSD-95 [70], enhanced dendritic growth [58], and increased progenitor cell survival [30] may also contribute to the enhanced benefits by promoting plasticity-associated changes in learning and memory. The observed attenuation of hippocampal CA3 cells in both the 8-OH-DPAT and EE treated groups suggests that neuroprotection may have also played a role in the observed benefits. Similar histological protection was observed after FP TBI as evidenced by reduced cortical lesion volumes that correlated with improved cognitive performance [75]. EE has also been shown to alter BDNF expression [12,42]. A recent study from our laboratory showed that following cortical impact injury there is a significant increase of BDNF in enriched female rats vs. sham controls [12], which did not correlate with improvement in a water maze task [97]. In contrast, an earlier study by Hicks and colleagues showed that BDNF was not increased after FP injury in EE rats vs. standard housed, but there was an improvement in cognitive performance [42]. Taken together, these two studies suggest that alterations in BDNF may not be the neural substrate mediating the improvements in cognitive function associated with EE after TBI.
The finding that a single systemic administration of 8-OH-DPAT and chronic EE after TBI confer neurobehavioral benefit when provided alone is clinically attractive, particularly because EE can be considered a reasonable animal correlate of the clinical rehabilitation process. Often after severe brain injuries, patients may receive a variety of therapies in an acute rehabilitation setting or they may receive convalescent care at home or at a skilled nursing facility. Although not well validated through rigorous clinical trials, the cognitive and physical stimulation associated with acute rehabilitation is thought to provide an enriching environment for patients, which may enhance recovery compared to a convalescent setting. During the rehabilitation phase of recovery, clinicians also utilize pharmacotherapies that may act in concert with the rehabilitation program in promoting recovery, which is not unlike the paradigm used in the current experiment. Although an additive effect was not observed in this study, which compared acute treatment with chronic rehabilitation after TBI, other approaches that are currently being investigated in our laboratory that may produce additive effects include assessments of chronic 5-HT1A receptor agonist treatments with either early or delayed EE. Determining an optimal time to introduce pharmacotherapy plus enrichment after brain injury could considerably augment clinical rehabilitation strategies.
Acknowledgements
This work was supported, in part, by National Institutes of Heath grants HD043851 and HD046700 awarded to AEK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alessandri B, Tsuchida E, Bullock RM. The neuroprotective effect of a new serotonin receptor agonist, BAY x3702, upon focal ischemic brain damage caused by acute subdural hematoma in the rat. Brain Res. 1999;845:232–35. doi: 10.1016/s0006-8993(99)01948-4. [DOI] [PubMed] [Google Scholar]
- 2.Baranova AI, Whiting MD, Hamm RJ. Delayed, post-injury treatment with aniracetam improves cognitive performance after traumatic brain injury in rats. J Neurotrauma. 2006;23:1233–40. doi: 10.1089/neu.2006.23.1233. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Bayir H. Reactive oxygen species. Crit Care Med. 2005;33:S498–501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 5.Bayir H, Clark RS, Kochanek PM. Promising strategies to minimize secondary brain injury after head trauma. Crit Care Med. 2003;31:S112–7. doi: 10.1097/00003246-200301001-00016. [DOI] [PubMed] [Google Scholar]
- 6.Boyeson MG, Harmon RL. Effects of trazodone and desipramine on motor recovery in brain-injured rats. Am J Phys Med Rehabil. 1993;72:286–93. doi: 10.1097/00002060-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Boyeson MG, Harmon RL, Jones JL. Comparative effects of fluoxetine, amitriptyline and serotonin on functional motor recovery after sensorimotor cortex injury. Am J Phys Med Rehabil. 1994;73:76–83. doi: 10.1097/00002060-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 9.Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–98. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- 10.Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–21. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 11.Busto R, Dietrich WD, Globus MY-T, Alonso O, Ginsberg MD. Extracellular release of serotonin following fluid-percussion brain injury in rats. J Neurotrauma. 1997;14:35–42. doi: 10.1089/neu.1997.14.35. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Li Y, Kline AE, Dixon CE, Zafonte RD, Wagner AK. Gender and environmental effects on regional BDNF expression after experimental traumatic brain injury. Neuroscience. 2005;135:11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 13.Clark RSB, Kochanek PM, Marion DW, Schiding JK, White M, Palmer AM, Dekosky ST. Mild posttraumatic hypothermia reduces mortality after severe controlled cortical impact in rats. J Cereb Blood Flow Metab. 1996;16:252–61. doi: 10.1097/00004647-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–21. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- 15.De Vry J, Dietrich H, Glaser T, Heine HG, Horvath E, Jork R, Maertins T, Mauler F, Optiz W, Scherling D, Schohe-Loop R, Schwarz T. BAY × 3702. Drugs of the Future. 1997;22:341–49. [Google Scholar]
- 16.De Vry J, Jentzsch KR. Discriminative stimulus properties of the 5-HT1A receptor agonist BAY × 3702 in the rat. Eur J Pharmacol. 1998;357:1–8. doi: 10.1016/s0014-2999(98)00503-2. [DOI] [PubMed] [Google Scholar]
- 17.De Vry J, Schohe-Loop R, Heine H-G, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T. Characterization of the aminomethylchroman derivative BAY × 3702 as a highly potent 5-hydroxytryptamine 1A receptor agonist. J Pharmacol Exp Ther. 1998;284:1082–94. [PubMed] [Google Scholar]
- 18.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 19.Dixon CE, Flinn P, Bao J, Venya R, Hayes RL. Nerve growth factor attenuates cholinergic deficits following traumatic brain injury in rats. Exp Neurol. 1997;146:479–90. doi: 10.1006/exnr.1997.6557. [DOI] [PubMed] [Google Scholar]
- 20.Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW. Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor Neurol Neurosci. 1999;14:285–94. [PubMed] [Google Scholar]
- 21.Dixon CE, Markgraf CG, Angileri F, Pike BR, Wolfson B, Newcomb JK, Bismar MM, Blanco AJ, Clifton GL, Hayes RL. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma. 1998;15:95–103. doi: 10.1089/neu.1998.15.95. [DOI] [PubMed] [Google Scholar]
- 22.Dunn-Meynell A, Pan S, Levin BE. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- 23.Faden AI. Comparison of single and combined drug treatment strategies in experimental brain trauma. J Neurotrauma. 1993;10:91–100. doi: 10.1089/neu.1993.10.91. [DOI] [PubMed] [Google Scholar]
- 24.Feeney DM. From laboratory to clinic: noradrenergic enhancement of physical therapy for stroke or trauma patients. Adv Neurol. 1997;73:383–94. [PubMed] [Google Scholar]
- 25.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–57. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 26.Feeney DM, Sutton RL. Pharmacotherapy for recovery of function after brain injury. Crit Rev Neurobiol. 1987;3:135–97. [PubMed] [Google Scholar]
- 27.Feeney DM, Weisend MP, Kline AE. Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J Neur Transplant Plast. 1993;4:199–13. doi: 10.1155/NP.1993.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feeney DM, Westerberg VS. Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can J Psychol. 1990;44:233–52. doi: 10.1037/h0084243. [DOI] [PubMed] [Google Scholar]
- 29.Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–26. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 30.Gaulke LJ, Horner PJ, Fink AJ, McNamara CL, Hicks RR. Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res Mol Brain Res. 2005;141:138–50. doi: 10.1016/j.molbrainres.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile AM, Beheshti Z. Enrichment versus exercise effects on motor impairments following cortical removals in rats. Beh Neural Bio. 1987;47:321–32. doi: 10.1016/s0163-1047(87)90435-3. [DOI] [PubMed] [Google Scholar]
- 32.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein M. Traumatic brain injury: a silent epidemic. Ann Neurol. 1990;27:327. doi: 10.1002/ana.410270315. [DOI] [PubMed] [Google Scholar]
- 34.Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury. Neurosurgery. 2005;56:590–604. doi: 10.1227/01.NEU.0000154060.14900.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guluma KZ, Saatman KE, Brown A, Raghupathi R, McIntosh TK. Sequential pharmacotherapy with magnesium chloride and basic fibroblast growth factor after fluid percussion brain injury results in less neuromotor efficacy than that achieved with magnesium alone. J Neurotrauma. 1999;16:311–21. doi: 10.1089/neu.1999.16.311. [DOI] [PubMed] [Google Scholar]
- 36.Hall ED, Kupina NC, Althaus JS. Peroxynitrite scavengers for the acute treatment of traumatic brain injury. Ann NY Acad Sci. 1999;890:462–68. doi: 10.1111/j.1749-6632.1999.tb08025.x. [DOI] [PubMed] [Google Scholar]
- 37.Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 38.Hamm RJ, Temple MD, OfDell DM, Pike BR, Lyeth BG. Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J Neurotrauma. 1996;13:41–47. doi: 10.1089/neu.1996.13.41. [DOI] [PubMed] [Google Scholar]
- 39.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–12. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Held JM, Gordon J, Gentile AM. Environmental influences on locomotor recovery following cortical lesions in rats. Beh Neurosci. 1985;99:678–90. doi: 10.1037//0735-7044.99.4.678. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez TD. Post-traumatic neural depression and neurobehavioral recovery after brain injury. J Neurotrauma. 2006;23:1211–21. doi: 10.1089/neu.2006.23.1211. [DOI] [PubMed] [Google Scholar]
- 42.Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience. 2002;112:631–37. doi: 10.1016/s0306-4522(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 43.Huger F, Patrick G. Effect of concussive head injury on central catecholamine levels and synthesis rates in rat brain regions. J Neurochem. 1979;33:89–95. doi: 10.1111/j.1471-4159.1979.tb11710.x. [DOI] [PubMed] [Google Scholar]
- 44.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increase in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurgery. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 45.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 46.Kline AE, Bolinger BD, Kochanek PM, Carlos TM, Yan HQ, Jenkins LW, Marion DW, Dixon CE. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002;937:22–31. doi: 10.1016/s0006-8993(02)02458-7. [DOI] [PubMed] [Google Scholar]
- 47.Kline AE, Chen MJ, Tso-Olivas DY, Feeney DM. Methylphenidate treatment following ablation-induced hemiplegia in rat: experience during drug action alters effects on recovery of function. Pharmacol Biochem Beh. 1994;48:773–79. doi: 10.1016/0091-3057(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 48.Kline AE, Dixon CE. Contemporary in vivo models of brain trauma and a comparison of injury responses. In: Miller LP, Hayes RL, editors. Head Trauma: Basic, Preclinical and Clinical Directions. John Wiley & Sons; NY: 2001. pp. 65–84. [Google Scholar]
- 49.Kline AE, Jenkins LW, Yan HQ, Dixon CE. Neurotransmitter and growth factor alterations in functional deficits and recovery following traumatic brain injury. In: Clark RSB, Kochanek P, editors. Brain Injury. Kluwer Academic Publishers; Norwell, MA: 2001. pp. 267–294. [Google Scholar]
- 50.Kline AE, Massucci JL, Dixon CE, Zafonte RD, Bolinger BD. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma. 2004;21:175–85. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- 51.Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712–22. doi: 10.1089/neu.2004.21.1712. [DOI] [PubMed] [Google Scholar]
- 52.Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–25. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- 53.Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163–66. doi: 10.1016/s0304-3940(00)00797-7. [DOI] [PubMed] [Google Scholar]
- 54.Kline AE, Yu J, Horvath E, Marion DW, Dixon CE. The selective 5-HT(1A) receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience. 2001;106:547–55. doi: 10.1016/s0306-4522(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 55.Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci Lett. 2002;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- 56.Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–51. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- 57.Koizumi H, Povlishock JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J Neurosurg. 1998;89:303–9. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- 58.Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Luttgen M, Elvander E, Madjid N, Ogren SO. Analysis of the role of 5-HT1A receptors in spatial and aversive learning in the rat. Neuropharmacology. 2005;48:830–52. doi: 10.1016/j.neuropharm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Lyeth BG, Liu S, Hamm RJ. Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 1993;617:69–75. doi: 10.1016/0006-8993(93)90614-s. [DOI] [PubMed] [Google Scholar]
- 61.Massucci JL, Kline AE, Ma X, Zafonte RD, Dixon CE. Time dependent alterations in dopamine tissue levels and metabolism after experimental traumatic brain injury in rats. Neurosci Lett. 2004;372:127–31. doi: 10.1016/j.neulet.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 62.Mauler F, Fahrig T, Horváth E, Jork R. Inhibition of evoked glutamate release by the neuroprotective 5-HT1A receptor agonist BAY × 3702 in vitro and in vivo. Brain Res. 2001;888:150–57. doi: 10.1016/s0006-8993(00)03074-2. [DOI] [PubMed] [Google Scholar]
- 63.Max W, Mackenzie EJ, Rice DP. Head injuries: costs and consequences. J Head Trauma Rehabil. 1991;6:76–91. [Google Scholar]
- 64.McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121:1155–64. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 65.McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–33. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- 66.Melena J, Chidlow G, Osborne NN. Blockade of voltage-sensitive Na+ channels by the 5-HT1A receptor agonist 8-OH-DPAT: possible significance for neuroprotection. Eur J Pharmacol. 2000;406:319–24. doi: 10.1016/s0014-2999(00)00688-9. [DOI] [PubMed] [Google Scholar]
- 67.Meneses A. 5-HT system and cognition. Neurosci Biobehav Rev. 1999;23:1111–25. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 68.Meneses A, Hong E. Role of 5-HT1B, 5-HT2A and 5-HT2C receptors in learning. Behav Brain Res. 1997;87:105–10. doi: 10.1016/s0166-4328(96)02266-8. [DOI] [PubMed] [Google Scholar]
- 69.Mignon LJ, Wolf WA. 8-hydroxy-2-(di-n-propylamino)tetralin reduces striatal glutamate in an animal model of Parkinson's disease. Neuroreport. 2005;16:699–703. doi: 10.1097/00001756-200505120-00009. [DOI] [PubMed] [Google Scholar]
- 70.Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol Learn Mem. 2004;81:200–10. doi: 10.1016/j.nlm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 72.Palmer AM, Marion DW, Botscheller ML, Bowen DM, DeKosky ST. Increased transmitter amino acid concentration in human ventricular CSF after brain trauma. NeuroReport. 1994;6:153–56. doi: 10.1097/00001756-199412300-00039. [DOI] [PubMed] [Google Scholar]
- 73.Palmer AM, Marion DW, Botscheller ML, Swedlow PF, Styren SD, DeKosky ST. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–24. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 74.Parton A, Coulthard E, Husain M. Neuropharmacological modulation of cognitive deficits after brain damage. Curr Opin Neurol. 2005;18:675–80. doi: 10.1097/01.wco.0000189872.54245.13. [DOI] [PubMed] [Google Scholar]
- 75.Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive and tissue integrity following severe traumatic brain injury in rats. Exp Neurol. 2001;168:373–84. doi: 10.1006/exnr.2000.7623. [DOI] [PubMed] [Google Scholar]
- 76.Phillips JP, Devier DJ, Feeney DM. Rehabilitation pharmacology: bridging laboratory work to clinical application. J Head Trauma Rehabil. 2003;18:342–56. [PubMed] [Google Scholar]
- 77.Plenger PM, Dixon CE, Castillo RM, Frankowski RF, Yablon SA, Levin HS. Subacute methylphenidate treatment for moderate to moderately severe traumatic brain injury: a preliminary double-blind placebo-controlled study. Arch Phys Med Rehab. 1996;77:536–40. doi: 10.1016/s0003-9993(96)90291-9. [DOI] [PubMed] [Google Scholar]
- 78.Prehn JH, Backhauss C, Karkoutly C, Nuglisch J, Peruche B, Rossberg C, Krieglstein J. Neuroprotective properties of 5-HT1A receptor agonists in rodent models of focal and global cerebral ischemia. Eur J Pharmacol. 1991;203:213–22. doi: 10.1016/0014-2999(91)90717-5. [DOI] [PubMed] [Google Scholar]
- 79.Prehn JH, Welsch M, Backhauss C, Nuglisch J, Ausmeier F, Karkoutly C, Krieglstein J. Effects of serotonergic drugs in experimental brain ischemia: evidence for a protective role of serotonin in cerebral ischemia. Brain Res. 1993;630:10–20. doi: 10.1016/0006-8993(93)90636-2. [DOI] [PubMed] [Google Scholar]
- 80.Raiteri M, Maura G, Barzizza A. Activation of presynaptic 5-hydroxytryptamine-like receptors on glutamatergic terminals inhibits N-methyl-D-aspartate-induced cyclic GMP production in rat cerebellar slices. J Pharmacol Exp Ther. 1991;257:1184–88. [PubMed] [Google Scholar]
- 81.Rasmuson S, Olsson T, Henriksson BG, Kelly PAT, Holmes MC, Seckl JR, Mohammed AH. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Mo Brain Res. 1998;53:285–90. doi: 10.1016/s0169-328x(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 82.Rose FD, Davey MJ, Love S, Dell PA. Environmental enrichment and recovery from contralateral sensory neglect in rats with large unilateral neocortical lesions. Beh Brain Res. 1987;24:195–202. doi: 10.1016/0166-4328(87)90057-x. [DOI] [PubMed] [Google Scholar]
- 83.Rose ME, Huerbin MB, Melick J, Marion DW, Palmer AM, Schiding JK, Kochanek PM, Graham SH. Regulation of interstitial excitatory amino acid concentrations after cortical contusion injury. Brain Res. 2002;943:15–22. doi: 10.1016/s0006-8993(02)02471-x. [DOI] [PubMed] [Google Scholar]
- 84.Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418–27. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- 85.Sakaue M, Somboonthum P, Nishihara B, Koyama Y, Hashimoto H, Baba A, Matsuda T. Postsynaptic 5-hydroxytryptamine (1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br J Pharmacol. 2000;129:1029–34. doi: 10.1038/sj.bjp.0703139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–27. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- 87.Semkova I, Wolz P, Krieglstein J. Neuroprotective effect of 5-HT1A receptor agonist, Bay × 3702, demonstrated in vitro and in vivo. Eur J Pharmacol. 1998;359:251–60. doi: 10.1016/s0014-2999(98)00634-7. [DOI] [PubMed] [Google Scholar]
- 88.Sinson G, Perri BR, Trojanowski JQ, Flamm ES, Mcintosh TK. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J Neurosurg. 1997;86:511–18. doi: 10.3171/jns.1997.86.3.0511. [DOI] [PubMed] [Google Scholar]
- 89.Smith DH, Okiyama K, Gennarelli TA, McIntosh TK. Magnesium and ketamine attenuate cognitive dysfunction following experimental brain injury. Neurosci Lett. 1993;157:211–14. doi: 10.1016/0304-3940(93)90739-8. [DOI] [PubMed] [Google Scholar]
- 90.Smith DH, Okiyama K, Thomas MJ, Claussen B, Mcintosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–69. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 91.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–57. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 92.Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. Expression of neurotrophin-3 mRNA in the rat visual cortex and hippocampus is influenced by environmental conditions. Neurosci Lett. 1996;218:107–10. doi: 10.1016/s0304-3940(96)13127-x. [DOI] [PubMed] [Google Scholar]
- 93.Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 94.Torup L, Møller A, Sager TN, Diemer NH. Neuroprotective effect of 8-OH-DPAT in global cerebral ischemia assessed by stereological cell counting. Eur J Pharmacol. 2000;395:137–41. doi: 10.1016/s0014-2999(00)00175-8. [DOI] [PubMed] [Google Scholar]
- 95.van Praag K, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev. 2000;1:191–98. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 96.Wagner AK, Chen X, Kline AE, Li Y, Zafonte RD, Dixon CE. Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp Neurol. 2005;195:475–83. doi: 10.1016/j.expneurol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 97.Wagner AK, Kline AE, Sokoloski J, Zafonte RD, Capulong E, Dixon CE. Intervention with environmental enrichment after experimental brain trauma enhances cognitive recovery in male but not female rats. Neurosci Lett. 2002;334:165–168. doi: 10.1016/s0304-3940(02)01103-5. [DOI] [PubMed] [Google Scholar]
- 98.Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–65. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- 99.Whyte J, Hart T, Schuster K, Fleming M, Polansky M, Coslett HB. Effects of methylphenidate on attentional function after traumatic brain injury. a randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76:440–50. doi: 10.1097/00002060-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Wilson MS, Hamm RJ. Effects of fluoxetine on the 5-HT1A receptor and recovery of cognitive function after traumatic brain injury in rats. Am J Phys Med Rehabil. 2002;81:364–72. doi: 10.1097/00002060-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp Neurol. 2006;197:309–17. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Yan HQ, Kline AE, Ma X, Hooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. NeuroReport. 2001;12:2323–27. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- 103.Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. NeuroReport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- 104.Yan HQ, Yu J, Kline AE, Letart P, Jenkins LW, Marion DW, Dixon CE. Evaluation of combined fibroblast growth factor-2 and moderate hypothermia therapy in traumatically brain injured rats. Brain Res. 2000;887:134–43. doi: 10.1016/s0006-8993(00)03002-x. [DOI] [PubMed] [Google Scholar]