Abstract
Although the behavioral-stimulant and reinforcing effects of cocaine and related psychomotor stimulants have been attributed to their actions at the dopamine transporter (DAT), the reinforcing effectiveness of these compounds varies. The properties that confer these differences are important considerations when developing agonist pharmacotherapies for the treatment of stimulant abuse. The present studies focused on the time course of action and pharmacological specificity of six 3-phenyltropane analogs of cocaine (RTI-112, RTI-126, RTI-150, RTI-171, RTI-177, and RTI-336) by observing their behavioral-stimulant, neurochemical, and reinforcing effects in squirrel monkeys. The faster-onset analogs (RTI-126, RTI-150, and RTI-336), and one of the slower-onset DAT selective analogs (RTI-177 and RTI-171) produced behavioral-stimulant effects, while the slower-onset nonselective analog RTI-112 did not. The time to the peak behavioral-stimulant effect and the peak caudate dopamine levels was strongly correlated, but the area under the curve of the time course of behavioral-stimulant effect and dopamine levels was not correlated. These results suggest that the rate of onset plays a more important role than duration of action in the stimulant effect of these analogs. In addition, the slower-onset nonselective analog (RTI-112) clearly did not exhibit any reinforcing effects while the faster-onset nonselective (RTI-126) and all the DAT-selective analogs showed robust reinforcing effects (RTI-150, and RTI-177) or showed trends towards reinforcing effects (RTI-336 and RTI-171). Hence, there was a general trend for compounds that had a faster onset and/or DAT selectivity to produce significant behavioral-stimulant and reinforcing effects.
Keywords: cocaine, dopamine, dopamine transporter, in vivo microdialysis, nonhuman primate, psychomotor stimulant, self-administration
INTRODUCTION
Cocaine is a nonselective inhibitor of monoamine transporters, including dopamine, serotonin, and norepinephrine, and also binds to sodium channels (Kennedy and Hanbauer, 1983; Madras et al., 1989; Reith et al., 1986; Schoemaker et al., 1985). However, the behavioral effects of cocaine have been attributed primarily to its actions at the dopamine transporter (DAT) (Ritz et al., 1987). DAT inhibitors from distinct chemical structural classes produce behavioral-stimulant (Cline et al., 1992; Gatley et al., 1999; Kuhar, 1993) and reinforcing (Bergman et al., 1989; Ritz et al., 1987; Wilcox et al., 2000) effects that correlate with their potency and occupancy at DAT. However, in order to produce equivalent increases in locomotor activity in rodents, selective DAT inhibitors must exhibit greater occupancy of DAT than does the nonselective monoamine transporter inhibitor cocaine (Rothman et al., 1992; Vaugeois et al., 1993). Neuroimaging studies in humans have found a significant correlation between DAT occupancy and the subjective high following administration of cocaine (Volkow et al., 1997) or methylphenidate (Volkow et al., 1999). However, DAT inhibitors differ in their effectiveness as positive reinforcers. For example, the selective DAT inhibitor GBR12909 maintains self-administration in animals (Bergman et al., 1989; Howell and Byrd, 1991), but it seems to be a less robust reinforcer than cocaine in maintaining behavior under a progressive-ratio schedule (Stafford et al., 2001). In addition, several local anesthetics are effective DAT inhibitors, but are weaker reinforcers than cocaine in maintaining behavior under progressive-ratio or second-order schedules (Wilcox et al., 2005; Wilcox et al., 2000). These studies suggest that DAT inhibition alone does not fully account for the reinforcing properties of cocaine and that neurotransmitters other than dopamine may influence the reinforcing effects of cocaine and related stimulants. For example, increasing serotonin activity attenuates cocaine-induced increases in dopamine, cocaine-induced behavioral-stimulant effects, and cocaine self-administration in nonhuman primates (Czoty et al., 2002; Howell and Byrd, 1995; Lindsey et al., 2004). Determining the pharmacological properties that influence the reinforcing effectiveness of behavioral stimulants can provide insights for medications development for treating stimulant abuse.
The use of agonist pharmacotherapies in the treatment of substance abuse has been successful, as shown with methadone maintenance for heroin dependence and nicotine replacement for tobacco use. These agonists provide positive subjective effects and improve compliance (Gorelick, 1998). These results support efforts to develop similar pharmacotherapeutic strategies to treat cocaine dependence (Grabowski et al., 2004; Howell and Wilcox, 2001; Mello and Negus, 1996). Several preclinical studies with DAT inhibitors provide evidence that substitute agonists may be used to reduce cocaine use (Lindsey et al., 2004; Mello and Negus, 1996; Rothman and Glowa, 1995). However, a possible limitation to the use of selective DAT inhibitors as medications for cocaine abuse is their potential for abuse. Both the phenyltropane analog, RTI-113 (Howell et al., 2000), and the phenylpiperazine derivative, GBR12909 (Bergman et al., 1989; Howell and Byrd, 1991), reliably maintain i.v. self-administration in nonhuman primates. A viable approach to limit the reinforcing effectiveness of DAT inhibitors is to manipulate their time course of action. The rate of onset plays an important role in the reinforcing effectiveness of a variety of drugs in animals and in humans. In rodents, drugs that occupied DAT more rapidly produced more robust behavioral effects (Desai et al., 2005; Pogun et al., 1991; Stathis et al., 1995). The more rapidly cocaine (Volkow et al., 2000) or methylphenidate (Volkow et al., 2002) enter the brain, the greater the reported “high” in humans. Orally-administered methylphenidate did not elicit as great of a high as did intravenously administered methylphenidate or cocaine, presumably due to the slow rate of uptake of the orally-administered drug in brain (Volkow et al., 2002). Accordingly, a slow onset of action may reduce the abuse liability of the medications (Gorelick, 1998; Sellers et al., 1989). In addition, a long duration of action is necessary to provide reasonable dosing schedules (Carroll et al., 1999). However, duration of action has not been found to be a critical factor in the reinforcing effects of opioid drugs in rodents (Panlilio and Schindler, 2000) or nonhuman primates (Ko et al., 2002).
The purpose of the present study was to characterize the time-course of the effects of six 3-phenyltropane analogs of cocaine on behavior and dopamine neurochemistry in squirrel monkeys. The cocaine analogs examined in the present study are more potent than cocaine at binding to the dopamine transporter (Carroll et al., 2006; Kuhar et al., 1999), and produce robust increases in locomotor activity when administered systemically to mice (Carroll et al., 2004; Kimmel et al., 2001). In these locomotor studies, the nonselective monoamine transporter inhibitor RTI-126 and the DAT-selective inhibitors RTI-150 and RTI-336 both had a faster rate of onset (30 min) and a short duration of action (4h). In contrast, the nonselective monoamine transporter inhibitor RTI-112 had a slower rate of onset (30–60 min) and a longer duration of action (10h). The DAT-selective inhibitors RTI-171 and RTI-177 also had slower rates of onset (30–120 min), but RTI-171 had a short duration of action (2.5 h) while RTI-177 had a very long duration of action (20 h). The behavioral-stimulant and neurochemical effects of the cocaine analogs were compared directly to their effectiveness to maintain i.v. drug self-administration. The hypothesis was that compounds with a faster rate of onset would exhibit increased behavioral-stimulant, neurochemical, and reinforcing effects.
MATERIALS AND METHODS
Subjects
Twenty-two adult male squirrel monkeys (Samiri sciureus) weighing 700–1200 g served as subjects. Animals lived in individual home cages and had daily access to food (Harlan Teklad monkey chow; Harlan Teklad, Madision, WI; fresh fruit and vegetables) and unlimited access to water. All monkeys had prior exposure to cocaine and other drugs with selective dopaminergic or serotonergic activity in various behavioral studies. Animal use procedures were in strict accordance with the National Institutes of Health “Guide for Care and Use of Laboratory Animals” (Publication No. 85–23, revised 1985) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Apparatus
During daily test sessions, each animal was seated in a Plexiglas chair within a ventilated, sound-attenuating chamber (MED Associates, Georgia, VT). The chair was equipped with stimulus lights and a response lever. For the studies using a fixed-interval schedule, equipment for delivering a mild electrical stimulus to the tail was attached to the chair. In contrast, for the drug self-administration studies, a motor-driven syringe pump (Harvard Apparatus, Holliston, MA) located outside the chamber was used to deliver self-administered drug injections. During drug self-administration experiments, Teflon and polyvinyl chloride tubing passed through a small hole in the chamber and connected the catheter to a syringe situated within a computer-controlled pump located outside the chamber. Behavioral test sessions lasted approximately 70 min each day, five days per week. During microdialysis experiments in a separate group of animals, subjects were seated in the chair and fitted with an adjustable Lexan neckplate that was positioned perpendicular to the medial plane of the body just above the shoulder. All subjects had been acclimated to the chair over several months. At least two weeks elapsed between microdialysis experiments.
Catheter Implantation
Eighteen monkeys were prepared with chronically indwelling venous catheters under sterile surgical conditions. Animals were initially anesthetized with a cocktail consisting of Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 3.0 mg), ketamine HCl (20 mg), and atropine (0.1 mg). Anesthesia was maintained with supplements of ketamine HCl. A catheter made of polyvinyl chloride tubing (0.38 mm i.d.; 0.76 mm o.d.) was inserted via the femoral or external jugular vein and passed to a point near the right atrium. The proximal end of the catheter was passed subcutaneously to the interscapular region of the monkey’s back where it exited the skin. The animal wore a nylon mesh vest (Lomir Biomedical, Inc., Malone, NY) at all times to protect the externalized end of the catheter. Veterinary staff prescribed preoperative antibiotics [Monocid (cefonicid), Rocephin (ceftriaxone)] and postoperative analgesics [Banamine (flunixin meglumine)]. Catheters were flushed with saline solution several times each week and were filled with heparinized saline and sealed with a stainless steel obturator when not in use.
Guide Cannula Implantation
A stereotaxic apparatus was used to implant CMA/11 guide cannulae (CMA/Microdialysis, Acton, MA) bilaterally to target both caudate nuclei of four monkeys as described previously (Czoty et al., 2000). Anesthesia was initiated with Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 3.0 mg) and atropine. Inhaled isoflurane (1.0–2.0%) was administered to maintain depth of anesthesia during the procedure. A stainless steel stylet was placed in the guide cannula when not in use. Analgesics [Banamine (flunixin meglumine)] and antibiotics [Rocephin (ceftriaxone)] were prescribed as necessary by veterinary staff. Animals were closely monitored during recovery from anesthesia, and a minimum of two weeks was allowed before microdialysis experiments were performed. Within two months following each guide cannula implantation, accurate placement was verified once in each monkey through use of magnetic resonance imaging (MRI) as described previously (Czoty et al., 2000). The guide provided a very specific path for the insertion of the probe such that the correct placement of the guide ensured correct placement of the probe.
Stimulus Termination Procedures
Six animals were trained under a fixed-interval (FI) 300-s schedule of stimulus termination. At the beginning of the testing session the behavioral chamber was illuminated with a red light for 300 s. When this interval had elapsed, the monkey had 3s to press the lever one time to terminate the red light, which was associated with an impending electrical stimulus. Upon termination of the red light, a white light was illuminated for 15 s, followed by a 60-s timeout. If the lever was not pressed during the 3-s period, the animal received a 3-mA stimulus for approximately 200 ms to the tail, followed by a 60-s timeout. Responding during timeout periods had no scheduled consequences. Animals were tested each day, five days per week, and each daily session consisted of thirteen FI components. When rates and patterns of responding had stabilized, venous catheters were implanted as described above. At least one week elapsed between catheter implantation and the initiation of procedures to establish baseline responses to intravenous saline and cocaine administration.
Drug experiments began when rates and patterns of responding to saline and cocaine stabilized (less than 10% variability in response rate for five consecutive test days). A single dose of drug or saline was administered through the intravenous catheter 5 s before the beginning of the experimental session. The exception to this dosing regimen was RTI-112, which was administered 30 min before the beginning of the experimental session. Pilot data suggested that the peak stimulant effect of RTI-112 occurred >30 min after administration. Animals were tested, but did not receive drug on Mondays and Wednesdays. They received a single dose of test drug or cocaine on Tuesdays and Fridays, and saline was administered prior to the session on Thursdays, as a control for the injection procedure. Animals received each dose of each drug in ascending order, then a second determination was performed with the doses administered in descending order. The reported data reflect an average of the two determinations. The baseline response for cocaine was reassessed between each set of dose-response determinations. The animals used in this study had a response rate of 0.54 ± 0.03 (group mean ± SEM) presses per second when given saline.
Self-Administration Procedures
Twelve animals were initially trained under a second-order schedule of stimulus termination. At the beginning of the testing session, the behavioral chamber was illuminated with a red light for 600 s. Completion of 20 responses (fixed ratio (FR) 20) produced a 2-s flash of white light FI 600-s (FR20:S). When the 600-s interval had elapsed, the monkey had 10s to complete an FR20 to terminate the red light, which was associated with an impending electrical stimulus. Upon termination of the red light, a white light was illuminated for 15 s, followed by a 60-s timeout. If an FR20 was not completed in the 10-s period, the animal received a 3-mA stimulus for approximately 200 ms to the tail, followed by a 60-s timeout. Responding during timeout periods had no scheduled consequences. Animals were tested daily, five days each week, and each daily session consisted of five FI 600-s (FR20:S) components. When rates and patterns of responding had stabilized, venous catheters were implanted as described above. Subsequently, the method of reinforcement was changed such that the termination of the red light resulted in the injection of 0.1 mg of cocaine. All other events and schedule parameters were identical to those of the second-order schedule of stimulus termination. Animals used in these studies had an average response rate of 1.00 ± 0.13 (group mean ± SEM) presses per second when self-administering 0.1 mg/inf cocaine.
Drug experiments began when rates and patterns of responding had stabilized (less than 10% variability in response rate for five consecutive test days). Patterns of responding were not used as stability criteria. Animals were allowed to self-administer the training dose for more than ten consecutive sessions. Subsequently, a range of doses of cocaine (0.03–1.0 mg/injection) was substituted for the training dose to establish a full dose-effect curve for cocaine self-administration. Doses of cocaine were evaluated in a counter-balanced order under the second-order schedule for a minimum of ten consecutive test days, and mean response rates at each dose were calculated by averaging daily mean response rates over the last five sessions. Periodically, extinction sessions with saline substitution were conducted on consecutive days until responding decreased to less than 20% of response rate obtained with the maintenance dose of cocaine (0.1 mg/injection) to ensure rapid extinction of responding in the absence of cocaine. Various doses of the phenyltropane analogs were evaluated in a counterbalanced order under the second-order schedule for a maximum of ten consecutive test days. If the response rate fell to or below 30% of responding for the training dose of cocaine, testing of the phenyltropane analog was discontinued, and cocaine-maintained responding was re-established. A stable response rate for cocaine was re-established for a minimum of five consecutive test days in between the substitution of each dose of a phenyltropane analog.
Microdialysis procedures
CMA/11 dialysis probes with a shaft length of 14 mm and active dialysis membrane measuring 4 mm long and 0.24 mm diameter were flushed with artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM Ca Cl2, 1.0 mM MgCl2 and 0.15 mM ascorbic acid, final pH=7.4–7.56) for at least 20 min. Probes were inserted into the guide cannulae and connected to a Harvard PicoPlus microinfusion pump via FEP Teflon tubing. Probes were perfused with artificial cerebrospinal fluid at 2.0 μl/min for the duration of the experiment. Samples were collected every 10 min in microcentrifuge tubes and immediately refrigerated. Following a 60-min equilibration, three consecutive 10-min samples were collected for determination of baseline dopamine concentration. Following collection of baseline samples, saline or a dose of a test drug was administered i.m. and 10-min samples were collected for an additional 120 min. Animals were tested a maximum of one time per week, and each site was accessed no more often than once every two weeks. This regimen of repeated access of the caudate has produced consistent responses to drug treatment without significant gliosis (Czoty et al., 2000).
High-performance liquid chromatography (HPLC) and electrochemical detection were used to quantify levels of dopamine. The HPLC system consisted of a small bore (3.2 mm X 150 mm, 3 micron) column (ESA, Inc., Chelmsford, MA) with a commercially available mobile phase (MD-TM, ESA, Inc.) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. After loading onto the refrigerated sample tray, samples (20 μl) were automatically mixed with 3 μl of ascorbate oxidase, and 18 μl of the mixture was injected into the HPLC system by an ESA Model 542 autosampler. Samples were analyzed within 12 hours of collection, remaining either in a refrigerator or in the refrigerated autosampler tray during this time. Electrochemical analyses were performed using an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential = 350 mV) and an ESA Coulochem II detector. The potential of channel 1 was set to −150 mV for oxidation, while the potential of channel 2 was set to 275 mV for reduction. A full range of dopamine standards (1 −50 nM) was analyzed both before and after each set of samples to evaluate possible degradation of dopamine. Levels of dopamine below 1 nM were considered below the limit of detection. A desktop computer collected data and chromatograms were generated by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA). The chromatograms were analyzed using the EZChrom software, comparing the experimental samples with the standards. The neurochemical effects of the drugs were compared with the neurochemical effects of saline and cocaine. Basal levels of dopamine were between 3–5 nM, unadjusted for probe recovery, as reported in earlier studies (Czoty et al., 2000). Before and after each in vivo experiment, probes were tested in vitro to determine suitability of the probes. Percent recovery was similar for all probes (10–20%).
Drugs
The full chemical name and molecular weight of each of the tropane analogs is given in Table 1. Based on published studies that determined the binding potency of each of the tropane analogs at the three monoamine transporters (DAT, NET, and SERT) in rat tissue (Kuhar et al., 1999), the compounds were classified as being DAT selective or nonselective (Table 2). RTI-126, RTI-112, RTI-150, RTI-171 (Research Triangle Institute, Research Triangle, NC) and cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) were dissolved in 0.9% saline. RTI-336 and RTI-177 were dissolved in 2% final volume 0.1 N HCl and 98% final volume water. Drug doses were determined as salts. For the stimulus termination and self-administration procedures, drug injections were administered through the intravenous catheter. For the microdialysis studies, drug injections were administered into the thigh muscle in a volume of 0.4 to 0.8 ml. Animals used in the microdialysis studies no longer had any viable veins for intravenous catheters, which necessitated the use of i.m. injections.
Table 1.
Tropane analogs of cocaine
| RTI-126 | MW = 324.35 | 3β-phenyl-2β-(1,2,4-oxadiazol-5-methyl)tropane hydrochloride (Carroll et al., 1993) |
| RTI-112 | MW = 344.27 | 3β-(3-methyl-4-chlorophenyl)-2β-carboxylic acid methyl ester hydrochloride (Carroll et al., 1992) |
| RTI-150 | MW = 358.89 | 3β-(4-methylphenyl)tropan-2β-carboxylic acid cyclobutyl ester hydrochloride (Carroll et al., 1995) |
| RTI-171 | MW = 332.86 | 3β-(4-methylphenyl)tropan-2β-(3-methylisoxazol-5-yl) hydrochloride (Kotian et al., 1996) |
| RTI-336 | MW = 429.38 | 3β-(4-chlorophenyl)tropane-2β-[3-(4-methylphenyl)isoxazol-5-yl] hydrochloride (Carroll et al., 2004) |
| RTI-177 | MW = 419.85 | 3β-(4-chlorophenyl)tropane-2β-(3-phenylisoxazol-5-yl) hydrochloride (Kotian et al., 1995) |
Full chemical name and molecular weight of each tropane analog used in these studies and references detailing the synthesis of each compound.
Table 2.
Characterization of tropane analogs
| Compound | DAT/NET ratio | DAT/SERT ratio | Selectivity |
|---|---|---|---|
| Cocaine | 0.0270 | 0.0856 | Nonselective |
| RTI-126 | 0.0127 | 0.0261 | Nonselective |
| RTI-112 | 0.0222 | 0.0762 | Nonselective |
| RTI-150 | 0.0008 | 0.0018 | DAT selective |
| RTI-336 | 0.0024 | 0.0007 | DAT selective |
| RTI-171 | 0.0035 | 0.0002 | DAT selective |
| RTI-177 | 0.0026 | 0.0005 | DAT selective |
Relative selectivity of binding to monoamine transporter ligands in rat tissue (Kuhar et al., 1999).
Data analysis and statistics
The overall rate data from the stimulus termination studies (Figure 1) and from the self-administration studies (Figure 5) were each analyzed using repeated-measures ANOVAs. When a significant main effect was observed, Tukey’s post-hoc multiple comparisons tests were used to determine statistical significance, defined at the 95% level of confidence (p<0.05). Time-course data from the stimulus termination studies (Figure 2) and the neurochemical studies (Figure 3) were each analyzed using a paired t-test, comparing the saline and drug data. For these analyses, the neurochemical data in Figure 3 was truncated at 80 min post-injection to better correspond to the behavioral-stimulant time-course data, with a final data point 78 min post-injection.
Figure 1.
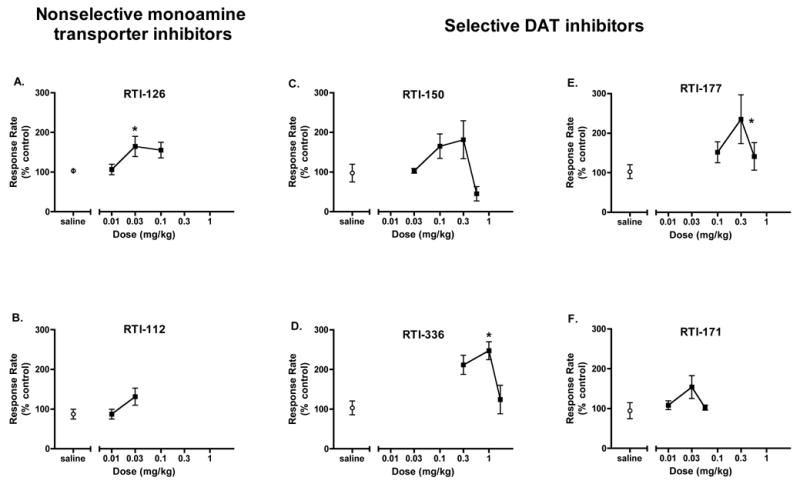
Dose-response curve of stimulant effects of nonselective monoamine transporter inhibitors (A, B) and selective DAT inhibitors (C–F) on responding maintained by an FI schedule in squirrel monkeys (n=3). Data points represent mean ± SEM rate of responding averaged across the 13-component session. Asterisks (*) represent individual points significantly different from saline control, based on Tukey’s post-hoc multiple comparisons tests.
Figure 5.
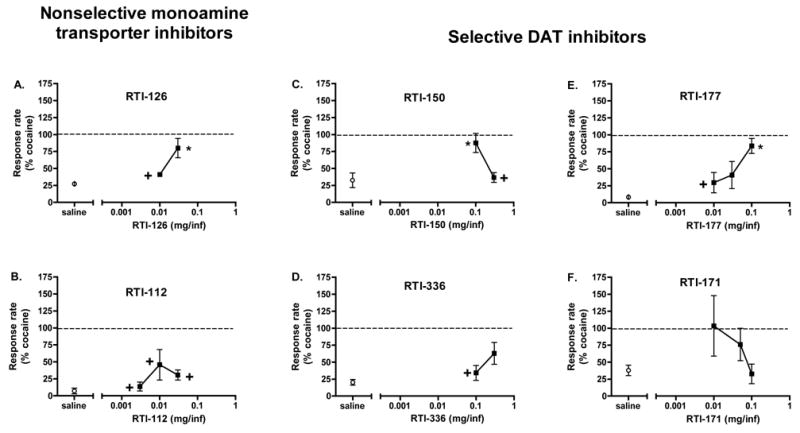
Effects of nonselective monoamine transporter inhibitors (A, B) and selective DAT inhibitors (C–F) on responding maintained by a second-order schedule of cocaine self-administration in squirrel monkeys (n=3). Data points represent mean ± SEM rate of responding. The dashed line represents responding maintained by 0.1 mg/inf of cocaine. Asterisks (*) represent individual points significantly different from saline control and crosses (+) represent individual points significantly different from cocaine, based on Tukey’s post-hoc multiple comparisons tests.
Figure 2.
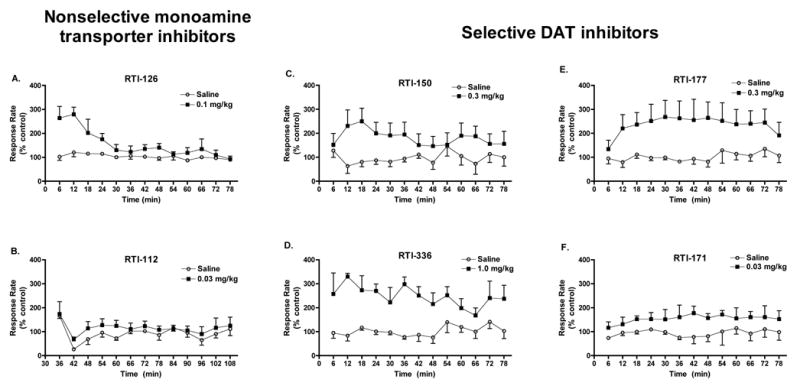
Time course of stimulant effects of nonselective monoamine transporter inhibitors (A, B) and selective DAT inhibitors (C–F) on responding maintained by an FI schedule in squirrel monkeys (n=3). Data points represent mean ± SEM rate of responding in each of 13 consecutive 5-min intervals. A 1-min timeout separated each 5-min FI component.
Figure 3.
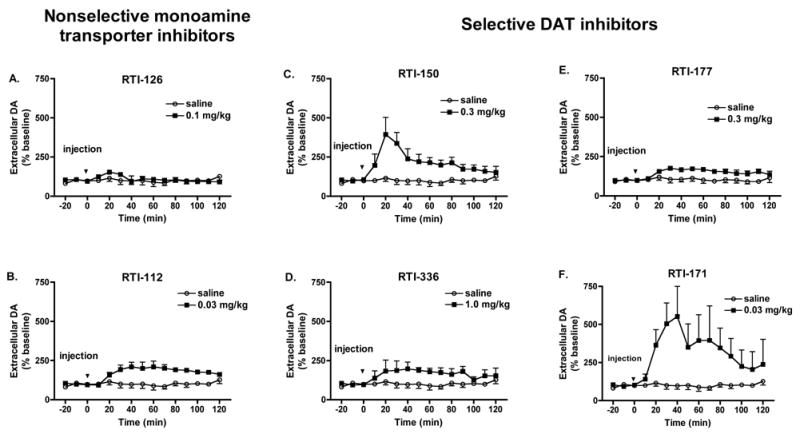
Effects of nonselective monoamine transporter inhibitors (A, B) and selective DAT inhibitors (C–F) on extracellular dopamine in the caudate nucleus of unanesthetized squirrel monkeys as determined with in vivo microdialysis (n=3). Drugs were administered i.m. at time point zero. Data points represent mean ± SEM dopamine levels as a percent of values obtained prior to drug administration.
The correlational analysis shown in Figure 4A compared the times to peak stimulus termination and neurochemical effect. The time to peak effect was deteremined for both the behavioral and neurochemical data in the same manner. When there was an obvious maximal effect, this data point was determined to be the peak. However, when consecutive data points increased in value until a plateau was observed, the peak point was determined to be the first data point on this plateau from which subsequent data points were not greater by at least 10% of that first plateau point. The correlational analysis in Figure 4B compared the area under the curve (AUC) for the stimulus termination and neurochemical data. The AUCs of the stimulus termination time-course data (Figure 2) and the microdialysis data (Figure 3) were determined using 100% of control as the baseline value and included peaks below the baseline. To facilitate AUC comparisons between these two data sets, the neurochemical data in Figure 3 was truncated at 80 min post-injection to better correspond to the behavioral-stimulant time-course data, with a final data point 78 min post-injection. Since RTI-112 did not produce significant behavioral-stimulant effects, these data were omitted from both correlational analyses.
Figure 4.
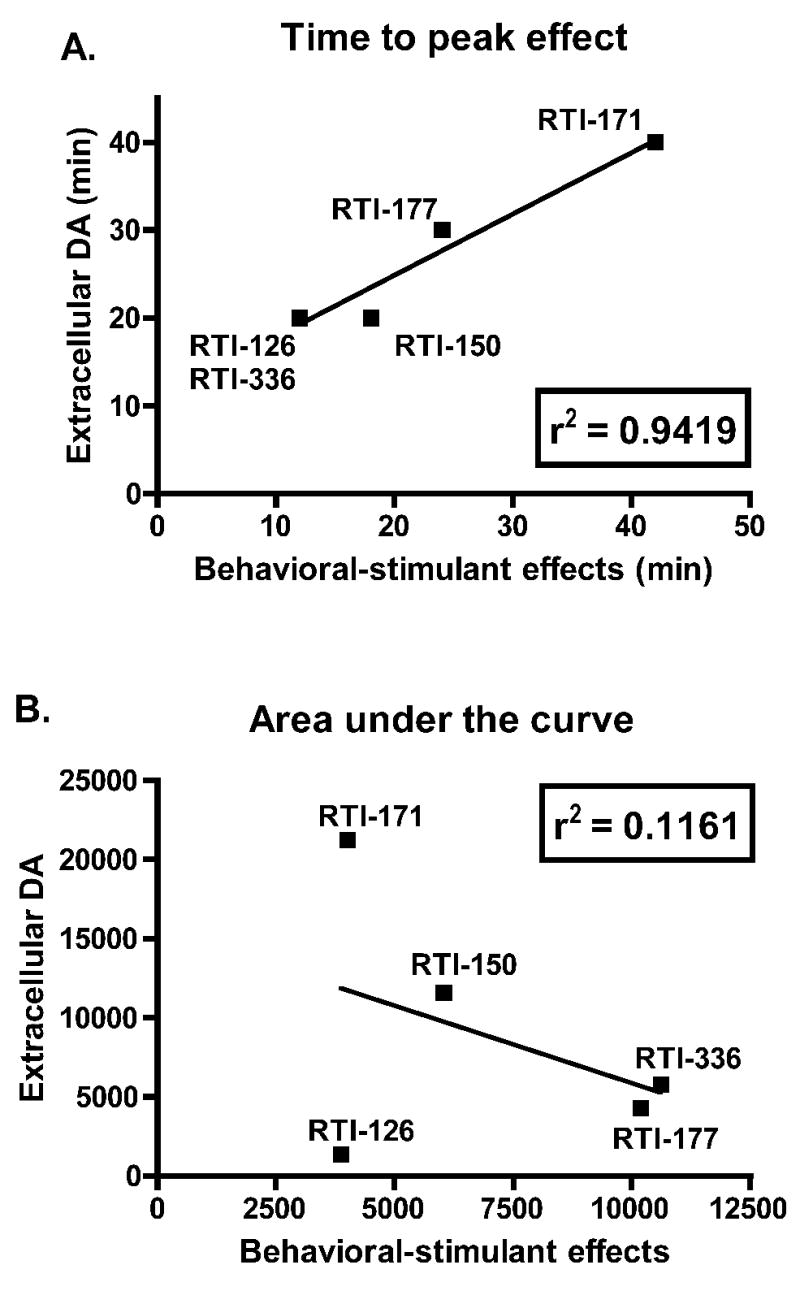
Correlations between the time to peak (A) and the area under the curve (B) of behavioral-stimulant effects and dopamine levels of each compound.
RESULTS
For each compound, a dose-response curve of the average response rate during the 13-component fixed-interval behavioral session was determined (Figure 1). With the exception of RTI-112, all of the compounds produced an inverted U-shaped dose-response curve that is typical of psychomotor stimulants. Doses of RTI-112 greater than 0.03 mg/kg produced adverse effects in the animals, including stereotypic behaviors and seizures, so higher doses were not tested. One-way repeated measures ANOVAs determined that, across the doses tested, RTI-126 [F(3,6)=6.965, p=0.022], RTI-150 [F(4,8)=8.632, p=0.005], RTI-336 [F(3,6)=6.214, p=0.029], and RTI-177 [F(3,6)=5.080, p=0.044] had a significant main effect, but that RTI-171 [F(3,6)=3.180, p=0.106] and RTI-112 [F(2,4)=5.513, p=0.071] did not. Individual points significantly different from saline control as determined by post-hoc tests are indicated by asterisks in Figure 1. Although RTI-150 had a main effect, post-hoc tests did not reveal a significant difference between any individual dose and saline. In summary, the three faster-onset analogs and one of the DAT-selective slower-onset analogs produced significant and dose-dependent behavioral-stimulant effects, while the other slower-onset analogs did not.
Figure 2 shows the time course of drug effects on responding maintained by an FI schedule for a single dose of each drug. The most effective or highest dose as determined by the mean data in Figure 1 was selected for this time course analysis. Paired t-test analyses revealed significant differences between drug and saline for RTI-126 [t(12)=−3.571, p=0.004], RTI-150 [t(12)=−7.096, p<0.001), RTI-336 [t(12)=−13.269, p<0.001], RTI-177 [t(12)=−11.915, p<0.001], and RTI-171 [t(12)=−11.218, p<0.001], but not for RTI-112 [t(12)=−1.284, NS]. In the RTI-112 studies, animals received saline or drug 30 min prior to the start of the session, rather than 5 sec as in the other panels. The first two time points of the RTI-112 data are highly variable likely due to the fact that the animals were seated in the test chair during the 30-min pretreatment period, without any stimulus lights. Compared to the other three analogs, RTI-126, RTI-150, and RTI-336 exhibit a faster rate of onset of behavioral-stimulant effects, peaking at 12, 18, and 12 min post-injection, respectively. In contrast, the behavioral effects of RTI-177 and RTI-171 reach a peak at 24 and 42 min post-injection, respectively. RTI-336, RTI-177, and RTI-171 all exhibit longer durations of action than do RTI-126 and RTI-150.
Figure 3 shows the time course of drug effects on extracellular dopamine in the caudate as measured by in vivo microdialysis for a single dose of each drug. The doses shown in the behavioral time course analyses in Figure 2 were selected for this analysis. Paired t-test analyses revealed significant differences between drug and saline for RTI-126 [t(10)=−3.042, p=0.012], RTI-112 [t(10)=−3.874, p=0.003], RTI-150 [t(10)=4.221, p=0.002), RTI-336 [t(10)=−4.723, p<0.001], RTI-177 [t(10)=−4.232, p=0.002], and RTI-171 [t(10)=−4.104, p=0.002]. As in the behavioral-stimulant studies, RTI-126, RTI-150, and RTI-336 had a faster onset, producing peak DA levels 20 min after drug administration. Peak DA levels were evident 30 min after RTI-112 and RTI-177 administration and 40 min after RTI-171 administration. These increases in DA levels were longer lasting after administration of RTI-112, RTI-336, RTI-177, and RTI-171 relative to RTI-126 and RTI-150.
The time to peak effect of behavioral-stimulant effects and dopamine levels were determined for each drug using the time-course data shown in Figures 2 and 3 and correlated in Figure 4A. In the case of a plateau of elevated behavior or dopamine levels, the first time point of this plateau was used as the peak. A linear regression analysis of these data results in an r2 value of 0.9419, and a p value of 0.0061. The area under the curve (AUC) of the behavioral-stimulant effects and DA levels are correlated in Figure 4B. A linear regression of these data results in an r2 value of 0.1661, and a p value of 0.4958.
The effects of drug substitution on responding maintained on a second-order schedule of cocaine self-administration are shown in Figure 5. The data in these figures are represented as a percent of the baseline response rate determined while self-administering the training dose of cocaine (0.1 mg/inf). Although the response rate engendered by this dose of cocaine varied from animal to animal, this dose produced peak responding on the cocaine dose-response curve. One-way repeated measures ANOVAs were performed on each data set to determine a main effect of the drug as compared to saline extinction data. This analysis showed that the following drugs had a main effect: RTI-126 [F(2,4)=11.275, p=0.023], RTI-150 [F(2,4)=20.858, p=0.008] and RTI-177 [F(3,6)=6.403, p=0.027] while RTI-112 [F(3,6)=2.956, p=0.120], RTI-336 [F(2,4)=1.758, p=0.283], RTI-171 [F(3,6)=2.190, p=0.190] did not. Doses greater than those shown for RTI-126, RTI-336, and RTI-177 produced adverse effects, such as stereotypies and seizures in these animals, so higher doses were not tested.
DISCUSSION
The present study in nonhuman primates compared the behavioral-stimulant, in vivo neurochemical, and reinforcing effects of several phenyltropane analogs of cocaine with varying time course of action and selectivity of binding to monoamine transporters. The time to the peak of drug-induced increases in extracellular dopamine indicated that RTI-126, RTI-150, and RTI-336 each had a faster rate of onset while RTI-112, RTI-177, and RTI-171 each had slower rates of onset. In this study, the rate of onset of dopaminergic effects after i.m. administration was predictive of the behavioral-stimulant effects of the cocaine analogs after i.v. administration in that the three faster-onset analogs (RTI-126, RTI-150, and RTI-336) produced significant behavioral-stimulant effects while two of the slower-onset analogs (RTI-112 and RTI-171) did not. The slower onset DAT-selective analog (RTI-177) produced significant behavioral-stimulant effects. In addition, the faster-onset analogs (RTI-126 and RTI-150) and the slower-onset DAT-selective analog (RTI-177) had robust reinforcing effects, whereas the slow-onset nonselective analog (RTI-112) did not maintain responding at rates comparable to that of cocaine. Although the reinforcing effects of the DAT-selective inhibitors RTI-336 and RTI-171 did not reach significance, there was a trend toward reliable self-administration. In summary, compounds that had a faster onset of action produced significant behavioral-stimulant effects, regardless of monoamine transporter selectivity. Compounds that had a faster onset of action or greater selectivity for DAT tended to function as more robust reinforcers.
Previous studies have characterized the behavioral and neurochemical effects of cocaine in squirrel monkeys under similar conditions as in the present studies. In subjects trained to lever press under a FI schedule identical to that employed in the present study, cocaine produced an inverted U-shaped dose-response curve typical of psychomotor stimulants (Howell and Byrd, 1991; Howell and Byrd, 1995). In separate studies, a dose of 1.0 mg/kg cocaine increased extracellular dopamine in the caudate nucleus to about 300–350% of baseline within the first 10–20 min after i.m. administration (Czoty et al., 2002; Czoty et al., 2000). In these studies, the rate of onset of the dopamine increases corresponded well with the rate of onset of behavioral-stimulant effects following cocaine administration reported previously (Howell et al., 2000), despite the fact that drugs were administered intravenously in the behavioral studies and intramuscularly in the microdialysis studies. In subjects trained to self-administer cocaine under a second-order schedule similar to the one used in the present studies, cocaine produced an inverted U-shaped dose-response curve (Czoty et al., 2002; Howell and Byrd, 1991), clearly indicating that cocaine functions as a robust reinforcer under the conditions employed in the present study. In summary, cocaine has a rapid rate of onset in producing stimulant effects and in increasing extracellular dopamine in squirrel monkeys, which is reflected by its robust reinforcing effects.
The rate of drug entry into the brain plays an important role in the reinforcing effectiveness of drugs in humans and nonhuman primates (Ko et al., 2002; Marsch et al., 2001). In humans, the rapid uptake of cocaine and methylphenidate into the brain more closely paralleled the perception of the “high”, rather than the duration of action of the drug itself (Volkow et al., 2002). In addition, routes of administration (i.e. smoking, intravenous) that result in a more rapid entry of the drug in brain produce greater “highs” than routes of administration (i.e. oral) resulting in slower drug uptake (Volkow et al., 2000). When cocaine was infused more slowly, it was found to be less effective as a reinforcer in nonhuman primates (Balster and Schuster, 1973; Panlilio et al., 1998). Similarly, the cocaine analog HD-23, which takes 60 min to attain significant binding was not as reinforcing in rhesus monkeys as were cocaine or methylphenidate (Lile et al., 2003). The piperazine analog (+)-CPCA binds to DAT more slowly than cocaine and was found to be a less robust reinforcer than cocaine in nonhuman primates (Woolverton et al., 2002). Woolverton et al. (2002) have suggested that reinforcing strength may be related to the rate of DAT occupancy over a time frame within 3 min after injection. The rate of drug onset also plays a role in determining its behavioral-stimulant effects, as evidenced by the strong correlation between the time to peak dopamine levels and peak behavioral-stimulant effects in the present study. RTI-171 was selective for DAT in rodents (Kuhar et al., 1999) and increased dopamine in the squirrel monkey caudate in the present studies, but it was not a very effective behavioral stimulant. One important characteristic of RTI-171 is that it had a slower rate of onset than the other DAT-selective tropane analogs in this study.
In addition to time-course of effects, the pharmacological specificity of the compounds may influence their stimulant and reinforcing effects. Although RTI-177 had a slower rate of onset than did RTI-126 and RTI-150, it still functioned as a reinforcer in the second-order self-administration protocol. A recent review suggests that a rapid onset of action is associated with the reinforcing effects of a drug but is not required for a drug to act as a reinforcer (Lile, 2006). Similarly, several other phenylpiperazine and phenyltropane DAT inhibitors function as positive reinforcers in nonhuman primates despite a slow onset of DAT binding (Bergman et al., 1989; Lile et al., 2003; Spealman and Kelleher, 1981). Indeed, decreasing the rate of cocaine infusion in rhesus monkeys decreased the reinforcing strength of the drug, but only to a certain point, suggesting a “floor” effect in the absence of alternate reinforcers (Woolverton and Wang, 2004). In the present studies, the nonselective monoamine transporter inhibitor RTI-112 did not have behavioral-stimulant effects, although the doses used in this study clearly increased dopamine levels in the caudate to approximately 200% of baseline. Published studies show that high doses of RTI-112 are associated with high DAT occupancy in rhesus monkeys (Lindsey et al., 2004), so the lack of behavioral-stimulant effects cannot be attributed to a lack of an effect at DAT. That RTI-112 did not maintain self-administration behavior when substituted for cocaine in the present study also corresponds well with earlier studies in rhesus monkeys showing that RTI-112 did not function as a reinforcer, ostensibly due to its serotonergic effects (Lindsey et al., 2004). Consistent with the latter interpretation, previous studies showed that increasing serotonergic activity reduced cocaine self-administration in rats (Richardson and Roberts, 1991) and in monkeys (Czoty et al., 2002; Kleven and Woolverton, 1993) as well as cocaine-induced increases in extracellular dopamine in nonhuman primates (Czoty et al., 2002). There is a growing literature that supports a negative modulatory role of serotonin on the behavioral-stimulant and reinforcing effects of DAT inhibitors.
In summary, the present data suggest that the rate of onset of drug effect and relative selectivity for monoamine transporters are important determinants of the behavioral-stimulant and reinforcing properties of the drug. Drugs with a faster rate of onset are more likely to exhibit behavioral-stimulant and reinforcing effects. Similarly, selectivity at DAT confers more robust behavioral-stimulant and reinforcing effects to these drugs. These behavioral effects of DAT inhibitors may be attenuated by actions at SERT. These are important considerations when developing substitute agonist medications to treat stimulant addiction.
Acknowledgments
This research was supported by U.S. Public Health Service grants DA00517 (LLH), DA12514 (LLH), DA15092 (HLK), DA13326 (FIC), and RR00165 (Division of Research Resources, National Institutes of Health). The authors would like to thank Paula D. Martin, Aeneas C. Murnane, and Janet M. Ojeda for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: Effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–29. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. Iii. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–5. [PubMed] [Google Scholar]
- Carroll FI, Gray JL, Abraham P, Kuzemko MA, Lewin AH, Boja JW, et al. 3-aryl-2-(3′-substituted-1′,2′,4′-oxadiazol-5′-yl)tropane analogues of cocaine: Affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters. J Med Chem. 1993;36:2886–90. doi: 10.1021/jm00072a007. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. Development of the dopamine transporter selective rti-336 as a pharmacotherapy for cocaine abuse. Aaps J. 2006;8:E196–203. doi: 10.1208/aapsj080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: Preclinical aspects. J Med Chem. 1999;42:2721–36. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Kotian P, Dehghani A, Gray JL, Kuzemko MA, Parham KA, et al. Cocaine and 3β-(4′-substituted phenyl)tropane-2β-carboxylic acid ester and amide analogues. New high afinity and selective compounds for the dopamine transporter. J Med Chem. 1995;38:379–88. doi: 10.1021/jm00002a020. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Kuzemko MA, Gao Y, Abraham P, Lewin AH, Boja JW, et al. Synthesis and ligand binding of 3β-(3-substituted phenyl)-and 3β-(3,4-disubstituted phenyl)tropane-2β-carboxylic acid methyl esters. Med Chem Res. 1992;1:382–7. [Google Scholar]
- Carroll FI, Pawlush N, Kuhar MJ, Pollard GT, Howard JL. Synthesis, monoamine transporter binding properties, and behavioral pharmacology of a series of 3beta-(substituted phenyl)-2beta-(3′-substituted isoxazol-5-yl)tropanes. J Med Chem. 2004;47:296–302. doi: 10.1021/jm030453p. [DOI] [PubMed] [Google Scholar]
- Cline EJ, Scheffel U, Boja JW, Carroll FI, Katz JL, Kuhar MJ. Behavioral effects of novel cocaine analogs: A comparison with in vivo receptor binding potency. J Pharmacol Exp Ther. 1992;260:1174–9. [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–7. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, gbr 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther. 2005;315:397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, et al. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl) 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Adv Pharmacol. 1998;42:995–7. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–64. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Characterization of the effects of cocaine and gbr 12909, a dopamine uptake inhibitor, on behavior in the squirrel monkey. J Pharmacol Exp Ther. 1991;258:178–85. [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–9. [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carroll FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor rti-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292:521–9. [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: Drug self- administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- Kennedy LT, Hanbauer I. Sodium-sensitive cocaine binding to rat striatal membrane: Possible relationship to dopamine uptake sites. J Neurochem. 1983;41:172–8. doi: 10.1111/j.1471-4159.1983.tb13666.x. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Ivy Carroll F, Kuhar MJ. Locomotor stimulant effects of novel phenyltropanes in the mouse. Drug Alcohol Depend. 2001;65:25–36. doi: 10.1016/s0376-8716(01)00144-2. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend. 1993;31:149–58. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Kotian P, Abraham P, Lewin AH, Mascarella SW, Boja JW, Kuhar MJ, et al. Synthesis and ligand binding study of 3β-(4′-substituted phenyl)-2β-(heterocyclic)tropanes. J Med Chem. 1995;38:3451–3. doi: 10.1021/jm00018a004. [DOI] [PubMed] [Google Scholar]
- Kotian P, Mascarella SW, Abraham P, Lewin AH, Boja JW, Kuhar MJ, et al. Synthesis, ligand binding, and quantitative structure-activity relationship study of 3β-(4′-substituted phenyl)-2β-heterocyclic tropanes: Evidence for an electrostatic interaction at the 2β position. J Med Chem. 1996;39:2753–63. doi: 10.1021/jm960160e. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ. Neurotransmitter transporters as drug targets: Recent research with a focus on the dopamine transporter. The Pharmacologist. 1993;35:28–33. [Google Scholar]
- Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Lile JA. Pharmacological determinants of the reinforcing effects of psychostimulants: Relation to agonist substitution treatment. Exp Clin Psychopharmacol. 2006;14:20–33. doi: 10.1037/1064-1297.14.1.20. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HM, et al. The reinforcing efficacy of psychostimulants in rhesus monkeys: The role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–66. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: Relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–69. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I [3h]cocaine binding sites in caudate-putamen. J Pharmacol Exp Ther. 1989;251:131–41. [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–65. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–8. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 2000;150:61–6. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- Pogun S, Scheffel U, Kuhar MJ. Cocaine displaces [3h]win35,428 binding to dopamine uptake sites in vivo more rapidly than mazindol or gbr12909. Eur J Pharmacol. 1991;198:203–5. doi: 10.1016/0014-2999(91)90622-w. [DOI] [PubMed] [Google Scholar]
- Reith MEA, Meisler BE, Sershen H, Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986;35:1123–9. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–40. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on gbr12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Grieg N, Kim A, DeCosta BR, Rice KC, Carroll FI, et al. Cocaine and gbr12909 produce equivalent motoric responses at different occupancy of the dopamine transporter. Pharmacol Biochem Behav. 1992;43:1135–42. doi: 10.1016/0091-3057(92)90493-y. [DOI] [PubMed] [Google Scholar]
- Schoemaker H, Pimoule C, Arbilla S, Scatton B, Javoy-Agid F, Langer SZ. Sodium dependent [3h]cocaine binding associated with dopamine uptake sites in the rat striatum and human putamen decrease after dopaminergic denervation and in parkinsons disease. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:227–35. doi: 10.1007/BF00501873. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Busto U, Kaplan HL. Pharmacokinetic and pharmacodynamic drug interactions: Implications for abuse liability testing. NIDA Res Monogr. 1989;92:287–306. [PubMed] [Google Scholar]
- Spealman RD, Kelleher RT. Self-administration of cocaine derivatives by squirrel monkeys. J Pharmacol Exp Ther. 1981;216:532–6. [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Rice KC, Glowa JR. A comparison of cocaine, gbr 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug Alcohol Depend. 2001;62:41–7. doi: 10.1016/s0376-8716(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Stathis M, Scheffel U, Lever SZ, Boja JW, Carroll FI, Kuhar MJ. Rate of binding of various inhibitors at the dopamine transporter in vivo. Psychopharmacology (Berl) 1995;119:376–84. doi: 10.1007/BF02245852. [DOI] [PubMed] [Google Scholar]
- Vaugeois JM, Bonnet JJ, Duterte-Boucher D, Costentin J. In vivo occupancy of the striatal dopamine uptake complex by various inhibitors does not predict their effects on locomotion. Eur J Pharmacol. 1993;230:195–201. doi: 10.1016/0014-2999(93)90802-o. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: Results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–66. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–30. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “High”. J Pharmacol Exp Ther. 1999;288:14–20. [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–8. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: Practical and theoretical concerns. Psychopharmacology (Berl) 2000;153:139–47. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ranaldi R, Wang Z, Ordway GA, Paul IA, Petukhov P, et al. Reinforcing strength of a novel dopamine transporter ligand: Pharmacodynamic and pharmacokinetic mechanisms. J Pharmacol Exp Ther. 2002;303:211–7. doi: 10.1124/jpet.102.037812. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–7. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]


