Abstract
Benign prostatic hyperplasia (BPH) is an extremely common disease of older men in which there is benign overgrowth of the prostatic transition zone, leading to obstruction of urine outflow. Fibroblast growth factor (FGF) 2, a potent growth factor for prostatic stromal and epithelial cells, is increased twofold in BPH and its concentration is correlated with stromal proliferation in this condition. Immunohistochemistry of normal and hyperplastic prostate revealed that FGF2-expressing stromal cells were present in higher numbers near the epithelial acini, implying that epithelial cells may express a factor that induces FGF2 expression by stromal cells. Conditioned medium from primary cultures of prostatic epithelial cells was capable of inducing increased expression of FGF2 by primary stromal cultures. Blocking experiments with neutralizing anti-interleukin (IL)-8 antibodies and pretreatment with lipopolysaccharide, which down-regulates the IL-8 receptor, show that this inducing activity is because of the presence of IL-8 in the epithelial-conditioned medium. Analysis of normal prostatic peripheral zone and BPH tissue by enzyme-linked immunosorbent assay reveals that IL-8 is present at increased levels in hyperplastic prostate. Therefore IL-8 produced by prostatic epithelial cells can induce FGF2, a potent stromal and epithelial growth factor, and in this manner promote the abnormal proliferation of the prostatic transition zone that is critical in the pathogenesis of BPH.
Benign prostatic hyperplasia (BPH) is an extremely common condition of older men, such that by 70 years of age the majority of men exhibit clinical symptoms of this disease. 1 The benign growth of the prostatic transition zone that is characteristic of BPH leads to obstruction of urine outflow and in this manner causes considerable morbidity. Thus BPH is of considerable medical importance and yet its pathogenesis is still obscure.
Prostate growth is controlled by a variety of polypeptide growth factors, including members of the fibroblast growth factor (FGF) gene family. 2 FGF2, FGF7, and FGF9 are all present in high concentrations in normal human prostate. 3-6 FGF2 is a potent growth factor for prostatic stromal cells. 4,6,7 In addition it can stimulate proliferation of primary prostatic epithelial cells in culture. 5,6 Thus FGF2 can act as both a paracrine growth factor for prostatic epithelial cells and as an autocrine growth factor for prostatic stromal cells.
The concentration of both FGF7 4 and FGF2 3,4 are significantly increased in hyperplastic prostate in comparison to normal peripheral and transition zone tissue. FGF2 is a potent mitogen for prostatic stromal cells and quantitative analysis of cellular proliferation by Ki67 immunohistochemistry of frozen sections of the tissue taken before protein extraction revealed a correlation between increased FGF2 tissue concentration and increased proliferation of stromal cells in BPH tissue, 4 consistent with a key role for this growth factor in driving the abnormal stromal proliferation in BPH.
Given that FGF2 protein is increased in BPH and that this overexpression has functional consequences for the proliferation of prostatic stromal cells, we sought to determine what factors control FGF2 expression in the human prostate. We report here that interleukin (IL)-8 is produced by prostatic epithelial cells and can act as a paracrine inducer of FGF2 production by prostatic stromal cells in vitro. In addition, we have found that IL-8 is present in substantial quantities in human prostate in vivo and that IL-8 concentration is elevated in BPH tissue. Thus stromal proliferation in BPH is controlled, at least in part, by IL-8 secreted by prostatic epithelial cells. This may ultimately lead to increased tissue mass in the prostatic transition zone, which is critical in the pathogenesis of BPH.
Materials and Methods
Tissue Acquisition and Analysis
Samples of the benign peripheral zone of the prostate and normal or hyperplastic transition zone were taken from radical prostatectomies. Tissues were received fresh and portions snap-frozen in liquid nitrogen or used to establish primary cell cultures (see below). The frozen tissues were then analyzed by frozen section to confirm the absence of carcinoma or high-grade prostatic intraepithelial neoplasia. Samples of hyperplastic transition zone were also harvested from suprapubic prostatectomies performed for the treatment of severe BPH.
Cell Culture, Production of Epithelial-Conditioned Medium, and Assay for FGF2 Induction
Primary epithelial and stromal cell cultures were established using prostatic tissue samples from the peripheral zone as described previously. 8 To prepare conditioned medium, primary epithelial cells were plated in 10-cm tissue culture dishes. When the cells were subconfluent epithelial growth medium was replaced with 8 ml of MCDB 153 medium containing insulin, transferrin, selenium, bovine serum albumin, and oleic acid (1% ITS+2; Sigma Chemical Co., St. Louis, MO). Conditioned medium was collected after 72 hours. The epithelial cells tolerated this treatment well and appeared healthy after this period. To assay for FGF2 induction 2 × 10 6 primary stromal cells were plated in 10-cm tissue culture dishes. The following day cells were placed in 4.5 ml of RPMI 1640 with 1% ITS+2. Cells were then treated with either 500 μl of epithelial conditioned medium or MCDB 153 with 1% ITS+2 as control. At intervals aliquots of medium were removed for analysis of FGF2 content by enzyme-linked immunosorbent assay (ELISA) as described below. Cell extracts were also prepared by lysing cells in buffer containing 20 mmol/L Tris, pH 8.2, 2 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L dithiothreitol, 0.1% Nonidet P-40, 250 mmol/L NaCl, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml benzamidine, and 1 mmol/L phenylmethyl sulfonyl fluoride. The cell lysate was cleared by centrifugation for 10 minutes in a microcentrifuge.
To determine whether IL-8 could induce FGF2 production, stromal cultures were plated as above and treated with either 167 pg/ml of recombinant IL-8 (R&D Systems, Minneapolis, MN) in RPMI 1640 with 1% ITS+2 and FGF2 content determined by ELISA on aliquots of medium removed at 6-hour intervals. To block IL-8 activity, 800 μl of conditioned medium was preincubated with 1.0 μg of neutralizing anti-IL-8 monoclonal antibody (mAb B208, R&D Systems) for 1 hour at 37°C. Control antibody was monoclonal anti-β actin antibody (A5441, Sigma Chemical Co.). A second approach to blocking IL-8 activity was to use lipopolysaccharide (LPS) treatment, which decreases IL-8 receptor mRNA by transcriptional 9 and posttranscriptional mechanisms. 9,10 Before addition of conditioned medium, stromal cultures were pretreated with LPS (Sigma Chemical Co.) at 10 ng/ml in growth medium for 24 hours. For both blocking experiments FGF2 concentration was determined by ELISA of stromal medium removed after 6 hours of treatment with conditioned medium.
Immunohistochemistry
Frozen tissue sections were fixed in acetone for 10 minutes and stored at −80°C. All sections were treated with Autoblocker (R&D Systems) to inhibit endogenous peroxidase and avidin/biotin (Vector Laboratories, Burlingame, CA) to block endogenous biotin. The sections were incubated with 100 ng/ml of anti-FGF2 mouse monoclonal antibody (GF22; Oncogene Research Products, Cambridge, MA) or rabbit polyclonal anti-IL-8 antibody (SC 7922; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 12 hours. After liberal washing with phosphate-buffered saline (PBS), pH 7.4, sections were then incubated with appropriate biotinylated secondary antibody at 7.5 μg/ml for 45 minutes at room temperature (Vector Laboratories). Sections were then washed with PBS containing 0.1% Tween 20 and incubated with avidin-biotin complex (Vectastain Elite, Vector Laboratories) for 15 minutes. The antigen-antibody reaction was demonstrated using diaminobenzidine as substrate and the sections then counterstained with hematoxylin.
Enumeration of FGF2-Expressing Cells
To determine the number of FGF2 cells that were located near epithelial acini or in stromal areas without adjacent epithelium a point-counting protocol was used as described previously. 11 A total of 10 periacinar and stromal fields were counted and the result individually summed for each slide. A total of six slides each from the normal peripheral zone, normal transition zone, and hyperplastic transition zone were examined.
ELISA
Cell extracts were prepared from snap-frozen tissues for ELISA as described previously. 6 Protein content of tissue and cell extracts was determined by the method of Bradford. 6 Determination of the FGF2 and IL-8 concentration in the media and cell extracts was performed by ELISA based on a quantitative sandwich immunoassay technique. For quantification of IL-8, 96-well enzyme immunoassay plates (Nunc, Rochester, NY) were coated with 0.5 μg/ml of capture antibody (mAb 208, R&D Systems) for 24 hours at room temperature. The plates were washed with PBS with 0.05% Tween 20 (wash buffer) to remove unbound antibodies. Plates were then blocked with PBS containing 1% bovine serum albumin, 5% sucrose, and 0.05% sodium azide for 2 hours at room temperature. This was followed by the addition of 10 to 100 μl of culture medium or tissue extract per well in a total final volume of 100 μl and incubation at room temperature for 2.5 hours. Recombinant IL-8 (R&D Systems) was used as standard. After multiple washes with wash buffer, the plates were incubated with biotinylated anti-human IL-8 antibody (BAF 208, R&D Systems) at 100 ng/ml for 2 hours at room temperature. Plates were washed liberally with wash buffer and 100 μl of diluted peroxidase-conjugated streptavidin (1:4000; Zymed Laboratories, San Francisco, CA) was aliquoted per well and incubated for 30 minutes at room temperature. The plates were then washed with wash buffer and incubated with 100 μl per well of substrate consisting of a 1:1 mixture of peroxidase solution and tetramethyl benzidine substrate (KPL, Gaithersburg, MD) for 15 minutes at room temperature. The enzymatic activity was stopped with 5 N sulfuric acid and optical density determined at 450 nm in a microplate reader. FGF2 ELISA was quantified by using a FGF2 ELISA kit from R&D Systems according to the manufacturer’s instructions. The IL-8 ELISA was linear from 60 to 2000 pg/ml whereas the FGF2 ELISA was linear from 1 to 64 pg/ml.
Northern Blotting and Quantitative Reverse Transcriptase-Polymerase Chain Reaction Analysis
Total RNA was extracted using Trizol reagent as per the manufacturer’s instructions (Life Technologies, Inc., Gaithersburg, MD). For Northern blotting, RNA (10 μg) was electrophoresed through 1.2% agarose gel at 50°C. The samples were then transferred to nylon membranes overnight, fixed by ultraviolet cross-linking, and hybridized overnight in Hybrisol I (Oncor, Gaithersburg, MD) at 42°C with 1 × 10 6 cpm/ml of 32P-labeled FGF2 cDNA probe prepared as described previously. 8 An equal amount of radiolabeled glyceraldehyde-3′-phosphate dehydrogenase probe (Clontech, Palo Alto, CA) was added 6 hours later. Filters were washed at 42°C for 20 minutes in 2× standard saline citrate containing 0.2% sodium dodecyl sulfate, followed by washing at 65°C for 40 minutes in 0.2% standard saline citrate containing 0.1% sodium dodecyl sulfate. The filters were exposed to Kodak XAR5 film (Eastman-Kodak, Rochester, NY) for 12 to 24 hours. Bands on the autoradiograms were quantified by densitometry using the Imagequant program on a Molecular Dynamics PhosphorImager. Reverse transcription and polymerase chain reaction with FGF2-specific primers was performed as described previously 8 except that reactions were performed for either 20 or 30 cycles. In addition, primers for β2 microglobulin were added as internal controls. The primer sequences for β2 microglobulin were 5′-ACCCCCACTGAAAAAGATGA (forward) and 5′-ATCTTCAAACCTCCATGATG (reverse). After the reaction was complete, 20 μl was analyzed by electrophoresis in a 2.5% agarose gel and stained with ethidium bromide.
Results
Increased Numbers of FGF2-Expressing Stromal Cells near Epithelial Acini
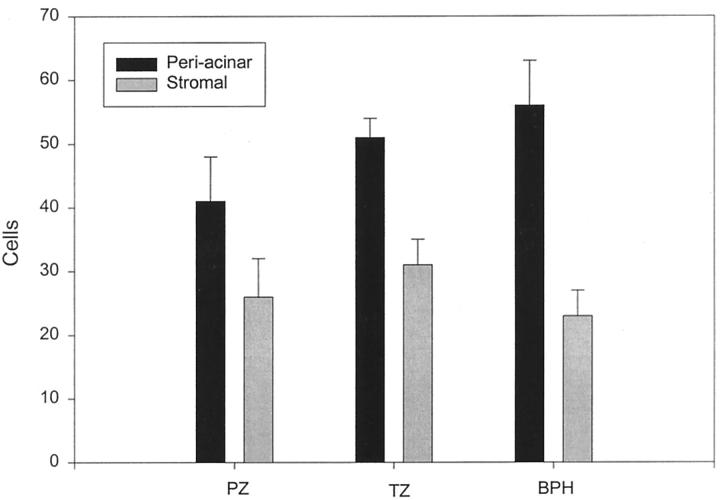
To determine the localization of the increased FGF2 in BPH we examined tissue sections from normal peripheral and transition zone and BPH tissues after immunohistochemistry with anti-FGF2 monoclonal antibody. FGF2 was expressed in stromal cells with fibroblastic morphology. In these sections we noted a definite tendency for the FGF2-positive cells to be located near the epithelial acini. Examples of such concentrations of FGF2-positive cells near epithelial acini are shown in Figure 1 ▶ as well as a control photomicrograph of an adjacent stromal area. It should be noted that this distribution was not uniform, in that some epithelial acini had large numbers of adjacent positive cells whereas others had relatively few. To try and quantitate this phenomenon a simple point-counting protocol was used to enumerate the number of cells staining positively on FGF2 immunohistochemistry in periacinar and stromal areas. At ×200 magnification, an ocular micrometer was aligned parallel to the base of epithelial acini chosen at random and all positively staining cells crossing the ocular micrometer were counted for a total of 10 measurements per slide. The same measurement was then performed with the ocular micrometer placed in a stromal area without adjacent epithelium. Six slides each from normal peripheral zone, normal transition zone, and BPH tissues were evaluated in this manner without knowledge of their origin. Results are shown in Figure 2 ▶ . For each type of tissue the number of FGF2-positive cells was significantly higher in the periacinar area (paired t-test, P < 0.04). In addition, there was a trend for higher cell counts in the periacinar areas of the normal transition zone and BPH tissues compared to the peripheral zone tissue, although this difference is not statistically significant. We could not identify any obvious association of these areas of increased numbers of FGF2-positive cells with atrophy, metaplasia, or inflammatory infiltrate.
Figure 1.
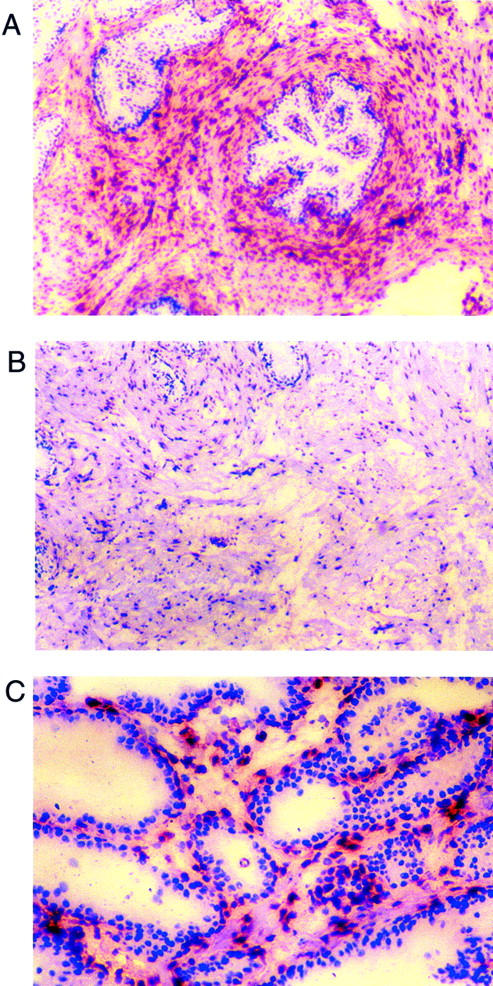
Localization of FGF2 expression in prostatic tissue by immunohistochemistry. Frozen sections of hyperplastic prostatic tissue were analyzed by immunohistochemistry using anti-FGF2 mouse monoclonal antibodies. A: Low-power view of periacinar area (original magnification, ×40). Note large numbers of FGF2-expressing cells near the epithelial acini. B: Low-power view of adjacent stromal area (original magnification, ×40). FGF2-expressing cells are present but the number of such cells is less than in the periacinar area C: High-power view of epithelial area (original magnification, ×400). In areas where several glands are adjacent, the number of FGF2-positive cells was often high in the intervening stroma, as shown here.
Figure 2.
Quantitation of the localization of FGF7-expressing cells in prostate tissue. Frozen sections of six normal peripheral zone, six normal transition zone, and six BPH tissues were analyzed by immunohistochemistry with anti-FGF2 monoclonal antibody. The number of cells located adjacent to epithelial acini (periacinar) was compared to the number of cells in stromal areas using a point-counting protocol. The mean counts in the 10 fields (± SEM) in each location for normal peripheral zone (PZ), normal transition zone, and hyperplastic transition zone (BPH) are shown. For all three types of tissue the difference between the periacinar and stromal tissues is statistically significant (P < 0.04, paired t-test).
Prostatic Epithelial Cells Secrete a Paracrine Factor that Stimulates FGF2 Secretion by Stromal Cells
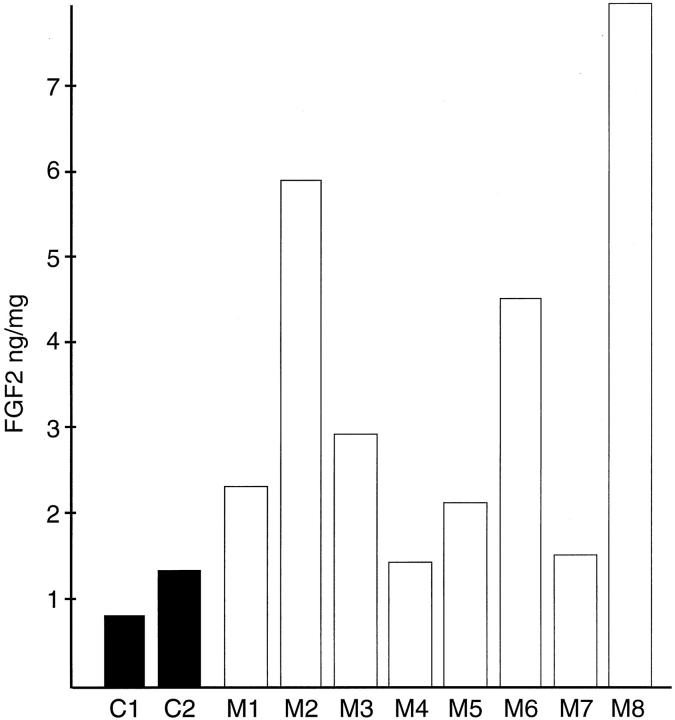
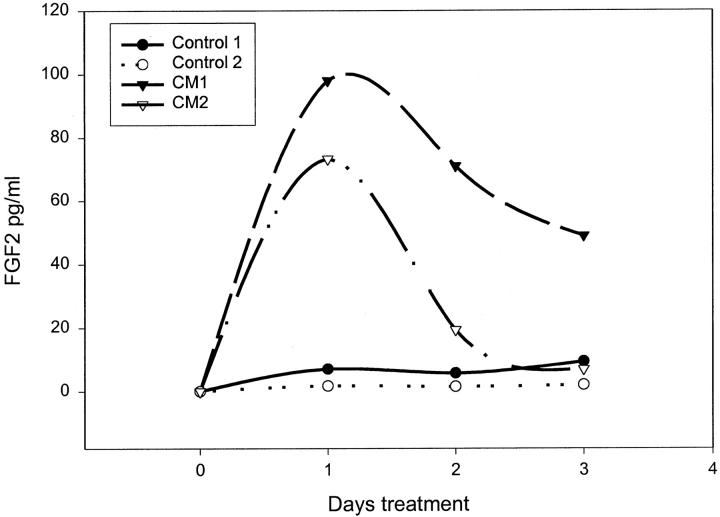
Given that the FGF2-positive cells are more common closer to the epithelial acini we wished to determine whether there was a paracrine factor or factors secreted by epithelial cells that induced FGF2 production by prostatic stromal cells. Such paracrine factors could play a role in normal growth if they are secreted into the stroma or might be released into the surrounding tissue if the epithelium is disrupted during inflammation. To determine whether such a factor(s) is secreted in vitro we collected conditioned medium from primary cultures of prostatic epithelial cells after 72 hours of incubation. The collection medium contained insulin as the only added growth factor to avoid the effect of epidermal growth factor and/or FGFs, which are normally present in the epithelial media, on the stromal cells. The eight different conditioned mediums were then added at a 1:10 dilution to primary cultures of prostatic stromal cells in RPMI 1640 containing insulin and cell extracts prepared after 48 hours of treatment. The FGF2 content of the extracts was then determined by ELISA. As can be seen in Figure 3 ▶ the cell extracts from stromal cells treated with conditioned medium contained 1.2- to 6.9-fold more FGF2 after 48 hours of treatment than control cells, with a mean increase of 3.1-fold. To determine whether the epithelial-conditioned media also were able to induce increased levels of FGF2 in the stromal medium, the stromal cells were treated with epithelial-conditioned medium and aliquots removed at intervals from 4 to 96 hours in various experiments. Aliquots were then assayed by ELISA for FGF2 content. The results of two such experiments are shown in Figure 4 ▶ . The FGF2 content of the stromal media was increased up to 14-fold by 24 hours after treatment and then declined throughout the next 48 hours. We have found a consistent increase in the FGF2 content of medium from the stromal cells treated with epithelial-conditioned medium, but the exact magnitude and kinetics of FGF2 accumulation were variable. The peak of FGF2 accumulation was between 6 and 48 hours and the increase relative to controls varied from 1.2- to 14-fold (mean, 8.8-fold). The reason for this variability is unclear. To determine whether the increased concentration of FGF2 in the cell extracts and conditioned medium was because of an increase in cell number or increased production of FGF2 per cell, the number of prostatic stromal cells was determined after 5 days of incubation with or without treatment with diluted conditioned medium. There was no significant difference in cell number between the treated and untreated stromal cells (data not shown). To rule out the possibility that the increased FGF2 in the stromal medium was because of release of preformed FGF2 because of toxicity caused by the epithelial conditioned medium, we evaluated the percentage of dead cells in treated and untreated stromal cultures by trypan blue exclusion. The percentage of cells taking up trypan blue was essentially the same (0.4 to 0.8%) at all time points tested in treated and untreated cells, so the conditioned medium was not toxic to the stromal cells.
Figure 3.
Stimulation of FGF2 production in stromal cells by epithelial-conditioned medium. Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a 1:10 dilution of one of eight different conditioned media (M1 to M8) prepared from primary epithelial cultures or not supplemented (C1 or C2). After 48 hours cell extracts were prepared and assayed for FGF2 content in duplicate by ELISA and protein content. The average FGF2 content per mg protein is shown. The average variability of duplicate determinations of FGF2 concentration was <10%.
Figure 4.
Stimulation of FGF2 release by epithelial-conditioned medium. Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with a 1:10 dilution of one of two different conditioned media (CM1 or CM2) prepared from primary epithelial cultures or not supplemented. At 24-hour intervals aliquots of media were collected and assayed for FGF2 content in duplicate by ELISA. The average variability of duplicate determinations of FGF2 concentration was <10%.
IL-8 is the Paracrine Factor Secreted by Prostatic Epithelial Cells that Stimulates FGF2 Expression
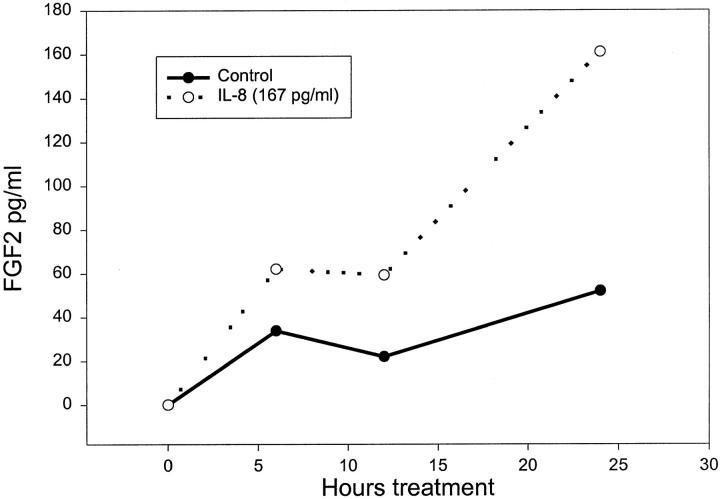
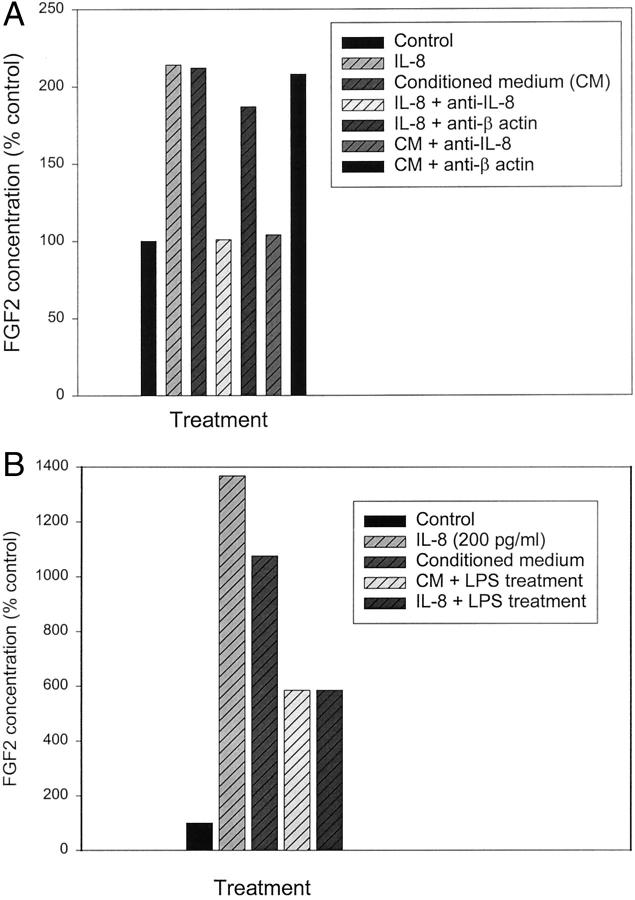
To try and determine the identity of the factor responsible for the FGF2 accumulation we assayed the epithelial-conditioned medium for a number of cytokines, because it has been shown that cytokines can induce FGF2 expression. 12,13 We have shown previously that IL-1α is present in conditioned medium from prostatic epithelial cells 11 but did not find significant induction of FGF2 expression in prostatic stromal cells by this cytokine at concentrations present in conditioned medium (data not shown). IL-6, which is produced by prostatic epithelial cells in culture, also did not significantly induce FGF2 accumulation at concentrations found in the epithelial conditioned medium (data not shown). IL-8 was present at an average concentration 2.4 ± 0.24 ng/ml (SEM, n = 14) in the conditioned mediums from the prostatic epithelial cells. Prostatic stromal cells also express detectable IL-8, but only at approximately one-twentieth the level expressed by epithelial cells. Using recombinant IL-8 at 167 pg/ml, approximately equivalent to the concentration of IL-8 found in the diluted conditioned medium, we found a threefold increase in FGF2 release by prostatic stromal cells by 24 hours after treatment (Figure 5) ▶ , similar to the level of induction by the conditioned medium. IL-8 also lead to a similar increase in accumulation of intracellular FGF2 in treated stromal cells (data not shown). We have tested the ability of IL-8 to induce FGF2 expression in 10 different experiments and although the exact magnitude of induction is somewhat variable there was induction of FGF2 expression in all cases. To verify that the FGF2-inducing activity of the conditioned medium was because of IL-8, we pretreated the conditioned medium with anti-IL-8 neutralizing antibody. As can be seen in Figure 6A ▶ , this pretreatment essentially eliminated the response of the stromal cells to the conditioned medium. This experiment has been repeated four times with similar results in all cases. Treatment with control antibody (anti-β actin) had no effect on FGF2 induction. LPS has been shown to down-regulate IL-8 receptor by both transcriptional 9 and posttranscriptional 9,10 mechanisms. When prostatic stromal cells were pretreated with LPS before addition of the conditioned medium, the FGF2-inducing activity was substantially reduced (Figure 6B) ▶ , similar to the effect of such pretreatment on FGF2 induction by recombinant IL-8. Finally, we noted that the degree of induction of FGF2 in stromal cell extracts (as shown in Figure 3 ▶ ) was proportional to the IL-8 concentration in the conditioned medium, with a correlation coefficient of 0.7 by linear regression for the six samples in which both values were available (data not shown). Thus, IL-8 is the major paracrine inducer of FGF2 production present in epithelial-conditioned medium.
Figure 5.
Stimulation of FGF2 release by IL-8. Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with 167 pg/ml of recombinant IL-8 or not supplemented. The concentration of FGF2 in the stromal media was then determined in duplicate by ELISA of aliquots removed at 6-hour intervals. Control (not supplemented), filled circles; IL-8 167 pg/ml, open circles.
Figure 6.
Inhibition of conditioned medium stimulation of FGF2 release by inhibitors of IL-8 activity. A: Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium and supplemented with recombinant IL-8 at 200 pg/ml, a 1:10 dilution of conditioned medium prepared from a primary epithelial culture, media containing IL-8 at 200 pg/ml preincubated with excess neutralizing anti-IL-8 antibody, media containing IL-8 at 200 pg/ml preincubated with anti-β actin antibody, diluted conditioned medium preincubated with excess anti-IL-8 neutralizing antibody, diluted conditioned medium preincubated with anti-β actin antibody or not supplemented (control). FGF2 concentration was determined in duplicate by ELISA in medium collected after 6 hours of treatment. B: Primary cultures of prostatic stromal cells were plated in RPMI 1640 with insulin, transferrin, and selenium. One half of the plates were then treated with LPS at 10 ng/ml for 24 hours. Cells were then supplemented with a 1:10 dilution of conditioned medium, IL-8 at 200 pg/ml, or not supplemented (control). FGF2 concentration was determined in duplicate by ELISA in medium collected after 6 hours of treatment. Data are expressed as a percentage of the unsupplemented control, not treated with LPS, which is considered 100%.
To determine whether IL-8 is produced by prostatic epithelial cells in vivo we performed immunohistochemistry on frozen sections of hyperplastic prostatic tissue with anti-IL-8 antibodies. As seen in Figure 7 ▶ , there is substantial expression of IL-8 by prostatic basal cells and weaker expression by the luminal secretory cells. Expression was also detected in some stromal cells with fibroblastic morphology. This is consistent with our observation that prostatic stromal cells in culture secrete IL-8, although at a much lower level than by cultured prostatic epithelial cells. As expected, inflammatory cells also expressed detectable IL-8.
Figure 7.
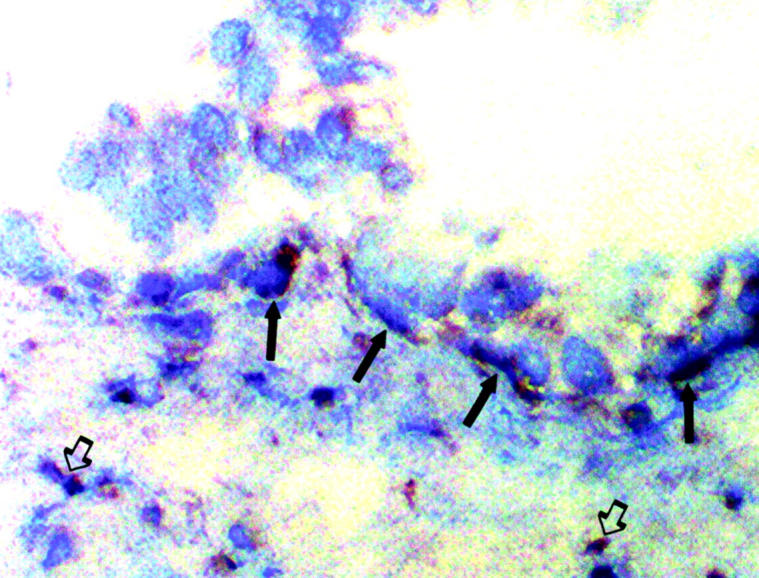
Localization of IL-8 expression in vivo by immunohistochemistry. Frozen sections of hyperplastic prostatic tissue were analyzed by immunohistochemistry using anti-IL-8 antibodies. Strong staining of basal cells (filled arrows) was seen as well as weaker staining of luminal epithelial cells. Staining of inflammatory cells (open arrows, bottom right) and some stromal cells (open arrows, bottom left) was also seen (original magnification, ×400).
IL-8 Increases FGF2 mRNA in Prostatic Stromal Cells
FGF2 protein levels have been shown to be controlled by alterations in transcription, 12-16 mRNA stability, 17 and protein translation 18 in different cell types. To determine whether IL-8 induced FGF2 mRNA in prostatic stromal cells, such cells were treated with recombinant IL-8, RNAs extracted, and Northern blotting performed using a FGF2 cDNA probe. RNA bands of ∼6.0, 4.2, and 3.1 kb were present, as has been described in cardiac myocytes. 19 As can be seen in Figure 8A, IL ▶ -8 induced increased levels of FGF2 mRNA as early as 6 hours after treatment. By densitometry, after normalization for variation in mRNA loading using control glyceraldehyde-3′-phosphate dehydrogenase hybridization a sevenfold increase in FGF2 mRNA levels was present at 6 hours, which decreased slightly by 24 hours. This induction of FGF2 mRNA by IL-8 was confirmed in three separate experiments (data not shown). This increase was also confirmed by semiquantitative reverse transcriptase-polymerase chain reaction. As can be seen in Figure 8B ▶ , RNA from stromal cells treated with IL-8 for 24 hours gave a visible band after 20 cycles of polymerase chain reaction using FGF2-specific primers, whereas RNA from untreated stromal cells did not give a visible band until 30 cycles of amplification. Bands for the control β2 microglobulin were equal in treated and untreated cells. Epithelial-conditioned medium also induced increased FGF2 mRNA levels by 2 hours after treatment (Figure 8C) ▶ , which by densitometry was approximately threefold. Thus the increase in FGF protein induced by epithelial-conditioned medium in stromal cell extracts is similar to the increase in FGF2 mRNA because of these treatments.
Figure 8.
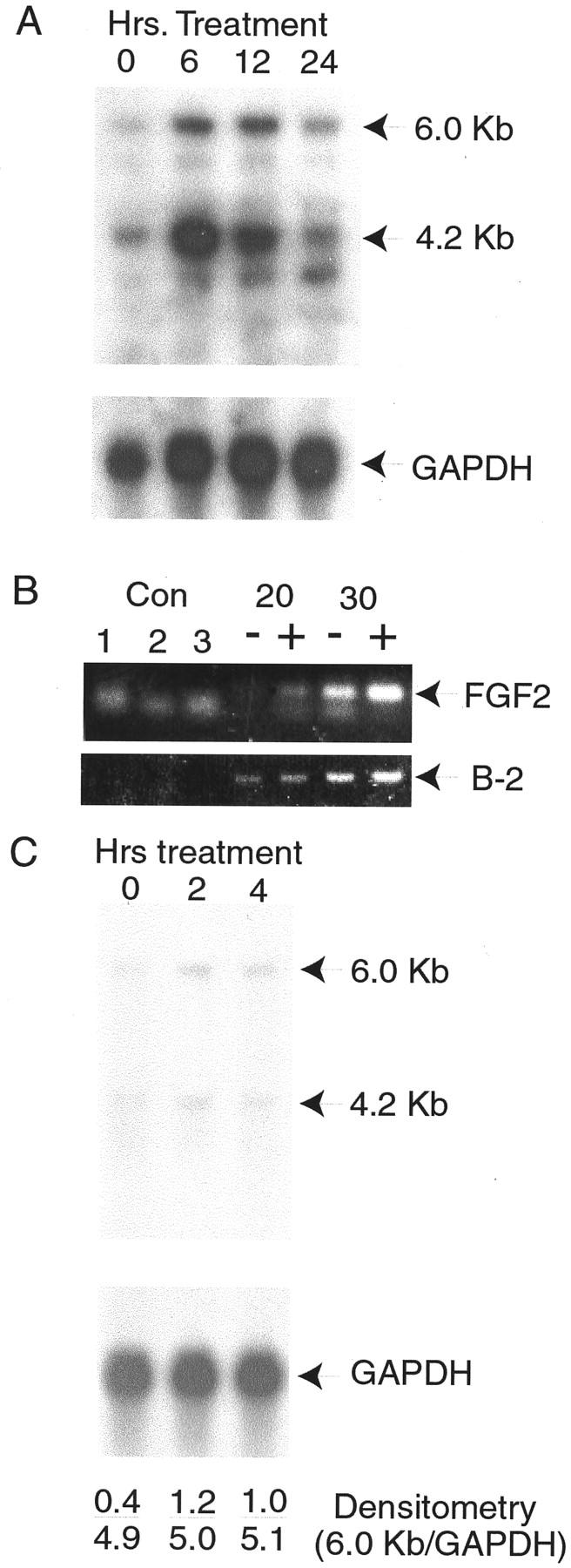
Induction of FGF2 mRNA by IL-8 and epithelial-conditioned medium. A: Primary stromal cells were treated for 6, 12, or 24 hours with 200 pg/ml of recombinant IL-8 or not treated (0 hours). RNAs were extracted and 10 μg/ml of total RNA from each treatment analyzed by Northern blotting with a FGF2 cDNA probe. After autoradiography major bands of 6.0 and 4.2 kb were detected (indicated by arrows), as well as a minor band of 3.1 kb. Control hybridization with glyceraldehyde-3′-phosphate dehydrogenase was performed to control for variation in RNA loading and transfer. B: Primary stromal cells were treated with recombinant IL-8 as described above, RNA isolated, and analyzed by reverse transcriptase-polymerase chain reaction. Control reactions were water (control 1), and RNAs from untreated or treated cells without reverse transcription (controls 2 and 3). Reverse-transcribed RNAs were subject to the indicated number of cycles of polymerase chain reaction before analysis by agarose gel electrophoresis. Reactions from RNAs of cells treated with IL-8 are indicated by (+), untreated samples by (−). Bands corresponding to the predicted size (349 bp) for the specific product from the FGF2 cDNAs are indicated. A smaller nonspecific band is also seen. The bands corresponding to the control β-2 microglobulin polymerase chain reaction products of 125 bp from the same reactions are also shown (B-2). C: Cells were treated with a 1:10 dilution of epithelial-conditioned medium for 2 or 4 hours or not treated (0 hours) and RNAs analyzed by Northern blotting as described in A above using FGF2 and control glyceraldehyde-3′-phosphate dehydrogenase cDNA probes. Results of densitometry using a Molecular Dynamics PhosphorImager are shown in each lane, expressed as the ratio of the intensity of 6 kb FGF2 band over the intensity of the glyceraldehyde-3′-phosphate dehydrogenase band.
Expression of IL-8 in Human Prostate in Vivo
Having established that IL-8 can act as an epithelial-derived paracrine inducer of FGF2 production by stromal cells in vitro we sought evidence that this paracrine stimulation might occur in vivo. We therefore assayed tissue extracts from normal transition zone and BPH tissue for IL-8 content by ELISA. The tissue content of IL-8 was quite variable, ranging from undetectable (<0.2 ng/g wet weight) to 128 ng/g wet weight. The mean IL-8 content of the normal transition zone was 16.4 ± 9.7 ng/g wet weight (SEM, n = 7) whereas BPH tissue contained 32.9 ± 7.7 ng/g (SEM, n = 16). The twofold higher IL-8 concentration in BPH tissue is the same as to the twofold higher level of FGF2 in BPH, consistent with a correlation between the expression of these two polypeptides. Thus IL-8 is expressed at biologically significant, but variable, levels in human prostate tissue in vivo, with higher levels present in BPH tissue.
Discussion
BPH is because of the slow but progressive growth of both epithelial and stromal cells within the prostatic transition zone tissue throughout many years. Quantitative immunohistochemical studies have shown both increased cellular proliferation and decreased apoptosis contribute to this excess growth. 20 FGF2 is elevated approximately twofold in BPH tissue 3,4 and FGF2 concentrations are correlated with stromal proliferation. 4 It should be noted that even relatively small perturbations of growth factor activity may be biologically significant in a disease characterized by subtle dysregulation of growth control throughout the course of 40 to 50 years, as is the case for BPH.
We have found that FGF2-producing stromal cells tend to be located near epithelial acini in the prostate and that epithelial cells release IL-8, which can induce FGF2 production in vitro. In addition, IL-8 is present in relatively high quantities in prostate tissue in vivo. The correlation between increased IL-8 and FGF2 levels in BPH tissues in vivo is consistent with the idea that IL-8 may act in a similar paracrine manner in vivo. The FGF2 induced in stromal cells by IL-8 can act as an autocrine growth factor for prostatic stromal cells. 4,6,7 FGF2 is also a growth factor for prostatic epithelial cells. 5,6 Thus it is possible that a double paracrine loop can be established in which IL-8 is secreted by epithelial cells, which then induces FGF2 expression by stromal cells, which can then induce epithelial proliferation and further increases in IL-8 expression, which in turn leads to increased FGF2 expression and so forth. This double paracrine loop is functionally equivalent to an autocrine loop and can result in increased and poorly controlled proliferation. Although it is clear that many factors may control FGF2 levels in the prostate, our data suggests that IL-8 may be an important regulator of FGF2 expression in vivo, with important functional implications for the pathophysiology of BPH.
FGF2 mRNA levels are increased by treatment of prostatic stromal cells with IL-8 or epithelial-conditioned medium. A twofold increase in FGF2 mRNA in BPH tissues has been observed by Mori and colleagues, 21 consistent with the idea that FGF2 expression is controlled primarily by the level of mRNA in vivo. FGF2 transcription is regulated by a variety of factors in different cell types 14-16 including cytokines. 12,13 Posttranscriptional regulation of FGF2 mRNA stability has also been described, 17 so it is also possible that increased mRNA stability may account for the increased FGF2 mRNA levels after IL-8 or conditioned medium treatment of stromal cells. The fact that the increase in the level of FGF2 mRNA is similar to the increase in FGF2 protein argues that alterations in FGF2 translational efficiency are probably not critical in the increased expression of FGF2 protein in BPH.
Given the highly variable levels of IL-8 in the human prostate in vivo it is likely that control of IL-8 expression in the prostate is complex. We have shown that IL-8 is produced and secreted by prostatic epithelial cells in vitro and expressed by prostatic basal cells and to lesser extent luminal epithelial cells in vivo. As shown by immunohistochemistry of prostatic tissue using anti-IL-8 antibodies, inflammatory cells also contribute to IL-8 levels in vivo, consistent with the known role of inflammatory cells in IL-8 production. 22 It is well known that normal prostate and BPH tissue often contain chronic inflammation, which can be extensive. 23,24 However, we did not observe any obvious correlation between FGF2-expressing cells detected by immunohistochemistry and infiltrates of chronic inflammatory cells, so the extent to which inflammation contributes to IL-8 and FGF2 expression in vivo is unclear. In addition, we do not know the extent to which the IL-8 produced by the epithelial cells is normally released onto the stroma or whether inflammation and atrophy may increase such release.
The finding that FGF2-expressing stromal cells are increased near epithelial acini and FGF2 can be induced by IL-8 released by prostatic epithelial cells is similar to our previously reported results in which FGF7-expressing stromal cells are increased adjacent to epithelial cells and can be induced by IL-1α, which is increased in BPH tissues. 11 FGF7 is an epithelial-specific growth factor and its level of expression is strongly correlated with increased epithelial proliferation in BPH. 4 Thus the expression of two different cytokines by prostatic epithelial cells seems to drive the expression of important epithelial and stromal growth factors, contributing to the abnormal proliferation in BPH. This raises the question of whether there may be a common underlying mechanism inducing the production of these cytokines in vivo that may contribute to the pathogenesis of BPH.
IL-8 has been demonstrated to be expressed by prostate cancer cells by immunohistochemistry. 25 In addition, the PC-3 prostate cancer cell line expresses IL-8 26,27 and inhibition of IL-8 activity decreases tumorigenicity 26,27 and metastasis 27 of PC-3 cells in vivo in SCID or nude mouse model systems. In agreement with these observations, direct analysis of 17 prostate cancer tissue extracts in our laboratory has shown that IL-8 is substantially increased in prostate cancer tissues (60.1 ng/g wet wet, unpublished observations). IL-8 is an angiogenic factor and part of its biological activity in promoting tumor progression is almost certainly because of its direct effect on endothelial cell proliferation and angiogenesis. However, we have shown previously that FGF2 is increased threefold in primary prostate cancer tissues, is expressed in the cancer stromal cells, and can act as a growth factor for prostatic epithelial cells. It is likely that the increased IL-8 expression by the cancer cells induces increased FGF2 production by adjacent stromal cells, which can then act a paracrine growth factor for the cancer cells, and in this manner contribute to prostate cancer progression.
In this report we have demonstrated that FGF2-expressing stromal cells tend to be located near epithelial acini and that epithelial cells can release IL-8, which can induce FGF2 expression. This finding has important implications for the pathophysiology of BPH and for prostate cancer progression. Further investigations are needed to understand the reason for increased IL-8 expression in BPH and cancer, with the goal of disrupting this abnormal expression to treat these two common diseases of older men.
Acknowledgments
We thank Dr. Douglas Mann for use of his microplate reader.
Footnotes
Address reprint requests to Michael M. Ittmann M.D., Ph.D., Research Service (151), Houston VAMC, 2002 Holcombe Blvd., Houston, Texas 77030. E-mail: mittmann@bcm.tmc.edu.
Supported by National Institutes of Health grant R01 DK54170.
References
- 1.Glynn R, Campion E, Bouchard G, Silbert J: The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol 1985, 121:78-83 [PubMed] [Google Scholar]
- 2.Culig Z, Hobisch A, Cronauer M, Radmayr C, Hittmair A, Zhang J, Thurnher M, Bartsch G, Klocker H: Regulation of prostatic growth and function by peptide growth factors. Prostate 1996, 28:392-405 [DOI] [PubMed] [Google Scholar]
- 3.Begun F, Story M, Hopp K, Shapiro E, Lawson R: Regional concentration of basic fibroblast growth factor in normal and benign hyperplastic prostates. J Urol 1995, 153:839-843 [PubMed] [Google Scholar]
- 4.Ropiquet F, Giri D, Lamb D, Ittmann M: FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol 1999, 162:595-599 [PubMed] [Google Scholar]
- 5.Giri D, Ropiquet F, Ittmann M: Alterations in expression of FGF2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res 1999, 5:1063-1071 [PubMed] [Google Scholar]
- 6.Giri D, Ropiquet F, Ittmann M: FGF9 is an autocrine and paracrine prostatic growth factor expressed by prostatic stromal cells. J Cell Physiol 1999, 180:53-60 [DOI] [PubMed] [Google Scholar]
- 7.Sherwood ER, Fong CJ, Lee C, Kozlowski JM: Basic fibroblast growth factor: a potential mediator of stromal growth in the human prostate. Endocrinology 1992, 130:2955-2963 [DOI] [PubMed] [Google Scholar]
- 8.Ittmann M, Mansukhani A: Expression of fibroblast growth factors (FGFs) and FGF receptors in human prostate. J Urol 1997, 157:351-356 [PubMed] [Google Scholar]
- 9.Lloyd AR, Biragyn A, Johnston JA, Taub DD, Xu L, Michiel D, Sprenger H, Oppenheim JJ, Kelvin DJ: Granulocyte-colony stimulating factor and lipopolysaccharide regulate the expression of interleukin 8 receptors on polymorphonuclear leukocytes. J Biol Chem 1995, 270:28188-28192 [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Rahimpour R, Ran L, Kong C, Biragyn A, Andrews J, Devries M, Wang JM, Kelvin DJ: Regulation of CCR2 chemokine receptor mRNA stability. J Leukoc Biol 1997, 62:653-660 [DOI] [PubMed] [Google Scholar]
- 11.Giri D, Ittmann M: Interleukin-1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol 2000, 157:249-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faris M, Ensoli B, Kokot N, Nel A: Inflammatory cytokines induce the expression of basic fibroblast growth factor (bFGF) isoforms required for the growth of Kaposi’s sarcoma and endothelial cells through the activation of AP-1 response elements in the bFGF promoter. AIDS 1998, 12:19-27 [DOI] [PubMed] [Google Scholar]
- 13.Wijelath ES, Carlsen B, Cole T, Chen J, Kothari S, Hammond WP: Oncostatin M induces basic fibroblast growth factor expression in endothelial cells and promotes endothelial cell proliferation, migration and spindle morphology. J Cell Sci 1997, 110:871-879 [DOI] [PubMed] [Google Scholar]
- 14.Detillieux KA, Meij JT, Kardami E, Cattini PA: Alpha1-adrenergic stimulation of FGF-2 promoter in cardiac myocytes and in adult transgenic mouse hearts. Am J Physiol 1999, 276:H826-H833 [DOI] [PubMed] [Google Scholar]
- 15.Hurley MM, Tetradis S, Huang YF, Hock J, Kream BE, Raisz LG, Sabbieti MG: Parathyroid hormone regulates the expression of fibroblast growth factor-2 mRNA and fibroblast growth factor receptor mRNA in osteoblastic cells. J Bone Miner Res 1999, 14:776-783 [DOI] [PubMed] [Google Scholar]
- 16.Ueba T, Kaspar B, Zhao X, Gage FH: Repression of human fibroblast growth factor 2 by a novel transcription factor. J Biol Chem 1999, 274:10382-10387 [DOI] [PubMed] [Google Scholar]
- 17.Touriol C, Morillon A, Gensac MC, Prats H, Prats AC: Expression of human fibroblast growth factor 2 mRNA is post-transcriptionally controlled by a unique destabilizing element present in the 3′-untranslated region between alternative polyadenylation sites. J Biol Chem 1999, 274:21402-21408 [DOI] [PubMed] [Google Scholar]
- 18.Kevil C, Carter P, Hu B, DeBenedetti A: Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene 1995, 11:2339-2348 [PubMed] [Google Scholar]
- 19.Weiner HL, Swain JL: Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci USA 1989, 86:2683-2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyprianou N, Tu H, Jacobs S: Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 1996, 27:668-675 [DOI] [PubMed] [Google Scholar]
- 21.Mori H, Maki M, Oishi K, Jaye M, Igarashi K, Yoshida O, Hatanaka M: Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate 1990, 16:71-80 [DOI] [PubMed] [Google Scholar]
- 22.Wuyts A, Proost P, Van Damme J: Interleukin 8 and other CXC chemokines. ed 3 Thomson A eds. The Cytokine Handbook, 1998, :pp 272-311 Academic Press, New York [Google Scholar]
- 23.Anim JT, Udo C, John B: Characterization of inflammatory cells in benign prostatic hyperplasia. Acta Histochem 1998, 100:439-449 [DOI] [PubMed] [Google Scholar]
- 24.Nickel JC, Downey J, Young I, Boag S: Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int 1999, 84:976-981 [DOI] [PubMed] [Google Scholar]
- 25.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL: Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 1998, 51:161-167 [DOI] [PubMed] [Google Scholar]
- 26.Moore BB, Arenberg DA, Stoy K, Morgan T, Addison CL, Morris SB, Glass M, Wilke C, Xue YY, Sitterding S, Kunkel SL, Burdick MD, Strieter RM: Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol 1999, 154:1503-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, Yano S, Bar-Eli M, Radinsky R, Pettaway CA, Dinney CP: Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res 2000, 6:2104-2119 [PubMed] [Google Scholar]