Abstract
Hepatic apoptosis has been shown to occur in both experimental and clinical alcoholic liver disease, but the signaling pathway remains unknown. This study was undertaken to examine specifically the involvement of the upstream signals, Fas and cytochrome c, in alcohol-induced caspase-3 activation and apoptosis in the liver. Male FVB mice were administrated intragastrically a single dose of alcohol at 6 g/kg, which has been shown to represent binge drinking in humans. Hepatic apoptosis was detected by a terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Active form of caspase-3 was identified by immunoperoxidase staining and confirmed by immunogold labeling and was found to be in the cytosol and nucleus. Enzymic assay further confirmed caspase-3 activation and nucleus localization. Systemic administration of caspase-3 inhibitor, Ac-DEVD-FMK, inhibited caspase-3 activity and abrogated apoptosis. Elevation of cytosolic cytochrome c was found by immunoperoxidase staining, immunogold labeling, and Western blot. Increased Fas ligand expression was detected by immunoperoxidase staining. Intravenous administration of a neutralizing Fas ligand monoclonal antibody resulted in suppression of caspase-3 activation and attenuation of apoptosis, but did not inhibit mitochondrial cytochrome c release. The results thus demonstrate that Fas/Fas ligand system-mediated caspase-3 activation plays a central role in the ethanol-induced hepatic apoptosis.
Ethanol consumption induces apoptosis in a variety of tissues including liver, 1 buccal mucosa, 2 salivary gland, 3 gastric mucosa, 4 brain, 5 thymus, 6 and spleen. 7 In particular, ethanol consumption-induced hepatic apoptosis has been widely recognized in rats, 8-13 mice, 14 minipigs, 15 and humans. 16,17 However, only limited information is available about the molecular mechanism of ethanol-induced liver apoptosis.
The cellular machinery involved in the execution of apoptosis includes a family of cysteine proteases termed caspases. 18 Although more than a dozen of caspases have been identified up to date, caspase-3 stands out because it is commonly activated in response to various death stimuli. 19-21 Two general conceptual pathways have been shown to lead to caspase-3 activation: 1) death signal and receptor systems such as Fas ligand (Fas L)/Fas and tumor necrosis factor (TNF)/TNF receptor (TNFR), and 2) intracellular stress signals such as mitochondrial cytochrome c release. 22,23 Although chronic ethanol administration was found to elevate caspase-3 activity and Fas L mRNA expression in the liver, 24,25 the signaling pathways of ethanol-induced apoptosis remain primarily unknown.
Systemic administration of specific caspase inhibitors has been widely used to investigate the role of caspases in apoptosis. Several reports have demonstrated that intravenous injection of caspase-3 inhibitors attenuates Fas- and ischemia/reperfusion-induced apoptosis. 26-29 Recently, systemic administration of a neutralizing Fas L monoclonal antibody was shown to effectively block the Fas/Fas L system and attenuate apoptosis. 30,31 By using these in vivo approaches for the investigation of apoptotic signaling pathway, the present study was undertaken to determine the role of caspase-3 in ethanol-induced hepatic apoptosis and to explore the possible upstream signals. Ethanol was administrated intragastrically with or without an intravenous injection of a caspase-3 inhibitor or a neutralizing Fas L monoclonal antibody. DNA fragmentation was determined using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay and immunogold electron microscopy. Caspase-3 activation, mitochondrial cytochrome c release, and Fas L expression were monitored by light and electron microscopy, Western blot, and enzymatic assay.
Materials and Methods
Chemicals and Reagents
ApopTag in situ apoptosis detection kit was purchased from Intergen Co. (Purchase, NY). Monoclonal hamster anti-mouse Fas ligand (no azide/low endotoxin), monoclonal mouse anti-cytochrome c, and polyclonal rabbit anti-active caspase-3 antibodies were purchased from PharMingen (San Francisco, CA). Polyclonal rabbit anti-Fas ligand antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Normal hamster IgG was purchased from ICN Pharmaceuticals, Inc. (Aurora, Ohio). Biotinylated rabbit anti-mouse IgG and goat anti-rabbit IgG antibodies and horseradish peroxidase (HRP)-streptavidin were obtained from Zymed Laboratories, Inc. (San Francisco, CA). Gold conjugates of protein A, goat anti-mouse IgG, and sheep anti-digoxigenin were obtained from BB International (Cardiff, UK). Caspase-3 inhibitor II (Z-DEVD-FMK), caspase-3 substrate I (Ac-DEVD-pNA), and p-nitroaniline were the products of Calbiochem Corp. (La Jolla, CA). Bicinchoninic acid protein assay kit was the product of Pierce (Rockford, IL). All other chemicals and reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
Animals
Male FVB mice, 10 weeks of age, were used in this study. The mice were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/dark cycle and free access to rodent chow and water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association of Accreditation of Laboratory Animal Care.
Experimental Treatments
A binge drinking model developed by Carson and Pruett 32 was followed for ethanol challenge with some minor modifications. This model was designed to achieve blood alcohol levels, behavioral effects, and physiological effects comparable to human binge drinking. Animals were randomly assigned into three treatments groups (5 to 7 mice in each group) along with appropriate controls: 1) ethanol, 2) caspase-3 inhibition, and 3) Fas L neutralization. After 6 hours of fasting, animals were administrated 25% (w/v) ethanol at a total accumulative dosage of 6 g/kg body weight by four equally divided gavages in 20-minute intervals. Control mice received the same volume of water. For caspase-3 inhibition, an irreversible caspase-3 inhibitor Z-DEVD-FMK was dissolved in 1% dimethylsulfoxide (DMSO). The inhibitor was injected into the tail vein at a dosage of 20 μg/g body weight before ethanol administration. Control mice received the same amount of 1% DMSO. For Fas L neutralization, a hamster anti-mouse monoclonal antibody was injected into the tail vein at a dosage of 5 mg/kg body weight before ethanol administration. Control mice received the same amount of normal hamster IgG. Twelve hours after ethanol administration, the mice were anesthetized with pentobarbital sodium (5 μg/g body weight), and liver tissues were taken for light and electron microscopy, Western blot analysis, and enzymatic assay.
Tissue Processing for Light and Electron Microscopy
For light microscopy, liver tissues were fixed with 10% formalin in 0.01 mol/L of phosphate-buffered saline (PBS), pH 7.4, and embedded in paraplast. Tissue sections of 5 μm were cut and mounted on silanized slides. For electron microscopy, liver tissues were cut into ∼1 mm 3 and fixed in 2% freshly depolymerized paraformaldehyde with 0.5% glutaldehyde in 0.1 mol/L of sodium cacodylate buffer, pH 7.4, for 2 hours. After rinsing in sodium cacodylate buffer, the samples were partially dehydrated with ethanol and embedded in LR White resin. Ultrathin sections were cut and collected on gold grids. Labeled ultrathin sections were observed with a Philip transmission electron microscope.
Assessment of Apoptosis by Light and Electron Microscopic TUNEL Assay
Apoptosis was assessed by detection of DNA fragmentation using in situ TUNEL assay with both light and electron microscopes. For light microscopic TUNEL, liver slides were processed with an ApopTag in situ apoptosis detection kit (Intergen Co.) according to the manufacture’s instructions. Briefly, liver tissue slides were pretreated with proteinase K and H2O2, and incubated with the reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and digoxigenin-conjugated dUTP for 1 hour at 37°C. The labeled DNA was visualized with HRP-conjugated anti-digoxigenin antibody with diaminobenzidine as the chromagen. Rat mammary gland tissue provided in the kit was used as positive control. For negative control, TdT enzyme was omitted from the reaction mixture. For electron microscopic TUNEL assay, the ultrathin sections were incubated with normal sheep serum for 30 minutes to block nonspecific reactions. The sections were then incubated in the presence of 0.25 U/μl TdT and 0.5 μmol/L of biotinylated dUTP in TdT buffer (0.5 mol/L potassium cacodylate, 2 mmol/L CoCl2, and 0.2 mmol/L dithiothreitol, pH 7.2) for 30 minutes at 37°C. After rinsing in immunogold buffer (0.01 mol/L PBS with 1% normal serum, 1% bovine serum albumin, 0.1% Tween 20, and 0.1% Na3N, pH 8.2), the ultrathin sections were labeled with 10-nm gold-conjugated sheep anti-digoxigenin for 1 hour. The ultrathin sections were then counterstained with uranyl acetate and lead citrate.
Immunoperoxidase Staining of Active Caspase-3, Cytochrome c, and Fas Ligand
After pretreatment, respectively, with 3% H2O2 and 5% normal serum, liver tissue sections were then incubated overnight at 4°C with polyclonal rabbit anti-active caspase-3 antibody, or monoclonal mouse anti-cytochrome c (clone 7H8.2C12) antibody or polyclonal rabbit anti-Fas ligand antibody. Sections were then incubated for 30 minutes in either biotinylated rabbit anti-mouse IgG antibody or biotinylated goat anti-rabbit IgG antibody, followed by incubation with HRP-streptavidin for 20 minutes. The antibody-binding sites were visualized by incubation with a diaminobenzidine-H2O2 solution using a diaminobenzidine kit. Finally, sections were counterstained with 0.5% methyl green.
Immunogold Labeling of Active Caspase-3 and Cytochrome c
Liver ultrathin sections were incubated with either monoclonal mouse anti-cytochrome c antibody or polyclonal rabbit anti-active caspase-3 antibody overnight at 4°C. After rinsing in immunogold buffer (0.01 mol/L PBS with 1% bovine serum albumin, 0.1% Tween, and 0.1% Na3N, pH 8.2), the ultrathin sections were incubated in either 10-nm gold-conjugated rabbit anti-mouse IgG antibody or 10-nm gold-conjugated protein A diluted in immunogold buffer for 1 hour. The ultrathin sections were then rinsed in distilled water and counterstained with uranyl acetate and lead citrate.
Enzymatic Assay of Caspase-3
Fresh liver tissues were homogenized with a Teflon homogenizer in the extraction buffer [25 mmol/L HEPES buffer, pH 7.4, containing 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 2 mmol/L dithiothreitol, and 0.1% CHAPS]. The homogenate was centrifuged at 20,000 × g for 30 minutes. The supernatant was diluted with the assay buffer (50 mmol/L HEPES, 10 mmol/L dithiothreitol, 1.0 mmol/L EDTA, 100 mmol/L NaCl, 0.1% CHAPS, and 10% glycerol, pH 7.4) and incubated at 37°C with 200 μmol/L caspase-3 substrate I (Ac-DEVD-pNA). p-Nitroaniline was used as the standard. Cleavage of the substrate was monitored at 405 nm and the specific activity was expressed in pmol of the product, nitroaniline, per minute per mg protein. For measurement of nuclear caspase-3 activity, hepatic nuclei were isolated as described previously. 33 Briefly, liver was perfused, removed, and minced in 2 volumes of ice-cold 0.32 mol/L sucrose in 25 mmol/L Tris-HCl buffer (pH 7.4, containing 3 mmol/L MgCl2, 25 mmol/L KCl, 2 mmol/L EDTA, 0.1 mmol/L spermine, 0.1% CHAPS, and 1% proteinase inhibitor cocktail). The tissue was homogenized with a glass Dounce homogenizer, and filtered through two layers of nylon mesh. The homogenate was centrifuged at 700 × g for 10 minutes. The pellet was resuspended in 2.4 mol/L of sucrose in 25 mmol/L of Tris-HCl buffer and centrifuged at 50,000 × g for 60 minutes. The nuclear pellet was washed in the extraction buffer, homogenized, and centrifuged at 20,000 × g for 30 minutes. The resulting supernatant was used for caspase-3 activity assay. Protein concentration was determined by using a Bicinchoninic acid protein assay kit (Pierce).
Western Blot Analysis of Cytochrome c
Fresh liver tissues were homogenized gently with a glass tissue grinder in a suspension buffer (20 mmol/L HEPES, pH 7.4, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1.0 mmol/L EDTA, 1.0 mmol/L EGTA, 1.0 mmol/L dithiothreitol, and 1% proteinase inhibitor cocktail) with 0.25 mol/L of sucrose. The crude homogenate was centrifuged at 750 × g for 10 minutes at 4°C and then at 8000 × g for 20 minutes at 4°C. The 8000 × g pellet was homogenized with Teflon homogenizer in the suspension buffer without sucrose and used as the mitochondrial fraction. The 8000 × g supernatant was further centrifuged at 100,000 × g for 60 minutes at 4°C and used as a cytosolic fraction. Aliquots containing 15 μg of protein were loaded on a 15% sodium dodecyl sulfate-polyacrylamide gel. After electrophoresis, protein was transferred to nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in Tris-buffered saline (pH 7.5) and probed with monoclonal mouse anti-cytochrome c. The membrane was then processed with HRP anti-mouse IgG. The protein bands were visualized by an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL) and quantified by Bio-Rad MultiAnalyst (Bio-Rad Laboratories, Richmond, CA).
Results
Liver Apoptosis by Acute Ethanol Treatment
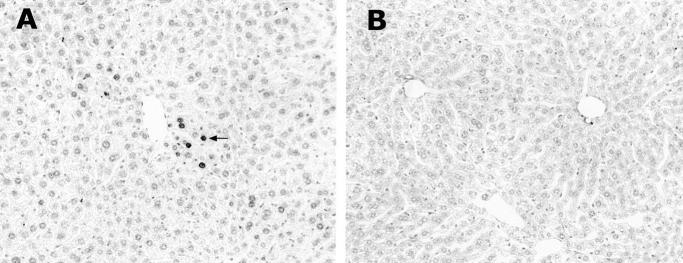
Ethanol-induced hepatic apoptosis was assessed by detection of DNA fragmentation using TUNEL assay under both light and electron microscopies. In comparison to the control mice (Figure 1A) ▶ , ethanol-treated mice showed positive staining in the liver (Figure 1B) ▶ under light microscopy. The positive nuclei were found mostly in the perivenous hepatocytes. The staining intensity was different among the positive cells. The hepatocytes closer to the central vein showed stronger staining. Under electron microscopy, only a few gold particles were seen in the nucleus of hepatocytes of control mice (Figure 1C) ▶ . However, numerous gold particles were found in the hepatic nucleus of ethanol-treated mice (Figure 1D) ▶ , indicating the occurrence of DNA fragmentation. Nuclear condensation of hepatocytes in ethanol-treated mice was apparent, and the heavy gold labeling was found in the region with a uniformed structure (Figure 1D) ▶ .
Figure 1.
Acute ethanol administration of an accumulative dose of 6 g/kg body weight induced apoptosis in the mouse liver as detected by TUNEL assay. Under light microscope, the control liver showed negative staining (A), and the ethanol-treated liver showed positive cells (arrows) around the central vein (B). Using immunogold TUNEL under electron microscope, only a few gold particles were seen in the nucleus of hepatocytes in control liver (C), whereas numerous gold particles (arrows) in the nucleus of hepatocytes in ethanol-treated liver were found (D), indicating severe DNA fragmentation. N, nucleolus. Original magnifications: ×130 (A and B); ×24,000 (C and D).
Inhibition of Caspase-3 Activation Attenuated Ethanol-Induced Liver Apoptosis
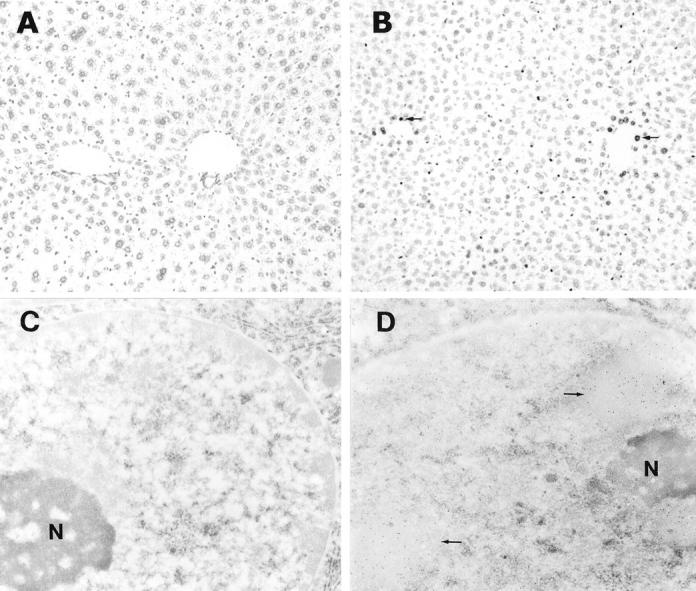
Caspase-3 activation associated with ethanol challenge was examined with a specific antibody to the active form of caspase-3 by both light and electron microscopies. Light microscopic staining demonstrated a negative result in the liver of control mice (Figure 2A) ▶ , but a positive result in the ethanol-treated mice (Figure 2B) ▶ . The distribution of the positive cells was perivenous. Electron-microscopic immunogold labeling showed that there was no active caspase-3 in either the cytoplasm (Figure 2C) ▶ or nucleus (Figure 2D) ▶ in control mice. However, positive labeling was demonstrated in both the cytoplasm (Figure 2E) ▶ and nucleus (Figure 2F) ▶ of the ethanol-treated mice, but mainly localized in the cytoplasm. The nuclear distribution of active caspase-3 was found in the region with a uniformed structure. To confirm the translocation of active caspase-3 into the nucleus, caspase-3 activity was determined in the isolated liver nuclei. A significant increase in the nuclear caspase-3 activity was found after acute ethanol administration (from 0.60 ± 0.41 to 1.61 ± 0.42 nmol/min/mg protein).
Figure 2.
Detection of active form of caspase-3 in the liver of ethanol-treated mice. Using light microscopic immunoperoxidase staining, no active caspase-3 was detectable in the control liver (A), and moderate staining was found in the liver of ethanol-treated mice (B). Under electron microscope, immunogold labeling of active caspase-3 showed negative results in both cytoplasm (C) and nucleus (D) of hepatocytes in the control liver. However, active caspase-3 was observed not only in the cytosol (arrows, E) but also in the nucleus (arrows, F) of hepatocytes in the ethanol-treated liver. M, mitochondria. Original magnifications: ×130 (A and B); ×43,000 (C–F).
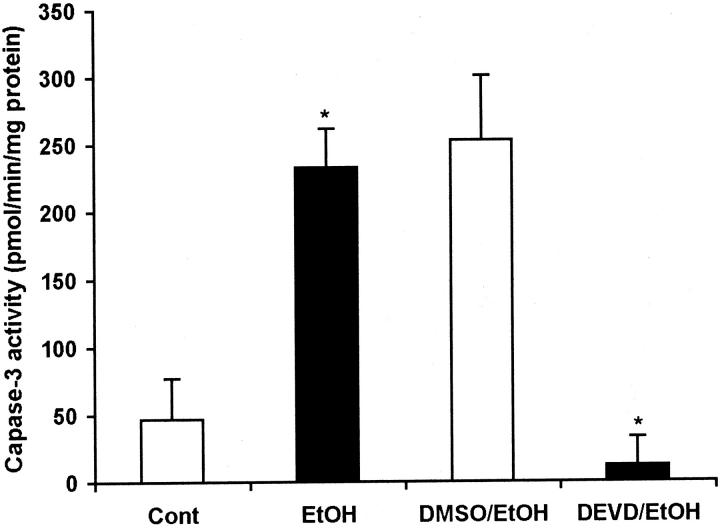
To elucidate the role of caspase-3 in the signaling pathway of ethanol-induced apoptosis, a caspase-3 inhibitor, Z-DEVD-FMK, was intravenously administrated before ethanol treatment. As shown in Figure 3 ▶ , acute ethanol treatment significantly elevated the caspase-3 activity in the liver and administration of Z-DEVD-FMK inhibited ethanol-induced caspase-3 activation. The TUNEL assay demonstrated that Z-DEVD-FMK attenuated ethanol-induced hepatic apoptosis (Figure 4) ▶ .
Figure 3.
Colorimetric assay of caspase-3 activity in the liver of mice treated with ethanol with or without systemic administration of caspase-3 inhibitor. Before administration of ethanol, a caspase-3 inhibitor, Z-DEVD-FMK (dissolved in 1% DMSO) was intravenously injected at a dosage of 20 μg/g body weight, and the same volume of 1% DMSO was injected as control. Ethanol induced a fivefold increase in caspase-3 activity in comparison to the control. Intravenous injection of Z-DEVD-FMK abrogated the ethanol-induced caspase-3 activation. EtOH, ethanol; DMSO, 1% DMSO; DEVD, Z-DEVD-FMK/1% DMSO. *, Significant difference between treatments and controls at P < 0.05 by Student’s t-test (n = 5).
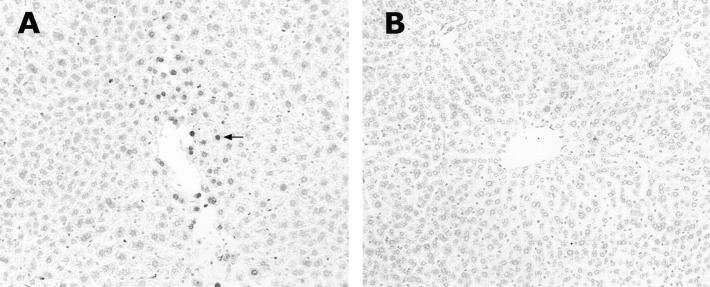
Figure 4.
Attenuation of hepatic apoptosis by systemic administration of caspase-3 inhibitor. Light microscopic TUNEL demonstrated apoptotic cells (arrow) in the liver of control mice treated with 1% DMSO and ethanol (A), but not in the liver treated with intravenous injection of caspase-3 inhibitor and ethanol (B). Original magnifications, ×130.
Elevation and Redistribution of Cytochrome c by Acute Ethanol Treatment
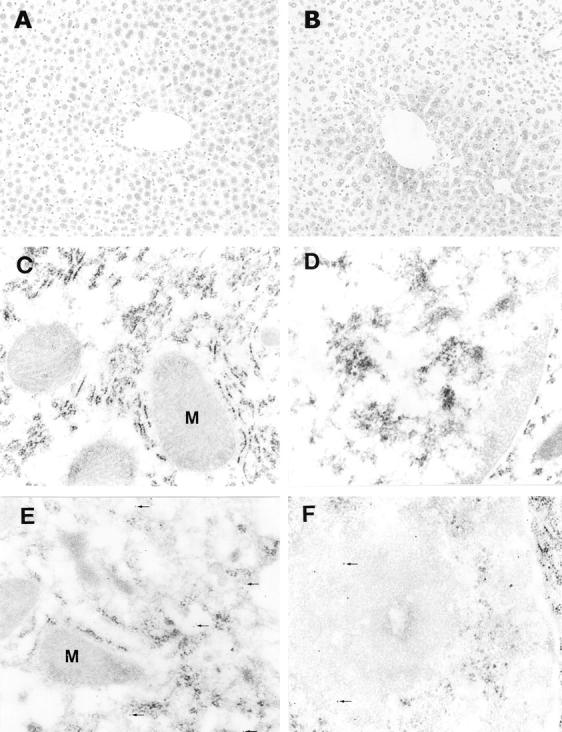
Cytochrome c is one of the upstream signals for caspase-3 activation. The effect of ethanol on hepatic cytochrome c was monitored by light and electron microscopic immunocytochemistry and Western blot. Under light microscopy, immunoperoxidase staining of cytochrome c showed moderate, uniform reactivity in the control liver (Figure 5A) ▶ and strong reactivity was found in the ethanol-treated liver (Figure 5B) ▶ . By immunogold labeling under electron microscopy, cytochrome c in the hepatocytes was found mainly in the mitochondria of hepatocytes in the control liver (Figure 5C) ▶ , whereas the cytosolic localization was prominent in the ethanol-treated liver (Figure 5D) ▶ . Using Western blot analysis, cytosolic elevation of cytochrome c was detected in the ethanol-treated liver (Figure 6) ▶
Figure 5.
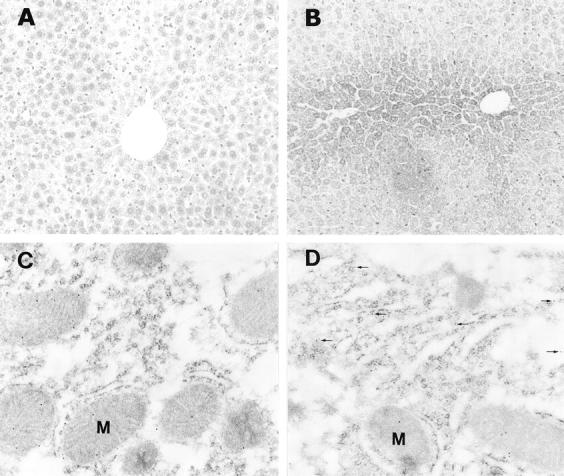
Detection of cytosolic cytochrome c elevation in the liver of ethanol-treated mice. Light microscopic immunoperoxidase showed moderate and uniform staining in the liver of control mice (A), but strong staining around central veins in the ethanol-treated liver (B). Using electron microscopic immunogold labeling, cytochrome c was found to be localized mainly in mitochondria and little in the cytosol of hepatocytes in the control liver (C), however, it was obviously apparent in the cytosol (arrows, D) in the ethanol-treated liver. M, mitochondria. Original magnifications: ×130 (A and B); ×43,000 (C and D).
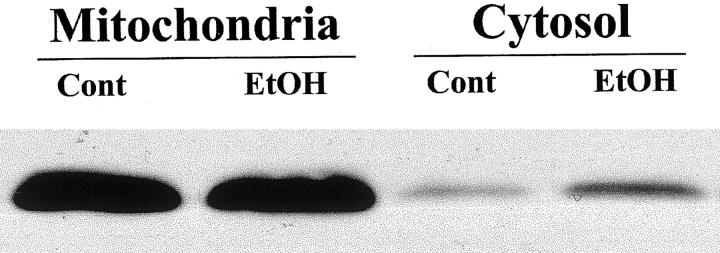
Figure 6.
Western blot analysis of cytochrome c elevation in the liver of ethanol-treated mice. Densitometric analysis indicated that a threefold elevation of the cytosolic cytochrome c was induced by ethanol. However, there was no detectable decrease in the mitochondrial cytochrome c content in ethanol-treated liver.
Neutralization of Fas L Inhibits Ethanol-Induced Caspase-3 Activation and Apoptosis
To demonstrate whether the Fas/Fas L system, another upstream signal for caspase-3 activation, is responsible for ethanol-elevated caspase-3 activity, Fas L protein expression in the liver was examined by light microscopic immunoperoxidase staining. In comparison to the weak staining in the liver of control mice (Figure 7A) ▶ , strong Fas L immunoreactivity was observed after acute ethanol administration (Figure 7B) ▶ . A neutralizing Fas L monoclonal antibody was applied to confirm the cause-and-effect relationship between the Fas/Fas L system and casapse-3 activation-mediated apoptosis. Neutralization of Fas L not only suppressed caspase-3 activation (Figure 8) ▶ , but also attenuated ethanol-induced apoptosis (Figure 9) ▶ . Western blotting analysis was then performed to clarify the possible linkage between the Fas/Fas L system and mitochondrial cytochrome c release. The result showed that neutralization of Fas L had no effect on ethanol-induced mitochondrial cytochrome c release (Figure 10) ▶ .
Figure 7.
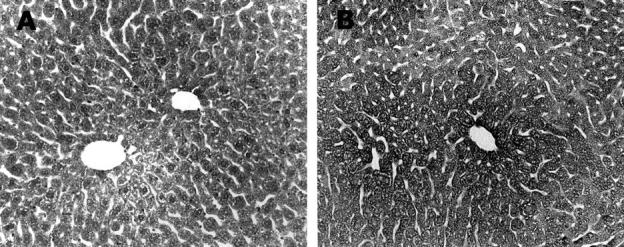
Detection of Fas ligand expression in the liver of ethanol-treated mouse. Light microscopic immunoperoxidase staining demonstrated a weak reactivity in the normal liver (A). Ethanol treatment markedly increased the Fas ligand reactivity in the liver (B). Original magnifications, ×130.
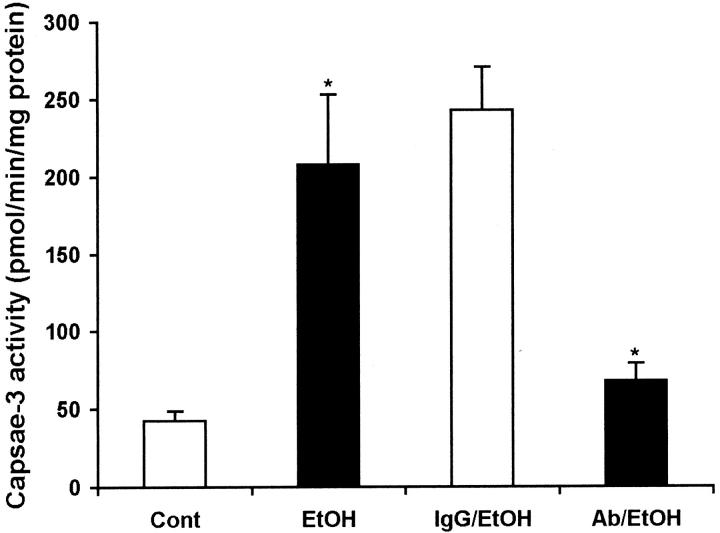
Figure 8.
Suppression of caspase-3 activity by systemic administration of a neutralizing Fas ligand antibody. Before administration of ethanol, the neutralizing Fas ligand monoclonal antibody was intravenously injected at a dosage of 5 mg/kg body weight, and normal hamster IgG was injected as control. High levels of caspase-3 activity in the ethanol-treated mouse liver with or without hamster IgG was observed. Intravenous injection of Fas ligand antibody significantly inhibited the caspase-3 activity. *, Significant difference between the treatments and controls at P < 0.05 by Student’s t-test (n = 5).
Figure 9.
Attenuation of hepatic apoptosis by systemic administration of a neutralizing Fas ligand antibody. Light microscopic TUNEL demonstrated apoptotic cells (arrow) in the liver of control mouse treated with normal hamster IgG and ethanol (A), but not in the mice treated with Fas ligand antibody and ethanol (B). Original magnifications, ×130.
Figure 10.
Western blot analysis of cytochrome c elevation after systemic administration of a neutralizing Fas ligand antibody. Ethanol induced cytosolic elevation of cytochrome c in the livers of mice pretreated with both normal hamster IgG and Fas ligand antibody. Densitometric analysis showed no detectable differences in either mitochondrial or cytosolic cytochrome c contents between the two groups. Cont, normal hamster IgG; EtOH, ethanol; Ab, Fas ligand antibody.
Discussion
The results obtained from this study demonstrate that acute ethanol administration causes hepatic apoptosis through the caspase-3 activation pathway. The cause-and-effect relationship between caspase-3 activation and apoptosis was demonstrated by the use of the caspase-3 inhibitor, Ac-DEVD-FMK. This inhibitor suppressed ethanol-mediated caspase-3 activation and attenuated ethanol-induced apoptosis, but did not affect the increase in cytosolic cytochrome c concentrations. Caspase-3 activation is correlated with both mitochondrial cytochrome c release and Fas L expression, up-stream signals for caspase-3 activation. However, the Fas/Fas L system likely plays a more important role than mitochondrial cytochrome c release because neutralizing Fas L attenuated caspase-3 activation and apoptosis, but did not inhibit mitochondrial cytochrome c release. This is thus the first study to demonstrate that ethanol-induced hepatic apoptosis involves activation of caspase-3. This activation is mainly mediated by the Fas/Fas L system although mitochondrial cytochrome c release may also play a role.
In the present study, we observed that the most TUNEL-positive cells are located around the central vein. However, only a few positive cells showed nuclear condensation, a characteristic morphological change of apoptosis. The TUNEL-positive nuclei with condensation appearance are considered to be the late stage of apoptosis. The condensed nuclei are referred to as apoptotic bodies by conventional light or electron microscopy in human alcoholic liver disease and chronic alcohol-feeding animal models. 8,9,12-14,16 The TUNEL-positive nuclei without condensation should be the early stage of apoptosis. In human alcoholic liver disease, the number of apoptotic cells detected by TUNEL assay were much more than the number of hepatocytes containing apoptotic bodies. 16 The TUNEL assay identifies DNA strand breaks that are seen in apoptosis. However, DNA strand breaks may also occur late in the terminal evolution of cell necrosis. The complexity of measuring apoptosis thus involves the difficulty of distinguishing apoptosis from necrosis. A fundamental difference between the two mechanisms of cell death is the morphological alteration of the cell. The nuclear modification of the apoptotic cells is accompanied by a preservation of the cytoplasmic structures of the cell. In contrast, immediate loss of membrane integrity occurs in the necrotic cells. This distinction thus far has made the electron microscopic evaluation of morphological changes a most reliable tool for determination of apoptosis. We thus used immunogold electron microscopy to examine hepatocyte apoptosis. In addition to the ultrastructural examination, the immunogold labeling of apoptotic nuclei in hepatocytes provided further evidence of ethanol-induced apoptosis in hepatocytes. Thus both conventional TUNEL and the immunogold electron microscopy demonstrated that acute ethanol administration indeed induced apoptosis in the liver.
Caspase-3 activation in the ethanol-treated liver occurred mostly in the hepatocytes and the immunoperoxidase staining intensity is higher in the cells around the central vein, which closely correlates with the location of apoptotic hepatocytes. Immunogold labeling further detected active caspase-3 in ethanol-treated hepatocytes. Colorimetric enzymatic assay showed a fivefold increase in the caspase-3 activity in the ethanol-treated liver. Activation of caspase-3 by ethanol has been reported previously. Chronic ethanol feeding caused a 37.6-fold enhancement in caspase-3 activity with a 7.9-fold increase in apoptosis in the buccal epithelial cells. 34 In the liver of chronic ethanol-treated rats, caspase-3 activity was significantly increased in three types of isolated hepatic cells, Kupffer cells, hepatocytes, and sinusoidal endothelial cells. 35 A novel aspect of the present study, however, is that the systemic administration of caspase-3 inhibitor dramatically blocked ethanol-induced caspase-3 activity as well as the DNA fragmentation, indicating caspase-3 is the key mediator of ethanol-induced hepatic apoptosis. Immunogold labeling and electron microscopic examination is a unique method to monitor subcellular localization of active caspase-3. It has been shown by immunogold labeling that procaspase-3 is located in the cytosol. 36 In the present study, we detected that active caspase-3 was distributed not only in cytosol but also in the nucleus of ethanol-treated hepatocytes. The nucleus localization of active caspase-3 identified by the immunogold electron microscopy was further confirmed by enzymatic assay using the nucleus fraction. The nuclear distribution of active caspase-3 provides direct evidence that caspase-3 locally cleaves nuclear substrates such as poly(ADP-ribose) polymerase.
The mitochondrial cytochrome c release-mediated caspase-3 activation pathway has been found in various cells in response to different death stimuli. 23 There is also evidence that cytochrome c is dispensable for caspase-3 activation and apoptosis. 37 In the present study, ethanol-induced cytosolic elevation of cytochrome c was detected by immunoperoxidase staining and immunogold labeling. Cytosolic elevation of cytochrome c has been shown to be accompanied with a decrease in mitochondrial cytochrome c concentrations. 23 However, both immunogold electron microscopic and Western blot analyses demonstrated that the elevation of cytosolic cytochrome c was not accompanied with an apparent decrease in the mitochondrial pool. Immunoperoxidase staining also showed stronger staining intensity in the ethanol-treated liver, therefore, indicating that an increase in the total amount of cytochrome c might occur in the ethanol-treated liver. It is thus possible that de novo synthesis of cytochrome c may take place under the treatment of ethanol in the liver.
Growing evidence suggests that Fas/Fas L system is one of most important signaling pathways that mediates caspase-3 activation and apoptosis in liver. Experimental activation of Fas by intraperitoneal injection of a Fas antibody to mice has led to liver failure and animal death as a result of massive hepatocyte apoptosis. 38,39 Studies with primary hepatocyte culture also showed that triggering the Fas/Fas L system induces hepatocyte apoptosis through processing and activation of caspase-3, -7, and -9. 40,41 Intravenous administration of caspase inhibitor, benzyloxycarbonyl-Val-Ala-Asp, blocked Fas antibody-induced caspase-3 and -7 activation and hepatic apoptosis in the mouse liver. 26 In the chronic ethanol-feeding animal model and human alcoholic liver disease, elevated expression of Fas and Fas L mRNA were detected in association with increased caspase-3 activity. 24,25 To elucidate the cause-and-effect relationship between Fas/Fas L system and caspase-3 activation and apoptosis, a neutralizing Fas L antibody was used to block the Fas/Fas L system. This method has been shown to attenuate apoptosis in vivo in some tissues. 30,31 The neutralization of Fas L in this study resulted in a significant inhibition of caspase-3 activation and attenuation of hepatic apoptosis. This result indicates that the Fas/Fas L system may act as a key signal in the ethanol-induced hepatic apoptosis. Fas/Fas L system-induced caspase-3 activation has been shown to be mediated by caspase-8. 41 Because caspase-8 can also cause mitochondrial cytochrome c release, 42 blocking the Fas/Fas L system might reduce the cytosolic elevation of cytochrome c. However, Western blot analysis in the present study demonstrated that mitochondrial cytochrome c release is independent of the Fas/Fas L system. In the presence of mitochondrial cytochrome c release, however, the hepatic apoptosis was attenuated by blocking the Fas/Fas L system. Therefore, mitochondrial cytochrome c release may not be an important pathway in the acute ethanol-induced hepatic apoptosis.
In conclusion, the data presented in this study indicate that the Fas/Fas L system-mediated caspase-3 activation signaling pathway plays a central role in ethanol-induced hepatic apoptosis. New therapeutic approaches may be expected based on the interference with Fas/Fas L or inhibition of caspase-3 activation.
Footnotes
Address reprint requests to Dr. Y. James Kang, University of Louisville School of Medicine, Department of Medicine, 511 South Floyd St., MDR 530, Louisville, KY 40202. E-mail: yjkang01@athena.louisville.edu.
Supported in part by the University of Louisville Hospital and the Jewish Hospital Foundation, Louisville, Kentucky.
References
- 1.Nanji AA: Apoptosis and alcoholic liver disease. Semin Liver Dis 1998, 18:187-190 [DOI] [PubMed] [Google Scholar]
- 2.Slomiany BL, Piotrowski J, Piotrowski E, Slomiany A: Induction of buccal mucosal apoptosis with chronic alcohol ingestion. Biochem Mol Biol Int 1998, 44:381-389 [DOI] [PubMed] [Google Scholar]
- 3.Slomiany BL, Piotrowski J, Slomiany A: Chronic alcohol ingestion enhances tumor necrosis factor-alpha expression and salivary gland apoptosis. Alcohol Clin Exp Res 1997, 21:1530-1533 [PubMed] [Google Scholar]
- 4.Piotrowski J, Piotrowski E, Slomiany A, Slomiany BL: Gastric mucosal apoptosis induced by ethanol: effect of antiulcer agents. Biochem Mol Biol Int 1997, 42:247-254 [DOI] [PubMed] [Google Scholar]
- 5.Zhang FX, Rubin R, Rooney TA: Ethanol induces apoptosis in cerebellar granule neurons by inhibiting insulin-like growth factor 1 signaling. J Neurochem 1998, 71:196-204 [DOI] [PubMed] [Google Scholar]
- 6.Wang J-F, Spitzer JJ: Alcohol-induced thymocyte apoptosis is accompanied by impaired mitochondrial function. Alcohol 1997, 14:99-105 [DOI] [PubMed] [Google Scholar]
- 7.Coiler SD, Wu WJ, Pruett SB: Endogenous glucocorticoids induced by a chemical stressor (ethanol) cause apoptosis in the spleen in B6C3F1 female mice. Toxicol Appl Pharmacol 1998, 148:176-182 [DOI] [PubMed] [Google Scholar]
- 8.Benedetti A, Brunelli E, Risicato R, Cilluffo T, Jezequel AM, Orlandi F: Subcellular changes and apoptosis induced by ethanol in rat liver. J Hepatol 1988, 6:137-143 [DOI] [PubMed] [Google Scholar]
- 9.Baroni GS, Marucci L, Benedetti A, Mancini R, Jezequel AM, Orlandi F: Chronic ethanol feeding increases apoptosis and cell proliferation in rat liver. J Hepatol 1994, 20:508-513 [DOI] [PubMed] [Google Scholar]
- 10.Yacoub LK, Fogt F, Griniuviene B, Nanji AA: Apoptosis and BcL-2 protein expression in experimental alcoholic liver disease in the rat. Alcohol Clin Exp Res 1995, 19:854-859 [DOI] [PubMed] [Google Scholar]
- 11.Kurose I, Higuchi H, Miura S, Saito H, Watanabe N, Hokari R, Hirokawa M, Takaishi M, Zeki S, Nakamura T, Ebimura H, Kato S, Ishii H: Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology 1997, 25:368-378 [DOI] [PubMed] [Google Scholar]
- 12.Sloamiany A, Piotrowski E, Grabska M, Piotrowski J, Sloamiany BL: Chronic ethanol-initiated apoptosis in hepatocytes is induced by changes in membrane biogenesis and intracellular transport. Alcohol Clin Exp Res 1999, 23:334-343 [PubMed] [Google Scholar]
- 13.Mi L-J, Mak KM, Lieber CS: Attention of alcohol-induced apoptosis of hepatocytes in rat livers by polyenylphosphatidylcholine (PPC). Alcohol Clin Exp Res 2000, 24:207-212 [PubMed] [Google Scholar]
- 14.Goldin RD, Hunt NC, Clark J, Wickramasinghe SN: Apoptotic bodies in a murine model of alcoholic liver disease: reversibility of ethanol-induced changes. J Pathol 1993, 171:73-76 [DOI] [PubMed] [Google Scholar]
- 15.Halsted C, Vilanueva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvili L, James SJ, Poirier L: Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology 1996, 23:497-505 [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z, Liu Y, Smith LO, Bonkovsky H, Baker S: Frequency and distribution of DNA fragmentation as a marker of cell death in chronic liver diseases. Virchows Arch 1997, 431:189-194 [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Laissue JA, Zimmermann A: TUNEL-positive hepatocytes in alcoholic liver disease. A retrospective biopsy study using DNA nick end-labeling. Virchows Arch 1997, 431:337-344 [DOI] [PubMed] [Google Scholar]
- 18.Cohen GM: Caspases: the executioners of apoptosis. Biochem J 1997, 326:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faleiro L, Kobayashi R, Fearnhead H, Lazebnik Y: Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J 1997, 16:2271-2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S: The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol 1997, 29:393-396 [DOI] [PubMed] [Google Scholar]
- 21.Porter AG, Janicke RU: Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999, 6:99-104 [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Yang J, Jones DP: Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1998, 1366:139-149 [DOI] [PubMed] [Google Scholar]
- 24.Deaciuc IV, Fortunato F, D’Souza NB, Hill DB, Schmidt J, Lee EY, McClain CJ: Modulation of caspase-3 activity and Fas ligand mRNA expression in rat liver cells in vivo by alcohol and lipopolysaccharide. Alcohol Clin Exp Res 1999, 23:349-356 [PubMed] [Google Scholar]
- 25.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L: Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med 1995, 182:1223-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandler JM, Cohen GM, MacFarlane M: Different subcellular distribution of caspase-3 and caspase-7 following Fas-induced apoptosis in mouse liver. J Biol Chem 1998, 273:10815-10818 [DOI] [PubMed] [Google Scholar]
- 27.Yaoita H, Ogawa K, Maehara K, Maruyama Y: Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 1998, 97:276-281 [DOI] [PubMed] [Google Scholar]
- 28.Farber A, Connors JP, Friedlander RM, Wager RJ, Powell RJ, Cronenwett JL: A specific inhibitor of apoptosis decreases tissue injury after intestinal ischemia-reperfusion in mice. J Vasc Surg 1999, 30:752-760 [DOI] [PubMed] [Google Scholar]
- 29.Cursio R, Gugenheim J, Ricci JH, Crenesse D, Rostagno P, Maulon L, Saint-Paul M, Fernard B, Auberger P: A caspase inhibitor protects rats lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J 1999, 13:253-261 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Ueda Y, Juan Y, Katsuda S, Takahashi H, Koh E: Fas-mediated apoptosis in adriamycin-induced cardiomyopathy in rats: in vivo study. Circulation 2000, 102:572-578 [DOI] [PubMed] [Google Scholar]
- 31.Suarez-Pinzon WL, Power RF, Rabinovitch A: Fas ligand-mediated mechanisms are involved in autoimmune destruction of islet beta cells in non-obese diabetic mice. Diabetologia 2000, 43:1149-1156 [DOI] [PubMed] [Google Scholar]
- 32.Carson EJ, Prutt SB: Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res 1996, 20:132-138 [DOI] [PubMed] [Google Scholar]
- 33.Buckley AR, Crowe PD, Russell DH: Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci USA 1988, 85:8649-8653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slomiany BL, Piotrowski J, Piotrowski E, Slomiany A: Activation of apoptotic caspase-3 and nitric oxide synthase-2 in buccal mucosa with chronic alcohol ingestion. Biochem Mol Biol Int 1998, 45:1199-1209 [DOI] [PubMed] [Google Scholar]
- 35.Deuciuc IV, Fortunato F, D’Souza NB, Hill DB, Schmidt J, Lee EY, McClain CJ: Modulation of caspase-3 activity and Fas ligand mRNA expression in rat liver cells in vivo by alcohol and lipopolysaccharide. Alcohol Clin Exp Res 1999, 23:349-356 [PubMed] [Google Scholar]
- 36.Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC: Immunohistochemical analysis of in vivo pattern of expression of CPP32 (caspase-3), a cell death protease. Cancer Res 1997, 57:1605-1613 [PubMed] [Google Scholar]
- 37.Tang DG, Li L, Zhu Z, Joshi B: Apoptosis in the absence of cytochrome c accumulation in the cytosol. Biochem Biophys Res Commun 1998, 242:380-384 [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugal T, Kitamura Y, Itoh N: Lethal effect of anti-Fas antibody in mice. Nature 1993, 364:806-809 [DOI] [PubMed] [Google Scholar]
- 39.Leist M, Gantner F, Kunstle G, Bohlinger I, Tiegs G, Bluethmann H, Wendel A: The 55-kD tumor necrosis factor receptor and CD95 independently signal murine hepatocyte apoptosis and subsequently liver failure. Mol Med 1996, 2:109-124 [PMC free article] [PubMed] [Google Scholar]
- 40.Jones RA, Johnson VL, Buck JN, Dobrota M, Hinton RH, Chow SC, Kass GN: Fas-mediated apoptosis in mouse hepatocytes involves the processing and activation of caspases. Hepatology 1998, 27:1632-1642 [DOI] [PubMed] [Google Scholar]
- 41.Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H: Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci 2000, 58:109-117 [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Kim CN, Yang J, Jemmerson R, Wang X: Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996, 86:147-157 [DOI] [PubMed] [Google Scholar]