Abstract
We previously showed that the expression of tenascin (TN-C), an extracellular matrix glycoprotein found in developing bone and atherosclerotic plaque, and matrix metalloproteinase-2 (MMP-2) are coordinated and interdependent in cultured vascular smooth muscle cells. In this study, we hypothesized that TN-C and MMP-2 are mechanistically involved in the pathobiology of calcific aortic stenosis. Human calcific aortic stenosis cusps demonstrated immunohistochemically prominent deposition of TN-C, MMP-2, and alkaline phosphatase activity, as well as MMP-2 gelatinolytic activity. Although far lesser amounts of TN-C were noted in several of the grossly non-calcified valve cusps, MMP-2 and AP were never detected. Further, when aortic valve interstitial cells (both sheep and human) were cultivated on collagen supplemented with TN-C, both MMP-2 mRNA expression and MMP-2 gelatinolytic activity (both pro and active forms), were up-regulated compared to control. These observations support the view that accumulation of first TN-C and then MMP-2 are associated with progression of calcification. The residual presence of these proteins in severe calcifications is indicative of their involvement in the pathogenesis.
The mechanism of calcific aortic stenosis has been investigated in clinical-pathological studies, that have demonstrated cuspal calcific deposits to be associated with mineralization of devitalized cells and subcellular vesicles, 1,2 as well as the deposition of extracellular matrix (ECM) proteins commonly present in bone. 3 A number of ECM proteins normally found in bone, including osteocalcin, osteopontin, osteonectin, matrix Gla protein, bone morphogenetic protein, matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9, are present in cardiovascular calcifications, including calcified valves, 3-9 but in general are not found in normal cardiovascular tissue. Bone morphogenetic protein-2 was also found in a cultured calcifying subpopulation of bovine aortic smooth muscle cells, 7 and calcifying aortic valve interstitial cells. 10 Tenascin-C (TN-C) is an ECM glycoprotein that is up-regulated during both development and pathological tissue remodeling. 11 Compelling evidence indicates that TN-C actively participates in normal mineralization. 12 Furthermore, developmental co-localization studies showed that TN-C is transiently expressed in heart valves. 13 Recently, TN-C was found to be present in macrophage-rich human coronary atherosclerotic plaque. 14 The role of TN-C in valvular calcification, however, has not been established.
TN-C is often co-expressed with MMPs, a family of zinc- and calcium-dependent extracellular matrix-degrading enzymes, in a variety of tissues. 15-17 In addition, it has been demonstrated, in vascular smooth muscle cells, that degradation of type I collagen by MMPs promotes TN-C expression at the transcriptional level. 18 Conversely, TN-C also up-regulates the expression of MMPs. 19 These findings are noteworthy because MMP-2 and MMP-9 expression/activity are associated with calcification of glutaraldehyde fixed heterograft bioprosthetic heart valves. 15 Recently, MMP-1, MMP-2, and MMP-9 were demonstrated to be present in calcific aortic stenosis cusps. 20
Our hypothesis focused on the relationship between TN-C and MMP-2. Based on the background just cited, we hypothesized that the presence of TN-C in aging or diseased valves was likely an early event in cuspal extracellular matrix degeneration, which is associated with up-regulation of MMP-2 in the progression of calcification. To examine this, we evaluated the presence and localization of TN-C and MMP-2 in human aortic valves with immunohistochemistry. Alkaline phosphatase (AP), well established as a crucial enzyme for bone formation and bone cell differentiation, was also studied. MMP zymography studies were carried out on fresh cusp retrievals obtained at the time of surgery for calcific aortic stenosis. We also studied TN-C effects on MMP-2 mRNA expression and gelatinolytic activity in primary cultures of sheep aortic valve interstitial cells (AVICs), and cells derived from aortic valve cusps from a case of human congenital aortic stenosis.
Materials and Methods
Retrieved Aortic Valve Tissues
Aortic valve specimens were obtained from patients undergoing aortic valve replacement for calcific aortic stenosis, and from cadaver hearts at autopsy. After collection, the tissue was rinsed with sterile saline, fixed in 10% neutral buffered formalin and subsequently embedded in paraffin. Heavily calcified clinical retrievals were decalcified in 10% hydrochloric acid before embedding.
Cell Culture
Normal mature, female sheep (Western Cross from Thomas Morris, Reisterstown, MD) hearts were removed, the aortic valve leaflets were dissected, and the endothelial cell layer removed. The remaining interstitial layers were minced and digested with 0.2% collagenase I (Sigma, St. Louis, MO) in M199 medium (Life Technologies, Inc., Gaithersburg, MD) containing 0.1% BSA at 37°C for 2 hours. Cells were then seeded and maintained on culture dishes with M199 medium containing 10% fetal calf serum (HyClone, Logan, UT), supplemented with penicillin and streptomycin (Life Technologies). Cells used in the experiments were between passages 3 and 10.
A human aortic valve specimen (congenital aortic stenosis and ventricular septal defect) was obtained from a 2-year-old female patient who underwent heart transplantation (specimen retrieval was approved by the I. R. B. of the Children’s Hospital of Philadelphia). The cusps from this specimen were used for tissue culture as described above.
Collagen gels were prepared as previously described. 21 To determine the effect of TN-C on MMP-2 expression, neutralized bovine dermal type I collagen (Cohesion Technologies, Inc., Palo Alto, CA) provided a model physiological microenvironment, and was supplemented with 15 μg/ml of human TN-C (Life Technologies) that was isolated and purified from the human Glioma cell line U-251MG. 22
Immunohistochemical Studies and Calcium Detection
Fixed tissues were embedded in paraffin and cut as 6-μm-thick sections. After deparaffinization and dehydration, immunohistochemistry was performed as previously described. 23 Mouse monoclonal antibodies against human TN-C (Life Technologies) and MMP-2 (Oncogene Research Products, Cambridge, MA) were used. After biotin-labeled secondary antibody incubation and peroxidase labeling, immunoreactive sites were visualized with diaminobenzidine, resulting in brown staining of immunopositive regions (Vector Laboratories, Burlingame, CA). As a control, a nonspecific mouse IgG (DAKO, Glostrup, Denmark) was substituted for the primary antibody. Alkaline phosphatase activity was detected as described. 24 X-phosphate and nitroblue tetrazolium solution were obtained from Boeheringer Mannheim. Calcium deposits were stained using Alizarin Red S (Sigma) as described previously. 10
Competitive Reverse-Transcription Polymerase Chain Reaction of MMP-2 Transcripts
MMP-2 expression in response to TN-C in cell culture was measured quantitatively by competitive reverse-transcription polymerase chain reaction (RT-PCR) as described. 25 Briefly, total RNAs were isolated, using Trizol (Life Technologies), from both sheep and human aortic valve interstitial cells cultivated on bovine type I collagen with or without supplemented TN-C for 48 hours in M199 medium with 0.5% fetal calf serum. The total RNAs were reverse transcripted into cDNA (RT products) with oligo dT primer. 25 The MMP-2 gene competitor (MMP-2M13) for the competitive RT-PCR was generated by PCR using pBluescript II SK (Stratagene, La Jolla, CA) as a template. The primers for generating MMP-2M13 competitor are: MMP2M13F: 5′ATG GCA TCG CTC AGA TCC GTG CTA AAA CGA CGG CCA GTG 3′ and MMP2M13R: 5′AGC TCA GCA GCC TAG CCA GTC GGT GCA GGA AAC AGC TAT GAC CAT G 3′. In addition, the primers for MMP-2 competitive RT-PCR are: MMP2F: 5′ATG GCA TCG CTC AGA TCC GTG 3′, and MMP2R: 5′AGC TCA GCA GCC TAG CCA GTC GG 3′. MMP2F and MMP2R are sequence-compatible for use with both the human and sheep MMP-2 genes. For MMP-2 competitive RT-PCR, both RT products and the primers for MMP2F and MMP2R were used, along with a series of dilutions of the MMP2M13 competitor added in different tubes. The PCR was performed for 45 cycles with 94° C, 55° C, and 72° C, for 40 seconds each. The PCR products were loaded onto a 3% agarose gel and electrophoresis was performed at 120 V for 2 hours. The intensities of the bands were analyzed using the Gel Doc System (Bio-Rad, Hercules, California), and the competitive RT-PCR data were calculated using SigmaPlot5.0 software (SPSS, Inc., Chicago, IL).
Detection of MMP-2
Conditioned medium was collected 48 hours after AVICs cultivation on collagen with or without supplemented TN-C. Cells were lysed in extraction buffer consisting of 0.1 mol/L Tris-HCl, 10 mmol/L EDTA, 1% Triton X-114 (pH 8.1), and fresh complete proteinase inhibitor CPI (Boehringer Mannheim). 26 Equal amounts of extracted proteins or concentrated medium were loaded per lane on 10% polyacrylamide-sodium dodecyl sulfate precast zymogram gels containing 0.1% gelatin (Bio-Rad, Hercules, CA). After electrophoresis, gels were renatured, developed, and stained according to manufacturer’s directions. Standard protein size markers (Bio-Rad) and human MMP-2 and MMP-9 zymography standards (Chemicon International, Inc., Temecula, CA) were used to determine the approximate size of bands indicating proteolytic activity. Five aortic valve cusps obtained in a fresh state at the time of heart valve replacement for calcific aortic stenosis were also studied. Cusps were homogenized in extraction buffer as described above. The lysates were centrifuged and equal amounts of protein were assayed for gelatinolytic activity.
Statistical Methods
Cell culture studies were carried out in triplicate, and data expressed as means ± SE. Differences between means were tested with Student’s t-test. RT-PCR data were subjected to correlation analyses.
Results
TN-C, MMP-2, and AP in Aortic Valve Cusps
Six clinically normal aortic valve cusps were documented to be non-calcified, as demonstrated by overall morphology and negative Alizarin Red S staining. Faint, diffuse TN-C immunopositivity was demonstrated in four of six of these normal aortic valves (Table 1 ▶ ; Figure 1A ▶ ). However, no MMP-2 immunostaining or AP activity were detected in any of these normal autopsied valves (Table 1) ▶ .
Table 1.
Clinical Pathologic Summary of Calcification, Alkaline Phosphatase, Tenascin-C, and Matrix Metalloproteinase-2 in Human Aortic Valve Cusps
| Case no. | Age (years) | M/F | Alizarin Red S | AP | TN-C | MMP-2 | |
|---|---|---|---|---|---|---|---|
| Autopsy (normal) | 1 | 68 | M | − | − | − | − |
| 2 | 46 | F | − | − | +* | − | |
| 3 | 60 | F | − | − | +* | − | |
| 4 | 34 | F | − | − | +* | − | |
| 5 | 55 | F | − | − | − | − | |
| 6 | 52 | F | − | − | +* | − | |
| Calcific aortic stenosis† | 7 | 83 | F | + | + | + | + |
| 8 | 58 | M | + | + | + | + | |
| 9 | 87 | F | + | + | + | + | |
| 10 | 62 | M | + | + | + | + | |
| 11 | 63 | M | + | + | + | + | |
| 12 | 79 | F | + | + | + | + |
Alizarin Red S, calcification; AP, alkaline phosphate histochemistry; TN-C, tenascin-C immunohistochemistry; MMP-2, matrix metalloproteinase-2 immunohistochemistry. +, present; −, absent.
*Faint, widespread, non-localized TN-C positivity without calcification.
†Intense TN-C and intense MMP-2 staining in the extracellular matrix surrounding the heavy calcific deposits.
Figure 1.
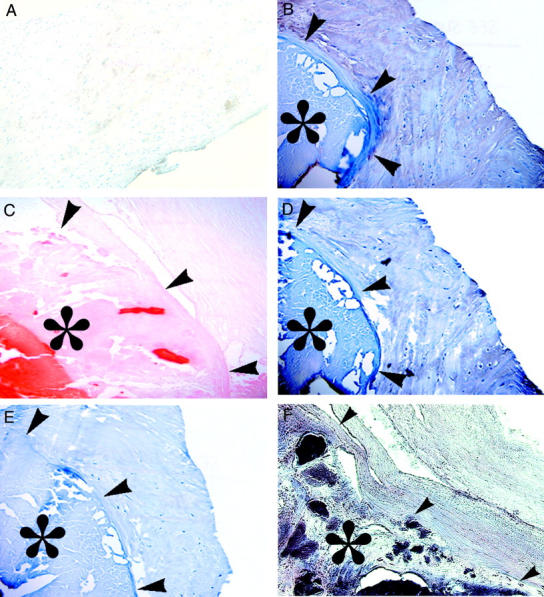
Representative photomicrographs of normal, noncalcific aortic valve cusps with faint, widespread TN-C by immunohistochemistry (brown staining) and hematoxylin counterstain (case 4 in Table 1 ▶ ) (A); Calcific aortic stenosis (* denotes calcified region, with arrows denoting the margin of the calcification) with intense TN-C immunopositive brown staining (case 10 in Table 1 ▶ ) (B); calcific aortic stenosis with Alizarin Red S (calcifications staining red, case 10 in Table 1 ▶ ) (C); calcific aortic stenosis with intense MMP-2 immunopositive staining (case 10 in Table 1 ▶ ) (D); IgG negative control for b and d shows a absence of peroxidase reactivity (case 10 in Table 1 ▶ ) (E); calcific aortic stenosis with intense alkaline phosphatase activity (purple stained region) localized within the calcific deposits (F), but not in the non-calcified extracellular matrix (case 7 in Table 1 ▶ ). Original magnification, ×200
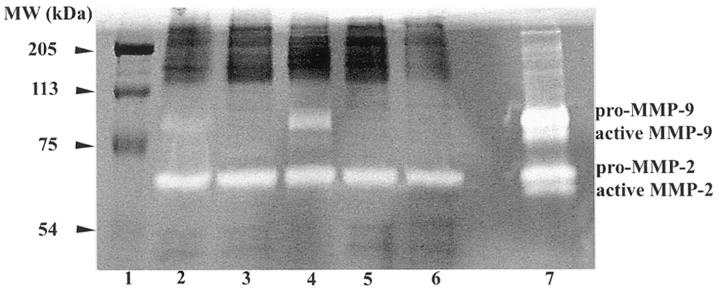
Calcific aortic stenosis cusps were characterized by dense mineral deposits, all of which were positive for AP activity. AP activity was observed to be present throughout the largely acellular calcific deposits, co-localizing with Alizarin Red S staining (Figures 1, F and C) ▶ . AP was not detectable in non-calcified regions of calcific aortic stenosis cusps, and was never detected in autopsied normal cusps (Table 1) ▶ . Calcific aortic stenosis valve cusps demonstrated intense TN-C immunopositive staining in all cases studied. TN-C positivity was distributed around, but not within, the massive calcific deposits (Figure 1B) ▶ . Intense MMP-2 immunopositivity (Figure 1D) ▶ was also observed in these calcific aortic stenosis cusps, with a distribution that was comparable to that of TN-C (Figure 1B) ▶ . MMP-2 activity was also detected by zymography studies of homogenates from aortic cusps samples obtained in a fresh state at surgery from 5 individual cases of calcific aortic stenosis (Figure 2) ▶ . However, only the pro form of MMP-2 was present, rather than the active form. Relatively smaller amounts of MMP-9 were also identified in three of these samples (Figure 2) ▶ .
Figure 2.
MMP-2 activity was present in individual extracts of five explanted aortic valve cusps obtained at surgery for calcific aortic stenosis. Gel zymography shows MMP-2 (pro form only) in all cases (lanes 2–6), and qualitatively lesser amounts MMP-9 in cases 1, 2, and 3 (lanes 2-4) as compared to MMP standards (lane 7). Lane 1 contains molecular weight standards.
TN-C Up-Regulates MMP-2 Gene Expression in Cultured Valvular Interstitial Cells
Experiments were carried out to examine the hypothesis that MMP-2 up-regulation in aortic valve interstitial cells was driven by the increased presence of TN-C in the cuspal extracellular matrix, comparable to MMP-2 up-regulation in arterial smooth muscle cells grown on a substrate of TN-C. To evaluate this hypothesis, sheep and human AVICs were separately cultivated on a substrate of type I collagen containing 15 μg/ml TN-C for 48 hours. The expression and activity of MMP-2 was evaluated using both competitive RT-PCR and gel zymography. Both sheep and human AVICs grown on collagen plus TN-C demonstrated an approximately sevenfold (sheep) to eightfold (human) increase in MMP-2 mRNA expression versus the collagen control (Figure 3A) ▶ . MMP-2 gelatinolytic activity was relatively greater in sheep AVICs cultivated on collagen with TN-C, compared to cultures on collagen only. MMP-2 is the major band identified in both conditioned medium and in cell lysate (Figure 3B) ▶ . Both pro-MMP-2 and the active form of MMP-2 were clearly demonstrated to be present in greater amounts in lysates of cells grown on collagen containing TN-C compared to control (Figure 3B) ▶ . However, no MMP-9 activity was observed in these cell culture studies. No obvious differences in MMP-2 activity were observed in conditioned medium, in comparisons of cells cultivated on collagen with or without TN-C supplementation. Also, in conditioned medium, MMP-2 was mainly in its pro form. Thus, in these cell culture studies, although basal MMP-2 activity was present, TN-C up-regulated MMP-2 and gave rise to an increased, cell-associated pro-form gelatinolytic activity, which may explain in part the association of TN-C and MMP-2 in calcific aortic stenosis.
Figure 3.
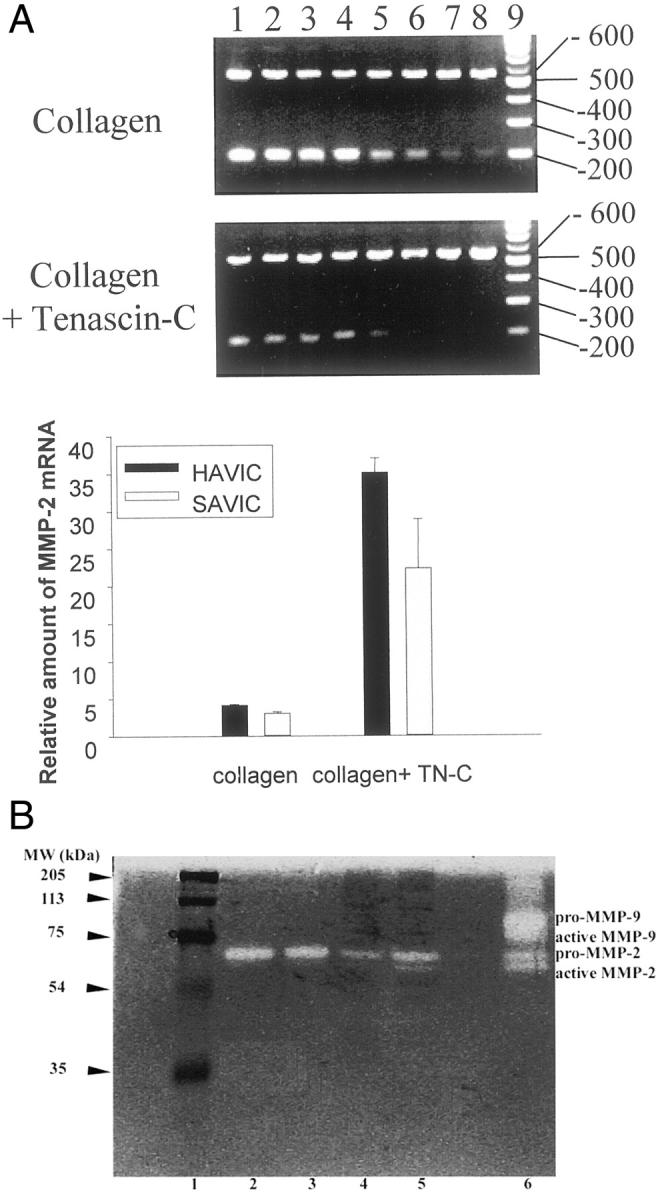
The results of aortic valve interstitial cell culture studies in which expression of matrix metalloproteinase-2 (MMP-2) is up-regulated by tenascin-C (TN-C). A: relative amounts of mRNA in sheep (SAVIC) and human aortic valve interstitial cells (HAVIC), evaluated by competitive RT-PCR. Cells were grown on a substrate of either bovine type I collagen or collagen with TN-C (15 μg/ml). A representative agarose gel (see Materials and Methods) is shown for SAVIC demonstrating greater amounts of MMP-2 RNA in the TN-C cultures. The top bands are derived from MMP-2 reverse transcriptase (RT) products, while the bottom bands are due to the MMP-2M13 competitors. The lanes from left to right represent PCRs with a series of dilutions of the MMP-2M13 competitor. The reduction in MMP-2M13 band intensity (bottom bands) occurs in conjunction with an increase in the upper bands (MMP-2 RT). The last lane is a 100 bp DNA ladder. Bars represent the mean ± SE of 3 independent measurements. TN-C significantly increased MMP-2 expression (P < 0.05). B: gel zymograms, demonstrating the results of SAVIC cultures, with greater MMP-2 activity, both pro-, and active-form, in the TN-C cell culture lysates (lane 5) compared to control (lane 4). MMP-2 levels in the conditioned media (detected only as the pro form) were comparable in the TN-C group (lane 3) versus control group (lane 2). No MMP-9 activity was detected in these cell culture studies. Lanes 1 and 6 are molecular marker and MMP gelatinase zymography standards, respectively.
Discussion
Intense immunohistochemical positivity for TN-C and MMP-2 in calcific aortic stenosis cusps was noted in all cases studied, in a distinct pattern around the calcific deposits (Figure 1, B and D) ▶ . In contrast, intense AP activity, which was only noted in calcific aortic stenosis, was observed only within calcific deposits (Figure 1F) ▶ . MMP-2 was never found in any of the valve cusps diagnosed as normal, and was never present without the co-association of TN-C. TN-C was present in several of the diagnostically normal valves, but at qualitatively low levels, in a distribution pattern that was distinctly different from observed in calcific aortic stenosis (Figure 1A) ▶ .
MMP-2/TN-C mechanisms in pathological calcification in aortic valve disease have not been investigated before this study. The role of TN-C in physiological mineralization is thought to involve cytokine activation, AP induction, and calcium binding. 27,28 This cascade of events results in new mineral formation in bone development and regeneration. Most cells do not express TN-C constitutively; however, its expression can be induced by a number of cytokines and other circumstances, including basic fibroblast growth factor, 28 transforming growth factor-β, 28 interleukin−4, 29 tumor necrosis factor-α, 29 and altered collagen structure 18 in a cell- and tissue-type-specific manner.
Our results, which demonstrate the presence of enzymatically active MMP-2, but only in the pro form, in homogenates of individual human calcific aortic stenosis cusps (Figure 2) ▶ , are of interest in view of other results in both these studies and the work of others concerning matrix metalloproteinases in cardiovascular disease. Variable amounts of MMP-9 activity were also present in several of our calcified cusp samples. However, MMP-9 was not detected in our cell culture studies (Figure 3B) ▶ , suggesting that AVICs may not be the source for MMP-9 via a TN-C association, or additional exogenous stimuli may be required for its induction. Both MMP-2 and MMP-9 were shown to be involved in the progression of atherosclerotic plaque 8 and in calcification of glutaraldehyde fixed heterograft bioprosthetic heart valves. 15 In our cell culture studies using AVICs cultured on a collagen gel in the presence of TN-C, we observed a seven- to eightfold increase in MMP-2 mRNA expression (Figure 3A) ▶ , and the gelatinolytic activity of MMP-2 was also increased (Figure 3B) ▶ , which suggests that the accumulation of TN-C around calcific regions may up-regulate the expression of MMP-2. The fact that most of the secreted MMP-2 (in conditioned medium) was in the pro form, and the active form of MMP-2 was associated with cells in cell lysates (Figure 3B) ▶ may help explain our observations in the clinical aortic valve retrievals, that most MMP-2 was in its pro form (Figure 2) ▶ . MMP-2 is secreted by cells in the latent form and must be activated by cell membrane-associated MMP, MT-1-MMP. 30 In our immunohistochemical studies, we showed that MMP-2 immunopositive staining in calcific aortic stenosis were prominently localized around the calcific deposits, regions that are also relatively hypocellular and probably lack the capacity to form MT-1-MMP. Thus the MMP-2 that we detected in the clinical aortic valve retrievals could have been secreted and laid down on the ECM many years before valve surgery and retrieval. Therefore, the presence of TN-C and MMP-2 in pathological specimens very likely reflects prior pathophysiologic events, with residual proteins at the site of disease development.
Several of the grossly normal human valve retrievals in our studies had low but definite amounts of TN-C demonstrable by immunohistochemistry, but were negative for MMP-2 (Table 1) ▶ . Based on our cell culture observations, one would hypothetically expect to find MMP-2 in association with TN-C in these cases. One explanation for these observations is that TN-C and MMP-2 typically participate in dynamic events involved in remodeling and development, and are usually transiently present. Thus, for these grossly normal valves, or for those with early microscopic calcifications, MMP-2 activity and TN-C interactions and turnover may be far more dynamic than in cell culture, and certainly more so than in acellular, diseased valve interstitium, thus explaining the absence of MMP-2 in non-diseased specimens. Only the pro form of MMP-2 was present in extracts from calcific aortic stenosis cusps. This may reflect accumulation of residual inactive MMP-2 in a dystrophic calcified region, thus representing a remnant of the pathogenesis.
Whereas TN-C and MMP-2 are both present around dense calcific deposits in calcific aortic stenosis, AP activity was noted to be present only within the actual valvular calcifications themselves. Others have shown that AP hydrolyzes phosphoesters and is thereby involved in initial events in physiological mineralization, 31,32 concerning the calcification of AP enriched, chondrocyte-derived, matrix vesicles. AP is also present during the initial events in pathological calcification of bioprosthetic heart valves. 33 AP-enriched matrix-vesicle-like structures were noted at sites of early mineralization in vascular tissue. 1 Because AP is up-regulated by TN-C, 27 we propose the following mechanism based on our results and the prior work of others: progression of aortic valve disease to calcific stenosis may occur through induction of TN-C by various factors, such as cytokines, or collagen damage, which then up-regulates the expression of MMP-2. The positive feedback by MMP-2 causes further accumulation of TN-C, leading to cellular aggregation and calcification with increased AP expression and deposition of calcium phosphates.
We conclude that the combined presence of MMP-2, TN-C, and AP in calcific aortic stenosis is a distinct characteristic of severe disease progression that has the following implications: 1) The association of TN-C, MMP-2, and AP in calcific aortic stenosis, with MMP-2 present in the pro form only, most likely represents residual extracellular matrix deposition that reflects either events involved in the progression of calcification or unsuccessful attempts at a healing response. 2) This view is further supported by our cell culture studies demonstrating both the active form of MMP-2 and pro form are up-regulated by TN-C in heart valve interstitial cells, demonstrating the dynamic effects of the TN-C/MMP-2 mechanism, not noted in the pathology specimens. 3) These results suggest a therapeutic strategy based on a TN-C/MMP-2 mechanism.
Acknowledgments
We thank Dr. Francis Gannon for providing human aortic valve specimens, Ms. Jeanne Connolly for both her scientific review of the manuscript and technical advice, and Ms. Pamela Lumpkin and Ms. Jennifer LeBold for their assistance in preparing the manuscript.
Footnotes
Address reprint requests to Robert J. Levy, MD, Children’s Hospital of Philadelphia, Abramson Research Building, 3416 Civic Center Boulevard, Philadelphia, PA 19104-4318; E-mail: levyr@email.chop.edu.
Supported in part by grants from the National Heart, Lung, and Blood Institute, RO1 HL38118, KO8 HL03974, T32 HL07915, and the William J. Rashkind Endowment of the Children’s Hospital of Philadelphia.
References
- 1.Kim KM: Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc 1976, 35:156-162 [PubMed] [Google Scholar]
- 2.Kim KM: Apoptosis and calcification. Scanning Microsc 1995, 9:1137-1178 [PubMed] [Google Scholar]
- 3.Srivatsa SS, Harrity PJ, Maercklein PB, Kleppe L, Veinot J, Edwards WD, Johnson CM, Fitzpatrick LA: Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J Clin Invest 1997, 99:996-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bini A, Mann KG, Kudryk BJ, Schoen FJ: Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 1999, 19:1852-1861 [DOI] [PubMed] [Google Scholar]
- 5.Mori K, Shioi A, Jono S, Nishizawa Y, Morii H: Expression of matrix Gla protein (MGP) in an in vitro model of vascular calcification. FEBS Lett 1998, 433:19-22 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM: Osteopontin is expressed in human aortic valvular lesions. Circulation 1995, 92:2163-2168 [DOI] [PubMed] [Google Scholar]
- 7.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL: Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 1993, 91:1800-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftus IM, Naylor AR, Goodall S, Crowther M, Jones L, Bell PR, Thompson MM: Increased matrix metalloproteinase-9 activity in unstable carotid plaques: a potential role in acute plaque disruption. Stroke 2000, 31:40-47 [DOI] [PubMed] [Google Scholar]
- 9.Mohler ER, III, Adam LP, McClelland P, Graham L, Hathaway DR: Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol 1997, 17:547-552 [DOI] [PubMed] [Google Scholar]
- 10.Mohler ER, III, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH: Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 1999, 8:254-260 [PubMed] [Google Scholar]
- 11.Mackie E: Molecules in focus: tenascin-C. Int J Biochem Cell Biol 1997, 29:1133-1137 [DOI] [PubMed] [Google Scholar]
- 12.Mackie E, Murphy L: The role of tenascin-C and related glycoproteins in early chondrogenesis. Microsc Res Tech 1998, 43:102-110 [DOI] [PubMed] [Google Scholar]
- 13.Hurle JM, Garcia-Martinez V, Ros MA: Immunofluorescent localization of tenascin during the morphogenesis of the outflow tract of the chick embryo heart. Anat Embryol 1990, 181:149-155 [DOI] [PubMed] [Google Scholar]
- 14.Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S, Sharifi BG: Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation 1999, 99:1284-1289 [DOI] [PubMed] [Google Scholar]
- 15.Simionescu D, Simionescu A, Deac R: Detection of remnant proteolytic activities in unimplanted glutaraldehyde treated bovine pericardium and explanted bioprotheses. J Biomed Mater Res 1993, 27:821-829 [DOI] [PubMed] [Google Scholar]
- 16.Talhouk R, Chin JR, Unemori EN, Werb Z, Bissell MJ: Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development 1991, 112:439-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowan K, Jones P, Rabinovitch M: Matrix metalloproteinase activity coincides with deposition of tenascin rich foci, colocalizing with proliferation in porcine pulmonary artery organ culture. Circulation 1997, (Suppl I):243 [Google Scholar]
- 18.Jones PL, Jones FS, Zhou B, Rabinovitch M: Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a β3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. J Cell Sci 1999, 112:435-445 [DOI] [PubMed] [Google Scholar]
- 19.Tremble P, Chiquet-Ehrismann R, Werb Z: The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol Biol Cell 1994, 5:439-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edep ME, Shirani J, Wolf P, Brown DL: Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol 2000, 9:281-286 [DOI] [PubMed] [Google Scholar]
- 21.Jones P, Crack J, Rabinovitch M: Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the α v β-3 intergrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 1997, 139:279-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erickson HP, Bourdon MA: Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol 1989, 5:71-92 [DOI] [PubMed] [Google Scholar]
- 23.Jones P, Cowan K, Rabinovitch M: Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 1997, 150:1349-1360 [PMC free article] [PubMed] [Google Scholar]
- 24.Balica M, Bostrom K, Shin V, Tillisch K, Demer LL: Calcifying subpopulation of bovine aortic smooth muscle cells is responsive to 17β-estradiol. Circulation 1997, 95:1954-1960 [DOI] [PubMed] [Google Scholar]
- 25.Sumida A, Fukuen S, Yamamoto I, Matsuda H, Naohara M, Azuma J: Quantitative analysis of constitutive and inducible CYPs mRNA expression in the HepG2 cell line using reverse transcription-competitive PCR. Biochem Biophys Res Commun 2000, 267:756-760 [DOI] [PubMed] [Google Scholar]
- 26.Connolly JM, Rose DP: Expression of the invasive phenotype by MCF-7 human breast cancer cells transfected to overexpression protein kinase C-a or the erbB2 proto-oncogene. Int J Oncol 1997, 10:71-76 [PubMed] [Google Scholar]
- 27.Mackie EJ, Ramsey S: Modulation of osteoblast behavior by tenascin. J Cell Sci 1996, 109:1597-1604 [DOI] [PubMed] [Google Scholar]
- 28.Smith GM, Hale JH: Macrophage/Microglia regulation of astrocytic tenascin: synergistic action of transforming growth factor-β and basic fibroblast growth factor. J Neurosci 1997, 17:9624-9633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latijnhouwers MA, Pfundt R, de Jongh GJ, Schalkwijk J: Tenascin-C expression in human epidermal keratinocytes is regulated by inflammatory cytokines and a stress response pathway. Matrix Biol 1998, 17:305-316 [DOI] [PubMed] [Google Scholar]
- 30.Jo Y, Yeon J, Kim HJ, Lee ST: Analysis of tissue inhibitor of metalloproteinases-2 effect on pro-matrix metalloproteinase-2 activation by membrane-type 1 matrix metalloproteinase using baculovirus/insect-cell expression system. Biochem J 2000, 345:511-519 [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson HC: Mechanism of mineral formation in bone. Lab Invest 1989, 60:320-330 [PubMed] [Google Scholar]
- 32.Wu LN, Sauer GR, Genge BR, Wuthier RE: Induction of mineral deposition by primary cultures of chicken growth plate chondrocytes in ascorbate-containing media: evidence of an association between matrix vesicles and collagen. J Biol Chem 1989, 264:21346-21355 [PubMed] [Google Scholar]
- 33.Schoen FJ, Levy RJ: Bioprosthetic heart valve calcification: membrane-mediated events and alkaline phosphatase. Bone Miner 1992, 17:129-133 [DOI] [PubMed] [Google Scholar]