Abstract
Nodal marginal zone B-cell lymphoma (NMZL) is actually considered as a distinct entity that must be distinguished from extra-nodal and splenic marginal zone lymphomas. To define the cell origin and the role of antigen stimulation we determined the nucleotide sequence of the tumor-related immunoglobulin heavy chain variable genes in 10 cases of NMZL. The results were also evaluated on the basis of the presence of chronic hepatitis C virus (HCV) infection. All 10 cases harbored VH somatic mutations with a sequence homology compared to the closest germline gene, ranging from 83.33 to 98.28%. Interestingly, different VH segments were preferentially used in HCV-positive and HCV-negative patients: three of five HCV-negative NMZLs used a VH4-34 segment joined with different D and JH segments whereas three of five HCV-positive NMZLs used a VH1-69 gene joined with a D3-22 and a JH4 segment, with very strong similarities in the CDR3s among the three different cases. These data indicate: 1) NMZL is derived from B cells that have experienced the germinal center reaction; 2) the preferential usage of a VH1-69 segment in the majority of the HCV-positive NMZL cases with similar CDR3s suggests the presence of a common antigen, probably a HCV antigen epitope, involved in the B-cell selection; and 3) the use of a VH4-34 segment suggests a role of yet unknown B-cell superantigen(s) in the selection of tumor B-cell precursors in HCV-negative NMZL.
Marginal zone B-cell lymphoma (MZL) is a distinct subtype of non-Hodgkin’s lymphoma (NHL) that has been recently recognized and defined by REAL and World Health Organization classifications. 1-3 It can be subdivided into three varieties: extra-nodal MZL (EMZL) that arises from mucosa-associated lymphoid tissue (MALT) and is indicated by World Health Organization classification as EMZL of MALT type, splenic MZL, and nodal MZL (NMZL). Because NMZL displays no appreciable differences in its cytological, architectural, and phenotypic features with EMZL, it has been debated whether MZL with primary nodal involvement has to be considered a distinct disease or merely represents the dissemination of EMZL of MALT-type. For this reason, the REAL classification has included MZL cases with primary lymph node involvement in a provisional category. Very recently, Nathwani and colleagues 4 identified several distinct clinical features between nodal and extra-nodal MZL. NMZL has a more frequent involvement of peripheral lymph nodes and an advanced disease stage at the presentation compared with EMZL of MALT-type. Moreover, NMZL has a shorter overall survival duration than EMZL. Therefore, from a clinical point of view, NMZL seems to be more similar to other low-grade NHLs such as follicular and lymphocytic lymphoma than EMZL, suggesting the presence of still unknown biological differences between EMZL and NMZL. For these reasons the World Health Organization classification has clearly recognized NMZL as a distinct disease that must be distinguished from EMZL of MALT-type with lymph node involvement and from other small B-cell NHLs. 3
Hepatitis C virus (HCV) is actually considered as the major etiological factor of type II essential mixed cryoglobulinemia, a disease associated with an underlying B-cell clonal proliferation that, in a minority of cases, may evolve into a frank NHL. 5,6 HCV is known to be both a hepatotropic and a lymphotropic virus and it has been suggested that it may play a role in the pathogenesis of clonal proliferation of B-cells. 7 Several studies have reported a higher prevalence of chronic HCV infection in patients with B-cell NHL compared with the general population, at least in certain geographical areas. 7-9 In particular, in a survey of HCV-positive NHL from the United States NMZL has been recognized as the most common histological type. 10
A role of chronic immunological stimulation in the development and maintenance of MZL, and in particular of EMZL of MALT-type, has been suggested by a variety of observations. MZL frequently originates in a setting of chronic inflammation triggered by chronic infection or autoimmune disorders, such as Helicobacter pylori gastritis, Sjögren’s syndrome, and Hashimoto’s thyroiditis. 11-13 The etiological link between gastric EMZL and H. pylori infection has also been shown by the regression of some cases by antibiotic therapy. 14,15 A role of chronic immunological stimulation is also suggested by sequence analysis of the tumor-related immunoglobulin gene rearrangement that identified a preferential use of the immunoglobulin heavy chain variable region (VH) genes associated with autoimmune disorders suggesting that tumor cells may arise from autoreactive marginal zone B-cells. 16,17 Moreover, the analysis of somatic mutations of VH genes revealed a variable but significant level of mutations supporting the hypothesis of a germinal center (GC) of a post-GC origin of MZL. 18,19 Nevertheless, these data have been obtained in a significant number of EMZL whereas the tumor-related immunoglobulin chain gene (IgH) status has not yet been determined in the recently identified subset of NMZLs.
In the present study we analyzed the nucleotide sequence of the tumor-related rearranged variable immunoglobulin heavy chain genes in 10 cases of NMZL. The cases examined have been well characterized from a clinical point of view to exclude any cases with extra-nodal involvement. Therefore, we considered this cohort of patients represented of true NMZL in accord to World Health Organization classification. Moreover, we also analyzed the data obtained on the basis of the presence of chronic HCV infection to identify peculiar aspects of the IgH genes distinguishing between HCV-positive and HCV-negative cases.
Materials and Methods
Patients
The 10 cases of NMZL evaluated in this study were selected among 18 cases, collected between 1990 and 1998, for which DNA or nitrogen liquid-frozen lymph node tissues were available for molecular studies. Diagnosis of monocytoid-marginal zone B-cell lymphoma was documented in all cases by morphology and immunohistochemistry performed on formalin-fixed paraffin-embedded tissue according to the proposed “Revised European-American Classification of Lymphoid Neoplasms.” 1 In particular, the presence of characteristic clear cells with a relatively abundant pale cytoplasm (positive for CD20 and CD79a and negative for CD5 antigens) recognized as marginal/monocytoid B cell was evident in all cases (Figure 1) ▶ . Moreover, bone marrow and peripheral blood double-immunofluorescence labeling with anti-CD5 and anti-CD19 was performed in all cases to exclude B-cell chronic lymphocytic leukemia. Complete clinical records and follow-up were available for all patients included in the study; as long as a case had involvement of a mucosal or other extra-nodal nonhemopoietic site by MZL it was classified as EMZL of MALT-type and discarded. On the basis of these criteria 8 out of the 18 cases initially selected were discarded from the study because of the presence of an extra-nodal localization of the lymphoma, in particular of a gastric involvement in seven cases and of the parotid gland in one case. All patients were evaluated on the basis of physical examination, endoscopy, and neck-thorax-abdominal computed tomographic scans.
Figure 1.
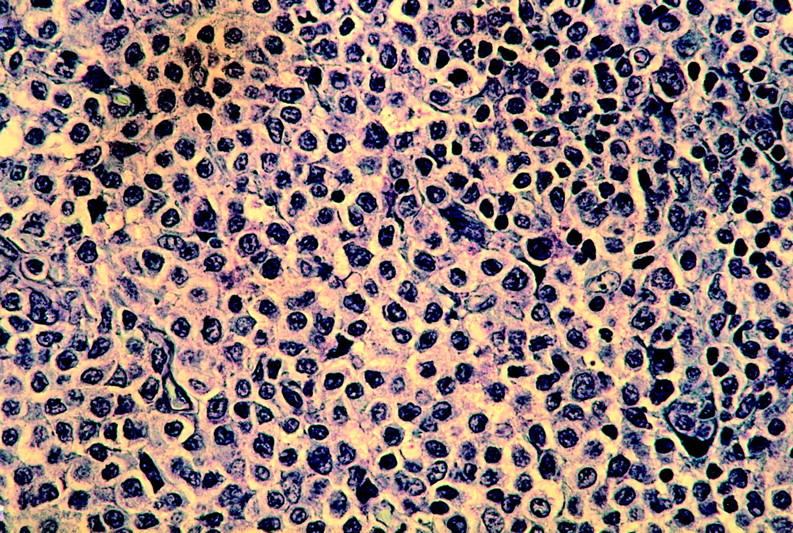
Lymph node from case 5 with NMZL. (Giemsa; original magnification, ×400).
Serum anti-HCV antibodies were detected in 5 out the 10 nodal MZLs examined by enzyme-linked immunosorbent assay (Chiron ELISA HCV; Chiron, Emeryville, CA) and confirmed in all cases by recombinant-based immunoblot test assay (Chiron RIBA HCV, Chiron). The serological assay was performed on the sera collected at the time of diagnosis. Patients found positive for the presence of anti-HCV antibodies underwent liver biopsy that showed the presence of a chronic liver disease in all cases. Abnormal serum levels of alanine aminotransferase were found in two HCV-positive patients. Cryocrit determinations and cryoglobulins composition were evaluated in all cases as described by Pozzato and colleagues, 6 although none of the patients presented clinical manifestations of cryoglobulinemia circulating monoclonal IgMk serum cryoglobulins (considered as positive as >1%) were present in one case (case 1).
Polymerase Chain Reaction (PCR) Amplification of the Rearranged IgH and IgK
DNA was extracted from lymph node specimens using a commercial kit (Easy-DNA; Invitrogen, Carlsbad, CA). One μg of DNA was added to PCR buffer (10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2), containing 200 μmol/L dNTPs, 100 nmol/L of each primer and 1.5 U of AmpliTaq Gold polymerase (Perkin Elmer, Norwalk, CT) in a total volume of 50 μl. Forty cycles of amplifications were performed at the following condition: 1 minute at 95°C, 1 minute at 60°C, and 1 minute at 72°C with 10 minutes of an initial denaturation step at 95°C and 7 minutes of a final extension step at 72°C. Initially, PCRs were performed in separate reactions with seven family-specific FR1 sense primers together with a 3′ primer complementary to a germline JH consensus sequence. If the region from FR1 to JH could not be amplified, the amplification experiments were performed at the same conditions using 5′ oligonucleotides specific for each leader sequence of the VH1 and VH7 families together with the same JH antisense primer. For each DNA sample a DNA-free control was used to check contamination. Each amplification experiment was performed in duplicate. After agarose gel electrophoresis, a predominant band was obtained in samples 1, 4, 7, 8, 9, and 10 using the VH FR1-specific family primers and in samples 2, 3, 5, 6, and 9 using VH leader-specific family primers. The segment initially obtained with FR1 primers in sample 9, belonging to VH3 family, was an out-of-frame rearrangement.
5′ oligonucleotides complementary to FR2 and FR3 consensus sequences were also used together with the 3′ JH primer at the same PCR conditions with the exception of an annealing temperature of 50°C in each cycle.
The light chain gene region was amplified using VK family-specific primers and a mixture of JK primers at the same PCR condition used for VH family-specific amplifications. The sequence of the oligonucleotides used for the PCR experiments is reported in Table 1 ▶ .
Table 1.
Primers Used for IgH and IgK Amplification
| Name | Sequence (5′/3′) |
|---|---|
| VH1 FR1 | CCTCAGTGAAGGTCTCCTGCAAGG |
| VH2 FR1 | TCCTGCGCTGGTGAAAGCCACACA |
| VH3 FR1 | GGTCCCTGAGACTCTCCTGTGCA |
| VH4a FR1 | GGTCCCTGAGACTCTCCTGTGCA |
| VH4b FR1 | TCGGAGACCCTGTCCCTCACCTGCA |
| VH5 FR1 | GAAAAAGCCCGGGGAGTCTCTGGA |
| VH6 FR1 | CCTGTGCCATCTCCGGGGACAGTG |
| VH1 leader | CCATGGACTGGACCTGGAGG |
| VH2 leader | ATGGACATACTTTGTTCCAC |
| VH3 leader | CCATGGAGTTTGGGCTGAGC |
| VH4 leader | ATGAAACACCTGTGGTTCTT |
| VH5 leader | ATGGGGTCAACCGCCATCCT |
| VH6 leader | ATGTCTGTCTCCTTCCTCAT |
| VH7 leader | TTCTTGGTGGCAGCAGCCACA |
| FR3a | ACACGGC(C/T)(G/C)TGTATTACTGT |
| FR2 | TGG(A/G)TCCG(C/A)CAG(G/C)C(T/C)(T/C)CNGG |
| JH | ACCTGAGGAGACGGTGACC |
| VK1 | ATGGACATGAGGGTCCCCGC |
| VK2 | ATGAGGCTCCCTGCTCAGCT |
| VK3 | ATGGAAACCCCAGC(G,T)CAGCT |
| VK4 | ATGGTGTTGCAGACCCAGGTC |
| VK5 | ATGGGGTCCCAGGTTCACCTC |
| VK6 | ATGTTGCCATCACAACTCATTG |
| JK124 | ACGTTTGAT(C,T)TCCA(C,G)CTTGGTC |
| JK3 | ACGTTTGATATCCACTTTGGTC |
| JK5 | ACGTTTAATCTCCAGTCGTGTC |
Sequencing of PCR Products and Sequencing Analysis
PCR products were excised from 1% low-melting agarose gel, further purified with Wizard PCR prep purification kit (Promega, Madison, WI) and directly sequenced using the BigDye terminator cycle sequencing kit and a DNA sequencer (ABI prism 310; PE Applied Byosystem, Foster City, CA). Sequencing reactions were performed with the same oligonucleotides used in the PCR amplifications by both sense and antisense primers. The nucleotide sequences of the amplification products representative of the IgH rearrangements were compared with the VBASE directory (VBASE Sequence Directory, I.M.; Tomlinson, MRC Center for Protein Engineering, Cambridge, UK; URL: www.mrc-cpe.cam.ac.uk/imt-doc) 20 using the DNAPLOT analysis software. A diversity (D) germline segment was assigned to the longest stretches with the highest nucleotide homology with a minimum of six successive matches or seven matches interrupted by one mismatch.
Somatic Mutation Analysis
Mutations in the variable region were identified by comparing the nucleotide sequence of each tumor with the closest germline VH sequence. Two nucleotide exchanges in one codon were considered as one replacement (R) mutation. Mutations at the joining site of the VH segment were not regarded as mutations. The number of expected replacement (R) mutations in the complementary determining region (CDR) or framework region (FR) was calculated using the formula: Exp R (CDR or FR) = n × (CDR Rf or Fr Rf) × (CDRrel or FRrel); where n is the total number of observed mutations, CDRrel or FRrel is the relative size of CDRs or FRs and Rf is the inherited replacement frequency to CDRs or FRs sequences. Rf value was calculated for each individual VH gene sequence using the InhsusCalc1.0 software, kindly provided by Dr Paolo Casali (Department of Pathology, Weill Medical College, Cornell University, NY). According to the binomial distribution model, the probability that excess or scarcity of R mutations in CDRs or FRs resulted on the basis of chance alone was calculated using the formula: p = {n!/[k!(n − k)!]} × qk × (1 − q)n-k; where k is the number of observed mutations in the CDRs or FRs and q is the probability that a R mutation will localize to CDRs or FRs (q = CDRrel × CDR Rf or FR rel × FR Rf). 21
Results
PCR Analysis of VH and VK Genes
IgH rearranged genes were amplified from DNAs extracted from lymph node biopsies using family-specific VH FR1 or, alternatively, family-specific VH leader primers coupled with a 3′ heavy-chain joining (JH) primer. After agarose gel electrophoresis and staining with ethidium bromide a predominant band was observed in all cases. All samples were analyzed in duplicate. These amplification products were directly sequenced on both strands with the same sense and antisense primers used in the PCR experiments. As anticipated by the PCR analysis, the VH segments involved in a productive VH-D-JH rearrangement in NMZLs belonged to the VH4 family in four cases, to the VH1 family in three cases, to the VH3 family in two cases, and to the VH2 family in one case. No rearranged segments of the VH5, VH6, and VH7 family were found to be involved in this series of NMZL cases. VH nucleotide sequences have been deposited in the GenBank database (accession numbers AF355605 to AF355614). All three of the VH1-family genes identified (cases 1, 4, and 5) were found to be closely related to the VH1-69 germline gene with a nucleotide homology ranging from 92.40 to 94.79%. Three of the four lymphoma VH4-family genes (cases 2, 3, and 6) seem to be related to the VH4-34 germline gene with a nucleotide identity ranging from 83.33 to 98.28%, whereas the fourth VH4-family case (case 9) was assigned to the VH4-30.4 germline segment. The two VH3-family lymphoma segments seemed to be related to the VH3-30.3 (case 7) and VH3-48 (case 10) whereas a rearranged VH2-05 germline segment was observed in case 8 (Table 2) ▶ .
Table 2.
VH, D, and JH Usage and HCV Infection in NlMZL
| Case no. | HCV | VH | DH | JH |
|---|---|---|---|---|
| 1 | Positive | VH1-69 | D3-22 | JH4 |
| 2 | Negative | VH4-34 | D6-19 | JH3 |
| 3 | Negative | VH4-34 | D7-27 | JH3 |
| 4 | Positive | VH1-69 | D3-22 | JH4 |
| 5 | Positive | VH1-69 | D3-22 | JH4 |
| 6 | Negative | VH4-34 | NI | JH6 |
| 7 | Positive | VH3-30 | D7-27 | JH4 |
| 8 | Negative | VH2-05 | D3-22 | JH4 |
| 9 | Positive | VH4-30.4 | NI | JH6 |
| 10 | Negative | VH3-48 | D3-3 | JH4 |
NI, not identified.
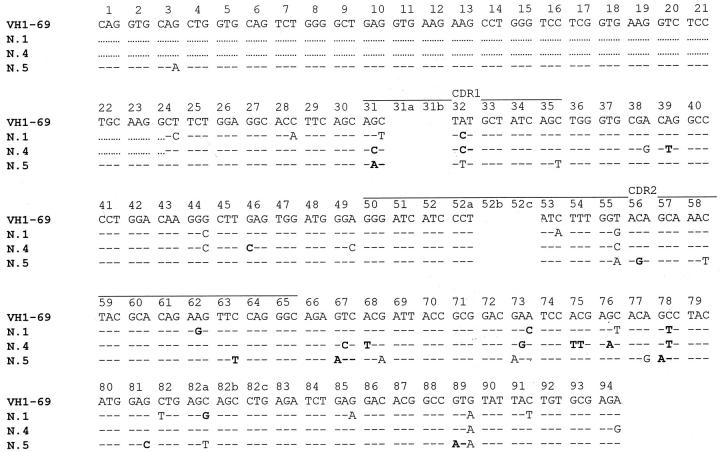
Assignment of D gene segments was based on the homology between CDR3 sequences and the germ line D genes. A D segment could be identified with confidence in 8 of the 10 lymphoma cases examined. A D3-22 segment was assigned in four cases. Surprisingly, the three nodal MZL cases with an involvement of the VH1-69 fragments used a D3-22 segment joined with a JH4 gene. In addition, the sequence of the CDR3 region found in the three different patients were very similar, both in length and in nucleotide composition. Moreover, the D3-22 segments were used in the same reading frame (Table 3) ▶ . Nevertheless, the sequence analysis of the VH1-69 segments revealed that the somatic mutations of the three VH1-69 cases were different, excluding a gross contamination between the different DNA samples (see Figure 3 ▶ ). Moreover, to confirm these data, amplification fragments of the VH-D-JH rearrangements were obtained with primers specific to FR3 and FR2 consensus regions of the VH segments. PCR experiments performed with a degenerate oligonucleotide specific for FR3 region revealed the presence of a clear band in cases 1 and 4 and of a smear with a faint band in case 5; of note all PCR fragments obtained were of the same length. In two out of the three cases (cases 4 and 5) a band of the same length was evident also with a degenerate primer specific for the FR2 region. In the third case (case 1) a smear was present probably because of the fact that the nucleotide sequence of the FR2 region of this case was not susceptible to amplification with this set of primers (Figure 2) ▶ . Sequence analysis of the DNA fragments obtained with FR2 and FR3 PCR experiments revealed a nucleotide composition identical to the sequence already determined with the family-specific amplification fragments in all cases. The three cases rearranging a VH1-69 segment were all positive for HCV infection. The other two HCV-positive cases rearranged a VH4-30.4 (case 9) or a VH3-30.5 segment (case 7). The three cases using a VH4-34 segment were all HCV-negative (Table 2) ▶ .
Table 3.
Determination of VH, D and JH Usage and Nucleotide Sequence of CDR3 Region in Nodal MZL
| Case no. | VH | N | D | N | JH |
|---|---|---|---|---|---|
| 1 | TGT GCGAGA | ggcccc | GATAGcAGTGGaTATTACTAC | tt | CTAC TGG |
| VH1-69 | D3-22 | JH4 | |||
| 2 | TGT GCGAGAGGT | agaaagggag | GCAGTGGCTtGTAC | ctgaaacat | ATGgTTTgGAcGTC TGG |
| VH4-34 | D6-19 | JH3 | |||
| 3 | TGT GCGAGAGGT | ggctatgatagtgatgg | CTccgTGGGGA | cgatggtc | GCTTTTGATATC TGG |
| VH4-34 | D7-27 | JH3 | |||
| 4 | TGT GCGAG | ggggccc | GATAGTAGTGcaTATTACTAC | ttt | TAC TGG |
| VH1-69 | D3-22 | JH4 | |||
| 5 | TGT GCGAGA | gggccc | GATAGTAGTGcaTATTACTA | ttt | CTAC TGG |
| VH1-69 | D3-22 | JH4 | |||
| 6 | TGT GCG | —— | actattcgttatcggcgccgcctatctttctttcggtat | —— | TACacCt TGG |
| VH4-34 | JH6 | ||||
| 7 | TGT GCGAAA | gaagggac | TTTTTGGAGTGG | ccc | CTcTGACTgg TGG |
| VH3-30.5 | D7-27 | JH4 | |||
| 8 | TGT GCACAT | tt | GTATTAtTATGATAGTgGTGtTTATTAC | cgcc | ACTTTGACTAC TGG |
| VH2-05 | D3-22 | JH4 | |||
| 9 | TGT GCCAGAGAT | —— | attcctttggtcgggagtataggtcg | —— | TACTGCGGTATGGACGTC TGG |
| VH4-30.4 | JH | ||||
| 10 | TGT GCGAGAGAT | cgattgacggtcg | ACGATTTTTGGAGTG | cca | ACTACTTaGACTAt TGG |
| VH3-48 | D3-3 | JH4 |
Nucleotides of N segments and mutations with respect to the germline sequence are indicated in lowercase.
In cases 6 and 9 a germline D segment was not assigned.
Figure 3.
Sequence analysis of the VH1-69 homologous segment in three HCV-positive NMZL cases (cases 1, 4, and 5). VH codons are numerated on the VH1-69 germ-line sequence. Bold letters: replacement mutations.
Figure 2.
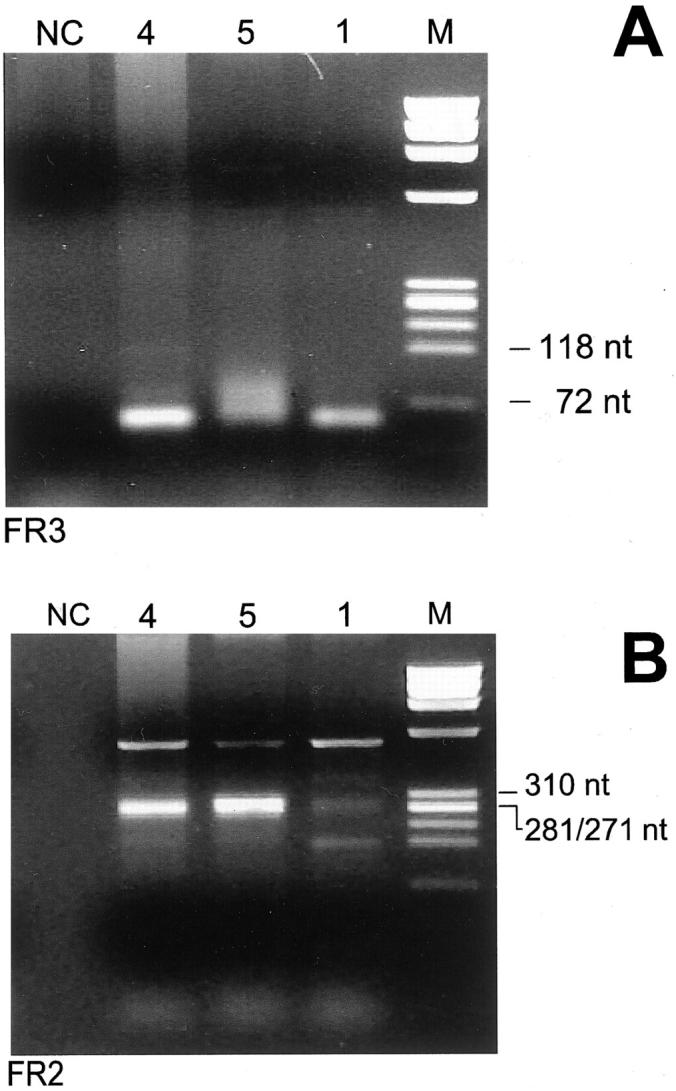
Gel electrophoresis of PCR amplifications of the CDR3s of cases 1, 4, and 5 obtained with primers specific for the FR3 (A) and FR2 (B) IgH regions. A: Amplicons of the same length were clearly present in patients 1 and 4 whereas in case 5 they were present as a smear with a faint band. B: Clonal bands of the same length were present only in cases 4 and 5. M, molecular weight marker ΦX174 HaeIII digested; NC, DNA-free negative control.
VK gene analysis was performed using VK family-specific sense primers coupled with a mixture of JK antisense primers. A clear predominant band was obtained in only six cases and in particular a VK2 segment in one case (case 7), a VK3 segment in two cases (cases 4 and 6), a VK4 segment in one case (case 2), and a VK5 segment in two cases (cases 3 and 5). Because it has been pointed out a relationship between VH1-69 heavy chain and Vk325 light chain segments, 19 PCR products obtained with VK3-specific primers were purified and subject to direct sequence analysis. A mutated Vk325 gene was identified in case 4 and a mutated Vk305 in case 6.
Somatic Hypermutations and Statistical Analysis
The rearranged VH sequences were found to carry somatic mutations in all cases of NMZL studied, with a sequence identity rate compared with the closest germline gene ranging from 83.33 to 98.28%. To establish if the pattern of somatic mutations were indicative of antigen selection, we determined the distribution of replacement (R) and silence (S) mutations in the FRs and CDRs regions using the method described by Chang and Casali. 21 A preferential cluster of R mutations in the CDRs region (P < 0.05) was found only in case 7. In FRs regions the number of R mutations was significantly lower than would be expected to arise solely by chance in five cases, probably as a result of selection against R mutations within the FRs to preserve structure of immunoglobulins. Among this group of patients cases 1, 5, and 7 were HCV-positive; cases 2 and 6 were HCV-negative (Table 4) ▶ .
Table 4.
Mutation Analysis of Rearranged VH Gene in Nodal Marginal Zone Lymphomas
| Case no. | Germline VH genes | Identity (%) | Obs R/S CDR | Obs R/S FR | Exp R CDR | Exp R FR | pCDR | pFR | HCV |
|---|---|---|---|---|---|---|---|---|---|
| 1 | VH1-69 | 92.4 | 2 /3 | 3 /8 | 3.89 | 9.04 | 0.144 | 0.002 | Positive |
| 2 | VH4-34 | 83.33 | 10 /4 | 9 /18 | 8.55 | 28.26 | 0.121 | <0.001 | Negative |
| 3 | VH4-34 | 98.28 | 2 /0 | 2 /1 | 0.88 | 2.87 | 0.173 | 0.253 | Negative |
| 4 | VH1-69 | 92.40 | 2 /1 | 8 /5 | 3.66 | 8.51 | 0.165 | 0.190 | Positive |
| 5 | VH1-69 | 94.79 | 3 /3 | 4 /4 | 2.62 | 8.69 | 0.242 | 0.015 | Positive |
| 6 | VH4-34 | 83.68 | 10 /2 | 15 /12 | 8.37 | 26.95 | 0.117 | <0.001 | Negative |
| 7 | VH3-30.5 | 92.54 | 7 /1 | 4 /4 | 3.12 | 9.93 | 0.018 | 0.003 | Positive |
| 8 | VH2-05 | 95.70 | 4 /0 | 4 /1 | 2.12 | 5.43 | 0.102 | 0.165 | Negative |
| 9 | VH4-30.4 | 94.33 | 4 /3 | 6 /3 | 3.03 | 8.90 | 0.180 | 0.07 | Positive |
| 10 | VH3-48 | 97.36 | 2 /0 | 1 /3 | 1.41 | 3.22 | 0.284 | 0.068 | ND |
VH genes, immunoglobulin heavy chain variable gene; R, replacement mutation; S, silent mutation; CDR, complementary determining regions; FR, framework regions; Obs R/S CDR and Obs R/S FR, number of the observed R and S mutations in the CDR and FR, respectively; Exp R CDR and Exp R FR, number of expected R mutation in the CDR and FR, respectively; pCDR and pFR, probability that excess or scarcity of the R mutations in the VH gene CDR or FR resulted from chance only; ND, not determined.
VH sequences were compared with germline VH sequences included in the VBASE sequence directory.
Discussion
The status of the immunoglobulin genes provides an indicator of the cell origin and of its clonal history in NHL. 22 Somatic hypermutations seem to be restricted to B cells proliferating within the microenvironment of the germinal center (GC). As a consequence, the presence of somatic mutations in the variable region of the rearranged immunoglobulin genes is actually considered the hallmark of B cells that have participated in a GC reaction. Moreover, the pattern of the distribution of somatic mutations and a preferential usage of immunoglobulin variable, diversity, and joining segments may reveal a role of antigens in driving B-cell proliferation. Clustering of nucleotide mutations leading to an amino acid substitution in the CDRs of VH segments is considered to indicate that the hypermutation process is driven by an antigen and a statistical method for assessing it is available. 21
In the cases examined we found that NMZLs harbor numerous point mutations with respect to the closest related germline gene sequences (Table 5) with a rate of homology ranging from 83.33 to 98.28% corresponding to a number of substitutions ranging from 5 to 41 nucleotides (average, 17.8). Because most of the human germline VH genes have been identified, it is unlikely that the majority of nucleotide substitutions found represent VH segments not yet identified or because of sequence polymorphism. 20 These data show that the tumor cells are likely to derive from mature B cells that had participated in a GC reaction. We cannot identify if tumor cells have to be considered of GC or of post-GC derivation because the molecular approach used (direct sequencing of PCR products) does not allow the assessment of nucleotide intraclonal variations because of an ongoing mutational process.
The distribution of R and silence S mutations of mutated VH sequence was analyzed by the method of Chang and Casali. 21 Somatic mutations of the our NMZLs were not distributed in a manner indicative of positive selection or of antigen-affinity maturation in the majority of cases. In fact, only one of the cases examined (case 7) showed a statistically significant evidence of accumulation of R mutations in the CDRs. Nevertheless, five cases showed evidence of negative selection of R mutations in the FRs (Table 4) ▶ . This aspect is consistent with a GC selection to preserve the immunoglobulin structure providing the scaffolding for the antigen-contacting cells.
Kuppers and colleagues 23 showed in four cases defined as monocytoid B-cell lymphoma a distribution of somatic mutations indicative of a clonal antigen-positive selection driven by antigens. Our data, obtained in a larger number of cases, contrast with these findings. Nevertheless, in their cases the primary localization of the lymphoma was not reported and at least in one case the tumor was localized in the stomach and therefore likely to be considered as an EMZL of MALT-type with monocytoid cells. Thus, the differences between our data and that of Kuppers may be because of different criteria in selecting lymphoma cases.
Zuckerman and colleagues 10 have recently reported that NMZL is the most common histological type found in a series HCV-infected NHL cases from the United States. They also found that ∼50% of the NMZL cases examined were HCV-infected. Therefore, the frequency of HCV chronic infection found in our NMZL cases, selected only on the basis of the availability of biological material adequate for molecular studies, confirms Zuckerman’s data. Interestingly, we also found that the VH gene usage among NMZL is not random. Different VH segments are preferentially used in HCV-positive and HCV-negative patients (Table 2) ▶ . Three out of five HCV-negative NMZLs (cases 2, 3, and 6) use the VH4-34 gene segment joined with different D and JH segments. In two cases, the VH4-34 segments appear to be heavily mutated, with nucleotide sequence homology with the VH4-34 germ-line gene of 83.33% in case 2 and 83.68% in case 6. In both these cases, despite the high frequency of somatic mutations, aspects for selection against R mutations in the FRs were present (Table 4) ▶ . The conservation of the amino acid sequence of the FRs is actually considered a feature that distinguishes functional from nonfunctional sequences suggesting that the immunoglobulins encoded by these VH4-34 MZL cells are functional. 24,25 Therefore, neoplastic cells have aspects indicating the origin from B lymphocytes that have undergone a selection process within the GC reaction. In case 3, the VH4-34 segment was slightly mutated (98.28% of homology) without a statistical significance of the pattern of distribution of R and S mutations. Nevertheless, the low rate of mutations found in this case determines that the statistical model used may be not informative. The VH4-34 gene is used in ∼5% of healthy adult B lymphocytes 26 and is frequently found in diffuse large-cell lymphoma, primary central nervous system lymphoma, B-chronic lymphocytic leukemia, and autoimmune disorders, but never in multiple myeloma. 27-32 Interestingly, the VH4-34 gene is found in virtually all cases of cold agglutinin disease 33-35 and the red blood cell I/i antigens bind to the FR1 domain of Ig. A restricted usage of VH genes, as well as binding of an immunoglobulin outside the CDRs, which are the sites that bind conventional antigens, are characteristic aspects of B-cell superantigens that are supposed to directly activate B cells. 36 In particular, certain portions of the FRs seems to be important for superantigen binding and these would be preserved in a superantigen selection pressure. 37 Interestingly, staphylococcal enterotoxins A and D, that function as a human B superantigen, are able to rescue B cell-expressing VH3 and VH4 (including VH4-34) genes inducing cell survival in in vitro experiments. 38,39 Therefore, the high frequency of the VH4-34 gene usage joined with different D and JH segments as well as the preservation of the FRs sequences, suggests a possible role of yet unknown B-cell superantigen(s) in driving HCV-negative NMZL development and proliferation.
Three out five HCV-positive NMZLs revealed the usage of the VH1-69 gene indicating a highly biased and nonrandom use of the VH segments in this subtype of tumors. Sequence analysis of the rearranged VH segments of these three cases revealed a substantial deviation from the germline VH1-69 sequence with a degree of mutations very similar among the different cases indicating that these cells have traversed the GC activating the somatic hypermutation mechanism. Statistical analysis of the pattern and distribution of R and S mutations between CDRs and FRs revealed a statistical significance in two cases (cases 1 and 5) in which the presence of R mutations in the FRs are lower than expected by chance only (Table 4) ▶ . This pattern is indicative of a selective pressure to conserve the functional structure of the immunoglobulin protein. Moreover, the VH1-69 segments were joined with a D3-22 and a JH4 segment in all of the three cases. A D3-22 segment joined with JH4 was also found in a HCV-negative case (case 8) assembled with a VH2-05 gene. As a consequence, the CDR3 region encoded for an amino acid sequence with very strong similarities between the three VH1-69 different cases. We exclude that this data may be because of cross contamination because the sequence analysis of the VH1-69 segments revealed different somatic mutations between the three samples (Figure 3) ▶ and the same CDR3 sequences were obtained with different sets of primers specific for FR3 and FR2 VH consensus sequences (Figure 2) ▶ . Therefore, these data indicate the role of a common antigenic epitope involved in the selection and in the expansion of the B-cell clone at the origin of neoplastic cells. The VH1-69 immunoglobulin segment is expressed in the restricted repertoire of fetal liver B lymphocytes and is thought to be involved in natural immunity. 40,41 A productive VH1-69 rearrangement is present in ∼1.6% of normal B lymphocytes in adults. 42 VH1-69 is rearranged in ∼10 to 20% of B-cell chronic lymphocytic leukemia 30,43 and a VH1-69 monoclonal rearrangement is present in the majority of patients with type II mixed cryoglobulinemia, a typical HCV-related disorder. 44,45 A preferential usage of VH1-69 was also found by Ivanovski and colleagues 46 in the majority of the HCV-positive lymphoplasmocytoid lymphoma/immunocytoma NHL subtypes secreting monoclonal IgM cryoglobulins. Very recently, a frequent usage of VH1-69 was also reported by Bahler and colleagues 19,47 in the subset of the salivary gland EMZL of MALT-type that arise in the course of lymphoepithelial scialoadenitis associated or not with Sjögren’s syndrome. In one case they also reported the use of a D3-22 joined with VH1-69 and JH4 segments. They also report that VH1-69 salivary gland lymphoma often has CDR3 regions with similar characteristics, ie, a preferential use of the JH4 segment, a length of 12 to 14 amino acids and the presence of some amino acids motifs at the VH-D and D-JH junctions, concluding that the salivary EMZL may be selected by similar antigen epitopes. Unfortunately, they did not report the status of HCV infection in the cases examined.
Therefore, in the subset of NMZLs with a chronic HCV infection we find the presence of the VH1-69 segment in the majority of cases examined, that is frequently expressed in other subsets of lymphoproliferative disorders, mainly associated with HCV infection. The almost equal sequences of the CDR3 region of the VH1-69 cases associated to the presence of a chronic HCV infection suggests, in first hypothesis, that a same HCV antigen epitope could be involved in the B-cell selection by a direct antigenic stimulation. This hypothesis is also sustained by the observation that B cells derived from a HCV-infected individual, immortalized as hybridomas, and selected for binding the HCV E2 envelope glycoprotein use the VH1-69 segment in the majority of cases. 48
Nevertheless, the possibility that the virus infects and induces the proliferation of a particular fraction of B lymphocytes cannot be excluded. Gastric EMZLs are associated with H. pylori infection in virtually all cases suggesting a possible H. pylori direct antigenic stimulation. 13 Nevertheless, in this subset of EMZL, tumor immunoglobulins seem to be autoantigen-related rather than specific to H. pylori and the neoplastic growth seem to be sustained by stimulation through a H. pylori-specific T-cell reaction. 49,50 We cannot exclude that HCV could play a role similar to that of H. pylori in gastric EMZL in the subset of HCV-positive NMZL.
Tierens and colleagues 18 recently reported an analysis of somatic mutations in a series of MZL occurring at different sites including four cases with primary lymph node localization in which the pattern of somatic mutations was not indicative of positive or negative antigen selection. However, they report in two out of the four cases of NMZL studied the presence of a case rearranging the VH1-69 gene joined with a D3-22 segment and a case with the rearrangement of a VH4-34 segment. Unfortunately, they did not report data about HCV infection in these cases.
In conclusion, these results indicate that NMZLs are originated from GC-experienced B lymphocytes with evidence of antigen selection in half of the cases. The presence of a VH1-69 segment in the subset of the HCV-positive NMZLs with similar CDR3 sequences strongly supports the hypothesis of a common antigen, probably a HCV antigen epitope, involved in the clonal B-cell selection. The involvement of a VH4-34 segment with different characteristics of the CDR3 regions suggests a role of unknown B-cell superantigen(s) in the selection of HCV-negative NMZL.
Footnotes
Address reprint requests to Giuseppe Torelli, M.D. Department of Medical Sciences, Section of Hematology, Policlinico, via del Pozzo 71.41100 Modena, Italy. E-mail: torelli.giuseppe@unimo.it.
Supported by a grant from the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy (to M. L.) and by the Associazione Italiana contro le Lucemie, Modena, Italy.
References
- 1.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of the lymphoid neoplasms. A proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Diebold J, Muller Hermelink HK: World Health Organization classification of neoplastic disease of the hematopoietic and lymphoid tissues. Am J Clin Pathol 1999, 111(suppl 1):S8-S12 [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD: World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the clinical advisory committee meeting—Airlie House, Virginia, November, 1997. J Clin Oncol 1999, 17:3835-3849 [DOI] [PubMed] [Google Scholar]
- 4.Nathwani BN, Anderson JR, Armitage JO, Cavalli F, Diebold J, Drachenberg MR, Harris NL, MacLennan KA, Müller-Hermelink HK, Ullrich FA, Weisenburger DD: Marginal zone B-cell lymphoma: a clinical comparison of nodal and mucosa-associated lymphoid tissue types. J Clin Oncol 1999, 17:2486-2492 [DOI] [PubMed] [Google Scholar]
- 5.Agnello V, Chung RT, Kaplan LM: A role of hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992, 327:1490-1495 [DOI] [PubMed] [Google Scholar]
- 6.Pozzato G, Mazzero C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sul faro S, Franzin F, Tulissi P, Moretti M: Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood 1994, 84:3047-3053 [PubMed] [Google Scholar]
- 7.Ferri C, Caracciolo F, Zignego AL, La Civita L, Monti M, Longobardo G, Lombardini F, Greco F, Capochiani E, Mazzoni A, Mazzaro C, Pasero GP: Hepatitis C virus infection in patients with non-Hodgkin’s lymphoma. Br J Haematol 1994, 88:392-394 [DOI] [PubMed] [Google Scholar]
- 8.Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, Russo D, Falasca E, Botta GA, Baccarani M: Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood 1996, 87:4296-4301 [PubMed] [Google Scholar]
- 9.Luppi M, Ferrari MG, Bonaccorsi G, Longo G, Narni F, Barozzi P, Marasca R, Mussini C, Torelli G: Hepatitis C virus infection in subsets of neoplastic lymphoproliferations not associated with cryoglobulinemia. Leukemia 1996, 10:351-355 [PubMed] [Google Scholar]
- 10.Zuckerman E, Zuckerman T, Levine AM, Douer D, Gutekunst K, Mizokami M, Qian DG, Velankar M, Nathwani BN, Fong TL: Hepatitis C virus infection in patients with B-cell non-Hodgkin lymphoma. Ann Intern Med 1997, 127:423-428 [DOI] [PubMed] [Google Scholar]
- 11.Hyjek E, Isaacson PG: Primary B-cell lymphoma of the thyroid and its relationship to Hashimoto’s thyroiditis. Hum Pathol 1988, 9:1315-1326 [DOI] [PubMed] [Google Scholar]
- 12.Hyjek E, Smith WJ, Isaacson PG: Primary B-cell lymphoma of salivary glands and its relationship to myoepithelial sialadenitis. Hum Pathol 1988, 19:766-776 [DOI] [PubMed] [Google Scholar]
- 13.Wotherspoon AC, Ortiz-Hildago C, Falzon MF, Isaacson PG: Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338:1175-1176 [DOI] [PubMed] [Google Scholar]
- 14.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, De Boni M, Isaacson PG: Regression of primary low-grade B-cell gastric lymphoma of mucosa associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342:575-577 [DOI] [PubMed] [Google Scholar]
- 15.Roggero E, Zucca E, Pinotti G, Pascarella A, Capella C, Savio A, Pedrinis E, Paterlini A, Venco A, Cavalli F: Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa associated lymphoid tissue. Ann Intern Med 1995, 122:767-769 [DOI] [PubMed] [Google Scholar]
- 16.Du M, Diss TC, Xu C, Peng H, Isaacson PG, Pan L: Ongoing mutation in MALT lymphoma immunoglobulin gene suggest that antigen stimulation plays a role in the clonal expansion. Leukemia 1996, 10:1190-1197 [PubMed] [Google Scholar]
- 17.Yumoto N, Furukawa M, Kurosu K, Mikata A: A particular characteristic of IgH complementary-determining region 3 suggests autoreactive B-cell origin of primary gastric B-cell lymphomas. Lab Invest 1998, 78:261-268 [PubMed] [Google Scholar]
- 18.Tierens A, Delabie J, Pittaluga S, Driessen A, De Wolf-Peeters C: Mutation analysis of the rearranged immunoglobulin heavy chain genes of marginal zone lymphomas indicate an origin from different marginal zone B lymphocytes subsets. Blood 1998, 91:2381-2386 [PubMed] [Google Scholar]
- 19.Bahler DW, Miklos JA, Swerdlow SH: Ongoing Ig hypermutation in salivary gland mucosa-associated lymphoid tissue-type lymphomas. Blood 1997, 89:3335-3344 [PubMed] [Google Scholar]
- 20.Cook GP, Tomlinson IM: The human immunoglobulin VH repertoire. Immunol Today 1995, 16:237-242 [DOI] [PubMed] [Google Scholar]
- 21.Chang B, Casali P: The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today 1994, 15:367-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuppers R, Klein U, Hansmann ML, Rajewsky K: Cellular origin of human B-cell lymphomas. N Engl J Med 1999, 341:1520-1529 [DOI] [PubMed] [Google Scholar]
- 23.Kuppers R, Hajadi M, Plank L, Rajewsky K, Hansmann ML: Molecular Ig gene analysis reveals that monocytoid B cell lymphoma is a malignancy of mature B cells carrying somatically mutated V region genes and suggest that rearrangement of the kappa-deleting element (resulting in deletion of the Ig kappa enhancer) abolish somatic hypermutation in the human. Eur J Immunol 1996, 26:1794-1800 [DOI] [PubMed] [Google Scholar]
- 24.Dorner T, Brezinschek H-P, Brezinschek RI, Foster SJ, Domiati-Saad R, Lipsky PE: Analysis of the frequency and pattern of somatic mutations within non productively rearranged human variable heavy chain genes. J Immunol 1997, 158:2779-2789 [PubMed] [Google Scholar]
- 25.Dorner T, Brezinschek H-P, Foster SJ, Brezinschek RI, Farner NL, Lipsky PE: Delineation of selective influences shaping the mutated expressed human Ig heavy chain repertoire. J Immunol 1998, 160:2831-2841 [PubMed] [Google Scholar]
- 26.Brezinschek HP, Brezinschek RI, Lipsky PE: Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol 1995, 155:190-202 [PubMed] [Google Scholar]
- 27.Hsu FJ, Levy R: Preferential use of the VH4 Ig gene family by diffuse large-cell lymphoma. Blood 1995, 86:3072-3082 [PubMed] [Google Scholar]
- 28.Thompsett AR, Ellison DW, Stevenson FK, Zhu D: VH gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cell with ongoing mutational activity. Blood 1999, 94:1738-1746 [PubMed] [Google Scholar]
- 29.Montesinos-Rongen M, Kuppers R, Schluter D, Spieker T, van Roost D, Schaller C, Reifenberger G, Wiestler OD, Deckert-Schluter M: Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the VH4-34 gene segment. Am J Pathol 1999, 155:2077-2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, Schulman P, Vinciguerra VP, Rai K, Rassenti LZ, Kipps TJ, Dighiero G, Schroeder HW, Jr, Ferrarini M, Chiorazzi N: Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptor. J Clin Invest 1998, 102:1515-1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascual V, Capra JD: VH4–21, a human VH gene segment over-represented in the autoimmune repertoire. Arthritis Rheum 1992, 35:11-18 [DOI] [PubMed] [Google Scholar]
- 32.Rettig MB, Vescio RA, Cao J, Wu CH, Lee JC, Han E, DerDanielian M, Newman R, Hong C, Lichtenstein AK, Berenson JR: VH gene usage in multiple myeloma: complete absence of the VH4.21 (VH4-34) gene. Blood 1996, 87:2846-2852 [PubMed] [Google Scholar]
- 33.Pascual V, Victor K, Spellerberg M, Hamblin TJ, Stevenson FK, Capra JD: VH restriction among human cold agglutinins. The VH4-21 gene segment is required to encode anti-I and anti-i specificities. J Immunol 1992, 149:2337-2344 [PubMed] [Google Scholar]
- 34.Silberstein LE, Jefferies LC, Goldman J, Friedman D, Moore JS, Nowell PC, Roelcke D, Pruzanski W, Roudier J, Silverman GJ: Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood 1991, 78:2372-2386 [PubMed] [Google Scholar]
- 35.Riboldi P, Gaidano G, Schettino EW, Steger TG, Knowles DM, Dalla Favera R, Casali P: Two acquired immunodeficiency syndrome-associated Burkitt’s lymphomas produce specific anti-i IgM cold agglutinins using somatically mutated VH4-21 segments. Blood 1994, 83:2952-2961 [PMC free article] [PubMed] [Google Scholar]
- 36.Levinson AI, Kozlowski L, Zheng Y, Wheatley L: B-cell-superantigens: definition and potential impact on the immune response. J Clin Immunol 1995, 15(suppl 6):26S-36S [DOI] [PubMed] [Google Scholar]
- 37.Silverman GJ, Pires R, Bouvet JP: An endogenous scialoprotein and a bacterial B cell superantigen compete with their VH family-specific binding interactions with human Igs. J Immunol 1996, 157:4496-4502 [PubMed] [Google Scholar]
- 38.Domiati-Saad R, Lipsky PE: Staphylococcal enterotoxin A induces survival of VH3-expressing human B cells by binding to the VH region with low affinity. J Immunol 1998, 161:1257-1266 [PubMed] [Google Scholar]
- 39.Domiati-Saad R, Attrep JF, Brezinsche HP, Cherrie AH, Karp DR, Lipsky PE: Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4-expressing B cells from apoptosis. J Immunol 1996, 156:3608-3620 [PubMed] [Google Scholar]
- 40.Schroeder HW, Jr, Hillson JL, Perlmutter RM: Early restriction of the human antibody repertoire. Science 1987, 238:791-793 [DOI] [PubMed] [Google Scholar]
- 41.Sasso EH, van Dijk KW, Bull AP, Milner ECB: A fetally expressed immunoglobulin VH1 gene belongs to a complex sets of alleles. J Clin Invest 1993, 91:2358-2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brezinschek HP, Brezinschek RI, Dorner T, Lipsky PE: Similar characteristics of the CDR3 of VH1-69/DP-10 rearrangements in normal human peripheral blood and chronic lymphocytic leukaemia B cells. Br J Haematol 1998, 102:516-521 [DOI] [PubMed] [Google Scholar]
- 43.Johnson TA, Rassenti LZ, Kipps TJ: VH1 genes expressed in B cell chronic lymphocytic leukemia exhibit distinctive molecular features. J Immunol 1997, 158:235-246 [PubMed] [Google Scholar]
- 44.Gorevic PD, Frangione B: Mixed cryoglobulinemia crossreactive idiotypes: implication for the relationship of MC to rheumatic and lymphoproliferative disease. Semin Hematol 1991, 28:79-94 [PubMed] [Google Scholar]
- 45.Agnello V: The etiology and pathophysiology of mixed cryoglobulinemia secondary to hepatitis C virus infection. Springer Semin Immunopathol 1997, 19:111-129 [DOI] [PubMed] [Google Scholar]
- 46.Ivanovski M, Silvestri F, Pozzatto G, Anand S, Mazzaro C, Burrone OR, Efremov DG: Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood 1998, 91:2433-2442 [PubMed] [Google Scholar]
- 47.Miklos JA, Swerdlow SH, Bahler DW: Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin VH genes show frequent use of V1-69 with distinctive CDR3 features. Blood 2000, 95:3878-3884 [PubMed] [Google Scholar]
- 48.Chan CH, Hadlock KG, Foung KH, Levy S: VH1-69 gene is preferentially used by hepatitis C virus-associated B cell lymphomas and by normal B cells responding to the E2 viral antigen. Blood 2001, 97:1023-1026 [DOI] [PubMed] [Google Scholar]
- 49.Hussel T, Isaacson PG, Crabtree JE, Spencer J: The response of cells from low-grade B-cell gastric lymphoma of mucosa associated lymphoid tissue to Helicobacter pylori. Lancet 1993, 342:571-574 [DOI] [PubMed] [Google Scholar]
- 50.Greiner A, Marx A, Heesemann J, Leebmann J, Schmausser B, Muller-Hermelink HK: Idiotype identity in a MALT-type lymphoma and B-cell in Helicobacter pylori associated chronic gastritis. Lab Invest 1994, 70:572-578 [PubMed] [Google Scholar]