Abstract
Interdigitating dendritic cells (IDC) of the human mesenteric lymph nodes (LN) were examined by two-color immunofluorescent microscopy and flow cytometry to clarify their exact localization, immunophenotype, and relationships with T and B cells. IDC were identified as HLA-DRbright large dendriform cells of the T cell areas co-expressing CD40, CD54 (ICAM-1), CD80 (B7/B7–1), CD83, and CD86 (B70/B7–2). The majority of IDC directly attached to a few IgD+ naive B cells as well as to numerous CD4+ T cells. When LN cells were singly suspended and briefly incubated in vitro, IDC formed clusters with IgD+ IgM+ naive B cells, but not with IgA+ or IgG+ B cells. When suspended LN cells were cultured, clustered B cells disappeared within 7 days, and on prolonged culture, some IDC developed into extensively dendriform cells forming stable complexes with several or sometimes numerous CD4+ IL-2R+ CD40L+ activated T cells. These findings indicate that resting naive B cells actually interact with IDC directly in T cell areas of human secondary lymphoid tissues. IDC have a non-antigen (Ag)-specific, strong affinity for resting naive B cells, but this affinity is transient and IDC cannot form stable complexes with B cells, although they can form stable complexes with activated T cells. It is suggested that the stable IDC/Ag-activated T cell complexes make it possible to capture and to stimulate rare Ag-specific resting naive B cells with high efficiency.
It is generally accepted that CD4+ T cells play a major role in the initial activation of resting naive B cells. However, the question as to where and how rare antigen (Ag)-specific resting naive B cells interact efficiently with rare Ag-specific CD4+ T cells remains to be answered. It is well established that dendritic cells (DC) have a unique capacity to stimulate Ag-specific resting naive T cells to proliferate, 1 but it has long been thought that DC are not directly involved in the regulation of B-cell-mediated immunity. A principal feature of DC located within the peripheral tissues is the capture of foreign Ag after tissue injury and subsequent initiation of immune responses. At the Ag/pathogen port of entry (e.g., mucosa, epidermis), immature DC such as Langerhans cells (LC) capture the Ag, and then migrate via the afferent lymph into the T cell areas of the proximal secondary lymph nodes (LN), where they mature into interdigitating dendritic cells (IDC), which represent a subset of mature DC located in the T-cell areas of secondary lymphoid tissues, and select and stimulate rare resting naive T cells specific for the Ag by presenting the Ag to them. 2
B cells enter LN via the same high endothelial venules (HEV) as do T cells, and thus transverse T cell areas before entering follicles. 3,4 It has been suggested that the site of primary B cell activation is within the T-cell areas of secondary lymphoid tissues. 5-7 Ag-specific activated CD4+ T cells stimulate Ag-specific naive B cells to proliferate and differentiate into germinal center founder cells or into short-lived plasma cells essentially producing IgM. 8 MacPherson et al 9-11 recently suggested that IDC act as matrix on which Ag-specific T and B cells interact efficiently. In addition, they suggested that IDC can deliver naive forms of antigens, which are essential for the initial activation of B cells, to Ag-specific resting naive B cells. 10,11 In recent years, several investigators have demonstrated that IDC are directly involved in regulating T-cell-mediated humoral immune responses in humans. 12,13 Bjorck et al 13 have demonstrated the morphological evidence indicating that naive B cells actually interact with DC in the human tonsils. However, little attention has been paid to the direct interactions between IDC and B cells, and little is known about this subject, particularly in the human immune system. In the present study, we investigated the direct morphological interactions of the human secondary lymphoid tissue IDC with T and B cells in situ and in vitro.
Materials and Methods
Antibodies
The expression of leukocyte cell surface markers was assessed by direct immunofluorescent staining using the following fluorescent isothiocyanate (FITC) or phycoerythrin (PE)-conjugated murine monoclonal antibodies (mAbs): anti-CD1a, anti-CD3, anti-CD4, anti-CD8, anti-CD11c, anti-CD14, anti-CD20, anti-CD22, anti-CD40, anti-CD54 (ICAM-1), anti-CD80 (B7/B7–1), anti-CD86 (B70/B7–2), all of which were purchased from DAKO (Glostrup, Denmark). FITC- and PE-conjugated anti-HLA-DR (class II MHC) were purchased from Becton Dickinson (Mountain View, CA). The PE-conjugated anti-CD83 was purchased from Immunotech (Marseilles, France), and PE-conjugated anti-IL-2R (CD25) and anti-CD40-ligand (CD40L; CD154) were purchased from DAKO. For three-color flow cytometry, PerCP-conjugated anti-HLA-DR was purchased from Becton Dickinson.
For the detection of immunoglobulin classes, we performed indirect immunofluorescent staining using FITC- or PE-congugated anti-rabbit IgG (DAKO) as a secondary antibody, and nonconjugated rabbit polyclonal antibodies to human IgA, IgD, IgG, or IgM (DAKO) as primary antibodies. For the detection of S-100b protein, we performed indirect immunoperoxidase method using rabbit anti-S-100 β subunit prepared as described previously, 14 and horseradish peroxidase (HRPO)-conjugated goat anti-rabbit IgG (DAKO).
Tissue Preparation
The mesenteric lymph nodes, which were devoid of cancer metastasis, were obtained with informed consent from five patients with colon cancer at the time of surgery. The age range of these patients was 55 to 75 years (mean age 62). Some LN were cut, and the fragments were immediately frozen in liquid nitrogen and stored at −80°C until cryostat sectioning. 7-mm-thick sections were placed on slides and air-dried at room temperature for 1 hour, then fixed with cold acetone L for 10 minutes. Sections were stored at −80°C until use.
Cell Preparation and Culture Conditions
LN were cut into 2-mm 3 cubes and put into a glass homogenizer (Pyrex, Tokyo, Japan) into which was added 10 ml of RPMI 1640 medium (Nissui, Tokyo, Japan) containing 10% heat-inactivated, low endotoxin (<6 pg/ml) fetal calf serum (Irvine Scientific, Santa Ana, CA). A single cell suspension was obtained by gentle pressure with a glass homogenizer without the addition of digestive enzyme. After the removal of dead cells and erythrocytes by Ficoll-Hypaque density gradient centrifugation, the cells were suspended in RPMI 1640 containing 10% fetal calf serum (FCS). The cells were then cultured at a concentration of 10 6 cells/ml in 5% CO2 in humidified air, and the culture media were half-changed every 7 days. Fresh or cultured LN cells (n = 105) were smeared on glass slides with a Cytospin (Sakura, Tokyo, Japan) at 1000 rpm for 5 minutes and air-dried. The smeared cells were then fixed with cold acetone for 10 minutes, air-dried, and examined without storage. For immunoperoxidase staining, smeared cells were fixed in 4% paraformaldehyde solution for 10 minutes without drying, rinsed in phosphate buffered saline (PBS), and air-dried.
Two-Color Immunofluorescent Staining
Frozen sections of LN and smeared suspended LN cells were incubated with 1:10-diluted normal goat serum (DAKO) for 15 minutes. The preparations were then incubated with both FITC-conjugated and PE-conjugated mAbs (20 μg/ml) for 30 minutes at room temperature. For the detection of immunoglobulin classes, the preparations were incubated with 1:200-diluted anti-IgD, 1:500-diluted anti-IgM, 1:1000-diluted anti-IgA, or 1:5000-diluted anti-IgG. The preparations were then washed with PBS and incubated with FITC- or PE-conjugated anti-rabbit IgG (1 μg/ml) for 30 minutes. The preparations were then rinsed with PBS and examined by fluorescent microscopy as described previously. 15
Immunoperoxidase Staining for S-100 Protein
The smeared LN cells fixed in paraformaldehyde were treated with 0.3% H2O2 in methanol for blocking endogeneous peroxidase activity, and then incubated with normal goat serum diluted at 1:10 in PBS for 10 minutes to block nonspecific staining. The smeared cells were then stained for S-100b protein by indirect immunoperoxidase method as described previously. 16 After the development of enzyme activity, the cells were counterstained with methyl green. The percentage of IDC in the smeared LN cells were determined by counting S-100+ dendriform cells/1000 LN cells.
Flow Cytometry
To determine the proportions of T cells and B cells in total LN cells, fresh LN cells prepared as described above or those cultured for 7 days were examined by two-color flow cytometry using FITC-conjugated anti-CD3 and PE-conjugated anti-CD22. Briefly, LN cells (n = 106) were incubated simultaneously with both of these mAbs (1 μg) for 30 minutes at 4°C.
The proportions of naive B cells and non-naive N cells in total LN cells were also determined by two-color flow cytometry. LN cells (n = 106) were incubated simultaneously with FITC-conjugated anti-CD20 (1 μg) and rabbit anti-human IgD (1 μg) for 30 minutes at 4°C. After washing with PBS, the cells were treated with PE-conjugated anti-rabbit IgG at a final concentration of 1 μg/ml for 30 minutes at 4°C.
For the detection of IDC, we performed three-color flow cytometric analysis using FITC-conjugated anti-CD40, PE-conjugated anti-CD83 or anti-CD86, and PerCP-conjugated anti-HLA-DR. As controls, FITC, PE, or PerCP-conjugated class-matched mouse immunoglobulin were used. Mesenteric LN cells (n = 106), which were obtained from one of the patients and cultured after washing with PBS, were fixed in 4% paraformaldehyde solution and examined by a FACScan as described previously.
Results
IDC in Situ
In the frozen sections, IDC were identified as HLA-DRbright dendriform cells located in the T cell areas of these LN. Almost all IDC co-expressed CD40, CD54, CD83, and CD86 strongly, and CD80 weakly, but not CD1a (Figure 1,a–d) ▶ . HLA-DR and CD40 were also detected weakly on B cells and macrophages; CD54 (ICAM-1) was detected on macrophages, endothelial cells of HEV, and follicular dendritic cells (FDC) in the germinal centers; and CD80 and CD86 were detected on a portion of the macrophages and B cells in the germinal centers.
Figure 1.
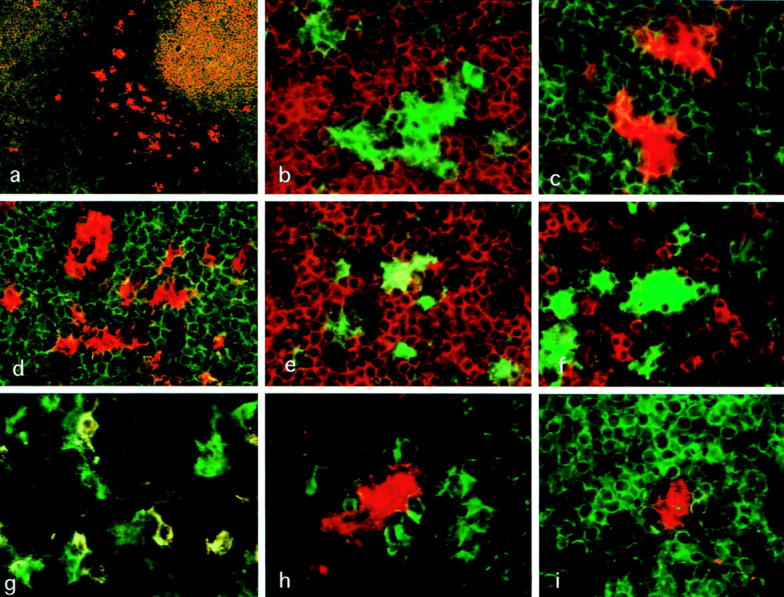
Two-color immunofluorescent microscopy applied on frozen sections of the human mesenteric lymph nodes. a: Note that IDC (CD40+ CD22−; red) are present mainly in T-cell areas but are present in the periphery of B cell (CD22+ CD40+; yellow)-rich areas in a small number. FITC-anti-CD22 and PE-anti-CD40. Magnification, ×40. b: IDC strongly expressing HLA-DR (green) are surrounded by numerous CD3+ T cells (red). FITC-anti-HLA-DR and PE-anti-CD3. Magnification, ×540. c: IDC strongly expressing CD86 (red) were surrounded by numerous CD4+ T cells (green). FITC-anti-CD4 and PE-anti-CD86. Magnification, ×540. d: CD54 (red) are also expressed on IDC and endothelial cells of HEV in T-cell areas. CD3+ T cells (green) are closely associated with IDC. FITC-anti-CD3 and PE-anti-CD54. Magnification, ×540. e: Some HLA-DR+ IDC express co-express CD4 (yellow). FITC-HLA-DR and PE-CD4. Magnification, ×540. f: A considerable number of CD8+ T cells (red) are present around HLA-DR+ IDC (green), but only a small number of them attach to IDC directly. FITC-anti-HLA-DR and PE-anti-CD8. Magnification, ×540. g: A considerable number of B cells (CD40+ CD22+; yellow) are scattered in T-cell areas, and about 70% of IDC (CD40+ CD22−; green) attach to a few B cells directly. FITC-anti-CD40 and PE-anti-CD22. Magnification, ×540. h: CD83+ IDC (red) attach to several IgD+ B cells (green) directly. FITC-anti-IgD and PE-anti-CD83. Magnification, ×650. i: A small number of CD83+ IDC (red) are present in B cell (CD20+; green)-rich areas. FITC-anti-CD20 and PE-anti-CD83. Magnification, ×540.
IDC were completely negative for monocyte/macrophage markers CD11c and CD14 (data not shown), B cell markers CD20 and CD22, and T-cell markers CD3 and CD8, although some IDC were weakly positive for helper T cell marker CD4 (Figure 1e) ▶ .
IDC usually exhibited extensively irregular contours and closely contacted numerous T cells, which were mostly CD4+ T cells (Figure 1e) ▶ . Although a considerable number of CD8+ T cells were present in the T cell areas, only a small number of them were found to attach to IDC (Figure 1f) ▶ . Only a small number of T cells attaching to IDC expressed IL-2R and CD40L (data not shown).
A considerable number of B cells were scattered in the T cell areas. Two-color immunofluorescent microscopy using FITC-anti-CD40 and PE-anti-CD22 indicated that approximately 70% of IDC in the T cell areas attach to a few CD22+ B cells (Figure 1g) ▶ . Interestingly, some of these attaching B cells were morphologically different from ordinary B cells in extending one or two cytoplasmic projections, through which some of B cells attached to IDC. B cells attaching to IDC were positive for IgD and IgM (Figure 1h) ▶ , but negative for IgA and IgG.
In addition, a small number of IDC, comprised of large dendriform cells positive for HLA-DR, CD40, CD54, CD83, and CD86, were found in B cell areas such as the periphery of the primary follicles (Figure 1i) ▶ . They were surrounded by numerous B cells and morphologically closely connected with neighboring B cells. However, no IDC were found within the germinal centers.
IDC in the Fresh LN Cell Suspension
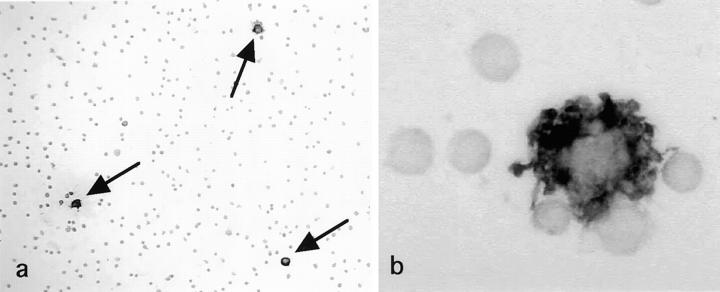
Because S-100b protein is a specific cytoplasmic marker present for IDC in the human lymphoid tissues, 14,16 the proportion of IDC in the fresh smeared LN cells was determined by S-100b immunoperoxidase method. As shown in Figure 2,a,b ▶ , IDC were clearly identified as S-100b+ large dendriform cells, which tended to form complexes with several small lymphocytes. The percentage of IDC in the fresh smeared LN cell was 0.6 (mean) ± 0.2 (SD). By immunofluorescent microscopy, IDC could be clearly distinguished from other types of cells as HLA-DRbright large cells with irregular contours co-expressing CD40, CD54, CD80, CD83, and CD86 (Figure 3,a–c) ▶ .
Figure 2.
S-100b-immunoperoxidase staining applied to fresh smeared mesenteric LN cells. S-100b protein is detected specifically in the cytoplasm of IDC. a: Approximately 0.6% cells corresponded to S-100b+ IDC, which were larger than lymphocytes and tend to attach several lymphocytes. Arrows indicate S-100b+ IDC. Magnification, ×100 b: A high power view of S-100b+ IDC. Note intense S-100b+ immunoreaction products in the cytoplasm of IDC attaching to a few lymphocytes. Magnification, ×1700.
Figure 3.
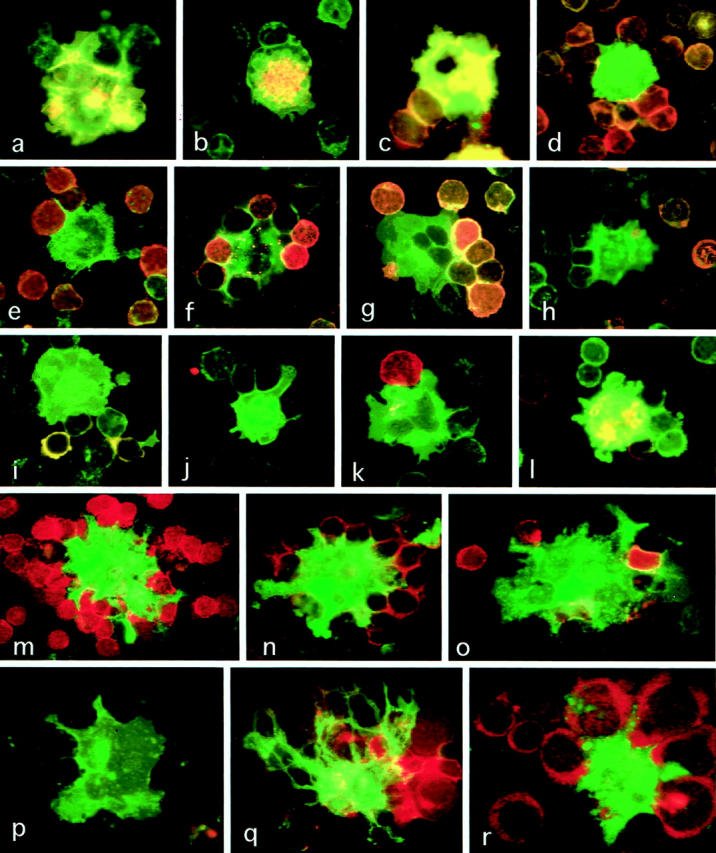
Two-color immunofluorescent microscopy applied to suspended cells obtained from the human mesenteric lymph nodes. a–l: Fresh mesenteric LN cells. a: IDC doubly positive for HLA-DR and CD86 (yellow) attaching to B cells singly positive for HLA-DR (green). FITC-anti-HLA-DR and PE-anti-CD86. Magnification, ×1200. b: HLA-DR+ IDC co-express CD83. FITC-anti-HLA-DR and PE-anti-CD83. Magnification, ×1200. c: HLA-DR+IDC co-express CD40. FITC-anti-HLA-DR and PE-anti-CD40. Magnification, ×1200. d: HLA-DR+ CD22-IDC (green) attaching to many CD22+ HLA-DR+ B cells (yellow-orange). FITC-anti-HLA-DR and PE-anti-CD22. Magnification, ×1200. e: HLA-DR+ CD20- IDC (green) attach to many CD20+ HLA-DR+ B cells (yellow-orange). FITC-anti-HLA-DR and PE-anti-CD20. Magnification, ×1200. f: HLA-DR+ IgD- IDC (green) attaching to many IgD+ HLA-DR+ B cells (orange) FITC-anti-HLA-DR and PE-anti-IgD. Magnification, × 1200. g: HLA-DR+ IgM- IDC (green) attaching to many IgM+ HLA-DR+ B cells (orange). FITC-anti-HLA-DR and PE-anti-IgM. Magnification, ×1200. h: IgA+ HLA-DR+ B cells (orange) are present outside the IDC-B cell cluster. FITC-anti-HLA-DR and PE-anti-IgA. Magnification, ×1200. i: IgG+ B cells (orange) are present outside the IDC-B cell cluster. FITC-anti-HLA-DR and PE-anti-IgG. Magnification ×1,200. j: T cells attaching to IDC are mostly negative for CD40L. FITC-anti-HLA-DR and PF-anti-CD40L. Magnification, ×1200. k: Only a small number of CD3+ T cells (red) are found to attach to HLA-DR+ IDC (green). FITC-anti-HLA-DR and PE-anti-CD3. Magnification, ×1200. l: only a small number of CD4+ T cells (red) attach to IDC, which are doubly positive for CD4 and HLA-DR (yellow). FITC-anti-HLA-DR and PE-anti-CD4. Magnification, ×1200. m–r: Mesenteric LN cells cultured for 7 days. m: HLA-DR+ IDC showing dendriform appearance (green) cluster numerous CD3+ T cells (red). FITC-anti-HLA-DR and PE-anti-CD3. Magnification, ×1200. n: HLA-DR+ IDC showing dendriform appearance (green) cluster numerous CD4+ T cells (red). FITC-anti-HLA-DR and PE-anti-CD4. Magnification, ×1200. o: Only a small number of CD8+ T cells (red) are found within the IDC-T cell cluster. FITC-anti-HLA-DR and PE-anti-CD8. Magnification, ×1200. p: B cells are not found within the IDC-T cell cluster. FITC-anti-HLA-DR and PE-anti-CD22. Magnification, ×1200. q: T cells attaching to IDC with extensive dendriform appearance are activated T cells expressing CD40L (red). FITC-anti-HLA-DR and PE-anti-CD40L. Magnification, ×1200. r: T cells attaching to IDC are activated T cells expressing IL-2R (red). FITC-anti-HLA-DR and PE-anti-IL-2R. Magnification, ×1200.
Morphological interactions of IDC with T and B lymphocytes were studied by two-color immunofluorescent microscopy using FITC-anti-HLA-DR and PE-anti-CD3, PE-anti-CD4, PE-anti-CD8, PE-anti-CD20, or PE-anti-CD22. IDC tended to form clusters with several or sometimes numerous lymphocytes. The majority (approximately 80%) of clustered lymphocytes were B cells, which expressed CD20, CD22, HLA-DR, and CD40 (Figure 3, d and e) ▶ . IDC often extended narrow slender cytoplasmic projections between two adjacent attaching B or T lymphocytes (Figure 3, e,f) ▶ . The immunoglobulin class of the clustered B cells was analyzed by two-color indirect immunofluorescent microscopy with anti-HLA-DR and anti-IgD, anti-IgM, anti-IgA, or anti-IgG. The results indicated that the cultured B cells were mostly IgD+ IgM+ naive B cells, and no IgA+ or IgG+ B cells were present within the clusters (Figure 3, f–i) ▶ . Although many T cells were present outside of the clusters, only a small number of T cells, which were mostly CD4+ IL-2R- CD40L- nonactivated T cells, were found within clusters (Figure 3, j–l) ▶ .
IDC in Cultured LN-Cell Suspension
The LN cells were cultured in 10% FCS-supplemented RPMI 1640 medium for 3 to 22 days. On culturing, IDC became larger and extensively dendritic in shape and formed clusters with several or sometimes numerous T cells, which were mostly CD4+ helper T cells, and CD8+ T cells were found in the clusters only in small numbers (Figure 3, m–o) ▶ . B cells within the clusters gradually decreased and almost completely disappeared within 7 days (Figure 3p) ▶ . These cultured T cells were mostly CD25+ CD40L+ -activated T cells (Figure 3, q and r) ▶ , and such IDC/activated T cell-clusters were stable for at least 22 days, whereas other nonclustered cells tended to die in prolonged culture, although cell death was not evident until day 10 in culture. The percentage of IDC in the LN cells cultured for 7 days was approximately 0.6 as determined by indirect immunoperoxidase method for S-100b protein (data not shown).
Flow Cytometric Analysis
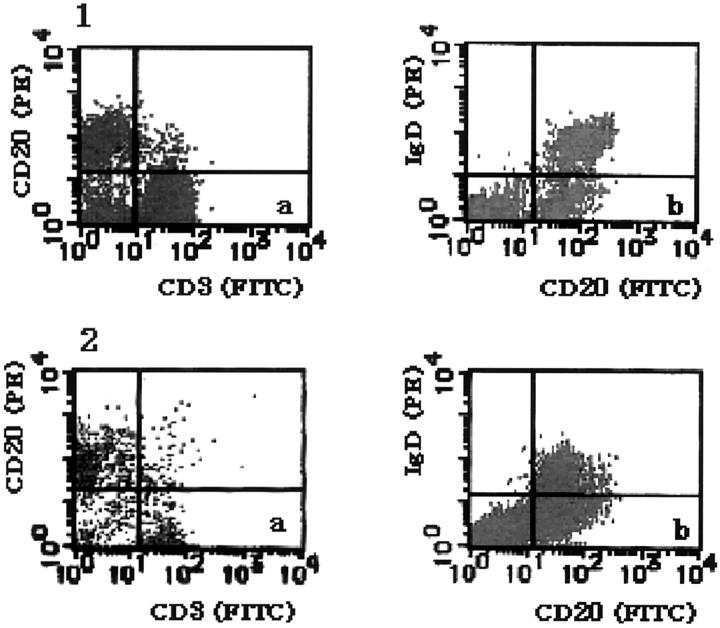
T cells, B cells, naive B cells, and non-naive B cells in the mesenteric LN cells were identified as CD3+, CD20+, IgD+/CD20+, or IgD-/CD20+ cells, respectively. The percentages of these subsets in fresh mesenteric LN cells obtained from the 5 patients were calculated by a FACScan. The results indicated that T cells were 58 ± 15.7% and B cells were 31.5 ± 11.7% (Figure 4, 1a ▶ ). Approximately 10% were non-T/non-B cells, which could not be further characterized. The percentages of naive B and of non-naive B cells were 9.2 ± 1.5 or 18.8 ± 8.8, respectively (Figure 4, 1b) ▶ ▶ .
Figure 4.
Two-color flow cytometry for T and B lymphocytes. Vertical and horizontal bars indicate negative borders determined by the class-matched controls of the gated lymphocyte fraction. Fresh (1) or 7-day-cultured (2) mesenteric LN cells. a: LN cells stained with FITC-conjugated anti-CD3 and PE-conjugated anti-CD20. In the fresh materials, T cells (CD3+) are 58 ± 15.7% and B cells (CD20+) are 31.5 ± 11.7% (1a). In the 7-day-cultured materials, T cells are approximately 47% or B cells are 42.5% (2a). b: LN cells stained with FITC-conjugated anti-CD20 (for B cells) and by indirect immunofluorescent method using rabbit anti-human IgD and PE-conjugated anti-rabbit IgG (for naive B cells). In the fresh materials, naive B cells (CD20+/IgD+) are 9.2 ± 1.5% and non-naive B cells (CD20+/IgD-) are 18.8 ± 8.8% (1b). In the 7-day-cultured materials, naive B cells are approximately 18% and non-naive B cells are approximately 20% (2b).
The proportions of these subsets in the mesenteric LN obtained from 2 patients were also estimated after culture for 7 days. The percentages of T cells and of B cells in mesenteric LN cells cultured for 7 days were approximately 40 or 55 respectively (Figure 4, 2a) ▶ ▶ . The percentages of naive B cells and of non-naive B cells were approximately 18 or 20, respectively (Figure 4, 2b) ▶ ▶ .
The proportion and immunophenotype of IDC in the mesenteric LN cell suspension was determined by three-color flow cytometry. Because IDC in the fresh non-cultured suspension hardly expressed DC-specific antigen CD83 on the surface, although they expressed this antigen in the cytoplasm, the proportion of IDC was determined in the mesenteric LN cells cultured for 7 days. IDC were clearly identified as CD83+/HLA-DRbright cells in the macrophage/IDC fraction (Figure 5) ▶ , which co-expressed CD40. The percentage of CD83+ IDC in total cells was 0.84%. Thus, CD40+ cells in the macrophage/IDC fraction mostly corresponded to IDC, which co-expressed CD86 and CD54, but negative for CD40L.
Figure 5.
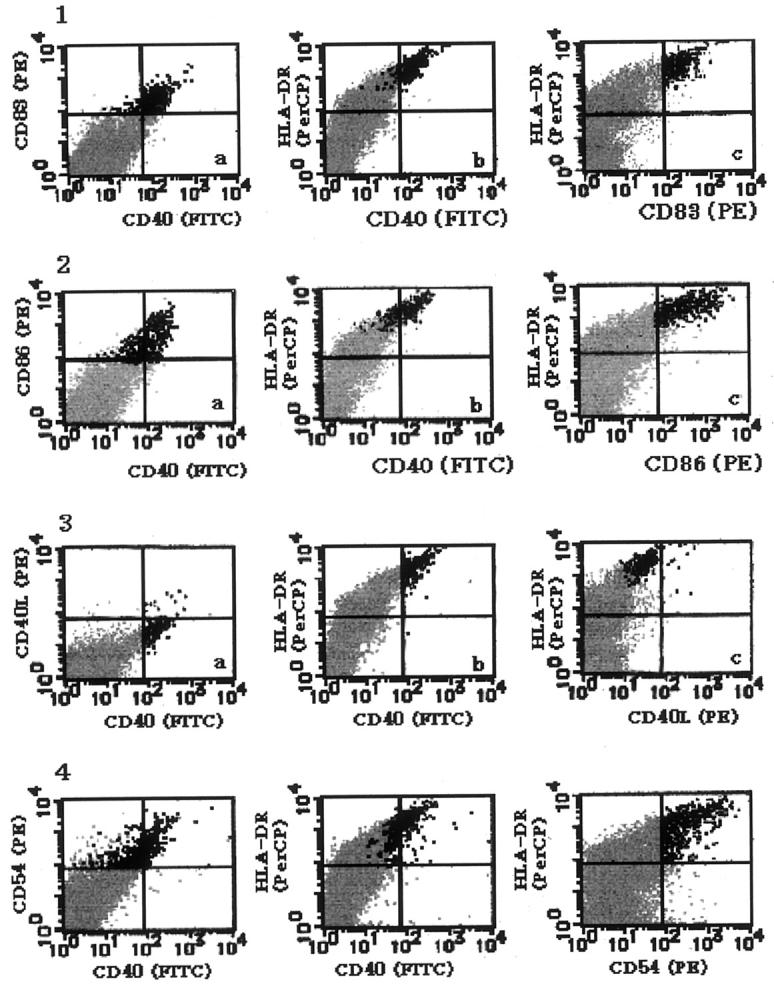
Three-color flow cytometry for IDC in the 7-day-cultured mesenteric LN cell suspension. Vertical and horizontal bars indicate negative borders determined by the class-matched controls of the gated macrophage/DC fraction. Therefore, positive cells in the lymphocyte-fraction are mostly indicated as negative. 1: LN cells stained with FITC-anti-CD40, PE-anti-CD83, and Per-CP anti-HLA-DR. Black dots indicate CD83+ IDC, which comprise 0.84% of total cells. Gray dots indicate the lymphocyte fraction. 2: The LN cells stained with FITC-anti-CD40, PE-anti-CD86, and PerCP-anti-HLA-DR. Black dots indicate CD86+ cells, which mostly co-express CD40 and HLA-DR. Gray dots indicate the lymphocyte fraction. 3: The LN cells stained with FITC-CD40, PE-CD40L, and PerCP-anti-HLA-DR. Black dots indicate CD40+ cells, and almost all of them are negative for CD40L. Gray dots indicate the lymphocyte fraction. 4. The LN cells stained with FITC-anti-CD40, PE-anti-CD54, and PerCP-anti-HLA-DR. Black dots indicate CD54+ cells, which mostly co-express CD40 and HLA-DR. Gray dots indicate the lymphocyte fraction.
Discussion
It is well established that DC play a major role in initiating T-cell mediated immune responses as sentinels of the immune system, capturing Ag in the periphery and activating Ag-specific resting naive T cells by presenting Ag to them after migration to the T cell areas of secondary lymphoid tissues. 1,2 In contrast, little is known about the role and function of DC in the regulation of B-cell mediated immune responses. It has been suggested that the site of primary B-cell activation is within the T-cell areas of secondary lymphoid tissues, 5-7 where numbers of IDC are present. Recently, Bjorck et al 13 have demonstrated in experimental animals that DC can cluster B cells in vivo and in vitro.They also reported that IDC directly interacted with IgD+ naive B cells in the human tonsils. 13 Based on these observations, it has been postulated that direct interaction of DC with B cells may elicit B-cell response. 17 However, little is known about the interactions between B cells and IDC especially in human lymphoid tissues. In the present study, we demonstrated that the majority of IDC in T cell areas of the human mesenteric LN attach to IgD+ naive B cells directly, while no IgG+ or IgA+ non-naive B cells were found to attach to IDC. Moreover, the present findings that naive B cells attaching to IDC tended to be morphologically different from ordinary B cells in extending one or two short cytoplasmic projections, and that naive B cells often attached to IDC through such cytoplasmic projections, implies that naive B cells are actively involved in DC/B cell association. These results confirm the report by Bjorck et al 13 that IDC actually interact with naive B cells directly in the T-cell areas of the human secondary lymphoid tissues, and support the hypothesis that the activation of resting naive B cells requires direct signals from DC in addition to the signals from helper T cells. It is well established that resting T cells have an Ag-nonspecific ability to adhere to IDC transiently. 18 T cells are thought to monitor the surface of DC for antigenic MHC-peptide complexes via unknown Ag-nonspecific, transient adhesion. 18,19 Kushnir et al 9 recently demonstrated that DC can participate in similar Ag-independent transient interactions with resting naive B cells independent of T cells. Consistently, when singly suspended human LN cells were incubated for a short time in vitro, IDC formed clusters with IgD+ IgM+ naive B cells preferentially, but no IgA+ or IgG+ B cells were found within the clusters. As shown in this study, naive B cells comprised approximately 10% of the mesenteric LN cells, whereas non-naive B cells comprised approximately 20%. Moreover, as shown in this study, one can often see narrow slender cytoplasmic projections of IDC between two adjacent attaching naive B cells. Taken together, it is reasonable to consider that attachment of naive B cells to IDC is not an in vitro artifact and that IDC have a special affinity for naive B cells, although this should be confirmed by further studies.
The molecular basis for specific attachment of naive B-cells to DC remains to be clarified, although it has been revealed that LFA-1 is partially involved in the cluster formation of B cells and DC. 9 It seems likely that certain cell adhesion molecules other than those specifically expressed on naive B cells mediate this attachment. Further studies should be done to clarify the molecular mechanism of DC/naive B-cell association.
The present finding that freshly suspended IDC clustered preferentially with naive B cells despite the presence of many T cells in the human mesenteric LN, suggests that the affinity of IDC for naive B cells is stronger than that for naive T cells, although this should be confirmed by further studies.
Kushnir et al 9 also demonstrated in experimental animals (rats and mice) that DC/B cell clusters break up within 24 to 48 hours. As shown in this study, in human LN culture, it took as long as 7 days for IDC/B cell-clusters to disappear completely. The present findings that the proportion of B cells, especially the proportion of naive B cells, did not reduce during culture for 7 days, indicate that this disappearance of IDC/B cell cluster was not due to the preferential death of B cells. It seems likely that human resting B cells adhere to IDC nonspecifically for a longer period of time than do rat or mouse B cells. Regardless of such detailed differences, the affinity between human IDC and resting naive B cells is also transient, and IDC never form a stable complex with B cells. The transient nature of the affinity between naive B cells and IDC may explain the fact that IDC are usually surrounded not by B cells but by T cells despite their strong affinity for naive B cells.
As shown in this study, although IDC initially contacted with a small number of CD4+ T cells, which were mostly IL-2R-CD40L-nonactivated T cells, some IDC developed into extensively dendriform cells expressing CD54, CD83, and CD86 strongly, which formed stable clusters with numerous activated CD4+ T cells expressing IL-2R and CD40L on prolonged culture. It is probable that these clustered activated T cells happened to be specific for Ag expressed on the IDC with which they were interacting, and that they were stimulated by the IDC to express these antigens. Once activated by DC, T cells express CD40L, which, in turn, signals the DC to up-regulate co-stimulatory molecules (CD54, CD80, CD86) and to secrete cytokines (IL-1, IL-6, IL-8, IL-12, TNF-α). 20,21 It is noteworthy that both IDC forming clusters with CD40L+ activated T cells and these clustered CD40L+ -activated T cells were continuously alive, while non-clustered cells tended to undergo apoptosis on prolonged culture, although apoptosis was not evident until day 10 in culture. These findings are consistent with those in a previous report in which CD40 ligation counteracted the apoptosis of in vitro-induced DC from CD34+ hematopoietic precursor cells. 22 Moreover, it is suggested that CD40 ligation also counteracts the apoptosis of the activated T cells.
It has been shown that Ag-specific activated helper T cells assist in the immunoglubulin synthesis and class-switching of Ag-specific resting naive B cells through CD40-CD40L interaction. It is probable that Ag-specific IDC/activated T cell complexes act as highly efficient and stable systems to capture rare Ag-specific resting naive B cells from enormous circulating resting naive lymphocytes, and to stimulate them to differentiate into germinal center founder cells or short-lived plasma cells secreting IgM.
In this study, we investigated the immunophenotype of IDC by two-color immuno-fluorescent microscopy. IDC have been previously examined mainly by single or double enzyme immunostaining, but it is very difficult to detect two different surface antigens on the same cells by double enzyme immunostaining, and the precise surface immunological properties of IDC could not be fully determined. In the present study, we demonstrated that almost all IDC, which are defined as HLA-DRbright large dendriform cells present in the T cell areas, co-express CD40, CD54, CD80, CD83, and CD86. Some IDC express CD4 weakly but do not express CD1a. Several investigators have reported that subsets of DC, such as peripheral blood DC and epidermal LC, express CD40L. 23,24 In this study, we demonstrated that almost all IDC in the human mesenteric LN are negative for CD40L. We failed to detect CD40L on the IDC of other LN examined, including axillary, cervical, and mediastinal LN (unpublished data). It seems likely that even though certain immature stages of DC express CD40L, DC lose this antigen during their maturation into IDC.
In our previous study, we detected a considerable number of CD1a+ immature (CD83− CD86−) DC, which are thought to be precursors of IDC derived from epidermal LC, in and/or around the lymph sinuses of the human axillary LN. 15 However, such cells were not observed in the human mesenteric LN in the present study. We also found that CD1a+ immature DC are present in superficial LN, but not in the deeply-located LN such as mesenteric, mediastinal, and para-aortic LN (unpublished data). It seems probable that IDC in such deeply located LN are derived from CD1a- precursors, the nature and origin of which remain unclear.
We recently found that the ability of IDC to cluster resting B cells largely depends on their anatomical location and that the ability of IDC in deeply located LN, which usually have well-developed follicles, tends to be strong, while that in superficial LN which usually have poorly developed or no follicles, tends to be weak (unpublished data), and we assume that the architecture of LN partially depends on the ability of IDC to cluster resting naive B cells.
As shown in this study, a considerable number of IDC, which comprised approximately 0.8% of total cells, could be detected in the mixture of LN cells prepared by the simple method using a glass homogenizer. It has been reported that it is very difficult to isolate IDC from the lymphoid tissues, particularly LNs, and we failed to isolate IDC from the mesenteric LNs. It seems likely that the difficulty in the isolation of IDC from the lymph nodes is largely due to their nature to form complexes with lymphocytes rather than their small number. We used the glass homogenizer method, because such simple methods probably minimize the loss of IDC during the preparation processes and the purification of IDC was not required. This method seems to be useful for the immunomorphological investigation of IDC, although it is clear that this method is not suitable for the functional analysis of IDC.
It is, however, noteworthy that the proportion of LN cells including T cells, B cells, and IDC was not significantly changed during 7-day culture. In short term culture, we recently found that if LN cells are cultured in mixture, the viability of LN cells, which included T cells, B cells, and IDC, is much better than the viability of LN cells when they are cultured separately (unpublished data). It seems likely that mixed LN cell culture provided a microenvironment suitable for the survival of LN cells.
Finally, it is noteworthy that a small number of IDC are present in B-cell-rich areas, such as the periphery of the mantle zones or primary follicles. IDC are not found within the germinal centers; they express the mature DC markers CD83 and CD86 but not CD11c and, therefore, are different from germinal center DC, which are reported to be present in the tonsil germinal centers and to express CD4 and CD11c but not CD83 or CD86. 25 The functional role of these IDC in B-cell-rich areas remains unclear, but the presence of such cells suggests that IDC are more deeply involved in the regulation of B-cell-mediated immunological responses than has been previously thought. Further studies should be performed to clarify the role of DC in the regulation of the B-cell-mediated immunity.
Footnotes
Address reprint requests to Kiyoshi Takahashi, M.D., Ph.D., Faculty of Health Science, Okayama University Medical School, 2-5-1 Shikata-cho, Okayama 700-8558, Japan. E-mail: dend@cc.okayama-u.ac.jp.
References
- 1.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1988, 392:245-252 [DOI] [PubMed] [Google Scholar]
- 2.Ingulli E, Mondino A, Khoruts A, Jenkins MK: In vivo detection of dendritic cell antigen presentation to CD4+ T-cells. J Exp Med 1997, 185:2133-2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford WL: Lymphocyte migration and immune responses. Prog Allergy 1975, 19:1-59 [DOI] [PubMed] [Google Scholar]
- 4.Young AJ: The physiology of lymphocyte migration through the single lymph node in vivo. Semin Immunol 1999, 11:73-83 [DOI] [PubMed] [Google Scholar]
- 5.Gray D: Recircuitment of virgin B cells into an immune response n restricted to activation outside lymphoid follicles. Immunology 1988, 65:73-79 [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob J, Kassir R, Kelsoe G: In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 1991, 173:1165-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ, Zhang J, Lane PJ, Chan FY, MacLennan IC: Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol 1991, 21:2951-2962 [DOI] [PubMed] [Google Scholar]
- 8.Liu YJ, Arpin C: Germinal center development. Immunol Rev 1997, 156:111-126 [DOI] [PubMed] [Google Scholar]
- 9.Kushnir N, Liu L, MacPherson G: Dendritic cells and resting B cells form clusters in vitro and in vivo: T cell independence, partial LFA-1 dependence, and regulation by cross-linking surface molecules. J Immunol 1998, 160:1774-1781 [PubMed] [Google Scholar]
- 10.Wykes M, Pombo A, Jenkins C, MacPherson GG: Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J Immunol 1998, 161:1313-1319 [PubMed] [Google Scholar]
- 11.MacPherson G, Kushnir N, Wykes M: Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev 1999, 172:325-334 [DOI] [PubMed] [Google Scholar]
- 12.Feyette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Banchereau J, Caux C, Briere F: Human dendritic cells skew switching of CD40-activated naive B cells towards IgA1 and IgA2. J Exp Med 1997, 185:1909-1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorck P, Flores-Romo L, Liu YJ: Human interdigitating cells directly stimulated D40-activated naive B cells. Eur J Immunol 1997, 27:1266-1274 [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Isobe T, Ohtsuki Y, Sonobe H, Takeda I, Akagi T: Immunohistochemical localization and distribution of S-100 proteins in the human lymphoreticular system. Am J Pathol 1984, 45:385-396 [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Asagoe K, Jin Z, Yanai H, Yoshino T, Hayashi K, Akagi T: Heterogeneity of dendritic cells in human superficial lymph node: in vitro maturation of immature dendritic cells into mature or activated interdigitating reticulum cells. Am J Pathol 1998, 153:745-755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Yamaguchi H, Ishizeki J, Nakajima T, Nakazato Y: Immunohistochemical and immunoelectron microscopic localization of S-100 protein in interdigitating reticulum cells of the human lymph node. Virchows Arch B Cell Pathol 1981, 45:385-396 [DOI] [PubMed] [Google Scholar]
- 17.Clark EA: Regulation of B lymphocytes by dendritic cells. J Exp Med 1997, 185:801-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Romani N, Steinman RM: An antigen-independent contact mechanism as an early T cell-proliferative response to dendritic cells. J Exp Med 1989, 170:527-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaba K, Steinman RM: Monoclonal antibodies to LFA-1 and to CD4 inhibit the mixed leukocyte reaction after the antigen-dependent clustering of dendritic cells and T lymphocytes. J Exp Med 1987, 65:403-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caux C, Burdin N, Galibert L, Herman P, Renard N, Sevet-Delprat C, Bancherau J: Functional CD40 on B lymphocytes and dendritic cells. Res Immonol 1994, 145:235-239 [DOI] [PubMed] [Google Scholar]
- 21.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lenzavecchia A, Alber G: Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 1996, 184:747-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorck P, Banchereau J, Flores RL: CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Intern Immunol 1997, 9:356-372 [DOI] [PubMed] [Google Scholar]
- 23.Pinchuk LM, Klaus SJ, Magaletti DM, Pinchuk GV, Norsen JP, Clark EA: Functional CD40 ligand expressed by human blood dendritic cells is up-regulated by CD40-ligation. J Immunol 1996, 157:4363-4370 [PubMed] [Google Scholar]
- 24.Salgado CG, Nakamura K, Sugaya M, Asahina A, Koyama Y, Irie S, Tamaki K: Functional CD40 ligand is expressed on epidermal Langerhans cells. J Leukoc Biol 1999, 66:281-285 [DOI] [PubMed] [Google Scholar]
- 25.Grouard G, Durand I, Filgueira L, Banchereau J, Liu Y-J: Dendritic cells capable of stimulating T cells in germinal centers. Nature 1996, 384:364-367 [DOI] [PubMed] [Google Scholar]