Abstract
During human pregnancy specialized placental cells of fetal origin, termed cytotrophoblasts, invade the uterus and its blood vessels. This tumor-like process anchors the conceptus to the mother and diverts the flow of uterine blood to the placenta. Previously, we showed that the expression of molecules with important functional roles, including a number of extracellular matrix integrin receptors, is precisely modulated during cytotrophoblast invasion in situ. Here we exploited this observation to study the role of the focal adhesion kinase (FAK), which transduces signals from the extracellular matrix and recruits additional signaling proteins to focal adhesions. Immunolocalization studies on tissue sections showed that FAK is expressed by cytotrophoblasts in all stages of differentiation. Because extracellular matrix-induced integrin clustering results in FAK (auto)phosphorylation on tyrosine 397 (Y397FAK), we also localized this form of the molecule. Immunolocalization experiments detected Y397FAK in a subset of cytotrophoblasts near the surface of the uterine wall. To assess the functional relevance of this observation, we used an adenovirus strategy to inhibit cytotrophoblast expression of FAK as the cells differentiated along the invasive pathway in vitro. Compared to control cells transduced with a wild-type virus, cytotrophoblasts that expressed antisense FAK exhibited a striking reduction in their ability to invade an extracellular matrix substrate. When cytotrophoblast differentiation was compromised (hypoxia in vitro, preeclampsia in vivo), Y397FAK levels associated with the plasma membrane were strikingly lower, although total FAK levels did not change. Together our results suggest that (auto)phosphorylation of Y397 on FAK is a critical component of the signaling pathway that mediates cytotrophoblast migration/invasion.
During tumorigenesis, precursor cells that presumably were once normal become cancerous. Clonal selection, which takes months to years, is an integral part of this process. In the end, only a subset of cells with favorable attributes, including unregulated proliferation, invasiveness, and the ability to acquire a blood supply, survive. In the interim it is difficult to define critical steps in the molecular transitions that select particular clones from heterogeneous mixtures of precursor cells. Identification of the individual changes that promote the metastatic phenotype in the context of the global nature of downstream consequences is a pivotal problem in modern tumor biology.
During development of the human placenta, the organ’s specialized epithelial cells, termed cytotrophoblasts (CTBs), encounter many of the same obstacles that tumor cells must overcome during clonal selection. Specifically, a subset of these CTBs must detach from the basement membrane, where they reside as a monolayer in the fetal component of the placenta, and invade the uterus, where they survive only if they access a supply of maternal blood. Because subsequent human development depends on the tumor-like properties of this subpopulation of CTBs, the molecular mechanisms that are involved have been carefully programmed. Consequently, the cells acquire the ability to invade as part of a tightly regulated, stepwise differentiation process (diagrammed in Figure 1A ▶ ). 1 Furthermore, this subset of CTBs stops proliferating once it initiates the program leading to an invasive phenotype. 2 Therefore, studying the process of CTB differentiation/invasion offers a unique opportunity to identify, by drawing functional analogies, molecules particularly likely to play important roles in promoting the invasive phenotype of tumor cells.
Figure 1.

Diagram (A) and histological section (B) of an anchoring chorionic villus at the fetal-maternal interface at the beginning of the second trimester of pregnancy (15 weeks of gestation). A: The anchoring villus (AV) functions as a bridge between the fetal and maternal compartments of the human placenta. Both AV and floating villi (FV) are covered with syncytiotrophoblasts (STB), contain fetal blood vessels (FBV), and are bathed by maternal blood in the intervillous space (IVS). CTBs in AV form cell columns (CC) that attach the fetal-placental unit to the uterine wall (UW). CTBs then invade decidua, myometrium, and uterine blood vessels (iCTB). Long arrow denotes direction of CTB migration/invasion. B: A histological section of the fetal-maternal interface diagrammed in (A) was stained with anti-cytokeratin (CK) to image all of the trophoblast populations. vCTB; villous CTB.
Much of our previous work focused on the role of adhesion molecules in CTB invasion. Initially we immunolocalized a battery of adhesion receptors and their ligands on tissue sections that contained CTBs in all stages of differentiation/invasion. These studies allowed us to phenotype differentiating cells in situ. The results showed that CTBs intricately modulate their expression of a wide repertoire of antigens that function in adhesion. This modulation process begins when the cells leave their basement membrane in the placenta, and ends when they colonize maternal blood vessels in the uterus. The onset of CTB differentiation/invasion is signaled by reduced staining for receptors characteristic of polarized CTB epithelial stem cells—integrin α6β4 and E-cadherin—and the onset of expression of adhesion receptors expressed on endothelium—VE-cadherin, Ig family members VCAM-1 and PECAM-1, and integrins αvβ3 and α1β1. 3-6 Cadherin-11 is also up-regulated on extravillus CTBs and on decidualizing endometrial stroma. 7,8 By using an in vitro model of CTB invasion that replicates the process of adhesion molecule switching in vivo, we assessed the contribution of individual adhesive interactions to the invasion process. In this regard, we found a particularly important role for integrin cell-extracellular matrix (ECM) receptors. A number of these molecules whose expression is up-regulated during differentiation promote invasion (eg, α1β1, αvβ3), whereas others are inhibitory (eg, α5β1). Thus, this highly regulated invasive program is governed by a system of checks and balances.
Together, our previous work suggests that the intracellular mechanisms that CTBs use to translate the information they receive via integrin receptors into signals is crucial to their ability to invade the uterus. Integrin interactions with ECM components lead both to occupancy of their ligand-binding sites and to clustering, which assembles multimolecular signaling complexes in focal adhesions, sites of cell-ECM interactions. 9,10 Thus, integrin signals are connected to multiple intracellular signaling pathways. 11 One important outcome of integrin clustering by ECM is the (auto)phosphorylation of focal adhesion kinase (FAK) on tyrosine 397 (pY397FAK). pY397FAK, in turn, serves as the backbone of a scaffold that recruits additional signaling proteins to focal adhesions. 12 FAK has been implicated in the transduction of survival signals from ECM 13-16 and in promoting cell migration. 17-22 Both processes are relevant to establishing a pool of cells with a migratory/invasive (metastatic) phenotype.
Several studies have attempted to determine whether there is a correlation between the levels of FAK expression and the acquisition of metastatic potential. Although FAK was overexpressed in some types of highly invasive tumors, the degree of FAK expression was not a significant prognostic factor. 23 Interestingly, comparative studies of normal breast tissue and invasive breast carcinoma revealed that FAK levels can be elevated 24 or reduced 25 in transformed versus normal tissue. From these studies it seems that regulation of total FAK levels is not crucial for promoting an invasive phenotype. Thus, the key might be regulation of its activity.
In the current study we have extended our work on the adhesion phenotype switching that occurs as CTBs differentiate/invade to address the putative role(s) of FAK in this unusual tumor-like behavior. Again, we have used a combination of in situ and in vitro approaches. Localization studies in placental tissues showed that CTBs in all stages of differentiation express FAK. However, only a subset of CTBs invade the uterine wall (Figure 1A) ▶ . Therefore, we hypothesized that some of these cells had high levels of (auto)phosphorylated FAK. To test this idea, we immunolocalized pY397FAK on tissue sections of the fetal-maternal interface. We also tested the effect of reducing CTB expression of FAK protein on the cells’ ability to invade a Matrigel substrate in vitro. Finally, we asked whether conditions that inhibit CTB invasion (hypoxia in vitro, preeclampsia in vivo) were associated with a reduction in FAK (auto)phosphorylation, as determined by measuring FAK kinase activity and by staining for pY397FAK. These results support the hypothesis that FAK (auto)phosphorylation on Y397 plays a key role in promoting the invasive behavior of normal CTBs and, by analogy, may also play an important role in tumor development.
Materials and Methods
Antibodies and Antibody Characterization
The 7D3 rat anti-human cytokeratin monoclonal antibody (mAb) was raised in this laboratory. 3 Several antibodies recognized both the phosphorylated and the nonphosphorylated forms of FAK: two mouse mAbs (Transduction Laboratories, Inc., San Diego, CA, and Chemicon, Temecula, CA); rabbit polyclonal antibodies A-17, C-20 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and JF1; 17 and goat polyclonal antibodies A-17 and C-20 (Santa Cruz Biotechnology Inc.). Anti-paxillin mAb was purchased from Zymed (South San Francisco, CA) and anti-phosphotyrosine 4G10 from UBI (Lake Placid, NY).
An antibody that recognized only FAK (auto)phosphorylated on Tyr397 (pY397FAK) was obtained from BioSource International Inc. (Camarillo, CA). To validate its use in immunolocalization studies, the specificity of this antibody was rigorously tested before initiation of the experiments reported here. Briefly, tissue sections cut from several organs of fak knockout mice and the parental wild-type animals were analyzed along with cultured cells from both strains. 17 The samples from fak-null animals, which served as negative controls, also had enhanced levels of potentially cross-reactive antigens, including proteins phosphorylated on tyrosine and the homologous (∼50%) FAK family member Pyk2. 26 Initial experiments showed that anti-pY397FAK antibody at a concentration of 2.5 μg/ml stained tissue sections and cells from wild-type, but not fak-null, animals. Staining was completely abolished when the antibody was preincubated with 100 μg/ml of the phosphopeptide used to generate the antibody, whereas addition of 100 μg/ml of the nonphosphorylated peptide had no effect. Preincubation with a phosphopeptide (100 μg/ml) that corresponds to the homologous sequences in Pyk2 had also no effect on the staining specificity of anti-pY397FAK antibody.
Tissue Sources for Immunolocalization Experiments
Control samples of chorionic villi and the portion of the uterine wall to which they attached were obtained from pregnancy terminations for nonmedical reasons and from uncomplicated spontaneous deliveries. Because of their relatively small size, samples from each first trimester placenta were processed in toto. Second and third trimester samples were from three to five randomly chosen sites. None of the samples had abnormalities that could be detected either grossly or histologically. Tissues were obtained from women whose pregnancies were terminated during the first trimester (52 samples), second trimester (7 samples), or at the time of delivery (8 samples). Conclusions were based on analysis of all samples in each group.
Samples of chorionic villi and the portion of the uterine wall to which they attached were also obtained by placental bed biopsy of women who received prenatal care at the University of California San Francisco (Moffitt/Long Hospital). Informed consent for the procedure was obtained during one of the late second/early third trimester clinic visits. The consent form and biopsy procedure were approved by the University of California San Francisco Committee on Human Research. Control samples were obtained from eight women who had no underlying medical conditions at 33 (n = 1), 35 (n = 4), and 36 (n = 3) weeks of gestation. Eight samples were obtained from preeclamptic patients at 32 (n = 1), 33 (n = 4), and 35 (n = 3) weeks of gestation. Preeclampsia was diagnosed according to the following criteria, recommended by Chesley 27 : nulliparity; no history of hypertension before pregnancy; increase in diastolic pressure of 15 mm Hg or systolic pressure of 30 mm Hg compared with blood pressure obtained before 20 weeks of gestation; proteinuria ≥0.3 g/24 hours (or 1+ on urine dipstick) in a catheterized specimen; hyperuricemia >5.5 mg/dl (or 1 SD greater than the normal mean value before term); return to normal blood pressure and resolution of proteinuria by 12 weeks postpartum. Severe preeclampsia was diagnosed according to the following criteria recommended by the American College of Obstetricians and Gynecologists: systolic blood pressure ≥160 mm Hg and/or diastolic pressure ≥110 mm Hg; proteinuria of ≥5 g in a 24-hour period or 3+ on urine dipstick; presence of cerebral or visual disturbances. Two patients were diagnosed with severe preeclampsia and were delivered by Cesarean section (32 and 33 weeks); six patients with preeclampsia had vaginal deliveries [33 (n = 3) and 35 (n = 3) weeks].
Immunolocalization
Placental tissues were processed for double indirect immunolocalization as previously described. 2,3 Briefly, tissues were fixed in 3% paraformaldehyde for 30 minutes, washed three times in phosphate-buffered saline (PBS), infiltrated with 5 to 15% sucrose followed by optimal cutting temperature (OCT) compound (Miles Scientific, Naperville, IL), and frozen in liquid nitrogen. Sections (6 μm) were prepared using a cryostat (Slee International, Inc., Tiverton, RI). Nonspecific antibody reactivity was blocked by using a commercially available kit (Vector, South San Francisco, CA). Sections were then incubated for 2 days at 4°C with a mixture of the three antibodies: 1) 2.50 μg/ml of ChromPure donkey whole IgG (Jackson ImmunoResearch, West Grove, PA); 2) 1 μg/ml of 7D3 anti-human cytokeratin; and 3) 1 μg/ml of either anti-FAK (JF1) or anti-pY397FAK. Then, the sections were rinsed three times in PBS for 10 minutes and incubated for 30 to 60 minutes at room temperature with the appropriate species-specific secondary antibodies conjugated to rhodamine (donkey anti-rat IgG) or biotin (donkey anti-mouse or anti-rabbit IgG), and washed three times in PBS for 10 minutes. Sections were then incubated for 15 minutes at room temperature with streptavidin/fluorescein isothiocyanate conjugate (Vector) and 10 μg/ml Hoechst 33342 (Molecular Probes, Eugene, OR), washed three times in PBS for 10 minutes, and mounted with Vectashield medium (Vector). Samples were examined with a Zeiss Axiophot Epifluorescence microscope (Thornwood, NY) equipped with filters to selectively view the rhodamine, fluorescein, and Hoechst 33342 fluorescence. When FAK was immunolocalized in cultured cells, fixation was followed by permeabilization (10 minutes in cold methanol). All other steps were performed using the protocol described for sections.
Several types of control incubations were included. Sera (preimmune or isotype-matched nonimmune) and PBS were substituted for the primary antibodies. In addition, fluorescein isothiocyanate-donkey anti-mouse and fluorescein isothiocyanate-donkey anti-rabbit IgG were substituted for biotin-conjugated reagents to detect binding of the primary antibody. Finally, the effects on blocking and control peptides were assessed as described above. Staining of control tissue sections and cells was never observed.
Cell and Tissue Culture
CTBs were isolated from pools of multiple placentas as described. 28 The placentas were obtained immediately after first trimester terminations and third trimester deliveries. Remaining leukocytes were removed by using a mAb to CD45 coupled to magnetic beads. The purified CTBs were cultured in DME H21 minimal essential medium containing 2% Nutridoma (Roche Molecular Biochemicals, Indianapolis, IN) and 50 μg/ml of gentamicin on two substrates. Routinely the cells were plated on Matrigel-coated tissue culture wells (Collaborative Biomedical Products, Bedford, MA). Co-cultures were also established with decidua (n = 4, 10 to 12 weeks of gestation).
Anchoring villi were prepared for culture as previously described. 29,30 Briefly, small fragments of placental tissue from the fetal-maternal interface were teased apart until they had the characteristic tree-like appearance of chorionic villi as viewed in a stereomicroscope. Anchoring villi were identified by the attached remnants of cell columns. Light microscopic examination of hematoxylin-stained sections of villus tissue preparations consistently showed the presence of floating and anchoring villi and the absence of endometrial contamination. Anchoring villi (wet weight 5 to 10 mg) were cultured on the surface of Matrigel-coated 12-mm Millicell-CM culture dish inserts (0.4 μm pores; Millipore Corp., Bedford, MA). The inserts were then placed into 24-well dishes in a mixture of F12 HAM/Dulbecco’s modified Eagle’s medium (1:1/v:v) culture medium (Sigma Chemical Co., St. Louis, MO) supplemented with an antibiotic/antimycotic mixture (100 U/ml penicillin, 100 μg/ml streptomycin, 1.25 μg/ml amphotericin B; Sigma) and 10% fetal calf serum. Each group (control and experimental) consisted of five explants. In toto, 12 placentas were individually analyzed.
Isolated CTBs, co-cultures and anchoring villi were maintained either under standard tissue conditions (5% CO2/95% air) or placed in a Bactron anaerobic incubator (Sheldon Manufacturing Inc., Cornelius, OR), where they were maintained in a 2%O2/93% N2/5% CO2 environment. Dissolved O2 at the cell-medium interface, measured using micro-oxygen electrodes (MI-730; Microelectrode Inc., Londonderry, NH), was 20% under standard tissue conditions and 2% in the Bactron anaerobic incubator.
BrdU Incorporation
Anchoring villi from 6- to 8-week-old placentas were cultured on Matrigel in either 20% O2 or 2% O2. After 48 hours, the original medium was replaced with fresh medium containing 1 μmol/L 5-bromo-2′-deoxyuridine (BrdU, Roche Molecular Biochemicals). Twenty-four hours later tissue was washed, fixed, embedded in OCT, and frozen in liquid nitrogen. Sections cut from these blocks were stained with fluorescein isothiocyanate-labeled anti-BrdU monoclonal antibody as recommended by the manufacturer (Roche Molecular Biochemicals).
Adenovirus Construction and Transduction
Adenoviruses were generated by using published methods. 31 The antisense FAK vector (pcDNA3 antisense FAK) was the kind gift of Drs. Y. Takeuchi and M. Suzawa from Kyoto University, Japan. 32 pEGFP-C1 was purchased from Clontech (Palo Alto, CA). NdeI-BamHI fragments of pcDNA3 antisense FAK and pEGFP-C1 were inserted into the pAdlox shuttle vector. The viruses were established by transfecting the ligated adenoviral genome constructs with replication-defective Ψ 5 virus into CRE8 cells that stably overexpressed Cre-recombinase. Positive clones were expanded in HEK293 cells. Adenoviruses that expressed either a FAK antisense oligonucleotide (AdASFAK) or GFP (AdcGFP) were plaque purified (1 × 10 10 pfu/ml) and isolated as described. 31 For transduction, explants or cells were infected with wild-type replication-defective Ψ 5 adenovirus, AdASFAK, or AdcGFP. Before plating, isolated CTBs and anchoring villus explants were incubated for 2 hours in medium containing 20 to 150 pfu/cell of virus. After one washing with PBS, the cells and villi were cultured as described above. At the end of the experiment FAK expression was monitored by immunostaining and immunoblotting.
Invasion Assays
Two assays were used. First, to quantify invasion, 2 × 10 5 CTBs were cultured in Matrigel-coated Transwell inserts (6.5 mm; Costar Corp.) containing polycarbonate filters with 8-μm pores. After 72 hours the cultures were rinsed in PBS, fixed, and stained with anticytokeratin antibodies as described below. To assay invasion, the filters were dissected from the inserts with a scalpel blade and mounted on poly-l-lysine-coated slides in a drop of mounting medium such that the underside of the filters faced upward. The number of cytokeratin-positive cell processes that penetrated the Matrigel and appeared on the underside of the filters was counted. In each of three independent experiments, three filters were used for each experimental condition. Second, CTBs were cultured with decidual explants. 33 Briefly, portions of the decidua parietalis, which does not contain CTBs before culture, were obtained at the time of pregnancy termination (10 to 12 weeks). The tissue was washed three times in ice-cold PBS and cut into 2-mm 3 pieces. After another three washes, five decidual pieces in 500 μl of medium (DME/2% Nutridoma) were plated on each of several Millicell inserts (12-mm diameter) coated with 200 μl of Matrigel. The inserts were transferred to 16-mm culture dishes containing 600 μl of medium and incubated for 6 hours, the amount of time required for the explants to attach to the ECM substrate. Then the medium was aspirated from the upper chamber, and 10 6 CTBs in 500 μl of medium was added. The cultures were maintained for 4 days in either a 2% or a 20% oxygen atmosphere, and medium was changed daily. Three co-cultures were set up for each experimental condition, and the experiments were repeated three times. The samples were fixed in 3% paraformaldehyde for 40 minutes, washed three times in PBS (4°C, 10 minutes), infiltrated with sucrose, embedded in OCT compound, and frozen in liquid nitrogen. Sections (6 μm) were cut using a cryostat (Slee International Inc., Tiverton, RI) and collected on poly-l-lysine-coated microscope slides. The sections were stained as described below. Samples were examined with a Zeiss Axiophot epifluorescence microscope equipped with proper filters and photographed with Kodak T-Max 400 film (Kodak, Rochester, NY).
Western Blots and FAK (Auto)phosphorylation Assay
To examine the efficiency of FAK antisense in reducing levels of the endogenous FAK protein, cells were lysed in modified RIPA buffer [1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 150 mmol/L NaCl, 10 mmol/L Tris/HCl, pH 7.4, 1 mmol/L ethylenediaminetetraacetic acid] containing freshly added protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride). Lysates were precleared with protein G-Sepharose (Amersham Pharmacia Biotech, Piscataway, NJ) and separated by SDS-polyacrylamide gel electrophoresis (PAGE) using 8% gels. Gels were transferred to nitrocellulose (Schleicher & Schuell, Keene, NH) and blotted using monoclonal anti-FAK antibody (Transduction Laboratories).
For kinase assays FAK was immunoprecipitated from cells lysed in modified RIPA buffer using a combination of two goat polyclonal anti-FAK antibodies, A-17 and C-20 (Santa Cruz Biotechnology). Immunoprecipitates were washed and then incubated in universal kinase buffer (10 mmol/L PIPES, pH.7.4, 10 mmol/L MnCl2) in the presence of [γ-32P]ATP for 30 minutes at 30°C. 34 Samples were separated using 8% SDS-PAGE and transferred to nitrocellulose. The membrane was blotted first with anti-FAK mAb (Transduction Laboratories). The bands were visualized using alkaline phosphatase-conjugated secondary antibodies and BCIP/NBT dye substrate (Roche Molecular Biochemicals). Dried membranes were exposed to BioMax MS film (Kodak) to detect incorporated radioactivity.
To assess total protein tyrosine phosphorylation, whole cell/Triton soluble lysates were prepared by solubilizing cells in a buffer containing 1% Triton X-100, 150 mmol/L NaCl, 10 mmol/L Tris/HCl, pH 7.4, 1 mmol/L ethylenediaminetetraacetic acid, and freshly added protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mmol/L phenylmethyl sulfonyl fluoride). Lysates were spun at maximum speed in a microfuge for 10 minutes at 4°C. Dissolving the pellets in modified RIPA buffer yielded a whole cell/Triton insoluble/RIPA soluble lysate. Lysates were spun at maximum speed in a microfuge for 10 minutes at 4°C. Dissolving the pellets in SDS-PAGE sample buffer yielded whole cell/Triton-insoluble/RIPA-insoluble lysates.
Results
Activated FAK Was Detected Only at Sites of CTB Invasion in Situ
Initially, we used immunolocalization techniques to evaluate FAK expression during human CTB invasion in situ. These studies focused on the fetal-maternal interface, which includes floating villi together with anchoring villi, their sites of uterine attachment, and the portion of the uterine wall that CTBs invade. The histological organization of this region is diagrammed in Figure 1A ▶ . A photomicrograph of a tissue section stained with anti-cytokeratin (CK), which labels syncytiotrophoblasts (STBs) and all CTB subpopulations, is shown in Figure 1B ▶ . In both panels the long arrow denotes the direction of migration/invasion.
CTBs in all locations showed high levels of FAK immunoreactivity (Figure 2) ▶ . In mononuclear villus CTBs (vCTB), FAK localized to the plasma membrane region (Figure 2A ▶ , short arrows). In multinucleate STBs, staining of the cytoplasm in a punctate pattern was often detected (Figure 2A ▶ , long arrows). However, the brightest immunoreactivity was found at the vCTB-STB border, suggesting that FAK expression is associated with the apical surfaces of the vCTB plasma membranes, the basal surfaces of STB plasma membranes, or both areas (Figure 2A ▶ , arrowheads). CK-positive invasive CTBs (iCTB) within the uterine stroma also stained for FAK in a pattern that was primarily plasma membrane-associated (Figure 2C ▶ , short arrows). All of the antibodies against FAK protein that were tested, including those recognizing epitopes in the C-terminal or N-terminal region, gave similar staining patterns.
Figure 2.
FAK is ubiquitously expressed in placental trophoblasts. Sections of the fetal-maternal interface (18 weeks) were stained with antibodies against cytokeratin (CK) (B and D) to image all of the trophoblast populations and with several antibodies that recognized all forms of the FAK. A: An example of the staining pattern of one FAK antibody (JF1). FAK staining was detected in all CTB subpopulations, primarily in association with the plasma membrane (short arrows). The cytoplasm of STB stained in a punctate pattern (long arrows). The brightest immunoreactivity was found at the villous CTB (vCTB)-STB border (arrowheads). C: Cytokeratin-positive CTBs that invaded the uterine wall (iCTB) showed diffuse cytoplasmic and bright cell-membrane staining for FAK, denoted by the arrows.
Data from numerous in vitro studies show that FAK (auto)phosphorylation on tyrosine 397 is involved in regulating this molecule’s activity during migratory processes. Accordingly, we stained tissue sections of the fetal-maternal interface with an antibody that recognizes only pY397FAK (Figure 3A) ▶ . vCTBs and STBs showed little or no staining with anti-pY397FAK (not shown). Interestingly, this antibody distinguished two CK-positive iCTB populations within the uterine wall. Strong staining for pY397FAK was seen in sites of initial invasion, CTBs near the surface of the uterine wall that borders the intervillous space, whereas CTBs that had penetrated more deeply did not stain (Figure 3B) ▶ . In pY397FAK-positive cells, immunoreactivity was either diffuse throughout the cytoplasm (Figure 3, D–F ▶ ; arrowheads) or localized to a very specific region (Figure 3, D ▶ -F; short arrows).
Figure 3.
pY397 FAK staining is detected on a subset of CTBs within the uterine wall (UW) in situ. A: Schematic diagram of FAK. The central kinase domain is flanked by large N- and C-terminal domains. The N-terminus contains a FERM (band 4.1, ezrin, radixin, moesin) domain and the C-terminus contains the focal adhesion targeting (FAT) domain. The major FAK (auto)phosphorylation site is on tyrosine Y397 (*Y397) near the junction of the FERM and kinase domains. Tissue sections included the superficial portion of the uterine wall that is adjacent to the intervillous space (IVS). C and E: Staining with anti-cytokeratin (CK) identified iCTBs. B and D: Staining with an antibody that recognized only FAK (auto)phosphorylated on tyrosine 397 (pY397FAK) distinguished two CK-positive iCTB populations within the uterine wall. Strong staining for pY397FAK was seen in CTBs near the uterine surface, adjacent to the intervillous space, whereas CTBs that penetrated more deeply did not stain. In pY397FAK-positive cells, immunoreactivity was either diffuse throughout the cytoplasm (arrowheads) or localized to a very specific region (short arrows).
Antisense FAK Expression Restricted CTB Migration/Invasion from Anchoring Villus Explants in Vitro
Next, we assessed the role of FAK in CTB migration/invasion in vitro. In the absence of a specific inhibitor of FAK (auto)phosphorylation on Y397, we used an adenovirus approach to deliver antisense oligonucleotides that were designed to lower the cells’ expression of FAK protein (AdASFAK). Two in vitro models were used. The first was an organ culture system in which explanted anchoring villi were cultured on Matrigel-coated wells. 29,30 In this model, villi attach to the Matrigel via the remnants of severed CTB columns. Subsequently, the area of the column that is in contact with the Matrigel gives rise to invasive CTBs that migrate out of the explant.
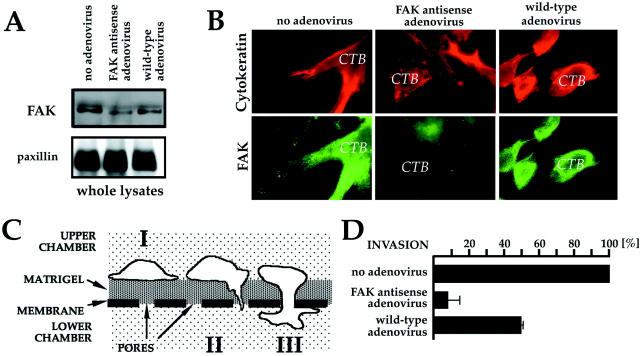
Initially, we used an adenovirus that was engineered to encode green fluorescent protein (AdcGFP) to determine which cells, among the many types found in the villus explant (see Figure 1 ▶ ), were infected. GFP was expressed primarily by CTBs in the cell columns (CC) (Figure 4A) ▶ . This finding is consistent with our previous observation that CTBs in this location up-regulate expression of αvβ3 integrin, 6 which is an adenovirus receptor. 35 GFP was expressed throughout the column, including at the expanded tips that contain outgrowths of migrating/invading CTBs (Figure 4, B and C ▶ ; arrow). In contrast, explants that were infected with AdASFAK (Figure 4D) ▶ showed a consistent reduction in CTB migration/invasion from the cell columns (Figure 4D ▶ , arrowhead). At the end of the experiment, tissue sections were prepared from control explants that expressed GFP and experimental explants that expressed antisense FAK. Immunolocalization showed that cell columns from the experimental explants stained only weakly for FAK as compared to cells in comparable regions of the control cultures (compare Figure 4E and 4F ▶ ).
Figure 4.
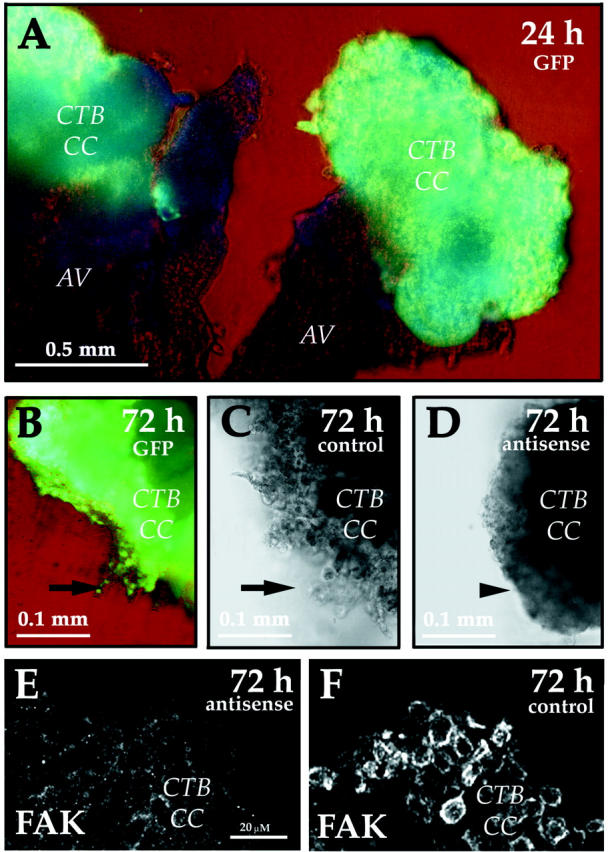
Anti-sense FAK inhibited the migration/invasion of CTBs from anchoring villi. FAK function was assessed using explanted anchoring villi (AV) cultured on Matrigel-coated wells. In this system, villi attach to the Matrigel and CTBs migrate from cell columns (CC) to invade the substrate. A and B: An adenovirus that was engineered to encode green fluorescent protein (GFP) was used to determine which cells in the villus explant were infected. GFP was primarily expressed by CTBs in the cell columns, including at the expanded tips that consist of migrating/invading CTBs (B, arrow). As compared to the control explants that were not exposed to virus (C), explants that were infected with an adenovirus that encoded anti-sense FAK showed a consistent reduction in CTB migration/invasion from the cell columns (D, arrowhead). At the end of the experiment tissue sections were prepared from control explants that expressed GFP and experimental explants that expressed anti-sense FAK. Immunolocalization showed that cell columns from the experimental explants stained only weakly for FAK (E) as compared to cells in comparable regions of the control cultures (F).
Expression of Antisense FAK Reduced FAK Protein Levels in Isolated CTBs and Suppressed Their Migration/Invasion in Vitro
We used a second tissue culture model to confirm and extend the results obtained in the villus explant system. When isolated vCTBs are plated on a Matrigel substrate, they execute the differentiation pathway that gives rise to iCTBs in vivo, including the adhesion molecule switching program. 1 Typically, this process takes 48 to 72 hours. Four different preparations of purified vCTBs were transduced with AdASFAK or control virus. Effects on FAK protein levels, FAK localization, and CTB invasion were quantified for each preparation. An immunoblot from one of the preparations comparing FAK protein levels in lysates of CTBs transduced with AdASFAK or control virus is shown in Figure 5A ▶ . After 72 hours in culture, densitometric analyses showed that transduction with AdASFAK reduced FAK expression by 48% relative to control CTBs that were either transduced with the parental, wild-type virus (AdWT) or not exposed to virus. As an additional control, the lower molecular mass region of the same blot was probed with an antibody specific for paxillin (<70 kd). Bands of equal intensity visible in all of the lanes were an independent confirmation that samples containing equal amounts of protein were loaded. Immunoblot analysis of two of the other preparations transduced with AdASFAK showed a reduction in FAK expression of 42 and 54%, respectively, relative to FAK levels in the control cells. In the fourth experiment, FAK protein was not detected after transduction with AdASFAK, whereas strong signals were observed in the control cell lysates.
Figure 5.
FAK expression is associated with CTB invasion. A, top: Immunoblot comparing FAK protein levels in cell lysates of control CTBs that were not exposed to virus, CTBs transduced with an adenovirus that encoded antisense FAK (AdASFAK), and CTBs transduced with the wild-type virus. After 72 hours in culture, densitometric analyses showed that transduction with AdASFAK significantly reduced (∼50%) FAK expression as compared to controls. A, bottom: The lower molecular mass region of the same blot was probed with an antibody specific for paxillin (<70 kd) as an independent confirmation that samples containing equal amounts of protein were loaded. B: Immunostaining also showed that transduction with AdASFAK reduced FAK expression. Staining with anti-cytokeratin was used to image all of the CTBs. C: Diagram of the invasion assay. Note that the size of the cells (∼15 to 20 μm) is not proportional to the depth of the Matrigel (100 μm). Isolated CTBs are plated on Matrigel-coated Transwell filter membranes (I). As the cells differentiate they invade the Matrigel substrate (II), pass through the filter pores and emerge on the opposite side of the membrane (II, III). Invasion is quantified after 48 to 72 hours by counting the number of cell processes on the underside of the filter. D: CTB invasion in cultures that were not exposed to virus was considered to be 100%. Transduction of CTBs with wild-type adenovirus and AdASFAK lowered invasion by 50 and 90%, respectively. Values are means of four experiments. Scale bars, SEM.
A portion of the CTBs from each of the four experiments described above was used for immunocytochemistry to detect FAK in individual cells. Figure 5B ▶ shows the results of the experiment in which transduction with AdASFAK reduced FAK expression by 48% as assessed by immunoblotting. Staining with anti-CK was used to image all of the CTBs. Control cells on the Matrigel substrate were well spread and extended numerous protrusions. In comparison, CTBs that were transduced with AdASFAK had a more rounded appearance. When control cells were stained with an antibody that recognizes all forms of FAK, high levels of immunoreactivity were detected throughout the cytoplasm and in association with the plasma membrane. In the AdASFAK-transduced samples, FAK staining was weak or undetectable in most CTBs.
The remaining CTBs from the four preparations were used to assess the effects of reducing FAK expression on their ability to invade. The assay is diagrammed in Figure 5C ▶ . Isolated CTBs are plated on Matrigel-coated Transwell filter membranes (Figure 5C, I) ▶ . As the cells differentiate, they invade the Matrigel substrate (Figure 5C, II) ▶ , pass through the filter pores and emerge on the opposite side of the membrane (Figure 5C ▶ , II and III). Invasion is quantified after 48 to 72 hours by counting the number of cell processes on the underside of the filter.
The data from four experiments are summarized in Figure 5D ▶ . In this analysis, the level of CTB invasion in cultures that were not exposed to virus was considered to be 100%. Transduction of the cells with wild-type adenovirus lowered invasion by ∼50% without affecting the number of cells that attached, the expression of several differentiation markers (eg, α5β1, HLA-G), or the rate of apoptosis (data not shown). Thus, transduction with adenovirus affected invasion by an unexplained mechanism, possibly by interfering with normal function of the αVβ3 integrin in adhesion. Nevertheless, transduction of CTBs with AdASFAK resulted in an additional four-fold reduction in the cells’ ability to invade, again without affecting attachment, differentiation, or apoptosis. Finally, we also assayed CTB migration on fibronectin-coated Transwell filters using methods we described previously. 36 The results showed that migration was inhibited to the same extent as invasion (data not shown).
Conditions that Restricted CTB Invasion in Vitro and in Vivo also Reduced pY397FAK Levels
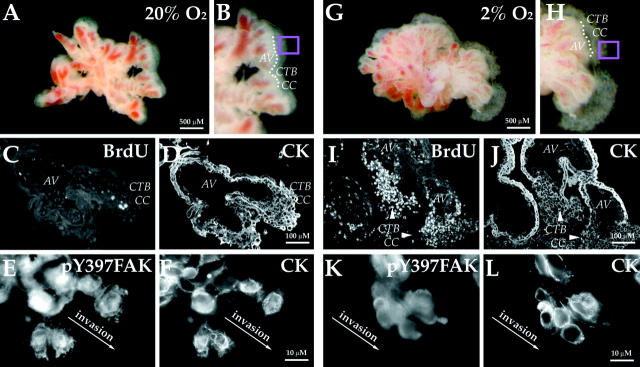
Because CTBs that penetrate the uterine wall in vivo must assume an invasive phenotype, we also examined FAK expression in situations in which their invasion is impaired. First, we investigated the effects of hypoxia, an important regulator of CTB invasion, on pY397FAK immunoreactivity. The morphology of control explants that were cultured for 72 hours under standard conditions (20% oxygen) is illustrated in Figure 6, A and B ▶ . As we showed previously, under these conditions CTBs in column remnants exit the mitotic cycle (Figure 6, C and D) ▶ and switch on the expression of stage-specific antigens that mediate migration and invasion (data not shown). 37 Here we found that CTBs at the edges of these explants stained brightly with antibodies that recognize all forms of FAK (data not shown) and pY397FAK. Particularly strong immunoreactivity for pY397FAK was noted in association with protrusions at the leading edge of cells at the periphery of explants (Figure 6, E and F) ▶ . The morphology of experimental explants that were cultured under hypoxic conditions (2% oxygen) is illustrated in Figure 6, G and H ▶ . As we showed previously, under these conditions CTBs proliferate as indicated by BrdU incorporation (Figure 6, I and J) ▶ . 37 Thus, the columns are larger than in control explants (compare Figure 6, A–D ▶ , with Figure 6, G–J ▶ ). Also in hypoxia, CTB invasion, assayed as diagrammed in Figure 5C ▶ , is impaired. 33,37,38 Here we found strong FAK staining in CTBs that were cultured in both 2% and 20% oxygen (data not shown). However, staining for pY397FAK was greatly reduced in CTBs cultured in hypoxia. In addition, the staining pattern changed. Much of the immunoreactivity was diffuse and cytoplasmic rather than plasma membrane-associated (compare Figure 6K to 6E ▶ ).
Figure 6.
Hypoxia suppresses generation of pY397FAK in column CTBs of anchoring villi. A and B: In standard culture conditions (20% oxygen), explanted anchoring villi (AV) give rise to CTB cell columns (CC). There is very little BrdU incorporation under these conditions (C). D: Cytokeratin staining of adjacent section. A portion of the border between the explant and the cell column is denoted by the dotted line. pY397FAK staining of the area in the pink box is shown in (E). Intense immunoreactivity was detected in association with CTBs at the ECM interface. F: Cytokeratin staining of the same cells. G and H: Hypoxia (2% oxygen) stimulates CTB mitosis. Therefore, anchoring villus explants maintained in hypoxic conditions have larger cell columns than those cultured in 20% oxygen 37 and BrdU incorporation dramatically increases (I). J: Cytokeratin staining of adjacent section. K: Staining for pY397FAK was greatly reduced in CTBs cultured in hypoxia as compared to 20% oxygen (compare with C). L: Cytokeratin staining of the same cells.
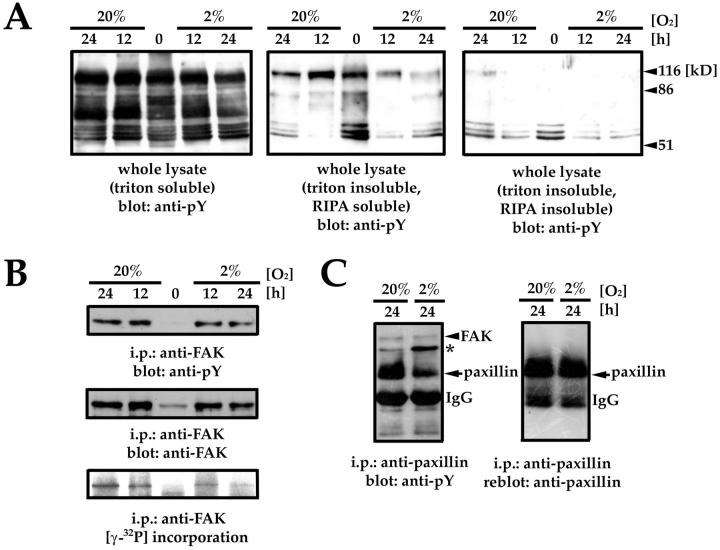
To determine whether conditions that impaired CTB invasion have an impact on levels of total tyrosine phosphorylation, we cultured CTBs for 12 and 24 hours in 20% or 2% O2. Overall, tyrosine phosphorylation in either the cytosolic fraction (Triton soluble) or the cytoskeleton-associated fraction (Triton insoluble, RIPA insoluble) did not change under the conditions tested. However, 100- to 130-kd proteins in the membrane-associated fraction (Triton insoluble, RIPA soluble) showed a decrease in tyrosine phosphorylation (Figure 7A) ▶ . To determine whether this decrease included FAK (125 kd) phosphorylation, we examined levels of total tyrosine phosphorylation and (auto)phosphorylation in FAK immunoprecipitates. Interestingly, there was very little difference in overall tyrosine phosphorylation of FAK, whereas (auto)phosphorylation as shown by [γ32P] incorporation was lower (Figure 7B) ▶ . Consequently, we examined tyrosine phosphorylation of paxillin, a putative FAK substrate. Lower levels of paxillin phosphorylation were observed in conditions associated with lower levels of FAK (auto)phosphorylation. Finally, this did not change the amount of FAK that co-immunoprecipitated with paxillin. However, either the amount or the phosphorylation of an unknown protein that associated with paxillin (asterisk) increased in hypoxia (Figure 7C) ▶ .
Figure 7.
pY397FAK and paxillin tyrosine phosphorylation decrease in hypoxia. A: Tyrosine phosphorylation in whole lysates of CTBs cultured at 20% O2 and 2% O2 for 0, 12, or 24 hours. B: Total tyrosine phosphorylation (top) and (auto)phosphorylation of FAK (bottom) in cells cultured under the same conditions. Immunoblotting with an antibody that recognizes total FAK demonstrates comparable levels of FAK in immunoprecipitates (middle). C: Tyrosine phosphorylation of paxillin decreased in CTBs cultured in 2% O2 for 24 hours. However, similar levels of FAK were detected in paxillin immunoprecipitates regardless of oxygen concentration and levels of paxillin phosphorylation. However, either the amount or the phosphorylation of an unknown protein that associated with paxillin (asterisk) increased in hypoxia.
We also studied the effects of hypoxia on pY397FAK expression by isolated CTBs. To simulate more closely conditions in vivo, the cells were co-cultured with decidua, a likely source of important paracrine signals that influence CTB invasion. In accord with our published work, 33 we found rapid invasion of CTB aggregates beneath the decidual surface in co-cultures that were maintained for 48 hours under standard tissue culture conditions (20% oxygen) (Figure 8A) ▶ . iCTBs in all locations showed strong staining for pY397FAK, primarily in association with the plasma membrane (Figure 8C) ▶ . Also in accord with our published work, we found that in hypoxia (2% oxygen) the CTB aggregates, which attached to the decidua, failed to invade below the surface (Figure 8D) ▶ . Staining with an antibody specific for pY397FAK showed only weak immunoreactivity in the cytoplasm of CTBs throughout the aggregates (Figure 8F) ▶ . This difference was noted despite the fact that hypoxia did not affect the staining patterns of antibodies that recognized all forms of FAK (data not shown).
Figure 8.
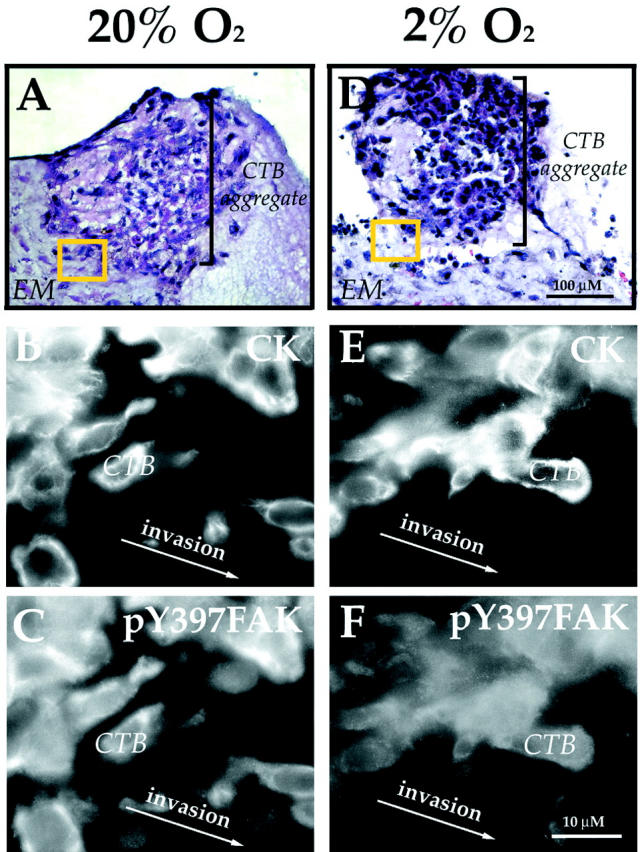
Hypoxia down-regulates (auto)phosphorylation of Y397FAK in isolated CTBs co-cultured with human decidual explants. Sections of first trimester CTBs co-cultured with decidual explants in either standard conditions (20%; A–C) or in hypoxia (2% oxygen; D–F) were stained with hematoxylin (A and D), anti-cytokeratin (CK: B and E), or anti-pY397FAK (C and F). A: In 20% oxygen, CTBs deeply invaded the decidua. pY397FAK staining of the area in the yellow box is shown in (C). Strong staining for pY397FAK was detected on CTBs particularly in the area of the plasma membrane. D: In hypoxia (2% oxygen), CTB invasion of decidua was inhibited and staining for pY397FAK was faint and diffuse (F).
Finally, we investigated whether placental hypoxia in vivo would have similar effects on CTB expression of FAK. As an example of placental hypoxia we studied tissue samples of the fetal-maternal interface that were obtained from women with preeclampsia—a pregnancy complication that is associated with shallow CTB invasion and, consequently, a reduction in blood flow to the placenta. 39,40 Control samples, matched for gestational age, were obtained from women with clinically normal pregnancies. The results are shown in Figure 9 ▶ . In the control samples, staining for all forms of FAK was detected in association with the plasma membrane of CTBs found near the surface of the uterine wall (Figure 9A) ▶ . A very similar staining pattern was observed in preeclampsia, except that the immunoreactivity was often more intense than in control samples (Figure 9B) ▶ . In contrast, we noted dramatic differences between the two groups in the immunolocalization of pY397FAK. Whereas bright staining was detected in association with the plasma membranes of CTBs in the control samples (Figure 9E) ▶ , little or greatly reduced immunoreactivity was detected in CTBs when the pregnancy was complicated by preeclampsia (Figure 9F) ▶ .
Figure 9.

Preeclampsia is associated with reduced staining for pY397FAK. A–H: Frozen sections of biopsies from the fetal-maternal interface were stained with anti-CK (C, D, G, and H) and either anti-FAK (A and B) or anti-pY397FAK (E and F). A: At week 35 of normal pregnancy, control CTBs stained for FAK. B: In preeclampsia, CTBs of the same gestational age also stained for FAK, usually with greater intensity than in control samples. E: Immunostaining of the control sample with anti-pY397FAK revealed groups of CTBs within the uterine wall that stained strongly, primarily at the cell surface. F: In comparison, CTB staining for pY397FAK was very weak when the pregnancy was complicated by preeclampsia.
We confirmed the results of the immunolocalization experiments by examining FAK (auto)phosphorylation and pY397FAK expression in vitro (Figure 10) ▶ . Control CTBs were isolated from the placenta of a patient who had a clinically normal pregnancy, and experimental CTBs were isolated from the placenta of a woman with preeclampsia. Then the cells were cultured under standard conditions for up to 48 hours. To assess (auto)phosphorylation, cell lysates were prepared at three time points: before plating, and after either 24 or 48 hours in culture. Then we assayed for incorporation of [γ]32P in FAK immunoprecipitates (see Material and Methods). Afterward the products were separated by SDS-PAGE and transferred to nitrocellulose. Immunoblotting with an antibody that recognized all forms of FAK showed that each of the lysates contained very similar levels of FAK protein (Figure 10A) ▶ . However, as compared to control cells, FAK (auto)phosphorylation on Y397 was not detected in CTBs isolated from the placenta of a patient diagnosed with preeclampsia (Figure 10A) ▶ .
Figure 10.
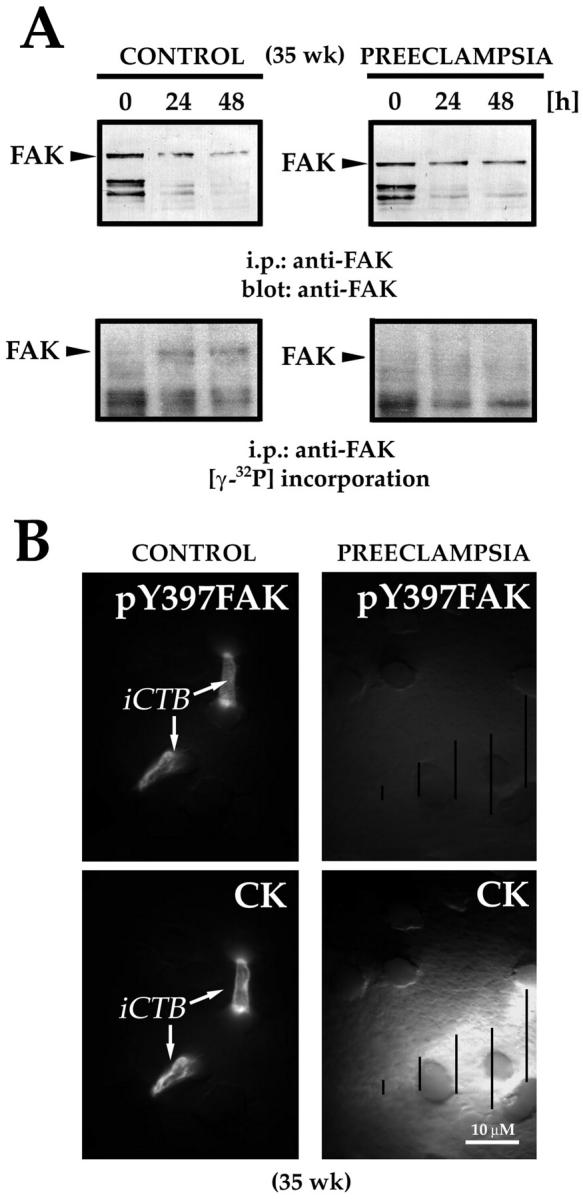
In preeclampsia, reduced FAK (auto)phosphorylation and staining correlated with impaired invasion. To assess (auto)phosphorylation, lysates of purified CTBs were prepared at the time points indicated. [γ-32P] incorporation into FAK was assayed as described in Materials and Methods. A, top: Immunoblotting with an antibody that recognized all forms of FAK showed that each of the lysates contained very similar levels of FAK protein. A, bottom: As compared to control cells, FAK (auto)phosphorylation on Y397, determined by [γ-32P] incorporation, was significantly reduced in CTBs isolated from the placenta of a patient with preeclampsia. CTBs isolated from the same placentas as those used in the FAK (auto)phosphorylation kinase assay were cultured on Matrigel-coated Transwell inserts, fixed, and stained with anti-cytokeratin (CK) and anti-pY397FAK. B, top: CTBs from the control placenta invaded extensively, as shown by the CK-positive cell processes on the underside of the filter. The tips of these processes stained brightly for pY397FAK. B, bottom: In preeclampsia CTB invasion was impaired, as shown by the absence of CK-positive processes on the underside of the filter (arrows). A bright halo of out-of-focus CK fluorescence (see lined markings) revealed the outlines of CTBs that remained on the upper Matrigel-coated surface. No reactivity with anti-pY397FAK was observed.
In four separate experiments, we also monitored the cells’ expression of pY397FAK as they invaded a Matrigel substrate, using the assay diagrammed in Figure 5C ▶ . To image the cell processes that make the initial tracks through the Matrigel, photomicrographs were taken from the underside of the filter. In control cultures, numerous cytokeratin-positive cell processes were visible. These stained brightly for pY397FAK (Figure 10B) ▶ . When the pregnancy was complicated by preeclampsia, no cell processes had emerged on the underside of the filter by 72 hours. Instead, anti-cytokeratin staining revealed only halos of CTBs that remained on the upper, Matrigel-coated surface (Figure 10B) ▶ . Similar halos of anti-pY397FAK immunoreactivity were not observed in association with these cells.
Discussion
Human placental development entails a novel differentiation process in which CTB stem cells that are attached to a basement membrane in floating chorionic villi form aggregates of nonpolarized cells that subsequently lose their association with one another and invade the uterine wall (Figure 1) ▶ . Our previous work showed that molecules whose expression is up-regulated coincident with uterine invasion in situ play important functional roles in culture models that replicate this process in vitro. 28,41,42 Subsequent gene deletion studies have confirmed that a number of these molecules play important roles in vivo during murine placentation. 43,44
Here we exploited the combination of approaches we have developed to study human placentation to understand the role of FAK during CTB invasion. In situ, FAK was highly expressed, primarily in a plasma membrane-associated pattern, at all stages of CTB differentiation. This observation was somewhat unexpected given our previous work that strongly suggested the importance of integrin function to the cells’ acquisition of a migratory and invasive phenotype. 41 This result prompted us to assess the staining pattern of an antibody that recognized only the form of the molecule that is phosphorylated on tyrosine 397, pY397FAK, the primary (auto)phosphorylation site that initiates further FAK activation. Although vCTBs and STBs showed little or no reactivity with this antibody, strong staining was observed in association with a subset of CTBs near the surface of the uterine wall. In vitro function-perturbation experiments showed that reducing FAK expression diminished CTB invasion. The inverse correlation also held: situations in which CTB invasion was down-regulated, such as hypoxia in vitro and preeclampsia in vivo, were associated with a dramatic reduction of pY397FAK levels in the cells especially in membrane-associated areas. Together our results suggest that (auto)phosphorylation of Y397 on FAK, rather than absolute FAK levels, is a critical component of the signaling pathway that mediates migration/invasion.
Our findings have interesting implications that are particularly relevant to current theories regarding tumor cell invasion, a prerequisite for metastatic spread, the most common cause of morbidity and mortality in various neoplastic diseases. 45 Invasion, the movement of cells beyond their normally circumscribed boundaries, is a complex process that requires coordination of many extracellular signals, including those generated by soluble factors, as well as contact with the ECM and other cells. Likewise, intracellular signals, such as those that lead to assembly and disassembly of the actin cytoskeleton, are also crucial. 46 The strongest evidence that FAK is a key point of convergence of both ECM- and growth factor-regulated signals that influence cell migration comes from studies of FAK-null mouse embryos. Specifically, the cells without FAK exhibit a reduced migration rate because of impaired focal adhesion turnover. 17,18,22 Conversely, overexpression of FAK leads to an increase in cell migration. 19 In light of the results of both types of experiments, it is not surprising that a number of groups report that FAK expression is up-regulated in cancer cells as compared to their normal counterparts. 24,47-51 Our results strongly suggest that (auto)phosphorylation of Y397FAK could be a critical determinant of the metastatic potential of tumor cells. This is supported by our analysis of a rare malignant iCTB tumor (placental site tumor) that we fixed according to the protocol that allowed immunolocalization of FAK and pY397FAK (Ilić and Genbačev, unpublished data).
Recent work from three laboratories shows that FAK also plays a role in cell proliferation, suggesting additional tie-ins to other cellular processes that are critical to tumor progression. 52-54 Prompted by these findings, we studied the relationship of FAK expression to migration versus proliferation in several cell lines at the population level. Immunoblotting showed that the cells, including the JAR choriocarcinoma (malignant vCTB) cell line, with the highest invasive potential and lowest proliferative rate had relatively lower levels of FAK in toto, but higher levels of pY397FAK. In comparison, other lines, including JEG (malignant vCTB) cells, with the lowest invasive potential and highest proliferation rate had higher levels of total FAK but less FAK (auto)phosphorylated on Y397 (Ilić and Genbačev, in preparation). In the tissue sections and culture models analyzed here, we failed to detect immunostaining for pY397FAK in proliferating cells—villus and column CTBs in situ and CTBs in explanted anchoring villi cultured under hypoxic conditions in vitro. Instead, expression was detected in migrating CTBs that had withdrawn from the cell cycle. 2 Together, our results suggest that it is possible to separate FAK signals that lead to proliferation from those that promote migration/invasion.
In conclusion, our studies of human placental CTB invasion both in situ and in vitro suggest that regulation of FAK (auto)phosphorylation, rather than FAK levels, is crucial to the cells’ ability to invade. This finding opens several interesting avenues of investigation, particularly with regard to tumor biology. One area is forecasting the outcome of premalignant conditions. For example, it will be interesting to determine whether increased staining for pY397FAK correlates with specific stages in tumor progression such as initial penetration of the cells’ own basement membrane. Additionally, some chemopreventive drugs may modulate integrin-mediated signals and reduce tyrosine (auto)phosphorylation of FAK, 55 either directly or via a myriad of interconnected pathways. In this case evaluation of pY397FAK levels could help determine treatment efficacy. These types of studies will add valuable information to our understanding of the consequences of increases in FAK activity and the relationship of this phenomenon to both normal and abnormal processes that involve the migration and invasion of cells.
Acknowledgments
We thank Drs. Yasuhiro Takeuchi and Miyuki Suzawa (Kyoto University, Japan) for the FAK antisense vector, Dr. David Schlaepfer (Scripps Institute, San Diego) for critical reading of the manuscript, and Evangeline Leash for expert editorial assistance.
Footnotes
Address reprint requests to Susan Fisher, University of California San Francisco, 513 Parnassus, HSW-604, San Francisco, CA 94143-0512. E-mail: sfisher@cgl.ucsf.edu.
Supported by the University of California at San Francisco Academic Senate and the National Cancer Institute Howard Temin Award (KO1 CA87652–01) (to D. I.); and by National Institutes of Health grants HD 30367, HD 26732, and HL 64597.
D. I. and O. G. contributed equally to this work.
Present address of F. G.: SUGEN, Inc., Oncology Targets, 230 E. Grand Ave., South San Francisco, California 94080.
References
- 1.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]
- 2.Genbačev O, McMaster MT, Fisher SJ: A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol 2000, 157:1337-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsky CH, Fitzgerald ML, Fisher SJ: Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992, 89:210-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vićovac L, Jones CJ, Aplin JD: Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta 1995, 16:41-56 [DOI] [PubMed] [Google Scholar]
- 5.Blankenship TN, Enders AC: Trophoblast cell-mediated modifications to uterine spiral arteries during early gestation in the macaque. Acta Anat 1997, 158:227-236 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Fisher SJ, Janatpour M, Genbačev O, Dejana E, Wheelock M, Damsky CH: Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997, 99:2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF, III: Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn 1996, 206:201-211 [DOI] [PubMed] [Google Scholar]
- 8.MacCalman CD, Getsios S, Chen GT: Type 2 cadherins in the human endometrium and placenta: their putative roles in human implantation and placentation. Am J Reprod Immunol 1998, 39:96-107 [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM: Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 1995, 131:791-805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM: Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol 1996, 135:1633-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giancotti FG, Ruoslahti E: Integrin signaling. Science 1999, 285:1028-1032 [DOI] [PubMed] [Google Scholar]
- 12.Schlaepfer DD, Hauck CR, Sieg DJ: Signaling through focal adhesion kinase. Prog Biophys Mol Biol 1999, 71:435-478 [DOI] [PubMed] [Google Scholar]
- 13.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY: Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol 1996, 134:793-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA: Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol 1996, 135:1383-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilić D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH: Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol 1998, 143:547-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida EA, Ilić D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH: Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol 2000, 149:741-754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S: Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995, 377:539-544 [DOI] [PubMed] [Google Scholar]
- 18.Ilić D, Kanazawa S, Furuta Y, Yamamoto T, Aizawa S: Impairment of mobility in endodermal cells by FAK deficiency. Exp Cell Res 1996, 222:298-303 [DOI] [PubMed] [Google Scholar]
- 19.Cary LA, Chang JF, Guan JL: Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci 1996, 109:1787-1794 [DOI] [PubMed] [Google Scholar]
- 20.Fox GL, Rebay I, Hynes RO: Expression of DFak56, a Drosophila homolog of vertebrate focal adhesion kinase, supports a role in cell migration in vivo. Proc Natl Acad Sci USA 1999, 96:14978-14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR: Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res 1999, 59:1655-1664 [PubMed] [Google Scholar]
- 22.Sieg DJ, Hauck CR, Ilić D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD: FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2000, 2:249-256 [DOI] [PubMed] [Google Scholar]
- 23.Kornberg LJ: Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 1998, 20:745-752 [DOI] [PubMed] [Google Scholar]
- 24.Weiner TM, Liu ET, Craven RJ, Cance WG: Expression of focal adhesion kinase gene and invasive cancer. Lancet 1993, 342:1024-1025 [DOI] [PubMed] [Google Scholar]
- 25.Glukhova M, Koteliansky V, Sastre X, Thiery JP: Adhesion systems in normal breast and in invasive breast carcinoma. Am J Pathol 1995, 146:706-716 [PMC free article] [PubMed] [Google Scholar]
- 26.Sieg DJ, Ilić D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD: Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J 1998, 17:5933-5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesley LC: Diagnosis of preeclampsia. Obstet Gynecol 1985, 65:423-425 [PubMed] [Google Scholar]
- 28.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ: 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991, 113:437-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genbačev O, Jensen KD, Powlin SS, Miller RK: In vitro differentiation and ultrastructure of human extravillous trophoblast (EVT) cells. Placenta 1993, 14:463-475 [DOI] [PubMed] [Google Scholar]
- 30.Genbačev O, Miller RK: Post-implantation differentiation and proliferation of cytotrophoblast cells: in vitro models. Placenta 2000, 21:S45-S49 [DOI] [PubMed] [Google Scholar]
- 31.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML: Construction of adenovirus vectors through Cre-lox recombination. J Virol 1997, 71:1842-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T: Differentiation and transforming growth factor-beta receptor down-regulation by collagen-α2β1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J Biol Chem 1997, 272:29309-29316 [DOI] [PubMed] [Google Scholar]
- 33.Genbačev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ: Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 1996, 97:540-550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanazawa S, Ilić D, Hashiyama M, Noumura T, Yamamoto T, Suda T, Aizawa S: p59fyn-p125FAK cooperation in development of CD4+CD8+ thymocytes. Blood 1996, 87:865-870 [PubMed] [Google Scholar]
- 35.Nemerow GR, Stewart PL: Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev 1999, 63:725-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janatpour MJ, McMaster MT, Genbačev O, Zhou Y, Dong J, Cross JC, Israel MA, Fisher SJ: Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 2000, 127:549-558 [DOI] [PubMed] [Google Scholar]
- 37.Genbačev O, Zhou Y, Ludlow JW, Fisher SJ: Regulation of human placental development by oxygen tension. Science 1997, 277:1669-1672 [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Genbačev O, Damsky CH, Fisher SJ: Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol 1998, 39:197-213 [DOI] [PubMed] [Google Scholar]
- 39.Brosens IA, Robertson WB, Dixon HG: The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972, 1:177-191 [PubMed] [Google Scholar]
- 40.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ: Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993, 91:950-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ: Integrin switching regulates normal trophoblast invasion. Development 1994, 120:3657-3666 [DOI] [PubMed] [Google Scholar]
- 42.Janatpour MJ, Utset MF, Cross JC, Rossant J, Dong J, Israel MA, Fisher SJ: A repertoire of differentially expressed transcription factors that offers insight into mechanisms of human cytotrophoblast differentiation. Dev Genet 1999, 25:146-157 [DOI] [PubMed] [Google Scholar]
- 43.Dubois B, Arnold B, Opdenakker G: Gelatinase B deficiency impairs reproduction. J Clin Invest 2000, 106:627-628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC: The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 2000, 25:311-314 [DOI] [PubMed] [Google Scholar]
- 45.Sporn MB: The war on cancer: a review. Ann NY Acad Sci 1997, 833:137-146 [DOI] [PubMed] [Google Scholar]
- 46.Ruoslahti E: Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999, 76:1-20 [DOI] [PubMed] [Google Scholar]
- 47.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG: Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 1995, 55:2752-2755 [PubMed] [Google Scholar]
- 48.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG: Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol 1996, 3:100-105 [DOI] [PubMed] [Google Scholar]
- 49.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S: Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer 1996, 68:164-171 [DOI] [PubMed] [Google Scholar]
- 50.Judson PL, He X, Cance WG, Van Le L: Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer 1999, 86:1551-1556 [DOI] [PubMed] [Google Scholar]
- 51.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L: Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res 2000, 6:2417-2423 [PubMed] [Google Scholar]
- 52.Zhao JH, Reiske H, Guan JL: Regulation of the cell cycle by focal adhesion kinase. J Cell Biol 1998, 143:1997-2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG: Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol 1999, 145:1461-1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D, Grammer JR, Cobbs CS, Stewart JE, Liu Z, Rhoden R, Hecker TP, Ding Q, Gladson CL: p125 focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci 2000, 113:4221-4230 [DOI] [PubMed] [Google Scholar]
- 55.Weyant MJ, Carothers AM, Bertagnolli ME, Bertagnolli MM: Colon cancer chemopreventive drugs modulate integrin-mediated signaling pathways. Clin Cancer Res 2000, 6:949-956 [PubMed] [Google Scholar]