Abstract
Automatic search for cytokeratin/mucin-1 double immunofluorescence was performed to detect and characterize circulating epithelial tumor cells in patients with advanced breast cancer. The peripheral blood samples in 8 of 19 patients (42.1%) presented with cytokeratin-positive and epithelial-type mucin-positive (CK+/MUC1+) tumor cells. Detailed microscopic analysis, however, suggested that the majority of the double immunopositive cells was apoptotic according to an “inclusion type” cytokeratin staining pattern and nuclear condensation. Furthermore, apoptosis-related DNA strand breaks could be demonstrated by applying the TdT-uridine nick end labeling assay in these cells. In 3 of 8 positive samples all of the CK+/MUC1+ cells displayed apoptotic features. We conclude that apoptotic cells significantly contribute to the circulating tumor cell fraction in breast cancer patients. As the predictive value of such cells for the outcome of the disease is unclear, they should be considered separately when analyzing tumor cell dissemination.
Disseminated tumor cells detectable in hematological samples are usually mentioned in conjunction with disease progression and unfavorable prognosis. 1-3 Immunocytochemical 4-6 and molecular biological 7-9 methods are increasingly applied for the specific demonstration of low frequency tumor cells in peripheral blood, bone marrow, and peripheral blood stem cell samples. In addition to the unambiguous detection and quantification of rare tumor cells in the background of hematopoietic cells, information on their functional status would be helpful to accurately determine the clinical impact of detectable disseminated tumor cells. One of the basic questions still to be answered is to determine to what extent tumor cells survive and maintain growth characteristics after entering the circulation. The high rate of positive samples from patients with favorable outcome suggest that some of the detected cells may lack metastatic potential. Apoptotic cells, occurring spontaneously or induced by cytotoxic therapy, surgery, etc., might be one source of such positivities. On the other hand, loss of cell-matrix adherence in epithelial cells directly triggers apoptosis, a recently described phenomenon termed “anoikis.” 10,11 The connection between tumor cell shedding and apoptosis has not been investigated in detail; however, by its demonstration a more precise interpretation of the findings on rare tumor cells in hematological samples could be achieved.
In the present study, circulating epithelial cells from the peripheral blood of breast cancer patients were analyzed for apoptosis-related features. Cytokeratin (CK) and epithelial-type mucin (MUC1)-expressing cells were detected by a novel automated fluorescence image analysis approach, enabling automatic search, exact quantification, and repositioning of the cells of interest in microscopic slides. 12 By this method, sensitive detection and quantification can be completed with simultaneous as well as sequential in situ multicolor analyses of the same rare cells. 13
Materials and Methods
Sample Preparation
We obtained 10 ml of peripheral blood from each of the 19 patients with stage 4 breast cancer. This was done by venipuncture in therapeutic intervals to reduce treatment-associated variables. In addition, peripheral blood from 10 healthy donors and from 15 oncology patients suffering with non-epithelial tumors was also analyzed as negative controls. Following the isolation of the mononuclear fraction by Lymphoprep gradient centrifugation (Nycomed Pharma, Oslo, Norway) approximately 1,000,000 cells were prepared to obtain standard cytocentrifuge preparations as described. 12
Double Immunofluorescence Demonstration of Circulating Breast Cancer Cells
Slides were fixed in 4% paraformaldehyde/phosphate buffered saline. Double immunofluorescence staining was performed (45 minutes at 37°C) by applying one of the three mouse-derived anti-cytokeratin cocktails; MNF116 (dilution 1:80, DAKO, Glostrup, Denmark), 5D3 (1:80, Novocastra, Newcastle, UK) or A45-B/B3 (1:100, Micromet, München, Germany) as well as the biotinylated BM2 antibody specific for epithelial-type mucin MUC1 (1:200, Medac Diagnostika, Vienna, Austria), diluted in 2% bovine serum albumin (Sigma, St. Louis, MO). The specific binding was detected by a FITC conjugated rabbit anti-mouse antibody (1:60, DAKO, Glostrup, Denmark) and the Cy3 conjugated streptavidin molecule (1:500, Jackson Laboratories, West Grove, PA) in the presence of 2% bovine serum albumin for 45 minutes at 37°C. The slides were mounted in glycerol-based Vectashield medium (Vector, Burlingame, CA) containing the DNA stain DAPI.
To prove the specificity of antibodies to tumor cells, species- and isotype-matched control antibodies (mouse IgG1; Sigma) were applied (dilution 1:100) instead of the cytokeratin cocktails in samples positive for tumor cells. Antibody binding to cells was detected as described above.
Automatic Fluorescence Microscopy
Selection and quantification of cytokeratin/FITC-MUC1/Cy3-immunolabeled and DAPI-stained cells were performed by the Metafer automatic fluorescence image analysis system (MetaSystems, Altlussheim, Germany). Cells were identified by segmentation according to prefixed fluorescence parameters in three colors. Digital images of the selected tumor cells for each search were displayed on the screen in the form of a gallery. The selected cells were automatically repositioned in the microscope for visual inspection and the cells were further classified according to their morphological appearance.
Demonstration of DNA Strand Breaks in Circulating Tumor Cells
Apoptosis related DNA strand breaks were demonstrated in the nuclei of CK+/MUC1+ cells in situ by the TdT-uridine nick end-labeling (TUNEL) assay as described 14 using the Apoptag kit (Intergen, Purchase, NY) in a sequential manner. The immunofluorescence was eliminated by proteolytic digestion (50 μg/ml pepsin, pH 1.5, 37°C) before the nick-end labeling. Targeted evaluation of the TUNEL assay was done following automatic repositioning.
Results and Discussion
Circulating breast cancer cells were identified according to their bright CK/MUC1 double positivity by automatic fluorescence image analysis in 8 of 19 peripheral blood samples (42.1%) originating from breast cancer patients. In contrast to this, no positive sample was obtained from the control group (0 of 25). Similarly, no specific immunofluorescence was detectable in either of the control samples reacted with isotype-matched IgG, which were found positive for tumor cells following the application of anti-CK antibodies.
In the positive samples, the number of the CK+/MUC1+ positive cells ranged from 1 to >1000 per 10 6 mononuclear cells. In addition to detection and quantification, the computer-assisted approach assured that each selected cell could be inspected morphologically following automatic relocation. While there was little variation in the appearance of the surface MUC1 expression, two types of cytokeratin- related staining patterns could be discriminated in the tumor cells with either of the used anti-CK antibodies. In addition to the filament-like intracytoplasmatic cytokeratin/FITC staining (Figure 1a) ▶ , which was related to intact tumor cells, an “inclusion” or “bubble” type cytokeratin labeling was frequently seen in somewhat smaller cells often with pycnotic and/or fragmented nuclei (Figure 1, b–d) ▶ . These features suggested an apoptotic phenotype. Moreover, a progressive transition from the intact into the inclusion type staining could be followed, displaying decreasing amounts of cytokeratin filaments replaced by inclusions increasing in size and fluorescence intensity. Finally, shrunken MUC1+ cells containing large intense CK+ inclusions could be seen (Figure 1d ▶ ). The TUNEL assay, which was performed to demonstrate apoptosis-related DNA-strand breaks, strongly supported the apoptotic nature of the latter cell type. None of the intact-appearing CK+/MUC1+ cells was found to be TUNEL-positive, whereas the majority of the cells with inclusion type CK staining did show an intense TUNEL fluorescence (Figure 1 ▶ c). Little or no TUNEL labeling was occasionally seen in conjunction with the inclusions (Figure 1b ▶ ). The nuclei of these cells still displayed a non-condensed chromatin structure, suggesting an early stage of apoptosis without detectable DNA fragmentation by the TUNEL method. No significant TUNEL labeling was observed in the bystanding mononuclear leukocytes; thus, it could be excluded that cell death was artificially triggered after blood sampling.
Figure 1.
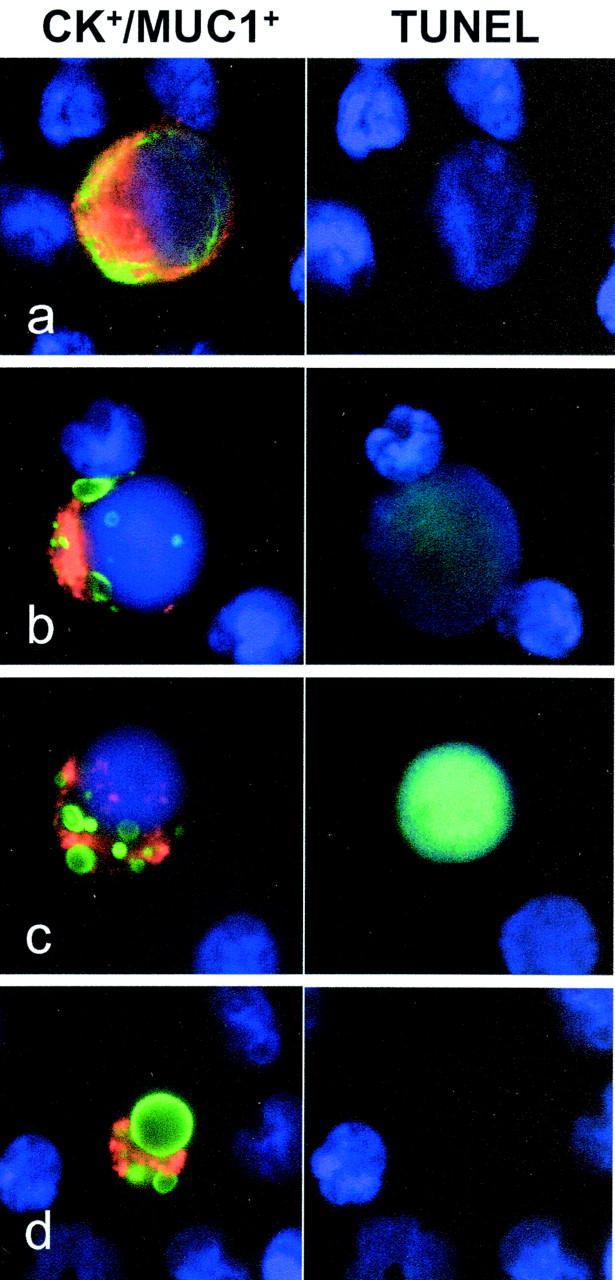
Circulating breast cancer cells detected by double immunofluorescence. Various cytokeratin/FITC (green) and MUC1/TRITC (red) immunofluorescence and DAPI staining (blue) patterns refer to circulating tumor cells in the peripheral blood of breast cancer patients. The intact intermediate filament network (a) is progressively replaced by cytokeratin inclusions (b–d), followed by chromatin condensation and loss, all characteristic of apoptosis. The appearance of apoptosis-related DNA strand breaks can be visualized in the majority of the epithelial cells displaying cytokeratin inclusions by sequentially performing the TUNEL assay (b, c). MUC1+ cell figures with large CK+ inclusions but lacking detectable amounts of chromatin represent the end phase of tumor cell apoptosis (d).
The absolute number of tumor cells with either of the cytokeratin staining patterns could be determined by the classification of the selected CK+/MUC1+ cells (Table 1) ▶ . Except for one patient (no. 19), who presented with a high number of intact tumor cells in the sample, the majority of the selected cells appeared with the inclusion type CK+ staining pattern and TUNEL positivity. Moreover, in 3 of 8 positive peripheral blood samples (37.5%), no intact tumor cells were found, whereas all CK+/MUC1+ cells displayed apoptosis related changes (nos. 12 to 14).
Table 1.
Frequency of Intact and Apoptotic CK+/MUC1+ Cells in Peripheral Blood
| Patient | Cells analyzed (×106) | Peripheral blood findings | |
|---|---|---|---|
| Intact CK+/MUC1+ | Apoptotic CK+/MUC1+ | ||
| 1 | 1.00 | 0 | 0 |
| 2 | 1.50 | 0 | 0 |
| 3 | 1.75 | 0 | 0 |
| 4 | 0.45 | 0 | 0 |
| 5 | 0.70 | 0 | 0 |
| 6 | 0.65 | 0 | 0 |
| 7 | 0.55 | 0 | 0 |
| 8 | 2.40 | 0 | 0 |
| 9 | 0.34 | 0 | 0 |
| 10 | 2.53 | 0 | 0 |
| 11 | 1.60 | 0 | 0 |
| 12 | 1.72 | 0 | 1 |
| 13 | 0.86 | 0 | 2 |
| 14 | 1.91 | 0 | 2 |
| 15 | 2.50 | 1 | 5 |
| 16 | 0.42 | 1 | 8 |
| 17 | 0.46 | 6 | 32 |
| 18 | 0.66 | 21 | 34 |
| 19 | 0.10 | 102 | 24 |
The peculiar redistribution of the filamentous cytokeratin network in epithelial cells according to phosphorylation and consecutive cleavage of low molecular weight keratins was recently described in early apoptosis. 15,16 Cleaved keratins were found to sequester into inclusions, forming complexes with catalytical subunits of caspase 3, the main effector molecule of the proteolytic cascade. 17 Moreover, the caspase-mediated cleavage of cytokeratins was reported to result in immunocytologically detectable apoptosis related neo-epitopes. 18 Common cytokeratin-specific antibody cocktails, usually reacting with cytokeratin 8, 18 and 19, recognize epithelial cells independent of their functional state. CK/MUC1 double immunofluorescence, in addition to improved target cell specificity, allows simple identification and quantification of both intact and preapoptotic/apoptotic tumor cells on the basis of the labeling pattern. The changes in the CK immunofluorescence pattern followed by chromatin fragmentation and shrinkage in the presence of relatively unaltered MUC1 cell surface staining were found to be characteristic for epithelial cells. The same features were demonstrated in experimentally induced tumor cell apoptosis in masses of floating cells of the otherwise adherent breast cancer cell lines MCF-7 and ZR75.1 (data not shown).
In summary, apoptotic figures were demonstrated in the peripheral blood of breast cancer patients with high frequency, exceeding the number of intact circulating tumor cells. In light of this observation, it would be interesting to analyze to what extent apoptotic tumor cells contribute to the clinical findings on disseminated tumor cells obtained by classical immunocytochemistry or by reverse transcription-polymerase chain reaction-based detection methods.
Footnotes
Address reprint requests to Gábor Méhes, M. D., CCRI, St. Anna Kinderspital, Kinderspitalgasse 6, A-1090 Vienna, Austria.
Present address of G.M. is Department of Pathology, University of Pécs Medical School, Szigeti út 12, H-7643 Pécs, Hungary.
References
- 1.Diel IJ, Kaufmann M, Costa SD, Hoole R, von Minkwitz G, Solomayer EF, Kaul S, Bastert G: Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst 1996, 88:1652-1658 [DOI] [PubMed] [Google Scholar]
- 2.Moss TJ, Reynolds CP, Sather HN, Romansky SG, Hammond GD, Seeger RC: Prognostic value of immunocytologic detection of bone marrow metastases in neuroblastoma. N Engl J Med 1991, 324:219-226 [DOI] [PubMed] [Google Scholar]
- 3.Braun S, Pantel K, Müller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnick A, Dimpfl T, Kindermann G, Riethmüller G, Schlimok G: Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000, 342:525-533 [DOI] [PubMed] [Google Scholar]
- 4.Schlimok G, Funke I, Pantel K, Strobel F, Lindemann F, Witte J, Riethmüller G: Micrometastatic tumor cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer 1991, 27:1461-1465 [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Izbicki J, Passlick B, Angstwurm M, Haussinger K, Thetter O, Riethmüller G: Frequency and prognostic significance of isolated tumor cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 1996, 347:649-653 [DOI] [PubMed] [Google Scholar]
- 6.Franklin WA, Glaspy J, Pflaumer SM, Jones RB, Hami L, Martinez C, Murphy JL, Shpall EC: Incidence of tumor cell contamination in leukapheresis products of breast cancer patients mobilized with stem cell factor and granulocyte colony stimulating factor (GCSF) or with G-CSF alone. Blood 1999, 94:340-347 [PubMed] [Google Scholar]
- 7.Burchill SA, Bradbury MF, Pittmann K, Southgate J, Smith B, Selby P: Detection of epithelial cancer cells in peripheral blood by reverse transcriptase polymerase chain reaction. Eur J Cancer 1995, 71:278-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori M, Mimori K, Ueo H, Tsuji K, Shiraishi T, Barnard GF, Sugimachi K, Akiyoshi T: Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription-polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 1998, 16:128-132 [DOI] [PubMed] [Google Scholar]
- 9.Funaki N, Tanaka J, Ohshio G, Maetani S, Imamura M: Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 1998, 77:1327-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisch SM, Francis H: Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 1994, 124:619-626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rytömaa M, Martins LM, Downward J: Involvement of FADD and caspase-8 signalling in detachment induced apoptosis. Curr Biol 1999, 9:1043-1046 [DOI] [PubMed] [Google Scholar]
- 12.Méhes G, Lörch T, Ambros PF: Quantitative analysis of disseminated tumor cells in the bone marrow by automated fluorescence image analysis. Commun Clin Cytometry 2000, 42:357-362 [DOI] [PubMed] [Google Scholar]
- 13.Ambros PF, Méhes G, Hattinger CM, Ambros IM, Luegmayr A, Ladenstein R, Gadner H: Unequivocal identification of disseminated tumor cells in the bone marrow by combining immunological and genetic approaches—functional and prognostic information. Leukemia 2001, 15:275-277 [DOI] [PubMed] [Google Scholar]
- 14.Gorzycza W, Gong J, Darzynkiewicz Z: Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 1993, 53:1945-1951 [PubMed] [Google Scholar]
- 15.Ku NO, Liao J, Omary MB: Apoptosis generates fragments of human type I keratins. J Biol Chem 1997, 272:33197-33203 [DOI] [PubMed] [Google Scholar]
- 16.Caulin C, Salvesen GS, Oshima RG: Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 1997, 138:1379-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacFarlane M, Merrison W, Dinsdale D, Cohen GM: Active caspases and cleaved cytokeratins are sequestered in cytoplasmic inclusions in TRAIL-induced apoptosis. J Cell Biol 2000, 148:1239-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jomvall H, Schutte B: Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 1999, 187:567-572 [DOI] [PubMed] [Google Scholar]


