Abstract
An antibody, GC-17, thoroughly characterized for its specificity for estrogen receptor-β (ER-β), was used to immunolocalize the receptor in histologically normal prostate, prostatic intraepithelial neoplasia, primary carcinomas, and in metastases to lymph nodes and bone. Comparisons were made between ER-β, estrogen receptor-α (ER-α), and androgen receptor (AR) immunostaining in these tissues. Concurrently, transcript expression of the three steroid hormone receptors was studied by reverse transcriptase-polymerase chain reaction analysis on laser capture-microdissected samples of normal prostatic acini, dysplasias, and carcinomas. In Western blot analyses, GC-17 selectively identified a 63-kd protein expressed in normal and malignant prostatic epithelial cells as well as in normal testicular and prostatic tissues. This protein likely represents a posttranslationally modified form of the long-form ER-β, which has a predicted size of 59 kd based on polypeptide length. In normal prostate, ER-β immunostaining was predominately localized in the nuclei of basal cells and to a lesser extent stromal cells. ER-α staining was only present in stromal cell nuclei. AR immunostaining was variable in basal cells but strongly expressed in nuclei of secretory and stromal cells. Overall, prostatic carcinogenesis was characterized by a loss of ER-β expression at the protein and transcript levels in high-grade dysplasias, its reappearance in grade 3 cancers, and its diminution/absence in grade 4/5 neoplasms. In contrast, AR was strongly expressed in all grades of dysplasia and carcinoma. Because ER-β is thought to function as an inhibitor of prostatic growth, androgen action, presumably mediated by functional AR and unopposed by the β receptor, may have provided a strong stimulus for aberrant cell growth. With the exception of a small subset of dysplasias in the central zone and a few carcinomas, ER-α-stained cells were not found in these lesions. The majority of bone and lymph node metastases contained cells that were immunostained for ER-β. Expression of ER-β in metastases may have been influenced by the local microenvironment in these tissues. In contrast, ER-α-stained cells were absent in bone metastases and rare in lymph nodes metastases. Irrespective of the site, AR-positive cells were found in all metastases. Based on our recent finding of anti-estrogen/ER-β-mediated growth inhibition of prostate cancer cells in vitro, the presence of ER-β in metastatic cells may have important implications for the treatment of late-stage disease.
Cellular differentiation and proliferation of prostatic epithelium has long been considered to be primarily mediated by androgens. In this regard, the majority of prostate cancers are initially responsive to anti-androgenic therapies but eventually become refractory to this form of treatment. 1,2 The first medical anti-androgenic therapy used to treat men with prostate cancer used estrogens, primarily acting indirectly at the hypothalamic level to down-regulate circulating levels of androgens, with resultant degenerative effects on neoplastic cells. 3 Paradoxically, despite its use as an anti-androgen, pharmacological doses of estrogens can also induce a marked proliferative alteration of prostatic epithelium termed squamous metaplasia, in the glands of a variety of mammals including humans. 4-8 The metaplastic change is initiated by the proliferation of basal cells that subsequently differentiate into squamous cells. 5,6,8 Importantly, it has been shown that estrogens alone can directly induce squamous metaplasia in the regressed prostates of castrated or hypophysectomized dogs. 5,6
Despite the proliferative effects of estrogens on basal cells, which are the purported progenitor cells of prostatic glandular epithelia, 9 the role that the hormone may play in the abnormal growth of the gland remains undefined. Results from a number of epidemiological 10,11 and experimental studies 10-15 have suggested that estrogens may be involved in this process.
Effects of estrogens on target tissues are now known to be mediated by ligand-specific transcription factor receptor proteins termed estrogen receptor-α and -β (ER-α and ER-β). 16-19 The two isoforms have highly homologous in DNA-binding domains but significant differences in acid amino sequences are found in the N-terminal, hinge region, ligand binding, and F domains. 17 Both receptors are present in many of the same tissues but differences in organ and tissue distribution as well as in levels of expression have been reported for the two isoforms. 17,20,21 In this regard, ER-β mRNA was found to be predominant over the ER-α isoform in the rat prostate 16 and it was also present in human gland albeit in lesser amounts than in the testis. 17 ER-β and ER-α were also shown to bind to the same ligands with different affinity. 21 In addition, after binding to estrogens and anti-estrogens, the two ER isoforms use different enhancer elements such as estrogen responsive element and AP1 sites in promoter regions of gene. 22 Taken together, these two studies suggest that ER-α and ER-β may mediate diverse downstream effects. 22-24
Although the precise biological function of the two ER isoforms in the prostate is currently undefined, 25 in one study, ER-β knockout mice have been reported to develop age-related prostatic hyperplasia, which suggests that the receptor may act to inhibit abnormal growth of the gland. 26 In support of this concept, Poelzl and colleagues 27 have recently reported that ER-β, but not the α isoform, specifically interacts with MAD2 the cell-cycle spindle assembly checkpoint protein. Moreover, Lau and colleagues 28 demonstrated that anti-estrogens down-regulate cell proliferation in human prostate cancer cells that only express the ER-β isoform. ER-β has also been found to be involved in mediating estrogen/anti-estrogen induction of quinone reductase via its interaction with the electrophile/anti-oxidant response element in the promoter region of the gene. 29 It has therefore been proposed that the receptor may be involved in regulating the expression of anti-oxidant enzymes and thus play a role in protecting cells against oxidative injury. 25,29
Before the discovery of ER-β, ER-α antibodies or in situ hybridization were used to study ER localization in normal, hyperplastic, and carcinomatous human prostate tissues. 30-32 Most of these reports showed that ER-α was predominately localized in the stroma of normal and hyperplastic prostates with the occasional detection of the receptor in basal cells and glandular epithelia. With the exception of one recent study, 33 ER-α immunostaining was not detected in primary or metastatic prostate cancers. To date, there is only one published report of ER-β localization in the normal human prostate. 34 Similarly, one study has reported ER-β protein expression in human breast tissues using an antibody developed by the investigators. 35
In the current investigation, we developed a novel antibody directed against the F domain of ER-β, a region that has no homology with the α receptor. 17 We demonstrate that this antibody does not cross-react with ER-α. This reagent was used to immunolocalize the receptor in morphologically normal glands from the three anatomical zones of the prostate, dysplasia (also termed “prostatic intraepithelial neoplasia”—the purported precursor of carcinoma), 36,37 and in primary and metastatic carcinoma. Laser-capture microdissection/reverse transcriptase-polymerase chain reaction (LCM/RT-PCR) was also used to study transcript expression of the receptor in dysplastic lesions and in grades 3 and 4/5 carcinomas. Results from the studies of ER-β were compared with the concomitant investigation of androgen receptor (AR) and ER-α expressions at both immunohistochemical and transcript levels. In this manner, changes in the expression of any of the three receptors could be evaluated as to how they may relate to the development and progression of prostatic carcinoma. Differences in receptor expression between grades 3 and 4/5 were emphasized in our study because it has recently reported that the percentage of grade 4/5 carcinoma in a prostatic neoplasm is highly predictive of disease progression. 38
To our knowledge, our report is the first in which antibodies specific for ER-β, ER-α, and AR were used together with LCM/RT-PCR to compare the expression of these receptors in normal human prostate, dysplastic lesions, and carcinoma.
We find that within the epithelial compartment of normal human prostate, ER-β is predominately localized in basal cells and to a lesser extent stromal cell nuclei. In contrast, ER-α was rarely detected in basal cells but was strongly expressed in stromal cell nuclei.
Expression of the β receptor was diminished in high-grade dysplasia and grade 4/5 carcinoma of the peripheral zone. A similar trend was found at the transcript level in microdissected tissues. The majority of metastases to bone and lymph nodes however contained ER-β-immunopositive carcinoma cells. In contrast to ER-β, ER-α expression at both protein and transcript levels was absent in all dysplasias but present in a few carcinomas of the peripheral zone. The α receptor was however expressed in metastases to two lymph nodes and in the majority of central zone dysplasias. AR expression remained consistently strong in all grades of dysplasias and primary carcinomas as well as in metastases.
In summary, we report that a down-regulation of ER-β expression occurs during prostatic carcinogenesis. This change may contribute to a loss in growth control processes mediated by the β receptor that could amplify the effects of persisting proliferative stimuli such as those mediated by AR.
The presence of ER-β as the predominant ER subtype in most metastases, together with our recent findings that anti-estrogens binding to the receptor inhibit proliferation of prostate cancer cells, 28 may be useful in devising new ligand-specific treatments for late-stage disease.
Materials and Methods
Generation of the GC-17 Polyclonal Antibody
The composition of the immunizing peptide used to generate the GC-17 rabbit anti-ER-β antibody was selected with the aid of the computer programs Protean (Dnastar, Inc., Madison, WI) and Peptool (BioTools, Inc., Edmonton, Alberta, Canada). A peptide sequence in the F domain of the human ER-β receptor (amino acids 449 to 465) was selected, because there is no homology with ER-α at this region. 17,39 The peptide was custom synthesized by Research Genetics (Huntsville, AL) with a format of 4-branch multiple antigenic peptide. Each rabbit (male New Zealand White (Panigen Corporation Inc., Blanchardville, WI), 2–3 kg was first inoculated with 0.5 mg of peptide antigen with complete Freund’s adjuvant, and then boosted with 0.25 mg of peptide plus incomplete Freund’s adjuvant at day 14, day 21, and every 2 weeks afterward until a satisfactory serum titer was obtained. A direct enzyme-linked immunosorbent assay (ELISA) was used to assess the immune responses to the peptide antigen. 40
Methods Used to Test the Specificity of the GC-17 Antibody
Competitive Inhibition ELISA Assay
The wells of an ELISA plate, Pro-Bind (Becton-Dickinson Labware, Franklin, NJ) were coated with a recombinant protein composed of the entire ER-β sequence (PanVera, Madison, WI) at a concentration of 5 μg/ml. The GC-17 antibody (1:6000) was then preincubated with the immunizing peptide at concentrations ranging from 4 mg to 4 μg/ml at room temperature for 30 minutes. In addition, 4 mg of a control peptide encompassing sequences in the N-terminal region of ER-β (Research Genetics) was preincubated with the GC-17 antibody (1:6000) at room temperature for 1 hour. The resulting antigen/antibody complexes were then incubated with the bound recombinant ER-β protein on the ELISA plate at 37°C for 2 hours. Alkaline phosphatase-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA) was used to recognize the GC-17 antibody that bound to recombinant ER-β protein on the plate. The whole complexes were visualized by incubation with p-nitrophenyl phosphate in 2-amino, 2-methyl, 1,3-propanediol buffer, pH 9.6. Results were quantified by optical density using the Microplate reader 550 (Bio-Rad, Richmond, CA). The entire assay was done four times.
Competitive Immunohistochemistry
Lau and colleagues 28 have recently demonstrated that DU145 and LNCaP cells, both derived from a metastatic prostate cancer, express abundant ER-β mRNA but not ER-α message. These cells were used to compliment and confirm that the GC-17 antibody reagent specifically detected ER-β but not ER-α by immunohistochemical staining. Using the GC-17 antibody and the anti-ER-α antibody (NCL-ER-6F11; Novocastra, Newcastle-upon-Tyne, UK) at the same dilutions as for tissue sections (see below), we performed immunohistochemical studies on 10% formalin-fixed cytospins of DU145 and LNCaP cells that had been routinely processed, embedded in paraffin, sectioned at 5 μm, and mounted on SuperFrost Plus slides (VWF Scientific, West Chester PA).
We performed peptide competition studies at the immunohistochemical level that approximated the conditions used in the ELISA assays described above. GC-17 antibody, at a dilution of 1:6000, was incubated with the immunizing ER-β peptide at concentrations of 400 and 40 μg at room temperature for 1 hour. In addition, competitive studies were conducted using ER-α recombinant peptide (400 and 40 μg; Affinity Bioreagents Inc., Golden, CO) on DU145 cells. Incubation conditions and time were identical to those used for the ER-β peptide competition studies. Deparaffinized sections of DU145 and LNCaP cells and human prostate tissue were then incubated with these mixtures at room temperature for 1 hour. Competition studies, done on prostate tissues, were identical to those performed on DU145 cells, except the peptide and antibody mixtures were incubated overnight and then applied to sections for 24 hours at room temperature. All of the remaining immunohistochemical and other staining procedures were identical to those used for tissue sections (see Immunohistochemical Procedures).
Western Blot Analysis
Four human normal prostate tissues from radical prostatectomies and one normal human testis tissue were used in this analysis. In addition, we used normal putative human prostate basal cells (PrEC; Clonetics, Walkersville, MD) and DU145 cells (ATCC, Rockville, MD) for these studies. Recombinant proteins, ER-α (RP310) and short form of ER-β (RP311) (Affinity Bioreagents Inc.) as well as long form of ER-β (PanVera), were included as controls. Tissues or cells were homogenized in radioimmunoprecipitation (RIPA) buffer containing 50 mmol/L Tris-HCl, pH 7.4, 1% Nonidet P-40 (Amaresco, Solon, OH), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L phenylmethylsulfonyl fluoride in isopropanol, 1 mmol/L activated sodium orthovanadate and 2× complete proteinase inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). Twenty-five μg of tissue protein extracts, 0.5 μg of recombinant ER-α protein, or 0.5 μg of recombinant ER-β protein were mixed with 2× SDS loading buffer (125 mmol/L Tris buffer, pH 6.8, 20% glycerol, 2% SDS, 2% β-mercaptoethanol and 1 μg/ml bromophenol blue) and electrophoresed onto a 10% SDS-polyacrylamide gel under reducing conditions. The separated proteins were transferred onto a PolyScreen polyvinylidene-difluoride transfer membrane (NEN, Boston, MA). The membrane was incubated for 1 hour in blocking buffer [phosphate-buffered saline (PBS) with 5% nonfat dry milk]. The primary antibodies were applied at 1:6000 for GC-17 ER-β antibody or 1:5 for 1D5 ER-α antibody (Biogenex, Mountainview, California) in PBS-T (PBS with 0.05% Tween-20) buffer with 0.1% bovine serum albumin for overnight at room temperature. After washing five times with PBS-T buffer, the membrane was incubated with horseradish peroxidase-linked donkey anti-rabbit IgG antibody (Amersham Pharmacia Biotech, Piscataway, NJ) for GC-17 or goat anti-mouse IgG antibody (NEN) for 1D5 at 1:2500 for 1 hour. The signals were visualized with the chemiluminescence ECL detection system (NEN) and autoradiography. All reagents were purchased from Sigma (St. Louis, MO) unless specified.
Prostate Tissues
Formalin-Fixed Radical Prostatectomy Specimens
Tissues studied were from 50 radical prostatectomy specimens collected by JEM at Stanford University Medical School, during the years 1995 to 1999. Patients ranged in age from 46 to 73 years of age and none had received any treatment before their undergoing prostatectomy. Prostates were fixed in 10% buffered formalin for 24 hours and then sectioned transversely. Tissues were dissected, fixed in 10% buffered formalin for 3 hours, routinely processed, and embedded in paraffin. A histopathological diagnosis was made by JEM on a hematoxylin and eosin (H&E)-stained section. The criteria used in the grading of the carcinomas were those described by Stamey and colleagues. 38 The slide, together with the corresponding paraffin block, was then sent to IL at Tufts University where immunohistochemical studies were performed. At least one section from each case was stained with H&E and reviewed by IL to assure that it matched the tissue components in the original slide. Paraffin sections were cut at 6 μm and mounted on SuperFrost Plus slides. Sections were left unbaked until used for immunohistochemical studies.
Among the 50 cases selected for study, 26 contained areas of carcinoma. Five of these were clear cell carcinomas of the transition zone whereas all of the remaining cancers were found in the peripheral zone. All of the peripheral zone cancers were composed of mixtures of grades 3 and 4/5 carcinomas. In contrast, all of the clear cell carcinomas were predominately grade 3 neoplasms. Twenty of the peripheral cancer specimens also contained varying amounts of low/moderate- to high-grade dysplastic lesions. Dysplasia of the peripheral zone was found in the absence of carcinoma in 6 of the 50 total cases we studied. Additionally, 7 of the 50 cases, were low/moderate-grade dysplasias of the central zone that did not co-exist with cancer. Among the 50 cases, two specimens each of lesion-free normal peripheral, central, and the transition zone were included in our study. Among the cases studied, 15 examples of benign prostatic hyperplasia were either commingled with other lesions 10 or occurred separately. 5
Bone and Lymph Node Metastases
In addition to the prostatectomy cases, archived paraffin blocks containing bone metastases were obtained from seven patients of MET that were treated at the University of Massachusetts Medical Center. The patients’ ages ranged from 59 to 74 and they were all clinically stage D2 at the time of diagnosis. All received anti-androgen treatment as follows: 1) four patients were orchectomized. One of these patients was given the luteinizing-hormone-releasing-hormone (LH/RH) agonist Lupron (TAP Pharmaceuticals Inc, Deerfield, IL) and the AR competitive inhibitor Eulixin (Flutamide; Schering Corp., Kenilworth, NJ) for 3 months, one treated with Eulixin for 24 months and the remaining two were not given any further anti-androgenic therapy. 2) Three patients were not orchectomized. Two were treated with Lupron for 8 months and the other with Lupron and Eulixin for 3 months. Following these anti-andogenic therapies for the periods noted above, it was determined that all seven patients were failing therapy. At those time points, biopsies of suspected bone metastases were obtained from the iliac crest of each patient. These samples were immediately fixed in 10% buffered formalin, routinely processed, embedded in paraffin, and 6-μm sections were placed on SuperFrost Plus slides and stained with H&E. Replicate sections of these lesions were used for immunohistochemical studies.
In addition, we also studied five archived examples of metastases to regional lymph nodes. Two cases were obtained from the Department of Pathology at the University of Massachusetts Medical School and the remaining three were from the University of Florida Medical School (a generous gift from Dr. William Murphy). The patients were 60 to 85 years of age. Regional lymph nodes (external iliac and pelvic) were obtained from all patients during radical prostatectomy. Only one patient had received any treatment before surgery (Lupron).
Frozen Tissues for LCM/RT-PCR
Eighteen separate specimens, derived from radical prostatectomies, were placed in cassettes containing Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, NC) and quick-frozen in liquid nitrogen by JEM and then shipped in dry ice to IL. Approximately 15 minutes elapsed from the surgical removal of the gland to the initiation of freezing. Formalin-fixed and paraffin-embedded tissue sections, immediately adjacent to the quick frozen specimens, were also taken for subsequent immunohistochemical studies (see below).
For diagnostic purposes and lesion selection, tissues were first cryostat sectioned and then fixed briefly in 70% ethanol and stained with H&E. The frozen tissue blocks were then stored at −70°C until they were used for microdissection. Before LCM, sections from these cases were found to contain varying amounts of grades 3 and 4/5 carcinomas as well as dysplastic and normal glands.
Procedures
Immunohistochemical Procedures
The following are the primary antibodies and the dilutions used in our studies: anti-ER-β, rabbit polyclonal antibody GC-17, diluted at 1:6000; anti-ER-α, mouse monoclonal antibody NCL-ER-6F11, diluted at 1:50 (Novocastra); anti-AR, rabbit polyclonal antibody, diluted to 22.7 μg/ml (Upstate Biotechnologies, Lake Placid, NY); anti-Mib5/Ki67, mouse monoclonal antibody, diluted at 1:50 (Immunotech, Westbrook, ME); and anti-high molecular weight cytokeratin, mouse monoclonal antibody 34βE12, diluted at 1:50 (Enzo Diagnostics, Farmingdale, NY). Immunostaining for prostatic-specific antigen (PSA) was done with a Nexus immunostainer (Ventana, Tucson, AZ) using prediluted reagents.
Five-μm thick sections were cut and mounted on SuperFrost Plus slides. Sections were left unbaked until immediately before use at which point they were baked for 1 hour at 60°C. After baking, sections were deparaffinized through three changes of xylene and rehydrated through graded alcohols into water. Heat-induced epitope retrieval was performed by boiling sections in citrate buffer pH 6.0 (pH 6.2 for ER-β) for 15 minutes on a laboratory hotplate. After boiling, sections were removed from the hotplate, allowed to cool at room temperature for 20 minutes, and were then rinsed thoroughly with water (sections stained for PSA did not require heat-induced epitope retrieval). Sections were then placed in 3% hydrogen peroxide for 15 minutes at room temperature to block endogenous peroxidase, washed with water, and placed in PBS (Sigma). Sections were then incubated with Power Block (Biogenex) nonspecific blocking reagent for 10 minutes at room temperature to reduce nonspecific staining, washed with water, and placed in PBS. Sections were then incubated with normal goat serum at 1:50 (Vector, Burlingame, CA) for 15 minutes at room temperature. The goat serum was then shaken off and sections were incubated with primary antibodies overnight at 4°C. After overnight incubation, each section received 20 seconds of washing with PBS, 20 seconds of washing with Biogenex Optimax detergent wash solution, followed by 10 minutes of washing in PBS on a rotator. Solutions were changed for every eight slides. After washing, sections were incubated with either Biogenex Mutlilink secondary antibody at a dilution of 1:20 for 20 minutes at room temperature or DAKO (Carpinteria, CA) ready to use secondary antibody for 10 minutes at room temperature. Sections were again washed according to the protocol described above. Sections were then incubated with either Biogenex streptavidin-conjugated horseradish peroxidase at a dilution of 1:20 for 20 minutes at room temperature or DAKO ready to use streptavidin-conjugated alkaline phosphatase for 10 minutes at room temperature. Sections were again washed as previously described. Immunostaining was visualized using either Biogenex liquid 3,3-diaminobenzidine (Biogenex) or DAKO’s New Fuchsin as the chromogen. After development, sections were rinsed in water, lightly counterstained with 10% Harris modified hematoxylin.
Positive controls for GC-17 included DU145 cells (see above and Results) and tissue sections of prostate that were previously shown to be consistently stained with the antibody. Positive tissue controls for ER-α were human breast cancers, shown to contain numerous immunostained cells. Morphologically normal human prostate sections served as positive controls for AR as well as for anti-high molecular weight cytokeratin and MIB5/Ki-67 stains. For all reagents, negative controls were performed by substituting the primary antibody with a class-matched isotype.
LCM and RT-PCR
In all instances, immunohistochemical studies for ER-β were performed on the paraffin tissue sections that were adjacent to the frozen sections used for microdissection and RT-PCR analysis. Frozen sections were cut on a cryostat at 5 μm and placed on precleaned glass slides (Fisher Scientific, Pittsburgh, PA) and immediately fixed in 70% ethanol for 5 seconds. The sections were then briefly dipped in distilled water, stained with 10% Harris hematoxylin for 15 seconds, dipped in distilled water, then successively placed in 70% ethanol for 30 seconds, briefly immersed in 1% eosin then placed in 95% ethanol for 1 minute, two changes of 100% ethanol for 1 minute each, and two changes of xylene for 30 seconds each. After air-drying for ∼30 minutes, tissues were microdissected using a Pixcell 2 LCM unit (Arcturus, Mountainview, CA). Eight to 10 normal acini were microdissected from each of three different cases. Similarly, 10 to 20 dysplastic glands were dissected from four separate cases of high-grade lesions, and approximately the same numbers of neoplastic glands were obtained from five cases of grade 3 and six different examples of grade 4/5 carcinomas. RNA was extracted from each sample and then separately subjected to RT-PCR analysis. Total cellular RNA was separately isolated using RNA Stat-60 reagent (Tel-Test Inc., Friendwood, TX) according to protocols provided by the manufacturer. The total isolated cellular RNA was reverse-transcribed using the GeneAmp RNA PCR kit (Applied Biosystems, Foster City, CA) in total 20-μl reaction mixture and 2 μl of the resulting cDNA was used in PCR on ER-α, AR, and GAPDH and 3 μl for PCR on ER-β. Hot start PCR using AmpliTaq Gold DNA polymerase (Applied Biosystems) was used in all amplification reactions. The enzyme was activated by preheating the reaction mixtures at 95°C for 6 minutes before to PCR. The PCR programs were 45 cycles for GAPDH and 55 cycles for ER-α, AR, and ER-β of 1 minute at 94°C, 1 minute at 60°C (annealing temperature), and 1 minute at 72°C. This protocol was chosen to minimize nonspecific product amplification. The primer sequences for ER-α, AR, and GAPDH were described in our previous study. 28 The primer set for ER-β was newly designed and the forward primer is 5′-GATGAGGGGAAATGCGTAGA-3′ and the reverse primer is 5′-CTTGTTACTCGCATGCCTGA-3′.
Results
Specificity of the GC-17 Antibody
Competitive ELISA
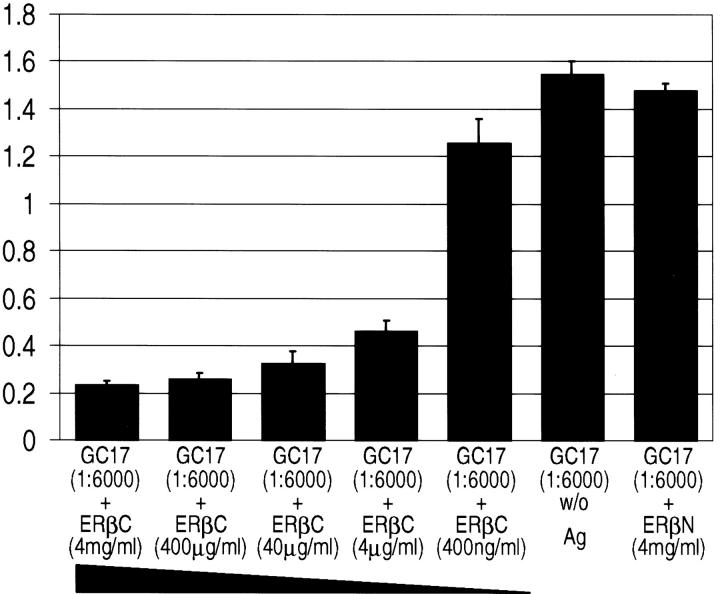
Preincubation of GC-17 with the immunizing peptide (C-terminus of ER-β, ERβC) successfully suppressed binding to the recombinant protein (Figure 1) ▶ . The suppression occurred in a concentration-dependent manner when compared to the control where the antibody was not preincubated with the immunizing peptide. In contrast, preincubation of GC-17 with the control N-terminus peptide of ER-β (ERβN) did not significantly suppress binding when compared with the control, indicating that the antibody was not cross-reactive with this region of the ER-β protein.
Figure 1.
Competitive ELISA. This graphic representation illustrates the concentration-dependent competition of the immunizing peptide (ERβC) with the GC-17 antibody. In addition, preincubation with 4 mg/ml of the control N-terminus peptide (ERβN) and GC-17 failed to compete out the binding to the recombinant ER-β peptide, demonstrating the specificity of the antibody. The GC-17 antibodies, bound to recombinant ER-β protein on ELISA plates, were recognized by alkaline phosphatase-conjugated anti-rabbit IgG antibodies. The whole complexes were visualized by incubation with p-nitrophenyl phosphate. The optical density at 405 nmol/L was measured. The entire assay was done four times. The column represents the mean value of optical density at 405 nmol/L of four measurements and the bar represents the SD. w/o, GC-17 preincubated without peptide antigen.
Competitive Immunochemistry
Strong nuclear immunostaining was detected in sections of DU145 and LNCaP cells that served as positive controls for the peptide competition studies (Figure 2A) ▶ . Preincubation of GC-17 with either 400 μg/ml or 40 μg/ml of the immunizing peptide ERβC totally abolished nuclear staining in sections of these cells, when compared with positive controls where the peptide was omitted (Figure 2B) ▶ . Identical results were obtained with sections of human prostate (Figure 2, C and D) ▶ . In contrast, preincubation of GC-17 with the recombinant ER-α protein failed to block ER-β immunostaining of DU145 cells by the antibody (data not shown). These studies confirmed that GC-17 does not cross-react with ER-α and supports data from our Western blot findings (see below). Thus, both the competitive ELISA and competitive immunohistochemistry showed GC-17 to be highly specific for binding to the ER-β protein.
Figure 2.
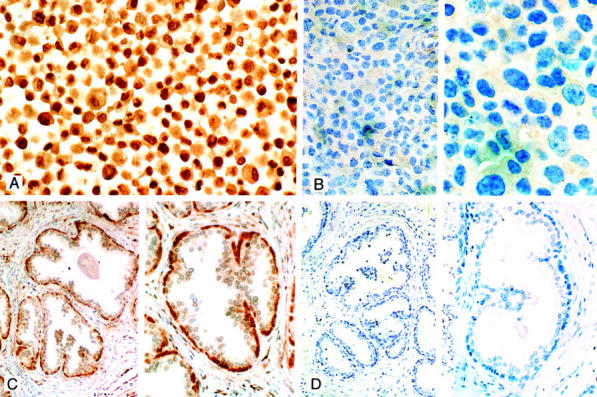
Competitive ER-β immunostaining DU145 cells (A and B) and normal prostate (C and D). A: DU145 cells immunostained in the absence of competing peptide are shown. Note the strong nuclear staining in the majority of cells (original magnification, ×265). B: After incubation of GC-17 with 40 μg of the immunizing peptide, there was almost a total absence of cells with positively stained nuclei (compare with A) [original magnifications: ×115 (left), ×350 (right)]. C: Tissue section of normal prostate. In the absence of competing peptide strong immunostaining of cells in the basal layer of glands is seen in this section of prostate (see also Figure 4, A and B ▶ ) [original magnifications: ×115 (left), ×230 (right)]. D: A replicate section of the prostate illustrated in C after preincubation of GC-17 with 40 μg of immunizing peptide. Note the absence of immunostaining in these sections [original magnifications: ×90 (left), ×280 (right)]. For details of the procedures used in these studies see Materials and Methods. All sections were counterstained with 10% Harris hematoxylin.
In addition, DU145 and LNCaP cells, that only express ER-β, 28 were negative when immunostained with the ER-α (NCL-ER-6F11) antibody. Positive staining of prostate tissues with the ER-α antibody was restricted to stromal cells (see Immunohistochemistry of Normal Prostate below).
Western Blot Analysis
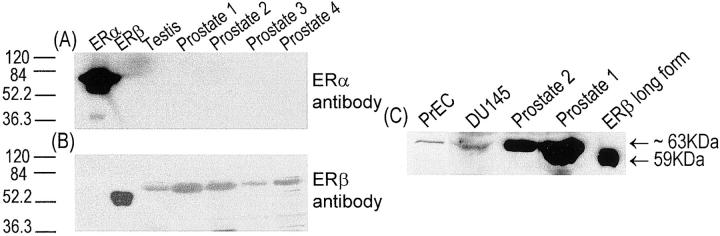
Using Western blot analysis, GC-17 was demonstrated to specifically recognize two commercially available recombinant ER-β proteins and show no cross-reactivity to an ER-α recombinant protein (Figure 3; A, B, and C ▶ ). The recombinant short-form ER-β protein (RP311) from Affinity Bioreagents Inc. has an estimated molecular size of 53 kd and represents a polypeptide (corresponding to amino acid residues 43 to 530) translated from the second initiation codon of the ER-β transcript. 16-18 The recombinant long-form ER-β protein (corresponding to amino acid residues 1 to 530) from PanVera represents the polypeptide translated from the first initiation codon of the transcript and has an estimated molecular size of 59 kd. Both short- and long-forms of recombinant receptor proteins were recognized by the GC-17 antibody as bands of their predicted sizes of 53 kd (Figure 3B) ▶ and of 59 kd (Figure 3C) ▶ in the Western blots. Interestingly, GC-17 recognized a protein of ∼63 kd in lysates prepared from the human prostatic cancer cell line DU145 (Figure 3C) ▶ , the putative human prostatic basal cell culture PrEC (Figure 3C) ▶ , as well as from normal human testis and prostate tissues (Figure 3, B and C) ▶ . In a previous study, 19 it was demonstrated that transfection of A549 cells with plasmids carrying a long-form ER-β nucleotide sequence or a short-form ER-β sequence the polypeptides expressed in cellulo had higher molecular sizes than those predicted from the numbers of their amino acid residues. In this regard, the in cellulo-translated polypeptide from the long-from coding sequence of ER-β had a molecular size of ∼63 kd that is larger than the predicted size of 59 kd from amino acid length estimation. Similarly, the in cellulo-translated polypeptide from the short-form nucleotide sequence was ∼56 kd instead of the predicted 53 kd. These data suggest posttranslational modifications of the ER-β molecule occur in cells. Importantly, our Western blot analyses with GC-17 revealed expression of only a 63-kd polypeptide in DU145 and PrEC cells as well as in normal prostate and testes tissues. These findings support the notion that the long-from ER-β may be the receptor form expressed in cells and tissues. The level of ER-α protein in human normal testis and prostate tissues was undetectable with the 1D5, the anti-human ER-α antibody (Figure 3A) ▶ when ECL-Western blots were exposed to X-ray films for 30 seconds. However, very weak signals derived from the ER-α protein could be detected when the blots were exposed to X-ray films for more than 10 minutes.
Figure 3.
Western blot analysis. These autoradiographs illustrate the binding ability and specificity of GC-17 to ER-β protein and its lack of cross-reactivity with ER-α protein. A and B: Shown from left to right are the ER-α recombinant protein, short form of ER-β recombinant protein, and tissue lysates from human testis and normal prostate (1 to 4). C: From left to right are cell lysates of PrEC and DU145 cells, tissue lysates from human normal prostate (1 and 2), and long form of ER-β recombinant protein. The recombinant proteins and cell or tissue lysates were separated with SDS-PAGE gel and the separated proteins were transferred onto PolyScreen polyvinylidene difluoride transfer membrane. The blot was incubated with the ER-α monoclonal antibody (A) or GC-17 ER-β polyclonal antibody (B and C) and the complexes were visualized by the chemiluminescence ECL detection system followed by autoradiography. Note that the antibody detects only the ER-α protein using ER-α monoclonal antibody and GC-17 ER-β polyclonal antibody does not detect the ER-α protein but clearly identifies single bands for both forms of ER-β recombinant proteins and for the cell or tissue lysates. These bands correspond to the reported size of the short and long forms of the receptor (see Results).
Immunohistochemistry of Prostate Tissues
Normal Prostate
In morphologically normal ducts and acini, nuclear ER-β expression was consistently densely localized in nuclei of basal cells as defined by anti-high molecular weight cytokeratin staining in replicate sections (Figure 4, A and B) ▶ . Strong nuclei staining was absent in secretory cells but frequently observed in stromal cells. Occasionally nuclear membrane staining for the receptor was also evident in a few luminal cells (Figure 4, A and B) ▶ . ER-α immunostaining was not present in secretory cells of normal ducts and acini but individual scattered receptor-positive basal cells were observed in less than 10% of all sections studied. The α receptor was however consistently found in stromal cell nuclei, especially in periglandular locations.
Figure 4.
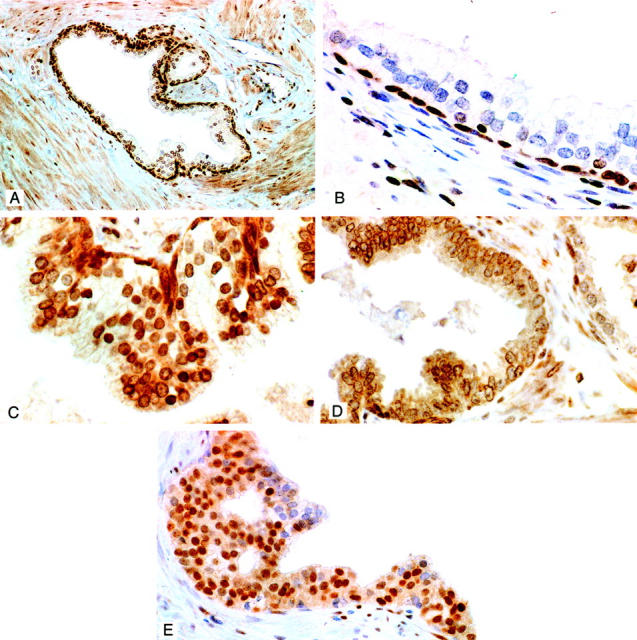
A and B: Immunolocalization of ER-β in the normal human prostate using the GC-17 antibody. Note the strong staining of cells in the basal layer and its virtual absence in the nuclei and cytoplasm of secretory cells (best seen in B). Some nuclear membrane staining was however evident in a few secretory cells (A and B). Nuclear staining is also evident in stromal cells. Original magnifications: ×100 (A), ×400 (B). C: ER-β immunostaining in low/moderate-grade dysplasia. Moderate to strong expression of the receptor is evident in dysplastic secretory cells. Note also the strongly stained cells in the basal layer (original magnification, ×400). D: ER-β-immunostained section of high-grade dysplasia. Nuclear staining is almost totally absent in this lesion. In some dysplastic cells light staining of nuclear membranes is evident. This area of the lesion is almost totally devoid of receptor-stained basal cells. A few residual-stained basal cells are however evident in the bottom left corner of the lesion. Note the presence of two positively stained basal cells in a portion of a normal gland on the right (original magnification, ×250). E: ER-α immunostaining in dysplasia of the central zone. The majority of dysplastic cells are immunostained for the receptor in this lesion. There was however great variation in the percentage of positive cells found in these central zone lesions. Intraluminal pseudo-gland formation seen here was common in dysplasias of the central zone (original magnification, ×200). All sections were counterstained with 10% Harris hematoxylin.
Pronounced nuclear staining for AR was a constant finding in secretory and stromal cell nuclei. In agreement with a past study, 39 variable immunostaining for AR was also observed in individual basal cells of normal glands. No difference was found in the cellular localization of the three steroid hormone receptors when the peripheral, transition, and central zones of the prostate were compared. Moreover, the same localization of the steroid hormone receptors, found in the three normal zones, was also seen in foci of benign prostatic hyperplasia.
The most consistent immunolocalization for the three receptors in basal cells was found within periurethral ducts.
Dysplastic Lesions
Immunohistochemical findings in dysplastic and carcinomatous lesions are summarized in Table 1 ▶ .
Table 1.
Immunohistochemical Findings in Dysplasias and Carcinomas
| Lesion | ER-α | ER-β | AR |
|---|---|---|---|
| Dysplasia/peripheral zone | |||
| Moderate grade* | − | + | + |
| High grade | − | − | + |
| Dysplasia/central zone* | + | + | + |
| Carcinoma/peripheral zone | |||
| Grade 3 | −/+ | + | + |
| Grade 4/5 | −/+ | −/+ | + |
| Carcinoma/transition zone | − | − | + |
| Metastatic carcinoma | |||
| Bone | − | + | + |
| Lymph nodes | −/++ | + | + |
*Data reflects positive staining in dysplastic and basal cells. Staining restricted to residual basal cells in high-grade dysplasias. +, Positive staining in >50% of cases; −/++, positive staining in 40% of cases; −/+, positive staining in <20% of cases. See text for estimates of the percent of positively stained cells in each lesion.
In the peripheral zone, a consistent pattern of ER-β expression was found in dysplastic lesions. A secretory cell localization for nuclear ER-β expression was commonly observed in low- to moderate-grade dysplastic lesions (Figure 4C) ▶ . The majority of both basal and dysplastic secretory cells in these lesions, contained moderate to strongly stained nuclei (Figure 4C) ▶ . A marked diminution to total absence of ER-β immunostained nuclei was a consistent feature in almost all dysplastic cells in the high-grade lesions we studied (Figure 4D) ▶ . Staining was however present in residual basal cells within these high-grade lesions. Thus, a loss of ER-β staining in high-grade dysplasias paralleled a decline in receptor-positive basal cells. In contrast to normal cells, the cytoplasm of dysplastic cells in lesions of all grades but especially in high-grade lesions were frequently stained by the GC-17 reagent, a feature not seen when the primary antibody was omitted from the incubation or with the use of any of the other anti-receptor reagents. Similarly nuclear membrane of cells in high-grade dysplastic cells also frequently stained with the GC-17 antibody (Figure 4D) ▶ . ER-α-positive dysplastic cells were not present within any of the peripheral zone lesions we studied.
In marked contrast, ER-α-stained cells were detected in five of seven (71%) of dysplasias in the central zone (Figure 4E) ▶ . There was however great variation in the numbers (10 to 90%) of immunopositive cells in any given central zone lesion. In replicate sections, six of these lesions contained dysplastic cells that were also positive for ER-β staining.
As previously reported, 41 AR was strongly expressed in the majority (>95%) of dysplastic cells irrespective of the origin or grade of the lesion.
Grade 3 and 4/5 Carcinomas
A transition from ER-β-positive to ER-β-negative staining of cells was observed in all 21 cases where cancer was found in the peripheral zone that paralleled the progression of the grade 3 carcinomas to the less differentiated grade 4/5 neoplasms. ER-β-positive cells were found in 13 of 15 (87%) grade 3 carcinomas of the peripheral zone. The spectrum of expression ranged from examples where all nuclei in an individual neoplastic gland were strongly stained (Figure 5A) ▶ to instances in which receptor immunostaining was weak and/or found in few cells within a given microscopic field. The latter examples were most often located in areas where a transition to higher grade carcinoma occurred (Figure 5B) ▶ . Unlike their counterparts in the peripheral zone, the vast majority of cells comprising grade 3 clear cell carcinomas in the transition zone were devoid of ER-β immunostaining. In two of five cases, scattered receptor-positive neoplastic cells were however found in a minority of glands (Figure 5C) ▶ .
Figure 5.
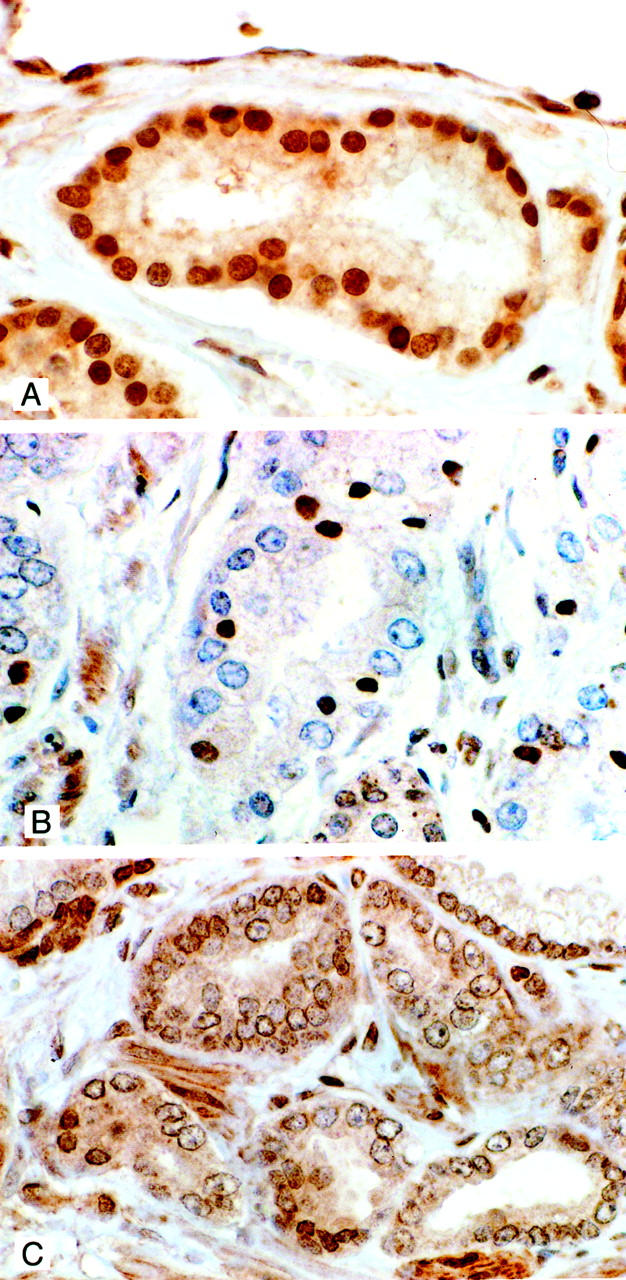
A: ER-β staining in grade 3 carcinoma. Strong nuclear immunostaining is evident in this grade 3 carcinoma. Light cytoplasmic staining of neoplastic cells is also present. Although positive immunostaining for the receptor was found in the majority of grade 3 cancers there was variation in the percentage of stained cells in any given lesion. This was especially the case in areas of transition from grade 3 to grade 4/5 carcinoma (see C) (original magnification, ×400). B: ER-β immunostaining of a clear cell carcinoma in the transition zone. Immunostaining for the receptor is absent in the majority of cells in this grade 3 clear-cell carcinoma. Scattered among the negatively stained cells are cells with small nuclei that are immunopositive for the receptor. The occurrence of these positive cells was uncommon in clear cell carcinomas. In most of these cancers all of the cells were unstained for the receptor. Interestingly, in replicate sections, these ER-β-positive cells were negative for AR immunostaining whereas the reverse was true for the majority of cells that were negative for the β receptor (original magnification, ×400). All sections were counterstained with 10% Harris hematoxylin. C: ER-β staining in an area of transition from grade 3 to grade 4/5 carcinoma. The majority of nuclei in this cancer are unstained. A few positively stained nuclei are however seen in two grade 4/5 glands (bottom left) and in a single cell in a grade 3 gland (bottom right). Light cytoplasmic staining is evident in most cells. Nuclear membrane staining is also present in many of these cells. Cytoplasmic staining was common in all grades of dysplasia and carcinoma. Nuclear membrane immunostaining was however only a feature of high-grade dysplasias and grade 4/5 carcinoma (original magnification, ×400).
Almost complete absence of ER-β nuclear staining was seen in all but 3 of 15 (20%) grade 4/5 carcinomas (Figure 5C) ▶ . In the majority of cases however staining of the nuclear membrane was frequently apparent. In the three cases, positive cells represented only 10% or less of the total cancer cells in a given lesion and the staining intensity was usually diminished.
Immunostaining for ER-α was detected in only 2 (7.6%) of the total 26 cases of primary carcinoma we studied. In these two instances, a few (<10%) weakly positive cells were found in both grades 3 and 4/5 carcinomas. Staining for the receptor was consistently absent in all clear cell carcinomas of the transition zone.
Irrespective of grade, strong nuclear AR immunostaining was a constant feature in the vast majority cells (>95%) comprising cancers of the peripheral zone. Nuclear AR immunostaining was also present in almost all grade 3 clear-cell carcinomas but it was less intense than that found in peripheral zone carcinomas. Interestingly, the occasional few cells that were ER-β-positive in these cancers were found to be negative for AR expression in replicate sections.
No change in the location or intensity of immunostaining for the three receptors in the stroma was evident in sections that contained carcinoma.
Metastatic Lesions
Nuclear ER-β immunostaining was present in metastatic carcinoma cells in bone lesions from all but one of the seven cases (Figure 6A) ▶ . The intensity of signal did however vary from strong to weak staining within cells comprising the lesions of individual case and/or among the cases. In three instances, carcinoma cells were surrounded by a prominent desmoplastic response in which ER-β staining was frequently found in the nuclei of fibroblasts. Nucleated hematopoietic marrow cells were also positive for the receptor whereas mature red cells were negative, a finding that served as a positive and negative internal tissue control for ER-β immunostaining in these lesions.
Figure 6.
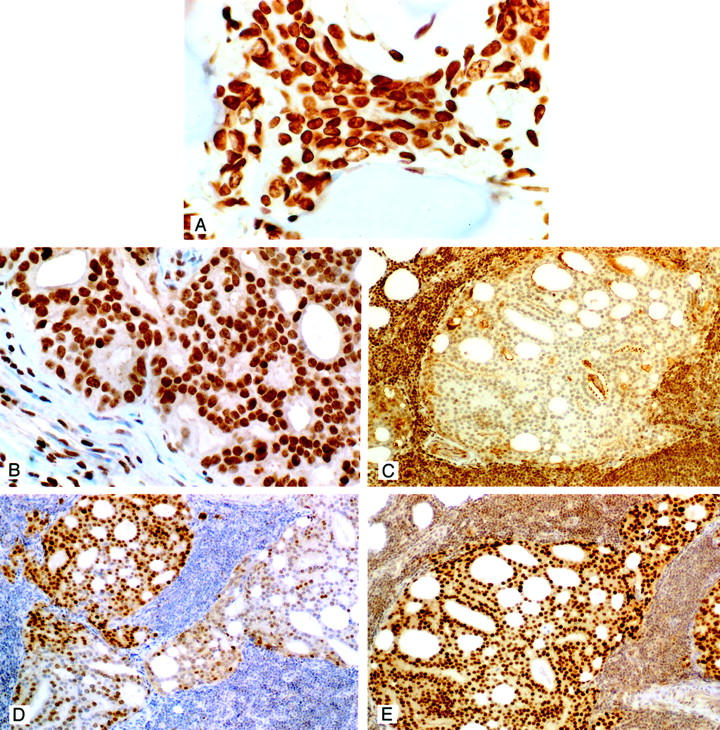
A: ER-β immunostaining in a prostatic carcinoma metastatic to bone. Note the strong nuclear immunostaining for the β receptor in this metastatic lesion. The neoplastic cells are localized between spicules of bone. Strong to moderate staining was present in the majority of metastases to bone (original magnification, ×400). B: ER-β immunostaining in a prostatic carcinoma metastatic to an internal iliac lymph node. Strong nuclear immunostaining is evident in this metastatic lesion. Strong PSA immunostaining of these cells were found (not illustrated). Note that the nuclei of several stromal cells in this lymph node are also positively stained for the receptor (original magnification, ×400). C: ER-β immunostaining of a prostatic carcinoma metastatic to a lymph node. In this example the metastatic cells were unstained for the receptor. Cells in this same cancer were however immunostained for ER-α and AR (see D and E). As was the case for the metastasis illustrated in B these cells were strongly PSA-positive. Note that lymphocytes are strongly stained for the receptor, which was a consistent finding and provided an internal positive control for immunostaining with the β antibody (original magnification, ×100). D: ER-α immunostaining of the same lesion illustrated in C. Immunopositive cells are seen scattered throughout this metastatic lesion. In one other case of lymph node metastasis a very few positive cells (<10%) were present. ER-β was however strongly expressed in that metastatic lesion. Note the absence of ER-α immunostaining of lymphocytes, a consistent finding that was in contrast to ER-β staining in these cells (original magnification, ×100). E: Representative section of AR immunostaining in metastatic lymph node lesions. Strong nuclear staining was uniformly found in the majority of cells that comprised these metastatic lesions. Cytoplasmic staining occurred only in metastases from the patient who had received anti-androgenic therapy. Lymphocytes were lightly stained for AR (original magnification, ×100). All sections were counterstained with 10% Harris hematoxylin.
In contrast to the finding of ER-β immunostaining in foci of all but one case of bone metastasis, no staining was observed for ER-α in these lesions. Despite the fact that all of the seven patients, including the one that lacked ER-β expression had been given anti-androgenic therapy, nuclear AR staining was observed in most metastatic cells in the bone biopsies. However AR staining was also consistently present in the cytoplasm as well as in the nucleus of metastatic cells, a finding observed in bone and lymph node lesions from all patients who received anti-androgenic therapy (see below).
PSA immunostaining was found in metastatic bone lesions in four cases but it tended to be scant especially when compared to lesions in lymph nodes from patients who did not receive anti-androgenic therapy (see following discussion).
Among the five cases of lymph node metastases, two contained a majority of neoplastic cells (>50%) that were uniformly strongly stained for ER-β (Figure 6B) ▶ . In one case immunostaining for the receptor was absent in the metastatic cells (Figure 6C) ▶ whereas in the remaining two cases a mix of negative and positively stained neoplastic cells were found. In one of the two cases, in which strong receptor expression was detected, the patient had been treated with the LH/RH agonist Lupron before surgery. Lymphocyte nuclei were consistently stained for the receptor and served as an internal positive tissue control in the two cases in which no receptor was detected in metastatic cells within the lymph nodes (Figure 6B) ▶ .
ER-α-immunostained carcinoma cells were found in two of five cases of lymph node metastases. Both patients were untreated before surgery. In one of these cases, ER-β staining was absent in metastatic cells (Figure 6C) ▶ . In one case, numerous α receptor-positive cells (>50%) were mixed with those in which receptor expression was absent (Figure 6D) ▶ . Very weak staining for ER-α was identified in a few cells (<10%) in the other case.
In all five cases, AR immunostaining was present in the vast majority (>95%) of metastatic cells (Figure 6E) ▶ . Nuclear AR expression was always strong in cancer cells and with the exception of the case where the patient received Lupron, cytoplasmic staining was not present in any of the metastatic cells. Strong PSA immunostaining was present in the cytoplasm of most metastatic cells in all cases including the one where the patient had received anti-androgenic therapy.
LCM/RT-PCR Analysis
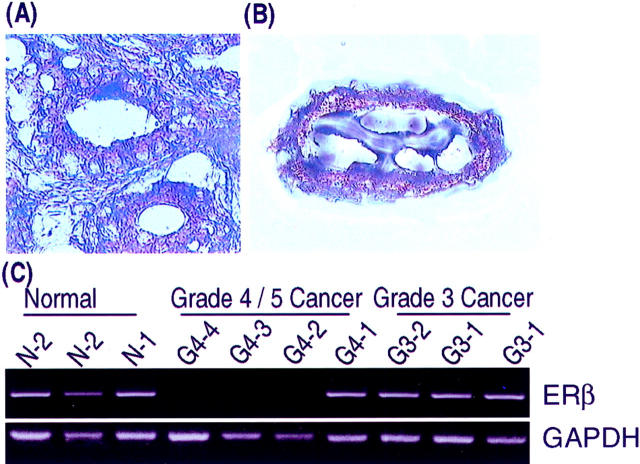
An example of a LCM specimen used in our study is seen in Figure 7, A and B ▶ .
Figure 7.
A and B: LCM dissection of a grade 3 carcinoma. A: The grade 3 neoplastic gland that was microdissected is seen in the center of this microscopic field (original magnification, ×325). B: The microdissected lesion is seen on the transfer cap (original magnification, ×325). Both sections were lightly stained with 10% Harris hematoxylin. C: Results of RT-PCR analysis of ER-β mRNA on microdissected human normal prostate acini (N-1 and N-2) and grade 3 (G3–1 and G3–2) and 4/5 (G4–1 to G4–4) carcinomas. Total RNAs were extracted from the microdissected tissues on transfer cap and reverse-transcribed. The resultant cDNAs were subjected to PCR analyses. The amplified products were run into 2% agarose gel with ethidium bromide. Representative fluorographs for ER-β and GAPDH RT-PCR analyses are shown.
Our findings with RT-PCR analysis for ER-β mRNA on LCM lesions approximated the results of immunohistochemical studies done on paraffin sections immediately adjacent to the frozen specimens used for RT-PCR as well as on other cases (Figure 7C) ▶ . ER-β transcripts were detected in two of the three samples of normal prostatic acini. In contrast, receptor message was found in only one of four microdissected samples of high-grade dysplasias. Sixty percent of grade 3 carcinomas contained ER-β transcripts whereas receptor message was detected in two of six (30%) of the grade 4/5 cancers. In two cases where ER-β message was present in grade 3 lesions, grade 4/5 carcinomas sampled within the same section lacked receptor mRNA expression. In close agreement with our immunohistochemical findings, AR mRNA was present in all normal glands, dysplasias, grade 3 cancers, and all but one of the grade 4/5 carcinomas. ER-α transcripts were not detected in any of the microdissected specimens we studied.
Discussion
In this study we developed and comprehensively demonstrated the specificity of a novel antibody, prepared against the F domain of ER-β. Using this reagent, together with antibodies for AR and ER-α, we immunolocalized the three receptors in normal human prostate, preneoplastic lesions, carcinoma, and in metastases. LCM/RT-PCR was used to assess the expression of the three receptors at the transcript level.
Using the GC-17 antibody reagent, we now show that ER-β is predominately immunolocalized in basal cells and to a lesser extent in stromal cells of the morphologically normal human prostate. In addition nuclear membrane localization of ER-β was occasionally observed in secretory cells. Our current studies have also confirmed past reports 30,31 that ER-α is detected in stromal cells and rarely in basal cells of the normal gland. As previously reported, 41-43 we found that AR was predominately localized in the nuclei of differentiated secretory cells and variably in basal cells of the normal acinar/duct unit as well as in stromal cells. The finding of all three receptors in stromal cells is compatible with the concept that they transduce steroid hormone signals to epithelial cells by a paracrine mechanism. 43 In this regard, Hall and colleagues 44 have reported that ER-β functions as a transdominant inhibitor of ER-α transcription and that it acts to decrease overall cellular sensitivity to estradiol. Therefore the β isoform, when co-localized with the α receptor in stromal cells, may play a critical role in regulating paracrine signaling from these cells to epithelia. However the presence of ER-β as virtually the sole ER subtype in basal cells, the purported precursor of secretory cells, 9 suggests that estrogens, acting through the receptor, may directly modulate the growth of these cells. In this context, it should also be noted that AR is variably expressed in basal cells. 41,42 Thus, proliferative signals mediated by AR in basal cells or by ER-α and AR in stromal cells may be opposed by the purported growth-inhibitory action of ER-β 25-28 localized in basal cells.
Before the discovery of ER-β 5,6 we reported that the separate administration of androgens and estrogens to hypophysectomized and castrated dogs induced marked proliferation of basal cells. Additionally, each hormone was found to direct distinct pathways of cell differentiation culminating in either squamous cells with estrogens or prototypic glandular cells with androgens. 6 Thus, although paracrine stimulatory signals emanating from stromal cells likely contributed to these effects, the direct action of the hormones mediated through their cognate receptors in basal cells may have also played a role. In either case results from these studies indicate that basal cells are major targets for the effects of steroid hormones in the prostate.
Mindful of these findings it was therefore of particular interest to trace the expression of ER-β, normally localized in basal cells, and AR during prostatic cancer development and progression.
The transition from normal to low/moderate dysplastic glands in the peripheral zone was marked by the appearance of ER-β homogeneously immunostained nuclei in secretory as well as basal cells with no changes in the localization of the other receptors.
The expression of ER-β was diminished in high-grade dysplasias when compared to normal glands and lower grade lesions. Because the receptor is predominately localized in basal cells in the normal gland, this finding is consistent with the reported depletion of these receptor-positive cells in the majority of high-grade dysplasias. 37 ER-β staining was present in the majority of grade 3 carcinomas of the peripheral zone but was greatly diminished or absent in most grade 4/5 carcinomas. Interestingly nuclear membrane staining was evident in many cells in high-grade dysplasias and grade 4/5 carcinomas. The precise meaning of this finding is currently undefined but seems to represent the localization of ER-β that occurs with neoplastic progression and could reflect alteration in receptor function. Recently, the promoter of human ER-β gene has been cloned and showed regions of CpG islands. 45 The diminution of ER-β expression in high-grade dysplasias and grade 4/5 cancers may be therefore related to the alteration of DNA methylation pattern in CpG islands of the promoter, resulting in down-regulation of the receptor at the transcriptional level.
While the underlying mechanism(s) for these findings in dysplasias awaits further investigation, based on the proposed anti-proliferative function of the receptor, 25-28 the presence of ER-β in secretory cells of low/moderate-grade lesions may represent a transient abortive attempt to counter growth of these cells. In contrast, the attrition of receptor-positive basal cells in the high-grade dysplasias may signify a continuing loss of growth inhibitory function mediated by ER-β in these precursor lesions. The reappearance of the β receptor in most grade 3 carcinomas and its possible effects on cancer growth is perplexing but may be related to the more favorable prognosis reported for these well-differentiated neoplasms. 38
Interestingly, in contrast to grade 3 carcinomas of the peripheral zone, expression of the β receptor was present only in a few scattered cells that comprised clear cell cancers of the transition zone. The differences in β receptor expression between the two grade 3 cancers likely reflect a distinct biology inherent in neoplasms with the clear cell phenotype.
Although absent from peripheral zone lesions, ER-α staining was however found in dysplastic cells in most lesions of the central zone. This finding may reflect suspected biological differences 46 as well as distinct pathways of hormone responsiveness between the zones of the human prostate that could play a role in the pathogenesis of these lesions. Interestingly, the central zone is rarely the site of origin for prostate cancer. 47
Our findings of RT-PCR analysis for ER-β mRNA expression on microdissected specimens approximated results from our immunohistochemical studies, indicating that receptor expression was down-regulated at both the transcriptional and translational levels in high-grade dysplastic and 4/5 grade neoplastic lesions. Our findings in prostate therefore differ from those reported for human colon cancer in which Folley and colleagues 48 demonstrated that a selective loss of ER-β protein but not receptor message expression occurs in these neoplasms. These authors attributed the loss of ER-β protein in colon cancers to be from posttranscriptional modifications of the receptor.
In our study ER-α expression was limited to only a small subset of dysplastic lesions in the central zone and to a very few primary carcinomas in the peripheral zone. Our findings therefore differed from those of Bonkhoff and colleagues 33 who found immunostaining for the receptor in high-grade dysplasias and grade 4/5 carcinomas. Using in situ hybridization these authors also reported that a high percentage of dysplasias and carcinomas in their study contained cells that expressed ER-α message. These results again differed from our findings as we did not detect ER-α message in any dysplasias or primary carcinomas we studied using the highly sensitive and specific LCM/RT-PCR analysis.
Unlike ER-β, AR was consistently strongly expressed at both the transcript and immunohistochemical levels, irrespective of grade, across the spectrum of preneoplastic lesions and carcinomas we studied. These results are in agreement with our past studies of AR in dysplasias 41 and those of others in both primary and metastatic carcinomas. 49 Importantly, results from a past study 50 demonstrated the presence of structurally intact AR that functionally binds androgen in the majority of both hormone-dependent and -independent carcinomas. Our current findings, together with those cited above, suggest that AR is expressed and functional in the majority of high-grade dysplasias and primary prostate cancers. It is therefore possible that continued androgen-mediated stimulation of dysplastic and tumor cells, coupled with the apparent loss of ER-β expression, may enhance carcinogenic progression as well as the growth of established prostatic carcinomas.
One of the most intriguing findings in our study was the high percentage of cases (83%) where the expression of ER-β was present in prostatic carcinoma cells metastatic to bone and regional lymph nodes. In marked contrast, ER-α staining was absent in all but two cases where receptor-positive cells were found in lymph node metastases.
Although 58% of the patients with metastatic lesions in our study had been given anti-androgen therapy, positive staining for ER-β was also present in cancer cells within the lymph nodes of three individuals that had not received this treatment. The presence of ER-β in metastatic cells from patients who did or did not receive anti-androgenic treatment suggests that blocking of androgen action was not a primary cause for the expression of the receptor in these sites.
It is tempting to speculate that the new tissue microenvironment in which these metastatic cells are found may have provided local factors that influenced the expression of ER-β and to a lesser extent ER-α in these sites. In this context, it has been reported that bone fibroblasts produced growth factors that induced human prostate cancer growth. 51 Alternatively, the metastatic process may, in some undefined manner, have favored the spread of cells with either ER isoform from the primary cancer.
In summary, we demonstrate that a consistent pattern of lost ER-β expression at both the transcriptional and translational levels occurs during prostatic carcinogenesis and tumor progression. This may signify the loss of an important role the receptor would normally play in inhibiting growth of the prostate that could contribute to neoplastic development. The continued expression of presumably functional AR throughout these processes, as well as other undefined factors, may therefore exert persistent unopposed growth stimulus acting on these cells.
The cause of ER-β expression and the effects that it may have on the growth of metastatic cells remains to be defined. The presence of the β isoform in these cancer cells may however have important ramifications for the treatment of patients with late-stage disease. In this regard, we recently reported that estrogens as well as anti-estrogens are potent growth inhibitors of human prostate cancer lines that express both ER isoforms. 28 In contrast, the growth of cells such as DU145, which only express ER-β, was markedly inhibited by the anti-estrogen ICI 182,807. These findings together with results from our current studies may therefore be helpful in devising new ligand-specific strategies for treating patients with metastatic prostate cancer.
Footnotes
Address reprint requests to Dr. Shuk-Mei Ho, 55 Lake Avenue North, S4-746, Department of Surgery, Division of Urology, University of Massachusetts Medical School, Worcester, MA 01655. E-mail: shuk-mei.ho@umassmed.edu.
Supported by United States Department of Defense grant DAMD17-98-1-8606.
References
- 1.Grayhack JT, Keeler TC, Kozlowski JM: Carcinoma of the prostate. Hormonal therapy. Cancer 1987, 60:589-601 [DOI] [PubMed] [Google Scholar]
- 2.Kozlowski JM, Ellis WJ, Grayhack JT: Advanced prostatic carcinoma. Early versus late endocrine therapy. Urol Clin N Am 1991, 18:15-24 [PubMed] [Google Scholar]
- 3.Huggins C, Hodges CV: Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972, 22:232-240 [DOI] [PubMed] [Google Scholar]
- 4.Helpap B, Stiens R: The cell proliferation of epithelial metaplasia in the prostate gland. An autoradiographic in vitro study. Virchows Arch B Cell Pathol 1975, 19:69-76 [DOI] [PubMed] [Google Scholar]
- 5.Leav I, Merk FB, Ofner P, Goodrich G, Kwan PW, Stein BM, Sar M, Stumpf WE: Bipotentiality of response to sex hormones by the prostate of castrated or hypophysectomized dogs. Direct effects of estrogen. Am J Pathol 1978, 93:69-92 [PMC free article] [PubMed] [Google Scholar]
- 6.Merk FB, Warhol MJ, Kwan PW, Leav I, Alroy J, Ofner P, Pinkus GS: Multiple phenotypes of prostatic glandular cells in castrated dogs after individual or combined treatment with androgen and estrogen. Morphometric, ultrastructural, and cytochemical distinctions. Lab Invest 1986, 54:442-456 [PubMed] [Google Scholar]
- 7.Mawhinney MG, Neubauer BL: Actions of estrogen in the male. Invest Urol 1979, 16:409-420 [PubMed] [Google Scholar]
- 8.Levine AC, Kirschenbaum A, Droller M, Gabrilove JL: Effect of the addition of estrogen to medical castration on prostatic size, symptoms, histology and serum prostate specific antigen in 4 men with benign prostatic hypertrophy. J Urol 1991, 146:790-793 [DOI] [PubMed] [Google Scholar]
- 9.De Marzo AM, Nelson WG, Meeker AK, Coffey DS: Stem cell features of benign and malignant prostate epithelial cells. J Urol 1998, 160:2381-2392 [DOI] [PubMed] [Google Scholar]
- 10.Ho SM, Lee KF, Lane K: Neoplastic transformation of the prostate. Naz RK eds. Prostate: Basic and Clinical Aspects. 1997, :pp 73-113 CRC Press Boca Raton [Google Scholar]
- 11.Bosland MC: The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr 2000, 2000:39-66 [DOI] [PubMed] [Google Scholar]
- 12.Santti R, Newbold RR, Makela S, Pylkkanen L, McLachlan JA: Developmental estrogenization and prostatic neoplasia. Prostate 1994, 24:67-78 [DOI] [PubMed] [Google Scholar]
- 13.Pylkkanen L, Makela S, Santti R: Animal models for the preneoplastic lesions of the prostate. Eur Urol 1996, 30:243-248 [DOI] [PubMed] [Google Scholar]
- 14.Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D: Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst 1988, 80:1045-1053 [DOI] [PubMed] [Google Scholar]
- 15.Bosland MC, Ford H, Horton L: Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd: SD rats treated with a combination of testosterone and estradiol-17 beta or diethylstilbestrol. Carcinogenesis 1995, 16:1311-1317 [DOI] [PubMed] [Google Scholar]
- 16.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA: Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996, 93:5925-5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosselman S, Polman J, Dijkema R: ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996, 392:49-53 [DOI] [PubMed] [Google Scholar]
- 18.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V: Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol 1997, 11:353-365 [DOI] [PubMed] [Google Scholar]
- 19.Bhat RA, Harnish DC, Stevis PE, Lyttle CR, Komm BS: A novel human estrogen receptor beta: identification and functional analysis of additional N-terminal amino acids. J Steroid Biochem Mol Biol 1998, 67:233-240 [DOI] [PubMed] [Google Scholar]
- 20.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA: Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 1997, 82:4258-4265 [DOI] [PubMed] [Google Scholar]
- 21.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA: Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138:863-870 [DOI] [PubMed] [Google Scholar]
- 22.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS: Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997, 277:1508-1510 [DOI] [PubMed] [Google Scholar]
- 23.Enmark E, Gustafsson JA: Oestrogen recept—an overview. J Intern Med 1999, 246:133-138 [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson JA: Estrogen receptor beta—a new dimension in estrogen mechanism of action. J Endocrinol 1999, 163:379-383 [DOI] [PubMed] [Google Scholar]
- 25.Chang WY, Prins GS: Estrogen receptor-beta: implications for the prostate gland. Prostate 1999, 40:115-124 [DOI] [PubMed] [Google Scholar]
- 26.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O: Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 1998, 95:15677-15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poelzl G, Kasai Y, Mochizuki N, Shaul PW, Brown M, Mendelsohn ME: Specific association of estrogen receptor beta with the cell cycle spindle assembly checkpoint protein, MAD2. Proc Natl Acad Sci USA 2000, 97:2836-2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau KM, LaSpina M, Long J, Ho SM: Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res 2000, 60:3175-3182 [PubMed] [Google Scholar]
- 29.Montano MM, Jaiswal AK, Katzenellenbogen BS: Transcriptional regulation of the human quinone reductase gene by antiestrogen-liganded estrogen receptor-alpha and estrogen receptor-beta. J Biol Chem 1998, 273:25443-25449 [DOI] [PubMed] [Google Scholar]
- 30.Wernert N, Gerdes J, Loy V, Seitz G, Scherr O, Dhom G: Investigations of the estrogen (ER-ICA-test) and the progesterone receptor in the prostate and prostatic carcinoma on immunohistochemical basis. Virchows Arch A Pathol Anat Histopathol 1988, 412:387-391 [DOI] [PubMed] [Google Scholar]
- 31.Schulze H, Claus S: Histological localization of estrogen receptors in normal and diseased human prostates by immunocytochemistry. Prostate 1990, 16:331-343 [DOI] [PubMed] [Google Scholar]
- 32.Ehara H, Koji T, Deguchi T, Yoshii A, Nakano M, Nakane PK, Kawada Y: Expression of estrogen receptor in diseased human prostate assessed by non-radioactive in situ hybridization and immunohistochemistry. Prostate 1995, 27:304-313 [DOI] [PubMed] [Google Scholar]
- 33.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K: Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol 1999, 155:641-647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor AH, Al Azzawi F: Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol 2000, 24:145-155 [DOI] [PubMed] [Google Scholar]
- 35.Fuqua SA, Schiff R, Parra I, Friedrichs WE, Su JL, McKee DD, Slentz-Kesler K, Moore LB, Willson TM, Moore JT: Expression of wild-type estrogen receptor beta and variant isoforms in human breast cancer. Cancer Res 1999, 59:5425-5428 [PubMed] [Google Scholar]
- 36.McNeal JE: Prostatic microcarcinomas in relation to cancer origin and the evolution to clinical cancer. Cancer 1993, 71:984-991 [DOI] [PubMed] [Google Scholar]
- 37.Bostwick DG: High grade prostatic intraepithelial neoplasia: the most likely precursor of prostate cancer. Cancer 1995, 75:1823-1826 [Google Scholar]
- 38.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM: Biological determinants of cancer progression in men with prostate cancer. JAMA 1999, 281:1395-1400 [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson JA: An update on estrogen receptors. Semin Perinatol 2000, 24:66-69 [DOI] [PubMed] [Google Scholar]
- 40.Harlow E, Lane D: Antibodies: A Laboratory Manual. 1988:pp 139-242 Cold Spring Harbor Laboratory, New York
- 41.Leav I, McNeal JE, Kwan PW, Komminoth P, Merk FB: Androgen receptor expression in prostatic dysplasia (prostatic intraepithelial neoplasia) in the human prostate: an immunohistochemical and in situ hybridization study. Prostate 1996, 29:137-145 [DOI] [PubMed] [Google Scholar]
- 42.Bonkhoff H, Remberger K: Widespread distribution of nuclear androgen receptors in the basal cell layer of the normal and hyperplastic human prostate. Virchows Arch A Pathol Anat Histopathology 1993, 422:35-38 [DOI] [PubMed] [Google Scholar]
- 43.Gleave ME, Chung LW: Stromal-epithelial interaction affecting prostate tumor growth and hormonal responsiveness. Endocrinol-Related Cancer 1995, 2:243-265 [PubMed] [Google Scholar]
- 44.Hall JM, McDonnell DP: The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 1999, 140:5566-5578 [DOI] [PubMed] [Google Scholar]
- 45.Li LC, Yeh CC, Nojima D, Dahiya R: Cloning and characterization of human estrogen receptor beta promoter. Biochem Biophys Res Commun 2000, 275:682-689 [DOI] [PubMed] [Google Scholar]
- 46.McNeal JE, Leav I, Alroy J, Skutelsky E: Differential lectin staining of central and peripheral zones of the prostate and alterations in dysplasia. Am J Clin Pathol 1988, 89:41-48 [DOI] [PubMed] [Google Scholar]
- 47.McNeal JE, Redwine EA, Freiha FS, Stamey TA: Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol 1988, 12:897-906 [DOI] [PubMed] [Google Scholar]
- 48.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW: Selective loss of estrogen receptor beta in malignant human colon. Cancer Res 2000, 60:245-248 [PubMed] [Google Scholar]
- 49.Culig Z, Hobisch A, Bartsch G, Klocker H: Androgen receptor—an update of mechanisms of action in prostate cancer. Urol Res 2000, 28:211-219 [DOI] [PubMed] [Google Scholar]
- 50.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, Verleun-Mooijman MC, Trapman J, Brinkmann AO, Santerse AB, Schroder FH, van der Kwast TH: Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol 1994, 144:735-746 [PMC free article] [PubMed] [Google Scholar]
- 51.Yomeda T: Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur J Cancer 1998, 34:240-245 [DOI] [PubMed] [Google Scholar]