Abstract
Metaplasia of subcoelomic mesenchyme has been implicated, but not proven, in the pathogenesis of common gynecological diseases such as endometriosis and rarer entities such as leiomyomatosis peritonealis disseminata and gliomatosis peritonei (GP). GP is associated with ovarian teratomas and is characterized by numerous peritoneal and omental implants composed of glial tissue. Two theories to explain the origin of GP have been proposed. In one, glial implants arise from the teratoma, whereas in the other, pluripotent Müllerian stem cells in the peritoneum or subjacent mesenchyme undergo glial metaplasia. To address the origin of GP, we exploited a unique characteristic of many ovarian teratomas: they often contain a duplicated set of maternal chromosomes and are thus homozygous at polymorphic microsatellite (MS) loci. In contrast, DNA from matched normal or metaplastic tissue (containing genetic material of both maternal and paternal origin) is expected to show heterozygosity at many of these same MS loci. DNA samples extracted from paraffin-embedded normal tissue, ovarian teratoma and three individual laser-dissected glial implants were studied in two cases of GP. In one case, all three implants and normal tissue showed heterozygosity at each of three MS loci on different chromosomes, whereas the teratoma showed homozygosity at the same MS loci. Similar results were observed in the second case. Our findings indicate that glial implants in GP often arise from cells within the peritoneum, presumably pluripotent Müllerian stem cells, and not from the associated ovarian teratoma. This finding has important implications for more common gynecological entities with debatable pathogenesis, such as endometriosis, by definitively demonstrating the metaplastic potential of stem cells within the peritoneal cavity.
Gliomatosis peritonei (GP), characterized by numerous peritoneal and omental implants composed of glial tissue, is a rare benign condition associated with both mature and immature teratomas of the ovary. 1-8 GP has also been reported in association with a gastric teratoma in a male infant 9 and an immature endometrial teratoma. 10 Two major theories regarding the pathogenesis of GP have been proposed. The first suggests that glial foci arise from the primary teratoma either through rupture of the capsule with subsequent implantation of tissue within the peritoneum, or via angiolymphatic spread as in carcinoma metastasis. 7 The second suggests that glial foci arise independently from pluripotent Müllerian stem cells in response to favorable intraperitoneal conditions, as a so-called “field effect.” 11
To address whether glial implants are genetically related to the associated ovarian teratoma or whether they arise independently, we exploited the unique genetic make-up of many ovarian teratomas. Approximately 65% of teratomas are derived from a single germ cell after the first meiotic division with subsequent failure of meiosis 2 or endoreduplication of a haploid ovum. 12 Teratomas arising through these mechanisms show homozygosity at most, if not all, polymorphic microsatellite (MS) loci. 13,14 In this study, we used MS loci demonstrating a heterozygous pattern in normal tissue and a homozygous pattern in the ovarian teratoma from the same patient to determine the origin of glial implants in GP. We presumed that if the glial implants showed a homozygous pattern similar to the teratoma, they were most likely related to the teratoma and arose via capsular rupture or angiolymphatic dissemination. If they demonstrated a heterozygous pattern, similar to normal tissue, they most likely arose via metaplasia of normal cells within the peritoneum.
Materials and Methods
Case Selection
Five cases of GP associated with ovarian teratomas were retrieved from the pathology archives of The Johns Hopkins Hospital, Baltimore, MD (two cases), The University of Michigan Hospital, Ann Arbor, MI (one case), and the Kumamoto University Hospital, Kumamoto, Japan (two cases). Hematoxylin and eosin (H&E)-stained tissue sections were reviewed for confirmation of diagnosis. Three cases were excluded from further analysis, one because DNA from the teratoma and matched normal tissue demonstrated identical alleles at all polymorphic MS loci tested, and two because amplifiable DNA could not be extracted from the available tissues. The two cases from Baltimore proved suitable for additional study. In one case GP was associated with a mature teratoma and in the second case, GP was associated with an immature teratoma (grade 2). This neoplasm contained a minor component of immature neuroepithelial tissue (2 of 19 slides). Four paraffin blocks were selected from each of these two cases: one containing ovarian teratoma, one containing nonneoplastic tissue (cervix or fallopian tube), and two containing either omentum or peritoneal surface with numerous (>50) glial implants.
DNA Extraction
Neoplastic (teratoma) and nonneoplastic (cervix or fallopian tube) tissue was isolated with a razor blade using an H&E-stained section as a dissection guide. DNA was extracted as previously described with slight modifications. 15 Briefly, 4-μm-thick wax sections were incubated in 300 μl of lysis buffer (200 μg/ml proteinase K, 50 mmol/L Tris, pH 8.3, 0.5% Tween-20, 100 μg/ml glycogen) for 48 hours at 60°C with the addition of 200 μg/ml proteinase K after the first 24 hours. The lysate was then extracted twice with phenol/chloroform (1:1) and allowed to incubate for 2 minutes after the addition of 36 μl of ethidium bromide (5 mg/ml). Phenol/chloroform (X1) and chloroform (X1) extractions were performed after the addition of one-half volume of 7.5 mol/L ammonium acetate; followed by the addition of one drop of Chelex-100 (Bio-Rad, Bethesda, MD) bead slurry. After a 5-minute incubation, DNA was ethanol-precipitated, washed with 70% ethanol, and resuspended in 10 to 50 μl of water. The same technique was used to extract DNA from glial implants stained and microdissected per the manufacturers’ protocol using an Arcturus laser-capture microscope (Mountain View, CA).
Genetic Analysis
DNA samples were subjected to polymerase chain reaction (PCR) amplification of multiple MS markers including D5S592 (5pter-5qter), D16S2624 (16q), and D17S1987 (17q). Primer pairs and annealing temperatures are shown in Table 1 ▶ . Each 10-μl PCR reaction contained 1× PCR buffer (10 mmol/L Tris-HCl, pH 9.2, 1.5 mmol/L MgCl2, 75 mmol/L KCl), 200 μmol/L dATP, 200 μmol/L dGTP, 200 μmol/L dTTP, 25 μmol/L dCTP, 2 μCi (3000 Ci/mmol) dCTP-32P, 0.1 μmol/L of each primer, and 1.0 U Taq polymerase. Input DNA varied from 1 to 4.8 μl as DNA obtained from the laser-captured glial implants required a higher input volume than DNA obtained from normal tissue and teratoma. After an initial denaturation step of 95°C for 5 minutes, DNA templates were amplified using 40 cycles of 95°C for 30 seconds, 55°C (D5S592, D16S2624), or 57°C (D17S1987) for 1 minute and 72°C for 1 minute, followed by a final extension step at 72°C for 7 minutes. PCR reactions were diluted with 5 to 10 μl stop buffer (20 mmol/L Tris-HCl, 10 mmol/L ethylenediaminetetraacetic acid, 0.1 mmol/L dCTP) and PCR products were resolved by electrophoresis on a 6% acrylamide-8 mol/L urea gel that was dried and subjected to autoradiography.
Table 1.
PCR Sequences and Annealing Temperatures
| Locus | Sequence (5′-3′) | Fragment size (bp) | Type of repeat | Annealing temperature |
|---|---|---|---|---|
| D5S592 (F) | AGACAGACAGAGAGATTAGA | 145–205 | Tetranucleotide | 55°C |
| D5S592 (R) | AGTAAAGTGAGTGGAGAGC | |||
| D16S2624 (F) | TGAGGCAATTTGTTACAGAGC | 143 | Tetranucleotide | 55°C |
| D16S2624 (R) | TAATGTACCTGGTACCAAAAACA | |||
| D17S1987 (F) | AAGAGCTGGGGGAGCTTAAG | 185–209 | Tetranucleotide | 57°C |
| D17S1987 (R) | AAGAACTTCTGCGGGTCAGA |
Results
In case 1, the teratoma was classified as a benign cystic teratoma that contained mature tissue derived from all three germ layers (Figure 1A) ▶ . The teratoma from case 2 displayed a similar histological picture but also included a minor component of immature neural tissue, leading to its classification as a grade 2 immature teratoma per the criteria of Thurlbeck and Scully. 16 All glial implants from both cases were composed of mature glial tissue (Figure 1B) ▶ . DNA was extracted from the teratoma, nonteratomatous normal tissue and three individual laser-captured microdissected glial implants for each of the two cases. A representative histological section stained with a modified H&E stain demonstrates a glial implant before microdissection (Figure 1C) ▶ , the same implant after microdissection (Figure 1D) ▶ and the cap onto which the tissue was captured (Figure 1E) ▶ .
Figure 1.
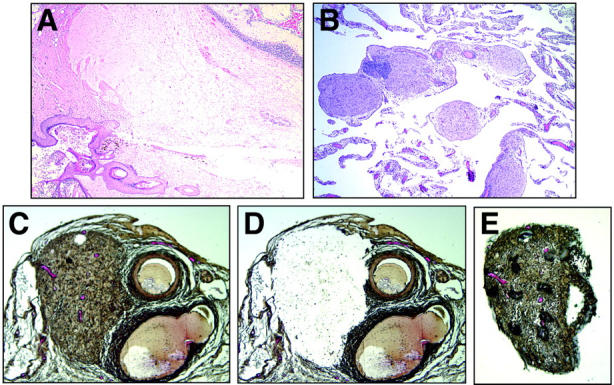
A: H&E-stained section of a mature teratoma containing keratinizing squamous epithelium and hair follicle (bottom left), mature glial tissue (center), and cerebellar tissue (top right) (original magnification, ×40). B: H&E-stained tissue section of omentum. Five individual foci of mature glial tissue are pink and round to ovoid in shape (original magnification, ×100). C: Modified H&E-stained section showing a glial implant (left) and adjacent blood vessels (right) before laser-capture microdissection (original magnification, ×200). D: Same section as shown in C after laser-capture microdissection (original magnification, ×200). E: Captured glial implant. The dark bubbles overlying the implant are an artifact of the capture membrane (original magnification, ×200).
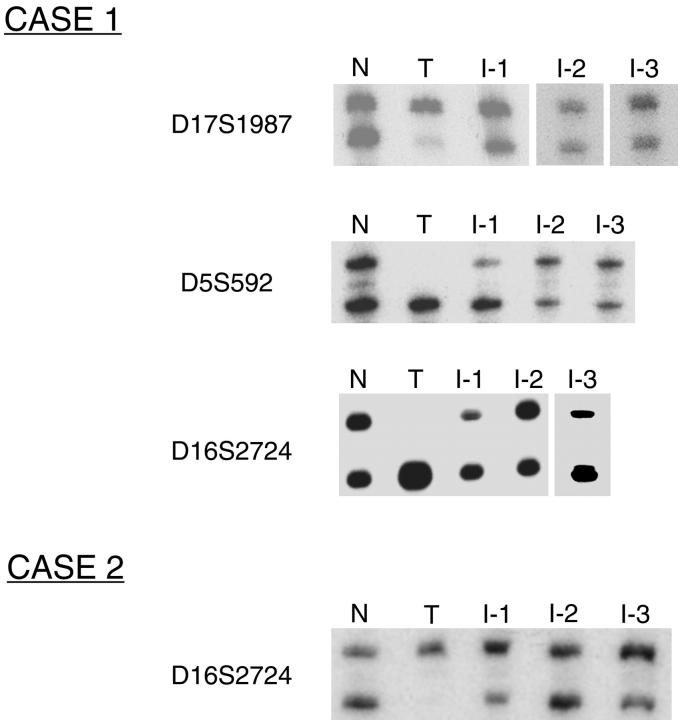
Polymorphic MS loci on three chromosomes (D5S592, D16S2624, and D17S1987) were amplified using these DNA samples as PCR templates. In both cases, DNA from normal tissue and individually microdissected glial implants demonstrated a heterozygous pattern at the polymorphic loci evaluated, whereas DNA from the matched teratoma showed a homozygous pattern at the same loci. Representative data from both cases are shown in Figure 2 ▶ .
Figure 2.
Representative data from analysis of polymorphic MS loci D5S592, D16S2624, and D17S1987 in two cases of GP. At all loci, nonteratomatous normal tissue (N) demonstrates a heterozygous pattern; teratoma (T) demonstrates a homozygous pattern and three individual glial implants (I-1, I-2, and I-3) demonstrate a heterozygous pattern, similar to normal.
Notably, although some of the PCR reaction products from implant DNA favor the allele present in the teratoma DNA, others show either allele amplification similar to that seen in the matched normal tissue, or even slight bias toward the allele absent in the teratoma DNA. Preferential amplification of one MS allele over the other is a well-recognized problem when amplifying very small quantities of template DNA, as would be expected from DNA extracted from small glial implants in GP. 17-19 In some cases preferential allele amplification can be so severe as to cause total allele drop out. The problem is further compounded by the availability for this study of only formalin-fixed, paraffin-embedded tissue, which is known to yield DNA more prone to PCR artifacts than DNA from frozen tissue. 20 Recognizing that preferential allele amplification might be encountered in PCR reactions using very small quantities of template DNA, we tailored our experimental approach to minimize the likelihood of being misled by PCR artifacts. First, we used meticulous laser capture microdissection rather than manual microdissection to minimize contamination of the implant samples by nonglial cells. We estimate that nonglial cells comprised <5% of the cells harvested for DNA extraction. Second, for several implants yielding sufficient quantities of DNA, results were verified with at least one and often two additional independent PCR reactions at a given locus. Third, we analyzed multiple implants from each case with as many markers as the small quantities of DNA extracted from individual implants would allow. Our findings strongly support the interpretation that the glial implants are derived from cells in the peritoneum and not from teratoma tissue contaminated by nonneoplastic cells.
Discussion
The differentiation capability of pluripotent Müllerian stem cells and their role in the pathogenesis of various gynecological diseases has been debated for decades. These cells have been implicated in the development of endometriotic foci, noninvasive implants of papillary serous tumors of low malignant potential, neoplastic foci in women with multifocal primary peritoneal carcinoma, smooth muscle foci in disseminated peritoneal leiomyomatosis, and glial implants in GP. 11 The unusual chromosomal composition of ovarian teratomas allowed us to use molecular tools to definitively evaluate the differentiation potential of normal cells within the peritoneum and subjacent mesenchyme in GP.
The origin of glial implants in GP and the factors responsible for the development of GP in association with selected teratomas has been the subject of much debate. One theory regarding the origin of glial tissue suggests that it is genetically related to the associated teratoma, being either extruded from the primary neoplasm through capsular defects or disseminated via angiolymphatic channels. Capsular defects have been described in resected teratomas and in some instances, teratomatous material has been observed protruding through these defects. 3-7 In addition, capsular rupture at the time of primary surgery has been described in cases where GP was only apparent at the time of second-look laparotomy. 1 In support of lymphatic dissemination, mature glial tissue has been found in mesenteric, para-aortic, and retroperitoneal lymph nodes in association with immature teratomas in the presence or absence of GP. 3,7,8,21,22 Moreover, cystic peritoneal masses composed of tissue from all three germ layers (including immature neural elements) have been described in addition to small glial foci in some cases of GP, suggesting that the former are true metastases. 2,3,6,7 It remains unclear whether these metastases arise through the same or different mechanism as the foci of mature glial tissue observed in typical GP.
An alternate theory, supported by our study, suggests that glial foci are genetically unrelated to the associated teratoma and arise from normal cells in response to favorable environmental conditions. The most likely candidate normal cells are pluripotent Müllerian stem cells on the peritoneal surface or in the subcoelomic mesenchyme. This hypothesis is based on the observation that many gynecological diseases seem to have a multifocal intraperitoneal origin and is further supported by reports of glial implants admixed with endometrial tissue. 2,3,4,23 Endometrial tissue is Müllerian in origin and is uncommon in teratomas. Given that we analyzed only two cases of GP and a limited number of implants, it is possible that some glial foci result from true implantation, whereas others arise via metaplasia of normal stem cells within the peritoneum.
Why some intraperitoneal stem cells (or tissues) differentiate along a glial pathway whereas others do not requires some speculation. The remarkable ability of stem cells derived from various organs to differentiate along divergent pathways has been the subject of multiple recent articles, including reports of studies demonstrating that bone marrow-derived stem cells can undergo glial differentiation. 24,25 It has been suggested that a stem cell’s microenvironment can induce a specific differentiation pathway or pathways, and it is possible that some teratomas with an abundant glial component secrete factors that induce glial differentiation in the peritoneum. Protein secretion by teratomas is a well-recognized phenomenon. For example, teratomas with a prominent thyroid component, such as struma ovarii and struma-carcinoid, have been shown to secrete thyroid hormone and calcitonin, respectively. 26,27 Notably, murine astrocyte cells and teratocarcinoma cell lines have been shown to secrete β-nerve growth factor in vitro. 28-30 Moreover, GP has been described in children without teratomas who have had ventriculoperitoneal shunts placed early in infancy. 31 Neural growth factors normally present in cerebrospinal fluid may enter the peritoneum through the shunt and induce glial differentiation in the same manner.
The results presented in this study demonstrate the ability of intraperitoneal cells, presumably pluripotent Müllerian stem cells, to undergo glial differentiation. This finding provides important insight into the pathogenesis of gynecological diseases characterized by intraperitoneal multifocality. Identifying the factors responsible for the intraperitoneal field effect in GP will be an important area of future investigation and may further advance our understanding of more common gynecological diseases such as endometriosis.
Acknowledgments
We thank Professor Hitoshi Okamura of the Kumamoto University School of Medicine for generously providing two cases of ovarian teratomas associated with gliomatosis peritonei; and the Laser Capture Microdissection Core, supported by the University of Michigan Cancer Center, for excellent technical assistance.
Footnotes
Address reprint requests to Kathleen R. Cho, M.D., Department of Pathology, University of Michigan, 4301 MSRB-III, Box 0638, 1150 West Medical Center Dr., Ann Arbor, MI 48109. E-mail: kathcho@umich.edu.
References
- 1.Gocht A, Lohler J, Scheidel P, Stegner H-E, Saeger W: Gliomatosis peritonei combined with mature ovarian teratoma: immunohistochemical observations. Path Res Pract 1995, 191:1029-1035 [DOI] [PubMed] [Google Scholar]
- 2.Calder CJ, Light AM, Rollason TP: Immature ovarian teratoma with mature peritoneal metastatic deposits showing glial, epithelial and endometrioid differentiation: a case report and review of the literature. Int J Gynecol Path 1994, 13:279-282 [DOI] [PubMed] [Google Scholar]
- 3.Harms D, Janig U, Gobel U: Gliomatosis peritonei in childhood and adolescence: clinicopathological study of 13 cases including immunohistochemical findings. Path Res Pract 1989, 184:422-430 [DOI] [PubMed] [Google Scholar]
- 4.Bassler R, Theele CH, Labach H: Nodular and tumorlike gliomatosis peritonei with endometriosis caused by a mature ovarian teratoma. Path Res Pract 1982, 175:392-403 [DOI] [PubMed] [Google Scholar]
- 5.Truong L, Juro S, III, McGavran HH: Gliomatosis peritonei, report of two cases and review of the literature. Am J Surg Path 1982, 6:433-449 [PubMed] [Google Scholar]
- 6.Neilsen SNJ, Scheithauer BW, Gaffey TA: Gliomatosis peritonei. Cancer 1985, 56:2499-2503 [DOI] [PubMed] [Google Scholar]
- 7.Robboy SJ, Scully RE: Ovarian teratoma with glial implants on the peritoneum: an analysis of 12 cases. Hum Pathol 1970, 1:643-653 [DOI] [PubMed] [Google Scholar]
- 8.Norris HJ, Zirkin HJ, Benson WL: Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer 1976, 37:2359-2372 [DOI] [PubMed] [Google Scholar]
- 9.Coulson WF: Peritoneal gliomatosis from a gastric teratoma. Am J Clin Pathol 1990, 94:87-89 [DOI] [PubMed] [Google Scholar]
- 10.Ansah-Boateng Y, Wells M, Poole DR: Coexistent immature teratoma of the uterus and endometrial adenocarcinoma complicated by gliomatosis peritonei. Gynecol Oncol 1985, 21:106-110 [DOI] [PubMed] [Google Scholar]
- 11.Dallenbach-Hellweg G: Critical commentary to “Gliomatosis peritonei combined with mature ovarian teratoma.” Pathol Res Pract 1995, 191:1037. [PubMed] [Google Scholar]
- 12.Surti U, Hoffner L, Chakravarti A, Ferrell RE: Genetics and biology of human ovarian teratomas. I. Cytogenetic analysis and mechanism of origin. Am J Hum Genet 1990, 47:635-643 [PMC free article] [PubMed] [Google Scholar]
- 13.Vortmeyer AO, Devouassoux-Shisheboran M, Li G, Mohr V, Tavassoli F, Zhuang Z: Microdissection-based analysis of mature ovarian teratoma. Am J Pathol 1999, 154:987-991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devoussoux-Shisheboran M, Vortmeyer AO, Silver SA, Zhuang Z, Tavassoli FA: Teratomatous genotype detected in malignancies of a non-germ cell phenotype. Lab Invest 2000, 80:81-86 [DOI] [PubMed] [Google Scholar]
- 15.Mutter GL, Chaponot ML, Fletcher JA: A polymerase chain reaction assay for non-random X-chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am J Pathol 1995, 146:501-508 [PMC free article] [PubMed] [Google Scholar]
- 16.Thurlbeck WM, Scully RE: Solid teratoma of the ovary: a clinicopathologic analysis of 9 cases. Cancer 1960, 1:804-811 [DOI] [PubMed] [Google Scholar]
- 17.Kimpton CP, Oldroyd NJ, Watson SK, Frazier RR, Johnson PE, Millican ES, Urquhart A, Sparkes BL, Gill P: Validation of highly discriminating multiplex short tandem repeat amplification systems for individual identification. Electrophoresis 1996, 17:1283-1293 [DOI] [PubMed] [Google Scholar]
- 18.Garvin AM, Holzgreve W, Hahn S: Highly accurate analysis of heterozygous loci by single cell PCR. Nucleic Acids Res 1998, 26:3468-3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluteau O, Legoix P, Laurent-Puig P, Zucman-Rossi J: PCR-based genotyping can generate artifacts in LOH analyses. Biotechniques 1999, 27:1100-1102 [DOI] [PubMed] [Google Scholar]
- 20.Sieben NL, ter Haar NT, Cornelisse CJ, Fleuren GJ, Cleton-Jansen AM: PCR artifacts in LOH and MSI analysis of microdissected tumor cells. Hum Pathol 2000, 31:1414-1419 [PubMed] [Google Scholar]
- 21.El Shafie M, Furay RW, Chablani LV: Ovarian teratoma with peritoneal and lymph node metastases of mature glial tissue: a benign condition. J Surg Oncol 1984, 27:18-22 [DOI] [PubMed] [Google Scholar]
- 22.Perrone T, Steiner M, Dehner LP: Nodal gliomatosis and α-fetoprotein production: two unusual facets of grade I ovarian teratoma. Arch Pathol Lab Med 1986, 110:975-977 [PubMed] [Google Scholar]
- 23.Dworak O, Knopfle G, Varchmin-Schultheiss K, Meyer G: Gliomatosis peritonei with endometriosis externa. Gynecol Oncol 1988, 29:263-266 [DOI] [PubMed] [Google Scholar]
- 24.Vogel G: Stem cells: new excitement, persistent questions. Science 2000, 290:1672-1674 [DOI] [PubMed] [Google Scholar]
- 25.Kopen GC, Prockop DJ, Phinney DG: Marrow stromal cells migrate throughout the forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999, 96:10711-10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simkin PH, Ramirez LA, Zweizig SL, Afonso SA, Fraire AE, Khan A, Dunn AD, Dunn JT, Braverman LE: Monomorphic teratoma of the ovary: a rare cause of triiodothyronine toxicosis. Thyroid 1999, 9:949-954 [DOI] [PubMed] [Google Scholar]
- 27.Blaustein A: Calcitonin secreting struma-carcinoid tumor of the ovary. Hum Pathol 1979, 10:222-228 [DOI] [PubMed] [Google Scholar]
- 28.Dicou E, Houlgatte R, Brachet P: Synthesis and secretion of β-nerve growth factor by mouse teratocarcinoma cell lines. Exp Cell Res 1986, 167:287-294 [DOI] [PubMed] [Google Scholar]
- 29.Furukawa S, Furukawa Y, Satoyoshi E, Hayashi K: Synthesis and secretion of nerve growth factor by mouse astrocytes in culture. Biochem Biophys Res Commun 1986, 136:57-63 [DOI] [PubMed] [Google Scholar]
- 30.Tarris RH, Weichsel ME, Jr, Fisher DA: Synthesis and secretion of nerve growth stimulating factor by neonatal mouse astrocyte cells in vitro. Pediatr Res 1986, 20:367-372 [DOI] [PubMed] [Google Scholar]
- 31.Hill DA, Dehner LP, White FV, Langer JC: Gliomatosis peritonei as a complication of a ventriculoperitoneal shunt: a case report and review of the literature. J Pediatr Surg 2000, 35:497-499 [DOI] [PubMed] [Google Scholar]