Abstract
Plakoglobin and its homologue β-catenin are cytoplasmic proteins that mediate adhesive functions by interacting with cadherin receptors and signaling activities by interacting with transcription factors. It has been suggested that plakoglobin can suppress tumorigenicity whereas β-catenin can act as an oncogene. We investigated the correlation between the expression pattern of N-cadherin, β-catenin, and plakoglobin and tumor behavior in primary tumors of 20 neuroblastoma patients of all stages and in 11 human neuroblastoma cell lines. N-cadherin and β-catenin were detected in 9 of 11 and 11 of 11 cell lines, respectively, whereas plakoglobin was undetectable or severely reduced in 6 of 11 cell lines. Tumor cells from 16 of 20 patients expressed N-cadherin and 20 of 20 patients expressed β-catenin at levels similar to those of normal ganglion cells. Plakoglobin was undetectable in 9 of 20 tumors. Plakoglobin deficiency in the primary tumors was significantly associated with adverse clinical outcome. Five of the patients with plakoglobin-negative tumors died whereas four patients are alive without evident disease. In contrast, all patients with plakoglobin-positive tumors are alive; 2 of 11 are alive with the disease and 9 of 11 are alive without evident disease. These results suggest that down-regulation of plakoglobin may be of prognostic value for neuroblastoma patients aspredictor of poor outcome.
Plakoglobin is a major component of the submembranal plaque of adherens junctions and desmosomes in mammalian cells. 1 Like its close homologue β-catenin, plakoglobin (or γ-catenin) interacts with cadherins to mediate cell-cell adhesion and associates with transcription factors of the LEF/TCF family to regulate the expression of target genes that are involved in cell fate determination and cell proliferation. 2,3 The transmembrane N-cadherin receptor is a Ca+2-dependent adhesion molecule that plays an important role in guiding morphogenetic events in neuronal tissues during embryogenesis. 4,5 In the embryo, disassociation and migration of the cells to the periphery follows reduction in N-cadherin levels, whereas a subsequent re-expression of N-cadherin is required for aggregation of neuroblasts into ganglia. 4,6 The α- and β-catenins and plakoglobin define the cytoplasmic aspect of the cadherin adhesive domain by anchoring cadherin to cytoskeletal elements at the submembrane plaque. The resulting junctional system controls adhesion, motility, growth, and differentiation. 7-9 Both plakoglobin and β-catenin are posttranscriptionally up-regulated in response to Wnt-1 in cultured cells. 10 However, although elevated β-catenin expression has been implicated in hyperproliferation and tumor formation, 11-13 overexpression of plakoglobin was shown to suppress cell proliferation and cell tumorigenicity in experimental animals. 14 Consistent with the ability of plakoglobin to act as a tumor suppressor are the findings that reduced plakoglobin expression was observed in tumor tissues and metastatic lesions of renal cells, 15 esophageal carcinomas, 16 and in skin carcinomas. 17 In addition, the plakoglobin gene displays loss of heterozygosity in some sporadic breast and ovarian cancers. 18
Neuroblastoma is one of the most common extracranial solid tumors in children. This neoplasm is comprised mostly of primitive neuroblasts derived from the neural crest cells, migrating toward their destined sympathetic ganglia and the adrenal medulla. The prognosis is highly dependent on the clinical stage of the tumor and the patient’s age at diagnosis; patients younger than 1 year with lesser tumor burden have the best prognosis. Certain genetic abnormalities in neuroblastoma tumor cells correlate with the clinical outcome. Established indicators of the aggressiveness of the tumor and poor outcome include deletion of the short arm of chromosome 1(1p), 19 the amplification of the N-myc gene 20 and near diploidy or tetraploidy. 19 Additional parameters are needed for identification and targeting of high-risk neuroblastoma patients with intensive therapeutic regimens that may allow an improvement in survival rates. Because impaired E-cadherin expression is frequently associated with the progression and metastasis in many types of carcinomas, 21 we have asked whether the expression level of N-cadherin and its associated molecules β-catenin and plakoglobin might serve as indicators of tumorigenicity in neuroblastoma. We analyzed by immunohistochemistry the expression of N-cadherin, β-catenin, and plakoglobin in paraffin sections of tumors derived from 20 neuroblastoma patients of all stages and compared them to normal ganglion cells. In addition, expression of these proteins was examined by Western blot analysis in a series of human neuroblastoma cell lines. The results showed normal levels of N-cadherin and β-catenin in the majority of tumors and cell lines, whereas approximately half of the primary tumors and cell lines exhibited plakoglobin deficiency. Moreover, the loss of plakoglobin expression in the primary tumors was significantly associated with poor clinical outcome. Collectively, these findings are compatible with the possibility that loss of plakoglobin may promote the tumorigenicity of neuroblastoma.
Materials and Methods
Tumors and Cell Lines
Tumor specimens were obtained from the tumor bank maintained by the Department of Pathology at Sheba Medical Center. Paraffin-embedded blocks of tumors from 20 neuroblastoma patients were studied. Three specimens of normal colon mucosa with ganglion cells and two ganglioneuroma specimens were used as positive controls expressing N-cadherin, β-catenin, and plakoglobin. Eleven human neuroblastoma cell lines were examined: SKNMC and SKNSH (HTB 10 and HTB 11; American Tissue Culture Collection, Rockville, MD), NBL-S 22 and NBL-WN 23 were kindly provided by SL Cohn, Northwestern University, Chicago, IL. The human neuroblastoma cell lines Kelly, LAN5, NUB7, NHB, and CHP126 were obtained from Y. Shilo, Tel-Aviv University, (Tel Aviv, Israel) and MB 24 was provided by Y. Wollman, Tel-Aviv University. The MBmm cell line was derived from the line MB after two cycles of subcutaneous growth in nude mice. The neuroblastoma cell lines were cultured in RPMI-1640 supplemented with 10% fetal calf serum (Life Technologies, Inc.), l-glutamine, penicillin, and streptomycin, at 37°C and with 5% CO2.
Immunohistochemical Analysis
Formalin-fixed paraffin-embedded sections were deparaffinized and rehydrated. For plakoglobin and N-cadherin staining the slides were pretreated twice for 5 minutes in a 750 W microwave oven, in 10 mmol/L of citrate buffer (pH 6.0). 25 All slides were rinsed with Tris buffer containing 0.05 mol/L Tris/HCL, pH 7.6, 0.1 mol/L NaCl, 0.1% bovine serum albumin, and 0.05% Tween 20. To reduce background signals, the slides were incubated at 20°C for 15 minutes with 10% nonimmune goat serum, followed by 30 minutes of CAS block (Zymed). The slides were then incubated for 16 hours at 4°C with monoclonal mouse anti-human plakoglobin antibody clone 11E4 or clone 15F11, 26 or with mouse anti-human N-cadherin antibody clone 13A9 27 (all kindly donated by MJ Wheelock, Toledo, OH). Incubation with rabbit anti-human β-catenin (Sigma) was for 45 minutes at 20°C. Staining was performed with labeled avidin-biotin. No staining was obtained when nonimmune serum was used instead of the primary antibodies, thus confirming the specificity of each primary antibody. We used a scoring system to evaluate semiquantitatively the extent and intensity of immunoexpression. Scoring was classified into the following four groups: 0, no immunoreaction; 0.5, mild immunoreaction; 1, marked immunoreaction equivalent to that of normal ganglion cells; and 2, high immunoreaction.
Immunoblotting
Cell extracts from equal number of cells from the different neuroblastoma cell lines were separated on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis minigels and transferred to nitrocellulose membranes. After blocking with bovine serum albumin, the blots were incubated with anti-human plakoglobin (11E4), anti-human β-catenin (5H10), and anti-human N-cadherin (13A9). The antigens were visualized by peroxidase-conjugated secondary antibody, and detection was done with Chemiluminescence blotting substrate kit (Boehringer Mannheim).
Results
Plakoglobin Deficiency Correlates with Poor Clinical Outcome in Neuroblastoma Patients
To determine whether alterations in the N-cadherin adhesive system may correlate with tumorigenicity of neuroblastoma, we compared the expression of N-cadherin, β-catenin, and plakoglobin in tumor specimens from 20 neuroblastoma patients presenting various stages of the disease (Table 1) ▶ . The patients were classified as having favorable or unfavorable histology based on the criteria used by the International Neuroblastoma Pathology Classification according to the modified Shimada system. 28 Unfavorable histology criteria included: patient older than 1.5 year at diagnosis, low tumor differentiation or lack of differentiation, high mitotic-karyorrhexis index, and poor schwannian stroma. According to the criteria of the International Neuroblastoma Staging System, three of the tumors were classified as stage I, two as stage II, three as stage III, nine as stage IV, and three as stage IVS. The median age of the patients at the time of diagnosis was 2 years. The median duration of follow-up for survivors was 55 months (range, 19 to 134 months). Of the 20 tumors, 10 were from infants younger than 1 year. Immunoreactivity of the indicated proteins in the tumors was analyzed by a semiquantitative procedure. Immunoreaction of N-cadherin, β-catenin, and plakoglobin in normal ganglion cells was scored as 1. In two benign ganglioneuroma specimens the three proteins were expressed at levels higher than those observed in normal ganglion cells (not shown). Figure 1 ▶ illustrates immunostaining for plakoglobin (left column), β-catenin (middle column), and N-cadherin (right column) of tumors from the patients designated in Table 1 ▶ as patients 4, 5, 10, and 11. The tumor cells of all four patients expressed N-cadherin and β-catenin whereas plakoglobin was expressed by the tumor cells of patients 10 and 11, but not by the tumors of patients 4 and 5. Patients 4 and 5 died of the disease, whereas patient 10 was alive and well without evident disease for 24 months and patient 11 was alive with the disease for 30 months.
Table 1.
Clinical and Pathological Data for Each Patient and Tumor Staining for N-Cadherin, β-Catenin, and Plakoglobin
| Patient no. | Stage | N-myc* | Histology† | Age‡ | Event-free survival§ | Survival¶ | PG | Expression β-cat | N-cad∥ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IV | Nd | FH | 3y | NR | DOD 13m | 0 | 1 | 0 |
| 2 | IV | Amp | UnF | 15m | NR | DOD 23m | 0 | 1 | 1 |
| 3 | IV | NA | FH | 3y | NR | DOD 30m | 0 | 1 | 0 |
| 4** | IV | Amp | UnF | 3y | NR | DOD 14m | 0 | 0.5 | 1 |
| 5** | IV-s | Amp | UnF | 4m | 24m | DOD 28m | 0 | 0.5 | 1 |
| 6 | IV-s | Nd | FH | 4m | 64m | NED 64m | 0 | 1 | 1 |
| 7 | III | Amp | UnF | 5y | 20m | NED 36m | 0 | 1 | 1 |
| 8 | III | Amp | UnF | 18m | 23m | NED 23m | 0 | 1 | 1 |
| 9 | II | NA | FH | 5m | 35m | NED 35m | 0 | 1 | 1 |
| 10** | IV | Amp | FH | 10m | 24m | NED 24m | 1 | 1 | 1 |
| 11** | IV | NA | FH | 5w | NR | AWD 30m | 2 | 2 | 2 |
| 12 | IV | NA | FH | 5y | 36m | AWD 41m | 1 | 1 | 0 |
| 13 | IV | NA | FH | 3y | 19m | NED 19m | 1 | 0.5 | 1 |
| 14 | IV-s | NA | FH | 4m | 65m | NED 65m | 1 | 1 | 1 |
| 15 | IV | NA | FH | 10y | 123m | NED 134m | 1 | 1 | 1 |
| 16 | III | NA | FH | 1w | 70m | NED 70m | 1 | 1 | 1 |
| 17 | II | Nd | FH | 20m | 77m | NED 77m | 1 | 0.5 | 1 |
| 18 | I | Nd | FH | 3m | 108m | NED 108m | 1 | 1 | 1 |
| 19 | I | NA | FH | 3m | 61m | NED 61m | 0.5 | 1 | 0.5 |
| 20 | I | NA | FH | At birth | 42m | NED 42m | 0.5 | 1 | 0 |
*N-myc amplification was determined in most tumors by interphase fluorescent in situ hybridization except in the tumors of patients number 9 and 11, in which it was determined by Southern blot analysis. ND, not done; Amp, N-myc amplified; NA, no N-myc amplification.
†Histology according to Shimada et al 28 ; FH, favorable histology; UnF, unfavorable histology.
‡Age at diagnosis; Y, years; M, months; W, weeks.
§Event-free survival; NR, no remission.
¶Survival following diagnosis; DOD, dead of the disease; NED, no evident disease; AWD, alive with the disease.
∥Expression of plakoglobin (PG), β-catenin (β-cat), and N-cadherin (N-cad) was determined by immunohistochemistry and scored as described in the Methods section.
**Shown in Figure 1 ▶ .
Figure 1.
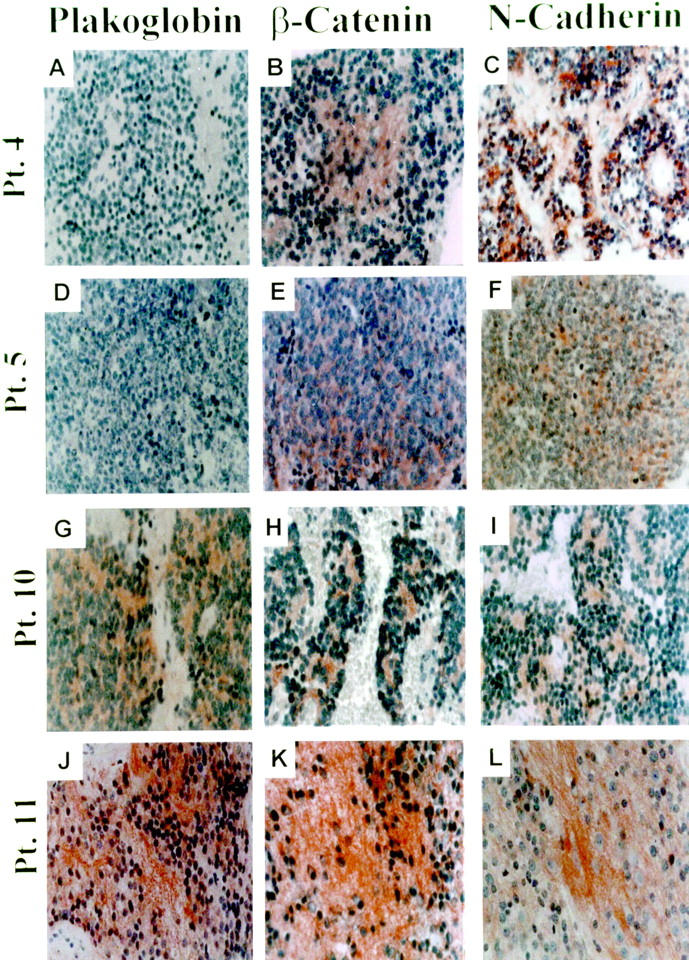
Examples of plakoglobin deficiency in tumors of neuroblastoma patients with bad outcome. Expression of plakoglobin (left), β-catenin (middle), and N-cadherin (right). Shown are stained sections of tumors from the patients (Pt.) designated in Table 1 ▶ as: number 4 (A, B, and C), number 5 (D, E, and F), number 10 (G, H, and I), and number 11 (J, K, and L). For histochemistry, monoclonal anti-human plakoglobin clone 11E4 (similar results were obtained with clone 15F11), rabbit anti-human β-catenin, and monoclonal anti-human N-cadherin clone 13A9 were used. N-cadherin and β-catenin were expressed in each of the four tumors. Plakoglobin was undetectable in the tumors of patients 4 and 5 who died of the disease 14 and 28 months after diagnosis. Plakoglobin was expressed in the tumor of patient 10 who is alive and well with no evident disease for 24 months and in the tumor of patient 11 who is alive with the disease for 30 months. Original magnification, ×400.
The results presented in Table 1 ▶ show that the majority of the neuroblastoma tumors tested expressed N-cadherin at levels comparable to those expressed by normal ganglion cells, whereas in four tumors N-cadherin was not detected. β-catenin was expressed in the tumor cells of all patients at medium to high levels (scored as 0.5 to 2, respectively). Plakoglobin, however, was undetectable in tumor cells of 9 of 20 patients. Among the nine plakoglobin-negative patients, four were diagnosed as stage IV, two as stage III, one as stage II, and two as stage IVS. Five of these plakoglobin-negative patients died of the disease and four were alive without evident disease (Table 1) ▶ . In four patients that were diagnosed as stage IV and died of the disease, remission was not achieved. Tumors from two stage IV patients that died of the disease were negative for both plakoglobin and N-cadherin. Tumor cells derived from 11 of 20 patients expressed medium to high levels of plakoglobin. In well-differentiated tumors, staining for plakoglobin was seen throughout the lesion. In tumors with mixed histology, undifferentiated and poorly differentiated areas usually showed no staining and weak staining, respectively, whereas the better-differentiated areas generally showed high staining. Nine of the patients with plakoglobin-positive tumors were alive without evident disease, the other two patients were alive with the disease 30 and 41 months after diagnosis. The plakoglobin-positive patients included five that were diagnosed as stage IV, among them two that were alive with the disease and three with no evident disease for 19, 24, and 134 months after diagnosis. These observations suggest that stage IV patients whose tumor cells express plakoglobin may have a better outcome than the plakoglobin-negative patients.
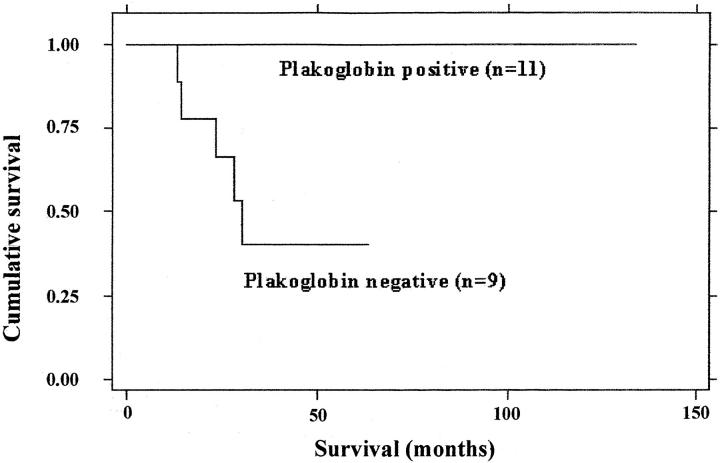
Figure 2 ▶ displays the Kaplan-Meier survival curve according to plakoglobin expression in the tumors. It shows that among the examined 20 patients those with plakoglobin-positive tumors survived for longer periods. Survival analysis of the results by the Mantel-Haenszel log-rank procedure (in which P < 0.05 indicates significant association), showed that loss of plakoglobin expression was significantly associated with poor survival (P < 0.005). The relationship between plakoglobin expression and established bad prognostic factors of the disease was examined by the Fisher exact test (Table 2) ▶ . In this test P < 0.05 indicates significant association between the investigated groups, namely plakoglobin loss and each of the clinical or genetic characteristics examined. The deficiency in plakoglobin was significantly associated with unfavorable histology (P < 0.01) and with N-myc amplification (P < 0.04). The loss of plakoglobin expression was however not significantly associated with stage IV disease (stage IV in comparison with all other stages combined), nor with an age of more than 1 year at presentation.
Figure 2.
Kaplan-Meier survival curve of patients according to plakoglobin expression in the tumors.
Table 2.
Clinical and Genetic Characteristics of 20 Patients with Neuroblastoma According to Plakoglobin Expression
| Characteristic | Plakoglobin expression, Number of patients (%) | P value* | |
|---|---|---|---|
| Negative (n = 9) | Positive (n = 11)† | ||
| Tumor stage | |||
| I, II, and IV-s | 3 (33) | 5 (45) | |
| III | 2 (22) | 1 (9) | |
| IV | 4 (44) | 5 (45) | NS‡ |
| Age at diagnosis | |||
| <1 year | 3 (33) | 7 (64) | |
| >1 year | 6 (66) | 4 (36) | NS |
| Histopathology | |||
| Favorable | 4 (44) | 11 (100) | |
| Unfavorable | 5 (56) | 0 (0) | <0.01 |
| N-myc gene | |||
| Single copy | 2 (29) | 8 (89)§ | |
| Amplified | 5 (71) | 1 (11) | <0.04 |
| Not evaluated | 2 | 2 | |
*The Fisher exact test with two tails was used. P <0.05 indicates significant association between plakoglobin deficiency and the indicated clinical or genetic parameter.
†Number of patients with plakoglobin-negative or plakoglobin-positive tumors.
‡NS, not significant (stage IV in comparison with all other stages combined).
§Percentage of tumors with N-myc amplification includes all patients who could be evaluated.
Expression of N-Cadherin, β-Catenin, and Plakoglobin in Neuroblastoma Cell Lines
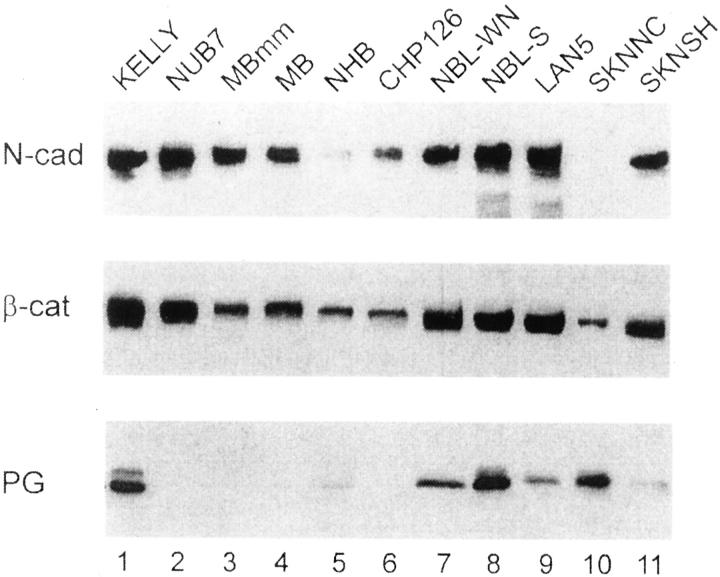
To further assess the expression of the cadherin-catenin system in neuroblastoma tumors, the levels of N-cadherin, β-catenin, and plakoglobin were determined by Western blot analysis in 11 cell lines that originated from human neuroblastoma tumors. Considerable levels of N-cadherin were detected in 9 of 11 of the neuroblastoma cell lines and all of the tested cell lines expressed β-catenin. In contrast, plakoglobin was undetectable in 4 of 11 cell lines, and was very low in 2 of 11 cell lines (Figure 3) ▶ . Taken together, our analyses showed that the majority of human neuroblastoma tumors and cell lines expressed N-cadherin, all expressed β-catenin, but plakoglobin was not expressed in a significant number of neuroblastomas.
Figure 3.
Western blot analysis of N-cadherin, β-catenin, and plakoglobin expression in 11 human neuroblastoma cell lines. The cell extracts from the same number of cells were separated by acrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and probed with the monoclonal antibodies (diluted 1:100), as indicated in the Materials and Method section. The names of the neuroblastoma cell lines are indicated on the top of the figure and the positions of N-cadherin (N-cad), β-catenin (β-cat), and plakoglobin (PG), are shown on the left.
Discussion
Previous work has indicated that the loss of E-cadherin that occurs as tumors progress to less-differentiated forms is an important determinant of their enhanced invasiveness in a variety of carcinomas. 21 N-cadherin is the predominant cadherin in neural cells and seems to play a major role in maintaining the multicellular structure of neural tissues. We therefore tested whether changes in the expression of N-cadherin and its cytoplasmic partner molecules β-catenin and plakoglobin are associated with progression of the childhood malignancy neuroblastoma. We found that N-cadherin and β-catenin are expressed in the great majority of neuroblastoma tumors and neuroblastoma cell lines at levels comparable to those expressed in normal ganglion cells. This observation is in agreement with a recent report demonstrating that N-cadherin was expressed in 11 specimens of neuroblastoma tumors. 29 It is also compatible with the results of Shinoura and colleagues 30 who reported that N-cadherin and α-catenin are highly expressed in invasive glioblastoma tumors. Thus, in contrast to the role played by E-cadherin in carcinomas, the expression of N-cadherin apparently does not restrict the invasion of neural malignancies including neuroblastoma. Moreover, the switch from E-cadherin to N-cadherin was recently shown to correlate with enhanced invasiveness. 31,32 We found that in contrast to N-cadherin and β-catenin, plakoglobin was undetectable in 9 of 20 primary neuroblastoma tumors, and undetectable, or dramatically reduced, in 6 of 11 neuroblastoma cell lines. Furthermore, the tumors of the five patients, who died of the disease, were all plakoglobin-negative. Thus, the plakoglobin deficiency in the primary tumor cells was significantly associated with poor survival (P < 0.005). To our best knowledge, this is the first report demonstrating a correlation between reduced expression of plakoglobin and poor survival in neuroblastoma. We have also asked whether the loss of plakoglobin expression in the primary neuroblastoma tumors is associated with other, established bad prognostic factors of the disease. We found that unfavorable histology of the tumor and N-myc amplification, but not stage IV disease, or the age of the patient (>1 year old), were associated with the plakoglobin deficiency. Moreover, our results suggest that among stage IV patients and patients that were older than 1 year of age at diagnosis, the patients with tumors expressing plakoglobin had a better outcome compared to those that were plakoglobin-negative.
By what mechanism could plakoglobin exert its suppressive effect on invasiveness of neuroblastomas? Our finding that plakoglobin deficiency generally occurred in tumors with unfavorable histology containing poorly differentiated cells that lack cell adhesion, is consistent with contribution of the adhesion function of plakoglobin to its tumor suppressor activity. Neuroblastomas do not express desmosomes and their plakoglobin is mostly associated with adherens junctions. Consequently, one possibility is that the decrease in expression of the adherens junctions may contribute to more invasive phenotype. In oral and pharyngeal squamous cell carcinomas, down-regulation of plakoglobin and desmoplakin is considered a reliable marker of extensive tumor growth and metastasis formation. 33 Similarly, in human skin carcinomas the expression of plakoglobin and desmoglein-1 is reduced or absent, suggesting that reduction of these molecules may contribute to invasiveness. 17 Other observations suggest that plakoglobin can suppress the tumorigenicity of carcinoma cells independently of the cadherin-catenin complex. Simcha and colleagues 14 reported that transfection of plakoglobin into a human renal carcinoma cell line that does not express cadherins, plakoglobin, α-catenin, and β-catenin resulted in suppression of tumor formation in nude mice. In these cells, plakoglobin did not exhibit junctional localization, but was diffusely distributed in the cytoplasm and the nucleus. These results suggest that the anti-tumorigenic activity of plakoglobin in these carcinoma cells could be associated with its signaling activity, rather than with its function in cell adhesion. Indeed, recent studies have indicated that plakoglobin and β-catenin differ in their signaling activities. 9,34,35 Our finding that invasive neuroblastoma tumors are deficient in plakoglobin, but express normal levels of N-cadherin and β-catenin is compatible with this interpretation. The cytochemical analyses could not detect plakoglobin or β-catenin staining in the nuclei of neuroblastoma. Because relatively small amounts of nuclear plakoglobin or β-catenin are sufficient for signaling, further studies will be required to directly link plakoglobin or β-catenin signaling to neuroblastoma invasiveness. In view of our finding that plakoglobin deficiency correlates with adverse outcome in neuroblastoma, it is interesting to note that the human plakoglobin gene was localized to chromosome 17q21, 18 and the gain of segment 17q21-qter is the most frequent cytogenetic abnormality in neuroblastoma cells. 36,37 Furthermore, this chromosomal gain is associated with advanced disease, patients that are older than 1 year old, deletion of chromosome arm 1p and amplification of the N-myc oncogene, all of which predict adverse outcome in neuroblastoma. 37
In conclusion, our results suggest that plakoglobin may have a tumor suppressor function in neuroblastoma patients. Future examination of plakoglobin expression in tumors of a higher number of patients and longer follow-up periods will show whether the loss of plakoglobin may be used as a marker for poor prognosis in neuroblastoma.
Acknowledgments
We thank R. Chen for statistical analysis; Z. Mark and N. Amariglio for Southern blot analysis; Y. Shilo, S. L. Cohn, and Y. Wollman for neuroblastoma cell lines; and M. J. Wheelock for antibodies.
Footnotes
Address reprint requests to Yael Kaufmann, Ph.D, Hematology Institute, The Chaim Sheba Medical Center, Tel-Hashomer, 52621, Israel. E-mail: kaufmann@post.tau.ac.il.
Supported by the Israel Cancer Research Fund fellowship (to R. A.).
Current address of B.D. is Department of Pathology, the Norwegian Radium Hospital, Oslo, Norway.
References
- 1.Cowin P, Karpell HP, Franke WW, Tamkun J, Hynes RO: Plakoglobin: a protein common to different kinds of intracellular adhering junction. Cell 1986, 46:1063-1073 [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ze’ev A, Geiger B: Differential molecular interactions of β-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol 1998, 10:629-639 [DOI] [PubMed] [Google Scholar]
- 3.Bullions LC, Levine AJ: The role of β-catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol 1998, 10:81-87 [DOI] [PubMed] [Google Scholar]
- 4.Hata K, Takeichi M: Expression of N-cadherin molecules associated with early morphogenetic events in chick development. Nature 1986, 320:447-449 [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga M, Hata K, Nagafuchi A, Takeichi M: Guidance of optic nerve fibers by N-cadherin adhesion molecules. Nature 1988, 334:62-64 [DOI] [PubMed] [Google Scholar]
- 6.Akitaya T, Bronner-Fraser M: Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn 1992, 194:12-20 [DOI] [PubMed] [Google Scholar]
- 7.Kemler R: From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 1993, 9:317-321 [DOI] [PubMed] [Google Scholar]
- 8.Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M: The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 1996, 135:767-779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhurinsky J, Shtutman M, Ben-Ze’ev A: Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J Cell Science 2000, 113:3127-3139 [DOI] [PubMed] [Google Scholar]
- 10.Papkoff J, Rubinfeld B, Schryver B, Polakis P: Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol 1996, 16:2128-2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korinek V, Barker N, Morin P, van Wichen D, de Weger R, Kinzler K, Vogelstein B, Clevers H: Constitutive transcriptional activation by a β-catenin-TcF complex in APC−/− colon carcinoma. Science 1997, 275:1784-1787 [DOI] [PubMed] [Google Scholar]
- 12.Morin P, Sparks A, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K: Activation of β-catenin-TcF signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 13.Peifer M: β-catenin as an oncogene: the smoking gun. Science 1997, 275:1752-1753 [DOI] [PubMed] [Google Scholar]
- 14.Simcha I, Geiger B, Yehuda-Levenberg S, Salomon D, Ben-Ze’ev A: Suppression of tumorigenicity by plakoglobin: augmenting effect of N-cadherin. J Cell Biol 1996, 133:199-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchner A, Oberneder R, Riesenberg R, Keiditsch E, Hofstetter A: Expression of plakoglobin in renal cell carcinoma. Anticancer Res 1998, 18:4231-4235 [PubMed] [Google Scholar]
- 16.Nakanisho Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S: Expression of E-cadherin, α-catenin, β-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology 1997, 54:158-165 [DOI] [PubMed] [Google Scholar]
- 17.Tada H, Hatoko M, Tanaka A, Kuwahara M, Muramatsu T: Expression of desmoglein I and plakoglobin in skin carcinomas. J Cutan Pathol 2000, 27:24-29 [DOI] [PubMed] [Google Scholar]
- 18.Aberle H, Bierkamp C, Torchard D, Serova O, Wagner T, Natt E, Wirsching J, Heidkamper C, Montagna M, Lynch T, Lenior GM, Scherer G, Fuenteun J, Kemler R: The human plakoglobin gene localizes on chromosome 17q21 and is subjected to loss of heterozygosity in breast and ovarian cancers. Proc Natl Acad Sci USA 1995, 92:6384-6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambros IM, Zellner A, Roald B, Amann G, Ladenstein R, Printz D, Gadner H, Ambros PF: Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med 1996, 334:1505-1511 [DOI] [PubMed] [Google Scholar]
- 20.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D: Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985, 313:1111-1116 [DOI] [PubMed] [Google Scholar]
- 21.Birchmeier W, Behrens J: Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 22.Cohn SL, Salwen H, Quasney MW, Ikegaki N, Cowan JM, Herst CV, Kennet RH, Rosen ST, DiGiuseppe JA, Brodeur GM: Prolonged N-myc protein half-life in a neuroblastoma cell line lacking N-myc amplification. Oncogene 1990, 5:1821-1827 [PubMed] [Google Scholar]
- 23.Foley J, Cohn SL, Salwen HR, Chagnovich D, Cowan J, Mason KL, Parysek LM: Differential expression of N-myc in phenotypically distinct subclones of a human neuroblastoma cell line. Cancer Res 1991, 52:6338-6345 [PubMed] [Google Scholar]
- 24.Wollman Y, Shahar I, Goldshtein M, Leibovici J: Malignant phenotype correlating with drug resistance in two human neuroblastoma cell lines. J Neurooncol 1994, 19:123-129 [DOI] [PubMed] [Google Scholar]
- 25.Goldberg I, Davidson B, Lerner-Geva L, Gotlieb WH, Ben-Baruch G, Novikov I, Kopolovic J: Expression of extracellular matrix proteins in cervical squamous cell carcinoma—a clinicopathological study. J Clin Pathol 1998, 51:781-785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, Wheelock MJ: P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res 1993, 207:252-260 [DOI] [PubMed] [Google Scholar]
- 27.Knudsen KA, Soler AP, Johnson KR, Wheelock MJ: Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol 1995, 130:67-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP: The international neuroblastoma pathology classification (the Shimada system). Cancer 1999, 86:364-372 [PubMed] [Google Scholar]
- 29.Shimono R, Matsubara S, Takamatsu H, Fukushige T, Ozawa M: The expression of cadherins in human neuroblastoma cell lines and clinical tumors. Anticancer Res 2000, 20:917-923 [PubMed] [Google Scholar]
- 30.Shinoura N, Paradies NE, Warnick RE, Chen H, Larson JJ, Simon M, Lynch RA, Kanai Y, Hirohashi S, Hemperly JJ, Menon AG, Brackenbury R: Expression of N-cadherin and α-catenin in astrocytomas and glioblastomas. Br J Cancer 1995, 72:627-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ: N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 1999, 147:631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazan RB, Phillps GR, Qiao RF, Norton L, Aaronson SA: Exogenous expression of N-cadherin in breast cancer cells, induces cell migration, invasion and metastasis. J Cell Biol 2000, 148:779-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depondt J, Shabana AH, Florescue ZS, Gehanno P, Forest N: Down-regulation of desmosomal molecules in oral and pharyngeal squamous cell carcinomas as a marker for tumor growth and distant metastasis. Eur J Oral Sci 1999, 107:183-193 [DOI] [PubMed] [Google Scholar]
- 34.Williams BO, Banish GD, Klymkowsky MW, Varmus HE: A comparative evaluation of beta-catenin and plakoglobin signaling activity. Oncogene 2000, 19:5720-5728 [DOI] [PubMed] [Google Scholar]
- 35.Zhurinsky J, Shtutman M, Ben-Ze’ev A: Differential mechanisms of LEF/TCF-dependent transcriptional activation of β-catenin and plakoglobin. Mol Cell Biol 2000, 20:4238-4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plantaz D, Mohapatra G, Matthay KK, Pellarin M, Seeger RC, Feuerstein BG: Gain of chromosome 17 is the most frequent abnormality detected in neuroblastoma by comparative genomic hybridization. Am J Pathol 1997, 150:81-89 [PMC free article] [PubMed] [Google Scholar]
- 37.Bown N, Cotterill S, Lastowska M, O’Neill S, Pearson ADJ, Plantaz D, Meddeb M, Danglot G, Brinkschmidt C, Christiansen H, Laureys G, Speleman F: Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med 1999, 340:1954-1961 [DOI] [PubMed] [Google Scholar]