Abstract
We have isolated a monoclonal antibody, clone βE11, which recognizes an antigen that is highly abundant on the surface of mitotic vascular endothelial cells and tumor cells. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting, expression of this 190-kd antigen is approximately threefold higher in mitotic versus interphase endothelial cells. Treatment of tumor cells with an antibody to the βE11 antigen inhibits their growth in a dose-dependent manner in vitro with maximal inhibition at an antibody concentration of 1 μg/ml. Different tumor cell lines demonstrate varying sensitivities to anti-βE11 with the following order of growth inhibition: colon > prostate = glioma > melanoma = fibroblast > breast > liver. Furthermore, the βE11 antigen localizes to regions of prostatic intraductal neoplasia in paraffin-embedded sections. Mass spectrometry of the cell-derived βE11 protein and V8-protease fingerprint analysis indicate that the βE11 antigen is nearly identical to the 4F2 heavy chain antigen, a cell surface protein that has been implicated in cell activation and proliferation. Expression of the βE11 antigen during mitosis functionally links it to a fundamental aspect of cell proliferation, and its spatial localization on the surface of both proliferating endothelium and tumor cells demonstrates its potential for tumor immunotherapy.
Cell growth is mediated by the concerted action of numerous positive and negative factors. The decision to progress through the cell cycle is driven by cyclin/cyclin-dependent kinase complexes in the nucleus that phosphorylate key regulators such as the retinoblastoma gene product to enable transcription of growth-promoting genes. 1 Cyclin-dependent kinase inhibitors such as p21/WAF1/Cip1 2 negatively regulate cyclin/cyclin-dependent kinase activity. In the cytoplasm, signal transduction via numerous pathways including those activated by MAP 3 and PI-3 4 kinases relays both stimulatory and inhibitory cues from the plasma membrane to nuclear effectors. Growth control signals originate at the plasma membrane with cytokine receptors, adhesion molecules, and integrins that receive extracellular stimuli and transmit regulatory signals to cytoplasmic signaling components. 5-7 Exquisite control over all these regulatory molecules ensures maintenance of normal cell growth.
When control over cell growth is no longer maintained as in cancer, persistent positively acting signals are produced, and unbridled proliferation ensues. This unchecked growth is often the result of overexpression of key growth-promoting molecules, including the products of oncogenes such as Ras 8 and Mdm2, 9 and the mutation of growth-inhibiting factors such as the tumor suppressors p53 10 and APC. 11 Alteration in cell surface components, in addition, often correlates with a tumorigenic cell phenotype. For example, overexpression of the p185 neu/c-erbB-2 receptor has been reported in various human cancers, 12 and induction of a deletion mutant of the epidermal growth factor (EGF) receptor in mouse fibroblasts results in an EGF-independent transformed phenotype. 13 Because many cell surface alterations are results of tumor progression, 14 they have been characterized as tumor-specific.
Tumor-specific cell surface antigens have been described in many different tissues. 14-17 For example, carcinomas of the lung, breast, colon, and ovary show abundant L6 surface antigen whereas normal cells demonstrate only limited expression. 18,19 Mucinous carcinomas of the colon, stomach, and ovary, but not normal tissues, highly express the carbohydrate antigens recognized by tumor-specific monoclonal antibodies B1 and B3. 20 Human breast tumor is the source of the BTAA glycoprotein to which circulating antibodies were discovered in breast cancer patients but not in normal women or patients with other carcinomas. 21 In prostate tissue, several tumor-specific antigens have been identified. 22-24 For example, both ductal epithelia and secretions of prostate adenocarcinoma are highly enriched in the mucin-like antigen recognized by monoclonal antibody PD41 whereas fetal or benign prostate specimens are devoid of this antigen. 22 In addition, androgen-independent rat prostate tumor cell lines and human prostate carcinoma, but not normal rat or human tissues or benign prostatic hyperplasia, express cell surface and cytoplasmic antigens recognized by monoclonal antibody MCA-R1. 23 Therefore, in a variety of cancers there seems to be expression of cell surface antigens that correlate with a tumorigenic phenotype.
Targeting of tumor-specific cell-surface proteins with antibodies or with immunotoxins 25 to eradicate tumors has demonstrated some success. For example, an immunotoxin to mesothelin, a differentiation antigen on the surface of mesotheliomas as well as ovarian and other human cancers, 26 demonstrates high cytotoxicity to mesothelin-expressing cells, and causes regression of mesothelin-expressing subcutaneous tumors in immunodeficient mice. 27 An immunotoxin against the interleukin (IL)-2 receptor, which is expressed on the surface of many leukemias and lymphomas but not on normal resting T cells, 28 causes complete regression of IL-2 receptor-bearing subcutaneous tumor xenographs. 29 Furthermore, an immunotoxin comprised of IL-4 fused to a fragment of Pseudomonas exotoxin substantially reduces or completely eliminates established subcutaneous acquired immune deficiency syndrome Kaposi’s sarcoma tumors in immunodeficient mice in a dose-dependent manner. 30 Limited success has been attained in phase I clinical trials of immunotoxins: the RFB4 immunotoxin, which targets CD22, mediated partial remission of tumors in 40% of treated B-cell lymphoma patients, 31 and the LMB-1 immunotoxin that utilizes the B3 antibody described above significantly reduced epithelial tumors in 5 of 38 patients who failed conventional therapy. 32 The importance and clinical efficacy of targeting these tumor-associated antigens has thus been demonstrated. Enrichment of tumors versus normal tissues with these antigens defines cancerous cell targets, and expression of these antigens on the cell surface makes them highly accessible to tumor-specific antibodies and immunotoxins.
Although considerable progress has been made in identifying proteins enriched in tumor populations that can be used as therapeutic targets for cancer treatment, only limited success has been achieved in eradicating tumors in the clinic. 30-32 At best, only partial remission or significant reduction of human tumors has been achieved with therapeutic agents such as immunotoxins in only a subset of treated patients. The limited efficacy of these tumor immunotoxins in treating human cancer demonstrates the need to find an alternative, more effective strategy for targeting and destroying cancerous cells.
Another approach to finding a tumor-specific antigen is to relate its presence on a cancer cell in a functional sense to a fundamental aspect of a tumor. A protein that is highly expressed during mitosis, for example, is functionally associated with a tumor cell because of its capacity for uncontrolled cell division. The importance of mitosis-specific protein regulation is underscored by the HER2-neu growth factor receptor. Similar to the EGF receptor, 33 HER2-neu shows markedly decreased tyrosine kinase activity in mitosis coincident with hyperphosphorylation on serine and threonine residues. 34 Moreover, a point mutation in HER2-neu that renders it unresponsive to this mitosis-specific regulation generates a protein with potent transforming ability. Mitosis-specific protein modification thus has important consequences for normal cell growth.
We have been interested in understanding how cell surface proteins on vascular endothelial cells generate signals that regulate normal growth and the manner in which perturbation of these signals results in vascular pathologies such as tumor angiogenesis and diabetic retinopathy. We have isolated a monoclonal antibody, clone βE11, that recognizes an antigen that is highly abundant on the surface of mitotic endothelial cells as well as many different tumor cells. Furthermore, we have demonstrated that the βE11 antibody inhibits the growth of tumor cells and, to a limited extent, endothelial cells, in a dose-dependent manner in vitro. We show here that the βE11 antigen is nearly identical to the 4F2 antigen, a glycoprotein originally characterized to be expressed on the surface of activated, but not resting, lymphocytes and shown to be associated with cell proliferation. 35-37 These results confirm the idea that targeting an antigen associated with mitosis and proliferation can inhibit tumor cell growth in vitro and raise the possibility that anti-βE11 can target and inhibit tumor progression via its dual effect on a tumor and its associated growing vasculature in vivo.
Materials and Methods
Cell Culture
Bovine retinal cell cultures were established as previously described. 38 Bovine retinal endothelial cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% bovine calf serum, 2 mmol/L l-glutamine, and pen/strep/fungizone. Bovine retinal pericytes were grown in DMEM supplemented with 10% bovine calf serum, 2 mmol/L l-glutamine, and pen/strep/fungizone. Tumor cell lines were provided by Dr. Patricia D’Amore (Harvard Medical School, Boston, MA). Human LNCaP, human HEPG2, human MCF7, rat C6 glioma, NBE rat prostate, murine B16 BL6 melanoma, murine Lewis Lung carcinoma, murine embryonic and brain endotheliomas, and murine 3T3 fibroblast cell lines were grown in DMEM supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, and pen/strep/fungizone. Human urinary bladder and astrocytoma cell lines were grown in RPMI supplemented with 10% fetal bovine serum, 2 mmol/L l-glutamine, and pen/strep/fungizone. Murine B16 BL6 melanoma and C6 rat glioma cell lines were adapted to grow in Gibco Hybridoma serum-free medium (Life Technologies, Inc., Grand Island, NY), and murine B16 BL6 cells were also adapted to grow in DMEM supplemented with HL-1 completely defined serum substitute (Biowhittaker, Walkersville, MD) for metabolic labeling.
Antibodies
Murine βE11 antibody was obtained from a hybridoma created by the fusion between SP20 plasmacytomas and spleen cells from mice immunized with a cell lysate that contained membrane as well as cytosolic proteins derived from growing pericyte cultures. 39 The βE11 hybridoma cell line was initially grown in 10% fetal bovine serum/DMEM but was weaned out of serum and propagated in Gibco serum-free medium. βE11 antibody was obtained from hybridoma-conditioned medium by precipitation with ammonium sulfate. 40 Murine 4F2 antibody was obtained from the conditioned medium of hybridoma clone 4F2C13 purchased from American Type Culture Collection (Manassas, VA) and maintained according to the manufacturer’s instructions. Affinity isolated βE11 and 4F2 antibodies were obtained as described below. Purified mouse IgG1 (kappa light chain) was obtained from Zymed (South San Francisco, CA) or from Sigma (St. Louis, MO). Goat anti-mouse-tetramethylrhodamine B isothiocyanate (TRITC) was purchased from Zymed or Jackson Immunoresearch (West Grove, PA), and goat anti-mouse IgG-horseradish peroxidase (HRP) was purchased from Chemicon (Temecula, CA). Goat anti-mouse IgG(Fc) was purchased from Jackson Immunoresearch.
Affinity Isolation of Antibody from Hybridoma Supernatant
Fifty ml of 5 to 9 day βE11-conditioned medium was incubated with 1.5 mg of goat anti-mouse IgG(Fc) conjugated to 7.5 ml Sepharose 4B (Sigma) overnight at 4°C with constant rotation. The next day the antibody-Sepharose complexes were poured into a 2.6-cm diameter column and were washed with 150 ml phosphate-buffered saline (PBS)-azide. Antibody was eluted with 200 mmol/L glycine, pH 2.8, that was collected directly into 1/10 volume of 1.0 mol/L Tris, pH 8.0. Typically 20 ml of glycine was eluted into 2 ml of Tris. The eluate was dialyzed against two exchanges of PBS-azide overnight and was concentrated ∼100-fold using 10-kd cutoff centrifuge filters (Millipore, Bedford, MA). Antibody concentration was measured by enzyme-linked immunosorbent assay, and antibody was stored at 4°C.
In Vitro Growth Assays
The cell lines mentioned above were trypsinized and plated at ∼2000 to 5000 cells/well in 24-well culture plates (Costar, Cambridge, MA) in DMEM supplemented with 5% fetal bovine serum (day 0). The next day (day 1), the cells were refed, with the same medium supplemented with either βE11 antibody or murine IgG1 control at doses ranging from 60 ng/ml to 1 μg/ml. Every other day the cells were trypsinized and counted ( model ZF; Coulter Electronics, Hialeah, FL), and the remaining cells were refed with 5% fetal bovine serum/DMEM +/− antibody. Growth assays were performed for 5 to 7 days.
Immunofluorescence Microscopy
Staining of cells was performed as previously described. 41 Briefly, cells were plated in 8-chamber culture slides (Becton Dickinson, Franklin Lakes, NJ) or on glass coverslips in the bottom of 24-well culture plates and were allowed to adhere at least 24 hours before fixation and permeabilization. Cells were fixed with 4% paraformaldehyde/DMEM for 5 minutes at room temperature and permeabilized for 90 seconds in the following buffer: 0.1% Triton X-100, 40 mmol/L HEPES, pH 7.15, 50 mmol/L PIPES, pH 6.90, 75 mmol/L KCl, 1 mmol/L MgCl2, 0.1 mmol/L EGTA. For cell surface staining, Triton was omitted from the buffer. For primary antibody staining, cells were incubated in ∼20 to 40 μg/ml of mouse anti-βE11 or murine IgG control for 1 hour at room temperature. For secondary antibody staining, cells were incubated with a 1:200 dilution of goat anti-mouse IgG-TRITC for one. Nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI) or Hoescht (both kindly provided by Dr. David Albertini, Tufts University, Boston, MA) added to the mounting medium to a final concentration of 1 μg/ml.
Immunohistochemistry
Paraffin-embedded sections from radical human prostatectomy specimens were obtained Tufts University Department of Pathology tissue bank and were stained using the Vectastain ABC kit as described by the manufacturer (Vector Laboratories, Burlingame, CA). Briefly, 5- to 10-μm paraffin-embedded tissue sections adhered to uncoated slides were deparaffinized with sequential 2 × 10 minute washes in xylene, 100% ethanol, and 95% ethanol. The slides were treated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidases and were blocked for nonspecific antibody reactivity with 1:200 normal horse serum:PBS. The sections were incubated overnight with ∼200 μl of anti-βE11 or mouse IgG control at 40 μg/ml, then for 30 minutes with ∼200 μl of 1 μg/ml biotinylated horse anti-mouse IgG. The avidin-biotin-peroxidase and alkaline-phosphatase solutions were prepared and added to the sections as described by the manufacturer, and the slides were monitored for color development under bright field of a microscope. The sections were counterstained with 10% Harris hematoxylin for 3 minutes and rinsed with dilute ammonium hydroxide. After the sections were dehydrated in sequential washes of 95% ethanol, 100% ethanol, and xylene substitute, they were mounted with xylene substitute mountant, dried, and photographed under ×33 or ×100 power using tungsten ektachrome film.
Extraction of Vascular Cells and Tumor Cells
Subconfluent cells grown in 150-mm plates were washed three times with PBS (room temperature) before extraction. For anti-4F2 versus anti-βE11 Western blotting comparisons of B16 BL6 melanoma serum-free cells, cells were solubilized in 1× sample buffer [4% sodium dodecyl sulfate (SDS), 10% β-mercaptoethanol, 10% glycerol, 100 mmol/L Tris, pH 7.5, 0.015% bromophenol blue]. For anti-βE11 Western blotting comparison of tumor cells lines grown in serum, the cells were extracted with 2 ml per 150-mm plate of lysis buffer containing the following: 40 mmol/L HEPES, pH 7.15, 50 mmol/L PIPES, pH 6.90, 75 mmol/L NaCl, 1 mmol/L MgCl2, 0.5 mmol/L EGTA, 0.1 mmol/L phenylmethyl sulfonyl fluoride, 5 μmol/L E-64 (l-trans-epoxysuccinyl-leucylamidino [4-guanidino] butane), 0.1 mg/ml soybean trypsin inhibitor, 2.5 mmol/L sodium orthovanadate, 0.1 mmol/L TPCK, 0.1 mmol/L TLCK, 0.1 mmol/L TAME, 0.1 mg/ml pepstatin, 0.1% Triton X-100, and 0.5% octyl glucoside. The detergent-soluble fraction was then clarified and dialyzed against two to three exchanges of the following buffer (dialysis buffer): 2 mmol/L Tris, pH 7.8, 0.2 mmol/L dithiothreitol, 0.2 mmol/L MgCl2, 0.5 mmol/L EGTA, and 0.02% azide. Protein concentration was determined with a Bradford assay kit (BioRad Laboratories, Hercules, CA).
Western Blotting
Samples (cell lysate or immunoprecipitate) were boiled in SDS sample buffer for 2 minutes before loading on 1.5-mm-thick polyacrylamide slab gels containing 0.1% SDS. The samples were separated by electrophoresis and were transferred to nitrocellulose (Schleicher and Schuell, Keene, NH) overnight at 200 mA using a TE series transphor electrophoresis unit (Hoeffer Scientific, San Francisco, CA). Western blotting was performed as described 42 by Amersham (Buckinghamshire, England). Briefly, blots were blocked with 5% nonfat dry milk in TBST (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 0.05% Tween 20) for at least 1 hour at room temperature or overnight at 4°C. For immunodetection, the blots were incubated with primary antibody at ∼5 μg/ml for at least 3 hours at room temperature and incubated 1 hour in ∼0.4 μg/ml of goat anti-mouse or goat anti-rabbit IgG-HRP. Detection was performed with Supersignal Western detection reagents (Pierce, Rockford, IL). For visualization of overall protein patterns gels were stained with 0.1% Coomassie blue in 50% methanol/10% acetic acid for a minimum of 1 hour, then destained in 50% methanol/10% acetic acid for a minimum of 2 hours. Images of stained gels were recorded with a model IS-1000 digital imager (Alpha Innotech, San Leandro, CA).
Immunoprecipitation
Cell lysis and immunoprecipitation were performed as previously described 43 with the following modifications. Cells were lysed in RIPA buffer containing150 mmol/L NaCl, 30 mmol/L Tris, pH 8.0, 0.1% SDS, 0.5% Na deoxycholate, and 1% Nonidet P-40. Approximately 8 μg of primary antibody (anti-βE11 or anti-4F2) was incubated with 10 μl of packed protein A/Sepharose beads (Pharmacia, Piscataway, NJ) for 1 hour at room temperature with gentle rotation. Meanwhile, 250 μl of lysate (∼400 μg protein) was precleared with 10 μl of protein A/Sepharose for 1 hour at room temperature. The precleared lysate was then incubated with antibody-protein A/Sepharose complex overnight at 4°C with gentle rotation. The beads were washed four times in RIPA buffer and one time in buffer containing 30 mmol/L Tris, pH 8.0, and 50 mmol/L NaCl. The beads were then boiled in 50 μl of 1× sample buffer for 3 minutes, and the supernatant was run on SDS-polyacrylamide gel electrophoresis (PAGE).
Two-Dimensional Gel Electrophoresis
Isoelectric focusing was performed essentially as described previously. 41 Briefly, samples indicated in the text were resuspended in isoelectric focusing sample buffer (8 mol/L urea, 0.08% SDS, 0.5% Nonidet P-40, 5% ampholines, pH 3 to 9; BioRad) and loaded on prefocused 4% acrylamide gels containing 8 mol/L urea, 0.08% SDS, 2% Nonidet P-40, and ∼2% ampholines (pH 3 to 9; BioRad). The gels were run at 350 V, 0 mA overnight at 4°C (total V-hours exceeded 6000). Gels were removed from the tubes and equilibrated in treatment buffer (62.5 mmol/L Tris, pH 7.0, 2% SDS, 10% glycerol, 5% β-mercaptoethanol). For second dimension separation, equilibrated gels were overlaid into the large well of a 5% polyacrylamide-0.1% SDS slab gel. Two identical tube gels were run into slab gels in the second dimension; one slab gel was stained with Coomassie blue for 15 minutes and extensively destained with H2O, and the other was processed for Western transfer as follows: incubated in 5% perchloric acid, 2 × 0.5 hour; fixed in 25% isopropanol, 10% acetic acid 1 × 0.5 hour, 2 × 1 hour, 1 × 0.5 hour; incubated in equilibration buffer (0.375 mol/L Tris-HCl, pH 8.8, 5% β-mercaptoethanol, 0.3% SDS) 2 × 0.5 hour. Gel was transferred to nitrocellulose 300 mA overnight. Western blotting was performed as described above. Immunoreactive Coomassie blue-stained gel spots were carefully excised from the gel, washed three times for 3 to 4 minutes/wash in 50% acetonitrile, and stored at −20°C. Tryptic fragments from the excised spots were analyzed by tandem mass spectrometry (MS/MS) as described 44 using a microcapillary HPLC-grade-quadrupole ion-trap mass spectrometer at the Harvard Microchemistry Facility, Cambridge, MA.
Metabolic Labeling
B16 BL6 melanoma cells were grown in HL-1 serum-free medium to a density of 2 to 2.5 × 10 6 cells per 100-mm dish. Metabolic labeling was performed essentially as described previously 41 with the following modifications. Cells were washed two times with PBS and were incubated with 4 ml of labeling medium per 100-mm dish. Labeling medium consisted of deficient DMEM (Irvine Scientific, Santa Ana, CA) supplemented with HL-1 serum substitute and physiological levels of l-leucine, l-lysine, and l-glutamine but with 1/100 the physiological level of l-methionine. 35S-cysteine/methionine [Tran35S-label reagent (ICN Biomedicals, Irvine, CA), label contains ∼70% 35S l-methionine and ∼15% 35S l-cysteine) was added to100 μCi/ml. Cells were incubated in labeling medium for the times indicated in the text and were extracted for immunoprecipitation as described above. For pulse-chase analysis, cells were incubated for the indicated times in labeling medium (pulse) then were washed two times with PBS and refed with complete DMEM supplemented with HL-1 serum substitute for the times indicated in the figure legends. Cells were then extracted for immunoprecipitation as described above. After running the immunoprecipitates on SDS-PAGE, the gel was fixed in 50% methanol/10% acetic acid at room temperature for a minimum of 30 minutes. The gel was then rinsed in water for 15 minutes and incubated for 1 hour at room temperature in Autofluor autoradiographic image enhancer (National Diagnostics, Atlanta, GA), dried for 1 hour at 60o under vacuum, and exposed to film at −80°C.
Enzymatic Treatments
PNGase Digestion
Metabolic labeling and immunoprecipitation was performed as described above. After antigen-bound antibody conjugates were washed, they were resuspended in 20 μl of 0.5% SDS and 1% β-mercaptoethanol and boiled for 5 minutes. Sodium phosphate, pH 7.8, and Nonidet P-40 were added to 50 mmol/L and 1%, respectively, and the samples were incubated with 1 U PNGase F (Sigma) for 16 hours at 37°C. Digested products were run on SDS-PAGE and processed for autoradiography.
Tunicamycin Treatment
Cells were incubated with normal growth medium plus 1:10,000 dilution of 10 mg/ml of tunicamycin (Sigma)/dimethyl sulfoxide for 16 to 18 hours at 37°C as previously described. 45 Metabolic labeling was performed as described above except using medium supplemented with tunicamycin.
V8 Protease-Limited Digests
Immunoprecipitation from serum-free B16 BL6 melanoma cells was performed as described above. After the beads were boiled in 50 μl of 1× sample buffer for 3 minutes, they were allowed to cool to room temperature. Limited protease digestion was performed as described previously. 46 Briefly, 2 μl of type XVII-B protease from Staphylococcus aureus strain V8 (Sigma) dissolved in 50 mmol/L NH4HCO3 was added to the beads to give the amounts of protease indicated in the text, and the digest was performed for 5 minutes at room temperature. Digested products were immediately separated by SDS-PAGE and processed for autoradiography as indicated above.
Results
The βE11 Antigen Is Highly Expressed on the Surface of Mitotic Versus Interphase Endothelial Cells
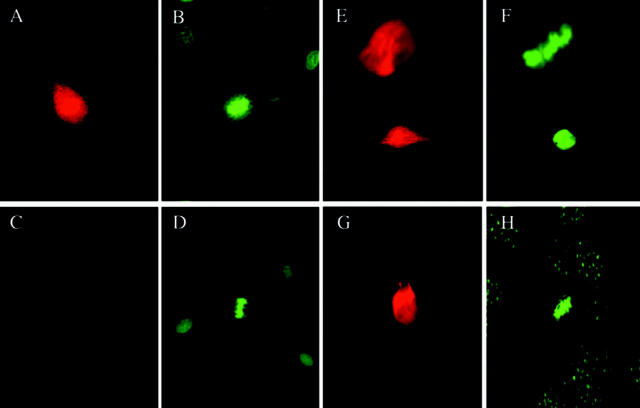
We first wanted to obtain antibodies that reacted with the cell surface of vascular endothelial cells. Hybridomas were produced by fusion of myeloma cells with spleen cells from mice immunized with a plasma membrane and cytoplasmic lysate derived from microvascular cell cultures (see Materials and Methods). Antibodies from individual clones were screened by immunofluorescence for reactivity with the cell surface of nonpermeabilized endothelial cells and pericytes. Antibodies from several hybridoma clones exhibited cell surface staining; however, one particular clone, designated βE11, produced an antibody that intensely stained the cell surface of only a subset of endothelial cells (Figure 1, A and B) ▶ . Closer inspection revealed that the βE11 antibody is localized to the surface of mitotic endothelial cells. Anti-βE11 strongly stains the surface of endothelial cells in metaphase, anaphase, and cytokinesis but not the surrounding interphase cells (metaphase/anaphase cell shown in Figure 1A ▶ ). In permeabilized endothelial cells, the same distinction can be made: mitotic cells are intensely stained by anti-βE11 whereas interphase cells are not (data not shown). Control mIgG demonstrates only background staining (Figure 1, C and D) ▶ .
Figure 1.
Anti-βE11 recognizes the surface of mitotic vascular endothelial cells and tumor cells. A–D: Anti-βE11 stains the cell surface of endothelial cells in mitosis. Anti-βE11 (A) or mIgG (C) immunofluorescence of formaldehyde-fixed, nonpermeablized bovine retinal endothelial cells; B and D are paired Hoescht-stained images. Cells in metaphase, anaphase, and cytokinesis are stained by anti-βE11; surrounding interphase cells are not stained. Shown in A is a metaphase/anaphase cell. E and F: Anti-βE11 recognizes both mitotic and interphase LNCaP tumor cells. E represents anti-βE11 immunofluorescence of formaldehyde-fixed, permeabilized LNCaP cells, and F is a paired Hoescht-stained image. G and H: Anti-βE11 stains C2 myoblasts in mitosis but not interphase. G shows permeabilized C2 myoblasts stained with anti-βE11 whereas H is the paired Hoescht-stained image. Original magnifications: ×40 (A–D), ×63 (E–H).
Western Blot Analysis of the βE11 Antigen in Endothelial Cells
By reducing SDS-PAGE and Western blotting the endothelial cell antigen recognized by anti-βE11 is ∼190 kd (Figure 3A) ▶ . Interestingly, subconfluent proliferating vascular endothelial cells express a higher level of the βE11 antigen by Western blotting than do confluent, quiescent endothelial cells growth arrested in a monolayer. By densitometric analysis of the Western blot, the level of βE11 antigen in proliferating endothelial cells is approximately threefold higher than in control growth-arrested cells (data not shown). These results confirm the immunofluorescence data indicating that mitotic or proliferating endothelial cells express a higher level of expression of the βE11 antigen than do interphase cells (Figure 1, A–F) ▶ .
Figure 3.
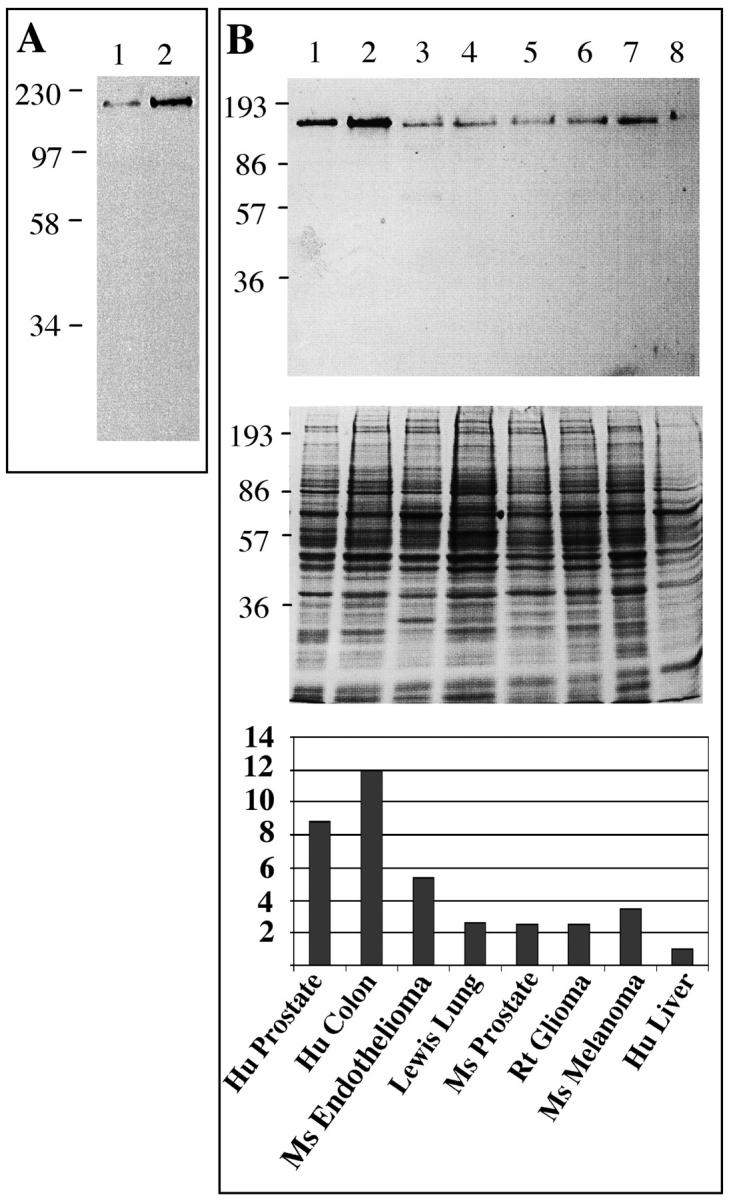
Western blotting of the βE11 antigen in primary endothelial cells and tumor-derived cell lines. A: Western blot of ∼500,000 cell equivalents from detergent lysates of bovine retinal endothelial cells growth arrested in a monolayer (lane 1) or at ∼50% confluency (lane 2) probed with anti-βE11 followed by goat anti-mouse IgG-HRP. B: ∼15 μg of tumor cell lysate blotted with anti-βE11 followed by goat anti-mouse IgG-HRP (top). Corresponding Coomassie blue-stained gel is shown in middle panel. Cell lysates are as follows: 1, LNCaP human prostate; 2, Cx.1 human colon carcinoma; 3, murine endothelioma; 4, Lewis lung; 5, murine prostate; 6, rat glioma; 7, B16 BL6 murine melanoma; 8, HEPG2 human liver. Molecular weight markers (in kDa) are indicated to the left of each panel.
Tumor Cell Expression and Localization of the βE11 Antigen
In Vitro
Because the βE11 antigen is highly expressed on cells undergoing mitosis, we hypothesized that cells with a high mitotic index such as tumor cells would possess abundant cell surface βE11 antigen. The localization of the βE11 antigen in tumor cells (Figure 1, E–H) ▶ parallels that seen in mitotic vascular endothelial cells. By immunofluorescence, nonpermeabilized LNCaP tumor cells demonstrate a bright, uniform staining whereas permeabilized cells reveal a reticular meshwork pattern that covers the entire surface of the cell (Figure 1, E–H) ▶ . Interestingly, no difference is seen in the localization patterns of the βE11 antigen between LNCaP tumor cells in M phase (Figure 1E ▶ ; upper cell, metaphase) and those in interphase (Figure 1E ▶ ; lower cell). Other cell lines such as the Cx.1 human colon carcinoma, 3T3 fibroblast, and B16 BL6 melanoma demonstrate a similar immunofluorescence pattern (data not shown). However, one cell line, the C2 myoblast, demonstrates mitosis-specific expression of the βE11 antigen. In permeabilized mitotic C2 myoblasts there is very intense expression of the βE11 antigen that may be associated with spindle microtubules or centrosomes (Figure 1, G and H) ▶ . Interphase cells, in contrast, show very faint βE11 expression in punctate form around the nucleus. The C2 myoblasts are the only tumor cells we studied that demonstrate such mitosis-specific immunolocalization of the βE11 antigen.
In Vivo
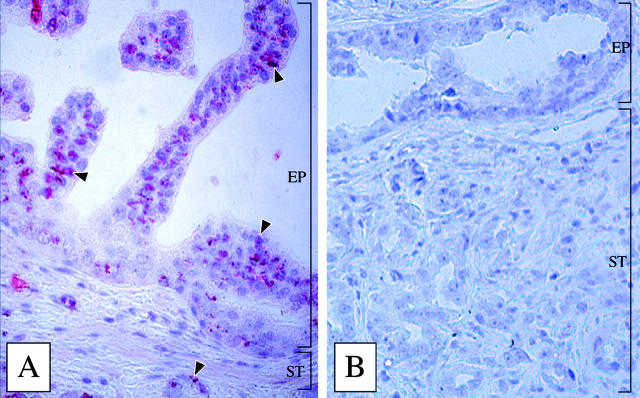
We have also localized the βE11 antigen to regions of prostatic tumor tissue. Anti-βE11 recognizes abundant antigen expression in regions of prostatic intraductal neoplasia (Figure 2A) ▶ . Most epithelial cell regions of the prostatic intraductal neoplasia lesion are intensely stained by anti-βE11 (Figure 2A ▶ , arrowheads). The surrounding stroma and smooth muscle layers show only background staining, and control mouse IgG antibody (Figure 2B) ▶ detects no antigen in the tissue sections. Interestingly, βE11 antigen is also localized to ductal epithelia embedded within the stromal layer. Areas of benign prostatic hyperplasia were only weakly stained by anti-βE11 (data not shown). Therefore, the βE11 antigen may define a highly dysplastic cell population in vivo.
Figure 2.
Anti-βE11 recognizes prostatic intraductal neoplasia lesions in human prostatic tumor tissue. Avidin-biotin-peroxidase immunohistochemistry was performed on a paraffin-embedded section of a radical human prostatectomy using anti-βE11 (A) or a murine IgG control (B) as described in Materials and Methods. Anti-βE11 intensely stains the epithelial cell layer (EP) in prostatic intraductal neoplasia lesions but does not stain stromal (ST) cells. Arrowheads indicate βE11 antigen localization in epithelial cells. Original magnification, ×100.
Western Blot Analysis of the βE11 Antigen in Tumor Cells
Reducing SDS-PAGE and Western blotting of detergent-solubilized cells shows that in all tumor cell lines examined the βE11 antibody recognizes an antigen of ∼190 kd (Figure 3B) ▶ similar to that recognized in endothelial cells (Figure 3A) ▶ . In Figure 3B ▶ the expression levels of the βE11 antigen in various cell lines are normalized to the total cell protein loaded on the gel. Because anti-βE11 immunoprecipitates an antigen from lysates of tumor cells treated with tunicamycin, an inhibitor of N-linked glycosylation (see Figure 8 ▶ ), it is unlikely that the βE11 antibody recognizes a carbohydrate antigen shared by several proteins of varied molecular weights. Therefore, anti-βE11 probably recognizes a protein epitope rather than a carbohydrate moiety.
Figure 8.
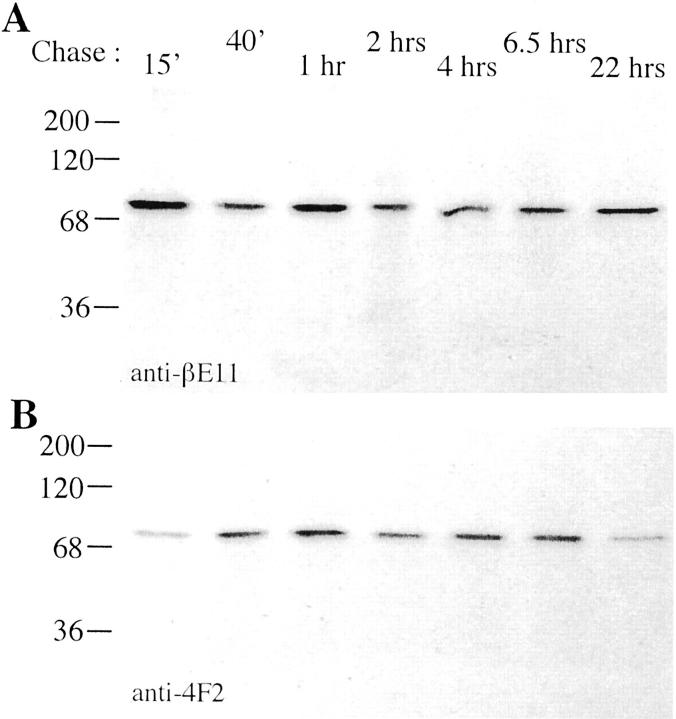
Pulse-chase analysis of βE11 and 4F2 antigens. B16 BL6 melanoma cells cultured in serum-free medium (Biowhittaker, Walkersville, MD) were pretreated with tunicamycin for 18 hours prior to pulse-labeling for 2 hours and chasing for the additional time periods indicated. Anti-βE11 (A) or anti-4F2 (B) immunoprecipitates were run on reducing 8.5% SDS-PAGE, and the gels were processed for autoradiography.
Anti-βE11 Inhibits Cell Growth
Immunolocalization of the βE11 antigen to the surface of nonpermeabilized cells suggests that it would be accessible to its cognate antibody added to intact, living cells. Because immunofluorescence (Figure 1; A, B, E–H ▶ ) and Western blotting (Figure 3, A and B) ▶ indicate that the βE11 antigen is correlated with proliferating cells, we wanted to determine whether anti-βE11 could inhibit cell growth in vitro. Primary cells, such as vascular endothelial cells and pericytes, as well as established tumor lines, such as Lewis lung, LNCaP human prostate, and B16 BL6 murine melanoma, were grown in vitro in the presence or absence of the βE11 antibody or an isotype-matched control. Anti-βE11 inhibits the proliferation of many different tumor cells such as those derived from human colon (80%; Figure 4A ▶ ) and human prostate (55%; Figure 4B ▶ ). However, anti-βE11 demonstrates little inhibition (15 to 20%) in primary cells and no inhibition in certain tumor cell lines such as HEPG2 hepatocarcinoma (Figure 4D) ▶ . Anti-βE11 thus has varied effects on the growth of different cell types in vitro. The inhibitory effect of the βE11 antibody is dose-dependent with maximal inhibition at 0.5 to 1.0 μg/ml for all cell lines tested. Anti-βE11 is not cytotoxic because gross inspection of the inhibited cells by phase-contrast microscopy shows no evidence of necrosis or apoptosis (data not shown). Furthermore, the inhibitory effect of anti-βE11 is reversible because cell proliferation resumes once the antibody is removed (Figure 4C) ▶ .
Figure 4.
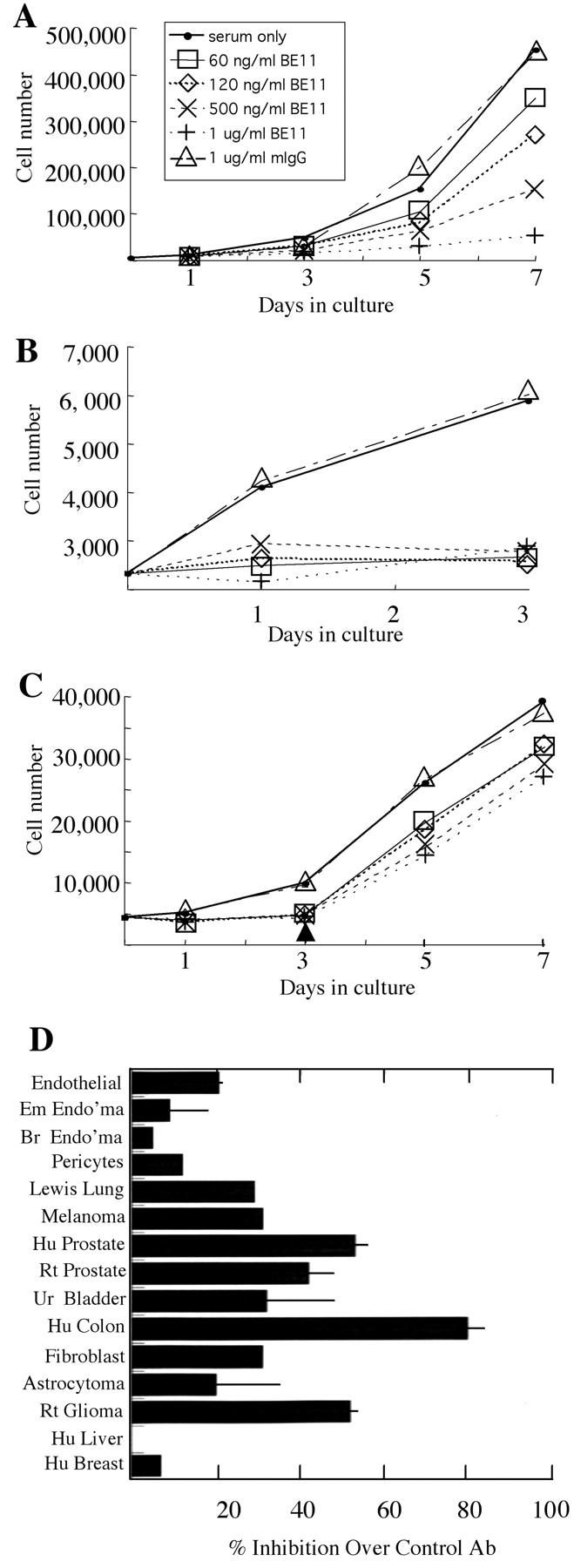
Anti-βE11 inhibits tumor cell growth in vitro. Primary and tumor cells were grown as described in Materials and Methods in the presence or absence of anti-βE11 or a murine IgG control for the number of days indicated. A: Cx.1 human colon carcinoma cells. B: LNCaP human prostate tumor cells. C: LNCaP cells were grown as in B for 3 days after which antibody was washed out of the medium (indicated by arrowhead). Legend in B and C is the same as that for A. D summarizes the maximal inhibition observed for each cell line used in the growth assays. For all cell lines, maximal inhibition occurred at days 5 to 7 and at a βE11 antibody concentration of 0.5 to 1.0 μg/ml. From top to bottom, cell lines are as follows: primary bovine retinal endothelial cells, murine embryonic endothelioma, murine brain endothelioma, primary bovine retinal pericytes, Lewis Lung carcinoma, B16 BL6 murine melanoma, LNCaP human prostate, rat prostate, human urinary bladder carcinoma, Cx.1 human colon carcinoma, murine 3T3 fibroblast, human astrocytoma, C6 rat glioma, HEPG2 human liver carcinoma, and MCSF7 human breast carcinoma.
Interestingly, in some cell lines, expression level of the βE11 antigen by Western blotting corresponds to inhibitory potential of the βE11 antibody in the in vitro growth assays. For example, the human colon carcinoma and the LNCaP human prostate tumor cells express abundant antigen by Western blotting (Figure 3B) ▶ and were also significantly inhibited in growth by the βE11 antibody (Figure 4, B and C) ▶ . However, such is not always the case; the rat glioma cells, which were inhibited to the same extent as the LNCaP tumor cells (∼55%), express ∼28% as much antigen as the LNCaP cells by Western blotting.
Identification of the Cell-Derived βE11 Antigen
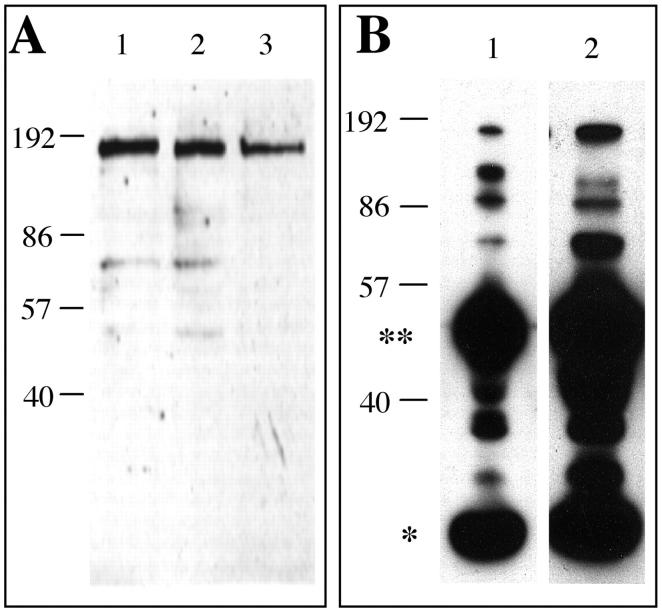
To circumvent problems of low abundance or N-terminal blockage commonly seen in protein sequence determinations of cell surface antigens, we chose to identify the βE11 antigen using two-dimensional gel electrophoresis and mass spectrometry. This technique is ∼100 to 1000 times as sensitive as Edman degradation and can yield sequence data from fmol amounts of digested peptide. 47,48 To avoid possible contaminating proteins from serum, we used a cell line grown in the absence of serum as a source for the βE11 antigen. Because the B16 BL6 melanoma was the only cell line that could be propagated in the absence of serum and expressed significant βE11 antigen by Western blotting (Figure 3B) ▶ , we used it as a cellular source of the βE11 antigen. Anti-βE11 recognizes its antigen in detergent lysates of B16 BL6 melanoma cells grown in the absence of serum virtually to the same extent as it recognizes its antigen in cell lysates derived from cells grown in the presence of serum (Figure 5A) ▶ . Furthermore, anti-βE11 immunoprecipitates an antigen of ∼190 kd (as well as smaller molecular weight species) from serum-free B16 BL6 melanoma cell lysates (Figure 5B) ▶ .
Figure 5.
The βE11 antigen is expressed in serum-free melanoma cells. A: Western blot of detergent lysates (∼10 μg total protein) from LNCaP cells grown in serum (lane 1) and B16 BL6 melanoma cells grown in the presence (lane 2) or absence (lane 3) of serum blotted with anti-βE11 followed by goat anti-mouse IgG-HRP. B: Immunoprecipitation from LNCaP cells grown in serum (lane 1) or B16-BL6 melanoma cells grown in the absence of serum (lane 2) was performed with serum-free affinity-isolated anti-βE11 and goat anti-mouse IgG(Fc)/Sepharose as described in Materials and Methods. The immunoprecipitated complexes were run on 8.5% SDS-PAGE and transferred to nitrocellulose. The blots were probed with anti-βE11 followed by goat anti-mouse IgG-HRP. Double asterisk indicates IgG heavy chain; single asterisk indicates IgG light chain. Molecular weight marker (kilodaltons) appear along left hand of each panel A, B.
We separated proteins derived from a detergent-solubilized extract of melanoma cells grown in the absence of serum by isoelectric focusing from pH 3 to 7 in the first dimension and then by SDS-PAGE in the second dimension. As shown in Figure 6 ▶ , two individual spots, both having a pI of ∼4.6 and molecular weights of ∼110 kd and 93 kd, were immunoreactive with anti-βE11 by Western blotting. Both spots were identified by Coomassie blue staining of the corresponding gel and were excised and analyzed by mass spectrometry as described in Materials and Methods. The ∼110-kd spot generated sequence for calnexin, an endoplasmic reticulum-resident membrane protein that transiently associates with many proteins shortly after synthesis, 49 and CBP-140, a heat-shock protein homologue. The ∼93-kd spot generated sequence for calnexin and the 4F2 cell-surface antigen heavy chain. Interestingly, the 4F2 antigen is a 125-kd cell-surface glycoprotein complex that was originally characterized to be a protein highly expressed on activated versus resting lymphocytes; 35-37 subsequently, it was found to be a protein complex that is expressed in all proliferating cells. 50
Figure 6.
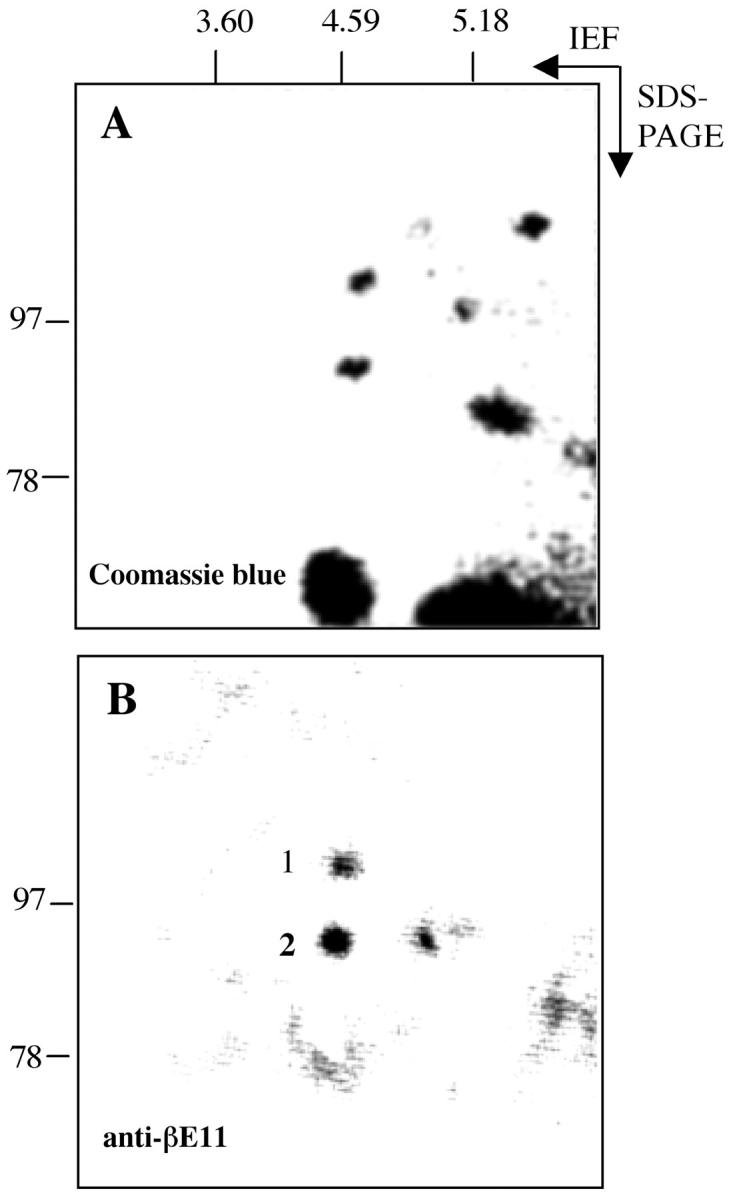
Two-dimensional gel electrophoresis and mass spectrometry yield protein sequence information for the βE11 antigen. Approximately 125 μg of total protein from serum-free B16 BL6 melanoma cells (see Materials and Methods) was separated by two-dimensional PAGE. First dimension was isoelectric focusing (+SDS) from pH 3 to pH 7, and second dimension was 5% reducing SDS-PAGE. A: Coomassie blue-stained-gel. B: Corresponding Western blot probed with anti-βE11 followed by goat anti-mouse IgG-HRP. Spots 1 and 2 were excised from the Coomassie blue-stained gel and were processed for mass spectrometry by the Harvard University Microchemistry Facility as described in Materials and Methods.
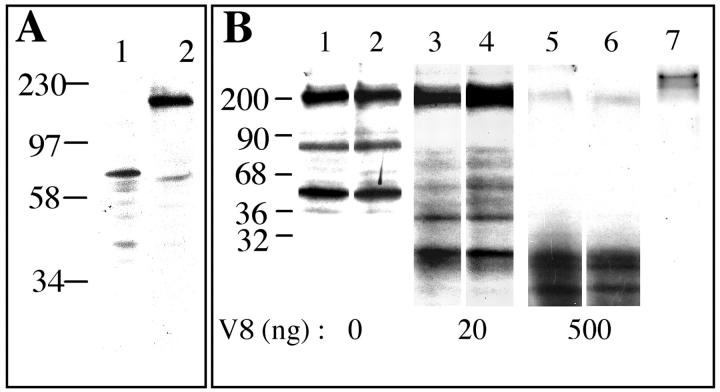
Because of the similarities of the 4F2 complex to the βE11 antigen in molecular weight, cell surface localization, and possible function in cell growth, it is likely that the two antigens are identical or closely related family members. To determine whether anti-βE11 and anti-4F2 recognize the same cell-derived antigen, staining patterns of anti-4F2 and anti-βE11 on Western blots were directly compared. In lysates of serum-free B16 BL6 melanoma cells, anti-βE11 recognizes an antigen most strongly at ∼190 kd and to a lesser extent an antigen at ∼75 kd (Figure 7A) ▶ . Under the same conditions, anti-4F2 primarily recognizes an antigen at ∼75 kd. Therefore, although antigens of similar molecular weights are identified by both antibodies on Western blots, the two antibodies also recognize distinct molecular weight species.
Figure 7.
Direct comparison of antigens recognized by anti-βE11 and anti-4F2. A: B16 BL6 melanoma cells were grown in serum-free medium and were lysed in sample buffer containing 20% SDS and 10% β-mercaptoethanol. Approximately 15 μg of lysate was Western blotted with either anti-βE11 (lane 1) or anti-4F2 (lane 2). B: V8 protease limited digest comparison of βE11 and 4F2 antigens. Complexes immunoprecipitated by anti-βE11 (lanes 1, 3, and 5) or anti-4F2 (lanes 2, 4, and 6) from radiolabeled B16 BL6 melanoma serum-free cells were incubated for 5 minutes with the indicated amounts of Staphylococcus V8 protease, and the peptides generated were separated on 10% reducing SDS-PAGE and visualized by autoradiography. Lane 7 shows the complexes immunoprecipitated by a murine IgG control.
The differences seen in Western blots probed with anti-βE11 versus anti-4F2 raised the possibility that anti-βE11 recognizes a protein that is similar to 4F2 but may be a distinct family member. To determine whether the βE11 antigen is distinct from 4F2, we performed limited protease digests on antigens immunoprecipitated by both antibodies. B16 BL6 melanoma serum-free cells were metabolically labeled as described in Materials and Methods, and the proteins immunoprecipitated by anti-βE11 and anti-4F2 were subjected to limited V8-protease digestion. Both anti-βE11 and anti-4F2 immunoprecipitate antigens of the same molecular weight (Figure 7B) ▶ . Furthermore, the fingerprints of peptides generated by V8 protease digestion of immunoprecipitated proteins are identical for both βE11 and 4F2 antigens. Lane 7 shows that the proteins digested with V8 protease are specifically recognized by antibodies to βE11 and 4F2 because they are not immunoprecipitated by a mouse IgG control. These results suggest that at this resolution, the βE11 antigen is identical to the 4F2 heavy chain.
βE11 and 4F2 Antigens in the Absence of N-Linked Glycosylation
To determine whether the synthesis and posttranslational processing and maturation of the βE11 and 4F2 antigens are similar, we performed pulse-chase analysis of B16 BL6 melanoma cells grown in the presence of tunicamycin and the absence of serum using radiolabeled cysteine and methionine. Melanoma cells grown in serum-free medium were pulsed for 2 hours with 35S-methionine as described in Materials and Methods, and after chasing with 35S-free complete medium for various times, the cells were lysed. Labeled proteins immunoprecipitated by anti-βE11 and anti-4F2 were directly compared (Figure 8) ▶ . Interestingly, bands at ∼200 and 45 kd are immunoprecipitated by both antibodies in the absence of tunicamycin (Figure 7B) ▶ ; but , in the presence of tunicamycin, an inhibitor of N-linked glycosylation, the 80 kd band is the only protein species immunoprecipitated (Figure 8) ▶ . The similarlity between the metabolically labeled antigens immunoprecipitated by both antibodies confirms the protease fingerprint analysis (Figure 7B) ▶ , suggesting that the βE11 and 4F2 are identical.
Discussion
We have identified an endothelial cell surface antigen that, by immunofluorescence with a monoclonal antibody (clone βE11), is highly expressed during M phase in vascular endothelial cells (Figure 1) ▶ . By Western blotting this 190-kd antigen is approximately threefold more abundant in proliferating versus quiescent endothelial cells, and it is highly expressed on a wide variety of tumor-derived cell lines (Figure 3) ▶ . Anti-βE11 inhibits cell proliferation in vitro in a reversible and dose-dependent manner with maximal effect at 1 μg/ml (Figure 4) ▶ . Different tumor cell lines demonstrate varying sensitivities to growth inhibition in the following order: colon > prostate = glioma > melanoma = fibroblast > breast > liver. Interestingly, the βE11 antigen is localized to regions of prostatic intraductal neoplasia in human prostatic tumor tissue (Figure 2) ▶ . Biochemical characterization and mass spectrometry analyses show that this protein is identical to the proliferation-associated antigen 4F2 (Figures 6, 7, and 8) ▶ ▶ ▶ .
βE11: A Functionally Defined Tumor Cell Antigen
Endothelial cells undergoing mitosis show robust staining with anti-βE11 (Figure 1, A and B) ▶ , and high expression of the βE11/4F2 antigen defines a cell population that is actively proliferating versus one that is growth arrested and quiescent (Figure 3A) ▶ . Previous reports have demonstrated cell-cycle-specific expression of 4F2 protein in G1 51 and mRNA in S phase, 52 and one group concludes that 4F2 mRNA levels remain constant throughout the cell cycle after rising sharply 3 to 6 hours after serum stimulation. 53 Our results suggest that high M-phase expression of the βE11 antigen could be because of transport of intracellular βE11/4F2 pools to the cell surface or to exposure of a masked or cryptic epitope. However, because anti-βE11 recognizes a protein, not a carbohydrate, moiety (Figure 8) ▶ , the M-phase-specific expression of the βE11/4F2 antigen is not because of exposure of an epitope created by glycosylation.
In addition to mitotic endothelial cells, all tumor cells studied exhibit abundant cell-surface expression of the βE11/4F2 antigen (Figure 1, E and F) ▶ . However, unlike endothelial cells, βE11/4F2 antigen expression seems to be similar in mitotic and interphase cells of most tumor lines. Interestingly, it has been suggested that a difference in the organization of the 4F2 antigen in the plasma membrane is responsible for high expression of the 4F2 antigen in neoplastic and embryonic versus normal adult cells, 56,57 and it has been proposed that overexpression of the 4F2 antigen results in malignant transformation. 55 Therefore, high expression of the 4F2 antigen may be a characteristic of neoplastic cells. Our results support this notion and suggest that tumor cells constitutively express the form of the βE11/4F2 antigen seen in mitotic primary cells. Moreover, as shown in Figure 2 ▶ , anti-βE11 targets prostatic intraductal neoplasia lesions of prostatic tumor tissue in histological sections. This result suggests that anti-βE11 may be able to target tumors in vivo.
Biochemical Characterization of the βE11 Antigen
SDS-PAGE and Western blot analysis indicates that the major antigen recognized by anti-βE11 in tumor cell lysates is ∼190 kd (Figure 3B) ▶ . However, three major antigens are immunoprecipitated by both anti-βE11 and anti-4F2 from metabolically labeled B16 BL6 melanoma cells (Figure 8) ▶ . One is a 69/80-kd doublet, another is a 200-kd species, and the third is a 45-kd antigen. The 200- and 45-kd antigens appear cyclically during the 22-hour chase period while the 69/80-kd doublet is fairly constant throughout.
The primary structure of the murine 4F2 heavy chain predicts a 526-amino-acid type-II glycoprotein, 53 and the 4F2 antigen has been well characterized to be a disulfide-linked complex of 125 kd that resolves into a 85-kd heavy chain and a 40-kd light chain on reducing SDS-PAGE 36,47 in lymphoid cells. In fibroblasts, the 4F2 heavy chain is a doublet of 73/85 kd. 59 Two pools of βE11/4F2 antigen may exist in the B16 BL6 melanoma cells used in our study. The 69/80-kd doublet may represent nonglycosylated βE11/4F2 heavy chain that remains unassociated with the 45-kd light chain. Perhaps this pool of heavy chain is sequestered by calnexin because calnexin peptides were discovered in the mass spectrometry analysis of two-dimensional gel spots immunoreactive with anti-βE11 (Figure 6) ▶ . The other pool of βE11/4F2 antigen is the 200-kd antigen that may represent a nonreduced complex of glycosylated heavy chain plus light chain that incorporates both species of the heavy chain doublet. Because the 45-kd βE11/4F2 light chain only appears in anti-βE11 or anti-4F2 immunoprecipitates containing the 200-kd antigen, it must be complexed to the heavy chain in the 200-kd species. N-linked glycosylation is necessary for the cyclical appearance of the 200-kd antigen and its association with the 45-kd light chain because in tunicamycin-treated cells only a single antigen of ∼80 kd is immunoprecipitated by anti-βE11 and anti-4F2 throughout the chase period (Figure 8) ▶ .
It is possible that creation of the 200-kd- and 45-kd-antigen complex exposes an epitope that is identified by anti-βE11 and anti-4F2 more efficiently than the epitope on the 69/80-kd heavy chain doublet. The 200- and 45-kd antigens are more abundant than both antigens in the 69/80-kd doublet (Figure 8) ▶ . Within the asynchronously growing melanoma cells may be waves of cells that expose this epitope. In primary endothelial cells, exposure of this epitope may account for the higher immunoreactivity with anti-βE11 in mitotic versus primary cells.
The cyclical disappearance of the 200- and 45-kd antigens could be because of shedding of the βE11/4F2 antigen from the cell surface. Indeed, a 200-kd radiolabeled antigen accumulates throughout time in immunoprecipitates of 0.2-μm-filtered cultured supernatants of metabolically labeled B16 BL6 melanoma cells (data not shown). Shedding of the βE11 antigen may be important in vivo as soluble βE11 antigen could be a diagnostic tool for monitoring tumor progression in cancer.
Identity with 4F2
4F2 antigens belong to a family of heterodimeric transmembrane proteins expressed on the surface of activated cells that are composed of different light chain subunits, of which six have been identified, disulfide-bonded to a common heavy chain. 50 Figure 7B ▶ shows that limited protease digest fingerprints of anti-βE11 and anti-4F2 immunoprecipitates are the same. Therefore, at this level of resolution, both heavy and light chains of the βE11 and 4F2 antigens are identical in these cells.
The differences between staining patterns of Western blots probed with anti-βE11 and anti-4F2 (Figure 7A) ▶ may be attributed to differences in affinity for various forms of the 4F2 antigen. Anti-βE11 may have a higher affinity for the complex of 4F2 heavy plus light chain at ∼200 kd, and anti-4F2 could have a higher affinity for the uncomplexed 4F2 heavy chain at 75 kd. On a Western blot, where the incubation time of antibody with antigen is only 3 to 6 hours, these differences in affinity are apparent. However, in the immunoprecipitations, where antibody is incubated with antigen overnight (14 to 18 hours), these differences are masked (Figures 7B and 8) ▶ ▶ because even low-affinity interactions have time to form.
Inhibition of Cell Growth
The 4F2 antigen targeted by anti-βE11 is likely to play an important role in regulating cell proliferation because anti-βE11 inhibits cell growth in vitro (Figure 4) ▶ . Gross examination of the inhibited cells by phase-contrast microscopy reveals that the antibody does not induce apoptosis or necrosis (R. Nayak, unpublished personal communication). Instead, it reversibly impedes proliferation, and the growth-inhibited cells do not seem to be arrested at a particular phase of the cell cycle.
The 4F2 antigen can transport neutral and cationic amino acids. 67-69 Because anti-βE11 inhibits tumor cell growth, transport of certain amino acids by the βE11/4F2 antigen may be necessary for cell proliferation. Early reports have demonstrated that significant amino-acid transport occurs in mitosis as well as in interphase. 73,74 Anti-βE11 probably inhibits amino acid transport during interphase and possibly mitosis. The gross observation that cells inhibited in growth by anti-βE11 do not arrest at a certain phase of the cell cycle suggests that amino acid transport mediated by the βE11/4F2 antigen is not limiting for cell cycle progression and growth. Therefore, anti-βE11 may reversibly inhibit cell growth because it impedes transport of necessary nutrients into the cell.
Previous work has demonstrated that antibodies to 4F2-like molecules reversibly inhibit bladder cancer and T-lymphoma cell growth in vitro in a dose-dependent manner. 59 Antibody concentrations used in these studies ranged from 1 to 5 μg/ml, at which inhibition was minimal, to a maximally inhibitive dose of 100 μg/ml. Other antibodies to 4F2 impede the growth of fibrosarcoma cells 56 and block DNA synthesis in activated peripheral blood mononuclear cells. 36 We have shown that an antibody that recognizes 4F2 not only inhibits tumor cell growth in vitro but is also capable of recognizing mitotic versus interphase cells. Our data, therefore, provides novel insight into how the 4F2 antigen is associated with proliferating and activated cells. High cell-surface expression during mitosis links it to a process inherent to dividing cells.
Antibody targeting of cell surface molecules other than 4F2, such as disialoganglioside GD3, 60 receptors for IL-2, 61 transferrin 62-64 and EGF, 65 and HLA-DR, 66 also inhibits cell growth in vitro and in vivo. Significant drawbacks to these studies, however, are the high concentrations of antibody needed to inhibit cell proliferation (6 to 50 μg/ml for anti-HLA-DR), the limited cell-type specificity of the antibodies, 60,61,65-66 and harmful in vivo side effects. 64 Anti-βE11, on the other hand, significantly inhibits the growth of a variety of tumor cells at submicrogram/ml doses. Furthermore, anti-βE11 inhibits the growth of primary endothelial cells in culture (Figure 4) ▶ , albeit to a limited extent. A hallmark of many growing tumors is extensive angiogenesis, 70 a process characterized by proliferation of endothelial cells that extend the vascular network so that nutrients may be effectively delivered to rapidly dividing tumor cells. 71-74 Anti-βE11 alone or conjugated to a toxin may not only inhibit the growth of tumor cells but may also attenuate angiogenesis. Therefore, in terms of tumor therapeutics, anti-βE11 may have distinct advantages over other monoclonal antibodies that have been used to inhibit tumor cell growth.
Acknowledgments
We thank Drs. Ramesh Nayak and Brad Shuster for early work in creating and characterizing the βE11 hybridoma; Suzanne Doblecki and Jaleel Shujath for performing the growth assays and immunohistochemistry; Dr. Irwin Leav for assistance with immunohistochemistry on tumor sections 72 ; and Dr. Rakesh Jain for his thoughtful comments and review of the manuscript.
Footnotes
Address reprint requests to Dr. Ira Herman, Department of Physiology, Tufts University School of Medicine, 136 Harrison Ave., Boston, MA 02111. E-mail: ira.herman@tufts.edu
Supported in part from a sponsored research award from Angio-Oncology Sciences, Inc.; and by National Institutes of Health grants EY 09033, GM 55110, and 5P30 DK34928 (GRASP Imaging Core) (to I. M. H.).
References
- 1.Cobrinik D, Dowdy SF, Hinds P, Mittnacht S, Weinberg RA: The retinoblastoma protein and regulation of cell cycling. Trends Biochem Sci 1992, 17:312-315 [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366:701-704 [DOI] [PubMed] [Google Scholar]
- 3.Davis RJ: The mitogen-activated protein kinase signal transduction pathway. J Biol Chem 1993, 268:14553-14556 [PubMed] [Google Scholar]
- 4.Toker A, Cantley LC: Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 1997, 387:673-6769192891 [Google Scholar]
- 5.Goustin AS, Leof EB, Shipley GD, Moses HL: Growth factors and cancer. Cancer Res 1986, 46:1015-1029 [PubMed] [Google Scholar]
- 6.Rosales C, O’Brien V, Kornberg L, Juliano R: Signal transduction by cell adhesion receptors. Biochim Biophys Acta 1995, 1242:77-98 [DOI] [PubMed] [Google Scholar]
- 7.Clark EA, Brugge JS: Integrins and signal transduction pathways: the road taken. Science 1995, 268:233-239 [DOI] [PubMed] [Google Scholar]
- 8.Bos JL: Ras oncogenes in human cancer: a review. Cancer Res 1989, 49:4682-4689 [PubMed] [Google Scholar]
- 9.Momand J, Zambetti GP, Olson DC, George D, Levine AJ: The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69:1237-1245 [DOI] [PubMed] [Google Scholar]
- 10.Baker SJ, Markowitz S, Fearon ER, Willson JKV, Vogelstein B: Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 1990, 249:912-914 [DOI] [PubMed] [Google Scholar]
- 11.Kinzler KW, Vogelstein B: Lessons from hereditary colorectal cancer. Cell 1996, 87:159-170 [DOI] [PubMed] [Google Scholar]
- 12.Dougall WC, Qian X, Peterson NC, Miller MJ, Samanta A, Greene MI: The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene 1994, 9:2109-2123 [PubMed] [Google Scholar]
- 13.Khazaie K, Dull TJ, Graf T, Schlessinger J, Ullrich A, Beug H, Vennstrom B: Truncation of the human EGF receptor leads to differential transforming potentials in primary avian fibroblasts and erythroblasts. EMBO J 1988, 7:3061-3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virji MA, Mercer DW, Herberman RB: Tumor markers in cancer diagnosis and prognosis. CA Cancer J Clin 1988, 38:104-127 [DOI] [PubMed] [Google Scholar]
- 15.Pastan I, Pai LH, Brinkmann U, FitzGerald D: Recombinant immuntoxins. Breast Cancer Res Treat 1996, 38:3-9 [DOI] [PubMed] [Google Scholar]
- 16.Carraway KL, Fregien N, Carraway K, III, Carraway CAC: Tumor sialomucin complexes as tumor antigens and modulators of cellular interactions and proliferation. J Cell Science 1992, 103:299-307 [DOI] [PubMed] [Google Scholar]
- 17.Wick B, Groner B: Evaluation of cell surface antigens as potential targets for recombinant tumor toxins. Cancer Lett 1997, 118:161-172 [DOI] [PubMed] [Google Scholar]
- 18.Hëllstrom ID, Horn D, Linsley P, Brown JP, Brankovan V, Hëllstrom KE: Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res 1986, 46:3917-3923 [PubMed] [Google Scholar]
- 19.Marken JS, Schieven GL, Hëllstrom I, Hëllstrom KE, Aruffo A: Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci USA 1992, :3503-3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastan I, Lovelace ET, Gallo MG, Rutherford AV, Magnani JL, Willingham MC: Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res 1991, 51:3781-3787 [PubMed] [Google Scholar]
- 21.Pal S, Sanyal U, Chattopadhyay U: Purification and characterization of a new 85-kDa glycoprotein antigen from human breast tumor. Int J Cancer 1995, 50:759-765 [DOI] [PubMed] [Google Scholar]
- 22.Beckett ML, Lipford GB, Haley CL, Schellhammer PF, Wright JGL: Monoclonal antibody PD41 recognizes an antigen restricted to prostate adenocarcinomas. Cancer Res 1991, 51:1326-1333 [PubMed] [Google Scholar]
- 23.Tjota A, Lee M-T, Isaacs JT, Kadohama N, Lee CL: Murine monoclonal antibodies reactive with a variety of androgen independent dunning rat prostate adenocarcinoma sublines also reactive with human prostate carcinoma. J Virol 1991, 146:205-212 [DOI] [PubMed] [Google Scholar]
- 24.Pastan I, Lovelace E, Rutherford AV, Kunwar S, Willingham MC, Peehl DM: A monoclonal antibody that reacts with an antigen on the surface of normal and malignant prostate cells. J Natl Cancer Inst 1993, 85:1149-1154 [DOI] [PubMed] [Google Scholar]
- 25.Pastan I, Willingham MC, FitzGerald DJ: Immunotoxins. Cell 1986, 47:641-648 [DOI] [PubMed] [Google Scholar]
- 26.Chang K, Pai LH, Batra JK, Pastan I, Willingham MC: Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res 1992, 52:181-186 [PubMed] [Google Scholar]
- 27.Chowdhury PS, Viner JL, Beers R, Pastan I: Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA 1998, 95:669-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldmann TA: The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science 1986, 232:727-732 [DOI] [PubMed] [Google Scholar]
- 29.Reiter Y, Kreitman RJ, Brinkmann U, Pastan I: Cytotoxic and antitumor activity of a recombinant immunotoxin composed of disulfide-stabilized anti-Tac Fv fragment and truncated Pseudomonas exotoxin. Int J Cancer 1994, 58:142-149 [DOI] [PubMed] [Google Scholar]
- 30.Husain SR, Kreitman RJ, Pastan I, Puri RK: Interleukin-4 receptor-directed cytotoxin therapy of AIDS-associated Kaposi’s sarcoma tumors in xenograft model. Nat Med 1999, 5:817-821 [DOI] [PubMed] [Google Scholar]
- 31.Vitetta ES, Stone M, Amlot P, Fay J, May R, Till M, Newman J, Clark P, Collins R, Cunningham D, Ghetie V, Uhr J, Thorpe PE: Phase I immunotoxin trial in patients with B cell lymphoma. Cancer Res 1991, 51:4052-4058 [PubMed] [Google Scholar]
- 32.Pai LH, Wittes R, Setser A, Willingham MC, Pastan I: Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat Med 1996, 2:350-353 [DOI] [PubMed] [Google Scholar]
- 33.Kiyakowa N, Lee EK, Karunagaran D, Lin S-Y, Hung MC: Mitosis-specific negative regulation of epidermal growth factor receptor, triggered by a decrease in ligand binding and dimerization, can be overcome by overexpression of receptor. J Biol Chem 1997, 272:18656-18665 [DOI] [PubMed] [Google Scholar]
- 34.Kiyokawa N, Yan D-H, Hung MC: Cell-cycle dependent regulation of p185neu: a relationship between disruption of this regulation and transformation. Proc Natl Acad Sci USA 1995, 92:1092-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenbarth GS, Haynes BF, Shroer JA, Fauci AS: Production of monoclonal antibodies reacting with peripheral blood mononuclear cell surface differentiation antigens. J Immunol 1980, 124:1237-1244 [PubMed] [Google Scholar]
- 36.Haynes BF, Hemler ME, Mann DL, Eisenbarth GS, Shelhamer J, Mostowski HS, Thomas CA, Strominger JL, Fauci AS: Characterization of a monoclonal antibody (4F2) that binds to human monocytes and a to a subset of activated lymphocytes. J Immunol 1981, 126:1409-1414 [PubMed] [Google Scholar]
- 37.Lüscher BM, Rousseaux M, Lees R, MacDonlad HR, Bron C: Glycoproteins involved in the stimulation of interleukin 1-dependent interleukin 2 production by a subline of el4 thymoma cells II. Structure, biosynthesis, and maturation. J Immunol 1985, 135:3951-3957 [PubMed] [Google Scholar]
- 38.Herman IM, D’Amore PA: Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol 1985, 101:43-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuster CB, Herman IM: Indirect association of ezrin with F-actin: isoform specificity and calcium sensitivity. J Cell Biol 1995, 128:837-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlow E, Lane D: Ammonium sulfate precipitation. Antibodies: A Laboratory Manual. 1988, :pp 298-299 NY, Cold Spring Harbor Laboratory Cold Spring Harbor [Google Scholar]
- 41.DeNofrio D, Hoock TC, Herman IM: Functional sorting of actin isoforms in microvascular pericytes. J Cell Biol 1989, 109:191-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amersham: ECL Western Blotting Protocols. 1994, pp 10–17
- 43.Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, Jahnsen T, Orstavik S: Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J 1999, 18:1858-1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chittum HS, Lane WS, Carlson BA, Roller PP, Lung F-DT, Lee BJ, Hatfield DL: Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry 1998, 37:10866-10870 [DOI] [PubMed] [Google Scholar]
- 45.Dixon WT, Sikora LKJ, Demetrick DJ, Jerry LM: Isolation and characterization of a heterodimeric surface antigen on human melanoma cells and evidence that it is the 4F2 cell activation/proliferation molecule. Int J Cancer 1990, 45:59-68 [DOI] [PubMed] [Google Scholar]
- 46.Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK: Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem 1977, 252:1102-1106 [PubMed] [Google Scholar]
- 47.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M: Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 1996, 379:466-469 [DOI] [PubMed] [Google Scholar]
- 48.Hunkapiller M, Kent S, Caruthers M, Dreyer W, Firca J, Griffin C, Horvath S, Hunkapiller T, Tempst P, Hood L: A microchemical facility for the analysis and synthesis of genes and proteins. Nature 1984, 310:105-111 [DOI] [PubMed] [Google Scholar]
- 49.Bergeron JJM, Brenner MB, Thomas DY, Williams DB: Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci 1994, 19:24-28 [DOI] [PubMed] [Google Scholar]
- 50.Deves R, Boyd CAR: Surface antigen CD98 (4F2): not a single membrane protein, but a family of proteins and multiple functions. J Membr Biol 2000, 173:165-177 [DOI] [PubMed] [Google Scholar]
- 51.Kehrl JH, Muraguchi A, Fauci AS: Differential expression of cell activation markers after stimulation of resting B lymphocytes. J Immunol 1984, 132:2857-2861 [PubMed] [Google Scholar]
- 52.Lumadue JA, Glick AB, Ruddle FH: Cloning, sequence analysis, and expression of the large subunit of the human lymphocyte activation antigen 4F2. Proc Natl Acad Sci USA 1987, 84:9204-9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parmacek MS, Karpinski BA, Gottesdiener KM, Thompson CB, Leiden JM: Structure, expression, and regulation of the murine 4F2 heavy chain. Nucleic Acids Res 1989, 17:1915-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suomalainen HA: The monoclonal antibodies TROP-4 and 4F2 detect the same membrane antigen that is exposed at an early stage of lymphocyte activation and is retained on secondary lymphocytes. J Immunol 1986, 137:422-427 [PubMed] [Google Scholar]
- 55.Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T: Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Comm 1999, 262:720-725 [DOI] [PubMed] [Google Scholar]
- 56.Azzarone B, Suarez H, Mingari M-C, Moretta L, Fauci AS: 4F2 monoclonal antibody recognizes a surface antigen on spread human fibroblasts of embryonic but not of adult origin. J Cell Biol 1984, 98:1133-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azzarone, Malpiece Y, Zaech P, Moretta L, Fauci AS, Suarez H: Analysis of the 4F2 surface antigen in normal and neoplastic fibroblastic human cells of embryonic and adult origin. Exp Cell Res 1985, 159:451-462 [DOI] [PubMed] [Google Scholar]
- 58.Hemler ME, Strominger JL: Characterization of the antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J Immunol 1982, 129:623-628 [PubMed] [Google Scholar]
- 59.Yagita H, Masuko T, Hashimoto Y: Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res 1986, 46:1478-1484 [PubMed] [Google Scholar]
- 60.Dippold WG, Knuth A, Meyer zum Bushenfelde KH: Inhibition of human melanoma cell growth in vitro by monoclonal anti-GD3-ganglioside antibody. Cancer Res 1984, 44:806-810 [PubMed] [Google Scholar]
- 61.Depper JM, Leonard WJ, Robb RJ, Waldmann TA, Greene WC: Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol 1983, 131:690-696 [PubMed] [Google Scholar]
- 62.Trowbridge IS, Omary MB: Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci USA 1981, 78:3039-3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trowbridge IS, Lopez F: Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci USA 1982, 79:1175-1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sauvage CA, Mendelsohn JC, Lesley JF, Trowbridge IS: Effects of monoclonal antibodies that block transferrin receptor function on the in vivo growth of a syngeneic murine leukemia. Cancer Res 1987, 47:747-753 [PubMed] [Google Scholar]
- 65.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J: Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res 1984, 44:1002-1007 [PubMed] [Google Scholar]
- 66.Moretta A, Accolla RS, Cerottini J-C: IL-2-mediated T cell proliferation in humans is blocked by a monoclonal antibody directed against monomorphic determinants of HLA-DR antigens. J Exp Med 1982, 155:599-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mastroberardino L, Spindler B, Pfeiffer R, Skelly P, Loffing J, Shoemaker CB, Verrey F: Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998, 395:288-291 [DOI] [PubMed] [Google Scholar]
- 68.Torrents D, Estevez R, Pineda M, Fernandez E, Lloberas J, Shi Y-B, Zorzano A, Palacin M: Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. J Biol Chem 1998, 273:32437-32445 [DOI] [PubMed] [Google Scholar]
- 69.Bertran J, Magagnin S, Werner A, Markovich D, Biber J, Testar X, Zorzano A, Kuhn LC, Palacin M, Murer H: Stimulation of system y+-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci USA 1992, 89:5606-5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkman J: What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1989, 82:4-6 [DOI] [PubMed] [Google Scholar]
- 71.Folkman J: Tumor angiogenesis: therapeutic implications. N Engl J Med 1971, 285:1182-1186 [DOI] [PubMed] [Google Scholar]
- 72.Leav I, McNeal J, Ziar J, Alroy J: The localization of transforming growth factor alpha and epidermal growth factor receptor in stromal and epithelial compartments of developing human prostate and hyperplastic, dysplastic, and carcinomatous lesions. Hum Pathol 1998, 29:668-675 [DOI] [PubMed] [Google Scholar]
- 73.MacMillan JC, Wheatley DN: Amino acid pools in mitotic and interphase HeLa cells. Exp Cell Res 1981, 133:470-475 [DOI] [PubMed] [Google Scholar]
- 74.Threlfall RJ, Thomas AJ: Fluctuations in proline and other free amino acids during the mitotic cycle of the myxomycete Physarum polycephalum. Eur J Biochem 1979, 93:129-133 [DOI] [PubMed] [Google Scholar]