Abstract
p62 is a RNA-binding protein that was isolated by immunoscreening a cDNA expression library with autoantibodies from patients with hepatocellular carcinoma (HCC). This autoantigen binds to mRNA encoding insulin-like growth factor II, which has been found to be overexpressed in HCC and is tumorigenic in transgenic animals. Immunohistochemical analysis of HCC liver showed that 33% (9 of 27) exhibited readily detectable staining of p62 protein in the cytoplasm of all malignant cells in cancer nodules, whereas it was undetectable in adjacent nonmalignant liver cells. In addition one of two patients with cholangiocarcinoma expressed p62 in malignant bile duct epithelial cells. p62 expression was also detected in scattered cells in cirrhotic nodules in contrast to uniform expression in all cells in HCC nodules. In HCC nodules, p62 mRNA was also detected by reverse transcriptase-polymerase chain reaction analysis. Nine normal adult livers did not contain detectable p62 mRNA or p62 protein whereas five fetal livers were all positive for mRNA and protein. The observations show that p62 is developmentally regulated, expressed in fetal, but not in adult liver, and aberrantly expressed in HCC and could be playing a role in abnormal cell proliferation in HCC and cirrhosis by modulating expression of growth factors such as insulin-like growth factor II.
Liver cancers are one of the most common cancers in the world, especially in developing countries, with hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCC) being the most frequent types of liver malignancy. Liver cirrhosis related to chronic viral hepatitis (hepatitis B and C) has been recognized as one of the major risk factors leading to the development of HCC, but the genetic defects and proteins involved in the development of liver cancer are not completely understood. 1
Using serum antibody from a patient with HCC to screen a cDNA expression library we recently identified a 62-kd RNA-binding protein that elicited a humoral immune response in ∼20% of HCC patients. 2 p62 contains one set of the RNA recognition motif 3 and four hnRNP K homology (KH) domains 4-6 and belongs to the family of IMPs (insulin-like growth factor II mRNA-binding proteins). 7 The human IMPs have high sequence identity with and similar RNA-binding domain distribution as other RNA-binding proteins such as the Xenopus Vg1RBP/Vera protein, 8,9 the chicken zipcode-binding protein-1, 10 and the mouse c-myc-coding region instability determinant-binding protein. 11 p62 represents a splice variant of IMP-2 and lacks 43 amino acids between the KH 2 and KH 3 domains, but the functional significance of the alternative splicing is currently unknown. These RNA-binding proteins have been implicated in posttranscriptional events such as RNA localization, stability, and translatability. Intracellular localization of Xenopus Vg1 and chicken β-actin mRNAs takes place via cis-elements in their 3′UTR that associate with Vg1RBP/Vera and zipcode-binding protein-1, respectively. The instability of mouse c-myc mRNA is partly mediated through endonucleolysis of an element in the coding region that is shielded from degradation by coding region instability determinant-binding protein. Finally, the human Koc (K homology protein overexpressed in cancer) protein, that is identical to IMP-3, was originally isolated by screening for genes differentially expressed in pancreatic cancer. 12
In this study we have examined the role of p62 in hepatic tumorigenesis. We show that p62 is expressed at high levels in fetal liver but is not detectable in adult liver. However, p62 is aberrantly and uniformly expressed in malignant cells of HCC nodules and in some cells in cirrhotic nodules. The results indicate that p62 has features associated with oncofetal proteins and its role in tumorigenesis could be by way of regulation of mRNA stability.
Materials and Methods
Patients and Tissue Specimens
Liver tissue from 27 HCC patients were obtained from Henan Medical University, Henan Province, People’s Republic of China and from the Department of Pathology, the Scripps Clinic, La Jolla, CA. Biopsies or surgically removed specimens were fixed in 10% formalin and embedded in paraffin for routine histological examination. The clinical data (20 males and 7 females; mean age, 49.3 years; range, 27 to 82 years) and pathological diagnoses are summarized in Table 1 ▶ . HCC grading criteria were according to those described. 13 Additional paraffin blocks from the Department of Pathology, Scripps Clinic, La Jolla, consisted of nine liver specimens from normal donors, two from patients with poorly differentiated CCC (see Table 1 ▶ for clinical data), and 23 from patients with liver cirrhosis. Patients with liver cirrhosis included 18 males and 5 females ranging from 30 to 66 years (mean age, 53.9 years). Five fetal livers ranging from 50 to 125 days were obtained from the Central Laboratory for Human Embryology (University of Washington, Seattle, WA), which is a National Institutes of Health funded center for collecting fetal embryos for research purposes. Hematoxylin and eosin (H&E)-stained sections of all specimens including cancer and noncancer cases were examined by two senior pathologists followed by separately conducted immunohistochemical analysis by other authors in this report.
Table 1.
Clinical Pathological Data on Patients with HCC and CCC and Immunohistochemistry of Liver Tissue
| Case | Age | Sex | Diagnosis | AFP (μg/ml) | Grading | Anti-p62C IHC* |
|---|---|---|---|---|---|---|
| 1 | 50 | M | HCC | >400 | III | + |
| 2 | 39 | M | HCC | >400 | II | − |
| 3 | 34 | M | HCC | 40 | II | − |
| 4 | 42 | F | HCC | <20 | I | − |
| 5 | 42 | M | HCC | >400 | III | + |
| 6 | 67 | F | HCC | <20 | II | + |
| 7 | 51 | M | HCC | >400 | II | − |
| 8 | 58 | F | HCC | 104 | II | + |
| 9 | 60 | M | HCC | >400 | III | + |
| 10 | 29 | M | HCC | >400 | II | − |
| 11 | 58 | M | HCC | >400 | III | − |
| 12 | 27 | F | HCC | >400 | II | − |
| 13 | 54 | M | HCC | >400 | I | + |
| 14 | 47 | M | HCC | >400 | II | − |
| 15 | 46 | F | HCC | >400 | III | − |
| 16 | 46 | M | HCC | >400 | I | − |
| 17 | 38 | M | HCC | 200 | IV | − |
| 18 | 48 | M | HCC | <20 | III | − |
| 19 | 41 | M | HCC | >400 | II | − |
| 20 | 51 | M | HCC | 400 | III | − |
| 21 | 51 | M | HCC | N/A | II | − |
| 22 | 62 | M | HCC | N/A | II | − |
| 23 | 46 | M | HCC | N/A | I | + |
| 24 | 37 | F | HCC | N/A | IV | − |
| 25 | 82 | M | HCC | N/A | III | + |
| 26 | 66 | F | HCC | N/A | III | − |
| 27 | 60 | M | HCC | N/A | II | + |
| 28 | 69 | M | CCC | N/A | + | |
| 29 | 50 | M | CCC | N/A | − |
HCC, hepatocellular carcinoma; CCC, cholangiocarcinoma; AFP, alpha fetoprotein; N/A, data not available.
Cases 1 to 20 were tissue specimens from patients in China and 21 to 29 from patients in the United States.
*IHC: immunohistochemical analysis.
Antigen Retrieval and Immunofluorescence Staining
Paraffin-embedded liver tissue specimens were sectioned at 5 μm and picked up on Superfrost plus slides (Fisher Scientific, Pittsburgh, PA). Antigen retrieval was performed by microwave-heating methods in a citrate-based antigen retrieval solution (BioGenex, San Ramon, CA) according to the manufacturer’s recommendation. Briefly, the sectioned slides were deparaffinized using xylene (Fisher Scientific), hydrated by dipping 20 times in each concentration of ethyl alcohol (100, 95, and 75%), and rinsed in distilled water several times. The slides were put into boiling citrate-based antigen retrieval solution and heated in a microwave oven for 15 minutes with 5- to 10-second sudden boiling at 20- to 30-second intervals. The heated slides were cooled down to room temperature in a water bath, rinsed with distilled water, and washed three times with phosphate-buffered saline (PBS) (10 mmol/L KH2PO4, 2 mmol/L NaH2PO4, 140 mmol/L NaCl, 40 mmol/L KCl, pH7.4). The sections were reacted with anti-p62C antibodies (diluted at 1:100 in PBS) and stained with fluorescein isothiocyanate-conjugated secondary antibody (Caltag, Burlingame, CA). Immunofluorescence was observed in an Olympus epifluorescence microscope BHT (Olympus Optical Co., Tokyo, Japan) using a ×20 or ×50 water immersion lens. Confocal microscopy was performed with a Zeiss LSM510 confocal laser-scanning microscope.
Protein Expression, Antibodies, and Western Blot Analysis
IMP-1 and Koc/IMP-3 cDNAs were inserted into pET28 vectors as described. 2 p62 and golgin-97 expression vectors, the latter encodes a Golgi autoantigen used as an unrelated control, 14 were constructed as previously described. 2 The expression vectors were transformed into Escherichia coli BL21 (DE3) cells and recombinant proteins were expressed by induction with IPTG for 4 hours. The recombinant proteins were affinity-purified on a Ni-NTA column and eluted with a 7 mol/L-urea-containing solution according to the manufacturer’s instructions (Qiagen, Santa Clarita, CA). Rabbit anti-p62C was made against a peptide containing the C-terminal 10 amino acids of p62 (PQGVASQRSK), which shares no amino acid homology with IMP-1 and Koc/IMP-3, and anti-golgin-97 was made against full-length golgin-97. Female New Zealand White rabbits were immunized with 100 to 250 μg of synthesized peptide or purified recombinant protein in Freund’s complete adjuvant, and boosted 1 month later with the same amount of peptide or protein in Freund’s incomplete adjuvant. Antisera were collected 10 days after the booster injection. One hundred ng of recombinant protein was run on each lane of 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% skim milk for 30 minutes, incubated with diluted rabbit anti-p62C (1:1500) for 1 hour, followed by horseradish peroxidase-conjugated secondary antibody (Caltag, Burlingame, CA). Reactivities were detected by the chemiluminescence method using enhanced chemiluminescence reagents (Amersham Life Science, Buckinghamshire, UK).
Antibody Absorption
Five hundred μg of recombinant proteins (p62 or golgin-97) were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes. The membranes containing only the recombinant protein were cut into small slices. The slices were blocked in 5% milk overnight, and divided into three parts. Five hundred μl of diluted anti-p62C antibody was incubated with the first part of these membrane slices for 8 hours, the absorbed supernatant recovered, and repeated absorption performed with the other two membrane sections in an identical manner. The absorbed antibody was kept in a −20°C freezer until use for immunofluorescent staining.
RNA from Liver Tissues
The method for RNA extraction was a modification of a method previously described. 15 Twenty sections of 5-μm thick paraffin-embedded liver specimens were used and areas were selected that contained at least 80% tumor hepatocytes as determined by examining adjacent H&E-stained sections. To avoid contamination, a separate razor blade was used to dissect out the tumor nodules of each liver specimen. The sections were transferred to 1.5-ml Eppendorf tubes, deparaffinated in three changes of 1.0 ml xylene (Fisher Scientific) and three washes of 1.0 ml ethanol. After brief drying, tissues were incubated with 300 ml of lysis buffer (10 mmol/L Tris-HCl, pH 8.0, 20 mmol/L ethylenediaminetetraacetic acid, 2% sodium dodecyl sulfate), and homogenized. The homogenized tissues were mixed with 15 μl of proteinase K solution (100 mg/ml) and incubated in 55°C water bath overnight. The solution was cooled to room temperature and 1 ml of Trizol reagent (Life Technologies, Inc., Gaithersburg, MD) was added. Total RNA was extracted according to the manufacturer’s suggestions. The RNA pellet was dissolved in 20 μl of distilled water and one-tenth aliquots were used for quantitation.
Poly(A)+ mRNAs from human fetal and adult livers were purchased from Clontech (Palo Alto, CA). Fetal liver RNA was reported by the manufacturer to have been extracted from a pool of 32 tissue specimens at age 18 to 24 weeks, whereas adult liver poly(A)+ RNA was extracted from a pool of two tissue specimens at age 15 and 35 years.
Reverse Transcription and Polymerase Chain Reaction (PCR)
Actin and p62 primers were synthesized as follows: actin forward primer AGGCCAACCGCGAGAAGATGACC, actin reverse primer GAAGTCCAGGGCGACGTAGCAC, p62FL forward primer 5′-TTGAATTCGCCATGGTGAACAAGCTTTACATCGGGAACC-3′, p62FL reverse primer 5′-TTTATGTCGACGGTGTTGGAAGGGCTACATT-3′, p62SL forward primer 5′-ATTGAACATGAAACAGGGACC-3, and p62SL reverse primer 5′-ACGTCTGGGCCTTCCGCAGG-3′. The p62FL primers were previously used for the amplification of the full-length coding region of p62 and generated a 1.7-kb PCR product. 2 The p62SL primers were designed to readily distinguish between p62 and IMP2 splice variants. The p62 cDNA does not contain the extra 129-bp sequence present in the longer splice variant IMP2 cDNA. 2,7 For p62-type and IMP2-type splice variants using the p62SL primers, the expected PCR products are 386 bp and 515 bp, respectively. Reverse transcription and PCR were performed as described with slight modifications. 16 Briefly, half μg of poly(A) + RNA or 1 μg of total RNA was reverse-transcribed in 20-μl volume containing 20 pmol oligo(dT)12–18 primer. Two μl of cDNA aliquot was pretreated with 5 U of RNase-free DNase (Boehringer Mannheim, Mannheim, Germany) at room temperature and heated at 95°C for 5 minutes. The reaction was then used for PCR in a 50-μl volume containing 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 0.01% (w/v) gelatin, and 0.2 μg of each pair of PCR primers. The PCR conditions used were 25 cycles with denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 2 minutes. The PCR products were run in 1 or 1.5% agarose gel and stained with ethidium bromide.
Results
p62 Is Expressed in Tumor Hepatocytes of HCC Nodules, but Not in Adjacent Nonmalignant Hepatocytes
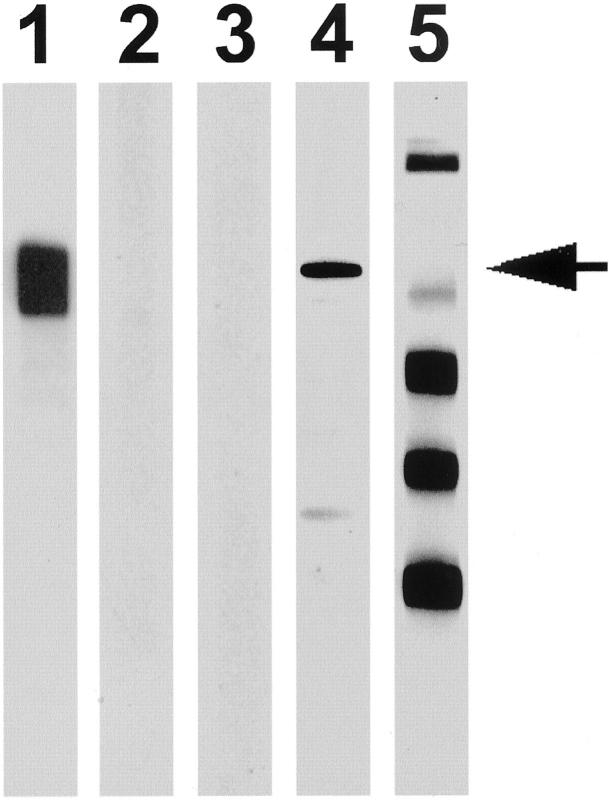
Specificity of anti-p62C raised against its C-terminal peptide was first tested using Western blots. Anti-p62C specifically recognized recombinant p62 protein (Figure 1 ▶ , lane 1), but had no cross-reactivity with recombinant IMP-1 and Koc/IMP-3 (lanes 2 to 3). Anti-p62C also reacted with native 62-kd protein in HepG2 cells with a weak reaction with a lower molecular size band that was unidentified (lane 4). It was not reactive with many other cellular antigens in the HepG2 cell extract as shown by the multiple reactivity of a lupus autoimmune serum (lane 5). Human HCC liver specimens were subsequently examined by immunohistochemical analysis. The cancer nodule from a liver specimen with a poorly differentiated carcinoma (Figure 2A) ▶ , uniformly showed strong reactivity to anti-p62C antibody (Figure 2, B and C) ▶ , but not to preimmune rabbit sera (data not shown). Nonmalignant liver epithelial cells adjacent to the HCC nodule and fibrous and connective tissue cells showed absent or barely detectable fluorescence so that the borders between tumor nodule and nontumor tissue could be clearly demarcated. The nuclei of tumor hepatocytes were stained with 4,6-diamidino-2-phenylindole (DAPI) (Figure 2D) ▶ to distinguish between nuclear and cytoplasmic domains. Comparison of signals in Figure 2, C and D ▶ , indicated that p62 was expressed mostly in the cytoplasm of tumor hepatocytes and visualized most clearly with confocal microscopy comparing negative contrast and immunohistochemistry (Figure 3) ▶ . The cytoplasmic localization of p62 protein in tumor hepatocytes was consistent with expression in cultured HEp-2 cells and with that of two homologous proteins (IMP-1 and IMP-3/Koc) in cultured NIH 3T3 cells as previously reported. 2,7 Anti-p62C antibody also recognized the cytoplasmic p62 protein in tumor hepatocytes of a well-differentiated carcinoma (Figure 4, A and B) ▶ . Antibody absorption experiments were performed to confirm the specificity of anti-p62C antibody. The anti-p62C antiserum preabsorbed with recombinant p62 protein lost reactivity to p62 protein (Figure 4C) ▶ , whereas the antiserum preabsorbed with control, irrelevant recombinant golgin-97 (a Golgi protein) retained reactivity (Figure 4D) ▶ . A total of 27 HCC liver specimens (20 from China and 7 from the United States) were examined using anti-p62C antibodies (Table 1) ▶ . Six of 20 Chinese specimens and 3 of 7 United States specimens (total of 33% in the combined groups) were shown to have p62 expressed in HCC nodules. These results were confirmed using anti-p62F antibody that was raised against the full-length p62 protein (data not shown). Statistically, there was no correlation of p62 expression with age, sex, and α-fetoprotein expression level (Table 1) ▶ .
Figure 1.
Specificity of anti-p62C antibodies in Western blot. Recombinant proteins of p62, IMP-1, and Koc/IMP-3 were expressed in E. coli and purified. Twenty ng of p62, IMP-1, and Koc/IMP-3 proteins were run on lanes 1, 2, and 3 in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and 100 μg of cell extract from HepG2 cells were run in each of lanes 4 and 5. The proteins were transferred onto nitrocellulose membranes. The membrane strips were reacted with anti-p62C (1:1000 dilution) in lanes 1 to 4 and with human autoantibody from serum of a patient with SLE autoimmune disease in lane 5 followed by HRPO-conjugated secondary antibody. Position of the 62-kd protein is marked by the arrow. Anti-p62C recognized a 62-kd band and showed a weak reaction with a 50-kd product from HepG2 cells. Anti-p62C was specific for p62, had no cross-reaction with IMP-1 and Koc/IMP-3, and did not react with other cellular antigens present in HepG2 cell extracts recognized by the lupus serum (lane 5).
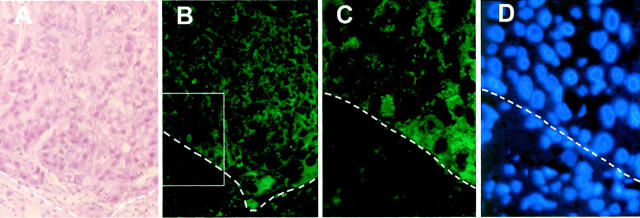
Figure 2.
p62 detection in the cytoplasm of tumor hepatocytes of a poorly differentiated HCC specimen (case 1). A: H&E staining showing HCC nodule of case 1 (Table 1) ▶ . B: Immunohistochemical analysis for p62. The section adjacent to that used for H&E staining was antigen-retrieved in a citrate-based solution, and subjected to immunofluorescent staining with the anti-p62C antibody (1:100 dilution), followed by fluorescein isothiocyanate-conjugated secondary antibody. Original magnification, ×200. C: Enlargement (original magnification, ×500) of the fluorescent area boxed in B to show that cytoplasm of cancer cells were positive and not nuclei. D: Counterstaining with DAPI showing the staining of nuclei in the same section as in C. The demarcation between tumor nodule and nontumor tissue is outlined by dashed lines. C and D represent enlargement of area boxed in B.
Figure 3.
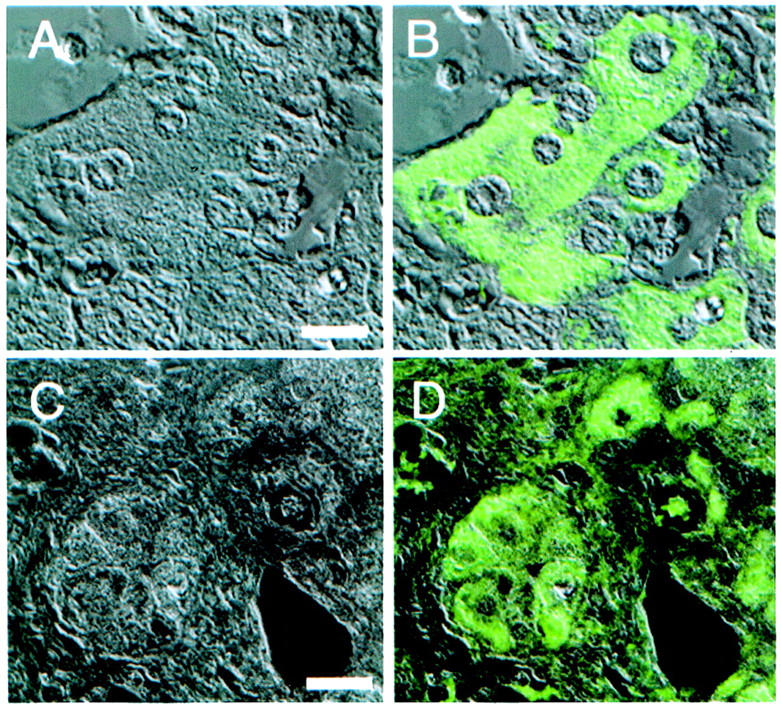
Subcellular localization of p62 in hepatic carcinoma. Paraffin-embedded sections of hepatic tissue were stained with anti-p62 antibody and subcellular distribution was examined by confocal microscopy. A and C show the Nomarski contrast pictures and cytoplasmic p62 is shown as an overlay in B and D. The immunofluorescent B and D show different areas of cancer nodules illustrating both the intensity of fluorescent signals for p62 and its distribution in cell cytoplasm. Scale bars: 14 μm (A and B), 20 μm (C and D).
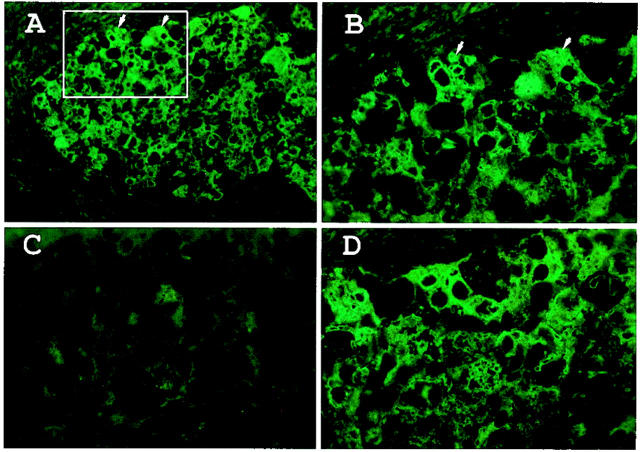
Figure 4.
Anti-p62C absorbed with recombinant p62 or with golgin-97, a Golgi autoantigen used as a control, in a well-differentiated HCC specimen (case 13). A: p62 was detected in the cytoplasm of cells in the cancer nodule by immunohistochemistry. B: Enlargement (original magnification, ×500) of the area boxed in A. C: Anti-p62C antiserum was absorbed with recombinant p62 protein. The fluorescent signal was primarily removed whereas staining was not removed by absorption with recombinant golgin-97 (D). Arrows in A and B point to cells in the same area.
Nine liver specimens from normal adult donors were also examined for p62 expression. No staining with either anti-p62F or anti-p62C antibodies was observed in all cases (data not shown). Taken together, these data demonstrated that p62 gene is down-regulated or not expressed in differentiated hepatocytes of adult normal livers but aberrantly expressed in the tumor hepatocytes of one-third of HCC patients investigated.
p62 Gene Is Expressed in Epithelial Cells of Bile Ducts in CCC
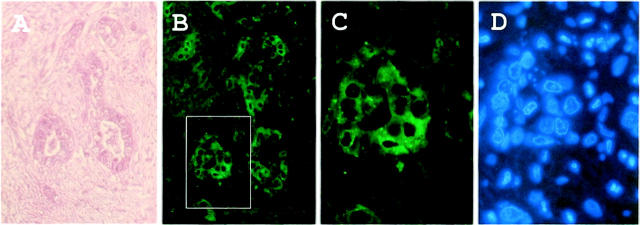
To examine whether p62 is expressed in other liver cancers, we performed immunohistochemical analysis on two available liver specimens with CCC using anti-p62C antibodies. Proliferating bile ducts were abundant in the specimen depicted in Figure 5A ▶ , and as shown in Figure 5, B to D ▶ , p62 was expressed in the cytoplasm of epithelial cells of bile ducts (case 28 in Table 1 ▶ ). However, p62 was not detectable in the epithelial cells of bile ducts in case 29.
Figure 5.
p62 expression in epithelial cells of proliferating bile ducts of CCC. A: H&E staining showing an area of proliferating bile ducts of case 28 (Table 1) ▶ . B: Immunofluorescent staining with anti-p62C as described in Figure 2B ▶ (original magnification, ×200). C: High magnification (original magnification, ×500) of boxed area in B. D: Staining with DAPI of the same section as in C.
Expression Patterns of p62 in Liver Cirrhosis
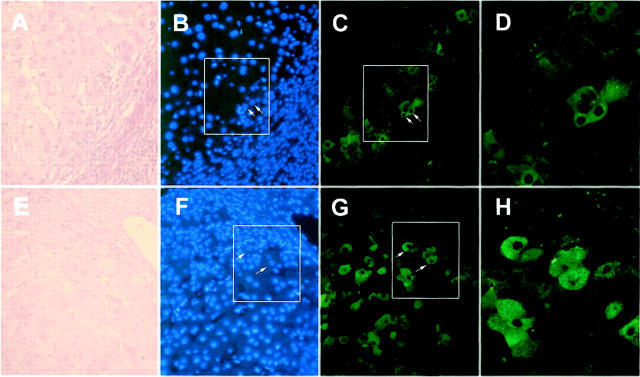
Liver cirrhosis is considered a high-risk precursor for development of HCC. Twenty-three specimens from patients with liver cirrhosis were examined for the expression of p62. As shown in the upper panels of Figure 6 ▶ , cytoplasmic staining was observed in ∼5% of hepatocytes in the periphery of cirrhotic nodules. This pattern of expression was observed in eight cases and designated as low focal positivity (Table 2) ▶ . The focal expression pattern of these cirrhotic livers was in clear contrast to the uniform expression of p62 in HCC (Figures 2 and 4) ▶ ▶ . Three other cirrhotic liver specimens displayed p62 expression not only in hepatocytes in the periphery of nodules but also in hepatocytes located in the centers of cirrhotic nodules that were not observed in the low focal positive cases (Figure 6 ▶ , bottom panels). The level of p62 expression varied from high to low intensity in different cells and the total number of p62-expressing cells varied from 20 to 50%. This expression pattern in the three cirrhotic livers was designated as high focal positivity (Table 2) ▶ .
Figure 6.
p62 expression in liver cirrhosis. Top: Indicated are one type of p62 expression in ∼5% hepatocytes mostly located at the periphery of a cirrhotic nodule from a patient with liver cirrhosis (low focal positivity), whereas bottom panels showed the other type of p62 expression in 20 to 50% of hepatocytes that can be located at the periphery or in other regions of cirrhotic nodules as shown here (high focal positivity). A and E: H&E staining showing cirrhotic nodules (original magnification, ×200). C and G: Immunofluorescent staining using anti-p62C (original magnification, ×200). B and F: DAPI staining of the same areas as in C and G, respectively. D and H: Enlargement (original magnification, ×500) of the areas boxed in B and F, respectively. Arrows in C and G point to cells showing p62 in cell cytoplasm.
Table 2.
Immunohistochemistry of p62 on Liver Tissue
| Total number of cases | Immunohistochemistry | |||
|---|---|---|---|---|
| Uniform fluorescence of all nodular cells | High focal positive fluorescence | Low focal positive fluorescence | ||
| HCC | 27 | 9 | 0 | 0 |
| CCC | 2 | 1 | 0 | 0 |
| Liver cirrhosis | 23 | 0 | 3 | 8 |
| Normal liver | 9 | 0 | 0 | 0 |
p62 in Fetal Livers
To determine whether the p62 gene was developmentally regulated, five human liver specimens at different fetal stages (days 50 to 125) were examined for the expression of p62 gene at the protein level. As shown in Figure ▶ 7, A to D, p62 protein was detectable in hepatocytes in a day 50 fetal liver. Similar to the day 50 fetus, p62 was expressed in the other four fetal livers ranging from day 50 to day 125. In contrast, p62 protein was undetectable in nine normal adult livers (Table 2) ▶ . These data indicated that p62 gene is expressed in human fetal livers and seems to be down-regulated in adult hepatocytes during development.
p62 Transcripts in Liver
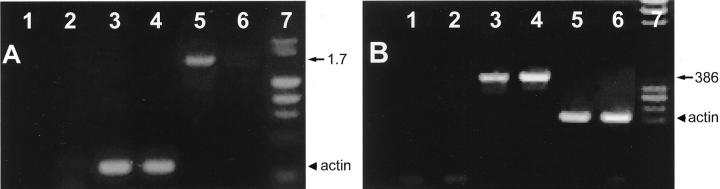
To investigate whether the p62 gene is regulated at the transcriptional level, RT-PCR was performed using an equal amount of poly(A)+ mRNA from either human fetal or adult livers. A 1.7-kb PCR product containing the open reading frame of p62/IMP-2 cDNA from fetal livers could be abundantly amplified with p62FL primers (Figure 8 ▶ , lane 5), whereas the product from adult livers was barely detected (lane 6). As a control, an actin fragment amplified from the same batch of cDNA showed no difference in amount of cDNA (Figure 8A ▶ , lanes 3 and 4). The 1.7-kb PCR DNA product from fetal livers was subcloned and five recombinant clones were identified. The nucleotide sequences of all five recombinant clones were identical to those of original p62 cDNA, 2 a splice variant that does not contain the extra 129-bp nucleotide present in IMP-2 cDNA, 7 suggesting that the shorter p62 rather than the longer IMP-2 form was selectively expressed in the fetus.
Figure 8.
Detection of p62 transcripts in livers. A: Expression of p62 gene was examined in human fetal and adult livers. Five hundred ng of poly(A)+ mRNA from either human fetal or adult livers were reverse-transcribed into cDNA. No template (lanes 1 and 2), one-tenth of cDNA aliquots from fetal livers (lanes 3 and 5), and from adult livers (lanes 4 and 6) were amplified by using actin primers (lanes 1, 3, and 4), or p62FL primers (lanes 2, 5, and 6) by PCR. One-third of each PCR product was run on a 1% agarose gel and stained with ethidium bromide. Lane 7 was a DNA marker. The position of amplified p62 (1.7 kb) and actin DNA fragments are indicated with an arrow and an arrowhead, respectively. B: Expression of p62 gene in HCC specimens. Total RNA was extracted from paraffin-embedded normal adult liver (lanes 1 and 5), case 3 (lane 2, p62-negative immunohistochemical staining in Table 1 ▶ ), case 9 (lanes 3 and 6, p62-positive immunohistochemical staining in Table 1 ▶ ), and case 25 (lane 4, p62-positive immunohistochemical staining in Table 1 ▶ ). Procedures of reverse transcription and PCR were similar to those described in A except usage of 1 μg of total RNA. p62SL primers were used in lanes 1 to 4 and actin primers in lanes 5 to 6. PCR products were run on a 1.5% agarose gel. Lane 7 was a DNA marker. Arrow indicates 386-bp PCR fragment of p62, whereas arrowhead represents actin products.
We further performed RT-PCR using total RNA extracted from paraffin-embedded tissues. Using the p62SL primers, a 386-bp p62 PCR product rather than the 515-bp IMP-2 product was amplified from each of two immunohistochemically p62-positive HCC tissues (Figure 8B ▶ , lanes 3 and 4). No PCR fragment was detected from a normal adult liver and a p62-negative HCC tissue (lanes 1 and 2, respectively). Thus, the data from RT-PCR were consistent with those from immunohistochemical analysis and also indicated that p62 rather than IMP-2 is the dominant splice form transcribed in HCC tissues.
Discussion
We recently found that p62, an insulin-like growth factor II ( IGF-II) mRNA-binding protein elicited a humoral immune response in 21% of HCC patients. 2 The observations reported here show that p62, a splice variant of IMP-2, was a developmentally regulated gene expressed in fetal liver and not in adult liver and aberrantly expressed in HCC. The frequency of p62 expression in HCC tissue (33%) is higher than the frequency of humoral immune response (21%), but this is not surprising because many factors modulate immune responses. The lower frequency of autoimmune responses to cancer-related antigens has also been observed in melanoma where it has been reported that only one-half of patients with melanomas positive for NY-ESO-1 antigen mounted circulating autoantibodies. 17
The human IGF-II gene is generated from several mRNAs with different 5′UTRs each associated with distinct promoters P1 to P4, but all having identical coding regions and 3′UTRs. 18 Promoters P2, P3, and P4 are active in fetal tissues and transformed cells where they generate mature transcripts of 5.0, 6.0, and 4.8 kb, respectively, whereas promoter P1 is only used in adult liver giving rise to a transcript of 5.3 kb. Cariani and colleagues 19 found a 40- to 100-fold increase in IGF-II mRNA in 9 of 40 liver cancer samples and this was primarily related to expression of two fetal (6.0 and 5.0 kb) species. It would be of interest to know whether this remarkable increase in fetal IGF-II mRNA in HCC is in some way modulated by expression of the p62 form of RNA binding protein.
Carcinogenesis is a multistep process involving not only genetic mutations in oncogenes and tumor-suppressor genes but also other complex factors conferring tumorigenic traits. 20,21 It is possible that one of these traits could be related to overexpression or increased stability of a growth factor such as IGF-II. The fact that we found p62 expression in some cells in cirrhotic nodules might indicate that p62 is associated with hyperproliferating cells. At least two lines of evidence indicate that the periphery and other areas of cirrhotic nodules are regions of proliferative activity. Proliferating cell nuclear antigen has been shown to be expressed in these regions as well as hepatocyte growth factor. 22-25 p62 expression pattern was not present to the extent found in HCC in which all cells exhibited cytoplasmic p62 immunoreactivity. The question arises whether this expression of p62 in a number of liver cirrhosis patients might be of prognostic value because this might be a marker of subsequent malignant transformation. However, it was not possible to do follow-up studies to determine whether any of the three patients with high focal expression of p62 might have ultimately developed HCC, because of constraints in revealing patient identity because of the fact that these studies were restricted to archival specimens and knowledge of patient identity was prohibited.
The most widely studied humoral autoimmune response in cancer has been related to the p53 tumor-suppressor protein. Autoantibodies to p53 have been found in general in ∼20% of patients with cancer and have been shown to be primarily associated with p53 accumulation in the tumor mostly related to p53 gene missense mutations. 26 Although the sensitivity was low, the specificity of anti-p53 for cancer was ∼96%. It has been reported that in individuals who were at high risk for cancer such as workers exposed to environmental mutagens or heavy smokers, the presence of anti-p53 might be a predictive factor in cancer development. 26
There is growing evidence that a number of intracellular proteins with RNA-binding motifs are associated with cancer. p62 belongs to a family of proteins that have two types of RNA-binding motifs, the RNA recognition motif and the heterogeneous nuclear RNP-K homology or KH domain. 27,28 Mueller-Pillasch and colleagues 12 have shown that a highly homologous protein to p62 called Koc is preferentially expressed in pancreatic and other cancers. Ross and colleagues 10 have shown that a murine protein, also highly homologous to p62 binds to c-myc mRNA. This murine protein binds to a sequence in the coding region of c-myc mRNA called the instability determinant and binding of the protein to this region of c-myc mRNA shields it from endonucleolytic cleavage. Of further interest is the fact that the human homologs of this group of proteins have been demonstrated by Nielsen and colleagues 7 to bind in a highly specific manner to leader 3 fetal mRNA encoding IGF-II. This fetal IGF-II mRNA has a specific 5′ sequence that is bound by these proteins. The importance of this finding with relationship to cancer is the fact that IGF-II has been shown to be overexpressed in human cancer 19,29-31 and mice transgenic for IGF-II have been shown to develop malignancy and organomegaly of different tissues. 32,33 In a SV40 large T-antigen transgene model, pancreatic tumors were noted to be associated with IGF-II mRNA expression. 34 In a model of HCC in hepatitis B virus envelope transgenic mice, 35 examination for abnormalities in structure and expression of a large panel of oncogenes and tumor suppressor genes, including ras, myc, fos, abl, src, Rb, and p53, only overexpression of IGF-II was found.
Aberrant expression of other RNA-binding proteins in human cancer has been increasingly recognized. The Hu proteins are RNA-binding proteins that contain three RNA recognition motifs. 36-39 These proteins have been shown to bind the AU-rich regions of 3′UTRs in mRNAs encoding early response genes such as c-myc, c-fos, and GM-CSF. 39,40 The binding of Hu proteins to the AU-rich resulted in the stabilization and increased translatability of the mRNA transcripts. 40-42 The Hu proteins are expressed in cancers from lung, breast, ovary, and testis where the expression is aberrant because the Hu proteins are primarily neuronal proteins. Certain cancer patients with aberrant expression of Hu proteins have sensory neuropathies called paraneoplastic neurological disorders and produce autoantibodies to Hu. 37 In a similar vein, the nucleocytoplasmic shuttling protein hnRNP A2/B1, encompassing N-terminal RNA recognition motifs, exhibits a characteristic spatiotemporal expression during mammalian lung development, 43 and is also expressed in large amounts in lung cancer. 44 Therefore, several families of RNA-binding proteins exhibit an oncofetal behavior to accommodate a state of increased cellular proliferation. The role of aberrantly regulated RNA-binding proteins in promoting tumorigenesis could be at the posttranscriptional level by way of modulating the stability or function of certain crucial mRNAs.
From the perspective of the production of human autoantibody and autoimmunity, the present report on the enhanced expression of p62 in 33% of liver cancer nodules examined, together with our early observation that some patients developed novel autoantibodies—including anti-p62—during the transition from chronic liver diseases to HCC, 2 suggest that anti-p62 antibodies may be produced in response to the enhanced expression of p62 in cancer cells. It is not clear at the present time whether the aberrant expression of a fetal protein such as p62 in the adult organism is by itself sufficient to induce an autoimmune response or whether other additional factors are required.
Figure 7.
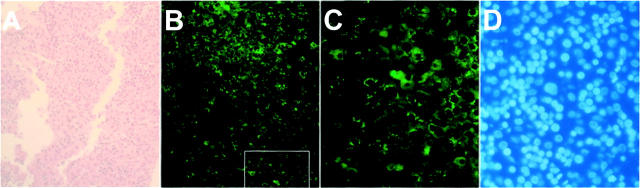
p62 expression in fetal human liver. A 50-day fetal liver was subject to immunohistochemical analysis using anti-p62C. A: H&E staining. B: p62 immunohistology. C: High magnification (original magnification, ×500) showing the area boxed in B. D: DAPI staining of the same area as in C.
Acknowledgments
We thank the histology laboratory of The Scripps Research Institute for sectioning tissue specimens and the Stein DNA Core Facility for providing help.
Footnotes
Address reprint requests to Dr. Eng M. Tan, Department of Molecular and Experimental Medicine, The Scripps Research Institute, 10550 North Torrey Pines Rd., La Jolla, CA 92130. E-mail: emtan@scripps.edu.
Supported by National Institutes of Health grant CA56956 and the Danish Cancer Society.
This is publication 13911-MEM from The Scripps Research Institute.
References
- 1.Berk PD, Okuda K, Wands JR: Hepatocellular carcinoma. Semin Liver Dis 1999, 19:233-311 [DOI] [PubMed] [Google Scholar]
- 2.Zhang JY, Chan EKL, Peng XX, Tan EM: A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med 1999, 189:1101-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Query CC, Bentley RC, Keene D: A common RNA recognition motif identified within a defined U1 RNA-binding domain of the 70K U1 snRNP protein. Cell 1989, 57:89-101 [DOI] [PubMed] [Google Scholar]
- 4.Siomi H, Matunis MJ, Michael WM, Dreyfuss G: The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res 1993, 21:1193-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siomi H, Choi M, Siomi MC, Nussbaum RL, Dreyfuss G: Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell 1994, 77:33-39 [DOI] [PubMed] [Google Scholar]
- 6.Adinolfi S, Bagni C, Castiglione Morelli MA, Fraternali F, Musco G, Pastore A: Novel RNA-binding motif: the KH module. Biopolymers 1999, 51:153-164 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC: A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol 1999, 19:1262-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshler JO, Highett MI, Abramson T, Schnapp BJ: A highly conserved RNA-binding protein for cytoplasmic mRNA localization in vertebrates. Curr Biol 1998, 8:489-496 [DOI] [PubMed] [Google Scholar]
- 9.Havin L, Git A, Elisha Z, Oberman F, Yaniv K, Schwartz SP, Standart N, Yisraeli JK: RNA-binding protein conserved in both microtubule- and microfilament-based RNA localization. Genes Dev 1998, 12:1593-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH: Characterization of a beta-actin mRNA zipcode-binding protein. Mol Cell Biol 1997, 17:2158-2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle GAR, Betz NA, Leeds PF, Fleisig AJ, Prokipcak RD, Ross J: The c-myc coding region determinant-binding protein: a member of a family of KH domain RNA-binding proteins. Nucleic Acids Res 1998, 26:5036-5044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller-Pillasch F, Lacher U, Wallrapp C, Micha A, Zimmerhack F, Hameister S, Varga G, Friess H, Bucher M, Geger HG, Vila MR, Adler G, Gress M: Cloning of a gene highly overexpressed in cancer coding for a novel KH-domain containing protein. Oncogene 1997, 14:2729-2733 [DOI] [PubMed] [Google Scholar]
- 13.Edmondson HA, Steiner PE: Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954, 7:462-503 [DOI] [PubMed] [Google Scholar]
- 14.Griffith KJ, Chan EKL, Lung CC, Hamel JC, Gao X, Miyachi K, Fritzler MJ: Molecular cloning of a novel 97-Kd Golgi complex autoantigen associated with Sjogren’s syndrome. Arthritis Rheum 1997, 40:1693-1702 [DOI] [PubMed] [Google Scholar]
- 15.Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T: Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol 1998, 153:1807-1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu M, Johnson RR, Iatrou K: Trans-activation of a cell housekeeping gene promoter by the IE1 gene product of baculovirus. Virology 1996, 218:103-113 [DOI] [PubMed] [Google Scholar]
- 17.Stockert E, Jager E, Chen Y, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ: A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med 1998, 187:1349-1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardone G, Romano M, Calabro A, Pedone PV, de Sio I, Persico M, Budillon G, Bruni CB, Riccio A, Zarrilli R: Activation of fetal promoters of insulin-like growth factors II gene in hepatitis C virus-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Hepatology 1996, 23:1304-1312 [DOI] [PubMed] [Google Scholar]
- 19.Cariani E, Lasserre C, Seruin D, Hamelin B, Kemeny F, Franco D, Czech MP, Ullrich A, Brechot C: Differential expression of insulin-like growth factor II mRNA in human primary liver cancers, benign liver tumors, and liver cirrhosis. Cancer Res 1988, 48:6844-6849 [PubMed] [Google Scholar]
- 20.Vogelstein B, Kinzler KW: The multistep nature of cancer. Trends Genet 1993, 9:138-141 [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000, 100:57-70 [DOI] [PubMed] [Google Scholar]
- 22.Kawakita N, Seki S, Sakaguchi H, Yanai A, Kuroki T, Mizoguchi Y, Kobayashi K, Monna T: Analysis of proliferating hepatocytes using a monoclonal antibody against proliferating cell nuclear antigen/cyclin in embedded tissues from various liver diseases fixed in formaldehyde. Am J Pathol 1992, 140:513-520 [PMC free article] [PubMed] [Google Scholar]
- 23.Ojanguren I, Ariza A, Llatjos M, Castella E, Mate JL, Navas-Palacios JJ: Proliferating cell nuclear antigen expression in normal, regenerative, and neoplastic liver: a fine-needle aspiration cytology and biopsy study. Hum Pathol 1993, 24:905-908 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Nawa K, Ichihara A: Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA 1986, 83:6489-6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi K, Nalesnik MA, Michalapoulos GK: Hepatocyte growth factor mRNA in human liver cirrhosis as evidenced by in situ hybridization. Scan J Gastroenterol 1996, 31:921-927 [DOI] [PubMed] [Google Scholar]
- 26.Soussi T: p53 antibodies in the sera of patients with various types of cancer: a review. Cancer Res 2000, 60:1777-1788 [PubMed] [Google Scholar]
- 27.Keene JD: RNA recognition by autoantigens and autoantibodies. Mol Biol Rep 1997, 23:173-181 [DOI] [PubMed] [Google Scholar]
- 28.Siomi H, Dreyfuss G: RNA-binding proteins as regulators of gene expression. Curr Opin Genet Dev 1997, 7:345-353 [DOI] [PubMed] [Google Scholar]
- 29.Reeve AE, Eccles MR, Wilkins RJ, Bell GI, Millow LJ: Expression of insulin-like growth factor-II transcripts in Wilms’ tumor. Nature 1985, 317:258-259 [DOI] [PubMed] [Google Scholar]
- 30.Scott J, Cowell J, Robertson ME, Priestley LM, Wadey R, Hopkins B, Pritchard J, Bell GI, Rall LB, Graham CF: Insulin-like growth factor-II gene expression in Wilms’ tumor and embryonic tissues. Nature 1985, 317:260-263 [DOI] [PubMed] [Google Scholar]
- 31.Osborne CK, Coronado EB, Kitten LJ, Arteaga CI, Fuqua SA, Ramaharma K: Insulin-like growth factor-II: a potential autocrine/paracrine growth factor for human breast cancer acting via the IGF-I receptor. Mol Endocrinol 1989, 3:1701-1709 [DOI] [PubMed] [Google Scholar]
- 32.Rogler CE, Yanng D, Rossetti L, Donohoe J, Alr E, Chang CJ, Rosenfeld R, Neely K, Hintz R: Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem 1994, 269:13779-13784 [PubMed] [Google Scholar]
- 33.Bates P, Fisher R, Ward A, Richardson L, Hill DJ, Graham CF: Mammary cancer in transgenic mice expression insulin-like growth factor II (IGF-II). Br J Cancer 1995, 72:1189-1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christofori G, Naik P, Hanahan D: A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 1994, 369:414-417 [DOI] [PubMed] [Google Scholar]
- 35.Pasquinelli C, Bhavani K, Chisari F: Multiple oncogenes and tumor suppressor genes are structurally and functionally intact during hepatocarcinogenesis in hepatitis B transgenic mice. Cancer Res 1992, 52:2823-2829 [PubMed] [Google Scholar]
- 36.Antic D, Keene JD: Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am J Hum Genet 1997, 61:273-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalmau J, Graus F, Rosenblum MK, Posner JB: Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuropathy. Medicine 1992, 71:59-72 [DOI] [PubMed] [Google Scholar]
- 38.Levine TD, Gao F, King PH, Andrews LG, Keene JD: Hel-N1: an autoimmune RNA-binding protein with specificity for 3′ uridylate-rich untranslated regions of growth factor mRNAs. Mol Cell Biol 1993, 13:3494-3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H: Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem 1996, 271:8144-8151 [DOI] [PubMed] [Google Scholar]
- 40.Fan CX, Steitz JA: Overexpression of the Hu R, a nuclear cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 1998, 17:3448-3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng SS, Chen CY, Xu N, Shyn AB: RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J 1998, 17:3461-3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keene JD: Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc Natl Acad Sci USA 1999, 96:5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montuenga LM, Zhou J, Avis I, Vos M, Martinez A, Cuttitta F, Treston AM, Sunday M, Mulshine JL: Expression of heterogeneous nuclear ribonucleoprotein A2/B1 changes with critical stages of mammalian lung development. Am J Respir Cell Mol Biol 1998, 19:554-562 [DOI] [PubMed] [Google Scholar]
- 44.Tockman MS, Mulshine JL, Piantadosi S, Erozan YS, Gupta PK, Ruckdeschel JC, Taylor PR, Zhukov T, Zhou WH, Qiao YL, Yao SX: Prospective detection of preclinical lung cancer: results from two studies of heterogeneous nuclear ribonucleoprotein A2/B1 overexpression. Clin Cancer Res 1997, 3:2237-2246 [PubMed] [Google Scholar]