Abstract
NPHS1 has recently been identified as the gene whose mutations cause congenital nephrotic syndrome of the Finnish type. The respective gene product nephrin is a transmembrane protein expressed in glomerular podocytes and primarily localized to the glomerular slit diaphragm. This interpodocyte junction functions in the glomerular filtration by restricting the passage of plasma proteins into the urinary space in a size-selective manner. The functional role of nephrin in this filtration process is so far not very well understood. In this study, we show that nephrin associates in an oligomerized form with signaling microdomains, also known as lipid rafts, and that these localize to the slit diaphragm. We also show that the nephrin-containing rafts can be immunoisolated with the 27A antibody recognizing a podocyte-specific 9-O-acetylated GD3 ganglioside. In a previous study it has been shown that the in vivo injection of this antibody leads to morphological changes of the filtration slits resembling foot process effacement. Here, we report that, in this model of foot process effacement, nephrin dislocates to the apical pole of the narrowed filtration slits and also that it is tyrosine phosphorylated. We suggest that lipid rafts are important in the spatial organization of the glomerular slit diaphragm under physiological and pathological conditions.
One of the main functions of the kidney is formation of the primary urine in the glomerulus by plasma ultrafiltration. The structural correlate of ultrafiltration is the glomerular capillary wall with its distinctly layered filtration barrier. It consists of the fenestrated vascular endothelium, the glomerular basement membrane, and a layer of morphologically highly specialized epithelial cells, podocytes. Podocytes form a tight network of multiple interdigitating cellular extensions, called foot processes, which are bridged by so-called “slit diaphragms.” 1 The filtration barrier is freely permeable for water, small solutes, and ions but does not allow passage for proteins larger than albumin and other large molecules. The charge selectivity in this process has been attributed to the glomerular basement membrane whereas the slit diaphragm supposedly plays a major role in the size selectivity. 2,3
The importance of the filtration barrier is reflected by a number of human diseases in which the filtration barrier function is disrupted resulting in the loss of plasma proteins into the urine. Patients with persistent proteinuria ultimately develop a nephrotic syndrome with a variety of symptoms including edema, hypoalbuminemia, and hyperlipidemia. 4
Because of the lack of precise molecular targets, the filtration barrier, and the slit diaphragm in particular, has not been easy to approach experimentally. This has, however, changed remarkably by the identification of NPHS1 as the gene responsible for the massive proteinuria in patients with congenital nephrotic syndrome of the Finnish type. 5 NPHS1 encodes for nephrin, a transmembrane protein of the immunoglobulin superfamily, expressed particularly in glomerular podocytes. It was subsequently localized primarily to the slit diaphragm by immunoelectron microscopy. 6-8 Together with the fact that congenital nephrotic syndrome of the Finnish type patients lacking functional nephrin fail to develop intact slit diaphragms, these findings have led to the conclusion that nephrin is a key component of the slit diaphragm. 9
The function of nephrin at the slit diaphragm is so far not very well understood. Based on its structure, nephrin has been proposed to oligomerize in a homophilic manner between neighboring foot processes. 7 Furthermore, the existence of tyrosine residues in the intracellular domain implicates a role of nephrin in signal transduction events. But experimental data are lacking. Likewise the involvement of nephrin in mechanisms leading to foot process effacement, the stereotypical morphology in proteinuric states, has not been addressed in a detailed manner so far. In rat models, these pathological events can be induced very rapidly. For instance, perfusion of the kidney with polycations such as protamine sulfate can induce foot process effacement including the conversion of slit diaphragms into tight junctions and proteinuria within minutes. 10 The slit diaphragms have therefore been proposed to represent very dynamic structures within the podocyte plasma membranes. 11,12
As dynamic assemblies in cell membranes, the so-called lipid rafts have gained great significance in recent years. 13-15 Lipid rafts are enriched in glycosphingolipids, cholesterol, GPI-anchored proteins, and a variety of signaling molecules. By compartmentalizing cell membranes, they function in a variety of cell biological processes, such as exocytosis, endocytosis, signal transduction, and cell adhesion.
In this study, we addressed the question whether the slit diaphragm as a specialized membrane domain may be organized by lipid rafts. To ensure in vivo relevance of the data, whole rat kidneys or isolated rat glomeruli from normal control rats and from rats with foot process effacement induced by intravenous 27A IgG injection, 16 were used in all experiments.
Materials and Methods
Animals
In all experiments, female Sprague-Dawley rats (weighing 120 to 170 g) were used. They were provided by the Animal Facilities of the European Molecular Biology Laboratory, Heidelberg, Germany; the University of Heidelberg, Heidelberg, Germany; and the University of Helsinki, Helsinki, Finland. All experimental studies were approved by the respective ethical committees.
Preparation of Total Glomerular Membranes and TX-100 Flotation Experiments from Isolated Rat Glomeruli
Glomeruli from kidneys taken from eight rats were isolated as previously described. 16 After pelleting, they were taken up in 3 ml of ice-cold homogenization buffer (20 mmol/L Tris-HCl, pH 7.4, 50 mmol/L NaCl plus the proteinase inhibitors chymostatin, leupeptin, anti-pain, and pepstatin), resuspended 20 times with a 22-gauge needle and homogenized in a Dounce homogenizer on ice. Pure glomerular membranes were prepared from the homogenate by density gradient centrifugation as described. 17 After centrifugation for 4 hours (24,000 rpm, 4°C, SW40 rotor; Beckman, Fullerton, CA), the light membrane fraction (500 μl) from the 5%/30% interface from the 5%/30%/40% Optiprep (Nycomed, Oslo, Norway) step gradient was collected and used for detergent extraction. For this, 250 μl was extracted by addition of 250 μl of 0.4% TX-100 in homogenization buffer on ice for 30 minutes. The TX-100 extract was then split into two TLS-55 tubes (Beckman), adjusted to 40% Optiprep, overlaid with 1.25 ml 30% and 200 μl 0% Optiprep containing 0.2% TX-100 and centrifuged for 2 hours (55,000 rpm, 4°C, TLS 55 rotor; Beckman). Six fractions (each 350 μl) were collected from top to the bottom. For further pelleting experiments, the fractions were spun in a TLA-100 ultracentrifuge (Beckman) in a TLA-100 rotor for 20 minutes at 80,000 rpm at 4°C (Figure 1B) ▶ , or the fractions were precipitated directly with 10% trichloroacetic acid and taken up in sample buffer. Equal volumes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting. The same protocol was also used for cultured mouse podocytes 18 transiently transfected with the Fugene-6 transfection method (Roche, Mannheim, Germany) with a cDNA construct containing rat nephrin. 19 Cholesterol depletion was performed by pretreating the total glomerular membranes with 0.2% saponin (Sigma, St. Louis, MO) for 10 minutes on ice before TX-100 extraction. 20
Figure 1.
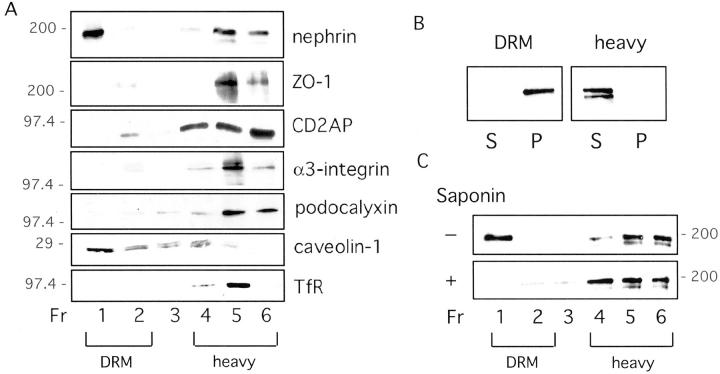
Nephrin is associated with DRM fractions in a cholesterol-dependent manner. A: Rat glomerular membranes were extracted with TX-100 and fractionated in an Optiprep step gradient. Immunoblotting with antibodies against podocyte proteins reveals enrichment of nephrin in DRM fractions. 1,2 Molecular weight markers are shown in kd. B: DRM fraction and heavy fraction were pelleted at 80,000 × g for 20 minutes. Only DRM-associated nephrin is found in the pellet demonstrating its detergent-resistance. C: Cholesterol depletion of glomerular membranes with 0.2% saponin releases nephrin from the DRM fractions in the TX-100 flotation gradients.
Sedimentation Velocity Centrifugation
The first [detergent-resistant membrane (DRM)] containing fraction and the fifth (soluble) fractions of the TX-100 flotation gradients were adjusted to 0.4% SDS and loaded onto a linear 5 to 30% sucrose gradient in homogenization buffer containing 0.2% TX-100 and 0.4% SDS. 21 After centrifugation in a SW60 rotor (Beckman) for 16 hours at 40,000 rpm, 500-μl fractions were collected from the top. For Western blot analysis, fractions were precipitated with trichloroacetic acid, resuspended in sample buffer, and separated by SDS-PAGE.
Endoglucosidase H (Endo H) Digestion
One hundred fifty μl of incubation buffer (50 mmol/L phosphate buffer, pH 5 to 6) was added to either 50 μl of the soluble gradient fraction or 50 μl of total glomerular membranes and boiled for 5 minutes. After cooling down to room temperature, 300 μl of 0.75% Nonidet P-40 was added, boiled again for 1 minute, 100 mU Endo H (Roche) was added to the cooled sample and incubated overnight at 37°C in the presence of chymostatin, leupeptin, anti-pain, and pepstatin. Digested proteins were trichloroacetic acid-precipitated with trichloroacetic acid and analyzed by SDS-PAGE and subsequent Western blotting.
Immunoblotting
The following primary antibodies were used: rabbit polyclonal anti-rat nephrin antiserum with or without Protein A purification as described previously, 19 rabbit polyclonal anti-human ZO-1 (Zymed Laboratories, San Francisco, CA), rabbit polyclonal anti-mouse CD2AP (courtesy of Dr. Andrey Shaw, Washington University, St. Louis, MO), rabbit polyclonal anti-human podocalyxin, 22 rabbit polyclonal anti-rat α3-integrin (Chemicon Int., Temecula, CA), rabbit polyclonal anti-human caveolin 1 (Santa Cruz Biotechnology, San Diego, CA), mouse monoclonal anti-human transferrin receptor (Zymed Laboratories), and mouse monoclonal anti-phosphotyrosine antibody PY20 (Transduction Laboratories, Lexington, KY). All antibodies have been shown to react with the respective rat proteins. The horseradish peroxidase-conjugated secondary antibodies were against rabbit and mouse (Bio-Rad Laboratories, Hercules, CA). Proteins separated by SDS-PAGE were transferred to nitrocellulose filters using a semi-dry blotting system (Amersham Pharmacia, Uppsala, Sweden) and after blocking and incubation with the respective antibodies detected by enhanced chemiluminescence (Amersham Pharmacia).
In Vivo Injection of Antibodies, Immunohistochemistry, and Immunoprecipitation
Three mg of either the mouse monoclonal 27A IgG3 or control mouse IgG3 (Sigma Chemical Co.) were intravenously injected into rats via the tail vein as described previously. 23 At 1 hour after injection, kidneys were removed and processed for immunohistochemistry, immunoprecipitation, or immunoelectron microscopy.
For immunohistochemistry, small pieces of the kidneys were frozen in liquid nitrogen and stored at −70°C until used. Tissues were cut at 4 μm and processed for the antibody stainings as previously described. 24 27A IgG3 bound to the surface of podocytes was detected with rhodamine-conjugated IgG (Dynatech, Denkendorf, Germany).
For immunoprecipitation, glomeruli were isolated and homogenized as described above, but in the presence of 0.5 mmol/L of sodium vanadate (Sigma) to block tyrosine phosphatases. Two hundred fifty μl of total glomerular membranes from the 27A-treated and the control-treated kidneys containing equal amounts of proteins were detergent-extracted by the addition of 250 μl of homogenization buffer containing 0.2% TX-100 and 0.5 mmol/L vanadate. Then, they were immunoprecipitated with anti-nephrin antibody overnight at 4°C. Protein A-Sepharose 4B beads (Zymed) were added and incubated for 1 hour at 4°C. The beads were washed three times with the same buffer and resuspended in sample buffer. Immunoblot analysis was performed as above.
For the immunoprecipitation with the 27A IgG3 to isolate rafts from total glomerular membranes or from TX-100 flotation gradient fractions, the same protocol was used.
Electron Microscopy
For unlabeled transmission electron microscopy, isolated rat glomeruli were extracted with 0.2% TX-100 for 30 minutes on ice with or without pretreatment with saponin. After pelleting (10,000 rpm at 4°C), the glomeruli were fixed with 2% glutaraldehyde in phosphate-buffered saline (PBS) for 1 hour at room temperature. After washing with PBS, a modified postfixation and staining technique was used, which minimizes treatment with OsO4 and uses tannic acid as a contrast agent, followed by subsequent dehydration of the specimens in cold acetone. 25 All tissues were then embedded in Epon 812 according to standard procedures.
For immunogold labeling, the isolated rat glomeruli were fixed with 2% paraformaldehyde in PBS for 30 minutes at room temperature or rat kidneys were perfusion-fixed in situ. Ultrathin frozen sections were prepared from fixed tissue and then transferred to nickel grids as described previously. 24 The anti-nephrin rabbit polyclonal antibody was used at a dilution of 1:100 and goat anti-rabbit IgG coupled to 10-nm colloidal gold (Biocell, Marburg, Germany) at 1:100. The sections were postfixed with 2% glutaraldehyde for 10 minutes and contrasted with OsO4, uranyl acetate, and polyvinyl alcohol. All ultrathin sections were observed under a Philips EM 301 transmission electron microscope.
Results
Nephrin Associates with Rafts in a Cholesterol-Dependent Manner
To determine which podocyte proteins associate with lipid raft microdomains, rafts were isolated from total rat glomerular membranes based on their insolubility in TX-100 and low buoyant density in Optiprep gradients. In these gradients, raft-associated proteins float to the top as DRM fractions, whereas detergent-soluble proteins or detergent-insoluble protein complexes associated with the cytoskeleton are found in the heavy bottom fractions.
Six fractions of the gradient were collected from the top to the bottom and analyzed by Western blotting. The slit diaphragm protein nephrin was found to a significant extent in the DRM fractions, but also in the heavy fractions, as shown in Figure 1A ▶ . The effect of TX-100 was further tested by subsequent pelleting of the DRM and the heavy fractions. Only the DRM-associated nephrin was pelletable (Figure 1B) ▶ .
Peripheral membrane proteins such as CD2AP and ZO-1 that are located to the intracellular aspect of the slit diaphragm 26,27 were also present in the DRM fractions but not in the same amounts as nephrin. Especially in the case of CD2AP, the amount of protein found in the DRM fractions varied between different preparations. Podocalyxin and α3-integrin, transmembrane podocyte proteins with no relation to the slit diaphragm, were mostly found in the heavy fractions. The quality of the gradients was confirmed by the proper localization of the raft marker protein caveolin-1 and the nonraft marker protein transferrin receptor, 17 respectively (Figure 1A) ▶ .
To further characterize the raft association of nephrin, we performed the same experiments after pretreating rat glomerular membranes with saponin. Saponin is a cholesterol-depleting agent, which destabilizes DRM structures rendering them sensitive to detergents such as TX-100. 20 Under these conditions, nephrin was released from the DRM fractions (Figure 1C) ▶ . These results show that nephrin associates with rafts in a cholesterol-dependent manner.
The Endoplasmic Reticulum (ER) Form of Nephrin Does Not Associate with Rafts
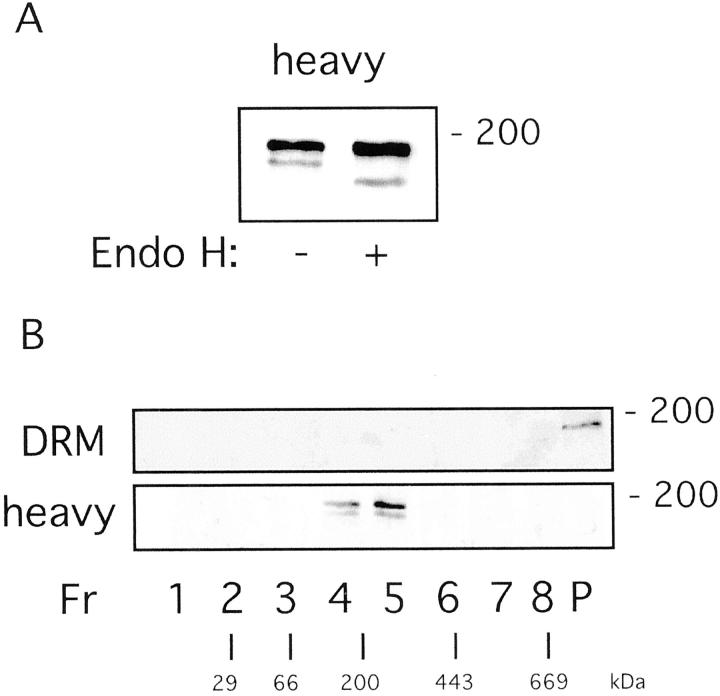
In all of the flotation gradients, nephrin showed an interesting band pattern. In the heavy fractions, a double band was seen in contrast to a single band in the DRM fractions. A similar double band in the heavy fractions of a detergent flotation gradient prepared from Madin-Darby canine kidney cells has been reported previously for the raft protein prominin. 28 In the case of prominin, the upper band represented the cell-surface form and the lower band the ER form of the protein, as determined by Endo H treatment. This enzyme only cleaves N-linked sugar residues synthesized in the ER whereas more complex N-linked sugar residues added in later compartments of the secretory pathway are Endo H-resistant. As lipid rafts first assemble at the level of the Golgi apparatus, the final site of glycosphingolipid synthesis, Endo H-sensitive proteins are usually not present in rafts. Because multiple potential N-glycosylation sites are present in the nephrin sequence, 5 we treated the double band-containing heavy fraction with Endo H. A significant band shift was seen for the lower band, whereas only a small band shift occurred for the upper band (Figure 2A) ▶ . From these results we conclude that the lower band of the doublet found in different species with different antibodies, 6-8,29 represents the Endo H-sensitive ER form of nephrin. This form is detergent-soluble supposedly because it has not yet been exposed to rafts.
Figure 2.
Demonstration of an Endo H-resistant oligomerized form of nephrin. A: The lower band of the nephrin double band only seen in heavy fractions from TX-100 flotation gradients is Endo H-sensitive indicating ER localization. B: DRM-associated nephrin is found in large protein complexes > 600 kd, whereas detergent-soluble nephrin was monomeric (180 kd), as determined by sedimentation velocity centrifugation. The large nephrin complex is resistant to 0.2% TX-100 and 0.4% SDS.
The Raft-Associated Form of Nephrin Is Found in Large Protein Complexes
It has been suggested that nephrin ectodomains oligomerize through a homophilic head-to-head assembly at the plasma membrane level of the slit diaphragm. 7 To address the question whether it really is the slit diaphragm-associated nephrin that partitions into rafts, we tested whether the raft-associated fraction of nephrin is oligomeric. Sedimentation velocity centrifugation in a linear sucrose gradient in the presence of 0.2% TX-100 and 0.4% SDS was used, which separates protein complexes into several size classes. 21 When DRM fractions and heavy fractions from TX-100 flotation gradients were analyzed in this manner, nephrin from the DRM fraction sedimented to the pellet of the gradient corresponding to a protein complex size of >600 kd (Figure 2B) ▶ . With regard to its resistance against SDS, it can be assumed that the complex is held together by strong protein-protein interactions. In contrast, nephrin from the heavy fractions was found in the middle of the gradient containing protein complexes with approximately the size of monomeric nephrin (∼180 kd).
The Slit Diaphragm Itself Is Detergent-Resistant in a Cholesterol-Dependent Manner
Next, we wanted to show morphologically that the slit diaphragm itself behaves similarly as nephrin toward detergents such as TX-100. For this purpose, we extracted isolated rat glomeruli with 0.2% TX-100 for 30 minutes at 4°C, with or without pretreatment with saponin, as we had been treating total glomerular membranes earlier. The extracted tissue was then processed for transmission electron microscopy. As has been shown previously, 1,26,29,30 the slit diaphragms exhibited a remarkable resistance against TX-100 treatment. Although most podocyte foot processes detached from the glomerular basement membrane and most areas on the podocyte plasma membrane were extracted, the slit diaphragms remained mostly intact (Figure 3B) ▶ . The situation dramatically changed after saponin pretreatment, which caused a disruption of the slit diaphragm and overall foot process architecture (Figure 3D) ▶ . Consistent with a recent study, 29 nephrin was still present at the slit diaphragm of TX-100-extracted rat glomeruli as revealed by immunoelectron microscopy (Figure 3C) ▶ . We suggest that the nephrin-based slit diaphragm is TX-100 resistant in a cholesterol-dependent manner.
Figure 3.
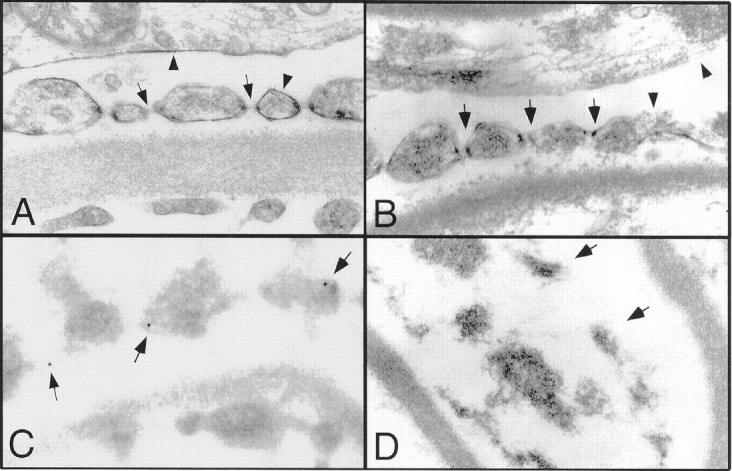
Transmission electron microscopy of isolated rat glomeruli. A: The mock-treated rat glomeruli show intact cell membranes (arrowheads) and podocyte slit diaphragms (arrowheads). B: TX-100 extraction of glomeruli primarily extracts cell membranes (arrowhead) but leaves slit diaphragms intact (arrowheads). A podocyte primary process and multiple foot processes are shown. The arrows mark the intact slit diaphragm. Also note that unlike in A the endothelial cells are disrupted. C: Immunogold labeling of nephrin (arrowheads) in TX-100-extracted glomeruli demonstrates that nephrin is not extracted from the detergent-resistant slit diaphragms. D: Saponin pretreatment before TX-100 extraction disrupts the slit diaphragms leading to an overall disruption of the slit diaphragm architecture with disconnected foot processes (arrows) (original magnifications, ×34,000).
Nephrin-Containing Rafts Can Be Immunoisolated with a Podocyte-Specific Anti-Ganglioside Antibody
Another biochemical method for the isolation of rafts is co-immunoprecipitation with anti-ganglioside antibodies. 31 Gangliosides are sialic acid-containing glycosphingolipids and, as such, important components of lipid rafts. For the isolation of rafts the buffer conditions were chosen that left the rafts intact. Using the 27A antibody against the podocyte-specific 9-O-acetyl GD3 32 and a cold TX-100-containing buffer, it was possible to co-immunoprecipitate the surface form of nephrin (Figure 4A) ▶ . 27A co-immunoprecipitated also other raft proteins such as caveolin-1, but not nonraft proteins such as the transferrin receptor (Figure 4A) ▶ . Consistent with these data, immunogold-labeling of the foot processes with the 27A antibody showed occasional labeling of the slit diaphragm but also elsewhere in the podocyte plasma membrane (data not shown). To test whether the interaction of nephrin and 9-O-acetyl GD3 takes place in rafts, the DRM fraction and the heavy fraction of the TX-100 flotation gradient were co-immunoprecipitated with the 27A antibody. Nephrin was only present in the precipitates from the DRM fraction and cholesterol depletion disrupted the interaction between nephrin and 9-O-acetyl GD3. After saponin treatment, neither in the DRM nor in the heavy fraction was nephrin co-immunoprecipitated by the 27A antibody (Figure 4B) ▶ . Hence, the interaction between nephrin and 9-O-acetyl GD3 is raft-dependent.
Figure 4.
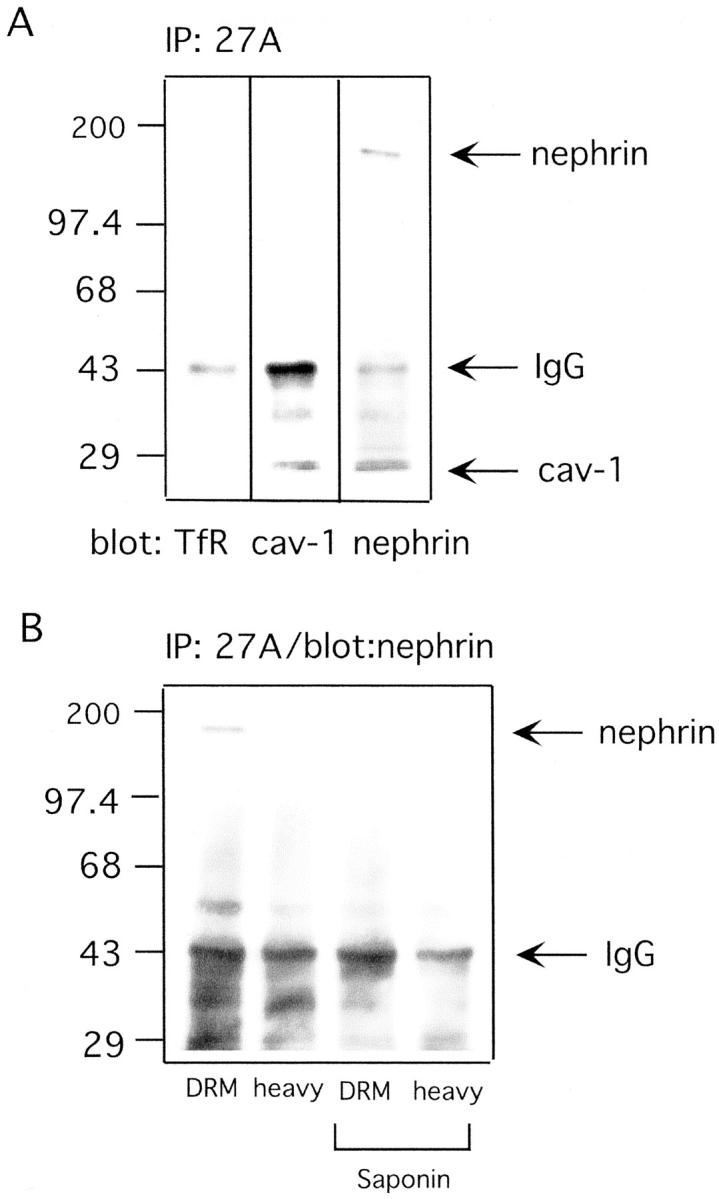
Nephrin interacts with 9-O-acetyl GD3 in raft microdomains. A: Immunoprecipitates obtained with 27A IgG3 from pure rat glomerular membranes contain nephrin and the raft marker caveolin-1 but not the nonraft marker transferrin receptor. The order of lanes from the left to the right represents the order of the reprobing of the blotting membrane. This explains the appearance of a caveolin-1 band in the nephrin lane. B: Only immunoprecipitates from DRM fractions in TX-100 flotation gradients contain nephrin. Note that in A and B only the surface form of nephrin was co-immunoprecipitated by 27A IgG3. Molecular weight markers are shown in kd, and nonspecific high- and low-molecular mass IgG bands are marked as IgG. Saponin pretreatment prevents nephrin pull-down by 27A, proving that the nephrin:9-O-acetyl GD3 interaction is raft-dependent.
In Vivo Injection of 27A IgG Causes Apical Dislocation and Tyrosine Phosphorylation of Nephrin
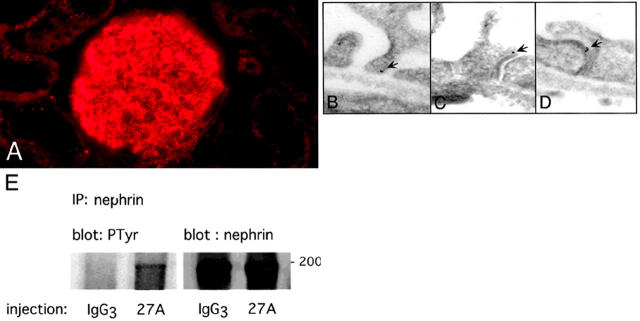
Intravenous injection of the 27A IgG3 into rats leads to severe morphological changes of podocytes including effacement-like changes of foot processes and filtration slits. 23 As described before, 23 injection of the antibody induced swelling of the foot processes and obliteration of the slit diaphragms within 1 hour. The binding of 27A IgG3 to the podocytes was confirmed by immunocytochemistry (Figure 5A) ▶ . By immunogold-labeling we detected the dislocation of nephrin to the apical pole of the narrowed filtrations slits (Figure 5D) ▶ . In addition, the intravenous injection of 27A IgG3 into rats induced tyrosine phosphorylation of nephrin after 1 hour (Figure 5E) ▶ . Injection of control mouse IgG3 did not cause tyrosine phosphorylation of nephrin (Figure 5E) ▶ .
Figure 5.
In vivo injection of 27A IgG causes apical dislocation and tyrosine phosphorylation of nephrin. A: One hour after intravenous injection of 27A IgG3, the antibody binds to the podocytes. In contrast to the IgG3-injected rats (data not shown), bound 27A is detected in the glomerulus by rhodamine conjugated anti-mouse IgG (A). B, C, and D: The effacement-like morphological changes at the podocyte filtration slits are shown in C and D. At these altered filtration slits, nephrin is found at the apical pole and often in clusters as demonstrated by the 15-nm gold particles (C and D). For comparison, nephrin at normal wide filtration slits of the control-injected rat is shown (B). Original magnifications, ×34,000. E: The apical dislocation is accompanied by tyrosine phosphorylation of nephrin. One hour after injection of 27A and control IgG3, glomerular membrane fractions were co-immunoprecipitated with anti-nephrin and labeled with anti- phosphotyrosine. Tyrosine phosphorylated nephrin was detected for the 27A-injected rats but not for the control animals. Equal loading of nephrin after immunoprecipitation was shown by probing with anti-nephrin antibody (right lanes).
Discussion
Attempts to study molecular mechanisms of plasma ultrafiltration in the kidney glomerulus have been difficult in the past because of the lack of appropriate molecular targets. With the identification of nephrin 5 the experimental situation has changed. Using nephrin as a target molecule for the slit diaphragm it is now possible to obtain insight into the molecular composition of the slit diaphragm. Likewise, molecular mechanisms maintaining normal glomerular filtration or their disturbances under pathological conditions can now be accessed more readily.
In this study, we provide evidence for the association of nephrin with signaling domains on the plasma membrane called lipid rafts in an oligomerized form suggesting slit diaphragm localization. Ultrastructural studies revealed that the slit diaphragm is detergent-resistant in a cholesterol-dependent manner. Furthermore, we immunoisolated nephrin-containing rafts with an antibody against the podocyte-specific 9-O-acetyl GD3 ganglioside. Finally, we showed that the in vivo injection of this antibody not only induced morphological changes of the filtration slits but also apical dislocation and tyrosine phosphorylation of nephrin.
The assembly of nephrin complex commences when the podocyte precursors transdifferentiate from typical polarized epithelial cells of the S-shaped body to more mature mesenchymal cells of the capillary loop stage. 8,29 The hallmark of these events is the downward migration of apical tight junctions along with their conversion into slit diaphragms. 1,26 A retrograde conversion is observed in pathological situations in humans extensively studied with the use of rat experimental models. 4,10-12,16,33-35 In particular in the protamine sulfate and the puromycin aminonucleoside models, these events were shown to depend on energy, calcium, and most importantly, signaling events including tyrosine phosphorylation. 11
In the bi-directional modulation of slit diaphragms to tight junctions, raft involvement may be important. A recent report proposed a role for lipid rafts in the organization of tight junctions and the regulation of paracellular permeability. 36 DRM fractions of TX-100 flotation gradients from an intestinal epithelial cell line were shown to contain major pools of ZO-1 and occludin. In contrast to Madin-Darby canine kidney cells, 36 ZO-1 that localized to the slit diaphragms and tight junctions of podocytes was not found to be as enriched in the DRM fraction in the present study. Similar to CD2AP, ZO-1 may partly disassociate from the nephrin complex under the detergent conditions used here. This might also explain why nephrin in the heavy fractions of our gradients was shown to be soluble in TX-100 despite its suggested linkage to the cytoskeleton via CD2AP (Figure 1B) ▶ . 27,37
A large number of signaling events have been shown to occur in the lipid raft environment. 15 Lipid rafts serve as building blocks for the establishment of signal transduction platforms favored by many kinases, in particular doubly acylated kinases of the src family. 15 A recurring theme in the building up of these platforms is the oligomerization of proteins. The T-cell receptor is a well-studied example for this. 38 Stimulation of T-cell receptor by antigen-presenting cells triggers its oligomerization. This process is accompanied by the recruitment of the T-cell receptor into raft microdomains leading to the engagement into higher order protein complexes. These complexes contain co-stimulatory molecules as well as multiple kinases and their phosphorylation substrates. Whether the functional role of nephrin at the slit diaphragm has similarities with the dual role of co-stimulatory molecules at the immunological synapse in severing cell-cell contacts and transducing signals remains to be explored. Given its structure, nephrin, in fact, has been suggested to play such a dual role. 7 As a member of the Ig superfamily, like most of the co-stimulatory molecules, the extracellular part of nephrin contains a number of modules important for cell adhesion function. The intracellular part contains eight tyrosine residues potentially involved in signaling.
We also found in this study that the oligomerized form of nephrin associates with lipid rafts. We do not know at present whether the oligomerization occurs within the raft or is a prerequisite for being recruited into the raft. The latter is supported by in vitro studies using cultured podocytes (data not shown). When overexpressing rat nephrin in these cells we were not able to detect raft association of nephrin. In these cells, nephrin was not found in protein complexes but only in a monomeric form.
Another interesting question is whether the oligomerization takes place only once early in development with the formation of the slit diaphragms by the maturing podocytes. 1 Alternatively, nephrin oligomers could assemble and disassemble continuously as a means of regeneration and cleansing of the glomerular filter.
The dynamic assembly and disassembly of nephrin oligomers would require control of activity and energy, which could occur via tyrosine phosphorylation of nephrin as detected after binding of 27A IgG3 to the surface of podocytes. We assume that the 27A antibody effect lies in the cross-linking of raft components as reported for other anti-ganglioside antibodies or other antibodies against raft components. 31,39 The cross-linking could induce oligomerization of nephrin regulated by kinases with affinity for nephrin. The signaling effect of the 27A antibody has been confirmed previously using cultured human peripheral blood mononuclear cells as a model in which the src family kinase, syk, was found to become activated. 40
The pathological changes of podocyte morphology induced by the anti-9-O-acetyl GD3 antibody are difficult to interpret. Because the injected rats did not develop significant proteinuria, 23 the relation of the effacement seen after binding of the antibody and the effacement produced by the toxic agents protamine sulfate and puromycin aminonucleoside or in human glomerulopathies remains to be established. In any case, apical dislocation similar to that of nephrin after injection of 27A is also seen for other junction proteins such as ZO-1 in PAN or protamine sulfate nephrosis. 12 Therefore, it is possible that the manipulation of this raft microdomain, by antibody binding or by charge, represents a general mechanism for foot process effacement. As exampled by congenital nephrotic syndrome of the Finnish type, genetic mutations of protein components within this microdomain lead to similar effects. 9 Podocin is another example for a protein that seems to be indispensable for the function of the slit diaphragm. Podocin is a recently identified protein of the raft-associated stomatin family 41 whose gene NPHS2 is mutated in families with autosomal-recessive steroid-resistant nephrotic syndrome. 42 Interestingly, podocin localizes to the slit diaphragm and associates with the nephrin-containing raft (K Schwarz, M Simons, J Reiser, W Kriz, LB Holzman, AS Shaw, and P Mundel, submitted for publication). It will be interesting to see what other molecules, eg, kinases, can be found in this specialized microdomain and how their function and organization is affected in different pathological situations.
Acknowledgments
We thank Hiltraud Hosser, Riitta Väisänen, and Eva Höyri for skillful technical assistance; Anu Pätäri for her help with the graphics; Andrey Shaw for providing the CD2AP antibody; the members of the Simons and the Holthöfer laboratories for helpful advice; and Kai Simons for his generous ongoing support and critical reading of the manuscript.
Footnotes
Address reprint requests to Harry Holthöfer, M.D., Ph.D., Molecular Medicine, University of Helsinki, Haartmaninkatu 8, PB 63, FIN-00014 University of Helsinki. E-mail: harry.holthofer@helsinki.fi., or Peter Mundel, M.D., Division of
Supported by the European Union grant “Quality of Life and Management of Living Resources,” a grant from the University of Helsinki, and a grant from the Deutsche Forschungsgemeinschaft (SFB352). M. S. was a recipient of an EMBO short-term fellowship.
P.M. and H.H. contributed equally to this work.
References
- 1.Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol 1995, 192:385-397 [DOI] [PubMed] [Google Scholar]
- 2.Daniels BS, Deen WM, Mayer G, Meyer T, Hostetter TH: Glomerular permeability barrier in the rat: functional assessment by in vitro methods. J Clin Invest 1993, 92:929-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drumond MC, Deen WM: Structural determinants of glomerular hydraulic permeability. Am J Physiol 1994, 266:F1-F12 [DOI] [PubMed] [Google Scholar]
- 4.Smoyer WE, Mundel P: Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med 1998, 76:172-183 [DOI] [PubMed] [Google Scholar]
- 5.Kestilä M, Lenkkeri U, Männikö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvasson K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1999, 1:575-582 [DOI] [PubMed] [Google Scholar]
- 6.Holthofer H, Ahola H, Solin ML, Wang S, Palmen T, Luimula P, Miettinen A, Kerjaschki D: Nephrin localizes at the podocyte filtration slit area and is characteristically spliced in the human kidney. Am J Pathol 1999, 155:1681-1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 1999, 96:7962-7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzman LB, St. John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR: Nephrin localizes to the slit pore of the glomerular epithelial cell. 1999, 56:1481-1491 [DOI] [PubMed] [Google Scholar]
- 9.Patrakka J, Kestila M, Wartiovaara J, Ruotsalainen V, Tissari P, Simons K, Toomre D: Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 2000, 58:972-980 [DOI] [PubMed] [Google Scholar]
- 10.Seiler MW, Venkatachalam MA, Cotran RS: Glomerular epithelium: structural alterations induced by polycations. Science 1975, 189:390-393 [DOI] [PubMed] [Google Scholar]
- 11.Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 1995, 268:F514-F524 [DOI] [PubMed] [Google Scholar]
- 12.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG: The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 1992, 141:805-816 [PMC free article] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen I: Functional rafts in cell membranes. Nature 1997, 387:569-572 [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, London E: Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998, 14:111-136 [DOI] [PubMed] [Google Scholar]
- 15.Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000, 1:31-40 [DOI] [PubMed] [Google Scholar]
- 16.Holthofer H, Reivinen J, Solin ML, Haltia A, Miettinen A: Decrease of glomerular disialogangliosides in puromycin nephrosis of the rat. Am J Pathol 1996, 149:1009-1015 [PMC free article] [PubMed] [Google Scholar]
- 17.Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R: EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 1999, 22:511-524 [DOI] [PubMed] [Google Scholar]
- 18.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997, 236:248-258 [DOI] [PubMed] [Google Scholar]
- 19.Ahola H, Wang SX, Luimula P, Solin ML, Holzman LB, Holthofer H: Cloning and expression of the rat nephrin homolog. Am J Pathol 1999, 155:907-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT: Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J 1998, 17:5334-5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E: Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol 1998, 140:795-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen A, Solin ML, Reivinen J, Juvonen E, Väisäinen R, Holthofer H: Podocalyxin in rat platelets and megakaryocytes. Am J Pathol 1999, 154:813-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekan G, Miettinen A, Schnabel E, Farquhar MG: Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection: localization of antigens and bound IgGs by immunoelectron microscopy. Am J Pathol 1990, 137:913-927 [PMC free article] [PubMed] [Google Scholar]
- 24.Mundel P, Gilbert P, Kriz W: Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem 1991, 39:1047-1056 [DOI] [PubMed] [Google Scholar]
- 25.Mbassa G, Elger M, Kriz W: The ultrastructural organization of the basement membrane of Bowman’s capsule in the rat renal corpuscle. Cell Tissue Res 1988, 253:151-163 [DOI] [PubMed] [Google Scholar]
- 26.Schnabel E, Anderson JM, Farquhar MG: The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 1990, 111:1255-1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner J: CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol 2000, 279:F785-F792 [DOI] [PubMed] [Google Scholar]
- 28.Roper K, Corbeil D, Huttner W: Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol 2000, 2:582-592 [DOI] [PubMed] [Google Scholar]
- 29.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F: Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 2000, 57:1949-1961 [DOI] [PubMed] [Google Scholar]
- 30.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 1990, 137:929-944 [PMC free article] [PubMed] [Google Scholar]
- 31.Kasahara K, Watanabe K, Takeuchi K, Kaneko H, Oohira A, Yamamoto T, Sanai Y: Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J Biol Chem 2000, 275:34701-34709 [DOI] [PubMed] [Google Scholar]
- 32.Reivinen J, Holthofer H, Miettinen A: A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int 1992, 42:624-631 [DOI] [PubMed] [Google Scholar]
- 33.Ryan GP, Karnovsky MJ: An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int 1975, 8:219-232 [DOI] [PubMed] [Google Scholar]
- 34.Caulfield JP, Reid JL, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis: evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 1976, 34:43-59 [PubMed] [Google Scholar]
- 35.Kerjaschki D: Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli: the effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest 1978, 39:430-440 [PubMed] [Google Scholar]
- 36.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara J: Tight junctions are membrane microdomains. J Cell Sci 2000, 113:1771-1781 [DOI] [PubMed] [Google Scholar]
- 37.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 1999, 286:312-315 [DOI] [PubMed] [Google Scholar]
- 38.Janes PW, Ley SC, Magee AI, Kabouridis PS: The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol 2000, 12:23-34 [DOI] [PubMed] [Google Scholar]
- 39.Harder T, Scheiffele P, Verkade P, Simons K: Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 2000, 141:929-942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reivinen J, Holthofer H, Miettinen A: Tyrosine phosphorylation of p72syk induced by anti-9-O-acetyl GD3 antibodies in human peripheral blood mononuclear cells. Scand J Immunol 1998, 48:615-622 [DOI] [PubMed] [Google Scholar]
- 41.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 2000, 24:349-354 [DOI] [PubMed] [Google Scholar]
- 42.Snyers L, Umlauf E, Prohaska R: Association of stomatin with lipid-protein complexes in the plasma membrane and the endocytic compartment. Eur J Cell Biol 1999, 78:802-812 [DOI] [PubMed] [Google Scholar]