Abstract
Human tissue xenograft models are currently the only tool for conducting in vivo analyses of intact human tissue. The goal of the present study was to develop reliable methods for successful generation of short-term primary tissue xenografts from benign and tumor-derived human prostate tissue. Primary human prostate xenografts were established in athymic nu/nu mice from eight of eight benign and five of five prostate cancer tissues, collected from a total of 10 patients who underwent radical prostatectomy for the treatment of prostate cancer. An average of 13 xenografts was established per specimen. Two tissue specimens were cryopreserved for >1 month before successful generation of prostate xenografts. After 1 month in vivo, xenograft tissues were harvested and examined regarding: gross evidence of vascularization; tissue morphology; proliferation; apoptosis; and expression of androgen receptor, prostate-specific antigen, and high molecular weight cytokeratins specific for basal cells in the prostate. Direct comparison of the original tissue specimen and the 1-month xenografts revealed similar histology; similar apoptotic and proliferative fractions in most cases; and comparable expression levels and expression patterns of androgen receptor, prostate-specific antigen, and high molecular weight cytokeratins. These data demonstrate that primary human prostate xenografts, benign and malignant, can be established routinely from human prostate tissue surgical specimens, and that the xenografts maintain tissue architecture and expression of key prostatic markers. The development of this methodology, including the technique for cryopreservation of human tissue, will allow multiple (successive) analyses of human prostate tissue to be conducted throughout time using a tissue sample derived from a single patient; and simultaneous analysis of human prostate tissues derived from a cohort of patients.
There are few suitable animal models for prostate cancer, because the only mammals that develop the disease with age are dogs and primates. In 1980, Hoehn and colleagues 1 established the PC-82 in vivo human prostate xenograft model (PC-82); they were the first to demonstrate that human prostate tissue could be transplanted and maintained as a xenograft in the subcutaneous environment of immunocompromised mice. Subsequently, a limited number of additional prostate cancer xenograft models have been established, 2-7 and have lead to significant advancements in the study of human prostate cancer. However, there are limitations to the use of xenografts that include the frequency and reliability with which they can be established and the possibility that they become mosaics of human and host tissue throughout time. Successful growth of primary prostate cancer tissue in nude mice has been reported in the literature, but the percent engraftment ranges from <5% to a maximum of 60%. 4,6
Prostate xenografts offer several advantages over the use of prostate cancer cell lines. In vivo studies with prostate cancer cell lines typically involve subcutaneous injection of a clonal epithelial cell line or co-injection of epithelial and stromal cells to generate tumors. However, the histology of the tumors rarely recapitulates the histology of the original tissue from which the cell lines were derived, and cell lines that are not tumorigenic in vivo cannot be analyzed in this way. In contrast, xenografts allow analysis of intact tissue containing all of the cell types present in the prostate (basal cells, epithelial cells, stromal cells, endothelial cells, neuroendocrine cells) within an intact tissue microenvironment, and benign tissue can be compared in vivo alongside tumor tissue.
The goal of this study was not to generate additional prostate cancer xenografts for serial passage in vivo, but to develop a technique that would allow for reproducible engraftment of benign and tumor-derived human prostate tissue in athymic nude mice. We have established a protocol that allows fresh, or previously cryopreserved, benign and tumor-derived human prostate tissue transplanted into the subcutaneous environment of male mice to become vascularized and remain morphologically similar to the original tissue, maintaining similar histology, apoptotic rate, and proliferative rate. This model represents a novel tool for the study of prostate cancer, because specimens can be collected from multiple patients, cryopreserved, and used to conduct controlled in vivo studies in hosts that can be manipulated experimentally. Potential therapeutic approaches can be evaluated in vivo in a large cohort of benign and tumor-derived human prostate tissues, thus providing a more reliable predictor of the efficacy of a particular treatment in the human population.
Materials and Methods
Animals
Three-month-old athymic male mice (Hsd: athymic nude/Nu) were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Serum testosterone levels were maintained at ∼4 ng/ml by subcutaneous implantation of 12.5 mg sustained-release testosterone pellets (Innovative Research of America, Sarasota, FL). All experiments involving laboratory animals were performed in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at the University of North Carolina in Chapel Hill.
Tissue Harvest, Cryopreservation, and Implantation
Human prostate tissue, designated as excess tissue, was obtained from 10 patients at the time of prostatectomy. All tissues were collected in accordance with National Institutes of Health guidelines on the use of human subjects, with approval by the Internal Review Board of the University of North Carolina Hospitals. Whenever possible, both benign and tumor tissue were collected from an individual patient, based on gross morphological assessment of tumor margins by the surgeon (JLM). Subsequent histological analysis of the collected tissues by a urological pathologist (SJM) allowed each specimen to be defined as benign (B) or tumor (T). A total of eight tissues were defined histologically as benign, and five defined as tumor. Tissue was harvested aseptically and immediately submerged in ViaSpan organ preservation solution (DuPont, Wilmington, DE) on ice. Tissue samples were cut into wedge-shaped pieces ∼3 to 4 mm in length and 2-mm thick at the broadest end. Wedges were either transplanted immediately into athymic nu/nu mice or cryopreserved in a solution consisting of prostate growth media 8 and 10% dimethyl sulfoxide. Wedges were thawed on ice and rinsed 3× in sterile ViaSpan before transplantation. For transplantation, small (∼3 mm) slits were made in the skin of the right and left flank of a nude mouse anesthetized with Domitor, and one wedge of tissue per flank was dipped in Matrigel and inserted subcutaneously. Wounds were closed with tissue glue. Mice were observed weekly after tissue implantation.
Tissue Evaluation
One month after implantation, mice were euthanized and the tissue harvested to evaluate engraftment. Tissue pieces were removed, formalin-fixed, paraffin-embedded, and subjected to hematoxylin and eosin (H&E) histological analysis. Proliferation was assessed in the tissue preparations by detection of Ki67 using MIB-1 monoclonal antibodies (Immunotech via Beckman Coulter, Inc., Fullerton, CA), and apoptosis was evaluated with the ApopTag kit (Intergen, Purchase, NY) using the manufacturer’s instructions. Percentages of proliferating and apoptotic cells were established by counting the number of MIB-1 or ApopTag-labeled nuclei in three 400× microscopic fields for each tissue. At least 2000 cells were counted per specimen, and the numbers shown represent the average from three counts. Expression of androgen receptor (AR) and prostate-specific antigen (PSA) was evaluated with anti-AR antibodies (Biogenex, San Ramon, CA) and anti-PSA antibodies (DAKO, Carpinteria, CA). Benign tissue was confirmed by the presence of basal cells that were identified with anti-345βE12 antibodies (Enzo Diagnostics, Farmingdale, NY) that detect high molecular weight cytokeratins (HM-CK).
Results
Successful Establishment of Short-Term Benign and Tumor Prostate Xenografts
Human prostate tissue was obtained from 10 patient specimens, with both benign and tumor tissue taken whenever possible. A total of eight benign and five tumor specimens were used. One hundred percent of the specimens (eight of eight benign and five of five tumor) remained engrafted successfully 1 month after transplantation (representative cases are shown in Figure 1 ▶ ). The average number of wedges transplanted per patient specimen was 13 ± 1.3, and the average percent viable at the time of harvest was 100%. Specimens 4B and 4T were cryopreserved for >1 month before transplantation. At the time of removal from the mouse host, two to three macroscopic subcutaneous host vessels were connected to the xenograft tissues. In most cases, the pathologist’s histological evaluation of the 1-month xenograft was similar or identical to the histological evaluation of the original patient tissue specimen (Table 1) ▶ . Minor histological differences included inflammation (10B), squamous metaplasia (2T, 3B), and transitional metaplasia (1B). Tumor tissues showed identical Gleason’s scores in the original patient tissue and the matched xenografts.
Figure 1.
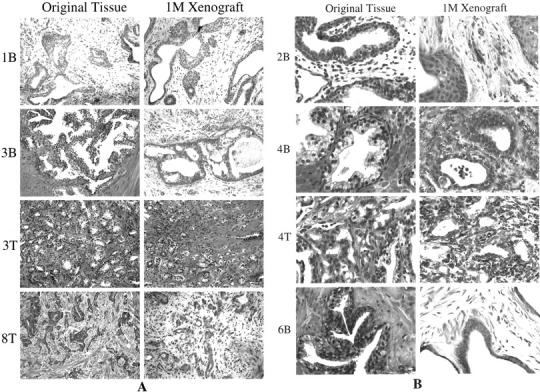
Histological features of original tissues and corresponding 1-month xenograft tissues are similar, as shown by H&E analysis of representative cases. A: Low-power images (original magnifications, ×1000) were composed so that overall tissue architecture may be evaluated. B: Composed of higher power images (original magnifications, ×2000) for observation of cellular features. Although the majority of glands within each xenograft recapitulated the original tissue, some xenografts contained isolated glands characterized by transitional cell metaplasia (1B) or squamous cell metaplasia (2B, 2T, 3B). Basal cell hyperplasia was also a common feature of the benign xenografted tissues.
Table 1.
Original Patient Prostate Tissues and Corresponding 1-Month Xenograft Tissues Were Evaluated Histologically by a Urological Pathologist and Identified as Benign Prostate or Prostate Cancer (CaP)
| Sample no. | Pathology evaluation of original tissue | Pathology evaluation of 1-month xenograft |
|---|---|---|
| 1B | Benign prostate | Benign prostate with some transitional metaplasia |
| 2B | Benign prostate | Benign prostate with some squamous metaplasia |
| 2T | CaP* (Gleason grade 6, some benign glands present) | CaP (Gleason grade 6)/some benign glands present; with some squamous metaplasia |
| 3B | Benign prostate | Benign prostate with some squamous metaplasia |
| 3T | CaP (Gleason grade 6) | CaP (Gleason grade 6) |
| 4B | Benign prostate | Benign prostate |
| 4T | CaP (Gleason grade 8) | CaP (Gleason grade 8) |
| 5T | CaP (Gleason grade 6) | CaP (Gleason grade 6) |
| 6B | Benign prostate | Benign prostate |
| 7B | Benign prostate | Benign prostate |
| 8T | CaP (Gleason grade 8) | CaP (Gleason grade 8) |
| 9B | Benign prostate | Benign prostate |
| 10B | Benign prostate | Benign prostate with inflammation |
*CaPs were further characterized by the assignment of a Gleason grade.
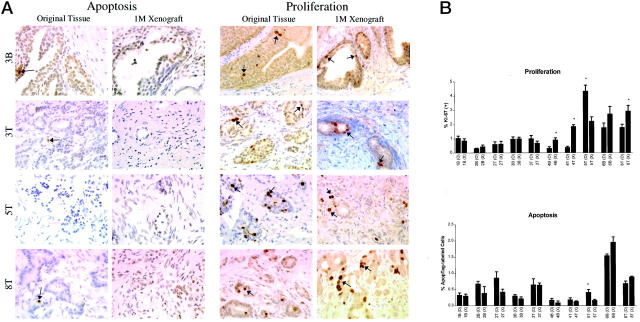
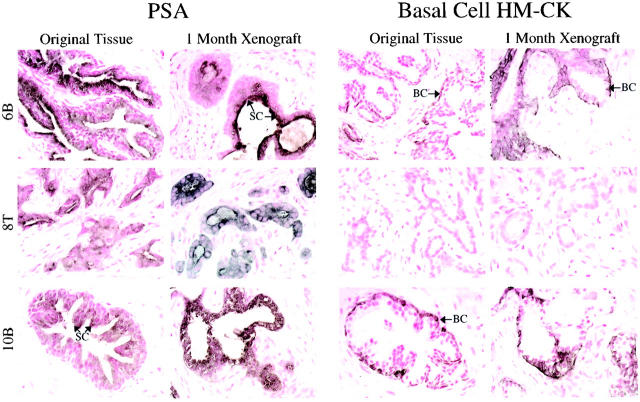
Immunohistochemical analysis with anti-Ki67 antibodies and ApopTag analysis revealed that the numbers of proliferating and apoptotic cells seemed similar between matched original patient tissue and 1-month xenografts (Figure 2A) ▶ . Quantitative analysis of the Ki67(+) and ApopTag(+) cells in matched original and xenograft tissues found statistically significant differences in proliferative indices in 4 of 10 cases, and a significant difference in apoptotic index in one case (Figure 2B) ▶ . In general, proliferation was slightly higher and apoptosis slightly lower in the 1-month xenograft compared to the original tissue. Immunodetectable AR protein levels were comparable between original tissues and matched xenografts, with respect to both the staining intensity and the staining pattern (Figure 3) ▶ . The original tissues and matched xenografts were characterized further regarding the expression of PSA and basal cell-specific HM-CK. Benign tissues and xenografts were characterized by expression of PSA by the differentiated tall columnar luminal epithelial cells and expression of HM-CK by basal cells (Figure 4) ▶ . CaP tissues and xenografts were characterized by heavy expression of PSA by glandular epithelium and the absence of HM-CK(+) basal cells (Figure 4) ▶ .
Figure 2.
Apoptosis and proliferation were evaluated in the original tissues and corresponding 1-month xenograft tissues. Representative samples are shown in A. The percentages of Apoptag-labeled cells (dark-brown nuclei) were equivalent in 9 of 10 matched original and xenograft tissues (B). Proliferative cells, as identified by antibodies to Ki67 (dark-brown nuclear staining), were detected in matched original and xenograft tissues. The proliferative fraction was significantly higher in 3 of 10 xenografts when compared to matched original tissue, and was significantly lower in one xenograft.
Figure 3.
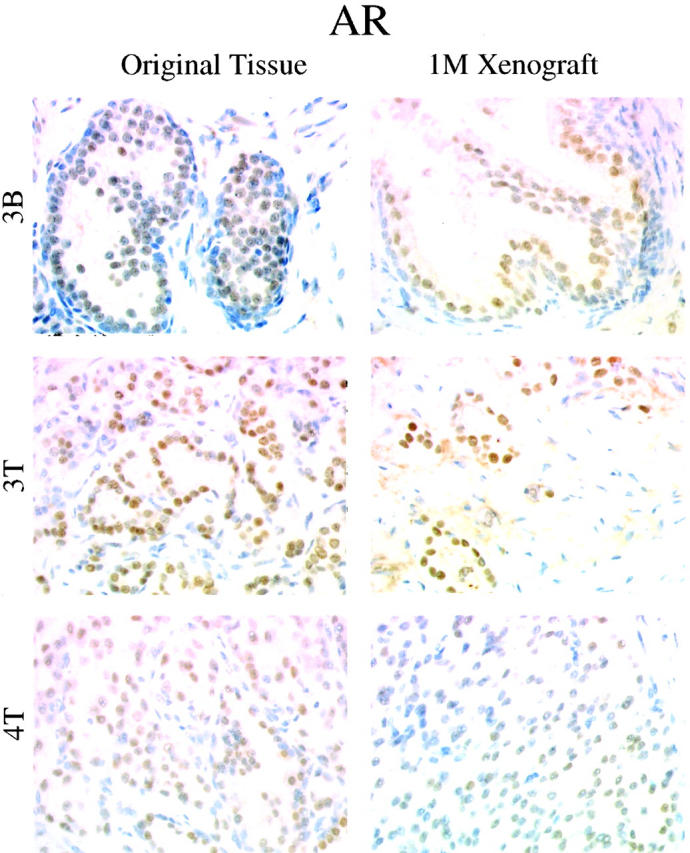
Original tissues and corresponding 1-month xenografts were characterized regarding AR expression. Representative samples are shown. Benign original tissues and xenografts were characterized by moderate AR positivity in the nuclei of secretory epithelial cells. CaP tissues and xenografts were characterized by a more heterogeneous expression of AR by epithelial cells. In each case, AR staining intensity and expression pattern of the original tissue were recapitulated in the 1-month xenograft.
Figure 4.
Expression of PSA and basal cell-specific cytokeratins (HM-CK) were evaluated in the original tissues and matched 1-month xenograft tissues. Representative samples are shown. Benign prostate tissues and xenografts (6B, 10B) were characterized by expression of PSA by the secretory epithelial cells (SC) (dark-brown to black cytoplasmic staining), and distinct expression of HM-CK by the basal cells (BC) lining the glandular structures (brown cytoplasmic staining). Original CaP tissues and corresponding xenografts (8T) were characterized by strong PSA expression by epithelial cells, and a lack of HM-CK (+) basal cells.
Discussion
Few animal models exist in which experiments can be conducted in viable benign and cancer tissue derived from the same human host. Animal models often do not recapitulate human diseases accurately, and many dynamic questions cannot be answered by studying archival human tissue specimens. The establishment of long-term, propagable, in vivo xenograft models of human prostate cancer (such as CWR22) represents a significant advancement in the tools available for the study of prostate cancer. However, it is not clear whether the native human tissue architecture remains stable throughout serial xenograft passage in vivo. The goal of this study was not to generate additional long-term prostate cancer xenografts for serial passage in vivo, but to establish reliable methods by which primary human prostate-tissue specimens could be used as a source of human tissue for routine establishment of short-term subcutaneous xenografts. Although there are several valid concerns about the use of xenograft models, including the effects of host cells on interspecies transplants, 9 they are currently the only experimental mechanism for studying normal biology and disease pathogenesis in intact human tissues. The use of short-term primary xenografts generated from patient material may offer an advantage regarding host cell infiltration, because the opportunity for ingrowth of host cells increases with serial passage in vivo. Our data show clearly that it is possible to achieve 100% engraftment of benign prostate tissue and prostate cancer tissue using the methodology described in this report. The efficiency with which our short-term xenografts remain viable is much higher than the efficiencies reported in the literature by others. Factors that may contribute to the difference include: 1) immediate placement in Viaspan solution after surgical removal; 2) maintenance of the tissue at 4°C until transplantation; and 3) consistency in size and shape of transplanted tissue. We hypothesize that nutrients are able to enter the newly-transplanted tissue wedges through simple diffusion at the tapered end, which facilitates survival until the development of vascular connections with the host. As demonstrated in Figures 1 to 4 ▶ ▶ ▶ , the tissue morphology; levels of apoptosis and proliferation; and expression of AR, PSA, and basal cell-specific cytokeratins, are very similar between the original patient tissue and the matched established xenograft. It is obvious in the H&E-stained sections that the xenografts are not necrotic or fibrotic, and vascularization is evidenced by the presence of small vessels and red blood cells. Thus, these methods provide a means of tissue homeostasis; the proliferation/apoptotic rates recapitulate the original tissue. An additional advantage to this short-term xenograft model is the persistence of the prostate tissue architecture during in vivo culture. The importance of stromal-epithelial interaction in the prostate is well documented, and stromal cells are key in regulating differentiation of prostate epithelial cells, possibly through AR-mediated events. Furthermore, there is ample evidence to suggest that stromal cells play an active, rather than passive, role in prostate carcinogenesis. 10-14 This is reflected in the observation that the HUNC-E prostate tumor epithelial cell line requires co-inoculation of a prostate stromal cell line for tumor development in vivo. 8 Models in which tissue is minced finely or cell slurries are prepared from heterogeneous human prostate cancer tissue offer an alternative to the injection of clonogenic cell lines, but re-establishment of tissue architecture, particularly glandular structures, from such suspensions is limited. By inserting an intact piece of tissue, the architecture of the glands is preserved, as well as the spatial relationship between the stromal and epithelial cell compartments.
In summary, the methods outlined in this report will enable researchers to establish in vivo xenograft cultures from human prostate specimens with great efficiency. The ability to cryopreserve the tissue before transplantation increases the flexibility of the model significantly and allows for more experimental control. As shown in Figure 1 ▶ , the histology of benign and tumor-derived human prostate tissue is preserved in the xenografts, even when the tissue was cryopreserved before transplantation (see samples 4B and 4T). Using the techniques described in this report, it will be possible to generate mice bearing multiple xenografts derived from different patient samples, so that experiments can be conducted in a large cohort of patient tissues using just a few mice. Likewise, mice can be generated that contain multiple xenografts from the same specimen, allowing grafts to be sequentially harvested for analysis at multiple time points during an experiment. For example, the in vivo response of benign and tumor-derived human prostate tissue can be evaluated after modulation of host hormone levels or treatment with anti-cancer compounds; efficacy can be evaluated in multiple patient tissues simultaneously, and tissue can be analyzed at multiple time points using a small number of animals. Furthermore, these methods for successful engraftment of prostate tissue in vivo may be useful in establishing xenografts of a wide variety of human tissues, normal and diseased.
Footnotes
Address reprint requests to Gary J. Smith, Ph.D., Department of Pathology and Laboratory Medicine, CB#7525 Brinkhous-Bullitt Bldg., University of North Carolina at Chapel Hill, Chapel Hill, NC 27599. E-mail: cellsort@med.unc.edu.
Supported by National Institutes of Health grants CA 77739 and CA 64865.
References
- 1.Hoehn W, Schroeder FH, Riemann JF, Joebsis AC, Hermanek P: Human prostatic adenocarcinoma: some characteristics of a serially transplantable line in nude mice (PC-82). Prostate 1980, 1:95-104 [DOI] [PubMed] [Google Scholar]
- 2.Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH: Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: luCaP 23. Clin Cancer Res 1996, 2:1039-1048 [PubMed] [Google Scholar]
- 3.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON: Progression of metastatic human prostate cancer to androgen-independence in immunodeficient SCID mice. Nat Med 1997, 3:402-408 [DOI] [PubMed] [Google Scholar]
- 4.Pretlow TG, Giaconia JM, Edgehouse NL, Pretlow TP: Primary human prostatic carcinomas grow as serially transplanted xenografts in nude mice. Proc Am Assoc Cancer Res 1993, 34:248 [Google Scholar]
- 5.Pretlow TG, Wolman SR, Micale MA, Pelley RJ, Kursh ED, Resnick MI, Bodner DR, Jacobberger JW, Delmoro CM, Giaconia JM, Pretlow TP: Xenografts of primary human prostatic carcinoma. J Natl Cancer Inst 1993, 85:394-398 [DOI] [PubMed] [Google Scholar]
- 6.Van Weerden WM, De Ridder CMA, Verdaasdonk CL, Romijn JC, Van der Kwast TH, Schroder FH, van Steenbrugge GJ: Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol 1996, 149:1055-1061 [PMC free article] [PubMed] [Google Scholar]
- 7.Wright GL, Haley CL, Csapo Z, van Steenbrugge GJ: Immunohistochemical evaluation of the expression of prostate tumor-association markers in the nude mouse human prostate carcinoma heterotransplant lines PC-82, PC-EW, and PC-EG. Prostate 1990, 17:301-316 [DOI] [PubMed] [Google Scholar]
- 8.Presnell SC, Borchert KM, Glover WJ, Gregory CW, Mohler JL, Smith GJ: Isolation and characterization of propagable cell lines (HUNC) from the androgen-sensitive Dunning R3327H rat prostatic adenocarcinoma. Carcinogenesis 1998, 19:585-590 [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Liu A, Dougherty C, Chen X, Guzman R, Nandi S: Beware of contaminating mouse cells in human xenografts from nude mice. Anticancer Res 2000, 20:1635-1640 [PubMed] [Google Scholar]
- 10.Byrne RL, Leung H, Neal DE: Peptide growth factors in the prostate as mediators of stromal epithelial interactions. Br J Urol 1996, 77:627-633 [DOI] [PubMed] [Google Scholar]
- 11.Chung LW: The role of stromal-epithelial interaction in normal and malignant growth. Cancer Surv 1995, 23:33-42 [PubMed] [Google Scholar]
- 12.Hayward SW, Baskin LS, Haughney PC, Foster BA, Cunha AR, Dahiya R, Prins GS, Cunha GR: Stromal development in the ventral prostate, anterior prostate and seminal vesicle of the rat. Acta Anat 1996, 155:94-103 [DOI] [PubMed] [Google Scholar]
- 13.Hayward SW, Rosen MA, Cunha GR: Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol 1997, 79:18-26 [DOI] [PubMed] [Google Scholar]
- 14.Gleave ME, Hsieh JT, von Eschenbach AC, Chung LWK: Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol 1992, 147:1151-1159 [DOI] [PubMed] [Google Scholar]