Abstract
We have examined the role of mechanical tension in myofibroblast differentiation using two in vivo rat models. In the first model, granulation tissue was subjected to an increase in mechanical tension by splinting a full-thickness wound with a plastic frame. Myofibroblast features, such as stress fiber formation, expression of ED-A fibronectin and α-smooth muscle actin (α-SMA) appeared earlier in splinted than in unsplinted wounds. Myofibroblast marker expression decreased in control wounds starting at 10 days after wounding as expected, but persisted in splinted wounds. In the second model, granuloma pouches were induced by subcutaneous croton oil injection; pouches were either left intact or released from tension by evacuation of the exudate at 14 days. The expression of myofibroblast markers was reduced after tension release in the following sequence: F-actin (2 days), α-SMA (3 days), and ED-A fibronectin (5 days); cell density was not affected. In both models, isometric contraction of tissue strips was measured after stimulation with smooth muscle agonists. Contractility correlated always with the level of α-SMA expression, being high when granulation tissue had been subjected to tension and low when it had been relaxed. Our results support the assumption that mechanical tension is crucial for myofibroblast modulation and for the maintenance of their contractile activity.
Myofibroblasts are present in certain normal tissues, in healing wounds, and in fibrocontractive diseases. 1-3 Wound contraction takes place when granulation tissue is populated with myofibroblasts 4 that are characterized by the expression of α-smooth muscle actin (α-SMA), 5 the actin isoform typical of vascular smooth muscle cells. 6 Incorporation of α-SMA into microfilament bundles or stress fibers has been suggested to mediate granulation tissue contraction in vivo, 3 and was shown to promote myofibroblast contraction in vitro. 7-9 Stress fibers have been proposed to function as contractile organelles 10,11 and their presence has been correlated with the production of isometric tension. 12 The factors regulating the transition between fibroblastic and myofibroblastic phenotypes are not fully identified. Transforming growth factor-β1 (TGF-β1) is accepted to be the major cytokine involved in α-SMA expression and myofibroblast differentiation 13,14 through the specific binding to TGF-β-receptor type II (TGF-β-RII). 15,16 This TGF-β effect depends on the presence of the fibronectin (FN) splice variant ED-A FN in the extracellular matrix. 17
Isometric tension has been shown to be important in vitro for the development of fibroblast contractile features such as stress fiber formation 18-20 and α-SMA expression. 21 Mechanical tension alters FN fiber assembly 22 and fibroblast response to growth factors; 23,24 it also prevents fibroblast apoptosis. 19 An altered mechanical load of the matrix stimulates fibroblasts to adjust their contractile activity to achieve tensional homeostasis. 25 Moreover, isometric tension modulates signaling mechanisms regulating fibroblast contraction. 26 Most previous studies on the effect of mechanical tension on fibroblastic cells have been restricted to the in vitro use of collagen substrates with different mechanical properties. 27 In this work, we have used an in vivo approach using two different rat models in view of evaluating: the role of mechanical load in myofibroblast modulation (α-SMA expression in particular) and in granulation tissue contractility. In the first model, the contraction of full thickness wound granulation tissue was prevented by splinting with a plastic frame. In the second model, the wall of rat granuloma pouches 4 was released from mechanical tension by evacuating the pouch exudate. In both models isometric contraction and expression of the myofibroblast markers F-actin, ED-A FN, α-SMA, TGF-β-RII, and TGF-β1 were evaluated. Our results indicate that mechanical tension is crucial for differentiation and maintenance of the myofibroblast phenotype. In addition, we show that tissue contractility correlates with the expression of α-SMA.
Materials and Methods
Preparation of Granulation and Granuloma Pouch Tissue
A total of 96 female Wistar rats (200 to 220 g) was used. After shaving the skin, full-thickness 25 × 25-mm wounds, including the cutaneous muscle, were made using surgical scissors in the middle of the dorsum on the first day of the experiments. Wounds were either allowed to heal spontaneously (unsplinted) or were subjected to mechanical tension (splinted) by fixing their edges on a 25 × 25-mm plastic frame using surgical thread (Supramid USP 2-0 DS 19; B. Braun-SSC AG, Emmenbrügge, Switzerland). Rats were sacrificed by CO2 anesthesia and tissues were dissected daily for 2 to 12 days after wounding. In a second series of experiments that investigated the effect of tension release, wounds were splinted for 7 days, the plastic frame was then removed and animals were sacrificed at 1 and 2 days thereafter. Granulation tissue from 8-day and 9-day unsplinted and splinted wounds, served as controls. Granulation tissues were cleaned from the scab and transferred to ice-cold Krebs-Henseleit solution (118.7 mmol/L NaCl, 4.7 mmol/L KCl, 1.17 mmol/L KH2PO4, 2.41 mmol/L MgSO4, 5.05 mmol/L CaCl2, 11.1 mmol/L d-glucose, 25.0 mmol/L NaHCO3), bubbling with 95% O2 and 5% CO2.
Granuloma pouches were induced in rats by injecting 15 ml of air into the dorsal subcutaneous tissue, followed by 1 ml of 1% croton oil in maize oil. 28,29 This treatment produces the formation of an abscess-like structure whose wall is rich in myofibroblasts 4,29,30 and has been used to study the effects of several smooth muscle contraction-inducing and -inhibiting agents on the isometric force development by a myofibroblastic tissue. 29,30 Pouches were either left intact or released from internal tension by evacuating ∼3 ml of the exudate after 14 days using an 18-gauge needle. Evacuation was repeated every 2 days to prevent new development of tension. To control whether the removal of exudate has other effects than tension release, we replaced it with an equal volume of physiological saline in an additional group. To study the role of TGF-β1 in granuloma pouch wall development, we injected daily 14-day-old granuloma pouches with 1 ml of soluble TGF-β-RII (final, 1 μg/ml muTGF-β-sR; gift of Dr. V. Koteliansky, Biogen Inc., Cambridge, MA) 31,32 for 7 days in another group. Tension-released granuloma pouches were dissected after 15, 16, 17, 18, and 21 days, intact pouches after 14 days and 21 days. Dissected pouches were transferred to ice-cold Krebs-Henseleit solution, bubbling with 95% O2 and 5% CO2, and thoroughly washed. Only tissue from the dorsal portion of granuloma pouches was used to standardize experiments. As control we used normal dermis and dorsal fascia covering the muscular layer. Fascia was exposed after full-thickness incision of skin and subcutaneous tissue and removed using fine forceps and scissors. Dermis was prepared by removing the epidermis and subcutaneous tissue with a scalpel blade. Fascia and normal dermis were further treated as described for granulation tissue and granuloma pouch.
Isometric Force Measurement
Dissected tissues were cut into strips of 5 × 10 mm in transversal orientation to the animal body axis. These were transferred into an organ bath containing 5 ml of Krebs-Henseleit solution bubbling with 95% O2 and 5% CO2 at 37°C and were mounted on the levers of an isometric force displacement transducer (FT03; Grass Instrument Co., West Warwick, RI). A resting tension of 1.5 g was applied in steps of 0.5 g with a 30-minute interval followed by 2 hours of equilibration. Tissue contraction was documented on an oscillograph writer (7WC16H; Grass Instrument Co.) and quantified by converting the analogue data with a MacLab 8/Chart digitization system (AD Instruments Pty. Ltd., Castle Hill, Australia). In a first series of experiments maximal tension development was determined after cumulative stimulation with various agonists of granulation tissue contraction in concentrations ranging from 10−10 mol/L to 10−4 mol/L. For all subsequent experiments agonists were applied at the following concentrations: angiotensin-II (AT-II; Bachem AG, Bubendorf, Switzerland), 10−5 mol/L; endothelin-1 (ET-1; Bachem), 10−7 mol/L; serotonin (Sigma Chemical Co., St. Louis, MO), 10−4 mol/L; histamine (Sigma Chemical Co.), 10−4 mol/L; KCl, 4 × 10−3 mol/L. Tension peaks were registered and mean values were calculated from four tissue strips per animal and three to five animals per experimental condition.
Immunohistochemistry, Confocal Laser-Scanning Microscopy, and Quantification of F-Actin by Fluorescence Image Analysis
Strips from granuloma pouch and wound granulation tissues were fixed in 4% neutral-buffered formalin and embedded in paraffin. Sections of 4 μm were stained with hematoxylin and eosin or with Masson’s trichrome. For immunohistochemistry deparaffinized sections were immersed in methanol containing 0.5% H2O2 for 10 minutes. Sections were incubated with primary antibodies either overnight at 4°C (anti-TGF-β-RII, rbAb; Santa Cruz Biotechnology Inc., Heidelberg, Germany) or 60 minutes at room temperature (anti-TGF-β1, rbAb; Santa Cruz), followed by 30 minutes of incubation with a secondary biotinylated goat anti-rabbit antibody (DAKO, Copenhagen, Denmark). Presence of the specific protein was evaluated by means of the streptavidin-biotin complex peroxidase method (DAKO) and peroxidase activity was visualized with 3-amino-9-ethylcarbazole (Sigma). Slides were counterstained with hematoxylin and mounted in Eukit. Pictures were acquired using a Zeiss Axiophot microscope equipped with Plan-Neofluar ×20/0.50 objective (Carl Zeiss Inc., Oberkochen, Germany) and with a digital color camera (Coolview; Photonic Science, London, UK).
For confocal laser-scanning microscopy tissue strips were snap-frozen in precooled liquid isopentane and embedded in OTC™ resin (Miles Scientific, Naperville, IL). Cryosections of 4 μm were stained for α-SMA (anti-αSM-1, IgG2a mAb), 6 F-actin (phalloidin-Alexa 488; Molecular Probes, Eugene, OR), ED-A FN (IST-9, IgG1 mAb; gift from Dr. L. Zardi, National Institute for Cancer Research, Laboratory of Cell Biology, Genoa, Italy), 33,34 desmin (D33, IgG1 mAb; DAKO), smooth muscle myosin heavy chain (SMMHC, rb polyclonal Ab 35 or BT-562, rb polyclonal Ab; Biomedical Technologies, Stoughton, MA), and non-muscle myosin heavy chain (NMMHC, rb polyclonal Ab) 35 and DNA [4,6-diamidino-2-phenylindole (DAPI)]. As secondary antibodies goat anti-mouse IgG2a tetramethylrhodamine B isothiocyanate-conjugated, goat anti-mouse IgG1 fluorescein isothiocyanate-conjugated, and goat anti-rabbit tetramethylrhodamine B isothiocyanate-conjugated (Jackson ImmunoResearch Laboratories, West Grove, PA) were used. All slides were mounted in buffered polyvinyl alcohol. Images were taken by means of confocal laser-scanning microscopy (LSM 410, Zeiss) using a Plan-Neofluar ×10/0.50 objective or a ×63/1.4 Plan-Neofluar oil immersion objective with a digitally extended focus depth of 1 μm, reconstructed from five optical sections at 0.2-μm z-resolution. All digital images were processed for printing using Adobe Photoshop and printed with a digital Fujifilm Pictrography 4000 printer (Fujifilm, Tokyo, Japan).
To quantify F-actin by fluorescence image analysis 4-μm sections of granulation tissue strips were fixed and triple-stained for F-actin (red), desmin (green), and cell nuclei (blue) as described above. For a given region of interest digital images with a resolution of 515 × 512 pixels were taken at red-, green-, and UV-light excitation, respectively, using a Plan-Neofluar ×20/0.50 objective (Zeiss), color-camera (Coolview, Photonic Science Ltd.), and image grabber software (ImageAccess V2.04K; Imagic Bildverarbeitung AG, Glattbrugg, Switzerland). Constant gain values and integration times were used for the digital camera to obtain similar exposure times for all specimens. To determine the level of F-actin, images were then automatically processed by KS400 software (Zeiss). First, the three 24-bit Red · Green · Blue-images per region were converted to 8-bit gray value images with pixel intensity values ranging from 0 (black) to 255 (white). Desmin staining, specific for vascular smooth-muscle cells, was automatically selected by a thresholding process and corresponding pixel values were set to 255. To exclude vessels from F-actin quantification, desmin-positive areas were then subtracted from F-actin and DAPI images. Second, the background of the desmin-corrected F-actin image was calculated from a manually chosen F-actin-negative area and subtracted from the mean pixel intensity of the whole image, thereby quantifying the level of F-actin expression in the region of interest. Finally, the F-actin expression level was related to the number of cells in the region, represented by the number cell nuclei calculated from the desmin-corrected DAPI image. Tissue was collected from three animals per experimental condition, five sections were prepared per animal, and five regions of interest were analyzed per section.
Western Blot Analysis
Dissected tissues were immediately frozen in liquid nitrogen, crushed, and dissolved in sample buffer: 10 mmol/L EGTA (Sigma), 1 mmol/L Nα-tosyl-l-arginine methyl ester hydrochloride (TAME; Fluka, Buchs, Switzerland), 1 mmol/L phenylmethyl sulfonyl fluoride (Fluka), 4% aprotinin (Bayer AG, Zurich, Switzerland), 0.5 mmol/L benzamidine (Sigma), and 1 mmol/L di-isopropylfluorophosphate (Fluka). Samples were sonicated (Branson Sonic Power Company, Danbury, CT), boiled for 3 minutes, and protein concentration was determined according to Bradford as described previously. 36 Equal amounts of total protein (5 to 20 μg) were loaded to 10 or 7% sodium dodecyl sulfate minigels (Bio-Rad Laboratories AG, Glattbrugg, Switzerland), separated by polyacrylamide gel electrophoresis, and transferred to nitrocellulose membrane (Protran; Schleicher & Schuell, Dassel, Germany). Membranes were then probed with the primary antibodies: anti-α-SMA, (αSM-1), β-actin (β74, rbAb), 37 total-actin (β74+AL-20), ED-A FN (IST-9), desmin (DAKO), SMMHC, NMMHC, 35 and TGF-β-RII (Santa Cruz). Secondary goat anti-mouse and anti-rabbit antibodies, conjugated with horseradish-peroxidase (Jackson ImmunoResearch Laboratories) were used and signals were detected by ECL chemiluminescence (Amersham, Rahn AG, Zürich, Switzerland). Subsequently, membranes were stripped of bound antibodies and reprobed for vimentin (mAb, clone V9; DAKO). Bands were digitized with a scanner (Arcus II; Agfa, Köln, Germany) and the ratio between all band densities of one blot was calculated by commercial computer software (ImageQuant V3.3l Molecular Dynamics, Sunnyvale, CA). Relative protein expression was normalized to the respective values for vimentin. Samples from three different animals per experimental condition were tested and protein/vimentin mean ratio was calculated from three scanned Western blots per animal sample.
Statistical Analysis
Quantitative results are presented as mean values ± SD. Mean values were tested for the likelihood to belong to the same numeric population by means of a two-tailed heteroscedastic Student’s t-test. Differences were considered to be statistically significant at values of P ≤ 0.01. In graphs, differences with statistical significance are indicated by filled symbols. In column charts P values ≤ 0.01 are indicated by an asterisk (*) and P ≤ 0.005 by a double asterisk (**). Positive linear correlation between isometric tissue contraction and expression of myofibroblast markers, quantified by Western blot analysis was statistically tested by calculating the square of the Pearson correlation product (r 2 value).
Results
Increased Mechanical Tension Enhances the Contractility of Wound Granulation Tissue
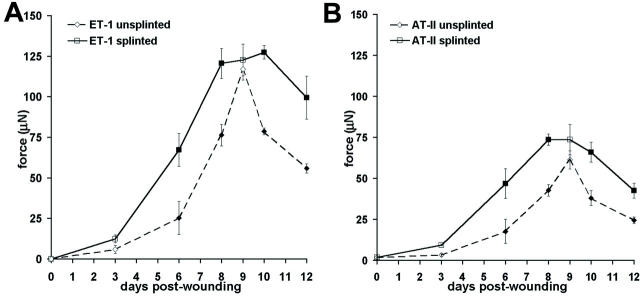
It has been shown that fibroblasts subjected to mechanical tension develop a contractile phenotype in vitro. 20,21 To test whether mechanical tension induces similar effects on fibroblasts in vivo, we have first increased the mechanical tension of rat granulation tissue by splinting wounds with plastic frames and compared the contractility of tissues from splinted and unsplinted wounds (Figure 1) ▶ . Isometric contraction was stimulated with various smooth muscle agonists in the following order of efficiency: ET-1 (10−7 mol/L), AT-II (10−5 mol/L), and serotonin (10−4 mol/L). Wound tissue contracted only weakly after stimulation with histamine (10−4 mol/L) and potassium (4 × 10−3 mol/L). Control tissues (0 days) did not show any significant response after stimulation with all agonists (not shown). The contraction of unsplinted and splinted granulation tissue increased from 3-day-old to 9-day-old tissue after stimulation with ET-1 (Figure 1A) ▶ ; moreover, splinted tissues contracted more strongly at 6 days (266%) and 8 days (185%) compared to unsplinted tissues. Surprisingly, splinted and unsplinted tissues exhibited comparable contraction after 9 days. At 10 days the contractile activity of unsplinted tissues decreased (67% compared to 9 days), whereas the contractile activity of splinted tissues was unchanged compared to 9 days. At 12 days unsplinted tissues showed a further decrease in contractility (71% compared to 10 days); splinted wounds also decreased their contractility compared to 10 days (78%) but maintained a higher contractility (168%) compared to unsplinted tissues. Results obtained after stimulation with AT-II (Figure 1B) ▶ and serotonin (not shown) were similar to those obtained after ET-1 stimulation.
Figure 1.
Isometric contraction of wound granulation tissue. Maximum contraction of tissue strips taken from 3- to 12-day-old wound granulation tissue and normal dermal tissue (0 days) was quantified after stimulation with ET-1 (A) and AT-II (B). Tissue from splinted wounds (continuous line) exhibits significantly higher contractility already at early stages of development (6 days) and maintains high levels of contractility up to 10 days after wounding compared to unsplinted granulation tissue (dashed line). Normal dermal tissue shows no significant contractility (0 days). Bars indicate SD of mean values. Filled squares indicate P ≤ 0.01 for comparison between unsplinted and splinted tissue of the same age.
Mechanical Tension Results in an Early Expression of Myofibroblast Markers in Wound Granulation Tissue
To assess whether mechanical tension stimulates myofibroblast differentiation in vivo, we compared the expression of myofibroblast markers such as F-actin, ED-A FN, α-SMA, TGF-β-RII, TGF-β1, desmin, SMMHC, and NMMHC in the different situations. F-actin was not present in fibroblasts of normal dermis (not shown), started to be visible in 3-day-old unsplinted wounds (Figure 2Aa ▶ ) and gradually increased (Figure 2, Ab to Ad ▶ ). Splinting induced noticeable accumulation of F-actin already at 1 day (not shown) and importantly enhanced F-actin levels throughout the following days (Figure 2, Ae to Ah ▶ ). Moreover, splinting improved the alignment of F-actin along the lines of mechanical tension, ie, parallel to the wound surface. F-actin bundles were devoid of α-SMA up until day 6 in unsplinted and until day 4 in splinted tissue, as evaluated by double staining (not shown). Quantification of Alexa 488-phalloidin fluorescence intensity confirmed higher levels of F-actin in splinted compared to unsplinted granulation tissues at any wound age (Figure 2B ▶ , between 376 and 226% increase). No significant changes of marker distribution were observed in the different wound locations. Quantification of granulation tissue cellularity by means of DAPI staining demonstrated a linear increase in cell number from 3- to 12-day-old granulation tissue (120%) and no difference between unsplinted and splinted tissue. ED-A FN was detected in low levels in 3-day-old unsplinted granulation tissue (Figure 2Aa ▶ ), expression increased between day 6 and day 9 (Figure 2, Ab and Ac ▶ ), and was reduced in 12-day-old granulation tissue (Figure 2Ad ▶ ). Splinting induced ED-A FN expression starting from day 1 (not shown) and enhanced its expression compared to unsplinted granulation tissue at all stages of wound healing (Figure 2, Ae to Ah ▶ ). No differences in vascularity were observed in splinted versus unsplinted wounds.
Figure 2.
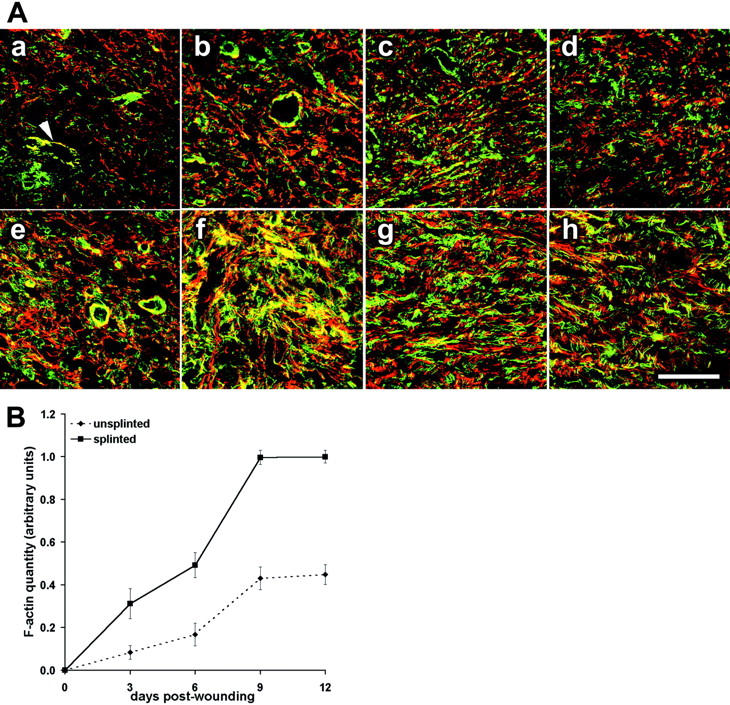
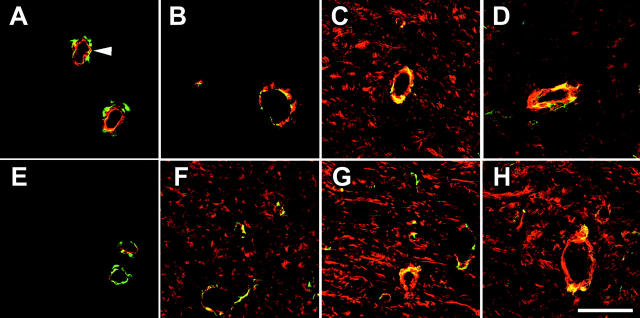
Mechanical tension enhances expression of F-actin and ED-A FN in wound granulation tissue. A: Sections of 3-day-old (Aa, Ae), 6-day-old (Ab, Af), 9-day-old (Ac, Ag), and 12-day-old (Ad, Ah) granulation tissue were double-stained for F-actin (green) and ED-A FN (red) and examined by confocal laser-scanning microscopy. Tissues from unsplinted wounds (Aa–Ad) are compared to those from splinted wounds (Ae–Ah). B: F-actin expression in unsplinted (dashed line) and splinted granulation tissue (continuous line) from 3 days to 12 days assessed by image analysis of immunostained tissue sections (normal dermis = 0 days). In 3-day-old unsplinted granulation tissue (Aa) fibroblastic cells exhibit low levels of de novo-appearing F-actin and ED-A FN whereas fibroblasts in 3-day splinted tissue (Ae) already show important expression and co-localization of the two proteins (yellow); co-localization is also seen in vascular smooth muscle cells (Aa, arrowhead). Expression of both proteins gradually increases and with partial co-localization at 6 days to 12 days after wounding. At any wound age fibroblastic cells of splinted wound granulation tissue exhibit higher expression of ED-A FN and F-actin compared to control (B). Scale bar, 50 μm.
α-SMA was not expressed in fibroblasts of normal dermis (not shown) and of 3-day-old granulation tissue (Figure 3, A and E) ▶ , and was not detected in fibroblasts of 6-day-old unsplinted granulation tissue (Figure 3B) ▶ . In unsplinted wounds, it was first detected after 7 days (not shown), was strongly expressed after 9 days (Figure 3C) ▶ , and expression was clearly reduced after 12 days (Figure 3D) ▶ . In contrast, splinting induced α-SMA expression by granulation tissue fibroblasts already at 4 to 6 days after wounding (Figure 3F) ▶ ; the level of α-SMA expression increased in splinted tissue to a maximum at 9 days (Figure 3; E to G ▶ ) and remained at an apparently similar level at 12 days (Figure 3H) ▶ . Three-day-old to 12-day-old splinted wounds exhibited increased expression of both TGF-β1 and TGF-β-RII; however, no clear differences were observed between splinted and unsplinted wound tissue by means of immunohistochemistry (not shown). Desmin and SMMHC were not expressed by granulation tissue fibroblasts, and expression of NMMHC did not change in any condition as tested by means of immunofluorescence and Western blotting (not shown).
Figure 3.
Wound splinting promotes early expression of α-SMA in granulation tissue. Sections of 3-day-old (A, E), 6-day-old (B, F), 9-day-old (C, G), and 12-day-old (D, H) granulation tissue were double-stained for α-SMA (red) and desmin (green). Protein expression in tissue from unsplinted wounds (A–D) is compared to that in splinted wound tissue (E–H). α-SMA is constitutively expressed by smooth muscle cells, co-localizing with desmin in vessels (A, arrowheads). Fibroblastic cells in unsplinted wound tissue express α-SMA at 9 days after wounding (C) and exhibit decreased expression levels after 12 days (D). Mechanical tension results in expression of α-SMA already at 6 days (E) that gradually increases up to 12 days (H). Scale bar, 50 μm.
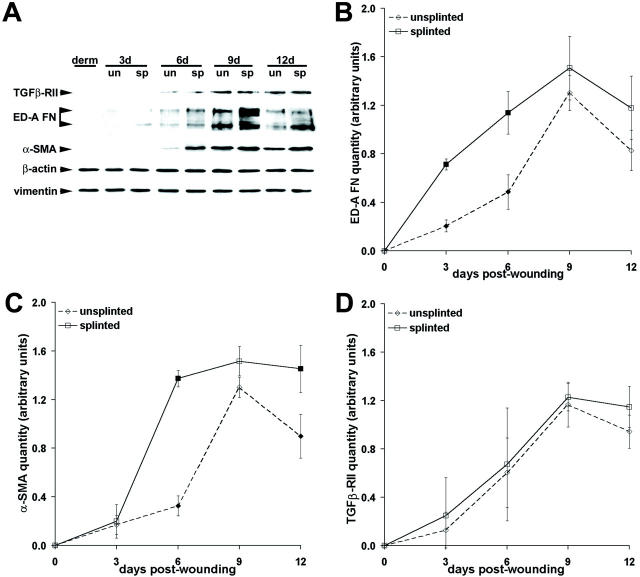
Analysis of myofibroblast marker expression in wound granulation tissue by Western blotting (Figure 4) ▶ essentially confirmed immunolocalization results. In unsplinted tissue ED-A FN expression was continuously increased from 3 days to 9 days (630%) and exhibited a reduction by 160% between 9 days and 12 days (Figure 4, A and B) ▶ . Splinting enhanced the amount of ED-A FN (Figure 4A) ▶ with the largest difference at 3 days (Figure 4B ▶ , 347%). Expression of α-SMA moderately increased from 3-day to 6-day unsplinted tissue (195%), steeply increased from 6 days to 9 days (400%), and was reduced by 145% between 9 days and 12 days (Figure 4, A and C) ▶ . Splinted tissue exhibited increased α-SMA expression levels at 6 days (Figure 4C ▶ , 420%) and showed similar α-SMA expression at 9 days compared to unsplinted granulation tissue. Splinting maintained high α-SMA expression levels at 12 days in contrast to the decreased expression in unsplinted wound tissue (Figure 4C) ▶ . TGF-β-RII expression increased from 0 to 12 days after wounding with no significant difference between splinted and unsplinted granulation tissue (Figure 4, A and D) ▶ . To relate changes in myofibroblast marker expression to enhanced tissue contractility during the evolution of unsplinted and splinted wound granulation tissue, we calculated correlation coefficients. Highest correlation was observed between the temporal course of ET-1-stimulated tissue contraction (Figure 1A) ▶ and expression of α-SMA (r 2 = 0.98). Lower correlation was calculated for TGF-β-RII (r 2 = 0.92), ED-A FN (r 2 = 0.90), and F-actin (r 2 = 0.84), no correlation for β-actin (r 2 = 0.69), NMMHC (r 2 = 0.61), desmin (r 2 = 0.57), and vimentin (r 2 = 0.55).
Figure 4.
Quantification of myofibroblast markers in granulation tissue by means of Western blot. A: Expression of β-actin, vimentin, and the myofibroblast markers TGFβ-RII, ED-A FN, and α-SMA was investigated. Extracts of 3- to 12-day-old granulation tissue from splinted wounds (sp) are compared to those of unsplinted wounds (un) and of normal dermis (derm). Expression of myofibroblast markers gradually increases in evolving granulation tissues up to 9 days after wounding. No change in expression of β-actin and vimentin is observed. B–D: Protein bands were scanned, quantified by image analysis, and normalized to vimentin. Expression of ED-A FN is significantly higher in 3 day and 6 day splinted tissue compared to the respective control (B), as observed for α-SMA expression at 6 days and 12 days (C). Expression of TGFβ-RII is similar in unsplinted and splinted wound granulation tissue at any age (D). Filled squares indicate P ≤ 0.01 for comparison between unsplinted and splinted tissue of the same age.
Loss of Mechanical Tension Decreases Tissue Contractility and Expression of Myofibroblast Markers
Because increased mechanical tension seems to induce myofibroblast differentiation and increase granulation tissue contractility, we sought to investigate whether reduction of tension may have the opposite effect. First, wounds were splinted for 7 days, then released from tension for 1 or 2 days and compared to granulation of the same age but still splinted. One-day-released granulation tissue contracted similarly compared to 8-day splinted tissue (96 ± 7%). However, tension release for 2 days significantly reduced contraction compared to 9-day splinted tissue to 63 ± 3% after stimulation with AT-II and to 63 ± 2% after stimulation with ET-1. Contraction of 2-day-released granulation tissue was also lower (71 ± 4%) when compared to 9-day-unsplinted granulation tissue. Characterization of released tissue by immunostaining and Western blotting revealed no changes after 1 day, but a decrease of F-actin and α-SMA after 2 days. Expression levels of ED-A FN, TGF-β-RII, TGF-β1, NMMHC, desmin, and vimentin were not significantly changed 2 days after release (not shown).
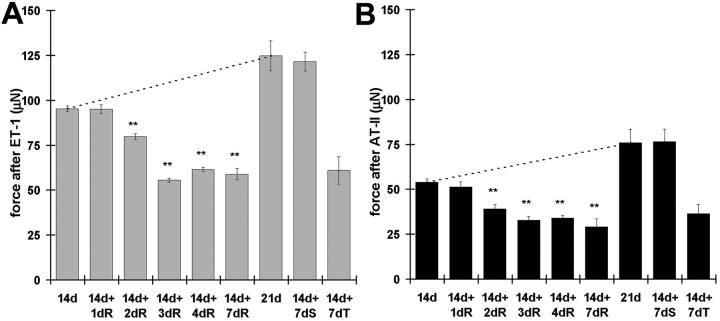
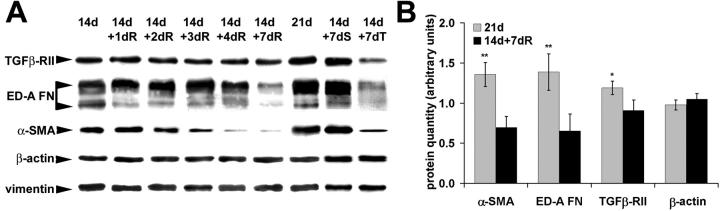
To investigate further the chronological relationship between tissue contraction and myofibroblast modulation after release of mechanical tension, we used the granuloma pouch model. 29 Tension was released in granuloma pouch tissue at 14 days by evacuating the exudate and isometric contraction was measured 1 to 7 days after release (Figure 5) ▶ . Contraction of 21-day control pouch tissue was maximally stimulated by ET-1 (10−7 mol/L) and by the following agonists in percentage of ET-1-response: AT-II (10−5 mol/L), 60%; serotonin (10−4 mol/L), 48%; potassium (4 × 10−3 mol/L), 45%; and histamine (10−4 mol/L), 6%. Results are presented for ET-1 (Figure 5A) ▶ and AT-II (Figure 5B) ▶ . Tissue contractility was higher in intact 21-day-old compared to 14-day-old pouches (Figure 5 ▶ , dashed lines), confirming previous observations. 30,38 Release of mechanical tension at 14 days decreased pouch tissue contraction beginning 2 days after release, exhibited a minimum at 3 days after release (14d+3dR), and kept this level until 7 days. Compared to intact pouches of the same age (21 days), tension release resulted in a significantly lower tissue contraction (38% for ET-1 and 46% for AT-II). As a control, we substituted the evacuated exudate with an identical volume of physiological saline for 7 days (Figure 5 ▶ , 14+7dS). Saline-injected pouch tissue contraction did not differ from intact pouches of the same age (21 days).
Figure 5.
Loss of mechanical tension decreases granuloma pouch tissue contraction. Maximum contraction of tissue strips from granuloma pouches was quantified after stimulation with ET-1 (A) and AT-II (B). Pouches were either left intact for 14 days and 21 days or were released from tension by removing exudate after 14 days for 1 to 7 days. A significant increase in tissue contraction is observed between 14-and 21-day-old intact pouches (dashed lines). Loss of tension decreases granuloma pouch tissue contractility time-dependently and starts 2 days after release (14d+2dR). Note the dramatic difference in tissue contractility between 21-day intact pouches (21 day) and 21-day-old pouches, which were released for 7 days (14d+7dR). Exchange of exudate by physiological saline shows no effect (14d+7dS), whereas injection of TGF-β-sRII significantly decreases pouch tissue contraction (14d+7dT). **, P ≤ 0.005 compared to tissue contraction mean values of 14-day intact pouches.
Alexa 568-phalloidin (Figure 6 ▶ , insets) revealed an intense F-actin staining in 14-day-old granuloma pouch fibroblasts, that was clearly decreased already 2 days after release (Figure 6B) ▶ ; this decrease was more important at 7 days after release (Figure 6C ▶ , inset). ED-A FN and α-SMA were co-localized and their expression increased in 21-day-old compared to 14-day-old intact granuloma pouch tissue (Figure 6, A and D) ▶ . Three days after tension release α-SMA expression was reduced (Figure 6B) ▶ ; 7 days after tension release, ED-A FN expression was also reduced and α-SMA was hardly detectable (Figure 6C) ▶ . TGF-β1, previously shown to be expressed in granuloma pouch tissue, 39,40 and TGF-β-RII were both predominantly expressed in the external collagen-rich layer of the pouch tissue. TGF-β1 and TGF-β-RII expression patterns in 7-day-released pouches did not differ significantly from 1- to 5-day-released tissue or 21-day intact pouch tissue, but the thickness of the collagen-rich layer decreased progressively during the 7-day period of tension release to ∼70% compared to 21-day intact pouches (not shown).
Figure 6.
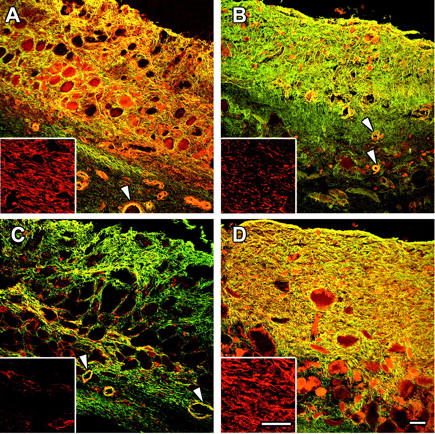
Loss of mechanical tension reduces myofibroblast differentiation in granuloma pouch tissue. Tissue sections of intact (A, D) and tension-released granuloma pouches (B, C) of different ages were double-stained for α-SMA (red) and ED-A FN (green) or stained for F-actin (insets, red) and examined by confocal laser-scanning microscopy. α-SMA and ED-A FN show important expression by fibroblastic cells and co-localization (yellow) in tissue from 14-day (A) and 21-day (D) intact granuloma pouches, as well as in vascular smooth muscle cells (arrowheads). Loss of tension in 14-day pouches results in decreased levels of F-actin and α-SMA expression 3 days after release (B) followed by decreased expression of ED-A FN 7 days after release (C). Note that expression of both proteins in small vessels is not affected by pouch relaxation. Scale bars, 50 μm.
As determined by Western blotting, α-SMA was significantly reduced 2 to 3 days after release, compared to 14-day-old intact pouch tissue, whereas decrease of ED-A FN expression started 4 days after release (Figure 7) ▶ . TGF-β-RII expression was only moderately diminished throughout the 7-day period after release and no change was observed for β-actin and vimentin expression (Figure 7A) ▶ . Compared to 21-day-old intact pouch tissue, 7-day tension release reduced α-SMA expression to 51%, ED-A FN to 47%, and TGF-β-RII to 81% (Figure 7B) ▶ . In contrast to wound granulation tissue fibroblasts, which did not express desmin and SMMHC, low expression of both proteins was observed in fibroblastic cells of 14-day and 21-day intact granuloma pouches. Tension release did not change desmin and NMMHC expression and only moderately diminished expression of SMMHC 7 days after release as evaluated by Western blotting and immunofluorescence (not shown). When removed exudate was replaced by physiological saline, no changes in protein expression were detected (Figure 7A ▶ , 14d+7dS).
Figure 7.
Quantification of myofibroblast markers in granuloma pouch tissue by means of Western blot. A: Protein expression in intact granuloma pouches (14d, 21d) was compared to 14-day-old pouches, which were subsequently released from mechanical tension for 1 to 7 days. Expression of α-SMA gradually decreases starting 2 days after release (14d+2dR), whereas decrease in ED-A FN expression occurs later (14d+7dR) after release. TGF-β-RII expression shows a moderate decrease after 7 days and no change is observed for β-actin and vimentin expression. Exchange of exudate by saline has no effect (14d+7dS), whereas injection of TGF-β-sRII significantly decreases expression of all myofibroblast markers (14d+7dT). B: Protein bands obtained from 21-day intact (21d) and 7-day-tension-released (14d+7dR) pouch tissue were scanned, their density was quantified by image analysis, and obtained values were normalized to vimentin. Tissue from 7-day-released pouches exhibits significantly reduced levels of α-SMA and ED-A FN expression, moderately reduced TGF-β-RII expression and no change in β-actin compared to 21-day intact pouches. *, P ≤ 0.01; **, P ≤ 0.005.
Injection of soluble TGF-β-RII into the granuloma pouch for 7 days decreased tissue contraction to 49% after stimulation with ET-1 (Figure 5A ▶ , 14d+7dT) and to 48% after AT-II stimulation (Figure 5B ▶ , 14d+7dT) compared to 21-day-old control pouch tissue. It reduced the expression levels of TGF-β, TGF-β-RII, ED-A FN, F-actin, and α-SMA significantly compared to 21-day-old control pouch tissue as demonstrated by Western blotting (Figure 7A) ▶ and immunofluorescence (not shown).
Discussion
It is accepted that mechanical tension plays an important role in the modulation of cultured fibroblasts into myofibroblasts by favoring the formation of stress fibers and the expression of α-SMA. 27 Here, we demonstrate that mechanical tension also influences myofibroblast differentiation in vivo. Fibroblasts populating the granulation tissue of splinted wounds exhibit earlier formation of stress fibers, expression of ED-A FN and of α-SMA compared to nonsplinted wounds. Inversely, release of tension in wound granulation tissue and in granuloma pouch leads to a sequential loss of stress fibers, α-SMA, and ED-A FN. Moreover, our study indicates a correlation between the level of α-SMA expression and contractility of the tissues obtained during the different experimental situations. The acquisition of contractile activity by granulation tissue on stimulation with smooth muscle agonists is a well-accepted phenomenon and has been suggested to represent a useful indication of the retractile potential of granulation tissue. 29,30,41 Acquisition of an increased contractile activity by wound splinting 42 has been correlated to the induction of microfilament bundles in fibroblasts. 18 Our results corroborate these observations and establish a correlation between changes in tissue contractility and cytoskeletal protein expression and/or organization after mechanical load modulation.
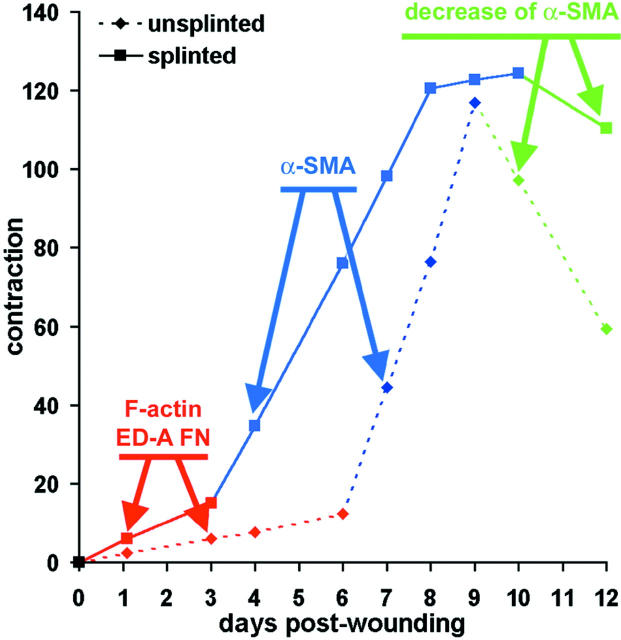
In both splinted and unsplinted wounds, the granulation tissue strip contractile activity evolves according to three phases, as depicted schematically in Figure 8 ▶ . During the first phase (red) the contractility of granulation tissue increases slowly, whereas during the second phase (blue), the increase is steep; the third phase (green) corresponds to a decrease of contractile activity. In the first phase there is an increase of F-actin and of ED-A FN expression that is clearly more important in splinted wounds. The onset of high contractile activity correlates always with the de novo expression of α-SMA, assembled into stress fibers. It is noteworthy that the first phase is shorter and the second phase is longer in splinted compared to unsplinted wounds and corresponds to the maximal level of α-SMA expression. Interestingly, the maximal level of contractile capacity is similar in both situations. Finally, a decrease in contractile activity takes place more slowly in splinted than in unsplinted wounds. The observed correlation between α-SMA expression and granulation tissue contractility is in agreement with the previously reported correlation between α-SMA expression and contraction of fibroblast-populated anchored collagen gels. 8,9 More recently we have shown that 3T3 fibroblasts exhibit a significantly higher contractile activity after transient and/or stable transfection with α-SMA cDNA compared to fibroblasts transfected with β-actin, γ-actin, or even α-cardiac actin cDNA in the absence of any change of myosin heavy chain expression. 7 All these data are compatible with the assumption that α-SMA expression alone plays a role in the enhancement of fibroblast contractility in vitro. Our results allow to hypothesize that also in vivo α-SMA expression plays a crucial role in the development of granulation tissue contractile capacity. In all situations, increased tension results in a greater expression of α-SMA and an increased force production, whereas decreased tension has the opposite effect. 2,21,43,44 Although in vivo and in vitro data indicate that α-SMA expression is stimulated by mechanical stress, it remains unclear how fibroblasts translate the physical signal into protein expression. In cells such as cardiomyocytes, endothelial and smooth muscle cells, mechanical stress was shown to stimulate the production and secretion of growth factors that mediate stress-induced cell responses. 45 In particular, expression of TGF-β1, the major inducer of α-SMA expression 13,14 and contractility in myofibroblasts, 8,21 is up-regulated in mechanically stressed mesangial cells. 46 Under our conditions the expression of TGF-β1 and of its receptor TGF-β-RII was gradually increased, but similarly in splinted tissue and unsplinted tissues. Nevertheless, blocking TGF-β1 by an excess of soluble receptor without changing the mechanical load reduced α-SMA expression and tissue contraction in our experimental conditions, implying a role of TGF-β1. Enhanced mechanical stress increases growth factor sensitivity 23,24,47 and loss of mechanical tension reduces the growth factor response of cultured fibroblasts. 48,49 It remains to be examined whether TGF-β1 activation changes in our experimental situations.
Figure 8.
Evolution of granulation tissue contractility. The chronological evaluation of granulation tissue contractile activity distinguished three phases characterized by: 1) slow increase (red), 2) steep increase (blue), and 3) decrease (green). These phases correlate with the onset of F-actin and ED-A FN expression (phase 1), de novo expression of α-SMA (phase 2), and decrease of α-SMA expression (phase 3) as indicated by the arrows. The first two phases clearly start earlier and the third phase is delayed in splinted (continuous line) compared to unsplinted wounds (dotted line).
Changes in mechanical stress may directly affect the level of extracellular matrix proteins. 50-52 Recently, it was shown that during wound healing ED-A FN expression 53,54 precedes the appearance of α-SMA-positive myofibroblasts and is essential to mediate TGF-β1-induced α-SMA expression. 17 Splinting induces ED-A FN expression in granulation tissue, suggesting a possible regulatory function of this protein during stress-induced myofibroblast differentiation. Regulation may occur at the level of FN expression, 55 alternative splicing, 56,57 fibril assembly, 22 or accessibility of functional residues in the ED-A domain of FN 58 as it has been demonstrated for plasma FN. 59 Integrins of focal adhesions are potential receptors and transducers of these extracellular signals. 60
When myofibroblasts were released from mechanical stress by removing the wound splint or by evacuating the granuloma pouch, disappearance of α-SMA preceded the decrease of ED-A FN and TGF-β1 levels. Thus, ED-A FN and TGF-β1 are crucial for the induction of α-SMA expression but are not sufficient to maintain myofibroblast differentiation in the absence of a mechanical stimulus. Taking into account the early formation of F-actin during wound healing and the rapid loss of actin stress fibers after load release, we suggest that the physical integrity of stress fibers 61 provides an important prerequisite for α-SMA expression and myofibroblast contraction. Mechanical stress induces actin polymerization and stress fiber formation when applied externally 62 or by increasing intracellular tension. 10,63 The ratio between G-actin/F-actin was shown to directly influence β-actin synthesis. 64,65 However, the role of the G-actin/F-actin ratio for the expression of other actin isoforms, such as α-SMA, has not been evaluated. It is conceivable that mechanical tension induces actin expression without discriminating among isoforms whereas factors such as TGF-β and ED-A FN exert a specific stimulation of α-SMA. 17 A similar phenomenon has been shown for the stress-dependent expression of α-skeletal actin by cardiomyocytes in the presence of AT-II. 45
In conclusion our results show that mechanical tension is a prerequisite for the development and maintenance of myofibroblast differentiation and hence of granulation tissue contraction. Given the reciprocal relationship between fibroblast contractility and the mechanical state of the matrix, the modulation of extracellular and intracellular tension may help to influence wound healing and development of fibrocontractive diseases.
Acknowledgments
We thank Drs. Victor Koteliansky (Biogen Inc., Cambridge, MA) for providing the soluble TGF-β-RII, Luciano Zardi (National Institute for Cancer Research, Laboratory of Cell Biology, Genoa, Italy) for providing IST-9 antibodies, and Jo de Mey (Department of Pharmacology, University of Maastricht, Maastrich, The Netherlands) for expert advice and teaching; A. Geinoz, M. Bacchetta, P. Henchoz, and S. Coutant-Zimmerli for technical assistance; J. C. Rumbeli and E. Denkinger for photographic work; and Mrs. S. Josseron for secretarial work.
Footnotes
Address reprint requests to Giulio Gabbiani, M.D. Ph.D., Department of Pathology, CMU, University of Geneva, 1 rue Michel-Servet, 1211 Geneva 4, Switzerland. E-mail: giulio.gabbiani@medecine.unige.ch.
Supported by the Swiss National Science Foundation Grants 31-61.336.00 and 31-54048.98.
References
- 1.Rønnov-Jessen L, Petersen OW, Bissell MJ: Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996, 76:69-125 [DOI] [PubMed] [Google Scholar]
- 2.Grinnell F: Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol 1994, 124:401-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serini G, Gabbiani G: Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999, 250:273-283 [DOI] [PubMed] [Google Scholar]
- 4.Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 [DOI] [PubMed] [Google Scholar]
- 5.Darby I, Skalli O, Gabbiani G: Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990, 63:21-29 [PubMed] [Google Scholar]
- 6.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G: A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986, 103:2787-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C: Alpha-smooth muscle actin expression up-regulates fibroblast contractile activity. Mol Biol Cell (in press) [DOI] [PMC free article] [PubMed]
- 8.Vaughan MB, Howard EW, Tomasek JJ: Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res 2000, 257:180-189 [DOI] [PubMed] [Google Scholar]
- 9.Arora PD, McCulloch CA: Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 1994, 159:161-175 [DOI] [PubMed] [Google Scholar]
- 10.Burridge K: Are stress fibres contractile? Nature 1981, 294:691-692 [DOI] [PubMed] [Google Scholar]
- 11.Katoh K, Kano Y, Masuda M, Onishi H, Fujiwara K: Isolation and contraction of stress fibers. Mol Biol Cell 1998, 9:1919-1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AK, Stopak D, Wild P: Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981, 290:249-251 [DOI] [PubMed] [Google Scholar]
- 13.Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993, 122:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rønnov-Jessen L, Petersen OW: Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 1993, 68:696-707 [PubMed] [Google Scholar]
- 15.Heldin CH, Miyazono K, ten Dijke P: TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390:465-471 [DOI] [PubMed] [Google Scholar]
- 16.Derynck R, Feng XH: TGF-beta receptor signaling. Biochim Biophys Acta 1997, 1333:F105-F150 [DOI] [PubMed] [Google Scholar]
- 17.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G: The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 1998, 142:873-881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Squier CA: The effect of stretching on formation of myofibroblasts in mouse skin. Cell Tissue Res 1981, 220:325-335 [DOI] [PubMed] [Google Scholar]
- 19.Grinnell F, Zhu M, Carlson MA, Abrams JM: Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res 1999, 248:608-619 [DOI] [PubMed] [Google Scholar]
- 20.Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB: Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec 1992, 232:359-368 [DOI] [PubMed] [Google Scholar]
- 21.Arora PD, Narani N, McCulloch CA: The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol 1999, 154:871-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliday NL, Tomasek JJ: Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp Cell Res 1995, 217:109-117 [DOI] [PubMed] [Google Scholar]
- 23.He Y, Grinnell F: Stress relaxation of fibroblasts activates a cyclic AMP signaling pathway. J Cell Biol 1994, 126:457-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingber DE, Folkman J: Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol 1989, 109:317-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M: Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol 1998, 175:323-332 [DOI] [PubMed] [Google Scholar]
- 26.Grinnell F, Ho CH, Lin YC, Skuta G: Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem 1999, 274:918-923 [DOI] [PubMed] [Google Scholar]
- 27.Grinnell F: Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol 2000, 10:362-365 [DOI] [PubMed] [Google Scholar]
- 28.Selye H: On the mechanism through which hydrocortisone affects the resistance of tissue to injury. J Am Med Assoc 1953, 152:1207-1213 [DOI] [PubMed] [Google Scholar]
- 29.Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G: Granulation tissue as a contractile organ. A study of structure and function. J Exp Med 1972, 135:719-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appleton I, Tomlinson A, Chander CL, Willoughby DA: Effect of endothelin-1 on croton oil-induced granulation tissue in the rat. A pharmacologic and immunohistochemical study. Lab Invest 1992, 67:703-710 [PubMed] [Google Scholar]
- 31.Komesli S, Vivien D, Dutartre P: Chimeric extracellular domain type II transforming growth factor (TGF)-beta receptor fused to the Fc region of human immunoglobulin as a TGF-beta antagonist. Eur J Biochem 1998, 254:505-513 [DOI] [PubMed] [Google Scholar]
- 32.Lin HY, Moustakas A, Knaus P, Wells RG, Henis YI, Lodish HF: The soluble exoplasmic domain of the type II transforming growth factor (TGF)-beta receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-beta ligands. J Biol Chem 1995, 270:2747-2754 [DOI] [PubMed] [Google Scholar]
- 33.Carnemolla B, Borsi L, Zardi L, Owens RJ, Baralle FE: Localization of the cellular-fibronectin-specific epitope recognized by the monoclonal antibody IST-9 using fusion proteins expressed in E. coli. FEBS Lett 1987, 215:269-273 [DOI] [PubMed] [Google Scholar]
- 34.Borsi L, Carnemolla B, Castellani P, Rosellini C, Vecchio D, Allemanni G, Chang SE, Taylor-Papadimitriou J, Pande H, Zardi L: Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol 1987, 104:595-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benzonana G, Skalli O, Gabbiani G: Correlation between the distribution of smooth muscle or non muscle myosins and alpha-smooth muscle actin in normal and pathological soft tissues. Cell Motil Cytoskeleton 1988, 11:260-274 [DOI] [PubMed] [Google Scholar]
- 36.Christen T, Bochaton-Piallat ML, Neuville P, Rensen S, Redard M, van Eys G, Gabbiani G: Cultured porcine coronary artery smooth muscle cells. A new model with advanced differentiation. Circ Res 1999, 85:99-107 [DOI] [PubMed] [Google Scholar]
- 37.Yao X, Chaponnier C, Gabbiani G, Forte JG: Polarized distribution of actin isoforms in gastric parietal cells. Mol Biol Cell 1995, 6:541-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Ramires FJ, Zhou G, Ganjam VK, Weber KT: Fibrous tissue and angiotensin II. J Mol Cell Cardiol 1997, 29:2001-2012 [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Sun Y, Zhang JQ, Ramires FJ, Weber KT: Appearance and regression of rat pouch tissue. J Mol Cell Cardiol 1999, 31:1005-1013 [DOI] [PubMed] [Google Scholar]
- 40.Appleton I, Tomlinson A, Colville-Nash PR, Willoughby DA: Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue. Implications for their role in tissue development and repair processes. Lab Invest 1993, 69:405-414 [PubMed] [Google Scholar]
- 41.Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR: Contraction of granulation tissue in vitro: similarity to smooth muscle. Science 1971, 173:548-550 [DOI] [PubMed] [Google Scholar]
- 42.Abercrombie M, James DW, Newcombe JF: Wound contraction in rabbit skin, studied by splinting the wound margins. J Anat 1960, 94:170-182 [PMC free article] [PubMed] [Google Scholar]
- 43.Dunphy JE: The healing of wounds. Can J Surg 1967, 10:281-287 [PubMed] [Google Scholar]
- 44.Delvoye P, Wiliquet P, Leveque JL, Nusgens BV, Lapiere CM: Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol 1991, 97:898-902 [DOI] [PubMed] [Google Scholar]
- 45.Sadoshima J, Izumo S: The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 1997, 59:551-571 [DOI] [PubMed] [Google Scholar]
- 46.Riser BL, Cortes P, Heilig C, Grondin J, Ladson-Wofford S, Patterson D, Narins RG: Cyclic stretching force selectively up-regulates transforming growth factor-beta isoforms in cultured rat mesangial cells. Am J Pathol 1996, 148:1915-1923 [PMC free article] [PubMed] [Google Scholar]
- 47.van Bockxmeer FM, Martin CE, Constable IJ: Effect of cyclic AMP on cellular contractility and DNA synthesis in chorioretinal fibroblasts maintained in collagen matrices. Exp Cell Res 1984, 155:413-421 [DOI] [PubMed] [Google Scholar]
- 48.Lin YC, Grinnell F: Decreased level of PDGF-stimulated receptor autophosphorylation by fibroblasts in mechanically relaxed collagen matrices. J Cell Biol 1993, 122:663-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin YC, Ho CH, Grinnell F: Decreased PDGF receptor kinase activity in fibroblasts contracting stressed collagen matrices. Exp Cell Res 1998, 240:377-387 [DOI] [PubMed] [Google Scholar]
- 50.Gruden G, Zonca S, Hayward A, Thomas S, Maestrini S, Gnudi L, Viberti GC: Mechanical stretch-induced fibronectin and transforming growth factor-beta1 production in human mesangial cells is p38 mitogen-activated protein kinase-dependent. Diabetes 2000, 49:655-661 [DOI] [PubMed] [Google Scholar]
- 51.Butt RP, Laurent GJ, Bishop JE: Mechanical load and polypeptide growth factors stimulate cardiac fibroblast activity. Ann NY Acad Sci 1995, 752:387-393 [DOI] [PubMed] [Google Scholar]
- 52.Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G: Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res 1993, 11:717-729 [DOI] [PubMed] [Google Scholar]
- 53.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L: Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol 1993, 142:793-801 [PMC free article] [PubMed] [Google Scholar]
- 54.French-Constant C, Van de Water L, Dvorak HF, Hynes RO: Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989, 109:903-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochitate K, Pawelek P, Grinnell F: Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp Cell Res 1991, 193:198-207 [DOI] [PubMed] [Google Scholar]
- 56.Mardon HJ, Sebastio G: Regulation of alternative splicing in the IIICS region of human fibronectin pre-mRNA encoding cell binding sites CS1 and CS5. J Cell Sci 1992, 103:423-433 [DOI] [PubMed] [Google Scholar]
- 57.Huh GS, Hynes RO: Elements regulating an alternatively spliced exon of the rat fibronectin gene. Mol Cell Biol 1993, 13:5301-5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao YF, Wieder KG, Classen JM, Van De Water L: Identification of two amino acids within the EIIIA (ED-A) segment of fibronectin constituting the epitope for two function-blocking monoclonal antibodies. J Biol Chem 1999, 274:17876-17884 [DOI] [PubMed] [Google Scholar]
- 59.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K: Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 1998, 141:539-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hynes RO: The dynamic dialogue between cells and matrices: implications of fibronectin’s elasticity. Proc Natl Acad Sci USA 1999, 96:2588-2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingber DE: Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 1997, 59:575-599 [DOI] [PubMed] [Google Scholar]
- 62.Wang N, Butler JP, Ingber DE: Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260:1124-1127 [DOI] [PubMed] [Google Scholar]
- 63.Hall A: Rho GTPases and the actin cytoskeleton. Science 1998, 279:509-514 [DOI] [PubMed] [Google Scholar]
- 64.Serpinskaya AS, Denisenko ON, Gelfand VI, Bershadsky AD: Stimulation of actin synthesis in phalloidin-treated cells. Evidence for autoregulatory control. FEBS Lett 1990, 277:11-14 [DOI] [PubMed] [Google Scholar]
- 65.Bershadsky AD, Gluck U, Denisenko ON, Sklyarova TV, Spector I, Ben-Ze’ev A: The state of actin assembly regulates actin and vinculin expression by a feedback loop. J Cell Sci 1995, 108:1183-1193 [DOI] [PubMed] [Google Scholar]