Abstract
Fibroblast growth factor-2 (FGF2) has neurotrophic effects in vitro and in vivo. It has been demonstrated to decrease photoreceptor cell death in rats exposed to constant light and in rats with an inherited defect in retinal pigmented epithelium (RPE) phagocytosis, but the effects of intravitreous injections of FGF2 in mice are equivocal. In this study, we used transgenic mice with increased expression of FGF2 in photoreceptors (rhodopsin promoter/FGF2 transgenics) to investigate the effects of sustained increased expression of FGF2 in mice with various types of photoreceptor degeneration, including rd mice that are homozygous for mutated phosphodiesterase β subunit, Q344ter mice that undergo photoreceptor degeneration because of expression of mutated rhodopsin, and mice exposed to 75% oxygen for 1 or 2 weeks. At P21, the outer nuclear layer was markedly reduced in rd mice or Q344ter mice regardless of whether they inherited the rhodopsin promoter/FGF2 transgene. However, after 2 weeks of exposure to 75% oxygen, outer nuclear layer thickness was significantly reduced in littermate control mice compared to FGF2 transgenic mice (P = 0.0001). These data indicate that increased expression of FGF2 in photoreceptors protects them from hyperoxia-induced damage, but does not decrease cell death related to expression of mutated proteins involved in the phototransduction pathway. This suggests that FGF2 protects photoreceptors from oxidative damage, which may play a role in complex genetic diseases such as age-related macular degeneration.
Blockade of fibroblast growth factor (FGF) signaling by expression of dominant-negative FGF receptor mutants in photoreceptors of transgenic mice results in slow degeneration of photoreceptors. 1 This suggests that one or more FGFs may act as a survival factor for photoreceptors. FGF2 is a good candidate, because intravitreous injections of FGF2 decrease photoreceptor cell death in Royal College of Surgeons (RCS) rats that have an inherited defect in phagocytosis in RPE cells 2,3 or rats exposed to constant light. 4
Early in the course of retinal degenerations, light exposure, or other types of insults such as trauma to the retina or cutting of the optic nerve, there is increased expression of FGF2 in the retina. 5-8 The increased expression of FGF2 may represent an attempt to limit cell death by increasing production of a survival factor. This hypothesis is supported by demonstrations in rats that several of these same insults that increase expression of FGF2, also decrease photoreceptor cell death from subsequent insults. 9-11
Thus, there is substantial evidence indicating that FGF2 is likely to be a trophic factor for photoreceptors, but there is one piece of evidence that is not supportive of this hypothesis; the survival-promoting effects of FGF2 in the retina seem to be quite different among species. Although intravitreous injections of FGF2 decrease photoreceptor cell death in the RCS rat model of inherited retinal degeneration or rats exposed to constant light, 2,4,10 they have little or no effect in mouse models. 12 This raises questions as to whether the findings in rats are predictive of the situation in humans, and mechanistic questions about how rats and mice might differ. However, because of the smaller size of mouse eyes compared to rat eyes, the reported lack of survival-promoting effects of FGF2 in mice may be because of the technical difficulty of intraocular injections in mice rather than a true biological difference. Furthermore, it is difficult to rule out a survival-promoting effect of Fgf2 in mice based on lack of efficacy of a single injection, because more sustained delivery may be needed to achieve a recognizable effect. Perhaps Fgf2 provides protection for both rat and mouse photoreceptors, but Fgf receptor density is somewhat greater in rat photoreceptors, so that a single intravitreous injection is sufficient to see an effect in rats, but not mice. In fact, this explanation has been suggested, because injury and light exposure cause a much smaller increase in Fgf receptor 1 mRNA expression in mice than rats. 6,12-14
To ensure sustained delivery of FGF2 to photoreceptors in mice, we used a genetic approach and made transgenic mice with a rhodopsin promoter/(human)FGF2 fusion gene. We have previously used this approach to explore the role of FGF2 in ocular neovascularization 15 and in this study we used it to investigate whether or not FGF2 promotes photoreceptor survival in various types of retinal degeneration.
Materials and Methods
Transgenic Mice that Overexpress FGF2 in Photoreceptors
The generation and characterization of rhodopsin promoter/Fgf2 transgenic mice (Rho/FGF2 mice) have been described. 15 These mice have been mated into a C57BL/6 background. Both homozygous and heterozygous Rho/Fgf2 mice have morphologically normal retinas.
Breeding Rho/Fgf2 Mice with rd Mice
FVB/NJ rd mice (Jackson Laboratories, Bar Harbor, ME), which are homozygous for a mutation in the β subunit of phosphodiesterase, were mated with homozygous Rho/Fgf2 mice. Offspring were genotyped by polymerase chain reaction on tail DNA with transgene-specific primers as previously described. 15 Mice that inherited a Rho/Fgf2 transgene and were heterozygous for the rd allele were mated with rd mice, and offspring were genotyped by polymerase chain reaction to identify mice carrying a Rho/Fgf2 transgene. The method of Pittler and Baehr 16 was used to identify mice with two mutant rd alleles. At P21 mice were sacrificed and eyes were removed and frozen in optimal cutting temperature embedding compound (OCT; Miles Diagnostics, Elkhart, IN). Frozen sections were stained with methyl green and outer nuclear layer thickness was examined by light microscopy.
Breeding Rho/Fgf2 Mice with Q344ter Mice
Transgenic mice that express a truncated rhodopsin because of a mutation resulting in a stop codon at position 344 develop retinal degeneration and are referred to as Q344ter mice. 17 Mice with a C57BL/6 background heterozygous for the Q344ter transgene were mated with mice heterozygous for the Rho/Fgf2 transgene. The offspring were genotyped and at P21 they were sacrificed, followed by the same procedures described above to examine outer nuclear layer thickness.
Hyperoxia-Induced Retinal Degeneration
We had previously noted that exposure of adult mice to 75% oxygen for longer than 1 week results in photoreceptor degeneration. 18 Mice heterozygous for the Rho/Fgf2 transgene were mated with wild-type C57BL/6 mice. Offspring were genotyped and after 6 weeks of age they were exposed to 75% oxygen for 0, 1, or 2 weeks and then sacrificed. Eyes were removed, frozen in OCT, and sections were stained with hematoxylin. Outer nuclear layer thickness was examined by light microscopy.
For quantitative analysis of outer nuclear layer thickness, eyes were marked for orientation and embedded in OCT. Ten-μm sections were cut serially along the vertical meridian, and the section through the center of the optic nerve was stained with methyl green. Sections were examined with an Axioskop microscope (Zeiss, Thornwood, NY) and images were digitized using a three-charge-coupled device color video camera (IK-TU40A; Toshiba, Tokyo, Japan) and a frame grabber. Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) was used to measure the thickness of the outer nuclear layer measured 100 μm from each edge of the optic nerve. The mean of the two measurements from each eye was used as a single experimental value.
Northern Analysis
RNA blot hybridization analysis was done as previously described 19,20 using 10 μg of total retinal RNA. The probe was a 0.8-kb cDNA fragment including most of exon 2 and all of exon 3 of the human FGF2 gene. The membrane was hybridized at 42°C and washed twice for 30 minutes at 60°C in 1× SSC, 0.2% sodium dodecyl sulfate, followed by two washes for 30 minutes each at 65°C in 0.2× SSC, 0.2% sodium dodecyl sulfate. Washed blots were exposed to XAR film (Kodak, Rochester, NY) for various times to obtain exposures within the linear range for autoradiographic signals. Blots were stripped and rehybridized with a probe for 18S ribosomal RNA to control for potential differences in RNA loading.
Immunohistochemistry
FGF2 transgenic mice and littermate controls were exposed to 75% oxygen for 1, 2, or 3 weeks and then sacrificed; their eyes were removed, fixed in 4% paraformaldehyde, and embedded in paraffin. Ten-μm ocular sections were stained immunohistochemically as previously described 19 with a 1:200 dilution of a rabbit anti-FGF2 peptide antibody generated against the 15 amino acids at the amino terminus of FGF2 (a gift from Dr. Leonard Hjelmeland, Davis, CA). This antibody has been extensively characterized and does not recognize FGF1. 21 Specificity of staining was assessed by comparison with staining using nonimmune serum for primary antibody.
Results
FGF2 Does Not Decrease Photoreceptor Cell Death in rd Mice
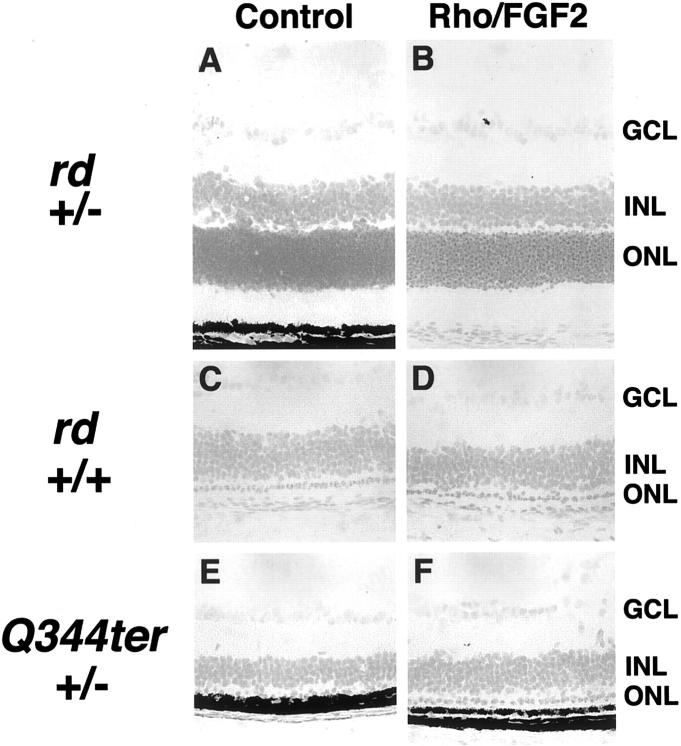
The retinal degeneration (rd) mouse is an animal model of autosomal-recessive retinitis pigmentosa in which there is a naturally occurring mutation in the β subunit of phosphodiesterase, which also occurs in some patients with retinitis pigmentosa. 22 Mice homozygous for the rd mutation undergo degeneration of photoreceptors that begins at P10 and is nearly complete by P21, whereas heterozygotes have normal retinas. As expected, mice that carried only one copy of the rd mutation, whether or not they carried the Rho/Fgf2 transgene, showed normal retinas with no evidence of retinal degeneration (Figure 1, A and B) ▶ . Mice that inherited two mutant rd alleles with or without a Rho/Fgf2 transgene showed extensive degeneration of photoreceptors with only one row remaining at P21 (Figure 1, C and D) ▶ . Extensive degeneration was a consistent finding in 10 rd mice that carried the Rho/Fgf2 transgene, indicating that expression of high levels of FGF2 in photoreceptors does not prevent photoreceptor cell death in rd mice.
Figure 1.
Increased expression of FGF2 in the photoreceptors of transgenic mice does not prevent mutant protein-induced photoreceptor degeneration. Mice that carried one mutant rd allele (rd +/−) showed no evidence of photoreceptor degeneration, whether they carried (B) or did not carry (A) a FGF2 transgene. Mice homozygous for the rd mutation (rd +/+) showed severe photoreceptor degeneration with only one row of photoreceptors remaining at postnatal day (P) 21 whether they carried (D) or did not carry (C) a FGF2 transgene. A total of 10 rd +/+, FGF2 +/− mice were evaluated and all showed a single row of remaining photoreceptors at P21. Mice that expressed a truncated rhodopsin protein in photoreceptors (Q344ter +/−) showed severe photoreceptor degeneration with only one row of photoreceptors remaining at P21 whether they carried (F) or did not carry (E) a FGF2 transgene. A total of 10 Q344ter +/−, FGF2 +/− mice were evaluated and all showed a single row of remaining photoreceptors at P21.
FGF2 Does Not Decrease Photoreceptor Cell Death in Q344ter Mice
A mutation that generates a stop codon at position 344 of rhodopsin results in a truncated protein and is found in some patients with autosomal-dominant retinitis pigmentosa. 23 Transgenic mice that carry one copy of the Q344ter transgene, develop retinal degeneration starting at P10, which is complete by P21. 17 Mice that inherited the Q344ter transgene, but not the Rho/Fgf2 transgene, showed severe retinal degeneration with only one row of photoreceptors remaining at P21 (Figure 1E) ▶ . Mice that inherited both transgenes showed equally severe degeneration and this was a consistent finding among a total of 10 mice (Figure 1F) ▶ . At P11, Rho/FGF2 transgenics showed high-level transgene expression whether or not they carried a Q344ter transgene (Figure 2) ▶ . By P14, there is substantial photoreceptor degeneration, and as expected Rho/FGF2-Q344ter-double transgenics showed less Fgf2 transgene mRNA compared to Rho/FGF2 mice. These data indicate that expression of high levels of FGF2 in photoreceptors before onset of degeneration does not prevent retinal degeneration because of expression of truncated rhodopsin.
Figure 2.
FGF2 transgene (FGF2tg) mRNA levels in Rho/FGF2-Q344ter double transgenics. A mouse carrying one Q344ter allele was mated with a mouse carrying two Rho/FGF2 alleles. Offspring were genotyped and two mice that carried both FGF2tg and Q344ter transgenes, and two that carried only a FGF2tg were sacrificed at each of two time points, P11 and P14. Because degeneration in Q344ter mice begins at P10 to P11, the P11 time point represents the very early stages of degeneration, whereas substantial degeneration has occurred by P14. Retinal RNA was isolated and reverse transcriptase-polymerase chain reaction was done using primers specific for the FGF2tg and S16 ribosomal protein to control for loading. At P11, there is good expression of FGF2tg mRNA in Rho/FGF2-Q344ter double transgenics that is very similar to that seen in Rho/FGF2 single transgenics. At P14, there is still a good signal for FGF2tg mRNA in double transgenics, but it is less than that seen in single transgenics, which is expected because at the P14 time point there has been substantial loss of photoreceptors in the double transgenics.
FGF2 Protects Photoreceptors from Hyperoxia-Induced Cell Death
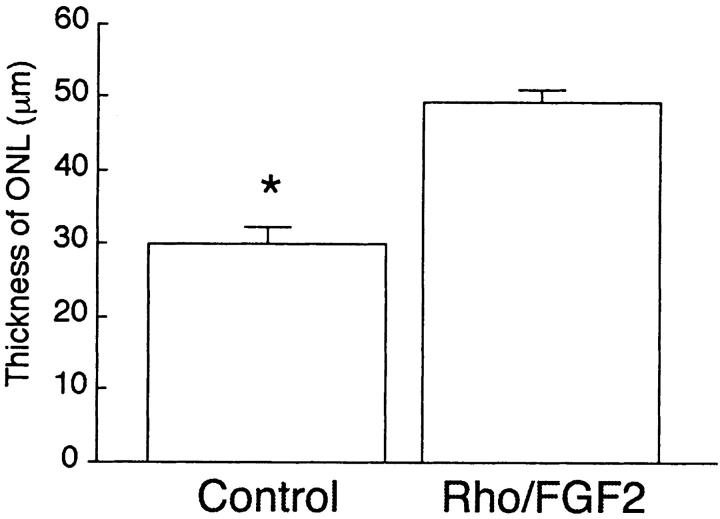
In a previously published study, we observed that exposure of adult mice to 75% oxygen for 2 or 3 weeks resulted in thinning of the outer nuclear layer. 18 This is consistent with a previous report of damaging effects on photoreceptors of hyperoxia, presumably because of oxidative damage. 24 After exposure to hyperoxia for 2 weeks, wild-type mice that did not carry the Rho/Fgf2 transgene, developed progressive thinning of the retina that was most marked in the posterior retina, adjacent to the optic nerve (Figure 3A) ▶ . In contrast, exposure to 75% oxygen for 2 weeks did not result in thinning of the outer nuclear layer in mice that carried the Rho/Fgf2 transgene (Figure 3C) ▶ . Figure 3B ▶ shows an ocular section from a transgene-negative mouse extending from the aura serrata on the left to the posterior retina on the right. It demonstrates that the outer nuclear layer has relatively normal thickness in the periphery, but becomes thinner posteriorly. In contrast, Figure 3D ▶ shows an ocular section from a Rho/Fgf2 transgene-positive mouse extending from the aura serrata on the left to the posterior retina on the right, and the outer nuclear layer becomes thicker scanning from the peripheral to posterior retina. Sections at 100 μm from the optic nerve were compared in Rho/Fgf2 transgene-positive and -negative mice after 0, 1, or 2 weeks of oxygen exposure. There was no significant difference in outer nuclear layer thickness in unexposed Rho/Fgf2 transgene-negative or transgene-positive littermates (Figure 3, E ▶ versus F) or those exposed to hyperoxia for 1 week (Figure 3, G ▶ versus H). However, after 2 weeks of oxygen exposure, transgene-negative mice showed significant thinning of the outer and inner nuclear layers, which in some mice was dramatic (Figure 3I) ▶ . The situation was strikingly different in Rho/FGF2 transgene-positive mice exposed to oxygen for 2 weeks in which the outer nuclear layer was consistently well-preserved (Figure 3J) ▶ . Measurement of outer nuclear layer thickness by image analysis with the examiner masked with respect to genotype (n = 5 in each group), confirmed that sustained expression of FGF2 in photoreceptors significantly protects them from hyperoxia-induced death (P = 0.0001; Figure 4 ▶ ).
Figure 3.

Prolonged hyperoxia results in degeneration of photoreceptors in the posterior portion of the retina that is prevented by increased expression of FGF2 in photoreceptors. A section through the posterior retina of a FGF2 transgene-negative mouse (control) that was exposed to 75% oxygen for 2 weeks shows marked thinning of the outer nuclear layer (A), whereas the posterior outer nuclear layer of a transgene-positive mouse (Rho/FGF2) exposed to 75% oxygen for 2 weeks appears normal (C). In the periphery of the retina of a transgene-negative mouse the outer nuclear layer is normal (B), but becomes progressively thinner toward the posterior portion of the retina (right). In contrast, the retina of a transgene-positive mouse has a normal outer nuclear layer in the periphery that becomes thicker toward the posterior retina (D). There was no significant difference in outer nuclear thickness 100 μm from the optic nerve in transgene-negative mice (E) compared to transgene-positive mice (F) that were not exposed to 80% oxygen, or those exposed to 75% oxygen for 1 week (G versus H). But transgene-negative mice exposed to 75% oxygen for 2 weeks showed marked thinning of the outer nuclear layer (I) compared to transgene-positive mice exposed to 80% oxygen for 2 weeks (J).
Figure 4.
Quantitative analysis of outer nuclear layer thickness demonstrates significant thinning in the posterior retina of wild-type littermates compared to FGF2 transgenics exposed to 75% oxygen for 2 weeks. FGF2 transgenics (Rho/FGF2; n = 10) and littermate controls (n = 10) were placed in 75% oxygen for 2 weeks and then outer nuclear layer thickness was measured by image analysis at 100 μm from the optic nerve. Statistical comparisons were made by the paired t-test.
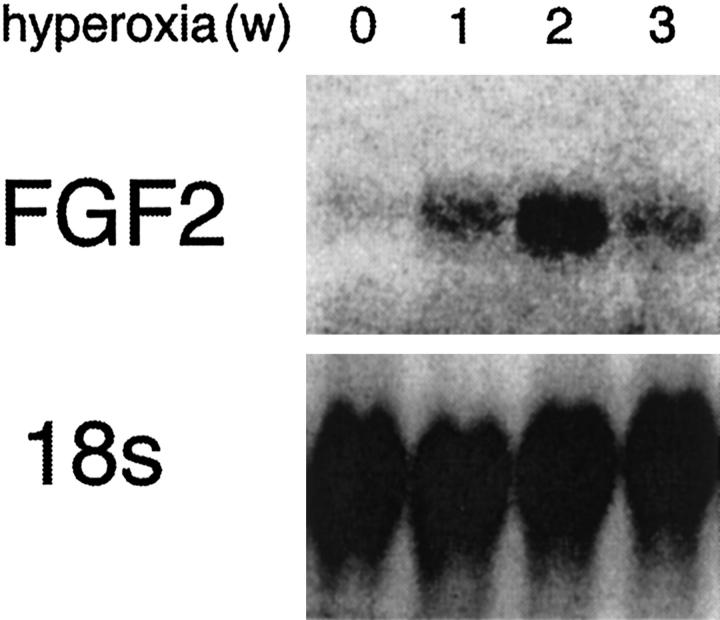
Hyperoxia Causes Increased Expression of Fgf2 mRNA in Wild-Type Mice
In rats, exposure to light or mechanical trauma causes increased expression of Fgf2 in the retina and preconditioning with such insults can decrease photoreceptor cell death from subsequent prolonged exposure to light. 6,14 Because ectopic FGF2 decreases hyperoxia-induced photoreceptor cell death in mice, we sought to determine whether hyperoxia caused increased expression of endogenous Fgf2, which could suggest normal control mechanisms meant to protect against oxidative damage in the retina. Northern analysis of retinas from wild-type mice exposed to hyperoxia showed increased expression of Fgf2 mRNA at 1 and 2 weeks, which decreased at 3 weeks (Figure 5) ▶ . Immunohistochemical staining for Fgf2 in the retinas of unexposed transgene-negative mice, showed faint staining throughout the retina (Figure 6A) ▶ , whereas unexposed transgene-positive mice showed dark staining in the outer nuclear layer and around retinal blood vessels (Figure 6B) ▶ . After 1 week of hyperoxia, staining for FGF2 was slightly decreased in both transgene-negative and transgene-positive mice (Figure 6, C and D) ▶ . After 2 weeks of hyperoxia, transgene-negative mice showed only one remaining row of photoreceptors, many of which were stained for Fgf2, as were cells in the inner nuclear layer (Figure 6E) ▶ . Transgene-positive mice exposed to hyperoxia for 2 weeks (Figure 6F) ▶ , showed no photoreceptor degeneration and retinal staining for Fgf2 was similar to that in the retinas of transgenics exposed to hyperoxia for 1 week, but less than that seen in unexposed transgenics. One possible interpretation as to why FGF2 staining is reduced after 2 weeks of hyperoxia, is that hyperoxia may result in increased Fgf2 turnover in an attempt to protect against greater oxidative stress. In transgenics, the high levels of FGF2 produced by the Rho/Fgf2 transgene may be sufficient to keep pace with the increased turnover so that although the steady-state level is lower than baseline, the dynamic FGF2 may be sufficient to prevent photoreceptor degeneration. In contrast, despite increased Fgf2 mRNA levels in wild-type mice, the increased endogenous Fgf2 expression may not be sufficient to keep pace with the higher turnover and therefore photoreceptors degenerate.
Figure 5.
Hyperoxia causes increased Fgf2 mRNA in the retina for 2 weeks, followed by a decrease thereafter. Wild-type C57BL/6 mice were exposed to 75% oxygen for 0, 1, 2, or 3 weeks and then 10 μg of retinal RNA for each time point was evaluated by Northern analysis for Fgf2 mRNA. The blot was stripped and reprobed for 18s ribosomal RNA. There is an increase in Fgf2 mRNA in the retina after 1 or 2 weeks of hyperoxia that decreases between 2 and 3 weeks.
Figure 6.
Immunohistochemistry for FGF2 in the retina of FGF2 transgenic (Rho/FGF2) and littermate control mice exposed to hyperoxia. In room air, littermate control mice show faint staining for FGF2 throughout the retina (A), whereas FGF2 transgenic mice show staining of photoreceptor cells and mild staining throughout the inner retina that is enhanced around blood vessels (B). After 1 week of exposure to 75% oxygen, staining for FGF2 appears somewhat decreased from baseline in both transgene-negative (C) and transgene-positive (D) mice. After 2 weeks of exposure to 75% oxygen, in transgene-negative mice, the outer nuclear layer has degenerated but some of the few remaining cells are positive for FGF2, as are several cells in the inner retina (E). Transgene-positive mice show similar staining for FGF2 after 1 (D) and 2 (F) weeks of hyperoxia suggesting that the FGF2 may have reached a steady-state level lower than baseline.
Discussion
There is substantial evidence suggesting that FGF2 is a survival factor for many types of neurons. It is up-regulated in various central nervous system neurons by sublethal injury, and injections of FGF2 or increased endogenous production of FGF2 decreases cell death from ischemia, neurotoxins, excitotoxicity, or nitric oxide. 25-28 The protection of many different types of neurons from many different types of insults suggests that FGF2 might be a universal neurotrophic factor that protects neurons from all types of damaging influences. Retinal photoreceptors offer a good system to test this hypothesis, because there are many animal models of photoreceptor degeneration.
Previous studies have demonstrated that FGF2 promotes photoreceptor survival in RCS rats, 2 which have a defect in phagocytosis of rod outer segments by RPE cells because of a mutation in the receptor tyrosine kinase gene Mertk. 3 In albino rats, FGF2 protects photoreceptors from the damaging effects of constant light. 4,10 However, intravitreous injections of FGF2 do not decrease photoreceptor degeneration from constant light or several different mutations in mice. 12 In this study, we have demonstrated that sustained increased expression of FGF2 also fails to rescue photoreceptors from a recessive inherited degeneration or a dominant inherited degeneration in mice. But, increased expression of FGF2 in photoreceptors protects them from cell death because of sustained increased inspired oxygen. This suggests that the mechanisms involved in cell death because of hyperoxia, which are likely to involve oxidative damage, and cell death because of mutations in photoreceptor-specific genes are different and that FGF2 provides protection against the former, but not the latter.
Why does FGF2 decrease photoreceptor cell death in RCS rats that have an inherited degeneration and FGF2 has no effect in rd or Q344ter mice? The mutation in RCS rats is in a gene coding for a receptor in RPE cells involved in phagocytosis and results in a defect in phagocytosis of rod outer segments and accumulation of debris between photoreceptors and RPE cells. 3,29 It is possible that photoreceptors die in RCS rats because the debris prevents trophic interaction between the RPE and photoreceptors and injection of FGF2 provides the missing trophic support thereby preventing photoreceptors from dying. In contrast, rd mice and Q344ter mice express mutant proteins in photoreceptor cells and whereas the mechanism by which the mutant proteins cause photoreceptors to die is unknown, it is likely to be something different from withdrawal of a trophic factor. Our data suggest that whatever the mechanism, increased expression of FGF2 in photoreceptors is unable to prevent the damage.
What is the evidence that FGF2 decreases photoreceptor cell death in rats expressing mutant proteins in photoreceptors? Intravitreous injection of FGF2 provides essentially no rescue of photoreceptors in several lines of transgenic rats expressing mutated rhodopsin (Matthew LaVail, PhD, personal communication, August, 2000); however, increased expression of FGF2 by intraocular injection of AAV.CMV-FGF2 decreases photoreceptor death in the same transgenic lines. 30 Therefore, sustained increased expression of FGF2 by gene transfer can prevent photoreceptor cell death from mutated rhodopsin in rats.
Why does increased expression of FGF2 by intraocular injection of AAV.CMV-FGF2 rescue photoreceptors from mutated rhodopsin-induced cell death in rats, whereas increased expression of FGF2 in photoreceptors of Rho/FGF2 transgenic mice does not? One possible explanation is that there is some type of species difference between rats and mice, either in the mechanism of photoreceptor cell death after expression of mutated proteins, or in the effectiveness of FGF2 as a survival factor. There are some data that suggest that the latter might be true. In rats, primary limited photoreceptor cell injury results in rescue of photoreceptors from several types of secondary insults, a phenomenon that has been referred to as an injury response. 6 One report has suggested that mice have little or no injury response. 13 However, another study using retinal whole mounts to quantitate photoreceptor cell number, found evidence of an injury response in mice, 31 and it may be that there is an injury response in both species, but it is less prominent in mice. One possible explanation is that although both rats and mice show increased expression of FGF2 in photoreceptors after trauma or other insults to the retina, rats also show increased expression of FGF receptors, whereas mice do not. 13 Also, although intravitreous injections of FGF2 increase survival of photoreceptors exposed to constant light in rats, 4 a similar effect is not seen in mice. 12
However, species differences may not be the only explanation. Gene transfer experiments in rats have suggested that the level of expression of FGF2 is critical for the rescue effect, with levels above or below an optimal range leading to little or no rescue (John Flannery, PhD, personal communication, August, 2000). Perhaps the level of expression of FGF2 in our transgenic mice is less than that achieved in some rats with FGF2 gene transfer and is insufficient to protect photoreceptors from mutation-induced cell death. Additional experiments are needed to investigate this possibility.
A third possibility that is more intriguing is that the location of increased expression may be critical. Intraocular injections of constructs in AAV.CMV vectors result in expression in other cells in addition to photoreceptors, particularly RPE cells. Perhaps expression of FGF2 in RPE cells results in production of a more effective neurotrophic factor that rescues photoreceptors.
Although it will be important to address the above issues in future studies and determine whether other experimental approaches to increase expression of FGF2 can prevent mutation-induced photoreceptor cell death in mice, our data clearly demonstrate that FGF2 can protect photoreceptors from oxidative damage in mice. This exciting finding is consistent with other recent observations. For instance, FGF2 protects cultured human RPE cells or vascular endothelial cells from oxidative damage. 20,32
Oxidative damage has been implicated in the pathogenesis of age-related macular degeneration, the most common cause of severe visual loss in patients older than the age of 60. 33 In this study, we have described a new model of hyperoxia-induced photoreceptor degeneration that mimics an important aspect of macular degeneration, predilection for involvement of the posterior (central) retina compared to the anterior (peripheral) retina. Perhaps the greater blood flow in the posterior portion of the choroid compared to the anterior portion results in more severe oxidative damage to the posterior retina. Elucidation of the exact mechanism of the topographical pattern of the degeneration in our model may provide new clues to help unlock the mystery of macular degeneration. However, our study also suggests that important species differences may exist with regard to the effect of growth factors on photoreceptors. Despite this note of caution, if the ability of FGF2 to inhibit oxidative damage occurs in human photoreceptors as well as those of mice, increased expression of FGF2 in photoreceptors and/or RPE cells may provide a viable therapeutic approach in diseases such as age-related macular degeneration.
Footnotes
Address reprint requests to Peter A. Campochiaro, M.D., Maumenee 719, Johns Hopkins University School of Medicine, 600 N. Wolfe St., Baltimore, MD 21287-9277. E-mail: pcampo@jhmi.edu.
Supported by a grant from Foundation Fighting Blindness, grant EY05951 and P30EY1765 from the National Eye Institute, a NRSA training grant (to N. B.), a Lew R. Wasserman Merit Award (to P. A. C.), a career development award (to D. J. Z.), and unrestricted funds from Research to Prevent Blindness, a grant from the Charles M. Moon, Jr. and Dr. P. Thomas Manchester Research Fund, a grant from Mrs. Harry J. Duffey, a grant from Dr. and Mrs. William Lake, a grant from the Steinbach Fund (to P. A. C. and D. J. Z.). P. A. C. is the George S. and Dolores Dore Eccles Professor of Ophthalmology.
Present address of Haruhiko Yamada and Eri Yamada: Kansai Medical University, Moriguchi, Osaka, Japan.
References
- 1.Campochiaro PA, Chang M, Ohsato M, Vinores SA, Nie Z, Hjelmeland L, Mansukhani A, Basilico C, Zack DJ: Retinal degeneration in transgenic mice with photoreceptor-specific expression of a dominant-negative fibroblast growth factor receptor. J Neurosci 1996, 16:1670-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM: Photoreceptor degeneration in inherited retinal dystrophy delayed by fibroblast growth factor. Nature 1990, 347:83-86 [DOI] [PubMed] [Google Scholar]
- 3.D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D: Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 2000, 9:645-651 [DOI] [PubMed] [Google Scholar]
- 4.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos G, Steinberg RH: Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA 1992, 89:11249-11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao H, Hollyfield JG: Basic fibroblast growth factor in retinal development: differential levels of bFGF expression and content in normal and retinal degeneration (rd) mutant mice. Dev Biol 1995, 169:168-184 [DOI] [PubMed] [Google Scholar]
- 6.Wen R, Song Y, Cheng T, Matthes MT, Yasumura D, LaVail MM, Steinberg RH: Injury-induced upregulation of bFGF and CNTF mRNAs in the rat retina. J Neurosci 1995, 15:7377-7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Hollyfield JG: Basic fibroblast growth factor: increased gene expression in inherited and light-induced photoreceptor degeneration. Exp Eye Res 1996, 62:181-189 [DOI] [PubMed] [Google Scholar]
- 8.Kostyk SK, D’Amore PA, Herman IM, Wagner JA: Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci 1994, 14:1441-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush RA, Williams TP: The effect of unilateral optic nerve section on retinal light damage in rats. Exp Eye Res 1991, 52:139-153 [DOI] [PubMed] [Google Scholar]
- 10.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, La Vail MM: Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci 1992, 12:3554-3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg RH: Survival factors in retinal degenerations. Curr Opin Neurobiol 1994, 4:515-524 [DOI] [PubMed] [Google Scholar]
- 12.LaVail MW, Yasamura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CK, Steinberg RH: Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci 1998, 39:592-602 [PubMed] [Google Scholar]
- 13.Cao W, Wen R, Li F, LaVail MM, Steinberg RH: Mechanical injury increases bFGF and CNTF mRNA expression in the mouse retina. Exp Eye Res 1997, 65:241-248 [DOI] [PubMed] [Google Scholar]
- 14.Wen R, Cheng T, Song Y, Matthes MT, Yasumura D, LaVail MM, Steinberg RH: Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat retina. Curr Eye Res 1998, 17:494-500 [DOI] [PubMed] [Google Scholar]
- 15.Ozaki H, Okamoto N, Ortega S, Chang M, Ozaki K, Sadda S, Vinores MA, Derevjanik N, Zack DJ, Basilico C, Campochiaro PA: Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization. Am J Pathol 1998, 153:757-765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittler SJ, Baehr W: Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA 1991, 88:8322-8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung C-H, Makino C, Baylor D, Nathans J: A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci 1994, 14:5818-5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada H, Yamada E, Hackett SF, Ozaki H, Okamoto N, Campochiaro PA: Hyperoxia causes decreased expression of vascular endothelial growth factor and endothelial cell apoptosis in adult retina. J Cell Physiol 1999, 179:149-156 [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA, Hackett SF, Vinores SA, Freund J, Csaky C, La Rochelle W, Henderer J, Johnson M, Rodriguez IR, Friedman Z, Derevjanik N, Dooner J: Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci 1994, 107:2459-2469 [DOI] [PubMed] [Google Scholar]
- 20.Hackett SF, C-L. S, Freund J, Gottsch JD, Bhargave S, Campochiaro PA: Neurotrophic factors, cytokines and stress increase expression of basic fibroblast growth factor in retinal pigmented epithelial cells. Exp Eye Res 1997, 64:865–873 [DOI] [PubMed]
- 21.Ishigooka H, Aotaki-Keen AE, Hjelmeland LM: Subcellular localization of bFGF in human retinal pigment epithelium in vitro. Exp Eye Res 1992, 55:203-214 [DOI] [PubMed] [Google Scholar]
- 22.Farber DB: From mice to men: the cyclic GMP phosphodiesterase gene in vision and disease. The Proctor Lecture. Invest Ophthalmol Vis Sci 1995, 36:263-275 [PubMed] [Google Scholar]
- 23.Sung C-H, Davenport CM, Hennessey JC, Maumenee IH J. JS, Heckenlively JR, Nowakowski R, Fishman G, Gouras P, Nathans J: Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci USA 1991, 88:6481–6485 [DOI] [PMC free article] [PubMed]
- 24.Noell WK: Visual cell effects of high oxygen pressures. Fed Proc 1955, 14:107-108 [Google Scholar]
- 25.Anderson KJ, Dam D, Lee S, Cotman CW: Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature 1988, 332:360-361 [DOI] [PubMed] [Google Scholar]
- 26.Otto D, Unsicker K: Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci 1990, 10:1912-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freese A, Finklestein SP, DiFiglia M: Basic fibroblast growth factor protects striatal neurons in vitro from NMDA-receptor mediated excitotoxicity. Brain Res 1992, 575:351-355 [DOI] [PubMed] [Google Scholar]
- 28.Maiese K, Boniece I, DeMeo D, Wagner JA: Peptide growth factors protect against ischemia in culture by preventing nitric oxide toxicity. J Neuroscil 1993, 13:3034-3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullen RJ, LaVail MM: Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science 1976, 192:799-801 [DOI] [PubMed] [Google Scholar]
- 30.Lau D, McGee L, Zhou S-Z, Rendahl K, Manning W, Escobedo J, Flannery J: Retinal degeneration is slowed in transgenic rats by AAV-mediated delivery of FGF-2. Invest Ophthalmol Vis Sci 2000, 47:3622-3633 [PubMed] [Google Scholar]
- 31.Frasson M, Picaud S, Leveillard T, Simonutti M, Mohand-Said S, Dreyfus H, Hicks D, Sahel J: Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci 1999, 40:2724-2734 [PubMed] [Google Scholar]
- 32.Yang W, de Bono DP: A new role for vascular endothelial growth factor and fibroblast growth factors: increasing endothelial resistance to oxidative stress. FEBS Lett 1997, 403:139-142 [DOI] [PubMed] [Google Scholar]
- 33.: The Macular Photocagulation Study Group: Argon laser photocoagulation for neovascular maculopathy: five year results from randomized clinical trials. Arch Ophthalmol 1991, 109:1109-1114 [PubMed] [Google Scholar]