Abstract
Thrombin, an important clotting factor, extravasates at sites of blood-retina barrier breakdown that is often associated with many retinal diseases. Here we investigated the effects of thrombin on human retinal pigment epithelial (HRPE) cells, monocytes, and HRPE cell/monocyte co-cultures. Thrombin induced secretion and mRNA expression of HRPE interleukin (IL)-8 and monocyte chemoattractant protein-1 (MCP-1). Thrombin also enhanced IL-8 and MCP-1 by HRPE cell/monocyte co-cultures, by apparently enhancing cell-cell contact mechanisms. The thrombin effects on IL-6 secretion were similar to those on chemokine secretion. Thrombin-induced chemokines by co-cultures were inhibited by anti-tumor necrosis factor-α (TNF-α) antibody, but not by anti-IL-1β antibody. TNF-α was detected in cell lysates of monocytes detached from HRPE cells after co-culture stimulation with thrombin. HRPE cells mainly produced these chemokines. However, thrombin generally potentiated exogenous IL-1β- and TNF-α-induced chemokine production by HRPE cells, monocytes, and co-cultures. Interferon-γ potentiated chemokine secretion by co-cultures with or without thrombin. Our results indicate that thrombin may cause leukocyte recruitment by inducing HRPE cell and monocyte chemokine and by enhancing HRPE cell/monocyte interactions, in part because of monocyte TNF-α induction, suggesting important mechanisms for ocular inflammation during blood-retina barrier breakdown and intra-ocular hemorrhage.
Human retinal pigment epithelial (HRPE) cells, situated between the neurosensory retina and the choroid, form the outer blood-retina barrier. Barrier breakdown is seen in ocular trauma as well as many other ocular diseases such as proliferative vitreoretinopathy, diabetic retinopathy, and age-related macular degeneration, leading to increased permeability to serum components. Intra-ocular influx of serum is associated with leukocytic infiltration that may promote cellular proliferation, leading to severe visual dysfunction. 1,2 Although the specific stimuli for leukocyte recruitment are unknown, breakdown of blood-retina barrier has been implicated, suggesting a role for serum-derived factors. 3 Derived from prothrombin, which may extravasate through breaches in the blood-retina barrier, 4 thrombin is produced by the action of tissue thromboplastin. Prothrombin in 1 ml of human plasma could convert into 15 to 38 U of thrombin that is concentrated in clotted blood. 4-6 Thrombin is formed in areas of hemorrhage and in diseased retinal tissue and has been demonstrated to be a potent stimulus for inflammation, aggravating the disease processes. 7,8 However, mechanisms describing interrelationships between extravasating serum components or hemorrhage and ocular inflammation have not been reported.
Thrombin has numerous activities. In its hemostatic roles, it converts fibrinogen to fibrin, and activates platelet adhesion, aggregation, and secretion. 9 Thrombin also elicits mitogenic responses in retinal pigment epithelial cells, retinal glial cells, vascular endothelial cells, vascular smooth muscle cells, and fibroblasts. 7,10,11 Thrombin has been tried as a therapeutic agent during intra-ocular surgery for diabetic retinopathy and ocular trauma to control bleeding and for macular holes as a mitogen of retinal cells, frequently resulting in severe ocular inflammation. 12,13 Therefore, it seems that thrombin may be a key factor in the clotting cascade that can also induce significant inflammation.
Interleukin (IL)-8, which is chemotactic for neutrophils and eosinophils, 14 and monocyte chemoattractant protein-1 (MCP-1), which directly attracts monocytes 15 and lymphocytes, 16 are the two chemokines responsible for the majority of HRPE-derived leukocyte chemotactic activity. 17-19 We reported that IL-8 and MCP-1 are present in eyes from patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy 20,21 and it has been shown that MCP-1 was detected in HRPE cells and monocytes in the eyes with age-related macular degeneration. 22 In addition, we found that direct interactions between HRPE cells and monocytes could result in chemokine induction. 23 Because monocytes are intimately associated with HRPE cells in the epiretinal membranes of proliferative vitreoretinopathy and in the subretinal neovascular membranes of age-related macular degeneration, 24,25 HRPE cell/monocyte interactions could induce further leukocyte accumulation and lead to the progression of these diseases by enhancing expression of IL-8 and MCP-1. Previous reports demonstrated that thrombin induces monocyte IL-8 and MCP-1, 26,27 which indicates that thrombin might play a role in inflammatory process by inducing chemokines. However, the effects of thrombin on chemokine production by ocular cells are unknown.
In this study we examined the effects of thrombin on chemokine secretion by HRPE cells and HRPE cell/monocyte co-cultures. In addition, we tested synergy between thrombin and the pro-inflammatory cytokines, IL-1β and tumor necrosis factor-α (TNF-α), on chemokine production, and the effects of interferon (IFN)-γ on chemokine secretion.
Materials and Methods
Reagents
Falcon Primaria flasks were purchased from Becton-Dickinson Inc. (Lincoln Park, NJ). Transwell chambers were purchased from Costar (Kennebunkport, ME). Human thrombin was purchased from ICN Pharmaceuticals, Inc. (Costa Mesa, CA). Recombinant human (rh) IL-8, rhMCP-1, rhIL-6, rhIL-1β, rhTNF-α, rhIFN-γ, monoclonal anti-IL-8 antibody (Ab), monoclonal anti-MCP-1 Ab, monoclonal anti-IL-6 Ab, monoclonal anti-IL-1β Ab, monoclonal anti-TNF-α Ab, biotinylated anti-IL-8 Ab, biotinylated anti-MCP-1 Ab, biotinylated anti-IL-6 Ab, and biotinylated anti-TNF-α Ab were purchased from R & D Systems (Minneapolis, MN). Dulbecco’s modified essential medium, Ca2+, Mg2+-free phosphate-buffered saline (PBS), fetal bovine serum, Leech recombinant hirudin produced by cDNA expressed in Saccharomyces cerevisiae, Triton X-100, sodium chloride, glycerol, magnesium chloride, ethylenediaminetetraacetic acid, sodium orthovanadate, sodium pyrophosphate, 4-(2-aminoethyl)-benzenesulfonyl fluoride, sodium fluoride, and aprotinin were purchased from Sigma Chemical Co. (St. Louis, MO), and leupeptin was purchased from Roche Molecular Biochemicals (Indianapolis, IN). Penicillin G, streptomycin sulfate, amphotericin B, and Moloney murine leukemia virus reverse transcriptase were purchased from Life Technologies, Inc. (Gaithersburg, MD), and Ampli Taq DNA polymerase was purchased from Perkin-Elmer (Norwalk, CT). Ficoll-Paque Plus was purchased from Amersham Pharmacia Biotechnologies (Uppsala, Sweden), and Fico-Lite monocytes was purchased from Atlanta Biologics (Atlanta, GA). Diff-Quick was purchased from Baxter (McGaw, IL), and the limulus amoebocyte lysate assay was purchased from BioWhittaker (Walkersville, MD).
HRPE Cell Culture
HRPE cells were isolated from donors who had healthy eyes within 24 hours of death as previously described, in accordance with the Helsinki agreement. 17 In brief, the sensory retina was separated gently from the HRPE monolayer, and the HRPE cells were removed from Bruch’s membrane using a 1 hour incubation with papain (5 μg/ml). Isolated HRPE cells were seeded into Falcon Primaria flasks in Dulbecco’s modified essential medium containing 15% fetal bovine serum, penicillin G (100 U/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (0.25 μg/ml). The HRPE monolayers exhibited uniform immunohistochemical staining for fibronectin, laminin, and type IV collagen in a chicken wire distribution, characteristic for these epithelial cells. Cells, grown in culture up to six passages, were used for all experiments.
Monocyte Culture
Human monocytes were isolated as previously described with modification, in accordance with the Helsinki agreement. 23,28,29 Peripheral blood was drawn into a heparinized syringe from healthy volunteers, diluted 1:1 in normal saline and mononuclear cells separated by density gradient centrifugation (Ficoll-Paque Plus). The cells were washed and then layered onto density gradient (1.068 g/ml) for the enrichment of monocytes (Fico-Lite monocytes). The isolated cells were then washed, cytospun onto a glass slide, stained with Diff-Quick, and differentially counted.
Cell Stimulation
Before experiments, HRPE cells were incubated in serum-free medium for 24 hours. HRPE cells and monocytes were incubated in control medium, Dulbecco’s modified essential medium, or in the same medium also containing thrombin. They were also incubated in the medium containing rhIL-1β (0.2 ng/ml), rhTNF-α (2 ng/ml), or rhIFN-γ (1000 U/ml), either alone or in combination with thrombin. For enzyme-linked immunosorbent assay (ELISA) of supernatants of HRPE cell/monocyte co-cultures, enriched monocyte populations (4 × 105/well) were layered onto HRPE monolayers (2 × 105/well) grown to confluency in a total of 400 μl of media in 12-well plates. In experiments in which monoclonal antibodies directed against IL-1β and TNF-α were used, they were incubated with HRPE cells and monocytes for 1 hour before addition of monocytes and maintained during stimulation. To detect whether cell contact was obligatory for chemokine production, HRPE cells and monocytes were co-incubated in the same cultures, but separated by porous polycarbonate filters. After experimental incubations, culture media were collected and then centrifuged to remove particulates. Cold Ca2+, Mg2+-free PBS was used to separate monocytes from HRPE cells as previously described. 28 The isolated cells were cytospun onto a glass slide, stained with Diff-Quick, and counted. The purity of the cells was >97%. To detect cell-associated cytokines, HRPE cells and monocytes in 60-mm culture dishes were lysed with 150 μl of lysing buffer containing 50 mmol/L Hepes (pH 7.4), 1% Triton X-100, 0.15 mol/L sodium chloride, 10% glycerol, 1.5 mmol/L magnesium chloride, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L sodium orthovanadate, 10 mmol/L sodium pyrophosphate, 1 mmol/L 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 mmol/L sodium fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Lysates were then incubated on ice for 15 minutes with shaking. Then, the extracts were centrifuged at 14,000 rpm for 10 minutes at 4°C. Supernatants were decanted into new tubes. Culture media and cell extracts were stored at −70°C until ELISA was performed. The ELISA values of chemokines were normalized so that they may be compared. Cytokines and reagents were negative for endotoxin contamination as determined by the limulus amoebocyte lysate assay method (<0.05 EU/ml).
ELISA
ELISA was performed on serial dilutions of HRPE cell, monocyte, and HRPE cell/monocyte co-culture supernatants and cell extracts. Antigenic IL-8, MCP-1, IL-6, and TNF-α were quantitated using a double-ligand ELISA method as described previously. 30 Standards included 0.5 log dilutions of rhIL-8, rhMCP-1, rhIL-6, or rhTNF-α from 5 pg to 100 ng/well. This ELISA method detected MCP-1, IL-6, and TNF-α concentrations of >15 pg/ml and IL-8 concentrations of >30 pg/ml.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Synthetic oligonucleotide primers based on the cDNA sequences of human IL-8, MCP-1, and β-actin were prepared: IL-8, 5′-AAGCTGGCCGTGGCTCTCTTG-3′ and 5′-AGCCCTCTTCAAAAACTTCTC-3′; MCP-1, 5′-GCTCATAGCAGCCACCTTCATTC-3′ and 5′-GTCTTCGGAGTTTGGGTTTGC-3′; and β -actin, 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGA TTTC-3′. PCR was performed in a semiquantitative manner, essentially as previously described. 21 First-strand cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase. One μg RNA was denatured at 65°C for 10 minutes and added to the reverse transcription mixture, as indicated by the manufacturer. After incubation at 37°C for 1 hour, cDNA from the reverse-transcription mixture was then subjected to PCR in a 10-μl volume containing 5 pmol of the primer pair and 0.5 U of Ampli Taq DNA polymerase using a DNA thermal cycler. Linearity range of the reaction was determined running 15 to 35 cycles. DNA was denatured for 5 minutes at 94°C, followed by 28, 26, and 20 PCR cycles for IL-8, MCP-1, and β-actin, respectively. Each cycle included a 1-minute denaturation at 94°C, a 1-minute primer annealing at 65°C, and a 2-minute polymerization at 72°C. Each RT-PCR reaction mixture was analyzed by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The intensity of the ethidium bromide luminescence was measured by an image sensor with a computer-controlled display.
Statistical Analysis
Individual experiments were performed in triplicate three times on three different HRPE cell lines and monocytes isolated from the blood of three different donors on separate days. Each cell line displayed similar fold-increases or decreases over control levels. Data are expressed as mean ± SEM. Various assay conditions were evaluated using analysis of variance test with a post hoc analysis (Scheff multiple comparison test); P values <0.05 were considered to be statistically significant.
Results
Thrombin Induces HRPE IL-8 and MCP-1 Synthesis
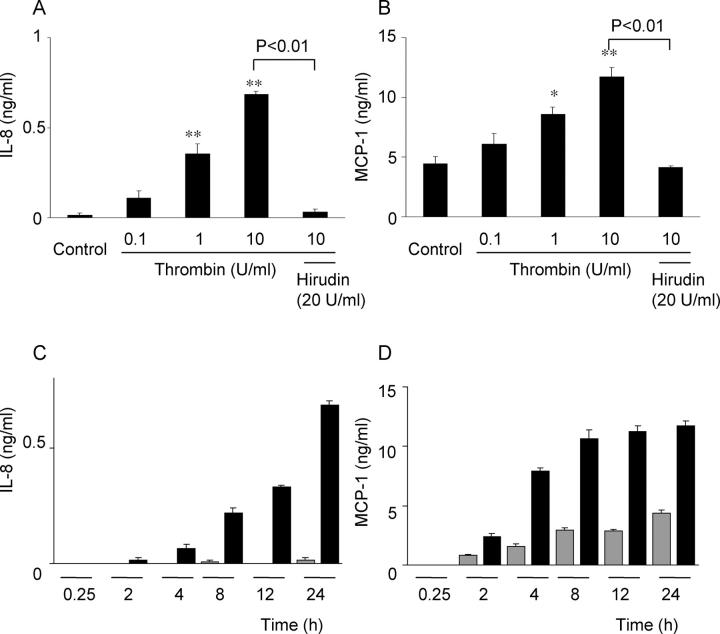
The effects of thrombin on HRPE chemokine secretion were investigated. Thrombin caused concentration-dependent IL-8 and MCP-1 release from HRPE cells reaching statistical significance at 1 U/ml (Figure 1, A ▶ and B). Hirudin (20 U/ml), a potent thrombin antagonist, binds to the anion-binding exosite and the enzymatic cleavage pocket of thrombin and prevents thrombin from binding and cleaving its receptor. This antagonist nearly completely blocked the effects of thrombin on chemokine induction. Higher doses of hirudin (50 and 100 U/ml) did not show more pronounced effects (data not shown). Thrombin-induced IL-8 and MCP-1 secretion was time-dependent, increasing for 24 hours (Figure 1, C and D) ▶ . IL-8 and MCP-1 mRNA levels were increased in thrombin-activated HRPE cells after 1 hour of stimulation, reaching a maximum after 4 hours (Figure 2, A and B) ▶ .
Figure 1.
Induction of HRPE IL-8 and MCP-1 secretion by thrombin. A and B: Thrombin induced HRPE IL-8 (A) and MCP-1 (B) in a dose-dependent manner. HRPE cells were incubated in medium containing various concentrations of thrombin (0 to 10 U/ml) or thrombin (10 U/ml) and hirudin (20 U/ml). After 24 hours, supernatants were collected and IL-8 and MCP-1 protein levels in supernatants were measured by ELISA. *, P < 0.05; **, P < 0.01, compared with control (n = 3). C and D: Thrombin-induced IL-8 (C) and MCP-1 (D) secretion was time-dependent. HRPE cells were incubated with (black bars) or without (gray bars) thrombin (10 U/ml) for different times and IL-8 and MCP-1 protein levels in supernatants were measured (n = 3).
Figure 2.
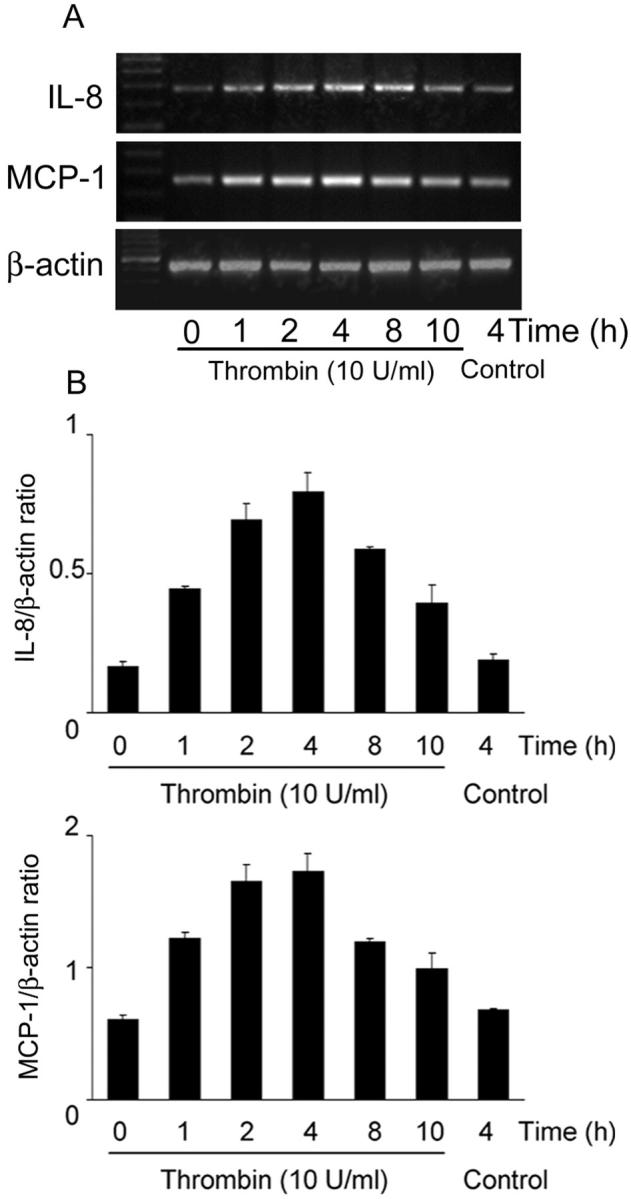
Thrombin induction of HRPE IL-8 and MCP-1 mRNA. A: HRPE cells were incubated with thrombin (10 U/ml) for different times, total RNA was extracted, and semiquantitative RT-PCR was performed. These representative data are from one of three independent experiments. B: Results are expressed as a ratio of each PCR product/β-actin band density. Values represent means ± SEM (n = 3).
Thrombin Enhances IL-8, MCP-1, and IL-6 Secretion by HRPE Cell/Monocyte Co-Cultures
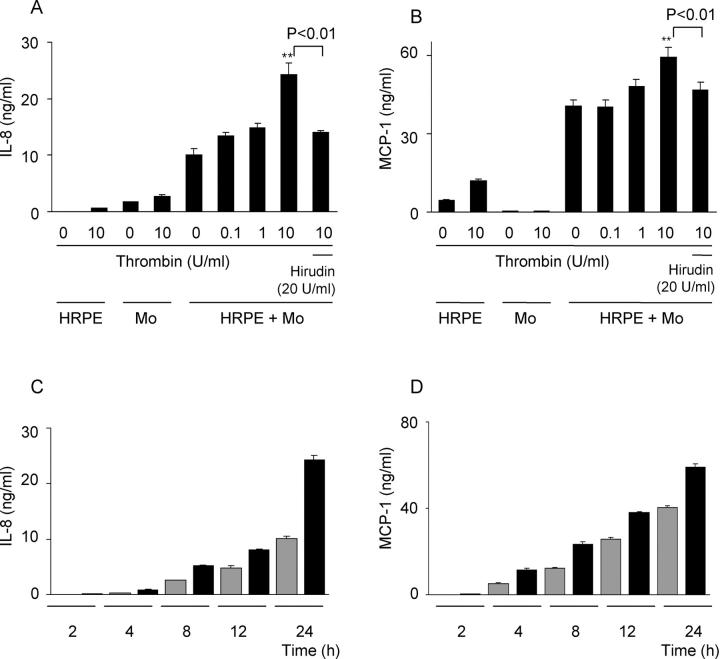
We studied the effects of thrombin on chemokine secretion because of HRPE cell/monocyte interaction. The direct overlay of monocytes onto HRPE cell cultures consistently resulted in increased IL-8 and MCP-1 production as we found previously (Figure 3, A and B) ▶ . 23 When added to HRPE cell/monocyte co-cultures, 10 U/ml of thrombin enhanced both IL-8 and MCP-1 production by 2.4-fold and 1.5-fold, respectively (Figure 3, A and B) ▶ . Thrombin-induced chemokines were inhibited by hirudin (Figure 3, A and B) ▶ . Although thrombin also induced elevated monocyte IL-8 and MCP-1 secretion, the synergistic increases in chemokine secretion by co-cultures exposed to thrombin were more than additive of the effects on HRPE cells and monocytes alone. Thrombin-induced IL-8 and MCP-1 secretion was time-dependent (Figure 3, C and D) ▶ .
Figure 3.
Effect of thrombin on IL-8 and MCP-1 secretion by HRPE cell/monocyte co-cultures. A and B: Thrombin enhanced IL-8 (A) and MCP-1 (B) secretion by HRPE cell/monocyte co-cultures. Various concentrations of thrombin (0 to 10 U/ml) or thrombin (10 U/ml) and hirudin (20 U/ml) were added to HRPE cell/monocyte co-cultures (HRPE + Mo). After 24 hours, supernatants were collected and IL-8 and MCP-1 protein levels in supernatants were measured by ELISA. *, P < 0.05; **, P < 0.01, compared with chemokine secretion from HRPE cell/monocyte co-cultures without thrombin (n = 3). HRPE, HRPE cells; Mo, monocytes. C and D: Thrombin-induced IL-8 (C) and MCP-1 (D) secretion was time-dependent. HRPE cells were incubated with (black bars) or without (gray bars) thrombin (10 U/ml) for different times and IL-8 and MCP-1 protein levels in supernatants were measured (n = 3).
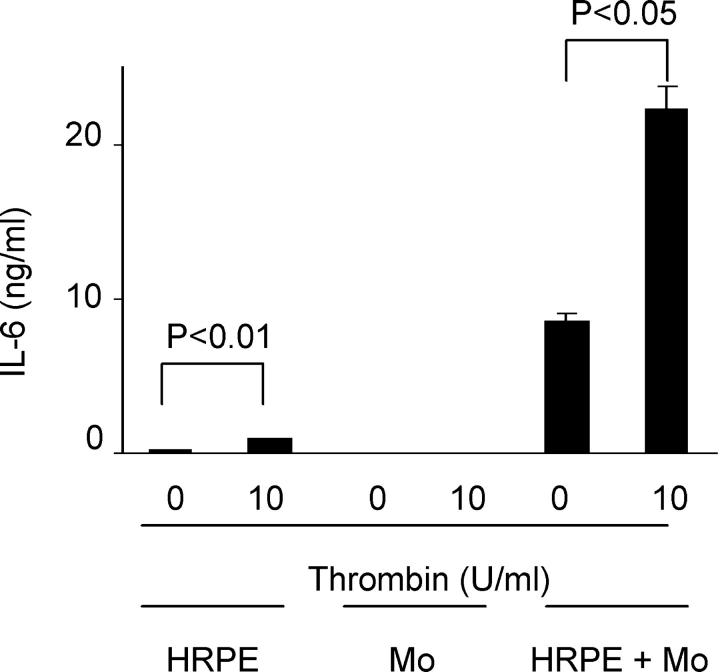
We also examined the effects of thrombin on IL-6 secretion to compare them with chemokine secretion. The direct overlay of monocytes onto HRPE cell cultures also induced IL-6 secretion (Figure 4) ▶ . Thrombin enhanced IL-6 secretion by HRPE cells and HRPE cell/monocyte co-cultures, whereas it had no effect on monocyte IL-6 secretion (Figure 4) ▶ .
Figure 4.
Effect of thrombin on IL-6 secretion by HRPE cell, monocytes, HRPE cell/monocyte co-cultures. Thrombin induced IL-6 secretion by HRPE cells and HRPE cell/monocyte co-cultures. HRPE cell, monocytes, and HRPE cell/monocyte co-cultures were incubated with thrombin (10 U/ml). After 24 hours, supernatants were collected, and IL-6 protein levels in supernatants were measured by ELISA (n = 3).
Thrombin Induces Cell-Associated IL-8 and MCP-1
Thrombin induced significant increases in cell-associated IL-8 and MCP-1 in cultured HRPE cells (Figure 5, a ▶ versus b and i versus j) and IL-8 in isolated monocytes (Figure 5A, c ▶ versus d). To detect the cells responsible for chemokine production during exposure of HRPE cell/monocyte co-cultures to thrombin, monocytes were separated from HRPE cells after co-incubation and HRPE cell and monocyte cell-associated chemokines were measured separately. After 4 hours of co-culture, cell-associated IL-8 and MCP-1 was induced in HRPE cells (Figure 5, a ▶ versus e and i versus m). More cell-associated IL-8 and MCP-1 was detected in HRPE cells in co-cultures than in monocytes (Figure 5 ▶ ; e versus g, 2.2-fold; and m versus o, 15.4-fold). Thrombin potentiated cell-associated IL-8 and MCP-1 in HRPE cells and IL-8 in monocytes after co-culture (Figure 5, e ▶ versus f, m versus n, and g versus h). Thrombin did not significantly induce monocyte MCP-1 (Figure 5B, k ▶ versus l) or significantly potentiate monocyte MCP-1 after culture with HRPE cells (Figure 5B, o ▶ versus p). HRPE cells also showed substantially more cell-associated IL-8 and MCP-1 than monocytes when co-cultures were stimulated with thrombin (Figure 5 ▶ ; f versus h, 2.5-fold; n versus p, 14.9-fold).
Figure 5.
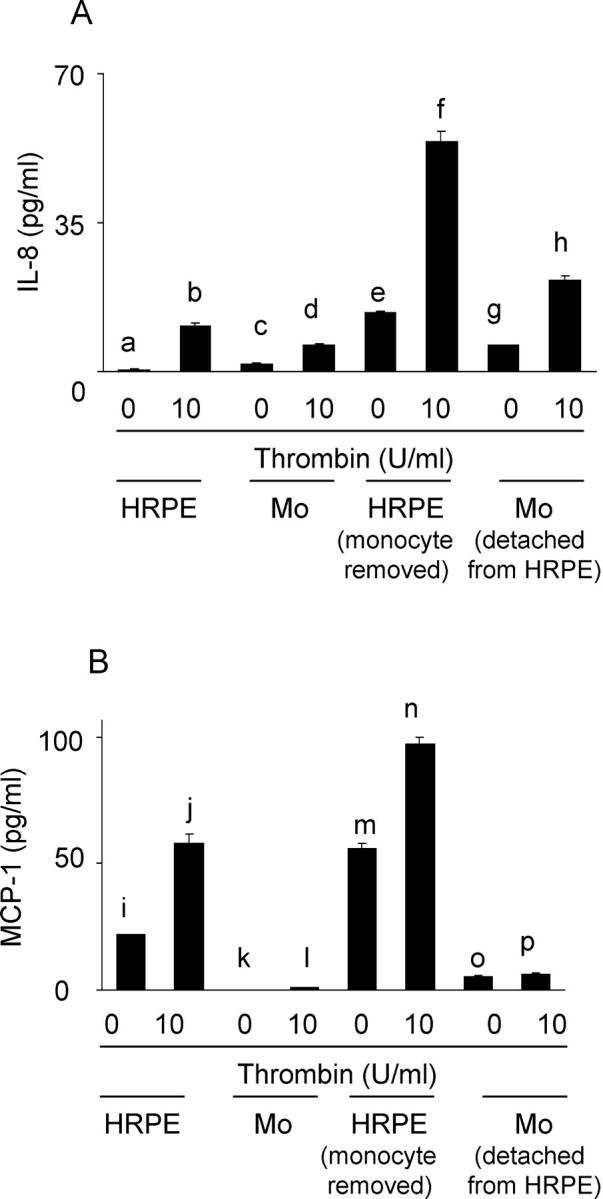
Thrombin modulated cell-associated IL-8 (A) and MCP-1 (B). HRPE cells (HRPE), monocytes (Mo), and HRPE cell/monocyte co-cultures were incubated with thrombin (10 U/ml) for 4 hours. HRPE cells and monocytes in the co-cultures were separated, and cells were lysed, as described in Materials and Methods. IL-8 and MCP-1 levels were measured by ELISA. c versus d, P < 0.05; a versus b, a versus e, e versus g, e versus f, f versus h, g versus h, i versus j, i versus m, m versus o, m versus n, and n versus p; P < 0.01 (n = 3).
HRPE Cell/Monocyte Contact Enhances Thrombin-Induced IL-8, MCP-1, and IL-6 Production
We examined whether cell contact was obligatory for IL-8, MCP-1, and IL-6 production. When HRPE cells were co-incubated with monocytes in the same cultures, but separated by porous polycarbonate filters, significantly less (P < 0.01) IL-8, MCP-1, and IL-6 were secreted into the supernatants (Figure 6, a ▶ versus c, e versus g, and i versus k). Thrombin induced significantly less (P < 0.01) IL-8, MCP-1, and IL-6 secretion into the culture supernatants from HRPE cell/monocyte co-cultures when the cells were separated by the filters than when monocytes were directly overlayed onto the HRPE monolayers (Figure 6, b ▶ versus d, f versus h, and j versus l).
Figure 6.
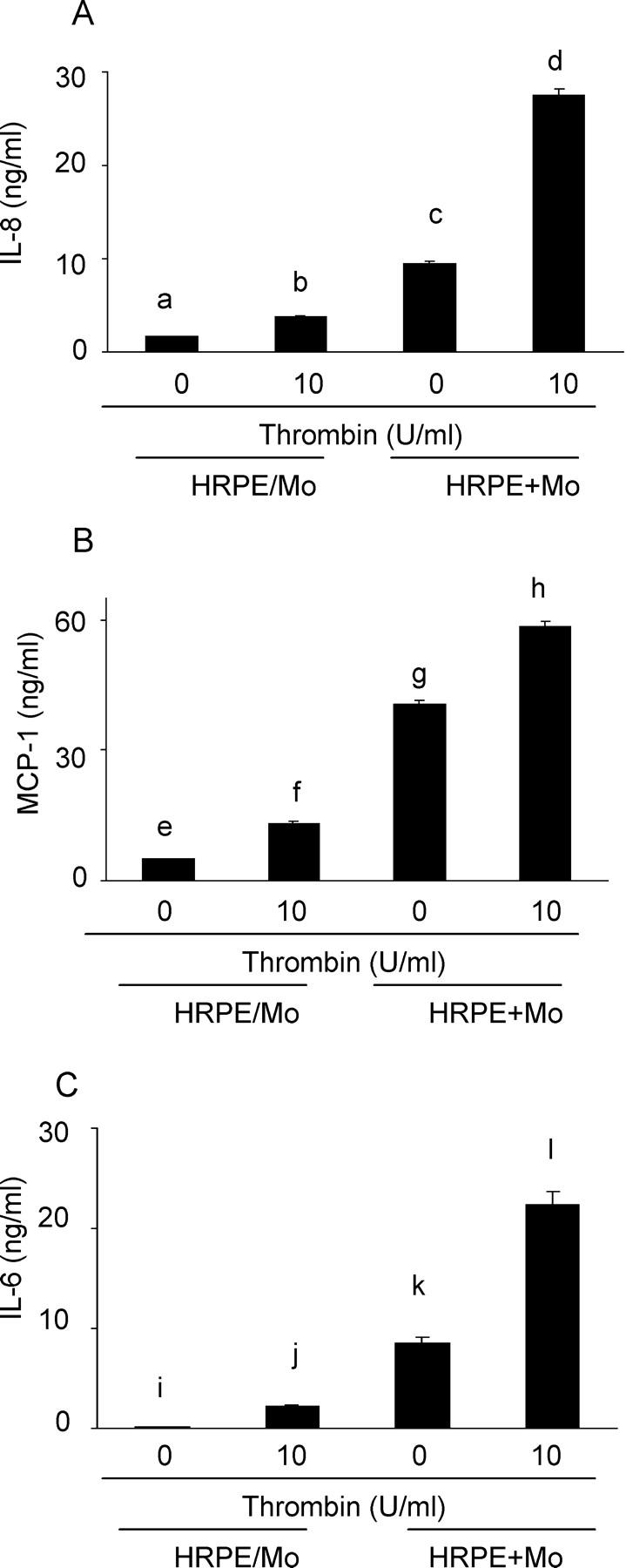
Cell-cell contact enhanced thrombin induction of IL-8 (A), MCP-1 (B), and IL-6 (C) secretion by co-cultures. HRPE cells co-incubated with monocytes in the same cultures, but separated by porous polycarbonate filters (HRPE/Mo), and HRPE cells overlayed with monocytes directly (HRPE + Mo) were incubated with thrombin (10 U/ml). After 24 hours, supernatants were collected and IL-8, MCP-1, and IL-6 protein levels in supernatants were measured by ELISA. a versus c, b versus d, e versus g, f versus h, i versus k, and j versus l; P < 0.01 (n = 3).
Anti-TNF-α, but Not Anti-IL-1β Ab, Inhibits Thrombin-Induced IL-8 and MCP-1 Secretion by HRPE Cell/Monocyte Co-Cultures
We examined the effect of neutralizing antibodies against IL-1β and TNF-α on IL-8 and MCP-1 production. Neither anti-IL-1β nor anti-TNF-α Ab had significant effects on IL-8 and MCP-1 secreted by HRPE cell/monocyte co-cultures (Figure 7, A and B) ▶ . In contrast, anti-TNF-α Ab, but not anti-IL-1β Ab, inhibited IL-8 and MCP-1 secretion by HRPE cell/monocyte co-cultures exposed to thrombin by 41 and 21%, respectively (Figure 7, A and B) ▶ . Thrombin-induced IL-8 and MCP-1 secretion by cultured HRPE cells or isolated monocytes alone was not inhibited by either anti-TNF-α Ab or anti-IL-1β Ab (data not shown)
Figure 7.
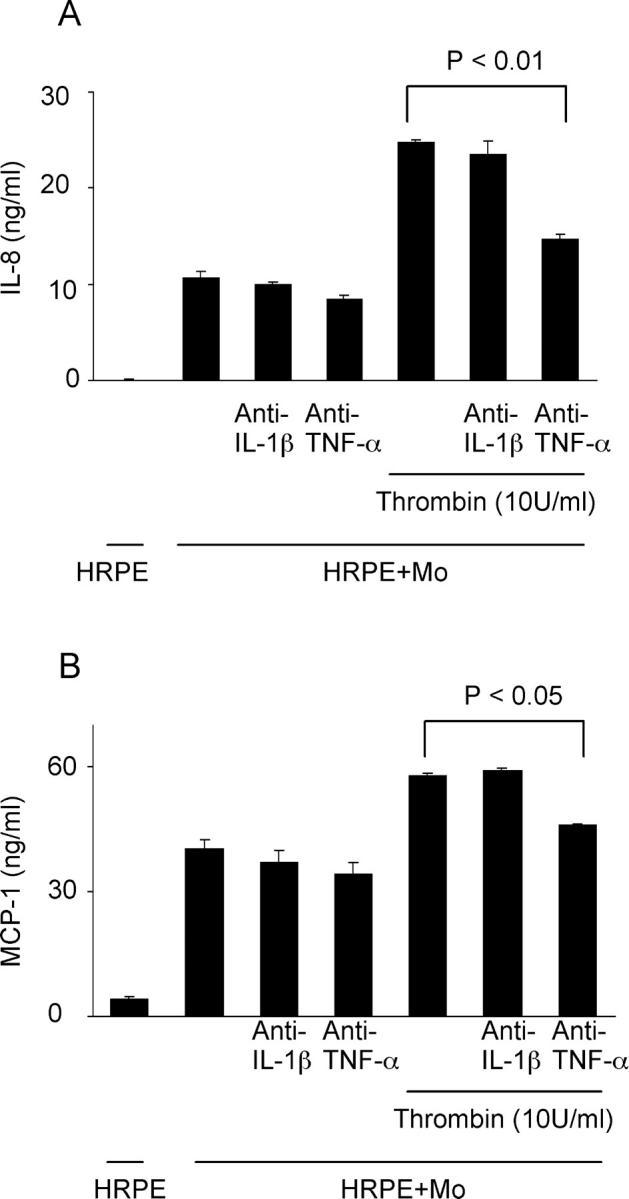
Anti-TNF-α Ab, but not anti-IL-1β Ab, inhibited thrombin-induced IL-8 (A) and MCP-1 (B) secretion by HRPE cell/monocyte co-cultures. HRPE cell/monocyte co-cultures (HRPE + Mo) were incubated with anti-TNF-α Ab or anti-IL-1β Ab in the presence or absence of thrombin (10 U/ml) for 24 hours. Supernatants were collected and IL-8 and MCP-1 protein levels in supernatants were measured by ELISA (n = 3).
Thrombin Induces TNF-α Synthesis in Monocytes during HRPE Cell/Monocyte Co-Cultures
Because anti-TNF-α Ab inhibited thrombin-induced chemokine secretion by HRPE cell/monocyte co-cultures, we tested whether thrombin could induce TNF-α synthesis by co-cultures. Although TNF-α was not detected in supernatants of HRPE cell/monocyte co-cultures stimulated with thrombin, thrombin did induce substantial levels of cell-associated TNF-α in monocytes after co-culture with HRPE cells (P < 0.01) (Table 1) ▶ . Only low levels (P > 0.05) of TNF-α were detected in monocytes co-cultured with HRPE cells in the absence of thrombin. No detectable TNF-α levels were observed in HRPE cells under any of the experimental conditions and in isolated monocytes with or without thrombin stimulation.
Table 1.
Cell-Associated TNF-α
| TNF-α (pg/ml) | |
|---|---|
| HRPE (NS) | 0 |
| HRPE (thrombin) | 0 |
| Mo (NS) | 0 |
| Mo (thrombin) | 0 |
| Mo/detached from HRPE (NS) | 18.3 ± 10.1 |
| Mo/detached from HRPE (thrombin) | 110.2 ± 7.4 |
| RPE/monocyte removed (NS) | 0 |
| RPE/monocyte removed (thrombin) | 0 |
HRPE, HRPE cells; Mo, monocytes; NS, no stimulant; thrombin, stimulated with thrombin (10 U/ml).
Thrombin Modulates rhIL-1β- and rhTNF-α-Induced IL-8, MCP-1, and IL-6 Secretion
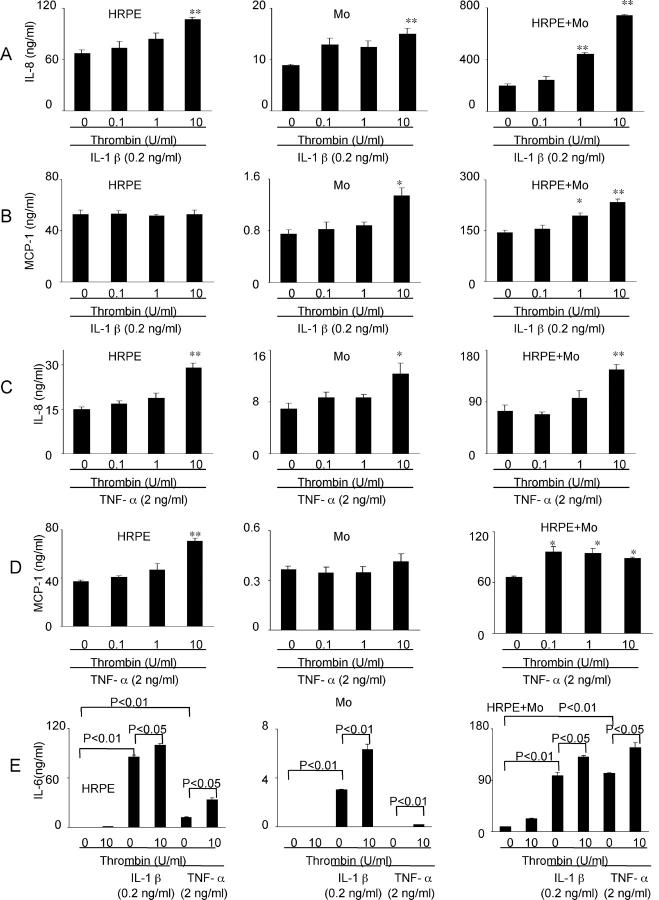
Since we previously reported that pro-inflammatory cytokine (rhIL-1β and rhTNF-α) potentiated IL-8 and MCP-1 secretion by HRPE cells, monocytes, and HRPE cell/monocyte co-cultures, 23 the effects of thrombin on these pro-inflammatory cytokine-induced chemokine secretion were examined. Co-incubation of cultured HRPE cells and isolated monocytes with IL-1β and thrombin potentiated IL-1β-induced IL-8 secretion by 1.6-fold and 1.7-fold, respectively, at the maximum concentration of thrombin (10 U/ml) (Figure 8A) ▶ . However, thrombin substantially synergized IL-1β-induced IL-8 secretion by HRPE cell/monocyte co-cultures 3.8-fold (Figure 8A) ▶ . Thrombin had no effects on IL-1β-induced HRPE MCP-1 secretion, but caused small, but significant increases in MCP-1 production by isolated monocytes exposed to IL-1β (Figure 8B) ▶ . As in the case of IL-8, thrombin significantly potentiated MCP-1 secretion by HRPE cell/monocyte co-cultures exposed to IL-1β by 1.7-fold (Figure 8B) ▶ .
Figure 8.
Effect of thrombin on rhIL-1β-induced (A and B) and rhTNF-α-induced (C and D) IL-8 (A and C), MCP-1 (B and D), and IL-6 (E) secretion. Thrombin had synergistic effects on rhIL-1β- and rhTNF-α-induced IL-8, MCP-1, and IL-6 secretion. HRPE cells (HRPE), monocytes (Mo), and HRPE cell/monocyte co-cultures (HRPE + Mo) were incubated in the medium containing rhIL-1β (0.2 ng/ml) or rhTNF-α (2 ng/ml), either alone or in combination with thrombin for 24 hours. Supernatants were collected and IL-8, MCP-1, and IL-6 protein levels in supernatants were measured by ELISA. *, P < 0.05; **, P < 0.01, compared with chemokine secretion from cells with rhIL-1β or rhTNF-α alone (n = 3).
Thrombin also significantly potentiated TNF-α-induced IL-8 secretion by HRPE cells, monocytes, and HRPE cell/monocyte co-cultures at maximum thrombin concentrations (10 U/ml) (Figure 8C) ▶ . Co-incubation of TNF-α and thrombin potentiated TNF-α-induced MCP-1 secretion by cultured HRPE cells and HRPE: monocyte co-cultures whereas it had no effects on MCP-1 production by isolated monocytes (Figure 8D) ▶ .
We also examined the effects of thrombin on pro-inflammatory cytokine stimulated IL-6 secretion. Thrombin enhanced IL-1β- and TNF-α-induced IL-6 secretion by HRPE cells and HRPE cell/monocyte co-cultures and IL-1β-induced IL-6 secretion by monocytes (Figure 8E) ▶ . Co-incubation of TNF-α and thrombin induced monocyte IL-6 secretion whereas TNF-α or thrombin alone had no effects on monocyte IL-6 secretion.
Recombinant hIFN-γ Modulates IL-8, MCP-1, and IL-6 Secretion
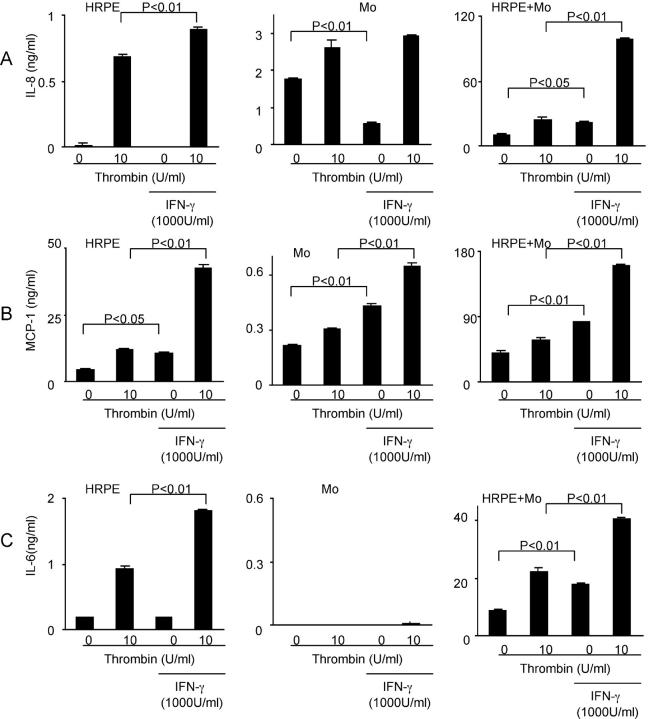
We examined the effects of IFN-γ on IL-8, MCP-1, and IL-6 secretion by HRPE cells, monocytes, and HRPE cell/monocyte co-cultures. IFN-γ had no effects on HRPE IL-8 and IL-6, and monocyte IL-6, and inhibited monocyte IL-8 whereas IFN-γ enhanced HRPE and monocyte MCP-1 (Figure 9) ▶ . IFN-γ potentiated thrombin-induced HRPE IL-8, MCP-1, and IL-6, and monocyte MCP-1, but had no effects on thrombin-stimulated monocyte IL-8 and IL-6. IFN-γ potentiated IL-8, MCP-1, and IL-6 secretion by co-cultures both in the absence and presence of thrombin.
Figure 9.
Modulation of IL-8 (A), MCP-1 (B), and IL-6 (C) secretion by IFN-γ. HRPE cells (HRPE), monocytes (Mo), and HRPE cell/monocyte co-cultures (HRPE + Mo) were incubated in the medium containing IFN-γ (1000 U/ml), either alone or in combination with thrombin for 24 hours. Supernatants were collected and IL-8, MCP-1, and IL-6 protein levels in supernatants were measured by ELISA (n = 3).
Discussion
There is strong evidence to support the concept that coagulation activation may be linked to inflammation, classically through its effects on complement activation. 31,32 Because clinical intra-ocular administration of thrombin induces severe inflammation, 12,13 the effects of thrombin on the cell types involved in ocular inflammatory processes may provide additional insight into mechanisms for the cross-talk between chemokine production and coagulation cascades in the eye.
In this study, we demonstrated that thrombin induced elevated HRPE IL-8 and MCP-1 mRNA and increased corresponding chemokine secretion in a dose- and time-dependent manner. These stimulatory effects of thrombin were significantly inhibited by the specific thrombin inhibitor, hirudin. We have found that cell to cell interaction of HRPE cells and monocytes induces IL-8 and MCP-1 production. 23 In this study, we also demonstrated that thrombin enhanced chemokine secretion by HRPE cell/monocyte co-cultures. This effect was also inhibited by hirudin. Thrombin has been shown to be directly chemotactic for monocytes and polymorphonuclear cells. 33,34 Our findings indicate that thrombin has also indirect chemotactic activity for monocytes and polymorphonuclear by inducing MCP-1 and IL-8 by HRPE cells, monocytes, and interacting HRPE cells and monocytes, revealing an important mechanism in the positive amplification of leukocyte recruitment. Thrombin effects on IL-6 secretion were generally similar to those on chemokines, which suggests that thrombin might also be involved in lymphoid immunomodulation by producing IL-6. 35
Thrombin action on IL-8, MCP-1, and IL-6 production seems to be enhanced by cell-to-cell contact, because co-incubation of HRPE cells and monocytes in the same cultures with thrombin, but separated by porous polycarbonate filters, did not induce the secretion of the high levels of these chemokines measured after direct overlay of human monocytes onto HRPE cells in the presence of thrombin. Therefore, HRPE-monocyte adhesion and/or ambient mediators subsequently produced seem to be involved in the chemokine production by HRPE cell/monocyte co-cultures stimulated by thrombin. Because we have found that both IL-8 and MCP-1 can be induced by stimulation of HRPE cells, monocytes, and HRPE cell/monocyte co-cultures with the proinflammatory cytokines, IL-1β or TNF-α, 23 we examined whether thrombin stimulation of chemokine production was mediated by these cytokines. Thrombin-induced secretion of IL-8 and MCP-1 by HRPE cell/monocyte co-cultures were inhibited by anti-TNF-α Ab, but not by anti-IL-1β Ab. Neither of these neutralizing antibodies had significant effects on thrombin-induced chemokine secretion by cultured HRPE cells or isolated monocytes alone. Thrombin did not induce the release of measurable levels of TNF-α in supernatants of cultured HRPE cells, isolated monocytes, or HRPE cell/monocyte co-cultures. However, TNF-α was detected in cell lysates of monocytes detached from HRPE cells after the co-cultures were stimulated with thrombin, but not in lysates of isolated monocytes or cultured HRPE cells exposed to thrombin independently. Because HRPE cells and monocytes were closely associated in our culture system, cell-associated TNF-α in monocytes could have effects on HRPE cells. Our results thus indicate that both thrombin treatment and interaction with HRPE cells might be required to induce monocyte TNF-α, and that the cell-associated TNF-α could, at least in part, play an important role in thrombin-induced HRPE-monocyte chemokine production. Our findings of cell-associated chemokines suggest that HRPE cells in co-cultures produce more IL-8 and MCP-1 than monocytes do. However, monocytes are likely to be responsible for HRPE cell activation during HRPE cell/monocyte interactions in the presence of thrombin. HRPE IL-8 and MCP-1 seem to be efficiently secreted as we previously reported, 19 because the levels of these chemokines in culture supernatants are much higher than those in cell lysates. Recent studies have shown that the direct adhesion of cells may be regulated by various adhesion molecules such as ICAM-1, VCAM-1, and integrin family receptors on the surfaces of interacting cells. 36-38 These reports, taken together with our findings, suggest that cell-to-cell interaction through adhesion molecules might induce chemokine production and work together with thrombin to induce TNF-α, which results in further chemokine production because of paracrine effects.
Pro-inflammatory cytokines such as IL-1β and TNF-α are considered to be important in the pathophysiology of the inflammatory component of numerous ocular disorders. They have been identified on epiretinal membranes removed surgically from patients with proliferative vitreoretinopathy and on epiretinal membranes produced experimentally. 39,40 In this study, thrombin generally potentiated exogenous IL-1β- and TNF-α-induced IL-8, MCP-1, and IL-6 production by HRPE cells, monocytes, and HRPE cell/monocyte co-cultures. Synergistic effects of thrombin and IL-1β on IL-8 production by HRPE cell/monocyte co-cultures were particularly prominent. The mechanism of this synergism has not yet been defined. However, Loppnow and colleagues 31 has hypothesized that thrombin might regulate IL-1 receptor expression or IL-1 might increase thrombin receptor expression. Accordingly, some cytokine enhancement of thrombin receptor expression and thrombin induction of cytokine receptors have been published. 41,42 In addition, in vascular endothelial cells thrombin potentiates TNF-α-induced nuclear factor-κB activity that is known to participate in the induction of IL-8, MCP-1, and IL-6 gene transcription. 43 Therefore, thrombin and cytokines may affect each others receptor expression and/or signaling pathways. The capacity of thrombin to potentiate cytokine-induced chemokine production is likely to be biologically important because it is likely that HRPE cells and monocytes in diseased retinal tissue are exposed to both inflammatory cytokines and thrombin, especially at sites of hemorrhage and breakdown of blood-retina barrier in the eyes.
IFN-γ potentiated IL-8, MCP-1, and IL-6 secretion by HRPE cell/monocyte co-cultures in the absence and presence of thrombin, but it had different effects on the secretion of these cytokines by HRPE cells and monocytes alone. IFN-γ has been shown to have immunomodulatory effects on HRPE cells and monocytes, including modulation of expression of cell surface molecules and cytokines, 44,45 which might enhance interaction between HRPE cells and monocytes.
The basal surfaces of HRPE cells are adjacent to the choroid which contains circulating leukocytes. HRPE cells exhibit polarized secretion of chemokines toward the basal side, suggesting that HRPE-derived chemokines may recruit leukocytes efficiently. 46,47 Our results show that once monocytes are recruited and contact with HRPE cells, IL-8 and MCP-1 secretion are enhanced, and further potentiated by thrombin. Therefore, the HRPE chemokine increases induced by thrombin are likely to have pathophysiologically significant effects.
Our findings suggest that chemokine induction by thrombin may exacerbate and perpetuate mechanisms of the blinding retinal diseases. This study may provide a novel molecular mechanism linking thrombosis and inflammation in the eye.
Footnotes
Address reprint requests to Victor M. Elner, M.D., Ph.D., Department of Ophthalmology, W. K. Kellogg Eye Center, University of Michigan, 1000 Wall St., Ann Arbor, MI 48105. E-mail: velner@umich.edu.
Supported by the National Institutes of Health (grants EY09441, EY007003), and Research to Prevent Blindness–Olga Keith Weiss Award (to V. M. E.).
References
- 1.Vinores SA, Amin A, Derevjanik NL, Green WR, Campochiaro PA: Immunohistochemical localization of blood-retinal barrier breakdown sites associated with post-surgical macular oedema. Histochem J 1994, 26:655-665 [DOI] [PubMed] [Google Scholar]
- 2.Bamforth SD, Lightman S, Greenwood J: The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol 1996, 91:624-632 [DOI] [PubMed] [Google Scholar]
- 3.Sen HA, Robertson TJ, Conway BP, Campochiaro PA: The role of breakdown of the blood-retinal barrier in cell-injection models of proliferative vitreoretinopathy. Arch Ophthalmol 1988, 106:1291-1294 [DOI] [PubMed] [Google Scholar]
- 4.Fenton JW, Fasco MJ, Stackrow AB: Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem 1977, 252:3587-3598 [PubMed] [Google Scholar]
- 5.Shuman MA, Majerus PW: The measurement of thrombin in clotting blood by radioimmunoassay. J Clin Invest 1976, 58:1249-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton JW, Landis BH, Walz DA, Finlayson JS: Human thrombins. Lundblad RL Fenton JW Mann KG eds. Chemistry and Biology of Thrombin. 1977, :pp 43-70 Michigan, Ann Arbor [Google Scholar]
- 7.Hackett SF, Singer JH, Leschey KH, Campochiaro PA: Thrombin is a stimulator of retinal pigment epithelial cell proliferation. Exp Eye Res 1991, 53:95-100 [DOI] [PubMed] [Google Scholar]
- 8.Chen LB, Buchanan JM: Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci USA 1975, 72:131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grand RJ, Turnell AS, Grabham PW: Cellular consequences of thrombin-receptor activation. Biochem J 1996, 313:353-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YQ, Li JJ, Karpatkin S: Thrombin inhibits tumor cell growth in association with up-regulation of p21(waf/cip1) and caspases via a p53-independent, STAT-1-dependent pathway. J Biol Chem 2000, 275:6462-6468 [DOI] [PubMed] [Google Scholar]
- 11.Puro DG, Mano T, Chan CC, Fukuda M, Shimada H: Thrombin stimulates the proliferation of human retinal glial cells. Graefes Arch Clin Exp Ophthalmol 1990, 228:169-173 [DOI] [PubMed] [Google Scholar]
- 12.Olsen TW, Sternberg P, Martin DF, Capone A, Jr, Lim JI, Aaberg TM: Postoperative hypopyon after intravitreal bovine thrombin for macular hole surgery. Am J Ophthalmol 1996, 121:575-577 [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Cho YS, Choi YJ: Intraocular hemocoagulase in human vitrectomy. Jpn J Ophthalmol 1994, 38:49-55 [PubMed] [Google Scholar]
- 14.Erger RA, Casale TB: Interleukin-8 is a potent mediator of eosinophil chemotaxis through endothelium and epithelium. Am J Physiol 1995, 268:L117-L122 [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard EJ: Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol 1989, 142:1956-1962 [PubMed] [Google Scholar]
- 16.Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, van Damme J, Oppenheim JJ: Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest 1995, 95:1370-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elner VM, Strieter RM, Elner SG, Baggiolini M, Lindley I, Kunkel SL: Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol 1990, 136:745-750 [PMC free article] [PubMed] [Google Scholar]
- 18.Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL: Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest 1991, 64:819-825 [PubMed] [Google Scholar]
- 19.Elner VM, Burnstine MA, Strieter RM, Kunkel SL, Elner SG: Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses. Exp Eye Res 1997, 65:781-789 [DOI] [PubMed] [Google Scholar]
- 20.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM: Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res 1995, 14:1045-1053 [DOI] [PubMed] [Google Scholar]
- 21.Yoshida A, Yoshida S, Khalil AK, Ishibashi T, Inomata H: Role of NF-kappaB-mediated interleukin-8 expression in intraocular neovascularization. Invest Ophthalmol Vis Sci 1998, 39:1097-1106 [PubMed] [Google Scholar]
- 22.Spandau U, Toksoy A, Holz F, Schrader W, Liesenhoff H: High expression of angiogenic chemokines in choroidal neovascularization membranes. Invest Ophthalmol Vis Sci (ARVO abstract) 2000, 41:S836 [Google Scholar]
- 23.Yoshida A, Elner SG, Bian Z-M, Kunkel SL, Lukacs NW, Elner VM: Differential chemokine regulation by Th2 cytokines during human RPE-monocyte co-culture. Invest Ophthalmol Vis Sci 2001, 42:1631-1638 [PubMed] [Google Scholar]
- 24.Jerdan JA, Pepose JS, Michels RG, Hayashi H, de Bustros S, Sebag M, Glaser BM: Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 1989, 96:801-810 [DOI] [PubMed] [Google Scholar]
- 25.Baudouin C, Peyman GA, Fredj-Reygrobellet D, Gordon WC, Lapalus P, Gastaud P, Bazan NG: Immunohistological study of subretinal membranes in age-related macular degeneration. Jpn J Ophthalmol 1992, 36:443-451 [PubMed] [Google Scholar]
- 26.Suk K, Cha S: Thrombin-induced interleukin-8 production and its regulation by interferon-gamma and prostaglandin E2 in human monocytic U937 cells. Immunol Lett 1999, 67:223-227 [DOI] [PubMed] [Google Scholar]
- 27.Grandaliano G, Valente AJ, Abboud HE: A novel biologic activity of thrombin: stimulation of monocyte chemotactic protein production. J Exp Med 1994, 179:1737-1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs NW, Strieter RM, Elner V, Evanoff HL, Burdick MD, Kunkel SL: Production of chemokines, interleukin-8 and monocyte chemoattractant protein-1, during monocyte: endothelial cell interactions. Blood 1995, 86:2767-2773 [PubMed] [Google Scholar]
- 29.Becker S, Soukup JM: Airway epithelial cell-induced activation of monocytes and eosinophils in respiratory syncytial viral infection. Immunobiology 1999, 201:88-106 [DOI] [PubMed] [Google Scholar]
- 30.Evanoff HL, Burdick MD, Moore SA, Kunkel SL, Strieter RM: A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest 1992, 21:39-45 [DOI] [PubMed] [Google Scholar]
- 31.Loppnow H, Bil R, Hirt S, Schonbeck U, Herzberg M, Werdan K, Rietschel ET, Brandt E, Flad HD: Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood 1998, 91:134-141 [PubMed] [Google Scholar]
- 32.Kaplanski G, Fabrigoule M, Boulay V, Dinarello CA, Bongrand P, Kaplanski S, Farnarier C: Thrombin induces endothelial type II activation in vitro: IL-1 and TNF-alpha-independent IL-8 secretion and E-selectin expression. J Immunol 1997, 158:5435-5441 [PubMed] [Google Scholar]
- 33.Naldini A, Sower L, Bocci V, Meyers B, Carney DH: Thrombin receptor expression and responsiveness of human monocytic cells to thrombin is linked to interferon-induced cellular differentiation. J Cell Physiol 1998, 177:76-84 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins AL, Howells GL, Scott E, Le Bonniec BF, Curtis MA, Stone SR: The response to thrombin of human neutrophils: evidence for two novel receptors. J Cell Sci 1995, 108:3059-3066 [DOI] [PubMed] [Google Scholar]
- 35.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL: Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest 2001, 107:861-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukacs NW, Strieter RM, Elner VM, Evanoff HL, Burdick M, Kunkel SL: Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1 alpha during monocyte-endothelial cell interactions. Blood 1994, 83:1174-1178 [PubMed] [Google Scholar]
- 37.Napoleone E, Di Santo A, Lorenzet R: Monocytes upregulate endothelial cell expression of tissue factor: a role for cell-cell contact and cross-talk. Blood 1997, 89:541-549 [PubMed] [Google Scholar]
- 38.Funayama H, Ikeda U, Takahashi M, Sakata Y, Kitagawa S, Takahashi Y, Masuyama J, Furukawa Y, Miura Y, Kano S, Matsuda M, Shimada K: Human monocyte-endothelial cell interaction induces platelet-derived growth factor expression. Cardiovasc Res 1998, 37:216-224 [DOI] [PubMed] [Google Scholar]
- 39.Armstrong D, Augustin AJ, Spengler R, Al-Jada A, Nickola T, Grus F, Koch F: Detection of vascular endothelial growth factor and tumor necrosis factor alpha in epiretinal membranes of proliferative diabetic retinopathy, proliferative vitreoretinopathy and macular pucker. Ophthalmologica 1998, 212:410-414 [DOI] [PubMed] [Google Scholar]
- 40.Limb GA, Earley O, Jones SE, LeRoy F, Chignell AH, Dumonde DC: Expression of mRNA coding for TNF alpha, IL-1 beta and IL-6 by cells infiltrating retinal membranes. Graefes Arch Clin Exp Ophthalmol 1994, 232:646-651 [DOI] [PubMed] [Google Scholar]
- 41.Ali H, Tomhave ED, Richardson RM, Haribabu B, Snyderman R: Thrombin primes responsiveness of selective chemoattractant receptors at a site distal to G protein activation. J Biol Chem 1996, 271:3200-3206 [DOI] [PubMed] [Google Scholar]
- 42.Nystedt S, Ramakrishnan V, Sundelin J: The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem 1996, 271:14910-14915 [DOI] [PubMed] [Google Scholar]
- 43.Anrather D, Millan MT, Palmetshofer A, Robson SC, Geczy C, Ritchie AJ, Bach FH, Ewenstein BM: Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J Immunol 1997, 159:5620-5628 [PubMed] [Google Scholar]
- 44.Elner SG, Elner VM, Pavilack MA, Todd RF, III, Mayo-Bond L, Franklin WA, Strieter RM, Kunkel SL, Huber AR: Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab Invest 1992, 66:200-211 [PubMed] [Google Scholar]
- 45.Boehm U, Klamp T, Groot M, Howard JC: Cellular responses to interferon-gamma. Annu Rev Immunol 1997, 15:749-795 [DOI] [PubMed] [Google Scholar]
- 46.Holtkamp GM, Van Rossem M, de Vos AF, Willekens B, Peek R, Kijlstra A: Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol 1998, 112:34-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holtkamp GM, De Vos AF, Peek R, Kijlsta A: Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin Exp Immunol 1999, 118:35-40 [DOI] [PMC free article] [PubMed] [Google Scholar]