Abstract
Oxidative stress is a prominent feature of the placenta in many complications of pregnancy, such as preeclampsia. The cause is primarily unknown, although ischemia-reperfusion injury is one possible mechanism. Our aim was to test this hypothesis by examining the oxidative status of human placental tissues during periods of hypoxia and reoxygenation in vitro. Rapid generation of reactive oxygen species was detected using the fluorogenic probe, 2′,7′-dichlorofluorescein diacetate, when hypoxic tissues were reoxygenated. The principal sites were the villous endothelium, and to a lesser extent the syncytiotrophoblast and stromal cells. Increased concentrations of heat shock protein 72, nitrotyrosine residues, and 4-hydroxy-2-nonenal were also observed in the villous endothelial and underlying smooth muscle cells, and in the syncytiotrophoblast. Furthermore, preloading placental tissues with the reactive oxygen species scavengers desferrioxamine and α-phenyl-N-tert-butylnitrone reduced levels of oxidative stress after reoxygenation. These changes are consistent with an ischemia-reperfusion injury, and mirror those seen in preeclampsia. Consequently, in vitro hypoxia/reoxygenation may represent a suitable model system for investigating the generation of placental oxidative stress in preeclampsia and other complications of pregnancy.
Preeclampsia is a major cause of maternal and perinatal morbidity and mortality, yet the cause remains unknown. Recent theories have implicated placental oxidative stress as a key intermediary event in the generation of the syndrome. 1,2 The mechanisms underlying the generation of the placental stress are however uncertain. Although the activities of the principal antioxidant enzymes and the expression of other antioxidant systems, such as thioredoxin, are decreased within the placental tissues, it is not clear whether these are primary effects or secondary to depletion through the increased generation of free radicals. 3,4 The most widely recognized predisposing factor for preeclampsia is deficient invasion of the endometrium by extravillous cytotrophoblast cells during the first trimester of pregnancy. 5 This results in incomplete conversion of the spiral arteries, such that the myometrial segments do not dilate and remain contractile. Consequently, these vessels display an abnormally high vascular resistance, and are associated with reduced uteroplacental perfusion as confirmed by Doppler flow velocimetric studies. 6-8 Hence, in the past it has generally been believed that the changes that characterize the preeclamptic placenta are the result of chronic hypoxia. However, comparison with morphological findings in other situations associated with low oxygen tensions suggests that hypoxia alone is insufficient to account for these changes. 9
An alternative hypothesis is that the retention of vasoreactivity in the incompletely remodeled arteries results in the maternal blood flow to the intervillous space being more variable than normal. The constancy of the placental perfusion may be a more important factor than the absolute rate of blood flow, for because both the fetus and the placenta extract considerable quantities of oxygen during mid to late gestation the placental tissues will soon become locally hypoxic during periods of vasoconstriction. When the maternal blood flow is re-established there will therefore be a rapid increase in tissue oxygenation, and such fluctuations in oxygen tension could provide the basis for an ischemia-reperfusion type insult. Depending on the severity and frequency of these insults the outcome might range from mild oxidative stress to severe tissue damage and frank infarction.
Ischemia-reperfusion injury is now a well-recognized consequence of malperfusion in many organ systems, and is mediated principally through the generation of cytotoxic reactive oxygen species (ROS). 10 “Reactive oxygen species” is a term used to describe a broad category of molecules that includes oxygen-containing radicals, such as superoxide, nitric oxide (NO), and hydroxyl radicals, and nonradical but reactive molecules derived from oxygen, for example hydrogen peroxide (H2O2), hypochlorous acid, and the peroxynitrite anion. 11 If the generation of ROS exceeds the capacity of the antioxidant defenses then oxidative stress results, in which there may be indiscriminate damage to lipids, proteins and DNA, leading to cell dysfunction and tissue damage. In the case of the placenta such injuries could account for the oxidative stress observed, and for the increased rates of infarction and syncytial necrosis. 12
The aim of the present study was to investigate the ischemia-reperfusion phenomenon in term placental tissues during the periods of hypoxia and reoxygenation (H/R) in vitro, to determine whether H/R may represent a suitable model system for investigating the generation of placental oxidative stress in preeclampsia and other complications of pregnancy. Our hypotheses to be tested were; first, that abundant ROS are generated during reoxygenation of hypoxic placental tissues; second, that there is evidence of oxidative stress in placental tissues after H/R; and third, that preloading of placental tissues with ROS scavengers reduces the levels of oxidative stress in hypoxia/reoxygenation.
Materials and Methods
Materials
Except where suppliers are stated individually, the materials and chemicals used in this study were purchased from Sigma Chemical Co., St. Louis, MO.
Tissue Collection
Term placentas (n = 15) were obtained from normal pregnancies with permission immediately after elective cesarean deliveries for repeat section before onset of labor. Villous samples (n ≥ 10, each ∼40 to 50 mg wet weight) were taken midway between the chorionic and basal plates, from five to seven lobules free of visible infarction, calcification, hematoma, or tears. After a brief rinse in cold phosphate-buffered saline (PBS), one sample was snap-frozen in liquid nitrogen as a time 0 control. The remaining samples were placed into culture medium (Medium-199 with 25 mmol/L HEPES, Earle’s salts, and l-glutamine; Life Technologies Ltd., Paisley, UK) equilibrated with 95% N2/5% CO2 (BOC, Guildford, UK) in a sealed glass bottle, and transferred to the laboratory on ice for individual experiments.
Establishment of Hypoxic Conditions
Using a special incubation bag with a moistened Anaerocult IS (Merck KgaA, Darmstadt, Germany) inside, villous samples were incubated in 4-well, flat-bottom culture plates (one sample per well) (Nunclon Delta Multidishes; Nalge Nunc International, Rochester, NY) with fresh medium that had been saturated at 37°C with 95% N2/5% CO2. The bag was flushed with the same gas mix for 1 minute, sealed, and transferred to a separate chamber (Desiccator Cabinet; Scientific Laboratory Supplies Limited, Nottingham, UK). The chamber was continuously flushed with 95% N2/5% CO2 to maintain a gas phase PO2 of <1 mmHg (OM-14 oxygen monitor; SensorMedics Corporation, Yorba Linda, CA). The partial pressure of oxygen in the culture medium of this hypoxic condition was monitored with a portable dissolved oxygen meter (model 9071; Jenway Inc., Princeton, NJ) and kept at 12 to 16 mmHg.
Conditions for Hypoxia-Reoxygenation
After 20 minutes of incubation under hypoxic conditions, villous tissues were randomly assigned to culture plates with medium that had been saturated at 37°C with either 5% O2/90% N2/5% CO2 or air/5% CO2, and maintained in separate humidified chambers continuously flushed with these respective gas mixes for up to 2 hours. Using these settings, the measured dissolved oxygen pressures in the media were 45 to 62 mmHg and 143 to 160 mmHg, respectively. In comparative experiments, placental tissues were cultured under hypoxic conditions throughout the 2-hour period. A total of six separate placentas were studied.
After culture, villous samples from individual experiments were snap-frozen in either precooled isopentane or directly in liquid nitrogen, and stored at −80°C for further immunohistochemistry or Western blot analysis.
Detection of ROS Generation with 2′,7′-Dichlorofluorescein Diacetate (DCFH-DA)
To detect the generation of ROS during the period of reoxygenation, placental tissues were cultured under hypoxic conditions for 20 minutes as previously described, then transferred to a microscope chamber. This was created from a 30-mm Petri dish with a 25-mm hole in the bottom, sealed with a 27 mm, no. 0 thickness coverslip using elastomeric glue (Sylgard; Dow Corning Corporation, Wiesbaden, Germany). The chamber contained medium that was saturated with air/5% CO2 at 37°C and 20 μmol/L of DCFH-DA, and was continuously gassed with air/5% CO2 and kept at 37°C on a heating stage. Using a Leica TCS SP-MP confocal microscope (Leica Microsystems, Heidelberg, Germany) the formation of the fluorescent product 2′,7′-dichlorofluorescein (DCF), as a result of oxidation of 2′,7′-dichlorofluorescein (DCFH) by ROS, was recorded in a time-lapse series with 30-second intervals. The dye was excited with a Tsunami T1/Sapphire laser tuned to 780 nm with a pulse width of 1.3 picoseconds and a repetition rate of 82 MHz. Emitted light was captured between 500 to 560 nm. Placental tissues kept in medium continuously gassed with 95% N2/5% CO2 served as controls. Each experiment was repeated in two separate placentas.
Immunohistochemical Staining of Inducible Heat Shock Protein 72 (HSP 72), Nitrotyrosine, and 4-Hydroxy-2-Nonenal (4-HNE)
Serial sections were cut at 10 μm, fixed in acetone (−20°C) for 10 minutes, washed with PBS, quenched with 3% H2O2 in methanol for 10 minutes, blocked with 10% normal goat serum, 0.2% Tween-20 in PBS (PBS-T) at room temperature for 1 hour, then reacted with the primary antibodies [1:2000 for rabbit polyclonal anti-HSP 72 antibody (Stressgen Biotechnologies Corp., Victoria, BC, Canada) 1:1000 for rabbit polyclonal anti-nitrotyrosine antibody (Upstate Biotechnology Inc., Lake Placid, NY)] diluted with 5% normal goat serum in PBS-T at 4°C overnight. The sections were incubated with biotinylated goat anti-rabbit IgG at 1:200 dilution for 30 minutes at room temperature, washed in PBS, and then incubated in avidin-biotin-peroxidase solution according to the instructions for the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) for another 30 minutes. Diaminobenzidine tetrahydrochloride at 0.5 mg/ml was used as the peroxidase substrate, and allowed to develop until optimal staining was achieved. Slides were then counterstained, dehydrated, and coverslipped. Omission of the primary antibodies served as the negative controls.
For detection of 4-HNE, sections were processed as described above except 10% normal horse serum in PBS-T was used to block nonspecific binding. Mouse monoclonal anti-4-HNE antibody (1:10; Japan Institute for the Control of Ageing, Shizuoka, Japan) and biotinylated horse anti-mouse IgG (Vector Laboratories) were used as the primary and secondary antibodies, respectively.
Double-Immunofluorescent Labeling for HSP 72, Nitrotyrosine, and 4-HNE
Double-immunofluorescent labeling was used to demonstrate the relative localization of HSP 72, nitrotyrosine, and 4-HNE. Serial sections were cut at 10 μm, fixed in acetone (−20°C) for 10 minutes, washed with PBS, blocked with 10% normal goat serum in PBS-T at room temperature for 1 hour, then reacted with the primary antibodies as described in immunohistochemistry except different working dilutions (1:1000 for anti-HSP 72 and 1:5 for anti-4-HNE, respectively) and a mouse monoclonal antibody to nitrotyrosine (1:20; Upstate Biotechnology Inc.) were used. After washing in PBS, the sections were incubated with a cocktail of fluorescein isothiocyanate-conjugated goat anti-rabbit IgG and Texas Red-conjugated goat anti-mouse IgG (CN Biosciences, Nottingham, UK) diluted at 1:50 with PBS at room temperature for 1 hour. The slides were observed by confocal microscopy (Leica TCS-NT; Leica Microsystems, Heidelberg, Germany), with simultaneous excitation and detection of both dyes. The superimposition of the two chromophores in the same image results in a green/red color scale, leading to a yellow color in case of co-localization.
Western Blot
Approximately 50 mg of placental tissue was homogenized in ice-cold distilled water with 0.05% Triton X-100, 1 mmol/L dithiothreitol, and Complete mini protease inhibitor cocktail (Roche Diagnostics Ltd., East Sussex, UK). Tissue homogenates were centrifuged at 13,000 rpm for 20 minutes and the supernatant removed. Protein concentrations were determined by the Peterson’s modification of microLowry method using a protein determination kit (from Sigma) and absorbance measured at 665 nm. Equal amounts of protein samples (40 μg per lane) were separated with the use of 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, proteins in one gel were transferred to a nitrocellulose membrane (Hybond ECL; Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, UK), blocked with 5% skimmed milk in PBS-T for 1 hour and subsequently probed with a rabbit polyclonal anti-HSP 72 (1:20,000; Stressgen Biotechnologies Corp.) or a mouse monoclonal anti-4-HNE antibody (1:10; Japan Institute for the Control of Aging) at 4°C overnight. Horseradish peroxidase-linked donkey anti-rabbit or sheep anti-mouse secondary antibodies (1:2000; Amersham Pharmacia Biotech UK Ltd.) were used in conjugation with enhanced chemiluminescence (SuperSignal West Pico, Pierce Chemical Company, Rockford, IL) to visualize the HSP 72 and 4-HNE bands on autoradiography films (BioMax Light; Eastman Kodak Company, Rochester, NY).
The membranes were stripped with a buffer containing 62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, and 100 mmol/L β-mercaptoethanol at 50°C for 50 minutes and reprobed with anti-β-actin monoclonal antibody (clone AC-15, 1:2000 dilution; Sigma Chemical Co.) to correct for loading variations. The duplicate gel was directly stained with Coomassie brilliant blue G250 (Bio-Rad Laboratories Ltd., Hertfordshire, UK) to confirm equal loading among the lanes. The relative intensity of protein signals was normalized to the corresponding β-actin density and quantified by densitometric analysis using the public-domain computer program (Scion Image, Beta Release 4.0.2; Scion Corp., Frederick, MD).
Effects of ROS Scavengers on the Expression HSP 72 and Production of 4-HNE after Hypoxia-Reoxygenation
Placental tissues were preloaded with different scavengers of ROS dissolved in PBS at various concentrations to test their effects on the expression of HSP 72 and the production of 4-HNE after H/R (1 hour of hypoxia then 2 hours of reoxygenation with air/5% CO2). These included desferrioxamine (0.1, 1, 10 mmol/L), α-phenyl-N-tert-butylnitrone (PBN; 1, 10, 100 mmol/L), and superoxide dismutase (SOD; 60 and 600 U/ml). Immunoblots of HSP 72 and 4-HNE were quantitatively analyzed as the above described. Densities of placental tissues administrated with PBS only were used as vehicle controls. The effects of these ROS scavengers were expressed as a ratio of controls. Each experiment was performed in triplicate from three separate placentas.
Statistical Analysis
All data are presented as mean ± SEM. They were tested for the homogeneity of variance (Bartlett’s test) and normality (Kolmogorov-Smirnov test) first, then computed with analysis of variance or nonparametric tests (Kruskal-Wallis test). Post hoc tests were performed if significant effects were determined. Pearson correlation analysis was used to test the correlation between the degree of protection that desferrioxamine, PBN, or SOD could offer, and the level of endogenous stress. Statistical significance was set at P < 0.05.
Results
Generation of ROS during Reoxygenation
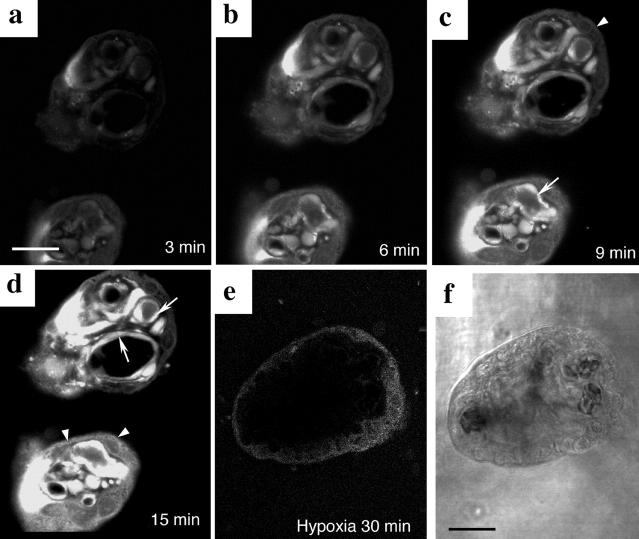
Formation of the fluorescent product DCF confirmed that ROS are generated rapidly in placental tissues on reoxygenation. Occasional cells were beginning to fluoresce during the inevitable period of delay while the chamber was positioned on the microscope stage, and focusing took place. Overall, the maximum interval between reoxygenation and the first image capture was ∼3 minutes. The intensity of the fluorescence rapidly increased, and saturation was achieved within 15 minutes of reoxygenation with air/5% CO2. The fluorescence was localized mainly in the villous endothelium, and to a lesser extent in the syncytiotrophoblast and stromal cells (Figure 1, a to d) ▶ . In contrast, no fluorescence was detected in control tissues kept hypoxic throughout (Figure 1e) ▶ .
Figure 1.
Detection of ROS using the fluorogenic probe, DCFH-DA. a–d: A time-lapse series of two terminal villi showing the generation of ROS, as revealed by the formation of fluorescent DCF, after reoxygenation of hypoxic placental tissues with air/5% CO2. The fluorescence was mainly localized in the villous endothelium (arrows) and to a lesser extent in the syncytiotrophoblast and stromal cells (arrowheads). e: Villous tissues kept under hypoxia as a control. f: Phase-contrast micrography of e. Scale bar, 50 μm.
Immunohistochemical Localization of HSP 72, Nitrotyrosine, and 4-HNE after Hypoxia-Reoxygenation
Expression of HSP 72 was greatly enhanced in placental tissues treated with H/R. After 20 minutes of culture under hypoxia, followed by 2 hours of incubation in media saturated with either air/5% CO2 or 5% O2/90% N2/5% CO2, intense immunostaining was observed principally in the villous endothelium and underlying smooth muscle cells, but also within stromal cells, cytotrophoblast cells, and the syncytiotrophoblast (Figure 2, c to e) ▶ . By contrast, only minimal staining was observed in control tissues frozen immediately after delivery or kept hypoxic throughout. This was mainly localized to the stromal cells (Figure 2, a and b) ▶ .
Figure 2.
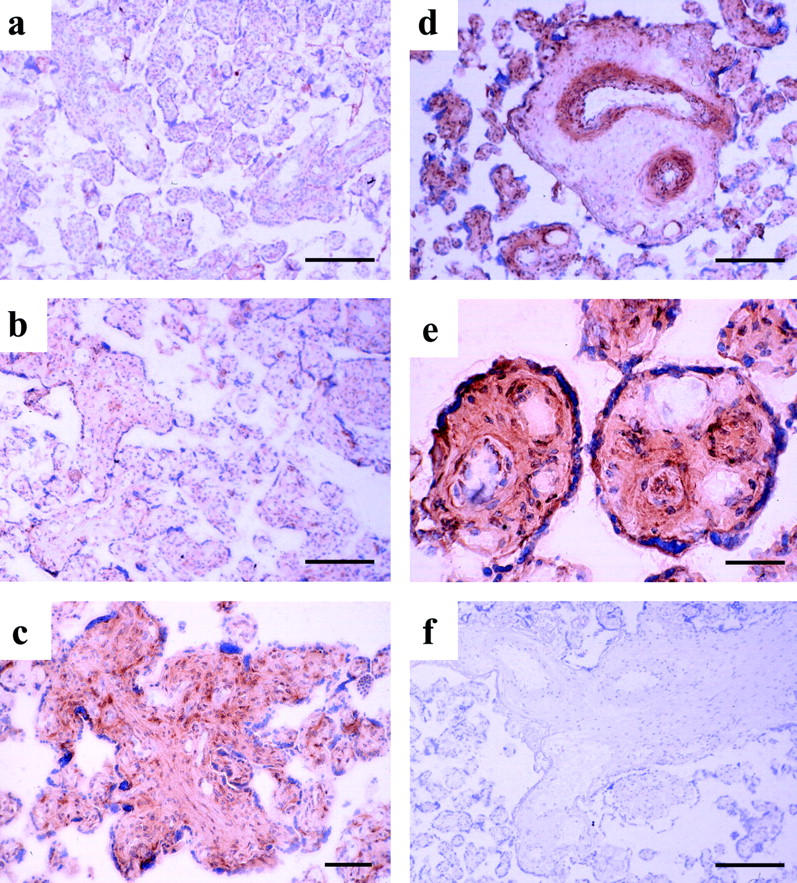
Immunoreactivity of HSP 72. Essentially, increasing immunoreactivity of HSP 72 was noted in the villous endothelium and underlying smooth muscle, but also in stromal cells, cytotrophoblast cells, and the syncytiotrophoblast after hypoxia-reoxygenation (c, d, and e). Villous tissues sampled immediately after delivery (a), kept in medium equilibrated with 95% N2/5% CO2 for 2 hours (b), and experiencing 20 minutes of hypoxia, followed by 2 hours of reoxygenation with either 5% O2/90% N2/5% CO2 (c) or air/5% CO2 (d and e). Omission of the primary antibody served as the negative control (f). Scale bars: 200 μm (a, b, d, and f), 100 μm (c), 50 μm (e). HSP 72, inducible heat-shock protein 72.
A generally similar pattern of immunolabeling was observed for nitrotyrosine residues. Strong labeling was found in the syncytiotrophoblast, villous endothelium and, to a lesser extent, the stromal cells and vascular smooth muscle layers in stem villi from placental tissues experiencing H/R (Figure 3, c to e) ▶ . However, there was virtually no labeling in control villous tissues that were frozen immediately after delivery or cultured under hypoxic conditions (Figure 3, a and b) ▶ .
Figure 3.
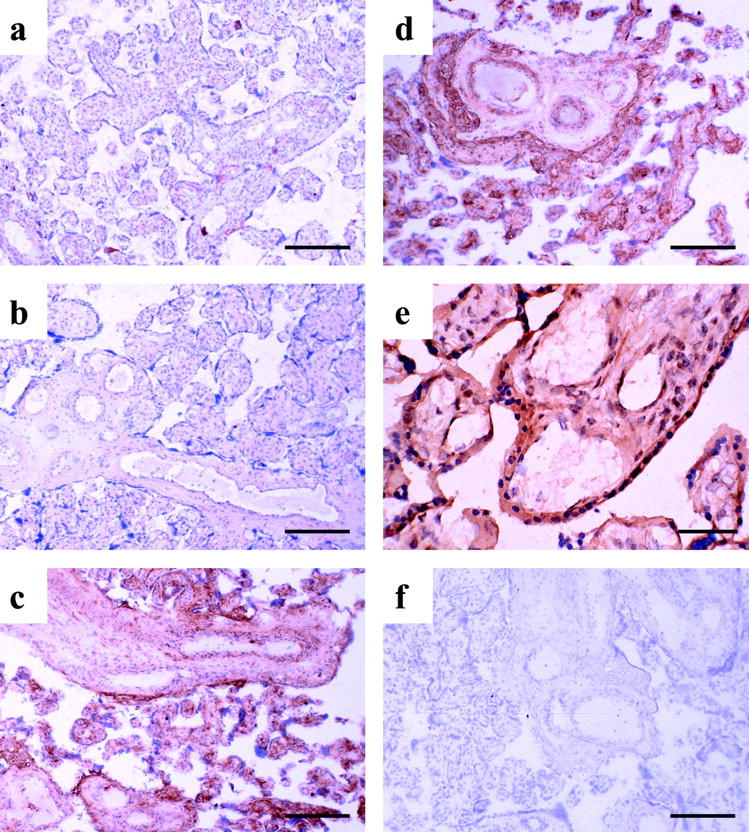
Immunostaining of nitrotyrosine residues. Generally, there was an increased immunoreactivity of nitrotyrosine localized in the villous endothelium, syncytiotrophoblast, and stromal cells in villous tissues undergoing hypoxia-reoxygenation (c, d, and e). Villous tissues sampled immediately after delivery (a), kept in medium equilibrated with 95% N2/5% CO2 for 2 hours (b), and experiencing 20 minutes of hypoxia, followed by 2 hours of reoxygenation with either 5% O2/90% N2/5% CO2 (c) or air/5% CO2 (d and e). Omission of the primary antibody served as the negative control (f). Scale bars: 200 μm (a–d, and f), 50 μm (e).
Formation of the toxic lipid peroxidation product, 4-HNE, was also observed, mainly within the villous endothelium after reoxygenation with either air/5% CO2 or 5% O2/90% N2/5% CO2 for 2 hours (Figure 4, c to e) ▶ . Again, there was virtually no immunoreactivity in control villous tissues (Figure 4, a and b) ▶ .
Figure 4.
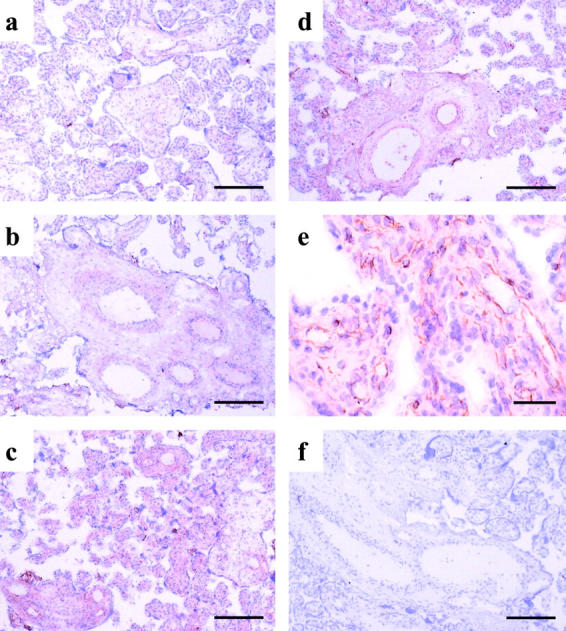
Immunoreactivity of 4-HNE. The 4-HNE immunostaining was mainly confined in the villous endothelium in villous tissues with hypoxia-reoxygenation (c,d, and e). Villous tissues immediately sampled after delivery (a), kept in medium equilibrated with 95% N2/5% CO2 for 2 hours (b), and experiencing 20 minutes of hypoxia, followed by 2 hours of reoxygenation with either 5% O2/90% N2/5% CO2 (c) or air/5% CO2 (d and e). Omission of the primary antibody served as the negative control (f). Scale bars: 200 μm (a–d, and f), 50 μm (e).
Double-Immunofluorescent Labeling for HSP 72, Nitrotyrosine, and 4-HNE
Information about the relative localization of HSP 72, nitrotyrosine, and 4-HNE was revealed by the use of double-immunofluorescent labeling (Figure 5) ▶ . HSP 72 seemed to be a more general marker for oxidative stress than nitrotyrosine and 4-HNE, which appeared primarily confined to the endothelium, adjacent smooth muscle cells, and the syncytiotrophoblast. In addition, there was also a mild increase in the fluorescent signal of 4-HNE in the syncytiotrophoblast after H/R that was not detected using the chromogenic technique (Figure 5, e and f) ▶ .
Figure 5.
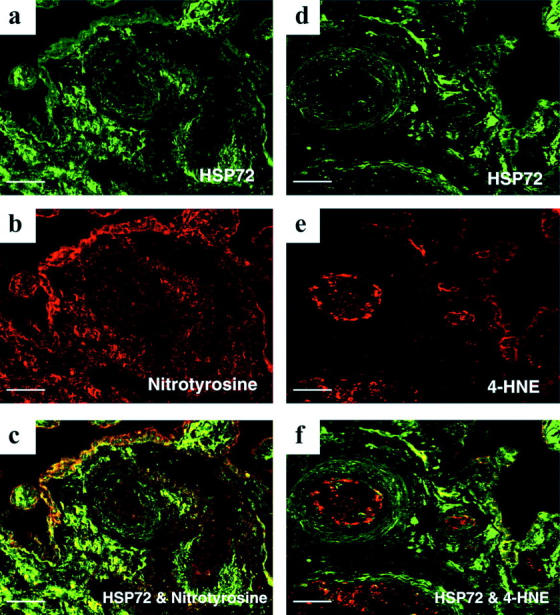
Double-immunofluorescent labeling showing the relative localization of HSP 72 and nitrotyrosine (a–c), and 4-HNE (d–f), respectively, in villous tissues experiencing 20 minutes of hypoxia, followed by 2 hours of reoxygenation with air/5% CO2. Note a mild increase in fluorescent signal of 4-HNE in the syncytiotrophoblast that was not disclosed by the chromogenic technique (e). Furthermore, HSP 72 is a more general marker of oxidative stress than nitrotyrosine and 4-HNE. Scale bar, 50 μm.
Effects of Hypoxia and Hypoxia-Reoxygenation on the Induction of HSP 72
To quantitate the cellular response to hypoxia and H/R, the induction of HSP 72 under different oxygen tensions and at different time intervals was monitored by immunoblotting.
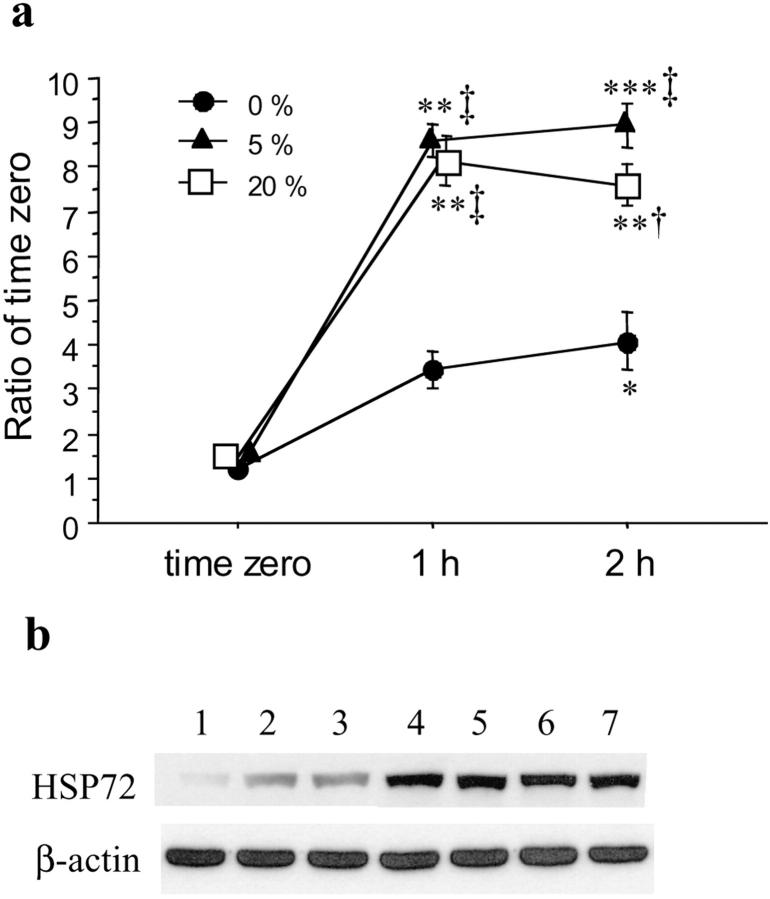
Significantly increased expression of HSP 72 was noted in placental tissues experiencing H/R as compared to those sampled immediately after delivery or kept under hypoxic conditions throughout (Figure 6) ▶ . There was no difference, however, between reoxygenation with air/5% CO2 or with 5% O2/90% N2/5% CO2. Equally, there was no difference between 1 hour and 2 hours of hypoxia or H/R treatment.
Figure 6.
Expression of HSP 72 in villous tissues response to hypoxia and hypoxia-reoxygenation. a: Levels of immunodetectable HSP 72 in homogenate from five different placentas. The values represent the mean ± SEM. P values based on repeated measures analysis of variance and the studentized range test: *, P < 0.05; **, P < 0.01; ***, P < 0.001, significantly different from placental tissues sampled immediately after delivery (time 0); †, P < 0.05; ‡, P < 0.01, significantly different from levels of placental tissues cultured under hypoxic conditions (95% N2/5% CO2). Filled circle, cultured under hypoxia; filled triangle, hypoxia-reoxygenation (5% O2/90% N2/5% CO2); open square, hypoxia-reoxygenation (air/5% CO2). b: The bands shown are the representatives of immunoblots performed on five sets of experiments. Lane 1, time 0; lanes 2 and 3, cultured under hypoxia at 1 hour and 2 hours, respectively; lanes 4 and 5, hypoxia-reoxygenation (5% O2/90% N2/5% CO2) for 1 hour and 2 hours; lanes 6 and 7, hypoxia-reoxygenation (air/5% CO2) for 1 hour and 2 hours, respectively. β-actin shown below was used to normalize for loading variability.
Effects of Desferrioxamine, PBN, and SOD on the Expression HSP 72 and Production of 4-HNE after Hypoxia-Reoxygenation
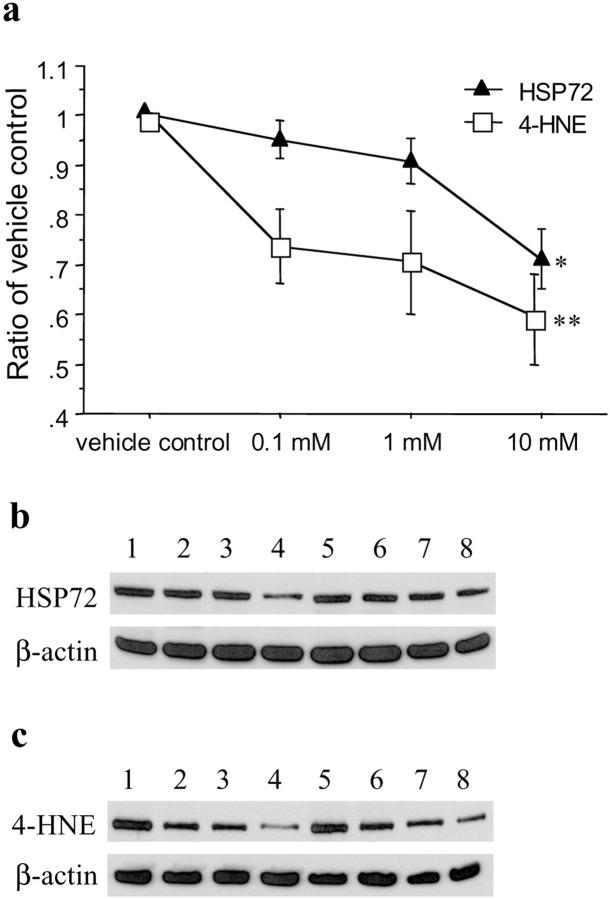
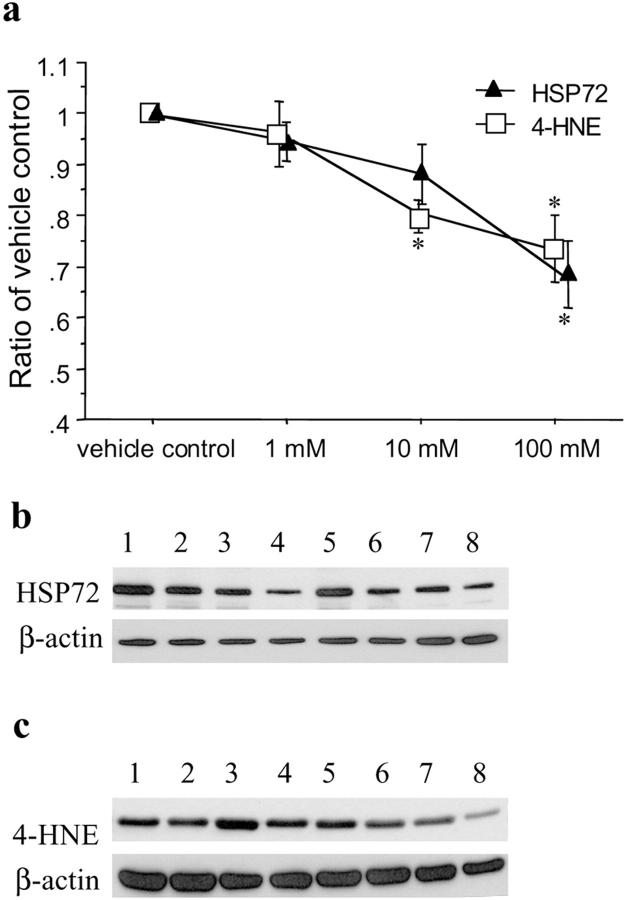
Concentration-dependent responses on the expression of HSP 72 and production of 4-HNE after H/R were observed in placental tissues preloaded with ROS scavengers. Desferrioxamine significantly reduced the expression of HSP 72 (H = 15.3, P = 0.0016) and the production of 4-HNE (H = 8.9, P = 0.03), particularly at a concentration of 10 mmol/L (P < 0.05 and < 0.01, respectively) (Figure 7) ▶ . Similarly, PBN attenuated the levels of HSP 72 (H = 11.9, P = 0.0076) and 4-HNE (H = 9.7, P = 0.02), with the maximal effect at 100 mmol/L, after H/R (Figure 8) ▶ . However, SOD did not provide significant protective effects against the expression of HSP 72 or production of 4-HNE (data not shown).
Figure 7.
Effects of desferrioxamine on the expression of HSP 72 and production of 4-HNE after hypoxia-reoxygenation (2 hours of air/5% CO2). a: A dose-response change was observed in placental tissues preloaded with desferrioxamine at a concentration ranging 0.1 to 10 mmol/L (Kruskal-Wallis test, H = 15.3, P = 0.0016 and H = 8.9, P = 0.03 for HSP 72 and 4-HNE, respectively). Data are presented as mean ± SEM from three different placentas. *, P < 0.05; **, P < 0.01 compared with vehicle control based on Dunnett’s post hoc test. Representative immunoblots for HSP 72 (b) and 4-HNE (c) from two placentas showing a maximal protective effect at a concentration of 10 mmol/L. Lanes 1 and 5, placental tissues preloaded with PBS only (vehicle control); lanes 2 and 6, 0.1 mmol/L; lanes 3 and 7, 1 mmol/L; lanes 4 and 8, 10 mmol/L desferrioxamine, respectively. β-actin shown below was used to normalize for loading variability.
Figure 8.
Effects of PBN on the expression of HSP 72 and production of 4-HNE after hypoxia-reoxygenation (2 hours of air/5% CO2). a: A dose-response change was observed in placental tissues preloaded with PBN at a concentration ranging from 1 to 100 mmol/L (Kruskal-Wallis test, H = 11.9, P = 0.0076 and H = 9.7, P = 0.02 for HSP 72 and 4-HNE, respectively). Data are presented as mean ± SEM from three different placentas. *, P < 0.05 compared with vehicle control based on Dunnett’s post hoc test. Representative immunoblots for HSP 72 (b) and 4-HNE (c) from two placentas showing a maximal protective effect at a concentration of 100 mmol/L. Lanes 1 and 5, placental tissues preloaded with PBS only (vehicle control); lanes 2 and 6, 1 mmol/L; lanes 3 and 7, 10 mmol/L; lanes 4 and 8, 100 mmol/L PBN, respectively. β-actin shown below was used to normalize for loading variability.
To exclude the possibility that desferrioxamine might adversely interfere with cellular function, leading to a reduced production of HSP 72, placental tissues were subjected to heat shock (45°C, 1 hour) in the presence of 10 mmol/L desferrioxamine. Increased HSP 72 expression was observed compared to controls with desferrioxamine alone (data not shown).
Correlation between the Degree of Protection Provided by Desferrioxamine and PBN and the Level of Endogenous Stress
During the course of these experiments it was noticed that there was variation between placentas in the level of HSP 72 expression in the samples frozen immediately after delivery, indicating differing levels of endogenous stress in vivo. To determine whether this influenced the degree of protection that the ROS scavengers could provide after H/R, correlation analysis was performed. The density of the immunoblot for HSP 72 expression associated with the maximal concentration of each scavenger was expressed as a ratio of that for the vehicle control, generating an index of the protection provided. This value was then correlated with the density of the immunoblot for the time 0 sample. The correlation coefficient was 0.91 (P = 0.001) and 0.86 (P = 0.006) for desferrioxamine and PBN, respectively (Figure 9) ▶ , showing that desferrioxamine and PBN are able to provide the greatest protection against H/R injury when levels of endogenous stress are low.
Figure 9.
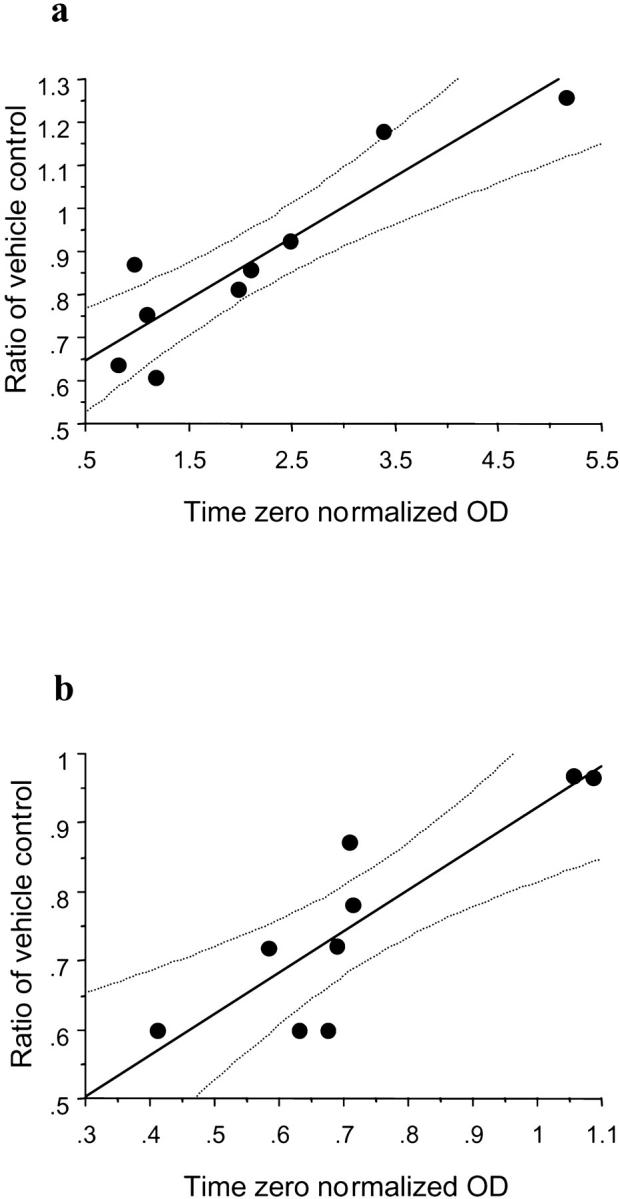
Correlation of endogenous stress levels and the maximal protective effects of desferrioxamine (a) and PBN (b). The correlation coefficient was 0.91 (P = 0.001) and 0.86 (P = 0.006) for desferrioxamine and PBN, respectively. The lines display the regression coefficient with 95% confidence for the mean.
Discussion
Our findings support the three stated hypotheses, and thus confirm that the human placenta is subjected to an ischemia-reperfusion injury when reoxygenated in vitro. First, the results with DCFH-DA show that ROS are generated rapidly in the villous endothelium, and to a lesser extent in the syncytiotrophoblast and stromal cells. Intracellular ROS, mostly superoxide radicals, can be produced by a number of pathways after ischemia-reperfusion. These include mitochondrial electron transfer processes, and a variety of enzymes such as NADPH oxidase and xanthine dehydrogenase/oxidase (XDH/XO). 10 Immunoreactivity of XDH/XO has been demonstrated in villous endothelium and the syncytiotrophoblast in placentas from uncomplicated pregnancies. 13 Under hypoxia both the synthesis and activity of XDH/XO increase, and conversion of the enzyme to the XO form is also enhanced. 14-16 This form of the enzyme transfers electrons to molecular oxygen, so generating superoxide radicals. During the period of hypoxia there is also a net catabolism of ATP, resulting in an increased concentration of hypoxanthine. Consequently, when oxygen is reintroduced XO will catalyze the accumulated hypoxanthine to produce superoxide and H2O2 formation. 17 The presence of transition-metal ions can promote the further generation of highly toxic hydroxyl radicals from these products.
Second, we have demonstrated that the expression of several well-characterized markers of oxidative stress is increased after reoxygenation. Much of the data derived from studies of ischemia-reperfusion injury at other sites are consistent with a role for both superoxide and NO as participants in the altered endothelium-dependent response. 18,19 The endothelial isoform of nitric oxide synthase (NOS) has been characterized in the human placenta, and immunohistochemically localized to the stem villous vessels and syncytiotrophoblast. 20 Immunoreactivity of the inducible isoform of NOS has also been detected, mainly in the villous stroma and to a lesser extent in the syncytiotrophoblast and vascular endothelium. 21 Recently, mRNA encoding the inducible isoform of NOS was found in the syncytiotrophoblast and villous arteriolar smooth muscle cells. 22 Under normal conditions, the flux of NO greatly exceeds the rate of superoxide production. However, within minutes after reperfusion of ischemic tissues the balance between NO and superoxide production is tipped in favor of superoxide as previously described. Superoxide reacts with NO much faster than the rate of superoxide dismutation, 23 thus producing the peroxynitrite anion that is a strong and long-lived oxidant. Peroxynitrite can inhibit mitochondrial electron transport, 24 oxidize proteins, initiate lipid peroxidation, 25 and affect signal transduction by nitrating aromatic amino acids such as tyrosine. 26 Our immunostaining of nitrotyrosine residues, a fingerprint of peroxynitrite, 27 shows that the stem villous vessels, including their smooth muscle layers, and the syncytiotrophoblast are the most vulnerable areas attacked by peroxynitrite during H/R.
Because of its stability, 4-HNE has been suggested as the most reliable index of free radical-induced lipid peroxidation. 28 4-HNE originates almost exclusively from phospholipid-bound arachidonic acid, and causes cellular damage mainly by the modification of intracellular proteins. 28 It may thus inhibit enzyme reactions and the synthesis of DNA, RNA, and proteins. Our results reveal that the principal sites of lipid peroxidation in placental tissues after H/R are the villous endothelium and the syncytiotrophoblast, consistent with our observations of ROS generation with DCFH-DA and immunostaining of nitrotyrosine residues. The syncytiotrophoblast is particularly vulnerable to oxidative stress as it is the endothelium-like surface layer lining the intervillous space, and so is the first to confront the fluctuations in oxygen tension. In addition, particularly in early gestation the syncytiotrophoblast contains much lower concentrations of the principal antioxidant enzymes than other villous tissues. 29,30 As a result it is selectively subjected to oxidative stress at the start of the second trimester when the oxygen tension in the intervillous space rises with onset of the maternal arterial circulation to the placenta. 31
The HSPs are known to serve as molecular chaperones to facilitate folding and prevent denaturation of other proteins during periods of stress. 32 In particular, HSP 72 is the major inducible member of the family and protein levels increase through activation of heat-shock transcription factor in response to a variety of exogenous stressors including heat shock, oxidative radicals, and inflammatory cytokines. 32,33 Oxidative stress can cause thermal stable proteins to become destabilized and undergo denaturation at physiological temperature, thus inducing the synthesis of HSP. 34 Up-regulation of HSP 72 has been found in conditions of H/R, rendering it a sensitive marker of ischemia-reperfusion injury. 35-37 Furthermore, substantial evidence indicates that increased expression of HSP 72 is capable of protecting cells or tissues from the subsequent noxious conditions, including hypoxia and ischemia-reperfusion. 32,33 Therefore, the expression of HSP 72 not only serves as a marker of oxidative stress, but more importantly represents an adaptive response aimed at preventing the aggregation of denatured proteins within the cytosol. The immunostaining of HSP 72 was compatible with the localization of nitrosylated or 4-HNE modified proteins, indicating the potential role of HSP 72 in helping these areas resist or recover from oxidative damage. HSP 72 may therefore play important roles in maintaining fetal vascular muscle tone and syncytiotrophoblast function in vivo.
Third, we have demonstrated that the ROS scavengers desferrioxamine and PBN significantly reduce the expression of HSP 72 and production of 4-HNE after H/R. Desferrioxamine is a membrane-permeable iron chelator that inhibits lipid peroxidation, and the generation of hydroxyl radicals from superoxide and H2O2 in the presence of iron ions. 38 The protective efficacy of desferrioxamine we demonstrated therefore implicates an important role for iron in the generation of hydroxyl radicals in the H/R injury.
PBN, a common spin-trapping compound, has been reported to protect against ischemia-reperfusion injury in animal studies. 39,40 However, in vitro characterization of PBN shows it to be a poor chain-breaking antioxidant and inhibitor of lipid peroxidation, and so its mechanism of action is uncertain. 38
Unexpectedly, we were unable to show protection by SOD against the expression of HSP 72 or formation of 4-HNE after H/R, even at a concentration 10 times higher than that reported in cultured rat hepatic sinusoidal endothelial cells. 41 This may be because SOD does not permeate biological membranes and is therefore not able to reduce the detrimental effects of superoxide anions, which are produced and remain injurious intracellularly. 42
Taken together, these data indicate that the key intermediaries required for an ischemia-reperfusion injury are present within human placental tissues, and that the villi are indeed vulnerable to such an insult in vitro. Furthermore, the patterns of immunostaining for 4-HNE and nitrotyrosine residues suggest there are parallels between the resultant oxidative stress and that observed in some complications of pregnancy, such as preeclampsia and fetal growth restriction. 43-45 Although conclusive evidence that an ischemia-reperfusion injury occurs in the preeclamptic placenta is lacking at present, the hypothesis is consistent with the recent finding that maternal supplementation with the antioxidant vitamins C and E has a beneficial effect. 46 The strong association of preeclampsia with failure of spiral artery remodeling also provides a plausible underlying mechanism. As confirmed in the present study, hypoxia alone is not sufficient to cause placental oxidative stress. However, retention of vasoreactivity in the spiral arteries may lead to intermittent perfusion of the intervillous space, and hence to fluctuating oxygen concentrations within the placenta. Similar assumptions regarding intermittent placental perfusion can be made in cases of maternal cocaine use and cigarette smoking in pregnancy, in which the transient but repetitive vasoconstrictive effects of both cocaine and nicotine on the uterine vasculature have been clearly demonstrated to cause decreased uteroplacental perfusion and fetal growth restriction. 47 Ischemia-reperfusion type injury may therefore provide a common pathway for the generation of placental oxidative stress in a number of diverse clinical conditions.
The consequences of the stress may be different between the different sites. As our immunohistochemical study revealed, villous endothelium together with underlying smooth muscle cells and the syncytiotrophoblast are the most vulnerable areas where peroxynitrite formation and lipid peroxidation occurred. ROS generated from this intermittent H/R may initially act as intracellular signaling molecules, mediating proliferation of the endothelial cells and hypertrophy of the vascular smooth muscle cells. 48 This is supported by observations on the ultrastructure of fetal stem arteries from preeclamptic placentas showing more prominent proliferation of endothelial and smooth muscle cells as compared to placentas from normal pregnancy. 49 If the condition of H/R repeats and persists, the amplitude of ROS production may overwhelm the capacity of the placental antioxidant defenses, and cellular dysfunction and tissue damage will result. This may be critical in the syncytiotrophoblast because of its lower antioxidant defenses. Further work is required to determine whether the insult is sufficient to exacerbate the apoptotic changes in the syncytiotrophoblast that have been proposed to underlie shedding of microvillous fragments. Shedding is increased in preeclampsia, and the resultant debris is thought to activate an enhanced inflammatory response in the mother, leading to generalized endothelial cell activation. 2
In summary, we have demonstrated that changes consistent with an ischemia-reperfusion injury occur when hypoxic placental tissues are reoxygenated in vitro. Moreover, the results have implications for our understanding of the generation of placental oxidative stress in complications of pregnancy such as preeclampsia and cigarette smoking.
Acknowledgments
We thank the staff of the Delivery Unit of the Rosie Hospital, Cambridge, for their help in obtaining the placental material; and the MultiImaging Center of the School of Biological Sciences, University of Cambridge, which was established with generous grants from the Wellcome Trust (055203/Z/98 and 039398/Z/93) where the imaging was performed .
Footnotes
Address reprint requests to Graham J Burton, Department of Anatomy, University of Cambridge, Cambridge CB2 3DY, United Kingdom. E-mail: gjb2@cam.ac.uk.
Supported by a grant from Chang Gung Memorial Hospital, Taiwan (CMRP 1034) and a Benefactor Scholarship from St John’s College, University of Cambridge (to T.-H. H.).
References
- 1.Roberts JM, Hubel CA: Is oxidative stress the link in the two-stage model of preeclampsia? Lancet 1999, 354:788-789 [DOI] [PubMed] [Google Scholar]
- 2.Redman CWG, Sargent IL: Placental debris, oxidative stress and pre-eclampsia. Placenta 2000, 21:597-602 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Walsh SW: Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and pre-eclamptic placentas. J Soc Gynecol Invest 1996, 3:179-184 [PubMed] [Google Scholar]
- 4.Sahlin L, Östlund E, Wang H, Holmgren A, Fried G: Decreased expression of thioredoxin and glutaredoxin in placentae from pregnancies with preeclampsia and intrauterine growth restriction. Placenta 2000, 21:603-609 [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Robertson WB, Dixon HG: The role of spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Ann 1972, 1:177-191 [PubMed] [Google Scholar]
- 6.Khong TY, De Wolf F, Robertson WB, Brosens I: Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986, 93:1049-1059 [DOI] [PubMed] [Google Scholar]
- 7.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A: A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994, 101:669-674 [DOI] [PubMed] [Google Scholar]
- 8.Aquilina J, Harrington K: Pregnancy hypertension and uterine artery Doppler ultrasound. Curr Opin Obstet Gynecol 1996, 8:435-440 [PubMed] [Google Scholar]
- 9.Burton GJ, Jauniaux E, Watson AL: Influence of oxygen supply on placental structure. O’Brien PMS Wheeler T Barker DJP eds. Fetal programming: influences on development and disease in later life. 1999, :pp 326-341 RCOG Press, London [Google Scholar]
- 10.Schachter M, Foulds S: Free radicals and the xanthine oxidase pathway. Grace PA Mathie RT eds. Ischaemia-Reperfusion Injury. 1999, :pp 137-147 Blackwell Science, London [Google Scholar]
- 11.Halliwell B, Gutteridge MC: Oxygen is a toxic gas—an introduction to oxygen toxicity and reactive oxygen species. Halliwell B Gutteridge MC eds. Free Radicals in Biology and Medicine. 1999, :pp 1-35 Oxford University Press, Oxford [Google Scholar]
- 12.Jones CJ, Fox H: An ultrastructural and ultrahistochemical study of the human placenta in maternal preeclampsia. Placenta 1980, 1:61-76 [DOI] [PubMed] [Google Scholar]
- 13.Many A, Westerhausen-Larson A, Kanbour-Shakir A, Roberts JM: Xanthine oxidase/dehydrogenase is present in human placenta. Placenta 1996, 17:361-365 [DOI] [PubMed] [Google Scholar]
- 14.Parks DA, Williams TK, Beckman JS: Conversion of xanthine dehydrogenase to oxidase in ischemic rat intestine: a reevaluation. Am J Physiol 1988, 254:G768-G774 [DOI] [PubMed] [Google Scholar]
- 15.McCord JM: Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 1985, 312:159-163 [DOI] [PubMed] [Google Scholar]
- 16.Hassoun PM, Yu FS, Shedd AL, Zulueta JJ, Thannickal VJ, Lanzillo JJ, Fanburg BL: Regulation of endothelial cell xanthine dehydrogenase xanthine oxidase gene expression by oxygen tension. Am J Physiol 1994, 266:L163-L171 [DOI] [PubMed] [Google Scholar]
- 17.Granger DN, Korthuis RJ: Physiologic mechanisms of postischemic tissue injury. Annu Rev Physiol 1995, 57:311-332 [DOI] [PubMed] [Google Scholar]
- 18.Carden DL, Granger DN: Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000, 190:255-266 [DOI] [PubMed] [Google Scholar]
- 19.Stewart AG, Barker JE, Hickey MJ: Nitric oxide in ischaemia-reperfusion injury. Grace PA Mathie RT eds. Ischaemia-Reperfusion Injury. 1999, :pp 180-195 Blackwell Science, London [Google Scholar]
- 20.Myatt L, Brockman DE, Eis AL, Pollock JS: Immunohistochemical localization of nitric oxide synthase in the human placenta. Placenta 1993, 14:487-495 [DOI] [PubMed] [Google Scholar]
- 21.Myatt L, Eis AL, Brockman DE, Kossenjans W, Greer I, Lyall F: Inducible (type II) nitric oxide synthase in human placental villous tissue of normotensive, preeclamptic and intrauterine growth-restricted pregnancies. Placenta 1997, 18:261-268 [DOI] [PubMed] [Google Scholar]
- 22.Baylis SA, Strijbos PJ, Sandra A, Russell RJ, Rijhsinghani A, Charles IG, Weiner CP: Temporal expression of inducible nitric oxide synthase in mouse and human placenta. Mol Hum Reprod 1999, 5:277-286 [DOI] [PubMed] [Google Scholar]
- 23.Huie RE, Padmaja S: The reaction of NO with superoxide. Free Radic Res Commun 1993, 18:195-199 [DOI] [PubMed] [Google Scholar]
- 24.Radi R, Rodriguez M, Castro L, Telleri R: Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 1994, 308:89-95 [DOI] [PubMed] [Google Scholar]
- 25.Radi R, Beckman JS, Bush KM, Freeman BA: Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 1991, 288:481-487 [DOI] [PubMed] [Google Scholar]
- 26.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS: Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 1992, 298:431-437 [DOI] [PubMed] [Google Scholar]
- 27.Beckman JS, Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996, 271:C1424-C1437 [DOI] [PubMed] [Google Scholar]
- 28.Esterbauer H, Schaur RJ, Zollner H: Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991, 11:81-128 [DOI] [PubMed] [Google Scholar]
- 29.Watson AL, Palmer ME, Jauniaux E, Burton GJ: Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta 1997, 18:295-299 [DOI] [PubMed] [Google Scholar]
- 30.Watson AL, Skepper JN, Jauniaux E, Burton GJ: Changes in concentration, localization and activity of catalase within the human placenta during early gestation. Placenta 1998, 19:27-34 [DOI] [PubMed] [Google Scholar]
- 31.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ: Onset of maternal arterial bloodflow and placental oxidative stress; a possible factor in human early pregnancy failure. Am J Pathol 2000, 157:2111-2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welch WJ: Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 1992, 72:1063-1081 [DOI] [PubMed] [Google Scholar]
- 33.Kiang JG, Tsokos GC: Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 1998, 80:183-201 [DOI] [PubMed] [Google Scholar]
- 34.Freeman ML, Borrelli MJ, Meredith MJ, Lepock JR: On the path to the heat shock response: destabilization and formation of partially folded protein intermediates, a consequence of protein thiol modification. Free Radic Biol Med 1999, 26:737-745 [DOI] [PubMed] [Google Scholar]
- 35.Akcetin Z, Pregla R, Darmer D, Heynemann H, Haerting J, Bromme HJ, Holtz J: Differential expression of heat shock proteins 70–1 and 70–2 mRNA after ischemia-reperfusion injury of rat kidney. Urol Res 1999, 27:306-311 [DOI] [PubMed] [Google Scholar]
- 36.Dienel GA, Kiessling M, Jacewicz M, Pulsinelli WA: Synthesis of heat shock proteins in rat brain cortex after transient ischemia. J Cereb Blood Flow Metab 1986, 6:505-510 [DOI] [PubMed] [Google Scholar]
- 37.Nishizawa J, Nakai A, Higashi T, Tanabe M, Nomoto S, Matsuda K, Ban T, Nagata K: Reperfusion causes significant activation of heat shock transcription factor 1 in ischemic rat heart. Circulation 1996, 94:2185-2192 [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B, Gutteridge MC: Ageing, nutrition, disease and therapy: a role for antioxidants? Halliwell B Gutteridge MC eds. Free Radicals in Biology and Medicine. 1999, :pp 784-859 Oxford University Press, Oxford [Google Scholar]
- 39.Nakai A, Asakura H, Taniuchi Y, Koshino T, Araki T, Siesjo BK: Effect of alpha-phenyl-N-tert-butyl nitrone (PBN) on fetal cerebral energy metabolism during intrauterine ischemia and reperfusion in rats. Pediatr Res 2000, 47:451-456 [DOI] [PubMed] [Google Scholar]
- 40.Pincemail J, Defraigne JO, Franssen C, Defechereux T, Canivet JL, Philippart C, Meurisse M: Evidence of in vivo free radical generation by spin trapping with alpha-phenyl N-tert-butyl nitrone during ischemia/reperfusion in rabbit kidneys. Free Radic Res Commun 1990, 9:181-186 [DOI] [PubMed] [Google Scholar]
- 41.Samarasinghe DA, Tapner M, Farrell GC: Role of oxidative stress in hypoxia-reoxygenation injury to cultured rat hepatic sinusoidal endothelial cells. Hepatology 2000, 31:160-165 [DOI] [PubMed] [Google Scholar]
- 42.Fridovich I: Superoxide radical and superoxide dismutases. Annu Rev Biochem 1995, 64:97-112 [DOI] [PubMed] [Google Scholar]
- 43.Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y: Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol 2000, 156:321-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F: Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension 1996, 28:488-493 [DOI] [PubMed] [Google Scholar]
- 45.Walsh SW, Vaughan JE, Wang Y, Roberts LJ: Placental isoprostane is significantly increased in preeclampsia. FASEB J 2000, 14:1289-1296 [DOI] [PubMed] [Google Scholar]
- 46.Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L: Effects of antioxidants on the occurrence of preeclampsia in women at increased risk: a ran- domised trial. Lancet 1999, 354:810-816 [DOI] [PubMed] [Google Scholar]
- 47.Pastrakuljic A, Derewlany LO, Koren G: Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta 1999, 20:499-512 [DOI] [PubMed] [Google Scholar]
- 48.Irani K: Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res 2000, 87:179-183 [DOI] [PubMed] [Google Scholar]
- 49.Heras JL, Haust MD: Ultrastructure of fetal stem arteries of human placenta in hypertensive disorders (toxemia) of pregnancy. Trophoblast Res 1988, 3:335-349 [Google Scholar]