Abstract
A novel Congo red-derived fluorescent probe (trans, trans),−1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB) that binds to amyloid plaques of postmortem Alzheimer’s disease brains and in transgenic mouse brains in vivo was designed as a prototype imaging agent for Alzheimer’s disease. In the current study, we used BSB to probe postmortem tissues from patients with various neurodegenerative diseases with diagnostic lesions characterized by fibrillar intra- or extracellular lesions and compared these results with standard histochemical dyes such as thioflavin S and immunohistochemical stains specific for the same lesions. These data show that BSB binds not only to extracellular amyloid β protein, but also many intracellular lesions composed of abnormal tau and synuclein proteins and suggests that radioiodinated BSB derivatives or related ligands may be useful imaging agents to monitor diverse amyloids in vivo.
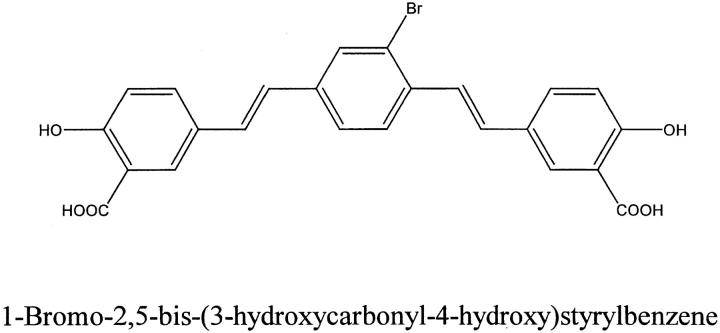
Alzheimer’s disease (AD) is the most frequent cause of dementia in the elderly. At this time there is no specific test available for a definite diagnosis at an early stage of the disease. Thus, efforts have been made to develop probes that bind AD amyloid plaques in brain for use as in vivo imaging agents. 1-3 For example, a Congo red (CR)-derived fluorescent probe, X-34, was developed recently that binds to AD brain lesions in tissue sections and has several desirable characteristics required for an in vivo amyloid imaging agent. 4 Another CR derived compound that binds to AD neurofibrillary tangles (NFTs), neuropil threads (NTs) and amyloid β-peptide (Aβ) deposits in plaques as well as to AD-like amyloid plaques in the brains of a transgenic mouse model of AD amyloidosis after in vivo injection. 1 However, this probe, (trans, trans), −1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene or BSB (Figure 1) ▶ , is not a specific ligand for fibrillar Aβ peptides, the major constituents of plaques in AD brains, since it also binds to NFTs and NTs both of which are composed of amyloid-like paired helical filaments (PHF) formed by hyperphosphorylated tau proteins. 1
Figure 1.
Schematic illustration of BSB.
Since there are two major species of Aβ in AD amyloid plaques, i.e., peptides ending at amino acid 40 (Aβx-40) or 42 (Aβx-42), the current study investigated whether BSB binds to all or a subset of Aβ plaques and whether it binds preferentially to plaques consisting mainly of either Aβx-40 or Aβx-42. 5 Further, since NFTs in AD are composed of six tau isoforms in about equal proportions, 6-10 but in other neurodegenerative diseases abnormal accumulations of tau proteins in neurons and/or glial cells are predominantly composed of either three or four microtubule (MT) binding repeat tau isoforms, we also asked if BSB binds all or a subset of tau inclusions. For instance, NFTs and glial inclusions in progressive supranuclear palsy (PSP), cortical basal degeneration (CBD), and certain tauopathies, i.e., frontotemporal dementia with parkinsonism (FTDP-17) are mainly composed of four repeat tau isoforms while neuronal inclusions in Pick’s disease are mostly composed of three repeat tau. 11 Finally, we investigated whether BSB binds to amyloid-like lesions containing α-synuclein including Lewy bodies (LB) in dementia with LB (DLB), Parkinson’s disease (PD), and neurodegeneration with brain iron accumulation type 1 (NBIA-1) as well as glial cytoplasmic inclusions (GCI) in multiple system atrophy (MSA). 12 We found that BSB labeled all of the above mentioned lesions although there were quantitative and qualitative differences when compared with thioflavin S (THIOS) and specific immunohistochemical stains for these lesions. Thus, BSB or related derivatives may be exploited as imaging agents for diverse amyloids including lesions formed by Aβ, tau, and α-synuclein.
Materials and Methods
Brain tissues from 23 patients were used in this study. Nineteen of these patients had neurodegenerative diseases and one control patient had no neurological disorder. Demographic data including the disease, age, and gender of these patients are listed in Table 1 ▶ in addition to other information on the brain samples. Also listed are the postmortem interval and the fixative used to preserve the tissues. Tissue blocks of interest were removed at the time of autopsy and fixed in either 70% ethanol containing 150 mmol/L sodium chloride or 10% neutral buffered formalin overnight and subsequently embedded in paraffin. Six-μm-thick serial sections were cut and adjacent sections were stained with BSB, THIOS, or antibodies to Aβ, tau, and α-synuclein (Table 2) ▶ . Immunohistochemistry was performed using ABC Kits (Vector Laboratories, Burlingame, CA) and diaminobenzidine as chromogen according to previously described procedures. 13;14
Table 1.
Demographic Data of Patients Used in this Study
| Patient no. | Diagnosis | Age | Gender | PMI (hours) | Fixative | Brain area |
|---|---|---|---|---|---|---|
| 1 | AD | 79 | F | 7 | NBF | Frontal cortex |
| 2 | AD | 80 | F | 7 | E | Frontal cortex |
| 3 | FAD/PS2 (N141I) = Volga Germans | 65 | F | 13.5 | E | Frontal cortex |
| 4 | DS | 33 | M | 8 | E | Frontal cortex |
| 5 | DS | 54 | M | 9 | E | Frontal cortex |
| 6 | FTDP-17/(P301L) | 62 | M | NA | NBF | Basal ganglia |
| 7 | FTDP-17/(exon 10+ 16) = familial progressive subcortical gliosis | 48 | M | 20 | E | Cerebellum |
| 8 | FTDP-17/(N279) = pallido-ponto-nigral degeneration | 50 | M | 6.5 | NBF | Frontal cortex |
| 9 | AD/LBv | 71 | M | 9 | E | Frontal cortex |
| 10 | DLB/PD | 85 | F | 6 | E | Cingulate cortex |
| 11 | PSP | 72 | M | 10 | E | Midbrain |
| 12 | PSP | 73 | M | 20.5 | E | Midbrain |
| 13 | PSP | 83 | F | 24 | E | Midbrain |
| 14 | Pick’s disease | 72 | M | 6.5 | NBF | Hippocampus |
| 15 | MSA | 43 | M | 20 | E | Cerebellum |
| 16 | MSA | 79 | M | 16 | E | Cerebellum |
| 17 | CBD | 77 | M | NA | NBF | Frontal cortex |
| 18 | CBD | 64 | F | NA | NBF | Frontal cortex |
| 19 | NBIA I | 29 | M | 6 | E | Hippocampus |
| 20 | PDC | 63 | M | NA | NBF | T, L Spin.c. |
| 21 | PDC | 64 | M | NA | NBF | T, L Spin.c. |
| 22 | PDC | 72 | M | NA | NBF | C Spin.c. |
| 23 | Normal control | 76 | F | 13 | E | Frontal cortex |
Abbreviations: AD/LBv, Alzheimer’s disease/Lewy body variant; C, cervical; CBD, cortico-basal degeneration; E, 80% ethanol with 150 mM sodium chloride; F, female; FAD/PS2, familial Alzheimer’s disease with a mutation in the PS2 gene; FTDP-17, fronto-temporal dementia with parkinsonism; L, lumbar; M, male; NBF, neutral buffered formalin; PDC, parkinsonism-dementia complex of Guam; PMI, post mortem interval; PSP, progressive supranuclear palsy; T, thoracic.
Table 2.
Antibodies and Immunostaining Conditions
| Antibody designation | Specificity | Species raised in | Dilution | Pretreatment for tissues fixed in ethanol | Pretreatment for tissues fixed in formalin | Reference no. |
|---|---|---|---|---|---|---|
| 17026 | Full length tau | Rabbit | 1:10000 | None | None | 15 |
| 4G8 | Aβ aa17–24 | Mouse | 1:10000 | None | Formic acid | 16 |
| BCO5 | Aβ aa38–42 | Mouse | 1:20000 | Formalin and formic acid | Formic acid | 5 |
| BA27 | Aβ aa36–40 | Mouse | 1:20000 | Formalin and formic acid | Formic acid | 5 |
| SYN303 | Synuclein aa1–89 | Mouse | 1:1000 | None | None | 17 |
Abbreviations: aa, amino acid; Aβ, amyloid beta protein.
Thioflavin S staining was carried out according to a protocol reported by Guntern et al. 18 BSB staining was performed as described. 1 Briefly, deparaffinized and hydrated tissue sections were immersed in a 0.01% BSB dissolved in 50% ethanol for 30 minutes. Then the sections were rinsed in a saturated aqueous solution of lithium carbonate. Finally, the sections were differentiated in 50% ethanol under microscope control. This process was stopped by immersion in distilled water. The sections were then coated with a thin layer of Vectorshield (Vector Laboratories) before coverslipping. The THIOS and BSB stained sections were viewed in an epifluorescence microscope using a fluorescent filter cube with an excitation filter of 405 nm and an emission filter of 435 nm. A fluorescein isothiocyanate (FITC) filter cube with an excitation filter of 350–390 nm and an emission filter of 530 ± 15 was used for comparison. Electronic images were obtained using a CoolSnap camera (Biovision, Exton, PA) mounted on a Nikon FXA microscope (Optical Apparatus, Inc., Ardmore, PA). These images were printed on a Fuji Pictrography 3000 printer (Mid City Camera, Philadelphia, PA).
Results
Aβ-Positive Lesions
Monoclonal antibody (MAB) 4G8 demonstrated Aβ plaques in a variety of neurodegenerative diseases: AD, Down’s syndrome (DS), familial AD (FAD), and DLB. The brains of the AD, DS, and FAD patients had the largest amounts of Aβ plaques. Thus, we chose consecutive tissue sections from the brains of each of these three patients and probed them with MAB 4G8, as well as with two other MAB specific for the x-40 (BA27) and x-42 (BCO5) forms of Aβ and also with the fluorescent dyes BSB and THIOS. BCO5 immunostained the largest numbers of Aβ plaques and BA27 the least although there was considerable regional variability in the number of BA27-positive plaques even within the same tissue section. These results are illustrated in Figure 2 ▶ . It is apparent that both BSB and THIOS stain larger numbers of plaques than BA27. However, by comparing many large BCO5 positive plaques with adjacent tissue sections treated with BSB and THIOS, it is evident that these two fluorescent dyes stain nearly as many plaques as BCO5 although some of the fluorescently stained plaques show a signal that is barely above background (Figure 2 ▶ , arrowheads in panels A, D, and E). Amyloid angiopathy was immunostained by BA27 as well as fluorescently labeled by BSB and THIOS.
Figure 2.
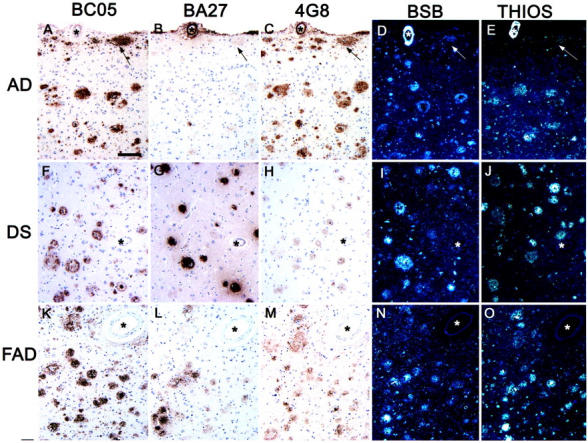
Neurodegenerative lesions composed of Aβ protein. This figure depicts tissue sections of frontal cortex immunostained with MAB. BCO5, first column; BA27, second column; 4G8, third column, or stained with BSB, fourth column; and THIOS, fifth column. The first row (A–E) shows an AD case, the second row (F–J) shows a DS case with AD, and the third row (K–M) shows a case of FAD with a mutation in PS2. The asterisks indicate the blood vessel that was used as landmark to identify the same area in each set of consecutive sections. The arrows point to the same subpial Aβ deposit. All images were taken at the same magnification. Scale bar, 100 μm.
Rare 4G8 positive Aβ plaques were observed in the brain of a 33-year-old DS patient, a Pick’s disease patient, a FTDP-17(P301L) patient and an elderly non-demented control patient. BSB did not stain plaques in any of these patients although weakly stained plaques could occasionally be demonstrated with THIOS (data not shown).
Tau-Positive Lesions
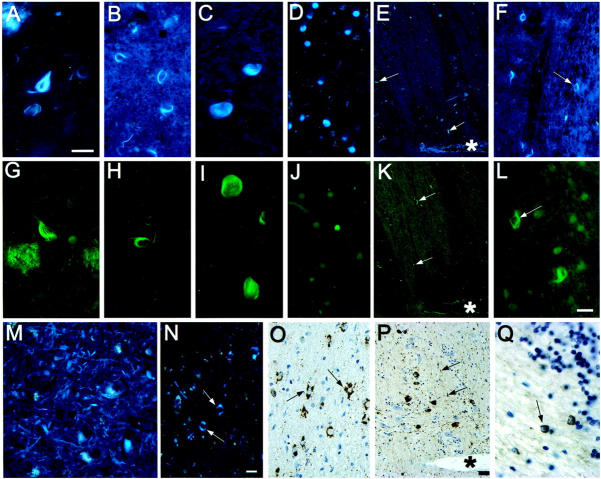
BSB stains NFTs and NTs in AD very prominently (Figure 3, A and G) ▶ . In PSP both globose tangles and coiled bodies were stained by BSB (Figure 3 B, C, H, and I ▶ ). Tau-positive oligodendrocytic inclusions in a case of familial progressive subcortical gliosis (PSG) were also stained by BSB and THIOS (Figure 3, F, L, and Q) ▶ . However, of the large numbers of tau-immunoreactive coiled bodies present in FTDP-17 (N279K) only a fraction was stained by either BSB or THIOS (not shown). Also, most of the tau-immunoreactive neurons from the same case were not stained by BSB or THIOS (Figure 3 ▶ , compare E, K, with P). The most frequently stained BSB and THIOS positive lesions in this case were NTs (arrowheads). Similarly, tau-immunoreactive neurons in CBD cases were not positive for BSB or THIOS (data not shown). However, BSB demonstrated extensive NT pathology in CBD in gray as well as in white matter (Figure 3M) ▶ . In another FTDP-17 case with the P301L mutation, perikaryal tau immunoreactivity was prominent in several neocortical areas and many large tau- positive cell bodies were present in the basal forebrain. None of these lesions were stained with BSB or THIOS. In this case many tau positive astrocytes with a characteristic crenated appearance were observed and a subset of these stained weakly with BSB (Figure 3, N and O) ▶ . The astrocytic nature of these lesions had been confirmed by double immunohistochemical staining with MABs to tau and glial fibrillary protein (unpublished observation). Pick bodies in Pick’s disease were stained, although weakly, with both BSB and THIOS (Figure 3, D and J) ▶ . Finally, NFTs in the cervical, thoracic and lumbar regions of the spinal cord of three Guamanian PDC patients stained with antiserum 17026, BSB, and THIOS (data not shown).
Figure 3.
Neurodegenerative lesions composed of tau protein. This figure shows tau immunoreactive lesions in various neurodegenerative diseases stained with BSB and THIOS. All sections in the first row (A–F) were stained with BSB and all sections in the second row (G–L) were stained with THIOS. In the third row the sections depicted in panels M and N are stained with BSB and in panels O to Q are immunostained with antiserum 17026. A and G: NFTs and Aβ plaques from an AD patient. B and H: Coiled bodies in the midbrain of a patient with PSP. C and I: Globose tangles in the midbrain of a patient with PSP. D and J: Pick bodies in the dentate gyrus of a patient with Pick’s disease. E, K, and P: Consecutive sections through the pons of a FTDP-17 (N279K) patient. P shows tau-positive neurons and neuropil threads. Both BSB and THIOS stain some neuropil threads in adjacent sections (E and K, respectively) but tau immunopositive neurons are not stained. Asterisks indicate the same blood vessel in these images and the arrows point to NTs. F, L, and Q: Coiled bodies in a patient with familial PSG (exon 10 + 16 mutation). M: NT pathology in a CBD patient. N and O: Tau and BSB stained glia (arrows) in the amygdala of a FTDP-17 (P301L) patient. A, C, G, I, and M are the same magnification; scale bar in A = 20 μm. B, H, L, and Q are the same magnification; scale bar in L = 10 μm. D, F, J, N, and O are the same magnification; scale bar in N = 10 μm. E, K, and P are the same magnification; scale bar in P = 50 μm.
α-Synuclein-Positive Lesions
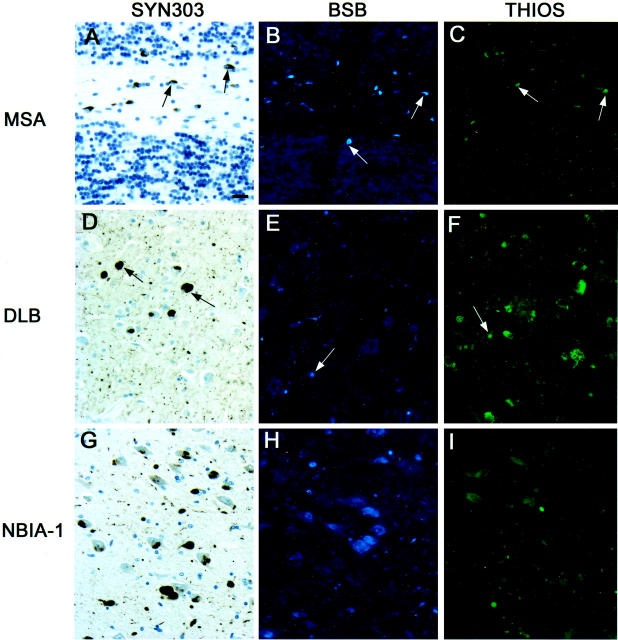
Both BSB and THIOS stained α-synuclein immunoreactive GCIs in MSA (Figure 4, A–C) ▶ . Cortical LBs in DLB stain strongly with anti-α-synuclein antibodies, but only occasional LBs are weakly stained with BSB and THIOS (Figure 4, D–F) ▶ . In a case of NBIA-1, many α-synuclein positive intraneuronal inclusion bodies (LBs and GCIs) were seen. These were especially abundant in area CA3 of the hippocampus, however neither BSB nor THIOS stained these inclusions (Figure 4, G– I) ▶ . The data of the BSB and THIOS staining of Aβ-, tau-, and α-synuclein-positive lesions are summarized in Table 3 ▶ .
Figure 4.
Neurodegenerative lesions composed of α-synuclein. The first column of this figure depicts tissue sections immunostained with Syn303. The second and third columns show tissue sections stained with BSB and THIOS, respectively. A to C: Cerebellar white matter flanked on the top and the bottom by granule cell layers of a patient with MSA. Arrows point to CGIs in each of the three panels. D: Several cortical LBs in the cingulate cortex of a patient with DLB/PD. In contrast to the α-synuclein antibody, BSB (E) and THIOS (F) stain only a few LBs in adjacent tissue sections. Arrows point to LBs in all three panels. G: A large number of α-synuclein-positive inclusions in the CA3 region of the hippocampus of a patient with NBIA-1. H and I were taken from adjacent tissue sections and show rare globules but no perikaryal inclusions. All panels are the same magnification; scale bar in A = 20 μm.
Table 3.
BSB and THIOS Stained Lesions in Various Neurodegenerative Diseases
| Antibody specificity | Cases | Lesions | BSB | THIOS |
|---|---|---|---|---|
| 4G8 (total Aβ) | AD, FAD, DS (54 yr) | Plaques | + | + |
| 4G8 (total Aβ) | AD, FAD, DS (54 yr) | Vascular amyloid | + | + |
| BA27 (Aβx-40) | AD, FAD, DS (54 yr) | Plaques | + | + |
| BA27 (Aβx-40) | AD, FAD, DS (54 yr) | Vascular amyloid | + | − |
| BCO5 | AD, FAD, DS (54 yr) | Plaques | + | + |
| 4G8 (total Aβ) | DS (33 yr) | Plaques | − | +/− |
| 4G8 (total Aβ) | Pick’s disease | Plaques | − | +/− |
| 4G8 (total Aβ) | FTDP-17 (P301L) | Plaques | − | +/− |
| 4G8 (total Aβ) | Elderly non-demented control | Plaques | − | +/− |
| 17026 (tau) | AD (all tau isoforms) | NFT | + | + |
| 17026 (tau) | AD (all tau isoforms) | NT | + | + |
| 17026 (tau) | PSP (4 repeat tau) | Globose tangles | + | + |
| 17026 (tau) | PSP (4 repeat tau) | Coiled bodies | + | + |
| 17026 (tau) | FTDP-17 cases (4 repeat tau): | |||
| 17026 (tau) | exon 10+ 16 | Oligodendrocyte inclusions | + | + |
| 17026 (tau) | N279K | Coiled bodies | +/− | +/− |
| 17026 (tau) | N279K | Tau+ neurons | − | − |
| 17026 (tau) | N279K | NT | + | + |
| 17026 (tau) | P301L | Neocortical perikaryal tau+ | − | − |
| 17026 (tau) | P301L | Basal forebrain tau+ cell bodies | − | − |
| 17026 (tau) | P301L | Tau+ astrocytes | +/− | − |
| 17026 (tau) | CBD (4 repeat tau) | Tau+ neurons | − | − |
| 17026 (tau) | CBD (4 repeat tau) | NT (gray and white matter) | + | − |
| 17026 (tau) | Pick’s disease (3 repeat tau) | Pick bodies | +/− | +/− |
| 17026 (tau) | Guamanian PDC | Spinal cord NFT | + | + |
| SYN303 (a-synuclein) | MSA | Glial cytoplasmic inclusions | + | − |
| SYN303 (a-synuclein) | DLB | Cortical LB | +/− | +/− |
Discussion
The data presented here indicate that BSB binds to a variety of amyloid-like lesions in neurodegenerative diseases that are formed by very different building block peptides or proteins including: Aβ peptides (amyloid plaques), hyperphosphorylated tau proteins (NFTs, NTs), and α-synuclein (LBs, GCIs). Each of these three proteins forms fibrils that deposit into amyloid-like lesions. The results of this study show that the binding properties of BSB are very similar to those of THIOS, but only BSB, and not THIOS crosses the blood brain barrier to label amyloid plaques in mice that model AD amyloidosis as reported earlier. 1 Indeed, the entry of BSB into brain was demonstrated further using a BSB derivative wherein the bromide was exchanged for iodine-125 and in vivo biodistribution data indicated that 0.27% of the injected compound infiltrated the brain 6 minutes after intravenous delivery in mice. 19 BSB binds not only to Aβ plaques in living tissue and Aβ aggregates in cultured cells but also in sections of frozen tissue. 1 While THIOS has been used in histology as an amyloid binding dye for several decades, the mechanisms whereby this and related histochemical stains bind to these fibrillar lesions is still under investigation. 20 Although the β-pleated sheet formation occurring in the fibrils formed by Aβ, hyperphosphorylated tau, as well as α-synuclein may be the substrate for BSB and THIOS binding, minor variations in the β-pleated sheet structures in the lesions described may account for the differences observed in the binding of BSB and THIOS to these lesions. 12,21 In addition, since fibrils formed by three or four microtubule-binding repeat tau isoforms had different affinities for BSB, variations in β-pleated sheet structures formed by these different tau isoforms also might contribute to variable BSB binding to tangles. For example, Pick bodies composed of three MT binding repeat tau stained weakly with BSB and THIOS, while PSP lesions composed of four MT binding repeat tau stained strongly with BSB and THIOS the 4-repeat lesions in CBD and FTDP-17 stained to variable degrees or not at all (Table 3) ▶ .
Although BSB binds to a number of fibrillar lesions occurring in neurodegenerative diseases tau- and α-synuclein positive lesions are much smaller and usually far fewer in numbers than Aβ plaques and would not pose a problem in prospective whole brain imaging of BSB bound to Aβ plaques. However, BSB did not stain the plaques in a young DS patient who was in the beginning stages of AD. Similarly, BSB did not stain the diffuse plaques of an elderly non-demented patient with pathological aging 22 which could be a preclinical stage of AD. Although BSB compound will not detect non-fibrillar Aβ accumulations that may be the earliest pre-clinical manifestations of AD, our data suggest that BSB or related compounds with the ability to readily cross the blood-brain barrier, such as the radioiodinated compound mentioned above, could be exploited to develop in vivo imaging agents to monitor the burden of diverse amyloid deposits in AD and other neurodegenerative disorders as well as the effects of novel therapeutic agents designed to reverse or ameliorate the accumulation of these lesions in the brains of living patients.
Acknowledgments
We thank all of the families that made brain tissue available for this study. We would also like thank Joe DiRienzi and I. Tsimberg as well as members of the Departments of Neurology, Psychiatry, Medicine, and Pathology and Laboratory Medicine for their help in the acquisition of the tissues.
Footnotes
Address reprint requests to John Q. Trojanowski, M.D., Ph.D., Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 36th and Spruce Streets, Philadelphia, PA 19104-4283. E-mail: trojanow@mail.med.upenn.edu.
Supported by grants AG-09215, AG-10124, AG-14449, and AG-11542 from The National Institute of Aging and grants from The Alzheimer Association, Oxford Foundation, and the Institute for the Study of Aging.
References
- 1.Skovronsky DM, Zhang B, Kung M-P, Kung HF, Trojanowski JQ, Lee VMY: In vivo detection of amyloid plaques in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 2000, 97:7609-7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klunk WE, Debnath ML, Pettegrew JW: Development of small molecule probes for the β-amyloid protein of Alzheimer’s disease. Neurobiol Aging 1994, 15:691-698 [DOI] [PubMed] [Google Scholar]
- 3.Klunk WE, Debnath ML, Pettegrew JW: Chrysamine-G binding to Alzheimer and control brain: autopsy study of a new amyloid probe. Neurobiol Aging 1995, 16:541-548 [DOI] [PubMed] [Google Scholar]
- 4.Styren SD, Hamilton RL, Styren GC, Klunk WE: X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer’s disease pathology. J Histochem Cytochem 2000, 48:1223-1232 [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y: Visualization of Aβ42(43)-positive and Aβ40-positive senile plaques with end-specific Aβ-monoclonal antibodies: evidence that an initially deposited Aβ species is Aβ1–42(43). Neuron 1994, 13:45-53 [DOI] [PubMed] [Google Scholar]
- 6.Buée-Scherrer V, Hof PR, Buée L, Leveugle B, Vermersch P, Perl DP, Olanow CW, Delacourte A: Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick’s disease. Acta Neuropathol (Berl) 1996, 91:351-359 [DOI] [PubMed] [Google Scholar]
- 7.Vermersch P, Robitaille Y, Bernier L, Wattez A, Gauvreau D, Delacourte A: Biochemical mapping of neurofibrillary degeneration in a case of progressive supranuclear palsy: evidence for general cortical involvement. Acta Neuropathol (Berl) 1994, 87:572-577 [DOI] [PubMed] [Google Scholar]
- 8.Dickson DW: Neurodegenerative diseases with cytoskeletal pathology: a biochemical classification. Ann Neurol 1997, 42:541-544 [DOI] [PubMed] [Google Scholar]
- 9.Flament S, Delacourte A, Verny P, Hauw J-J, Javoy-Agid F: Abnormal tau proteins in progressive supranuclear palsy: similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol (Berl) 1991, 81:591-596 [DOI] [PubMed] [Google Scholar]
- 10.Ksiezak-Reding H, Morgan K, Mattiace LA, Davies P, Liu W-K, Yen S-H, Weidenheim K, Dickson DW: Ultrastructure and biochemical composition of paired helical filaments in corticobasal degeneration. Am J Pathol 1994, 145:1496-1508 [PMC free article] [PubMed] [Google Scholar]
- 11.Hong M, Trojanowski JQ, Lee VMY: Tau-based neurofibrillary lesions. Clark CM Trojanowski JQ eds. Neurodegenerative Dementias. 2000, :pp 161-175 McGraw-Hill, [Google Scholar]
- 12.Giasson BI, Wilson CA, Trojanowski JQ, Lee VMY: Tau and α-synuclein in neurodegenrative diseases. Chesselet M-F eds. Contemporary Clinical Neuroscience: Molecular Mechanisms of Neurodegenerative Diseases. 2001, :pp 151-176 Humana Press, Totowa, NJ, [Google Scholar]
- 13.Schmidt ML, Carden MJ, Lee VMY, Trojanowski JQ: Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest 1987, 56:282-294 [PubMed] [Google Scholar]
- 14.Schmidt ML, Lee VMY, Saido TC, Perl DP, Schuck T, Iwatsubo T, Trojanowski JQ: Amyloid plaques in Guam amyotrophic lateral sclerosis/and parkinsonism-dementia complex contain species of Aβ similar to those found in the amyloid plaques of Alzheimer’s disease and pathological aging. Acta Neuropathol (Berl) 1998, 95:117-122 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt ML, Zhukareva V, Newell KL, Lee VMY, Trojanowski JQ: Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol (Berl) 2001, 101:518-524 [DOI] [PubMed] [Google Scholar]
- 16.Kim KS, Miller DL, Sapienza VJ, Chen CJ, Bai C, Grundke-Iqbal I, Currie JR, Wisniewski HM: Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosci Res Commun 1988, 2:121-130 [Google Scholar]
- 17.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VMY: Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 2000, 290:985-989 [DOI] [PubMed] [Google Scholar]
- 18.Guntern R, Bouras C, Hof PR, Vallet PG: An improved thioflavine S method for staining neurofibrillary tangles and senile plaques in Alzheimer’s disease. Experientia 1992, 48:8-10 [DOI] [PubMed] [Google Scholar]
- 19.Zhuang Z-P, Kung M-P, Hou C, Skovronsky DM, Gur TL, Plössl K, Trojanowski JQ, Lee VMY, Kung HF: Radioiodinated styrylbenzenes and thioflavins as probes for amyloid. J Med Chem 2001, 44:1905-1914 [DOI] [PubMed] [Google Scholar]
- 20.Friedhoff P, Schneider A, Mandelkow E-M, Mandelkow E-M: Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry 1998, 37:10223-10230 [DOI] [PubMed] [Google Scholar]
- 21.Giasson BI, Murray IV, IV, Trojanowski JQ, Lee VM: A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem 2001, 276:2380-2386 [DOI] [PubMed] [Google Scholar]
- 22.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau A, Davies P, Yen S-H, Aronson MK: Identification of normal and pathologic aging in prospectively studied nondemented elderly humans. Neurobiol Aging 1991, 13:179-189 [DOI] [PubMed] [Google Scholar]