Abstract
Interactions between specific cell-surface molecules, which include the urokinase receptor (uPAR) and integrins, are crucial to processes of tumor invasion and metastasis. Here we demonstrate that uPAR and β1-integrins may cluster at distinct sites at the cell surface of metastatic MDA-MB-231 breast cancer cells and form functional complexes. Attachment assays performed in the presence of a synthetic peptide (p25), which interferes with the formation of uPAR-integrin complexes, reveal that uPAR is able to regulate the adhesive function of integrins in breast cancer cells. On dissociation of the uPAR-integrin complexes by p25, tumor cell attachment to the extracellular matrix was either decreased (vitronectin) or increased (fibronectin). Moreover, the tumor cells display remarkable morphological changes when cultured on fibronectin in the continuous presence of p25, leading to increased cell spreading and attachment. In marked contrast to control conditions, increased cellular adhesion to fibronectin after p25 treatment was entirely β1-integrin-mediated. The role of uPAR-integrin complexes in tumor progression was studied in an in vivo bone xenograft model. Stably transfected MDA-MB-231 cells that overexpress p25 showed a significant reduction in tumor progression in bone (P ≤ 0.0001 versus mock-control). In line with these observations, continuous administration of p25 (25 μg/mouse/day, osmotic minipumps) for 28 days resulted in significantly reduced tumor progression of MDA-MB-231 cells in bone (P ≤ 0.005) when compared to scrambled control peptide. In conclusion, our data demonstrate that uPAR can act as an adhesion receptor in breast cancer and is capable of regulating integrin function. Our findings strongly suggest that adhesive and proteolytic events are tightly associated in metastatic breast cancer cells and that functional integrin-uPAR complexes are involved in tumor progression in vivo.
Tumor invasion and metastasis is a multistep process that depends on coordinated expression and temporal regulation of a series of proteolytic and adhesive events. 1-11 Integrins and the urokinase receptor (uPAR) are two cell-surface molecules that participate in adhesion, detachment, and proteolytic disruption of neighboring structures. 1-10 Integrins are noncovalently associated heterodimers that consist of α and β chains. The composition of α-β heterodimers determines the specificity of the integrin for its ligand, in most cases an extracellular matrix molecule. Integrins are expressed by a large variety of tumors, including breast cancer cells. 12 We and others have shown that malignant breast cancer cells express β1 and β3 integrins that are functionally involved in their adhesion to different extracellular matrix molecules. 13-17 Clinical studies have provided evidence that altered integrin expression patterns, especially those of the β1-integrin subfamily, correlate with metastatic behavior of cancer cells and poor prognosis in patients with breast cancer. 12,18-24
Additionally, invasion and metastasis requires proteolytic degradation of surrounding matrices. The plasminogen-activator/plasmin system and matrix metalloproteinases have been implicated in these processes in a large variety of tumors including breast cancer. 7 The generation of pericellular plasmin by urokinase (uPA) in tumor cells may lead to direct plasmin-mediated proteolysis or, indirectly, may lead to activation of other proteinases (eg, matrix metalloproteinases) that are capable of extracellular matrix breakdown. Both plasminogen and uPA bind to specific cell-surface receptors, localizing plasmin activity to the cell surface where it can be used by migrating cells to degrade tissue barriers during normal and pathological processes. 7,25-32 One of these receptors, uPAR, plays a crucial role in the activation of plasminogen into plasmin, and is thus primarily responsible for the initiation of the plasminogen activator/plasmin cascade. Accumulating clinical evidence indicates that uPA and uPAR are overexpressed in breast cancer and that high uPA/uPAR levels correlate with poor prognosis of patients with breast cancer. 5,25,27,28,33
Adhesive and proteolytic events may not be separate and independent processes in highly migratory cells. Wei and co-workers 34 provided the first direct evidence that uPAR, after binding to uPA, is not only involved in proteolysis, but may also act as a high-affinity receptor for vitronectin in embryonic kidney cells. More recently, it was shown that uPAR physically associates with integrins in highly migratory, uPAR-expressing cells. 1,31,35-43 uPAR can form a complex with activated integrins when attached to specific extracellular matrix molecules. It was shown that these uPAR-integrin complexes both inhibited the native integrin adhesive function and promoted the chemotaxis and adhesion to vitronectin via a ligand-binding site on uPAR. 35
The uPAR-integrin complexes could be specifically disrupted by a 17-amino acid peptide sequence (p25), isolated from a phage peptide library. 35 The p25 peptide interfered with uPAR-mediated adhesion resulting in decreased adhesion of uPAR-expressing cells to vitronectin, a substrate for uPAR when associated to integrins. These results support the notion that uPAR is capable of altering integrin function without affecting ligand binding of both cell surface receptors. 35 Recently, a critical non-I-domain-binding site for uPAR on CD11b (M25, residues 424 to 440) was identified by homology with this p25 phage display peptide. 41 The M25 peptide also blocked the association of uPAR with β1-integrins and impaired β1-integrin-dependent cellular spreading and migration on various substrates. 41 Moreover, recent observations verify that protease-independent functions of uPAR operate in vivo and identify uPAR as a potential target in certain pathological conditions. 42 Taken together, these experimental data indicate that uPAR associates with integrins directly and that disruption of this association broadly impairs integrin function, suggesting a novel strategy for regulation of integrins in the pathological processes.
In this study we demonstrate that uPAR-integrin complexes exist in malignant MDA-MB-231 breast cancer cells in vitro and in experimentally induced metastatic bone lesions in nude mice. It is shown that uPAR is able to regulate the adhesive function of integrins in breast cancer cells using in vitro attachment assays. Results from in vivo experiments described in this article strongly suggest that uPAR-integrin complexes are functionally involved in tumor progression because administration of p25 resulted in significantly reduced tumor progression.
Materials and Methods
Breast Cancer Cell Lines
The breast cancer cell line MDA-MB-231 was purchased from the American Type Culture Collection (Rockville, MD). This cell line was established from a single pleural effusion obtained from a 51-year-old white woman with poorly differentiated adenocarcinoma. 44 Cells were cultured in RPMI 1640, 10% fetal bovine serum, and penicillin/streptomycin (Life Technologies, Breda, the Netherlands) in a humidified incubator at 37°C at 5% CO2 until confluency. For attachment assays, tumor cells were cultured until ∼90% confluency and were dissociated into single cell suspensions from the tissue culture flasks using 0.125% w/v trypsin and 0.05% w/v ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) for 3 minutes (see Attachment Assays).
Proteins, Antibodies, and Reagents
Bovine serum albumin was purchased from Miles Scientific Inc. (Naperville, IL) Serum-free conditioned medium (SFCM) was obtained from confluent MC3T3E1 mouse osteoblasts (a kind gift from Dr. Merregaert, University of Antwerp, Belgium) that were incubated for 24 hours in serum-free medium supplemented with 0.5% v/v ITS+ (insulin, transferrin, 2.5 μg/ml selenium, 0.5 mg/ml bovine serum albumin, and 0.5 μg/ml linoleic acid; Collaborative Research, Inc., Bedford, MA). SFCM contains a mixture of various extracellular bone matrix components and reflects the diversity and composition of extracellular bone matrix in vivo as described earlier. 13,14
Human fibronectin and vitronectin were obtained from Life Technologies and were used in attachment assays (see below). The following antibodies were used in attachment assays and/or immunoprecipitation procedures (see below); mouse anti-human β1-integrin antibodies (P4C10, Life Technologies; mAb 1977, Chemicon International Inc., Temecula, CA), rabbit anti-human uPAR antibodies (399R; American Diagnostics, Greenwich, CT) and the mouse-anti-human uPAR monoclonal antibody H2. 36,45
Peptide Synthesis
Two synthetic peptides were used in attachment assays: p25, AESTYHHLSLGYMYTLN-NH2; and in scrambled order NYHYLESSMTALYTLGH-NH2 (scrambled control peptide, sc25) as described earlier. 35
Peptides (p25 and its scrambled control) were synthesized by solid-phase strategies on an automated multiple peptide synthesizer (AMS422; Abimed Analysen-Technik, Langenfeld, Germany) using Fmoc chemistry. The purity of the peptides was determined by reverse-phase HPLC.
Cell Attachment Assays; Proteins Coated onto Plastic
Cell attachment assays were performed in bacteriological 96-wells plates and dotted with glycoproteins or SFCM as described previously. 13,14
In brief, 10 μl of SFCM or extracellular matrix protein in PBS containing 1 mmol/L Ca2+ was applied, resulting in a protein-coated area (dot) of 0.12 cm2. After 16 hours at 4°C, the fluid was removed and 100 μl of 60% v/v methanol was added to each well for 2 hours at 4°C. The methanol fixation was found to improve the stability and durability of the protein-coated area without altering the biological activity of the substrate. 13,14 The methanol was removed and wells were washed for 30 minutes at 4°C with washing buffer (50 mmol/L Tris-HCl, pH 7.8, 110 mmol/L NaCl, 5 mmol/L CaCl2, 0.1 mmol/L phenylmethyl sulfonyl fluoride, 1% w/v bovine serum albumin, and sodium azide) to block unbound sites on the plastic. After removal of the washing buffer, the wells were washed three times with serum-free medium RPMI 1640 supplemented with glutamine and 0.5% v/v ITS+.
Cells were precultured in RPMI 1640 (and Hepes), 10% fetal bovine serum, and penicillin/streptomycin in a humidified incubator at 37°C at 5% CO2 until 90% confluency. For attachment assays tumor cells were dissociated from the flasks using 0.125% w/v trypsin, 0.05% w/v ethylenediaminetetraacetic acid solution in PBS (pH 7.2) for 3 minutes. Cells were washed and resuspended in serum-free medium (RPMI 1640 containing 0.5% v/v ITS+) and seeded at a density of 10,000/cm 2 (2800 cells/150 μl) on the matrix-coated 96 wells. The 96-well dishes were incubated at 37°C at 5% CO2 for a maximum of 3 hours. Each well was washed three times with serum-free medium and attached cells were fixed for 20 minutes with 80% methanol at 4°C and stained with AmidoBlack. Two nonoverlapping microscopic fields (2.54 mm2/field) within each protein-coated area were counted. Each group was performed in fourfold and experiments were repeated three times.
Breast cancer cell attachment to SFCM, vitronectin, and fibronectin was determined in the absence or continuous presence of the p25 peptide and/or function blocking anti-β1 antibody (P4C10). Scrambled peptide sequences and/or nonimmune IgGs were used as negative controls.
The scrambled peptide was used as a negative control for p25 and did not significantly alter the adhesion of the tumor cells compared to control conditions (no addition of peptide) at a concentration range between 0 to 250 μmol/L (results not shown).
To exclude the potential effect of trypsinization on removal of uPAR from the cell surface control experiments were performed (fluorescence-activated cell sorting analyses, attachment assays). Data from these experiments showed that uPAR is expressed (and functionally active) at the cell surface of MDA-MB-231 cells throughout the entire adhesion experiment (results not shown).
In Vivo Models of Experimental Metastasis
MDA-MB-231 breast cancer cells (10 5 cells/100 μl) were injected into the left heart ventricle of 8-week-old female BALB-c nu/nu mice according to the protocol described by Arguello and co-workers. 46 Subsequent to tumor cell administration, breast cancer growth of the cancer cells in the skeleton was assessed by radiographs (Kodak X-OMAT TL; Eastman Kodak Company, Rochester, NY) using a Hewlett-Packard X-ray systems Faxitron 43805. Five weeks after tumor cell inoculation the animals developed osteolytic bone metastases as expected 47,48 and at the end of the experiment mRNA was isolated from the bone/bone marrow of all long bones. The presence of tumor cells in the bone/bone marrow was checked with reverse transcriptase-polymerase chain reaction (RT-PCR) using human-specific β2-μ-globulin primers as described below. 49,50
Bone Xenograft Model for Breast Cancer Cells
We have established a bone xenograft model for breast cancer cells to study the role of uPAR-integrin complexes in tumor progression in bone as described earlier for prostate tumors. 51,52 Parental MDA-MB-231 breast cancer cells (10 5 cells/μl) were injected directly into the bone marrow cavity of the tibiae from BALB-c nu/nu mice and visible osteolytic lesions were formed after 3 weeks. Osteolytic lesions are because of the presence of numerous actively resorbing osteoclasts surrounding the metastatic lesions. 47-49
To establish the role of uPAR-integrin complexes in tumor progression, parental MDA-MB-231 breast cancer cells were stably transfected with a p25 expression construct (or empty pCR 3 vector) under a CMV promoter (see below). Subsequently, 10 5 p25-overexpressing MDA-MB-231 cells (MDA-p25) or mock-transfected cells (MDA-mock) were injected into the left and right tibiae of the same animal (8-week-old female BALB-c nu/nu mice), respectively. Subsequent to tumor cell administration, breast cancer growth of both cell lines in the skeleton of the same animal was assessed by radiographs as described above. mRNA was isolated from the bone/bone marrow of both tibiae 3 weeks after intra-osseous injections. The presence of tumor cells in the bone marrow was checked with RT-PCR using human-specific β2-μ-globulin primers as described below.
In addition, the progression of parental MDA-MB-231 breast cancer cells in the in vivo bone xenograft model was studied during continuous administration of 25 μg/mouse/day of p25 or a scrambled control peptide for 28 days with subcutaneously implanted osmotic minipumps (200 μl, 0.25 μl/hr, model 2004; Alzet Scientific Products, Alza Corporation, Mountain View, CA).
Detection of Tumor Cells and Expression of Tumor-Derived Factors Using RT-PCR
Total RNA was isolated from bone metastases or bone lesions according to the method of Chomczynski and Sacchi. 50 Tissues were homogenized in the lysis buffer using an Ultra-Turrax T25 (Janke & Kunkel, Staufen, Germany), extracted with phenol and chloroform, precipitated at −20°C with 100% isopropanol, resuspended in autoclaved denatured water, and stored at −80°C. RNA concentration was determined spectrophotometrically assuming 40 μg/ml per optical density at a wavelength of 260 nm (1-cm path length) and quality was checked on a 1% agarose gel containing 0.5 μg ethidium bromide (EtBr)/ml. The RNA was reverse-transcribed into cDNA using MMLV reverse transcriptase (MBI Fermentas, Vilnius, Lithuania) and used for PCR amplification with Goldstar DNA polymerase (Eurogentec, Seraing, Belgium). Expression of tumor-derived factors was determined using 10 ng of cDNA for each PCR reaction in an end volume of 25 μl per tube (32 cycles, 58°C annealing temperature, 2.0 mmol/L MgCl2) using a GeneAmp PCR system 9700 (Perkin Elmer, Branchburg, NJ).
Sense (S) and antisense (AS) primers were designed to specifically hybridize with tumor (human) transcripts and not with host transcripts (mouse) during the PCR reactions and the conditions specified above. This way we have been able to specifically amplify tumor-derived transcripts and not mouse (host) transcripts (see results). The following sense (S) and antisense (AS) primers were used in RT-PCR reactions: β1-integrin (S = 5′-AAT.GAA.GGG.CGT.GTT.GGT.AG-3′, AS = 5′-CTG.CCA.GTG.TAG.TTG.GGG.TT-3′, amplicon size 290 bp); tPA (S = 5′-TCT.TAG.ATT.TCG.TGT.GCC.AG-3′, AS = 5′-CTC.TGA.GCT.GTA.CTT.CCC. CG-3′, amplicon size 296 bp); uPA (S = 5′-GCC.ATC.CCG.GAC.TAT.ACA.GA-3′, AS = 5′-AGG.CCA.TTC.TCT.TCC.TTG.GT-3′, amplicon size 416 bp); uPAR (S = 5′-CTG.GAG.CTG.GTG.GAG.AAA.AG-3′, AS = 5′-TGT.TGC.AGC.ATT.TCA.GGA.AG-3′, amplicon size 406 bp); PAI-1 (S = 5′-ATA.CTG.AGT.TCA.CCA.CGC.CC-3′, AS = 5′-GTG.GAG.AGG.CTC.TTG.GTC.TG-3′, amplicon size 320 bp); PAI-2 (S = 5′-ATG.GTC.TAC.ATG.GGC.TCC.AG-3′, AS = 5′-CGC.AGA.CTT.CTC.ACC.AAA.CA-3′, amplicon size 285 bp); β2-μ-globulin (S = 5′-CCA.GCA.GAG.AAT.GGA.AAG.TC-3′, AS = 5′-GAT.GCT.GCT.TAC.ATG.TCT.CG-3′, amplicon size 268 bp).
Immunoprecipitation Protocol
MDA-MB-231 breast cancer cells were cultured in a 9-cm Petri dish until subconfluent and washed twice with PBS. Subsequently the cells were washed twice with PIPES buffer and 5 ml PIPES and 0.2% v/v Triton X-100 was added and incubated for 5 minutes on ice as described earlier. 35,53,54 The supernatant was transferred to a tube and 5 ml of 2× RIPA buffer was added. The insoluble fraction was used for immunoprecipitation as described earlier. 35
Immunoprecipitation was performed using either mouse anti-human β1-integrin antibodies (P4C10) or rabbit anti-human uPAR antibodies (399R).
After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the gel was blotted to an activated Immobilon membrane (Amersham Pharmacia Biotech, Essex, UK) and detection of β1-integrin (mAb 1977) and uPAR (399R) was performed using the enhance chemiluminescence-blotting and detection method for Western blotting according to the manufacturer’s protocol (Amersham Pharmacia Biotech).
In addition total cell lysates of cultures MDA-MB-231 cells were prepared as described earlier 55 and analyzed by Western blotting with two different antibodies for human uPAR (399R, H2) and β1-integrins (P4C10, mAb 1977).
Immunofluorescence
MDA-MB-231 cells were seeded on sterilized SFCM-coated glass coverslips at a density of 10,000 cells/cm2. After 2 days the coverslips were washed twice with PBS and cells were fixed for 30 minutes with buffered formalin at room temperature. Subsequently, the tumor cells were washed three times with PBS and stored in PBS until use.
After removal of PBS, the fixed cells were quenched for 10 minutes in 50 mmol/L NH4Cl. After three washes with PBS the cells were permeabilized for 4 minutes with 0.1% v/v Triton X-100 in PBS. After removal of the fluid, the cells were preincubated for 1 hour in 0.5% w/v Boehringer milk powder (BMP; Boehringer Mannheim, Mannheim, Germany) in PBS. Subsequently the cells were incubated overnight (18 hours) with primary antibody in 0.5% w/v BMP-PBS at 4°C. The following primary antibodies were used; P4C10 (mouse anti-human β1-integrin monoclonal antibody, 1:500) and 399R (rabbit anti-human uPAR polyclonal antibody, 1:200).
After overnight incubation, the glass coverslips were washed three times with 0.5% w/v BMP-PBS and subsequently the cells were incubated for 1 hour with 1:500 diluted Cy-Dyes (Amersham Pharmacia Biotech) at room temperature. The following fluorescent secondary antibodies were used: Cy-2 (anti-mouse) and/or Cy-3 (anti-rabbit). After 1 hour the secondary antibodies were removed and the cells were washed three times in distilled water and air dried. The stained cells were mounted in Fluormount (BDH; Brunschwig Chemie, Amsterdam, The Netherlands) and examined by confocal microscopy (Zeiss LSM 510) at excitation wavelengths of 488 nm (Cy2) and 543 nm (Cy3).
Stable Transfection of MDA-MB-231 Cells with cDNA for p25
A preproPTHrP-p25 cDNA was subcloned into pCR 3 (Invitrogen, San Diego, CA) expression vector. The preproPTHrP signal peptide, positions 6 to 130 (GenBank Accession no. M17183) was ligated to the p25-coding sequence containing a stop codon at position 176 to 178 bp of the expression construct (total length, 178 bp). The p25-coding sequence was derived from the peptide sequence A-E-S-T-Y-H-H-L-S-L-G-Y-M-Y-T-L-N 36 and included a stop codon at the 3′ end; GCT.GAA.TCT.ACA.TAT.CAC.CAT.CTG. TCC.CTT.GGC.TAC.ATG.TAT.ACA.CTC.AAC.TGA. The preproPTHrP-p25 or empty pCR 3 vector (mock) was transfected into MDA-MB-231 cells by calcium phosphate precipitation. Single clones were isolated by limiting dilution in the presence of the selective marker G418 (Sigma Chemical Co., St. Louis, MO). Clones were screened and selected by measuring mRNA expression of p25 using RT-PCR. The following PCR primers were used for screening of the MDA-MB-231 clones; sense, 5′-GAG.CGC.GAG.CGG.AGA.CGA.TG-3′; and antisense, 5′-CAT.GTA.GCC.AAG.GGA.CAG.ATG-3′ (expected amplicon size, 160 bp).
No differences in viability and growth rate of the parental, mock-transfected, and p25-expressing MDA-MB-231 cells were found (results not shown).
Statistics
Significant differences between the experimental groups within each experiment were calculated using a factorial one-way analysis of variance followed by a Fisher’s PLSD test.
Results
Expression of PA/Plasmin Members and Integrins in Bone Metastases
Figure 1 ▶ depicts the expression of t/uPA, uPAR, and PAI-1 and PAI-2, and β1-integrin transcripts by MDA-MB-231 cells derived from a bone metastasis that was formed 5 weeks after inoculation of the tumor cells into the left heart ventricle of nude mice. The steady-state mRNA levels were analyzed by semiquantitative RT-PCR using human-specific oligonucleotides (see Materials and Methods). Our data show that metastatic breast cancer cells strongly express uPA and other members of the PA/plasmin system including uPAR, tPA, and PAI-1 (Figure 1A) ▶ and β1-integrins in vivo (Figure 1B) ▶ . Similar results were found in cell cultures of MDA-MB-231 cells in vitro. 48
Figure 1.
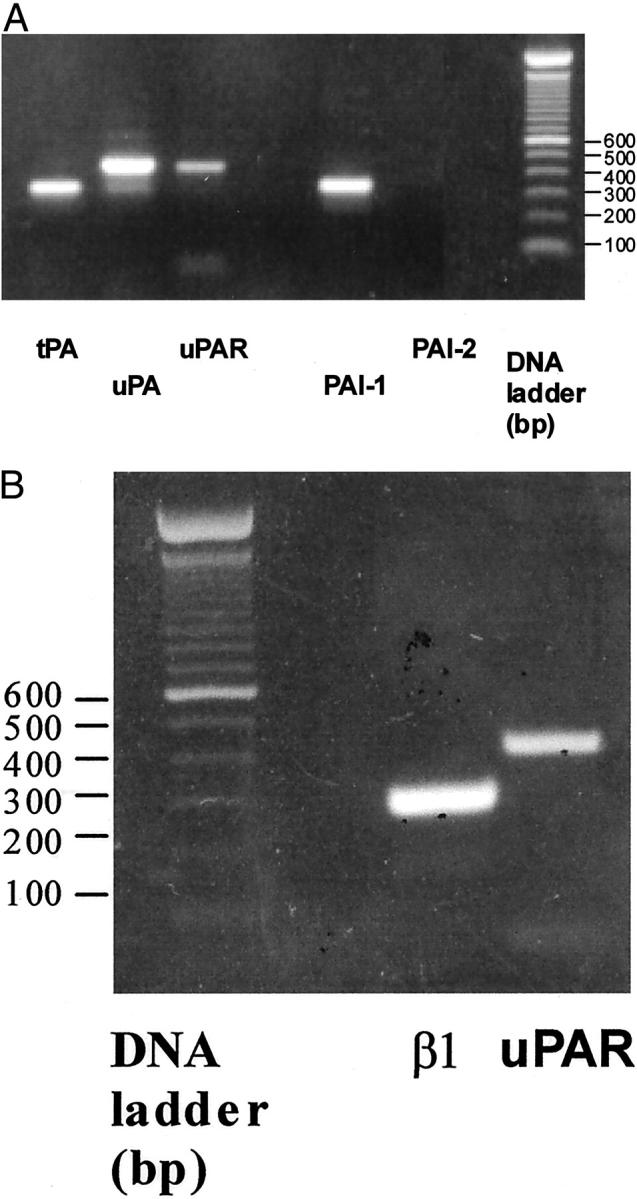
mRNA expression of members of the PA/plasmin system (t/uPA, uPAR, PAI 1, PAI 2 (A) and β1-integrin (B) in MDA-MB-231 cells derived from a skeletal metastasis from nude mice as determined by RT-PCR (10 ng cDNA, 32 cycles, 56°C annealing temperature).
uPAR-Integrin Complexes in Breast Cancer Cells
The presence of functional uPAR-integrin complexes in highly invasive breast cancer cells was studied using attachment assays, and immunohistochemical and immunoprecipitation procedures.
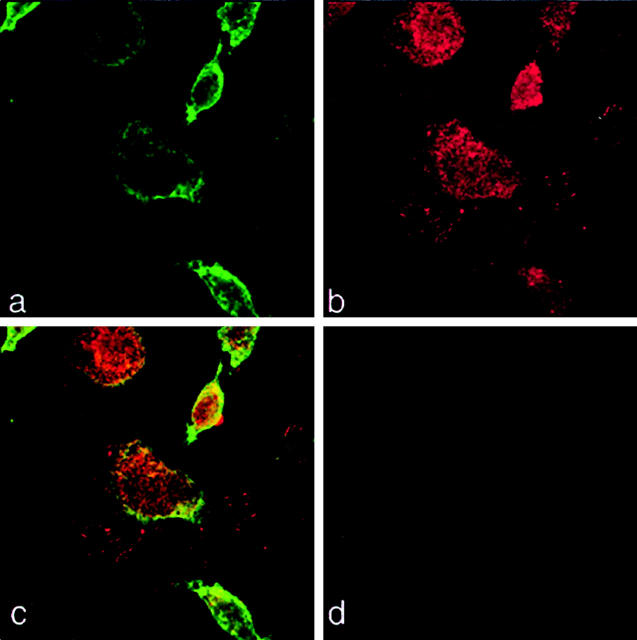
Figures 2 and 3 ▶ ▶ show the double-immunofluorescence staining of human MDA-MB-231 cells for β1-integrins and uPAR in vitro. Using confocal microscopy we found that uPAR and β1-integrins co-localize at the cell surface of MDA-MB-231 cells (Figure 3) ▶ .
Figure 2.
The urokinase receptor (uPAR) and β1-integrins co-localize (a–c). MDA-MB-231 cells were cultured for 3 days on glass coverslips in α-MEM plus 10% FCS, fixed in 2% paraformaldehyde, and subsequently stained with polyclonal uPAR (399R) antibody and β1-integrin (P4C10) monoclonal antibodies. Secondary antibodies were then used to visualize optical planes of the cells by confocal microscopy (Zeiss LSM 510): integrins (Cy2 = green, a), uPAR (Cy3 = red, b), and sites of co-localization (yellow, c). d: The negative control (no primary antibody added, Cy2 and Cy3 secondary antibodies). Original magnifications, ×400.
Figure 3.
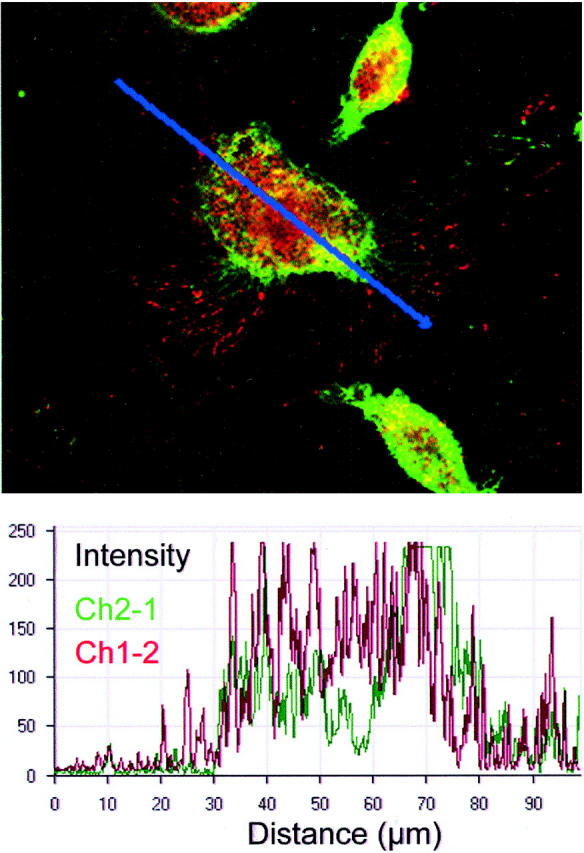
The urokinase receptor (uPAR) and β1-integrins co-localize. MDA-MB-231 cells were cultured for 3 days on glass coverslips in α-MEM plus 10% FCS, fixed in 2% paraformaldehyde, and subsequently stained with polyclonal uPAR (399R) antibody and β1-integrin (P4C10) monoclonal antibodies followed by Cy3-conjugated goat anti-rabbit antibodies (red) and Cy2-conjugated rat anti-mouse antibodies (green), respectively. Bottom: A graphic representation of the co-localization of both receptors (intensities of red and green) after measurement of both signals along the optical plane visualized by the blue line of the top. Intensity measurements were performed with Zeiss LSM 510 software.
In Western blot analyses of total MDA-MB-231 cell lysates (Figure 4A) ▶ , the anti-uPAR Abs (399R and H2) detected two forms of the uPAR as expected. 55 These bands may represent the intact uPAR (49 kd) and cleaved 2 + 3 domain form of uPAR (30 kd). 56 In addition, β1-integrins were detected in the same cell lysates using P4C10 and mAb 1977 antibodies (130 kd). When the membrane fraction of MDA-MB-231 cells was immunoprecipitated with an anti-β1-integrin antibody P4C10, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a Immobilon membrane, β1-integrins were detected on staining with the anti-β1-integrin antibody mAb 1977 (Figure 4B ▶ , lane 4), whereas no bands were detected in the negative control groups (Figure 4B ▶ , lanes 1 to 3). When the membrane fraction of MDA-MB-231 cells was immunoprecipitated with an anti-β1-integrin antibody P4C10 and subsequently separated and blotted, the 30-kd uPAR(2 + 3) was detected on staining with the rabbit anti-uPAR antibody 399R (Figure 4B ▶ , lane 5) suggesting co-precipitation of uPAR(2 + 3) with β1-integrins in metastatic breast cancer cells. In contrast, addition of p25 (100 μmol/L) to the cell lysates completely disrupted co-precipitation of uPAR(2 + 3) with β1-integrins (Figure 4B ▶ , lane 6), whereas a scrambled peptide sc25 (100 μmol/L) had no effect (Figure 4B ▶ , lane 7).
Figure 4.

A: Analysis of the detergent phase of Triton X-114 cell extracts from MDA-MB-231 cells by Western blot using two antibodies for uPAR (H2, 399R) and β1-integrins (P4C10, mAb 1977) as described previously. 55 Cleaved uPAR (30 kd) is present in cultured MDA-MB-231 cells as expected, 55,56 which may represent uPAR(2 + 3). B: Complex formation between uPAR and β1-integrins and the effect of p25. After immunoprecipitation with an anti-β1-integrin antibody (P4C10) the precipitate was separated using SDS-PAGE. Subsequently, the precipitate was blotted onto an Immobilon membrane and stained with either the anti-β1-integrin antibody (P4C10) or an anti-uPAR antibody (399R) using the ECL method for Western blotting according to the manufacturer’s protocol (see Materials and Methods). Lane 1: Negative control (nonspecific primary antibody, nonimmune mouse IgG). Lanes 2 and 3: Negative controls (nonspecific secondary antibodies, nonimmune mouse and rabbit IgGs, respectively). Lane 4: Immunoprecipitation with the anti-β1-integrin antibody P4C10 and subsequent staining with the anti-β1-integrin antibody mAb 1977. Lane 5: Immunoprecipitation with an anti-β1-integrin antibody (P4C10) and subsequent staining with an anti-uPAR antibody (399R). Lanes 6 and 7: Same as lane 5 but now in the presence of 100 μmol/L p25 or scrambled control peptide sc25, respectively.
uPAR-Integrin Complexes and Cancer Cell Attachment
Attachment assays performed with MDA-MB-231 breast cancer cells using function blocking β1-integrin antibodies and a 17-amino acid peptide sequence (p25) are depicted in Figures 5 to 7 ▶ ▶ ▶ . The p25 peptide specifically disrupts uPAR-integrin complexes and was found to interfere with uPAR-mediated adhesion without affecting ligand binding of both cell surface receptors. 35 In the presence of increasing amounts of synthetic p25 peptide, adhesion of MDA-MB-231 cells to vitronectin was decreased dose dependently (Figure 5A) ▶ , suggesting the existence of uPAR-integrin complexes and the functional involvement of uPAR in adhesion. In contrast, attachment of MDA-MB-231 cells to the extracellular matrix component fibronectin was stimulated dose dependently in the presence of p25 (Figure 5B) ▶ .
Figure 5.
Attachment of MDA-MB-231 breast cancer cells to vitronectin (A) or fibronectin (B) in the presence of increasing concentrations of p25 peptide. A scrambled peptide was used as a negative control (concentration range, 0 to 100 μmol/L), and did not alter the attachment characteristics of the cancer cells (data not shown). Values are expressed as p25/scrambled peptide ratio × 100 (treatment/control ratio × 100). (Scrambled VN control at 100 μmol/L = 67.5 ± 3.7 cells/cm2, Scrambled FN control at 100 μmol/L = 114 ± 24.7 cells/cm2). *, P ≤ 0.05.
Figure 6.
Representative micrographs of the attachment of MDA-MB-231 cells to osteoblast extracellular matrix (OB), vitronectin (VN), and fibronectin (FN) in the absence or presence of anti-β1-integrin antibodies alone or in combination with p25.
Figure 7.

Attachment characteristics of MDA-MB-231 cells to osteoblast extracellular matrix (A), vitronectin (B), or fibronectin (C) in the presence/absence anti-β1-integrin antibody and/or p25. *, P ≤ 0.01.
The involvement of uPAR as an adhesion receptor in regulating integrin function was studied further in cell attachment assays in the absence or presence of anti-β1-integrin antibodies and p25. Figure 6, A through L ▶ , depicts representative micrographs of these experiments for three extracellular matrices (osteoblast extracellular matrix, vitronectin, and fibronectin, respectively). Addition of anti-β1-integrin antibodies during attachment of the breast cancer cells to fibronectin and osteoblast extracellular matrix inhibited both the number of adherent cells and cellular spreading (Figure 6, B and J) ▶ . In contrast, in the presence of p25 not only significantly more breast cancer cells adhered to fibronectin but cellular spreading on fibronectin was also strongly increased (Figure 6K) ▶ . Conversely, adhesion of the breast cancer cells to vitronectin was not significantly affected by anti-β1-integrin antibodies whereas with p25 the attachment was significantly decreased (Figure 6, F and G) ▶ . No additional effect was observed when anti-β1-integrin antibodies and p25 were added simultaneously during attachment of MDA-MB-231 cells to vitronectin (Figure 6H) ▶ .
Quantitative measurements of these attachment experiments are shown in Figure 7, A through C ▶ . Figure 7A ▶ shows that adhesion of MDA-MB-231 cells to bone matrix was inhibited by anti-β1-integrin antibodies (92% inhibition) and p25 peptide (70% inhibition). When anti-β1-integrin antibodies and p25 were added simultaneously during attachment (Figure 7A) ▶ , adhesion was almost completely inhibited (97% inhibition). In the presence of anti-β1-integrin antibodies, cellular attachment to vitronectin was not altered significantly. In contrast, adhesion of MDA-MB-231 cells was inhibited significantly in the presence of p25 (Figure 7B) ▶ , demonstrating that vitronectin binding, under these conditions, is mediated by uPAR rather than β1-integrins (see also Figure 5A ▶ ). When p25 and anti-β1-integrin antibodies were added simultaneously (Figure 7B) ▶ , adhesion of MDA-MB-231 cells to vitronectin was not significantly different from p25 alone.
Adhesion of MDA-MB-231 cells to fibronectin was not affected by anti-β1-integrin antibodies, but was strongly stimulated by p25 (303%, Figure 7C ▶ ), suggesting reactivation/participation of integrins in adhesion to fibronectin on addition of the p25 peptide. The latter was demonstrated further when anti-β1-integrin antibodies were added simultaneously to p25. Under these conditions, attachment of the breast cancer cells was almost completely inhibited when compared to p25 alone (Figure 6C ▶ ; p25 alone = 267 ± 7 versus p25 + anti-β1-integrin antibody = 21 ± 0.6, P ≤ 0.0001).
These data, therefore, suggest the involvement of uPAR-integrin complexes in the modulation of cellular adhesion to extracellular matrices. The ability of uPAR to act as an adhesion receptor for vitronectin in MDA-MB-231 cells was further investigated with the monoclonal antibody H2, which was shown previously to specifically recognize u-PAR, 36 prevent u-PA binding to its receptor on endothelial cells and significantly inhibits endothelial cell attachment to vitronectin. 45 In the presence of 10 μg/ml of monoclonal antibody H2, adhesion of MDA-MB-231 cells to vitronectin was significantly decreased (Figure 8) ▶ as shown previously for endothelial cells in an identical assay. 45
Figure 8.
The anti-uPAR monoclonal function-blocking antibody H2 (10 μg/ml) inhibits adhesion of MDA-MB-231 cells to vitronectin. *, P ≤ 0.01.
Taken together, these in vitro results suggest the existence of functional uPAR-integrin complexes on malignant breast cancer cells and indicate that uPAR can act as an adhesion receptor for vitronectin and is capable of regulating integrin function.
Involvement of uPAR-Integrin Complexes in Tumor Progression in Vivo
The putative involvement of uPAR-integrin complexes in tumor progression in vivo was investigated using stably transfected p25-overexpressing MDA-MB-231 cells (MDA-p25) in a bone xenograft model (Figure 9) ▶ . For comparison, the right and left tibiae of the same animal were injected with 10 5 MDA-mock and MDA-p25, respectively (Figure 9A) ▶ . Three weeks after intra-osseous inoculation, cancer growth in the skeleton was assessed by radiographs (Figure 9A) ▶ and revealed extensive tumor progression and large osteolytic lesions in control MDA-mock cells (arrows) but not p25-overexpressing MDA-p25 cells in the same animal. PCR analyses of tumor-derived β2-μ-globulin steady-state mRNA levels from both tibiae confirmed the presence of viable MDA-mock and MDA-p25 cells in the bone microenvironment (Figure 9B) ▶ and demonstrated strong expression of p25 by MDA-p25 cells (Figure 9C) ▶ . Tumor burden was significantly reduced in the MDA-p25 group when compared to MDA-mock (Figure 9D) ▶ . It is important to note that surface expression of uPAR and β1-integrin as determined by fluorescence-activated cell sorting analyses did not differ between mock- and p25-transfected cell lines (results not shown). Moreover, no difference was observed in the proliferation of both cell lines in vitro (doubling time for both cell lines, 18 hours).
Figure 9.
Tumor progression after intra-osseous injection of MDA-MB-231 control cells (MDA-mock) and p25 overexpressing MDA-MB-231 cells (MDA-p25 stable transfectants). Tibiae of the same nude mouse were either injected with 10 5 MDA-mock (control cells, right tibia) or MDA-p25 (p25 expressing cells, left tibia). Three weeks after intra-osseous inoculation, cancer growth in the skeleton was assessed by radiographs (A) and reveal extensive tumor progression and large osteolytic lesions in control MDA-mock cells (arrows) but not p25-overexpressing MDA-p25 cells. RT-PCR analyses of steady-state mRNA levels for the human housekeeping gene β2-μglobulin reveal the presence of MDA-mock (lanes 1 to 4) and MDA-p25 cells (lanes 6 to 9) in the bone marrow compartment of the tibiae (B) (n = 4). Lanes 5 and 10 depict RT-PCR analyses of normal mouse bone marrow (no tumor cells) steady-state mRNA. C: RT-PCR analysis of steady-state mRNA expression of MDA-p25 cells. 1, Negative control, no reverse transcription; 2, cDNA from MDA-mock cells from right tibia; 3, cDNA from MDA-p25 cells from left tibia. Tumor areas (sq. pixels) was determined using image analysis software (NIH-Image 1.62b7 software) and show significant reduction of tumor volume (D) (*, P ≤ 0.0001; n = 8).
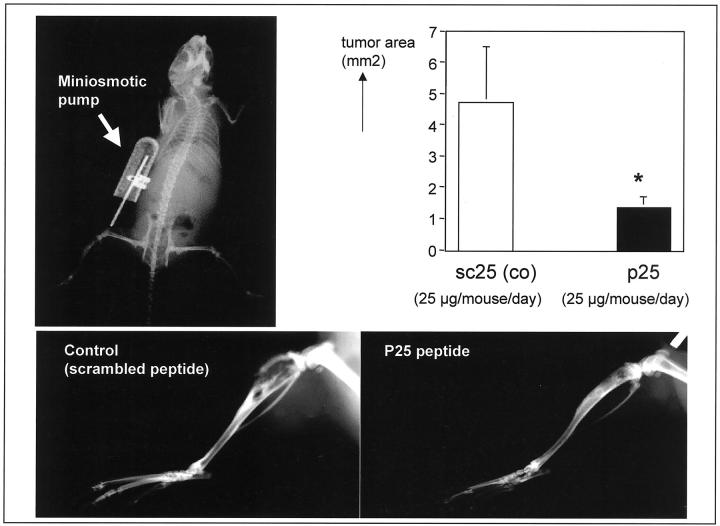
In an attempt to confirm the in vivo data, we have studied the tumor progression of parental MDA-MB-231 breast cancer cells in the in vivo bone xenograft model during continuous administration of 25 μg/mouse/day of p25 or a scrambled control peptide (sc25) for 28 days with subcutaneously implanted osmotic minipumps (model 2004; Alzet Scientific Products). Growth of the breast cancer cells in the bone/bone marrow microenvironment was significantly inhibited on administration of p25 when compared to the scrambled peptide control (P ≤ 0.05) (Figure 10) ▶ . Our data, therefore, strongly suggest that uPAR-integrin complexes can modulate tumor progression in vivo.
Figure 10.
Tumor progression after intra-osseous injection of parental MDA-MB-231 control cells. Tibiae of nude mice were injected with 10 5 MDA-MB-231 cells. After intra-osseous inoculation with 10 5 MDA-MB-231 breast cancer cells nude mice were treated with p25 or scrambled control peptide (25 μg/mouse/day) using osmotic minipumps (top left radiograph). Three weeks after intra-osseous inoculation, cancer growth in the skeleton was assessed by radiographs and reveal extensive tumor progression and large osteolytic lesions in the control group (scrambled peptide, sc25) whereas tumor burden in p25-treated animals was significantly decreased (P ≤ 0.05) (bottom right). Tumor areas (sq.pixels) was determined using image analysis software (NIH-Image 1.62b7 software) and show significant reduction of tumor volume (top right) (*, P ≤ 0.05; n = 8).
Discussion
The data presented in this study strongly suggest the existence of uPAR-integrin complexes in highly tumorigenic breast cancer cells. It was found that β1-integrins and uPAR are localized at the cell surface and cluster at focal contacts and that integrins and uPAR may form functional complexes in these tumor cells. Attachment assays revealed that uPAR is able to regulate the adhesive function of integrins on breast cancer cells. Overexpression of p25 by MDA-MB-231 cells resulted in a significant decrease in tumor progression in a bone xenograft model for breast cancer. Moreover, in vivo administration of p25 for 28 days (osmotic minipumps) significantly reduced the growth of breast cancer cells in vivo, strongly suggesting the functional involvement of uPAR-integrin complexes in tumor growth and invasion.
Cancer metastasis results from several interdependent processes that encompass a variety of adhesive and proteolytic events. The cell surface receptors uPAR and integrins have been implicated in these processes. We have shown previously that adhesion of MDA-MB-231 breast cancer cells to different extracellular matrix molecules was mediated, at least in part, by adhesion receptors from the integrin family. 13,14 In addition, our group reported earlier that uPA-uPAR pathway of plasmin activation are directly involved in the proteolytic breakdown of extracellular bone matrices by various tumor cell lines including MDA-MB-231. 48
In this article we provide evidence that uPAR and integrins form functional complexes in metastatic breast cancer. In line with previous studies, 55,56 we have found that MDA-MB-231 cells express two forms of uPAR that are anchored to the cell surface. Western blot analyses revealed that, in particular, the uPAR(2 + 3)-cleaved form on MDA-MB-231 cells produces functional complexes with β1-integrins. This may be because of the fact that β1-integrins only associate with uPAR(2 + 3) and that binding binding/interaction is shielded by domain 1 or that cleavage induces conformational changes that makes the binding site available in uPAR(2 + 3). Alternatively uPAR(2 + 3), which is more abundantly expressed at the cell surface than full-length uPAR, may compete for β1-integrin binding. Attachment assays performed with a synthetic peptide (p25), which interfered with the formation of uPAR-integrin complexes by affecting ligand binding of both receptors, 35 revealed that the attachment characteristics can change dramatically on dissociation of the uPAR-integrin complex. Furthermore, we found that changes in adhesion depended on the nature of the extracellular matrix. Adhesion of breast cancer cells to vitronectin, a ligand for uPAR when associated to integrins at the cell surface, 34 was inhibited dose dependently in the continuous presence of p25. These data indicate that uPAR acts as an adhesion receptor for vitronectin in these tumor cells. In contrast, adhesion of breast cancer cells to another extracellular matrix molecule, fibronectin, was strongly increased after p25 treatment. When attached to fibronectin, the tumor cells demonstrated a remarkable change in their morphology in that spreading of MDA-MB-231 cells was strongly increased on p25 treatment. Addition of function-blocking anti-β1-integrin antibodies in the presence of p25 completely blocked tumor cell attachment to fibronectin. These results suggest that β1-integrins become reactivated on p25 treatment because of dissociation of uPAR-integrin complexes and, subsequently, mediate the adhesion of MDA-MB-231 cells to fibronectin.
The data presented in this article suggest a functional role of uPAR-integrin complexes in tumor progression in vivo, because in vivo administration of p25 and p25 overexpression by MDA-MB-231 cells strongly and significantly decreased the tumor load in bone. Our in vitro and in vivo data support the notion that tumor cell invasion depends on cooperation between adhesive and proteolytic events and that uPAR plays a central role in these processes. 1,4,7,10,11,31,32,34-38,40-43 There is ample evidence indicating that other adhesion receptors and proteolytic enzymes are tightly linked and may mutually influence each other. For instance, matrix metalloproteinase-2 may be localized to the surface of invasive cells through integrin receptors. 57,58 Moreover, it was found that specific CD44 splice variants are involved in tumor cell migration and MMP-9 association. 59 It seems, therefore, that invasion and metastasis depend on regulation of a variety of adhesive and proteolytic processes.
The results presented in this article suggest that uPAR is capable of regulating the activity and availability of integrins depending on the nature of the extracellular matrix. It seems that the interaction between β1-integrins and uPAR influences cellular attachment characteristics because formation of complexes between the two cell surface receptors promotes the adhesion to specific matrix proteins, vitronectin, and suppresses the normal adhesive function of the integrins (fibronectin). Our data are in line with observations in other nonmalignant uPAR-expressing embryonic kidney cells. 35 Wei and co-workers 35 demonstrated that uPAR is able to regulate integrin function in these cells stably transfected with uPAR. Xue and colleagues 1 showed that uPAR associates with certain members of the β1- and β3-integrin families in fibrosarcoma cells when adherent to certain matrix molecules. Our observations provide novel evidence that metastatic breast cancer cells form uPAR-integrin complexes, which determine the attachment characteristics of the tumor cells for various substrata.
The data presented here indicate that uPAR is not only involved in processes that are related to plasminogen activation but show that adhesive and proteolytic events are tightly associated in metastatic breast cancer. Our in vivo studies emphasize the importance of uPAR-integrin complexes in breast cancer progression. Further research is warranted to study the mechanism, involvement and regulation of uPAR-integrin complex formation during cancer progression and metastasis. Results from these studies may provide new insights in the treatment of neoplastic disease.
Acknowledgments
We thank Dr. Frans Prins (Leiden University Medical Center, Department of Pathology) for his help and assistance with the confocal microscope.
Footnotes
Address reprint requests to Dr. Gabri van der Pluijm, Leiden University Medical Center, Department of Endocrinology and Metabolic Diseases C4-R, Albinusdreef 2, 2333 ZA Leiden, The Netherlands. E-mail address: g.van_der_pluijm@lumc.nl.
Supported by a grant from the Royal Netherlands Academy of Arts and Sciences (Koninklijke Nederlandse Akademic van Wetenschappen).
References
- 1.Xue W, Mizukami I, Todd RF, Petty HR: Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res 1997, 57:1682-1689 [PubMed] [Google Scholar]
- 2.Albelda SM: Biology of disease: role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest 1993, 68:4-17 [PubMed] [Google Scholar]
- 3.Maemura M, Dickson RB: Are cellular adhesion molecules involved in the metastasis of breast cancer ? Breast Cancer Res Treat 1994, 32:239-260 [DOI] [PubMed] [Google Scholar]
- 4.Blasi F: uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today 1997, 18:415-417 [DOI] [PubMed] [Google Scholar]
- 5.Xing RH, Rabbani SA: Overexpression of urokinase receptor in breast cancer cells results in increased tumor invasion, growth and metastasis. Int J Cancer 1996, 67:423-429 [DOI] [PubMed] [Google Scholar]
- 6.Juliano RL: The role of β1 integrins in tumors. Semin Cancer Biol 1993, 4:277-283 [PubMed] [Google Scholar]
- 7.Andreasen PA, Kjøller L, Christensen L, Duffy MJ: The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 1997, 72:1-22 [DOI] [PubMed] [Google Scholar]
- 8.Gui GPH, Wells CA, Browne PD, Yeomans P, Jordan S, Puddefoot JR, Vinson GP, Carpenter R: Integrin expression in primary breast cancer and its relation to axillary node status. Surgery 1995, 117:102-108 [DOI] [PubMed] [Google Scholar]
- 9.Costantini V, Sidoni A, Deveglia R, Cazzato OA, Bellezza G, Ferri I, Bucciarelli E, Nenci GG: Combined overexpression of urokinase, urokinase receptor plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign malignant breast tissues. Cancer 1996, 77:1079-1088 [DOI] [PubMed] [Google Scholar]
- 10.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ: Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 1996, 134:1563-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nip J, Rabbani SA, Shibata HR, Brodt P: Coordinated expression of the vitronectin receptor and the urokinase-type plasminogen activator receptor in metastatic melanoma cells. J Clin Invest 1995, 95:2096-2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varner JA, Cheresh DA: Integrins and cancer. Curr Opin Cell Biol 1996, 8:724-730 [DOI] [PubMed] [Google Scholar]
- 13.van der Pluijm G, Vloedgraven H, Papapoulos S, Löwik C, Grzesik W, Kerr J, Gehron Robey P: Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest 1997, 77:665-675 [PubMed] [Google Scholar]
- 14.van der Pluijm G, Vloedgraven HJM, Ivanov B, Robey FA, Grzesik WJ, Gehron Robey P, Papapoulos SE, Löwik CWGM: Bone sialoprotein peptides are potent inhibitors of breast cancer cell adhesion to bone. Cancer Res 1996, 56:1948-1955 [PubMed] [Google Scholar]
- 15.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ: Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci 1995, 108:1945-1957 [DOI] [PubMed] [Google Scholar]
- 16.Zutter MM, Santoro SA, Staatz WD, Tsung YL: Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA 1995, 92:7411-7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui GP, Puddefoot JR, Vinson GP, Wells CA, Carpenter R: In vitro regulation of human breast cancer cell adhesion and invasion via integrin receptors to the extracellular matrix Br J Surg 1995, 82:1192-1196 [DOI] [PubMed] [Google Scholar]
- 18.Koukoulis GK, Virtanen I, Korhonen M, Laitinen L, Quaranta V, Gould VE: Immunohistochemical localization of integrins in normal, hyperplastic neoplastic breast. Am J Pathol 1991, 139:787-799 [PMC free article] [PubMed] [Google Scholar]
- 19.Pignatelli M, Cardillo MR, Hanby A, Stamp GW: Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol 1992, 23:1159-1166 [DOI] [PubMed] [Google Scholar]
- 20.Jonjic N, Lucin K, Krstulja M, Iternicka Z, Mustac E: Expression of beta-1 integrins on tumor cells of invasive ductal breast carcinoma Pathol Res Pract 1993, 189:979-984 [DOI] [PubMed] [Google Scholar]
- 21.Liapis H, Flath A, Kitazawa S: Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol 1996, 5:127-135 [DOI] [PubMed] [Google Scholar]
- 22.Friedrichs K, Ruiz P, Franke F, Gille I, Terpe HJ, Imhof BA: High expression level of alpha 6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res 1995, 55:901-906 [PubMed] [Google Scholar]
- 23.Natali PG, Nicotra MR, Botti C, Mottolese M, Bigotti A, Segatto O: Changes in expression of alpha6/beta 4 integrin heterodimer in primary and metastatic breast cancer. Br J Cancer 1992, 66:318-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S: Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res 1998, 4:407-410 [PubMed] [Google Scholar]
- 25.Duffy MJ, Reilly D, O’Sullivan C, O’Higgins N, Fennelly JJ, Andreasen P: Urokinase-plasminogen activator, a new and independent prognostic marker in breast cancer. Cancer Res 1990, 50:6827-6829 [PubMed] [Google Scholar]
- 26.Gaffney PJ, Cooke DA, Burnand KG: Urokinase and its receptor: marker of malignancy? Adv Exp Med Biol 1994, 360:187-191 [DOI] [PubMed] [Google Scholar]
- 27.Grondahl-Hansen J, Hilsenbeck SG, Christensen IJ, Clark GM, Osborne CK, Brünner N: Prognostic significance of PAI-1 and uPA in cytosolic extracts obtained from node-positive breast cancer patients. Breast Cancer Res Treat 1997, 43:153-163 [DOI] [PubMed] [Google Scholar]
- 28.Duffy MJ, Duggan C, Maguire T, Mulcahy K, Elvin P, McDermott E, Fennelly JJ, O’Higgins N: Urokinase plasminogen activator as a predictor of aggressive disease in breast cancer. Enzyme Protein 1996, 49:85-93 [DOI] [PubMed] [Google Scholar]
- 29.Duggan C, Kennedy S, Kramer MD, Barnes C, Elvin P, McDermott E, O’Higgins N, Duffy MJ: Plasminogen activator inhibitor type 2 in breast cancer. Br J Cancer 1997, 76:622-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly D, Andreasen P, Duffy MJ: Studies on plasminogen activator inhibitor 1 levels in human breast cancer. Biochem Soc Trans 1990, 18:354-355 [DOI] [PubMed] [Google Scholar]
- 31.Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA: Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest 1997, 100:58-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werb Z: ECM and cell surface proteolysis: regulating cellular ecology. Cell 1997, 91:439-442 [DOI] [PubMed] [Google Scholar]
- 33.Grondahl-Hansen J, Peters HA, van Putten WLJ, Look MP, Pappot H, Ronne E, Danø K, Klijn JGH, Brünner N, Foekens JA: Prognostic significance of the receptor for urokinase plasminogen activator in breast cancer. Clin Cancer Res 1995, 1:1079-1087 [PubMed] [Google Scholar]
- 34.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA: Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem 1994, 269:32380-32388 [PubMed] [Google Scholar]
- 35.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA: Regulation of integrin function by the urokinase receptor. Science 1996, 273:1551-1555 [DOI] [PubMed] [Google Scholar]
- 36.Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle UH, Majdic O, Bartke I, Knapp W, Stockinger H: Urokinase plasminogen activator receptor, beta 2-integrins Src-kinases within a single receptor complex of human monocytes. J Exp Med 1995, 181:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sitrin RG, Todd RF, Albrecht E, Gyetko MR: The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Invest 1996, 97:1942-1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon DI, Rao NK, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L, Chapman HA: Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood 1996, 88:3185-3194 [PubMed] [Google Scholar]
- 39.Xue W, Kindzelski AL, Todd RE, Petty HR: Physical association of complement type 3 receptor and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol 1994, 152:4630-4640 [PubMed] [Google Scholar]
- 40.Yebra M, Parry GCN, Stromblad S, Mackman N, Rosenberg S, Mueller BM, Cheresh DA: Requirement of receptor-bound urokinase-type plasminogen activator for integrin alphavbeta5-directed cell migration. J Biol Chem 1996, 271:29393-29399 [DOI] [PubMed] [Google Scholar]
- 41.Simon DI, Wei Y, Zhang L, Rao NK, Xu H, Chen Z, Liu Q, Rosenberg S, Chapman HA: Identification of a urokinase receptor-integrin interaction site promiscuous regulator of integrin function. J Biol Chem 2000, 275:10228-10234 [DOI] [PubMed] [Google Scholar]
- 42.Waltz DA, Fujita RM, Yang X, Natkin L, Zhuo S, Gerard CJ, Rosenberg S, Chapman HA: Nonproteolytic role for the urokinase receptor in cellular migration in vivo. Am J Respir Cell Mol Biol 2000, 22:316-322 [DOI] [PubMed] [Google Scholar]
- 43.Carriero MV, Del Vecchio S, Capozolli M, Franco P, Fontana L, Zannetti A, Botti G, D’Aiuto G, Salvatore M, Stoppelli MP: Urokinase receptor interacts with αvβ5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res 1999, 59:5307-5314 [PubMed] [Google Scholar]
- 44.Cailleau R, Young R, Olivé M, Reeves WJ: Breast tumor cell lines from pleural effusions. J Natl Cancer Inst 1974, 53:661-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroon ME, Koolwijk P, van Goor H, Weidle UH, Collen A, van der Pluijm G, van Hinsbergh VW: Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol 1999, 154:1731-1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arguello F, Baggs RB, Frantz CN: A murine model of experimental metastasis to bone and bone marrow. Cancer Res 1988, 48:6876-6881 [PubMed] [Google Scholar]
- 47.Sasaki A, Boyce BF, Story B, Wright KR, Chapman M, Boyce R, Mundy GR, Yoneda T: Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Res 1995, 55:3551-3557 [PubMed] [Google Scholar]
- 48.van der Pluijm G, Löwik C, Papapoulos S: Tumour progression and angiogenesis in bone metastasis from breast cancer: new approaches to an old problem Cancer Treat Rev 2000, 26:11-27 [DOI] [PubMed] [Google Scholar]
- 49.van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos S, Löwik C: Monitoring metastatic behavior of human tumor cells in mice with species-specific PCR: elevated expression of angiogenesis and bone resorption stimulators by breast cancer in bone metastases. J Bone Miner Res 2001, 16:1077-1091 [DOI] [PubMed] [Google Scholar]
- 50.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 51.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW: Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998, 77:887-894 [DOI] [PubMed] [Google Scholar]
- 52.Papapoulos SE, Hamdy NAT, van der Pluijm G: Bisphosphonates in the management of prostate cancer metastatic to the skeleton. Cancer 2000, 88:3047-3053 [DOI] [PubMed] [Google Scholar]
- 53.Klemke RL, Yebra M, Bayna EM, Cheresh DA: Receptor tyrosine kinase signaling required for integrin alpha v beta5-directed cell motility but not adhesion on vitronectin. J Cell Biol 1994, 127:859-866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stahl A, Mueller BM: The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol 1995, 129:335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solberg H, Rømer J, Brünner N, Holm A, Sidenius N, Danø K, Høyer-Hansen G: A cleaved form of the receptor for urokinase-type plasminogen activator in invasive transplanted human and murine tumors. Int J Cancer 1994, 58:877-881 [DOI] [PubMed] [Google Scholar]
- 56.Luther T, Magdolen V, Albrecht S, Kasper M, Riemer C, Kessler H, Graeff H, Müller M, Schmitt M: Epitope-mapped monoclonal antibodies as tools for functional and morphological analyses of the human urokinase receptor in tumor tissue. Am J Pathol 1997, 150:1231-1244 [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with alpha v beta 3. Cell 1996, 85:683-693 [DOI] [PubMed] [Google Scholar]
- 58.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA: Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 1998, 92:391-400 [DOI] [PubMed] [Google Scholar]
- 59.Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, Cardiff RD: CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol 1998, 176:206-215 [DOI] [PubMed] [Google Scholar]