Abstract
Decreased expression of the epithelial cell adhesion protein E-Cadherin occurs in several forms of human epithelial-derived cancers, including bladder cancers. We investigated the possibility that aberrant methylation of the CpG island flanking the 5′ transcriptional start site of the e-cadherin gene is responsible for the decreased expression of this gene in bladder cancer, similar to the relationship previously seen between e-cadherin methylation and gene expression in other types of human cancers. Using methylation-specific polymerase chain reaction, we found methylation of this CpG island in 20 of 47 cases (43%) of bladder neoplasms ranging from low-grade papillary neoplasms to advanced, invasive cancers. When methylation status was compared to immunochemical staining for E-Cadherin, we found significantly diminished levels of E-Cadherin expression in 14 of 15 cases (93%) with methylation of the gene. We also found decreased expression of E-Cadherin, although to a somewhat lesser extent, in a high percentage (77%) of the cases without methylation of the gene. Although these data suggest a relationship between e-cadherin CpG island methylation and decreased gene expression, it evident that other mechanisms also contribute to decreased expression of this gene in bladder neoplasia. Remarkably, we also found low levels of e-cadherin methylation in urothelial cells from three of nine (33%) histologically normal bladders, with all three of the normal bladder samples with methylated e-cadherin being from individuals older than 70 years of age. Thus, methylation of the e-cadherin CpG island may occur normally in this tissue with aging as well as in low-grade papillary neoplasms, and is not specific to cancer in the bladder. This finding of methylation in normal urothelial cells from elderly individuals is provocative with respect to a possible link between aging and increased risk for bladder cancer, but it suggests limitations on the usefulness of using methylation of e-cadherin as a molecular marker for detection of bladder cancer.
The E-Cadherin (E-Cad) transmembrane glycoprotein modulates calcium-dependent intercellular adhesion in a variety of epithelial tissues. The e-cadherin gene is mutated in the germline of some families with genetic predisposition to gastric cancers 1 and somatic mutations are common in lobular breast cancers and some gastric and gynecological cancers. 2-4 In many other common human cancers, including cancers of the breast, prostate, colon, stomach, esophagus, pancreas, thyroid, head and neck, and bladder, levels of E-Cad protein are greatly reduced compared to normal epithelial tissues. 5,6 The loss of E-Cad expression seems to be involved in invasive and metastatic properties of neoplastic cells, 7 consistent with the function of a tumor suppressor gene.
The structure of the e-cadherin gene is notable for a dense CpG island that flanks the 5′ transcriptional start site. Decreased expression of the e-cadherin gene has been linked to aberrant methylation of this CpG island in several common forms of human cancer 8-12 but, while E-Cad protein expression has been previously reported to be significantly decreased in bladder cancers, 13-17 no previously published study has investigated the potential role of methylation in causing loss of E-Cad expression in this form of cancer. Characterizing the extent of e-cadherin methylation in bladder cancer is of interest from the standpoint of understanding the pathogenesis of this disease, and also because aberrant methylation, detectable by sensitive, polymerase chain reaction-based methods, might be used as a marker for early detection of cancer in tissue and fluid specimens. 18 We therefore undertook an investigation to determine the extent of e-cadherin methylation in bladder neoplasia, including low-grade papillary lesions as well as malignant neoplasms, and to study the possible relationship of aberrant methylation to decreased expression of the gene.
Materials and Methods
Tissues and Microdissection
Genomic DNA was extracted from 47 formalin-fixed paraffin-embedded biopsy samples of primary bladder neoplasms, including 13 cases of papillomas and papillary neoplasms of low malignant potential, 10 cases of papillary cancers (low grade and high grade), 6 cases of invasive papillary cancer, 8 cases of flat urothelial carcinoma in situ, and 10 cases of invasive urothelial carcinoma (not otherwise specified). In addition, nine normal, nonneoplastic bladder samples were taken from autopsy cases. Histology sections were cut for each case at 5-μm thickness followed by standard hematoxylin and eosin staining. Mechanical microdissection was performed to remove contaminating stromal and inflammatory cells. 19
Methylation-Specific Polymerase Chain Reaction (MSP)
Cells recovered from microdissection were transferred into Nonidet P-40 tissue digestion buffer (0.5 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L Tris-HCl, 0.5% Nonidet P-40) containing 10 μl Proteinase K (20 mg/ml; Life Technologies. Rockville, MD) and incubated at 37°C for 16 hours. DNA was then isolated and bisulfite treated for 16 hours as previously described. 20 Sodium bisulfite-treated DNA was recovered using microcon centrifugal filter devices (Millipore Corp., Bedford, MA) according to manufacturer’s recommendations. Targeting the CpG island spanning the E-cadherin transcriptional start site, we used polymerase chain reaction (MSP) primers specific for methylated DNA (upstream, TAATTAGCGGTACGGGGGGC; downstream, CGAAAACAAACGCCGAATACG) and unmethylated DNA (upstream, TTAGTTAATTAGTGGTATGGGGGGTGG; downstream, ACCAAACAAAAACAAACACCAAATACA) to amplify the bisulfite-modified DNA. The MSP reactions were performed as previously described. 20
Sequencing Bisulfite-Modified DNA
Confirmatory sequencing of bisulfite-modified DNA was conducted after amplification with two sets of overlapping methylation-independent primer pairs (upstream no. 1, GTAGGTGAATTTTTAGTTAATTAG; downstream no. 1, AAACTCACAAAAACTTTACAATTC; upstream no. 2, GAATTGTAAAGTATTTGTGAGTTT; downstream no. 2, ACTCCAAAAACCCATAACTAAC) that were selected to avoid CpG sites. Polymerase chain reaction products were cloned (Invitrogen, Carlsbad, CA) followed by sequencing with standard reagents (USB, Cleveland, OH).
Immunohistochemistry
E-Cad protein expression was evaluated in formalin-fixed paraffin-embedded primary tumors by immunohistochemistry. Five-μm sections were treated with DAKO target retrieval solution (DAKO, Carpinteria, CA) for 30 minutes according to the manufacturer’s recommendations and then incubated for 2 hours with an anti-human-E-Cad mouse monoclonal antibody (Sigma, St. Louis, MO) at a concentration of 0.04 μg/ml, using an automated slide stainer (DAKO). Secondary reagents (LSAB2 secondary reagent system) were supplied by DAKO and used according to the manufacturer’s specifications. The extent of staining (ie, percentage of cancer cells staining positive) was scored as 4+ (>90%), 3+ (60 to 95%), 2+ (30 to 60%), 1+ (5 to 30%), or 0 (<5%). Intensity of staining in the cancer cells was scored relative to normal urothelial cells as 4+ (equal to normal), 3+ (decreased by < 30%), 2+ (decreased by 30 to 60%), 1+ (decreased by 60 to 95%), or 0 (no staining). The two scores were then averaged for a single score to represent E-Cad expression. Scoring was conducted in a blinded manner by two pathologist observers (SM and EG), and differences in scoring (never greater than one score difference) were resolved by a concurrent review and consensus score.
Results and Discussion
As we originally hypothesized, the e-cadherin gene is methylated in a significant number of bladder neoplasms and this methylation is associated with decreased E-Cad expression. Using MSP, we found 20 of 43 bladder neoplasm samples (43%) to have methylated alleles of e-cadherin. We validated results of the MSP assay by bisulfite sequencing of DNA cloned from three of the cases in which we detected hypermethylated e-cadherin. The sequenced clones, shown in Figure 1B ▶ , demonstrated dense hypermethylation of the CpG island, with aberrant methylation of CpG sites between the MSP primer sequences as well as those CpG sites within the primer sequences.
Figure 1.

Methylation of e-cadherin in bladder neoplasms. A–C: MSP analysis of methylation at the CpG island flanking the transcriptional start site of the e-cadherin gene. After bisulfite modification, each sample is amplified using primers specific for methylated (M) and unmethylated (U) sequences. A: The results of seven bladder neoplasms with methylation. B: The results of seven bladder neoplasms without methylation. C: The results of six normal control bladder samples. The MDA-MB231 breast cancer cell line was used as a positive control for a methylated gene. D: The bisulfite sequencing of clones of the e-cadherin promoter from two of the bladder cancers with methylation detected by MSP and from a normal unmethylated bladder e-cadherin gene. Arrows designate positions of cytosines in the sequence that, when unmethylated, are converted to thymidines by bisulfite.
Methylation of e-cadherin was found in lesions thought to represent early stages of bladder cancer progression, such as noninvasive papillary neoplasms and flat urothelial carcinoma in situ, as well as the more advanced invasive cancers. For example, methylation was seen in 4 of 13 (31%) lesions classified as papilloma or papillary neoplasia of low malignant potential, 3 of 10 (30%) papillary cancers (low grade or high grade) without invasion, and 3 of 6 (50%) papillary cancers with invasion. Because of the small number of invasive bladder cancers evaluated in this study, we cannot evaluate a possible trend of e-cadherin methylation increasing with malignant progression of bladder cancer, analogous to the situation we observed previously for malignant progression of breast cancer. 21 However, our data show convincingly that this epigenetic alteration is not limited to advanced levels of bladder neoplasia.
An unexpected finding in this study was the detection of methylation of e-cadherin in the normal control samples that were taken from the three oldest individuals tested, aged 71, 82, and 92 years (Figure 1C) ▶ . Only unmethylated forms of e-cadherin were detected in the other six apparently normal control samples, taken from individuals aged 27, 34, 43, 51, 60, and 67 years. These control samples were all examined histologically during the microdissection procedure, and no neoplastic lesions were recognized. Furthermore, no decrease in E-Cad expression was recognized by immunohistochemical staining of these samples. We interpret these findings to reflect age-related gene methylation of the e-cadherin gene in bladder epithelium, similar to what has been previously reported for the estrogen receptor (ER) and IGF2 genes in colonic mucosa of aged individuals. 22,23
These results are intriguing from the aspect of a possible link between aging, e-cadherin methylation, and increased risk for bladder cancer. However, all bladder cancer patients studied were relatively elderly (mean age, 79.8 years for cancer patients with methylated e-cadherin; mean age, 77.9 years for cancer patients without methylated e-cadherin) and thus it is not possible to determine whether methylation of e-cadherin in bladder neoplasms is restricted to neoplasms arising in elderly patients. Importantly, our finding of methylation of the e-cadherin gene in normal urothelium from elderly individuals suggests that this marker may not be sufficiently specific for use as a clinical assay to detect bladder cancer in urine, particularly because the methylation of apparently normal cells occurs in the same patient population that would be targeted by a screening test.
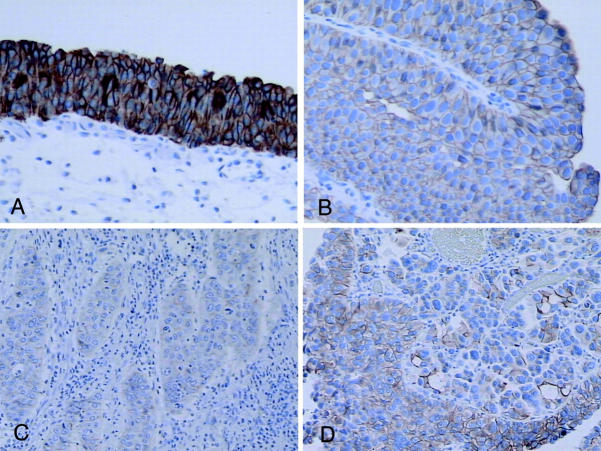
Expression of the E-Cad protein was measured by staining sections (41 cases) with specific E-Cad monoclonal antisera. This antibody selectively stains normal epithelium, including urothelial epithelium, in a characteristic membrane-specific pattern. All nine of the normal bladder mucosa samples, as well as normal urothelial epithelium adjacent to the neoplasms, studied showed strong, uniform staining for E-Cadherin (Figure 2A) ▶ . A similar level of strong, diffuse cell-membrane staining for e-cadherin was seen in only 2 of 41 samples of bladder neoplasms, both being cases with no detectable methylation of the e-cadherin gene. Although some of the bladder neoplasms studied have uniformly diminished staining for E-Cad (Figure 2, B and C) ▶ , many of the cancers have focal and incomplete loss of E-Cad expression. (Figure 2D) ▶ . Heterogeneous staining for E-Cad expression such as this has been reported previously for breast cancer, 24,25 but not for bladder cancer.
Figure 2.
Immunohistochemical staining for E-Cadherin in normal bladder epithelium (A) and bladder cancers (B–D). The papillary cancer in B was scored as 2+ (1+ for intensity and 3+ for extent) and the infiltrating cancer in C was scored as 1+ (both extent and intensity). D: An infiltrating cancer with heterogeneous staining for E-Cadherin (scored as 2+ for both extent and intensity). All neoplasms (B–D) have methylation of e-cadherin.
The comparison of MSP measurements of e-cadherin methylation and immunohistochemical measurements of E-Cad expression is summarized in Table 1 ▶ . Strong expression of E-Cad (3+ or 4+) is seen in 5 of 19 bladder neoplasms without methylation of the gene, but only in only 1 of 15 bladder neoplasms with methylation of e-cadherin. Conversely, nearly all bladder neoplasms with e-cadherin methylation (14 of 15) have recognizably diminished staining for E-Cad. Many bladder neoplasms without e-cadherin methylation (13 of 19) also have reduced expression of E-Cad, although to a significantly lesser extent than those cases with e-cadherin methylation (unpaired t-test significance of immunochemistry staining scores, assuming unequal variances, is 0.029). We did not note any significant correlation between methylation and tumor morphology, stage, or histological grade.
Table 1.
Immunohistochemistry-Staining Scores for Neoplasms with Methylated E-cadherin (A) and Only Unmethylated E-cadherin (B)
| IHC Score | 0–1 | 2 | 3 | 4 |
|---|---|---|---|---|
| A: Cases with methylation | ||||
| Papillary non-invasive | 4 | 2 | – | – |
| In situ flat | 2 | – | – | – |
| Papillary invasive | 3 | – | – | – |
| Invasive NOS | 2 | 1 | 1 | – |
| B: Cases without methylation | ||||
| Papillary non-invasive | 2 | 10 | 2 | 1 |
| In situ flat | – | – | – | – |
| Papillary invasive | 2 | – | – | – |
| Invasive NOS | 3 | 2 | 1 | 1 |
Notably, as seen in Figure 1A ▶ , methylation of e-cadherin in bladder neoplasia is heterogeneous; ie, unmethylated alleles, as well as methylated alleles, were seen in all of the samples with e-cadherin methylation. All tissues for our study were carefully microdissected before analysis and, although it is possible that DNA from contaminating normal stromal cells contributed to the unmethylated signals in some cases, it is unlikely that these strong unmethylated signals were entirely because of contaminating normal cells. Our results are therefore most suggestive of heterogeneous methylation of the e-cadherin promoter in bladder neoplasia.
Thus, our results indicate that both methylation of e-cadherin and expression of the E-Cad protein are heterogeneous in bladder neoplasia. This heterogeneity is similar to that previously observed in breast cancer, 26 suggesting that heterogeneous epigenetic alterations of e-cadherin may be even more common in neoplasia than uniform loss of functional protein resulting from gene mutations. Heterogeneous methylation of the e-cadherin promoter may reflect a plasticity of phenotype with respect to cell adhesion that is not possible when the gene is mutated. Supporting this hypothesis, our previous studies found increased e-cadherin methylation in cells selected for invasion in an in vitro assay, and dramatically decreased methylation when the same cells were subsequently cultured as spheroids. 26 By analogy, low expression of E-Cad could allow dissociation of individual cells from the primary tumor mass for invasion or metastasis, and restoration of E-Cad expression in the invasive or metastatic cells could allow these cells to re-establish cadherin-mediated intercellular adhesion and signaling.
Although our data are significant for the association of e-cadherin methylation with reduced E-Cad expression, the aberrant gene promoter methylation cannot explain all loss of E-Cadherin expression in bladder cancer. Indeed, this is also the case for other types of human cancers; loss of E-Cad expression in breast cancer, for example, occurs frequently even in neoplasms without e-cadherin methylation. 8 Recent studies with breast cancer cell lines have found a correlation between endogenous E-Cad expression and activity of the e-cadherin promoter in these cells as measured with a reporter gene construct. 27 Thus, defects in trans-activating pathways, as well as methylation of the gene promoter, seem to be capable of reducing levels of E-Cad expression.
Footnotes
Address reprint requests to Edward Gabrielson, M.D., Johns Hopkins Oncology Center, 418 N. Bond St., Baltimore, MD 21231. E-mail: egabriel@jhmi.edu.
References
- 1.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE: E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392:402-405 [DOI] [PubMed] [Google Scholar]
- 2.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H: E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994, 54:3845-3852 [PubMed] [Google Scholar]
- 3.Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, Cornelisse C: E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 1996, 13:1919-1925 [PubMed] [Google Scholar]
- 4.Risinger JI, Berchuck A, Kohler MF, Boyd J: Mutations of the E-cadherin gene in human gynecologic cancers. Nat Genet 1994, 7:98-102 [DOI] [PubMed] [Google Scholar]
- 5.Ohene-Abuakwa Y, Pignatelli M: Adhesion molecules in cancer biology. Adv Exp Med Biol 2000, 465:115-126 [DOI] [PubMed] [Google Scholar]
- 6.Behrens J: Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 1999, 18:15-30 [DOI] [PubMed] [Google Scholar]
- 7.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 8.Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, Baylin SB: E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 1995, 55:5195-5199 [PubMed] [Google Scholar]
- 9.Melki JR, Vincent PC, Clark SJ: Concurrent DNA hypermethylation of multiple genes in acute myeloid leukemia. Cancer Res 1999, 59:3730-3740 [PubMed] [Google Scholar]
- 10.Graff JR, Greenberg VE, Herman JG, Westra WH, Boghaert ER, Ain KB, Saji M, Zeiger MA, Zimmer SG, Baylin SB: Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle’s cell, and poorly differentiated human thyroid carcinoma. Cancer Res 1998, 58:2063-2066 [PubMed] [Google Scholar]
- 11.Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, Silverberg SG, Nishizuka S, Terashima M, Motoyama T, Meltzer SJ: E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst 2000, 92:569-573 [DOI] [PubMed] [Google Scholar]
- 12.Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S: Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995, 92:7416-7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakatsuki S, Watanabe R, Saito K, Saito T, Katagiri A, Sato S, Tomita Y: Loss of human E-cadherin (ECD) correlated with invasiveness of transitional cell cancer in the renal pelvis, ureter and urinary bladder. Cancer Lett 1996, 103:11-17 [DOI] [PubMed] [Google Scholar]
- 14.Syrigos KN, Krausz T, Waxman J, Pandha H, Rowlinson-Busza G, Verne J, Epenetos AA, Pignatelli M: E-cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer 1995, 64:367-370 [DOI] [PubMed] [Google Scholar]
- 15.Lipponen PK, Eskelinen MJ: Reduced expression of E-cadherin is related to invasive disease and frequent recurrence in bladder cancer. J Cancer Res Clin Oncol 1995, 121:303-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA: Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res 1993, 53:3241-3245 [PubMed] [Google Scholar]
- 17.Rebel JM, Thijssen CD, Vermey M, Delouvee A, Zwarthoff EC, Van der Kwast TH: E-cadherin expression determines the mode of replacement of normal urothelium by human bladder carcinoma cells. Cancer Res 1994, 54:5488-5492 [PubMed] [Google Scholar]
- 18.Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG: Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA 1998, 95:11891-11896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii H, Zhou W, Gabrielson E: Detection of frequent allelic loss of 6q23–q25.2 in microdissected human breast cancer tissues. Genes Chromosom Cancer 1996, 16:35-39 [DOI] [PubMed] [Google Scholar]
- 20.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB: Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996, 93:9821-9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, Graff JR: Aberrant methylation of the estrogen receptor and E-cadherin 5′ CpG islands increases with malignant progression in human breast cancer. Cancer Res 2000, 60:4346-4348 [PubMed] [Google Scholar]
- 22.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB: Methylation of the oestrogen receptor CpG island links aging and neoplasia in human colon. Nat Genet 1994, 7:536-540 [DOI] [PubMed] [Google Scholar]
- 23.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP: Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res 1998, 58:5489-5494 [PubMed] [Google Scholar]
- 24.Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ: Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol 1996, 105:394-402 [DOI] [PubMed] [Google Scholar]
- 25.Moll R, Mitze M, Frixen UH, Birchmeier W: Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol 1993, 143:1731-1742 [PMC free article] [PubMed] [Google Scholar]
- 26.Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG: Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 2000, 275:2727-2732 [DOI] [PubMed] [Google Scholar]
- 27.Ji X, Woodard AS, Rimm DL, Fearon ER: Transcriptional defects underlie loss of E-cadherin expression in breast cancer. Cell Growth Differ 1997, 8:773-778 [PubMed] [Google Scholar]