Abstract
The expression, cellular distribution, and activity of PIP2-specific phospholipase C (PLC) in healthy human gastric-mucosa cells have been recently studied in our laboratories and a direct evidence for an almost exclusive expression of PLC β isoforms, with the exception of PLC β4, has been provided. These results addressed our attention to possible modification of PLC expression and activity during neoplastic transformation of the human gastric mucosa. In the present article we present results indicating that PLC δ2 is markedly expressed in type II intestinal metaplasia and in the adenocarcinoma whereas traces of other PLC isoforms were sometime detected. Interestingly, we found that type I intestinal metaplasia was in the majority of the cases PLC δ2-negative, but when expressed, this type of metaplasia generally considered as benignant, always evolved toward neoplastic transformation. These results therefore readdress the question of surveillance of the patients with type I intestinal metaplasia and suggest that PLC δ2 expression might be a possible marker of gastric malignant transformation.
The involvement of phospholipase C (PLC) in the mechanisms regulating the activity of the gastric mucosa cells has been recently proposed. 1,2 Pharmacological trials have indicated that the modulation of inositol triphosphate production and consequently of Ca++ mobilization may influence gastric secretion. 3 In addition PLC has also been reported to play a role in the cytoprotection activated after damaging concentrations of deoxycholate in human gastric cells. 4 Moreover, studies performed on rabbit antrum have provided important clues toward the existence in gastric myocytes of hormone-specific receptors coupled to phosphoinositide signaling pathways. 5 Very recently we have investigated the expression and activity of PIP2-specific PLC in healthy human gastric-mucosa cells providing direct evidence for an almost exclusive expression of the PLC β family, with the exception of PLC β4, and at the same time supplying a cellular cartography of each represented isoform of this family. 6 These results prompted us to investigate whether the expression and the activity of the PLC isoforms vary during neoplastic transformation of the human gastric mucosa. Intestinalization of the gastric mucosa is a widely demonstrated reaction to external injury that may increase cancer risk at long term, therefore studies aimed at highlighting histochemical or cytochemical features implicated with such a risk would be of help because the identification of premalignant hallmarks might address early therapeutic strategies. Thus, the goal of this study was to investigate the expression of the PLC isoforms in the human metaplastic, dysplastic, and neoplastic gastric mucosa cells.
Materials and Methods
Tissue
Tissues were obtained from gastric biopsies performed in 16 healthy consenting donors and 45 informed patients, 25 of whom were affected by type I intestinal metaplasia (IM).
Protein Fractionation, Immunoprecipitation, and Western Blotting
Cell homogenates were resuspended in lysis buffer (10 mmol/L Tris-HCl buffer, pH 7.4, 1% Nonidet P-40, 150 mmol/L NaCl, 1 mg/ml bovine serum albumin, 1 mmol/L vanadate, 50 mmol/L sodium fluoride) and left on ice for 30 minutes. Cell lysates (20 μg of protein) were separated through 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and incubated for 1 hour at room temperature with rabbit polyclonal anti-PLC β1, β2, β3, β4, γ1, γ2, δ1, and δ2 antibodies (δ3 and δ4 not yet commercially available) (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). For anti-PLC immunoprecipitation, cell lysates (400 μg of proteins) were incubated at 4°C for 60 minutes with anti-PLC β1, β2, β3, β4, γ1, γ2, δ1, and δ2 antibodies previously coupled to magnetic beads coated with secondary antibodies. Immunocomplexes were collected by a magnet and washed several times with RIPA buffer (phosphate-buffered saline containing 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) in the presence of protease inhibitors.
PIP2-Specific PLC Activity Assay
PIP2-specific PLC activity was investigated using a [3H]-labeled PIP2 exogenous substrate containing equal amounts of dried cold PIP2 and ]3H[ PIP2 (specific activity, 30,000 dpm/nmol; Amersham). Briefly, 50 μg of protein from homogenated human gastric biopsies or, when required, equal amounts of immunocomplexes were incubated in 3 nmol [3H]-PIP2 (as exogenous substrate), 150 mmol/L NaCl, 10 mmol/L 2-(N-morpholino) ethane-sulfonic acid, 0.06% taurodeoxycholate, and 10 mmol/L CaCl2 at 37°C for 30 minutes. Inositol lipid extraction was performed in chloroform/methanol/chloric acid (1:2:0.1). After two washes in methanol/1 mol/L chloric acid (1:1), the lipid phase was chromatographed on silica gel plates in chloroform/methanol/ammonium hydroxide/water (45:35:2:8) and spots, identified using PIP2 standards, were scraped off and counted by liquid scintillation.
Immunohistochemical Analysis
Sections were prepared from biopsies performed in the gastric antrum. Diagnosis of dysplasia or neoplasia were made on the bases of the 1998 Padova and Sidney International Classifications, respectively. 7,8 Deparaffinized sections were hydrated into distilled water through a series of graded alcohols (ethanol 100, 95, and 70%). Endogenous peroxidases were inhibited by washing for 15 minutes in distilled water-H2O2 3%. The slides were then incubated with rabbit polyclonal IgG anti-PLC: β1, (G-12), β2 (Q-15), β3 (C-20), β4 (C-18), γ1 (530), γ2 (Q-20), δ1 (C-19), and δ2 (C-19) (Santa Cruz Biotechnology) at the dilution of 1:125 in TBS (10 mmol/L Tris-HCl, pH 8.0; 150 mmol/L NaCl) for 90 minutes at room temperature. TBS was used in negative controls instead of the primary antibodies. The immunoperoxidase assay was performed with an ultrastain polyvalent-horseradish peroxidase immunostaining kit and the antibody localization was detected by the AEC kit (YLEM, Rome, Italy), according to the manufacturer’s suggestions. The sections were washed with water, counterstained with Mayer’s hemallume for few minutes, and then rinsed copiously in tap water. Glycerinated gelatin (Sigma, St. Louis, MO) was used for coverslipping the sections. The histological diagnosis was blindly performed by three different observers.
Results
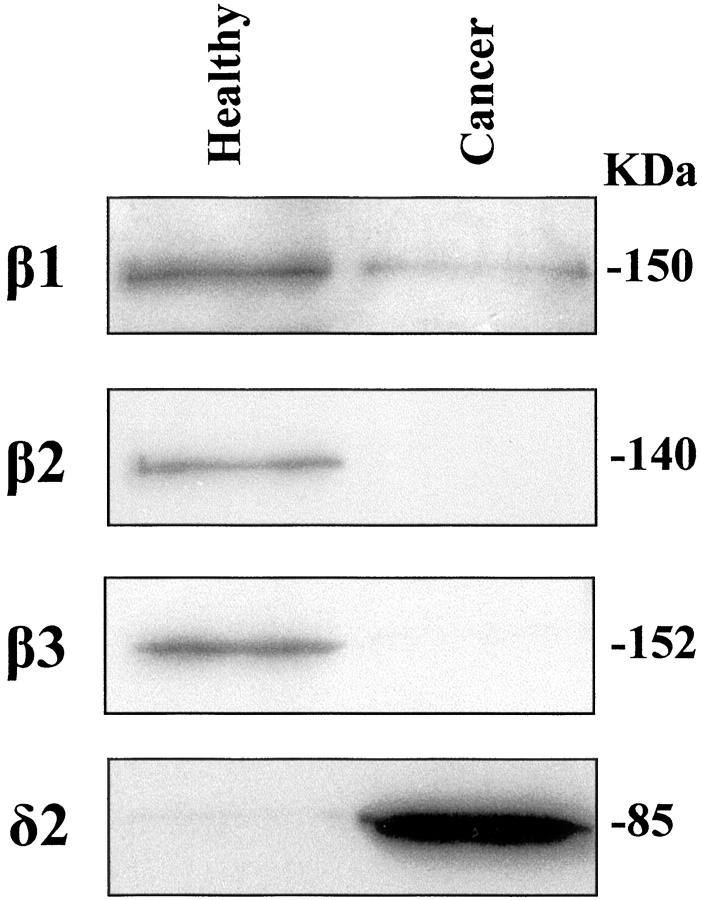
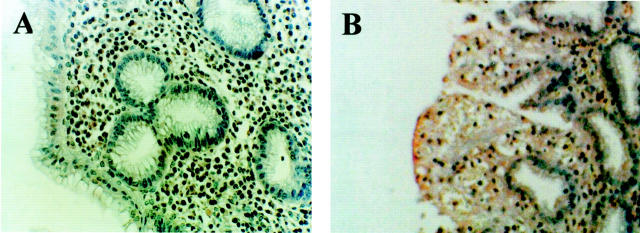
Because previous data obtained from an in vitro PIP2-specific PLC activity assay performed on human healthy whole mucosa homogenates indicated an evident involvement of the phosphoinositide machinery in gastric cell metabolism, 6 we sought to identify which PLC isoforms were involved in the hydrolytic activity in human neoplastic gastric cells. Therefore we performed an in vitro assay on gastric cell extracts after sequential immunoprecipitation of each PLC isozyme by means of appropriate antibodies. Results indicated that the hydrolytic activity in the PLC γ1/PLC γ2 or in the PLC β family-deprived extracts was highly comparable to that in total homogenates, whereas in the PLC β1/PLC β2-deprived extracts activity was nearly absent when compared to whole cell extracts (Table 1) ▶ . The same assay performed with collected immunoprecipitated PLC δ1/PLC δ2 isoforms yielded a level of hydrolytic activity very similar to the one measured in the total homogenates, whereas very scarce or no activity was noticed in PLC γ and in PLC β immunocomplexes. Further sequential extraction of PLC δ1 from PLC δ1/PLC δ2 immunocomplexes showed that the main contribution to the PLC activity was accounted for PLC δ2 (Table 2) ▶ . The histochemical analysis performed on the gastric mucosa from patients affected by type II IM dysplasia or adenocarcinoma revealed a dramatic shift of the PLC isoform expression when compared to the healthy samples. Indeed, in the human gastric mucosa undergoing neoplastic transformation only a marked PLC δ2 reaction was assessed (Figure 1E) ▶ . The specificity of the PLC δ2 reaction was strongly corroborated by the finding that in microscopic fields displaying both neoplastic and normal glands, PLC δ2 reaction was clearly assessed only in the neoplastic cells (Figure 1 ▶ ; B–E), essentially confined to the cytoplasmic compartment as evidenced by higher microscopic magnification (Figure 2) ▶ . These results were confirmed by the Western blot analysis that showed a high recovery of PLC δ2 in neoplastic gastric mucosa when compared to the expression of the other isoforms (Figure 3) ▶ . Of interest was the finding that the gastric mucosa affected by type II IM was PLC δ2-positive (Figure 1D) ▶ whereas the type I IM was PLC δ2-negative (Figure 1C) ▶ with the exception of eight patients that displayed an evident PLC δ2 expression (Table 3 ▶ ; Figure 1D ▶ ). To assess whether PLC δ2 was normally expressed in the human healthy intestinal mucosa we also looked for this PLC isoform in the duodenum mucosa and results confirmed that PLC δ2 is primarily represented in the intestinal mucosa (not shown). Of note, PLC δ2 was not expressed in the mucosa affected by inactive gastritis (Figure 4A) ▶ , whereas pictures of dysplastic gastritis (gastric ulcer) were found positive for PLC δ2 (Figure 4B) ▶ .
Table 1.
PIP2-Specific PLC Activity from Whole Cell Extracts and from PLC γ1, γ2, δ1, δ2, and β1, β2, β3, and β4-Deprived Extracts
| Nmol of 3H-PIP2 hydrolyzed/50 μg proteins | |
|---|---|
| Whole cell extracts | 415 ± 43 |
| PLC γ1, PLC γ2-deprived extracts | 379 ± 31 |
| PLC δ1, PLC δ2-deprived extracts | 61 ± 8 |
| PLC β1, β2, β3, β4-deprived extracts | 388 ± 47 |
Data are the mean of three separate experiments ±SD.
Table 2.
PIP2-Specific PLC Activity from PLC γ1, γ2, δ1, δ2, β1, β2, β3, and β4 Complexes Immunoprecipitated from 50-μg Protein Whole Cell Extract
| Nmol of 3H-PIP2 hydrolyzed/ immunocomplex | |
|---|---|
| PLC γ1, γ2 | 27 ± 4 |
| PLC δ1, δ2 | 361 ± 33 |
| PLC δ1 | 39 ± 5 |
| PLC β1, β2, β3, β4 | 47 ± 8 |
Data are the mean of three separate experiments ±SD.
Figure 1.
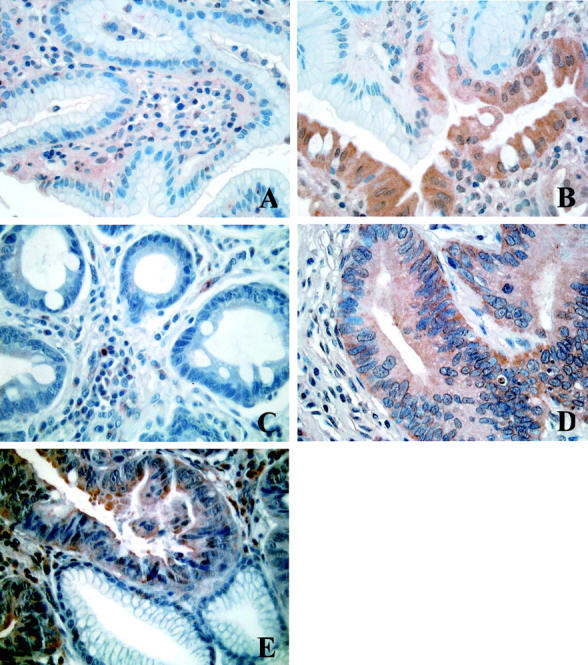
Immunohistochemical analysis of PLC δ2 expression in human gastric healthy (A), type I IM (PLC δ2-positive) (B), type I IM (PLC δ2-negative) (C), type II IM (D), and gastric adenocarcinoma (E). Note in B and E the specificity of the reaction in different glands of the same microscopic field.
Figure 2.
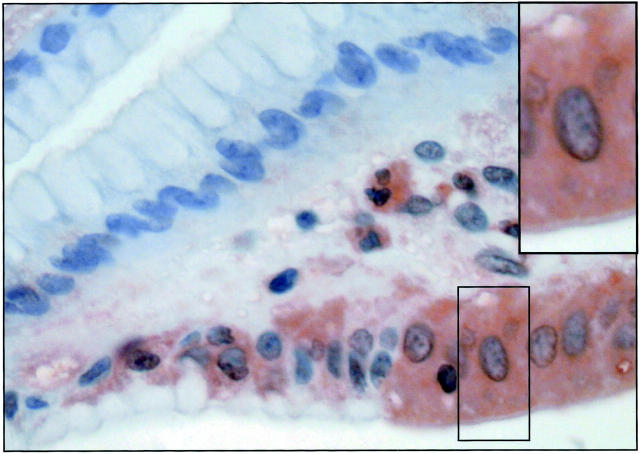
Magnified section of gastric cancer cells. Location of PLC δ2 is almost totally confined to the cytoplasmic compartment.
Figure 3.
Western blot analysis of PLC isoform expression in human healthy and cancer gastric homogenates (30 μg). PLC δ1, PLC γ1, PLC γ2, and PLC β4 were not detectable either in healthy or in cancer samples.
Table 3.
Gastric Antrum Biopsy
| No. | Age, yr | Male | Female | Expression of PLC-δ2 | Follow-up (three years) | |||
|---|---|---|---|---|---|---|---|---|
| 40–60 | 60–70 | 70–80 | ||||||
| Healthy donors | 16 | 5 | 7 | 4 | 12 | 4 | Negative | – |
| Type I IM | 17 | 9 | 6 | 2 | 12 | 5 | Negative | Type I IM (14), Gastric ulcer (3) |
| Type I IM | 8 | 4 | 3 | 1 | 6 | 2 | Positive | Carcinoma* |
| Type II IM | 10 | 3 | 4 | 3 | 8 | 2 | Positive | Carcinoma |
| Carcinoma | 7 | 1 | 4 | 2 | 5 | 2 | Positive | – |
*P < 0.0001.
Site of biopsy: gastric antrum. The site was documented by monitored endoscopy.
Figure 4.
Immunohistochemical analysis of PLC δ2 expression in human inactive gastritis (A) and in gastric ulcer (B). The expression of the enzyme is slightly but clearly detectable only in the ulcer tissue.
Discussion
The occurrence of a signal machinery regulated by receptor coupled via distinct G proteins to different effector enzymes, including PIP2-specific PLC has already been reported in gastric structures 2-5 and recently we have provided evidence that the PLC β is almost exclusively expressed and engaged in the healthy human gastric mucosa cells. 6 The PLC family includes three types (β, γ, and δ) and counts at least 10 isoforms whose expression and activity vary depending on cell type or tissue. 9-11 In this article we report for the first time to our knowledge, results indicating that the specific expression of the PLC β isoforms in the healthy human gastric mucosa is dramatically replaced by a unique expression of PLC δ2 during the neoplastic transformation. Investigations of histochemical alterations in the human gastric mucosa, with a view to identifying particular premalignant patterns provided, to date, conflicting results. The Padova Classification 7 of gastric dysplasia and related lesions have proposed five broad categories reflecting the degree of certainty that can be achieved with the histopathological material available: 1) negative for dysplasia, 2) indefinite for dysplasia, 3) noninvasive neoplasia, 4) suspicious for invasive carcinoma, and 5) invasive adenocarcinoma. The first group includes both normal tissue and IM. Although IM may increase cancer risk at long term, the metaplastic glands are generally not considered neoplastic. 12 Nevertheless, attention has been paid to possible features that may have implications concerning gastric cancer development. 13 Two types of IM are conventionally classified: type I also called “complete IM,” which is thought to need no requirement of surveillance of the patient and type II also named “incomplete IM,” which is generally believed to increase cancer risk. Our results demonstrate that PLC δ2 expression characterizes the metaplastic and neoplastic evolution of human gastric mucosa and readdress the question of the surveillance of the patient affected by type I IM. Indeed in a consistent number of patients (8 over 25) with areas of type I IM PLC δ2-positive, the follow-up revealed in all cases the development of a gastric cancer, whereas type I IM PLC δ2-negative sometime associated to gastric ulcer but thus far (3 years from the type I IM diagnosis), never to neoplastic evolution. These findings would indicate PLC δ2 as a possible hallmark predictive of neoplastic transformation in the human gastric mucosa. In this context, the absence of PLC δ2 expression in the inactive gastritis and the discrete expression of this enzyme in the gastric ulcer sharply could suggest for PLC δ2 a role of possible indicator of neoplastic evolution. The relationship between PLC overexpression and neoplasia has been already suggested in different cell types because PLC γ1 has been found overexpressed in human colorectal cancer 14 and familial adenomatous polyposis 15 even though the factors that cause such an overexpression remain uncovered, whereas PLC β3 has been implicated in neuroendocrine gastroenteropancreatic tumors. 16 In this regard a polymerase chain reaction analysis would be of great help in defining the genetic picture for the complete understanding of the role of PLC δ2 in gastric cancer, but, thus far, sequences of this isoform are not available. Nevertheless the results here presented highlight the almost unique involvement of PLC δ2 in the neoplastic transformation of the human gastric mucosa and in this context the possibility that the expression of this enzyme in type I IMs could represent a predictive marker of cancer evolution should be taken into account. The signaling pathway implicated with the PLC δ2 overexpression remains obviously to be defined. Certainly the current challenge is to clarify the nature and dynamics of the membrane-enzyme microinterface and their relation. In higher plants the δ isoforms are involved in the response to nutritional and environmental stresses, particularly cyclin-dependent growth control, but the details of what regulates this PLC are unknown. 17 The lack of information about the physiological meaning of this isoform, addressed our laboratories to work oriented to design PLC δ2 sequence in the attempt to develop early prognostic support and related therapeutic strategies.
Footnotes
Address reprint requests to Prof. Sebastiano Miscia, Department of Biomorphology, Via dei Vestini 6, 66100 Chieti, Italy. E-mail: s.miscia@morpho.unich.it.
Supported by Italian MURST Confin ’99.
References
- 1.Kitsukawa Y, Turner RJ, Pradan TK, Jensen RT: Gastric chief cells possess NK1 receptors which mediate pepsinogen secretion and are regulated by agents that increase cAMP and phospholipase C. Biochim Biophys Acta 1996, 13:105-116 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S, Okabe S: Stimulatory effects of sacralfate on secretion and synthesis of mucus by rabbit gastric mucosal cells. Involvement of phospholipase C. Dig Dis Sci 1996, 41:498-504 [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Gantz I, Del Valle J: Histamine H2 receptor activates adenylate cyclase and PLC via separate GTP-dependent pathways. Am J Physiol 1996, 271:613-620 [DOI] [PubMed] [Google Scholar]
- 4.Kokoska ER, Smith GS, Wolff AB, Deshpande Y, Rieckenberg CL, Banan A, Miller TA: Role of calcium in adaptive cytoprotection and cell injury induced by deoxycholate in human gastric cells. Am J Physiol 1998, 275:322-330 [DOI] [PubMed] [Google Scholar]
- 5.Rodier G, Magous R, Mochizuki T, le Nguyen D, Bali JP, Bataille D, Jarrousse C: Glicentin and oxyntomodulin modulate both the phosphoinositide and cyclic adenosine monophosphate signalling pathways in gastric myocytes. Endocrinology 1999, 140:22-24 [DOI] [PubMed] [Google Scholar]
- 6.Di Baldassarre A, Marchisio M, Felaco M, Antonucci A, Centurione L, Grilli A, Di Valerio V, Cutroneo G, Schiavone C, Miscia S, Ianetti G: Histochemical and biochemical analysis of phospholipase C isoforms in normal human gastric mucosa cells. Anat Rec 2001, 262:440-444 [DOI] [PubMed] [Google Scholar]
- 7.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddel RH, Sipponen P, Watanabe H: Gastric dysplasia. Am J Surg Pathol 2000, 24:166-176 [DOI] [PubMed] [Google Scholar]
- 8.Dixon MF, Genta RM, Yardley JH, Correa P, : and the participants in the International Workshop on the Histopathology of Gastritis, Houston 1994: Classification and grading of gastritis. Am J Surg Pathol 1996, 20:1161-1181 [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG, Bae YS: Regulation of phosphoinositide specific phospholipase C isozymes. J Biol Chem 1997, 272:15046-15048 [DOI] [PubMed] [Google Scholar]
- 10.Miscia S, Di Baldassarre A, Cataldi A, Rana R, Di Pietro R, Bosco D, Grilli A, Amerio G, Sabatino G: Immunocytochemical localization of phospholipase C isozymes in cord blood and adult T-lymphocytes. J Histochem Cytochem 1999, 47:929-935 [DOI] [PubMed] [Google Scholar]
- 11.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana R, Vitale M, Di Valerio V, Manzoli FA: Inefficient phospholipase C activation and reduced Lck expression characterize the signal defect of umbilical cord T lymphocytes. J Immunol 1999, 163:2416-2424 [PubMed] [Google Scholar]
- 12.Chen VW, Abu-Elyazeed RR, Zavala DE: Risk factors of precancerous lesions in a high Colombian population. I Salt Nutr Cancer 1990, 13:59-65 [DOI] [PubMed] [Google Scholar]
- 13.Rugge M, Leandro G, Farinati F: Gastric epithelial dysplasia. How clinicopathologic background relates to management. Cancer 1995, 76:376-382 [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Lee SD, Park JG, Kim CM, Ryu SH, Suh PG: Overexpression of phospholipase C-gamma-1 in colorectal carcinomas is associated with overexpression of factors that bind its promoter. J Biol Chem 1995, 270:16378-16384 [DOI] [PubMed] [Google Scholar]
- 15.Park JG, Lee YA, Kim SS, Park KJ, Noh DY, Ryn SH, Suh PG: Overexpression of phospholipase C gamma 1 in familial adenomatous polyposis. Cancer Res 1994, 54:2240-2244 [PubMed] [Google Scholar]
- 16.Oberg K: Biological aspect of neuroendocrine gastro-enteropancreatic tumors. Digestion 1996, 57:42-44 [DOI] [PubMed] [Google Scholar]
- 17.Rebecchi MJ, Pentyala SN: Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 2000, 80:1291-1335 [DOI] [PubMed] [Google Scholar]