Abstract
Previous publications demonstrated that elevated systemic levels of interleukin (IL)-8 decrease local neutrophil recruitment. We tested whether sustained, high plasma levels of IL-8 would prevent local inflammation after inflammatory insults. Mice carrying the transgene for human IL-8 were separated on the basis of their plasma levels of IL-8 into IL-8-positive (plasma levels >90 ng/ml) and IL-8-negative (IL-8 below detection). Presence of the IL-8 transgene did not improve survival or morbidity nor did it alter peritoneal neutrophil recruitment induced by the cecal ligation and puncture model of sepsis. In an acute lung injury model created by intratracheal injection of acid, IL-8-positive mice showed no reduction in alveolar neutrophil recruitment. There was no difference in the local recruitment of neutrophils when either thioglycollate or glycogen was injected intraperitoneally. We examined the chemotactic response to murine chemokines to test how neutrophil recruitment occurs in the setting of elevated plasma IL-8 and found that neutrophils from both IL-8-positive and -negative mice respond equally well to recombinant KC or macrophage inflammatory protein (MIP)-2. We measured KC and MIP-2 in the peritoneum after thioglycollate injection and demonstrated that IL-8-positive mice have significantly higher levels of the chemokines compared to the IL-8-negative mice. Antibody inhibition of KC and MIP-2 in the IL-8-positive mice significantly decreased peritoneal neutrophil recruitment in response to thioglycollate, clarifying their important role in the local neutrophil recruitment. Our data demonstrate that despite the presence of high plasma levels of IL-8, neutrophils may still be recruited to sites of local inflammation because of chemokine redundancy.
Interleukin 8 (IL-8) belongs to a class of cytokines referred to as chemokines. These are small molecular weight peptides, usually <12,000 d that are divided into broad categories based on their protein structure. The CXC chemokines have two pairs of cysteine residues separated by an intervening amino acid whereas the CC chemokines have the cysteine residues located adjacent to one another. Other members of the chemokine family include the CX3C and C groups. 1,2 The CXC chemokines serve to recruit neutrophils to local sites of inflammation. 3 The exact murine homolog of IL-8 has not been defined, but two candidate genes are KC and macrophage inflammatory protein 2 (MIP-2). 4
The chemokines have several functions but one of their major attributes is the ability to induce chemotaxis of neutrophils. 5,6 With this property chemokines serve to bring these cells to sites of acute inflammation, and also to retain them once they have arrived. A portion of the mechanism responsible for this recruitment is the generation of a chemotactic/haptotactic gradient to draw the cells into the local environment. 7 Previous reports have demonstrated that high intravascular levels of IL-8 will reduce neutrophil emigration to sites of local inflammation. 8-10
Mice carrying the human IL-8 transgene have extremely high plasma levels of this chemokine. 11 Rodent neutrophils will respond to human IL-8 by chemotaxis although a higher concentration is required. 12 We sought to test whether continuous high plasma concentrations of IL-8 would reduce neutrophil recruitment in four different animal models of acute inflammation. Given the redundancy of chemokines 1,2 we investigated whether the murine chemokines would become up-regulated to offset the high plasma IL-8 levels and allow recruitment of neutrophils to the sites of inflammation. To specifically test if local up-regulation of endogenous murine chemokines was responsible for neutrophil recruitment, we performed antibody inhibition studies.
Materials and Methods
Characterization of the Transgenic Mice
A breeding colony of mice was established from mice generously donated by Scott Simonet of Amgen, Inc. (Thousand Oaks, CA). These mice (HE8 line) carry the human IL-8 transgene and express high, systemic levels of IL-8 in the plasma. 11 Hybrid mice were obtained by crossing the IL-8 transgenic mice with BDF1 mice (Charles Rivers, Portage, MI). At ∼8 weeks of age the plasma levels of IL-8 were determined by obtaining a small sample of blood from the tail vein. IL-8 was determined using our previously described enzyme-linked immunosorbent assay (ELISA). 13 Mice were divided on the basis of their plasma levels of IL-8 into negative mice (IL-8-negative), which had undetectable levels of IL-8 (<200 pg/ml), and positive mice (IL-8-positive), which had >90,000 pg/ml of IL-8. A third group of mice was also evaluated, wild-type BDF1. In the unmanipulated mice, a complete blood count with differential was performed. Additionally, the lungs were homogenized and the myeloperoxidase (MPO) content of the lung was determined as a measure of neutrophil sequestration, as previously described. 14 Briefly, the chest cavity was exposed and the right side of the heart perfused with 0.9% isotonic saline (normal saline) to remove blood from pulmonary circulation. The right lung was then removed and placed on ice in MPO homogenization buffer, the lung was homogenized followed by sonication. After centrifugation the supernatant was mixed with assay buffer and read in a kinetics mode on an ELISA reader.
Models of Acute Inflammation
The experiments in this article were performed in accordance with National Institutes of Health guidelines and approval from the Animal Use Committee of the University of Michigan.
Cecal Ligation and Puncture (CLP)
CLP was performed as previously described with minor modifications. 15,16 Mice were anesthetized with ketamine/xylazine (87 and 13 mg/kg of body weight, respectively) and a small midline abdominal incision was made. The cecum was isolated and punctured three times with a 16-gauge needle that resulted in 60 to 70% lethality. The needle was removed and a small amount of the stool was expelled from the punctures to ensure patency. The mice were closed using 4.0 sutures for the abdomen and stainless steel removable wound clips for the skin. After the surgery, mice were injected subcutaneously with 1 ml of prewarmed isotonic saline before being placed into their cage. Mice were treated with subcutaneous antibiotics (23 mg/kg of imipenem diluted in D5W) at 2 hours after surgery. This dose was repeated every 12 hours for a total of 3 days so that mice were receiving both antibiotics and fluids. Mice were kept in a temperature-controlled room (25°C) with light and dark cycles every 12 hours.
Collection of Data
Mice were sacrificed 24 hours after CLP, before they received their third antibiotic injection. At the time of sacrifice heparin anti-coagulated plasma was collected and stored for later cytokine analysis. A complete blood count was performed with a Coulter Counter (model Zf; Coulter Electronic, Hialeah, FL). Peritoneal washes were performed by opening the peritoneum sterilely and washing with 1 ml of 1× Hanks’ balanced salt solution. This was followed with a separate wash of 15 ml with 1× Hanks’ balanced salt solution. The 1-ml wash was centrifuged and supernatant was frozen for later analysis. Cells from the centrifugation were then combined with cells from the 15-ml wash. White blood cell differentials were obtained by staining with Diff-Quick (Baxter, Detroit, MI). The right lung was harvested for MPO determination. Blood was obtained from the tail vein and an automated differential was performed using a Hemavet Mascot (CDC Technologies, Oxford, CT) as previously described. 17
Preparation of Recombinant Murine Chemokines and ELISAs
Recombinant murine KC and MIP-2 were prepared in a bacterial expression system and the recombinant protein isolated from glutathione-Sepharose columns (pGEX system; Pharmacia, Piscataway, NJ) as previously described. 18,19 The purified KC or MIP-2 protein was >95% pure as judged by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. These purified proteins will induce neutrophil chemotaxis in mouse or human neutrophils and they were used to raise rabbit or goat polyclonal antibodies by immunization with Freund’s complete adjuvant or Titermax (Sigma Chemical Co., St. Louis, MO). The antibodies were used to prepare ELISAs for protein quantification using our previously published methods. 13 These antibodies have specificity with no cross-reactivity in the ranges used. 18 A polyclonal antibody to KC was also raised in a goat as previously described. 19
Cytokine Analysis
Cytokines were measured in the peritoneal fluid, bronchoalveolar lavage, and plasma. Tumor necrosis factor (TNF) was determined by cytolytic activity directed against the WEHI 164 fibrosarcoma cells as previously described. 20,21 The concentration of TNF was determined by calculation from a standard curve of recombinant human TNF (Cetus, Emmeryville, CA). IL-6 was measured by the B9 cell proliferation assay as previously described 22 and the IL-6 concentrations determined from a standard curve of human recombinant IL-6. KC and MIP-2 were quantified by an ELISA developed in our laboratory according to our published methods. 13,18 Briefly, polyclonal antibodies were purified on protein A columns and used as capture antibodies. A portion of the same antibody was biotinylated and used for the detection antibody. Streptavidin-HRP (Jackson Immunoresearch, West Grove, PA) was used for amplification and the color was developed with TMB solution (Genzyme, Cambridge, MA). Neutrophil chemotaxis was performed using a 48-well chemotaxis chamber (Neuroprobe, Cabin John, MD) and previously described methods. 18,23 Neutrophils were prepared from the peripheral blood of either the IL-8-positive or IL-8-negative mice by dextran sedimentation and hypotonic lysis of residual red blood cells. Chemotaxis was expressed as the percentage of cells that migrated to 10−6 mol/L fMLP (Sigma), after background subtraction.
In Vitro Stimulation
Alveolar macrophages or peritoneal cells were harvested from either the IL-8-positive or IL-8-negative mice. The cells were incubated in RPMI 1640 with 1% fetal calf serum at a concentration of 1.5 × 10 5 cells/ml (alveolar macrophages) or 5 × 10 5 cells/ml (peritoneal cells). The cells were stimulated with 1 μg/ml of lipopolysaccharide and the supernatants collected after 24 hours.
Acid Lung Injury
Acid lung injury was induced by injection of 80 to 100 μl of diluted HCl, pH 1.5, following a previously described procedure. 24,25 Isotonic 0.9% saline was diluted 1:3 and the pH adjusted to 1.5 with concentrated HCl. Mice were anesthetized with ketamine/xylazine, the mice vertically suspended and the trachea exposed. Under direct visualization, the acid was instilled into the trachea using a 30-gauge needle. This was done with multiple small injections with the animal spontaneously breathing between each injection. Control mice had 0.9% isotonic saline (normal saline) injected in place of diluted acid. Immediately after surgery the neck was closed with a stainless steel surgical clip. At the time of sacrifice, heparin anti-coagulated plasma was obtained and saved for cytokine analysis. The lungs were then lavaged with 1 ml of warmed Hanks’ balanced salt solution in 0.2- to 0.3-ml aliquots. This bronchoalveolar lavage fluid was centrifuged and the supernatant saved for cytokine analysis. The lungs were lavaged with a second ml of Hanks’ balance salt solution and the cell pellets combined. A cell count was performed, and a differential done on a Diff-Quik-stained cytospins.
Glycogen or Thioglycollate Peritonitis
Glycogen was prepared fresh as a 2% solution (oyster shell glycogen, Sigma) in sterile isotonic saline. Thioglycollate (2.9%; Difco, Detroit, MI) was prepared and autoclaved. Either thioglycollate or glycogen was injected intraperitoneally in a 1- to 2-ml volume, as previously described. 18,19 Four hours later mice were sacrificed and the peritoneum washed as described in the CLP model. Heparin anti-coagulated plasma was also obtained.
Antibody Neutralization Studies
For these experiments mice were injected subcutaneously with 1 ml of 50:50 mixture of goat anti-KC plus rabbit anti-MIP-2. These antibodies have previously been described and will effectively neutralize murine chemokines in vivo. 19 For controls, mice were injected with 1 ml of control goat serum plus control rabbit serum. Two hours later, the mice were injected with 2 ml of thioglycollate. Samples were harvested 4 hours later and the total number of peritoneal neutrophils determined as described above.
Statistical Analysis
The values for the IL-8-positive mice were compared to the IL-8-negative mice using the Student’s t-test.
Results
Normal Parameters
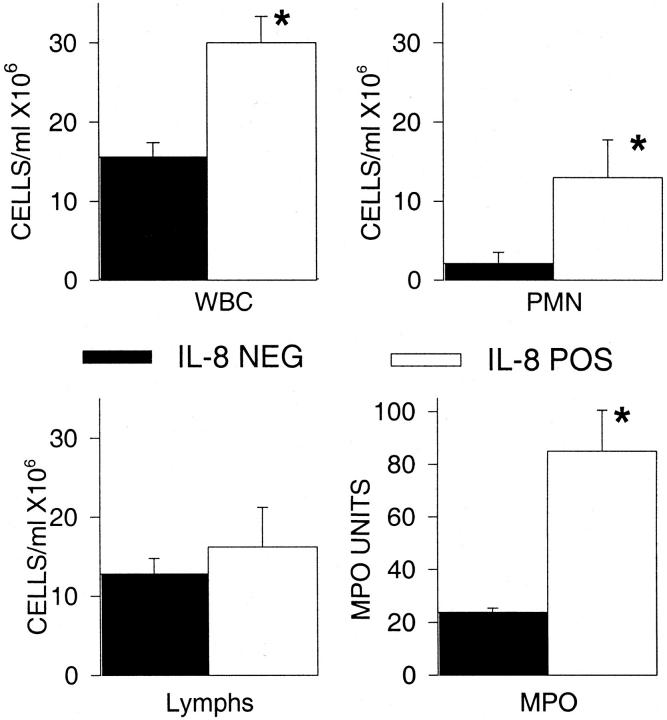
We evaluated several normal parameters to differentiate the IL-8-positive mice from the IL-8-negative mice. First, the differences in plasma levels of IL-8 were significantly different, but because this was the criterion used to separate the two populations this was the expected result. IL-8-positive mice had a significantly higher white blood cell count because almost exclusively of the increased numbers of neutrophils (Figure 1) ▶ . In a separate experiment, we examined the peripheral blood values of nontransgenic mice and IL-8-negative mice. The absolute number of peripheral blood neutrophils in the nontransgenic mice (2.37 ± 0.54 × 10 6 cells/ml) was nearly identical to the IL-8-negative mice (2.35 ± 0.41 × 10 6 cells/ml). Pulmonary MPO demonstrated substantially more neutrophils within the lung. Histological examination of the lungs showed that the neutrophils were within the blood vessels and not in the alveolar airspaces. These results confirm the previous report that the IL-8 transgenic mice have evidence of increased numbers of neutrophils both in the circulating blood and within the tissues. 11 They also indicate that mice will respond to the human IL-8 transgene because there are increased numbers of neutrophils.
Figure 1.
Pathophysiological differences between IL-8-positive and IL-8-negative mice. Mice were divided into positive and negative groups based on the plasma levels of IL-8. The IL-8-positive mice had a significantly higher WBC that was because of neutrophils. Additionally, there were increased neutrophils within the lungs as indicated by an elevated MPO. Each value is the mean ± SEM for 16 mice for the hematology parameters and 9 mice for the MPO. *, P < 0.05 IL-8-positive compared to IL-8-negative.
Effect of IL-8 Transgene on CLP Morbidity and Mortality
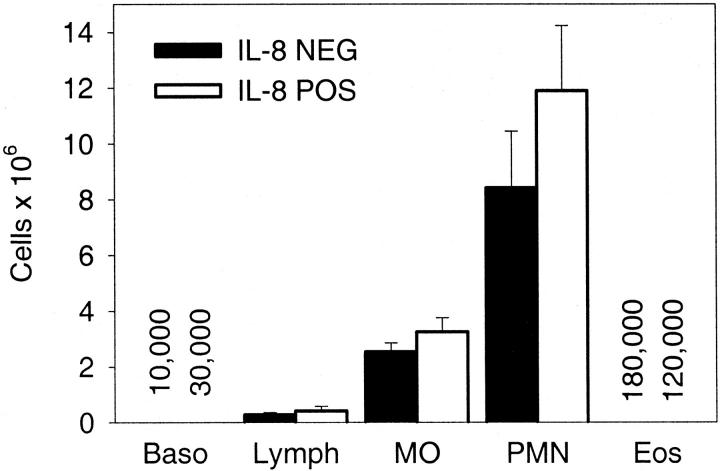
Mice were subjected to CLP and followed for 7 days. There was a slightly better survival in the IL-8-positive mice, but this was not significant based on log-rank survival analysis (P > 0.05). In the IL-8-positive mice there were 15 out of 34 survivors, whereas in the IL-8-negative mice there were 10 out of 37 survivors. Because neutrophils represent a critical component of the innate immune response to bacterial infections we determined if there was adequate recruitment of neutrophils into the peritoneum in response to severe inflammation. Mice were subjected to CLP, treated with antibiotics, and sacrificed at 24 hours. Figure 2 ▶ shows that there was no difference between the IL-8-positive and IL-8-negative mice with regards to the total number of recruited neutrophils. There was also no difference in the number of macrophages, lymphocytes, basophils, or eosinophils recovered from the peritoneum.
Figure 2.
Peritoneal cells 24 hours after CLP. The total number of cells in the peritoneum was determined in both the IL-8-positive and IL-8-negative mice. There was no difference in the number of neutrophils in the peritoneum. Each value is the mean ± SEM of 15 IL-8-negative mice and 18 IL-8-positive mice. There were very few basophils or eosinophils present in the peritoneum, so the actual number of cells is listed with the number on the left representing the IL-8-negative mice.
Acid Lung Injury
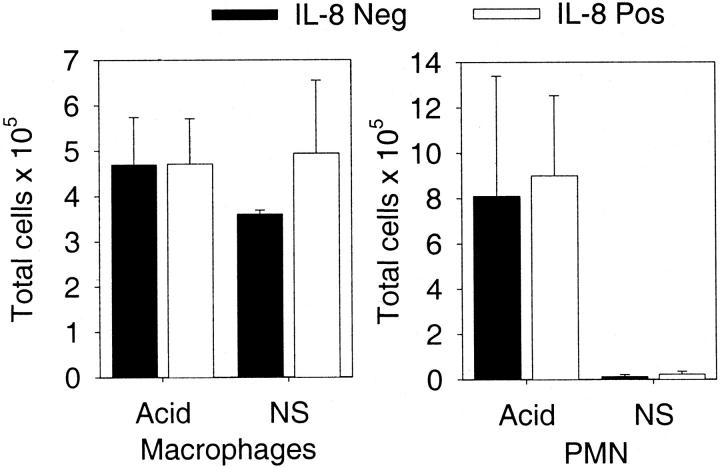
Aspiration of gastric contents may result in acute lung injury. 26 To mimic this scenario, we injected diluted HCl (pH 1.5) intratracheally and then determined the number of neutrophils recruited into the bronchoalveolar space. Examination of histological sections showed that neutrophils were in the alveolar space. Kinetics studies showed that in the first 8 hours there was a modest influx of neutrophils, but by 15 hours >40% of the cells in the lavage fluid were neutrophils. There were no significant differences between IL-8-positive and IL-8-negative mice (P > 0.05). At the 0-hour time point there were no neutrophils recovered in the bronchoalveolar lavage fluid. These findings are in contrast to the total lung MPO data that showed greater activity in the IL-8-positive mice (Figure 1) ▶ . Alveolar neutrophil recruitment was examined more closely in Figure 3 ▶ where the total number of cells in the lung was quantitated. The number of neutrophils was substantially greater in response to acid injection compared to isotonic saline (normal saline), but there was no difference between IL-8-positive and IL-8-negative mice.
Figure 3.
Pulmonary cellular constituents 15 hours after acid lung injury. The total number of macrophages or neutrophils in the lung was determined in response to intratracheal injection of either acid or isotonic saline (normal saline, NS). Acid resulted in a substantial increase in the number of neutrophils recovered in the lavage fluid, with no difference between the IL-8-positive and IL-8-negative mice. Each value is the mean ± SEM for eight IL-8-positive and eight IL-8-negative mice injected with acid and four IL-8-positive and three IL-8-negative mice injected with isotonic saline.
Peritoneal Neutrophil Recruitment to Thioglycollate or Glycogen
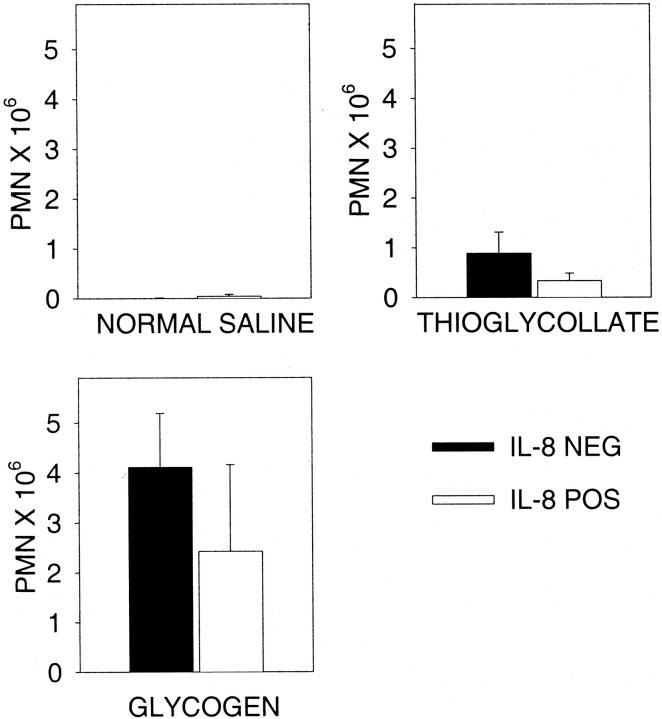
Intraperitoneal injection of thioglycollate has been widely used for the elicitation of neutrophils in rodents. 27 Mice were sacrificed 4 hours after intraperitoneal injection (control mice received isotonic saline) and the total number of peritoneal cells determined. Isotonic saline injection resulted in virtually no peritoneal neutrophil recruitment (Figure 4) ▶ . These data parallel the lung data in which intratracheal injection of isotonic saline did not result in pulmonary neutrophil recruitment. Additionally, no detectable IL-8 was present in the peritoneal lavage fluid from either the IL-8-negative or IL-8-positive mice. In contrast, injection of either glycogen or thioglycollate resulted in neutrophil recruitment. In both models there were fewer neutrophils recruited in IL-8-positive mice similar to the previous report using these mice, 11 but this difference was never statistically significant (P > 0.05). We have previously shown that the peritoneal recruitment of neutrophils in response to 3% thioglycollate is mediated by local production of both KC and MIP-2. 19 Thioglycollate injection did not cause any increase in the local levels of IL-8, which is expected because the IL-8 is under constitutive control.
Figure 4.
Peritoneal neutrophil recruitment in response to thioglycollate or glycogen. Mice were sacrificed 4 hours after injection of isotonic saline (normal saline), thioglycollate, or glycogen and peritoneal cells recovered. Both thioglycollate and glycogen recruited substantial numbers of cells to the peritoneum, with no difference between the IL-8-positive and IL-8-negative mice. Each value is the mean ± SEM for eight to nine mice for either thioglycollate or glycogen.
To investigate the inflammatory mediators produced after each of these inflammatory challenges, we measured TNF and IL-6 in both the plasma and at the site of inflammation. Table 1 ▶ displays the data and demonstrates that there was no difference between IL-8-negative and IL-8-positive mice. Table 1 ▶ also illustrates trends in these different models of inflammation. CLP represents the greatest inflammatory insult and has the highest levels of TNF and IL-6. It should be noted that these samples were obtained at different times after the onset of the injury and may not represent the peak values. In general, local cytokine levels were higher than systemic levels with the exception of CLP, as previously reported. 28,29
Table 1.
Systemic and Local Cytokine Levels after Injury
| TNF pg/ml | IL-6 ng/ml | |||||||
|---|---|---|---|---|---|---|---|---|
| Cytokines 24 hours after CLP* | ||||||||
| IL-8 | Blood | Peritoneum | Blood | Peritoneum | ||||
| Negative | 42 ± 19 | 29 ± 7 | 284 ± 111 | 105 ± 44 | ||||
| Positive | 118 ± 34 | 62 ± 16 | 260 ± 116 | 57 ± 25 | ||||
| Cytokines 15 hours after acid lung injury | ||||||||
| IL-8 | Blood | BAL | Blood | BAL | ||||
| Negative | 8.7 ± 4.2 | 7.0 ± 5.1 | 0.98 ± 0.26 | 2.2 ± 1.3 | ||||
| Positive | 6.5 ± 6.5 | 77 ± 68 | 0.45 ± 0.09 | 0.83 ± 0.3 | ||||
| Cytokines 4 hours after thioglycolate | ||||||||
| IL-8 | Blood | Peritoneum | Blood | Peritoneum | ||||
| Negative | BDL | BDL | 0.21 ± 0.09 | 3.3 ± 1.6 | ||||
| Positive | BDL | 89 ± 53 | 0.39 ± 0.17 | 11.2 ± 5.7 | ||||
*Cytokines were measured in the blood, peritoneal wash, or bronchoalveolar lavage as indicated in the tables. Each value is the mean ± SEM.
BDL, below detection limit (4 pg/ml).
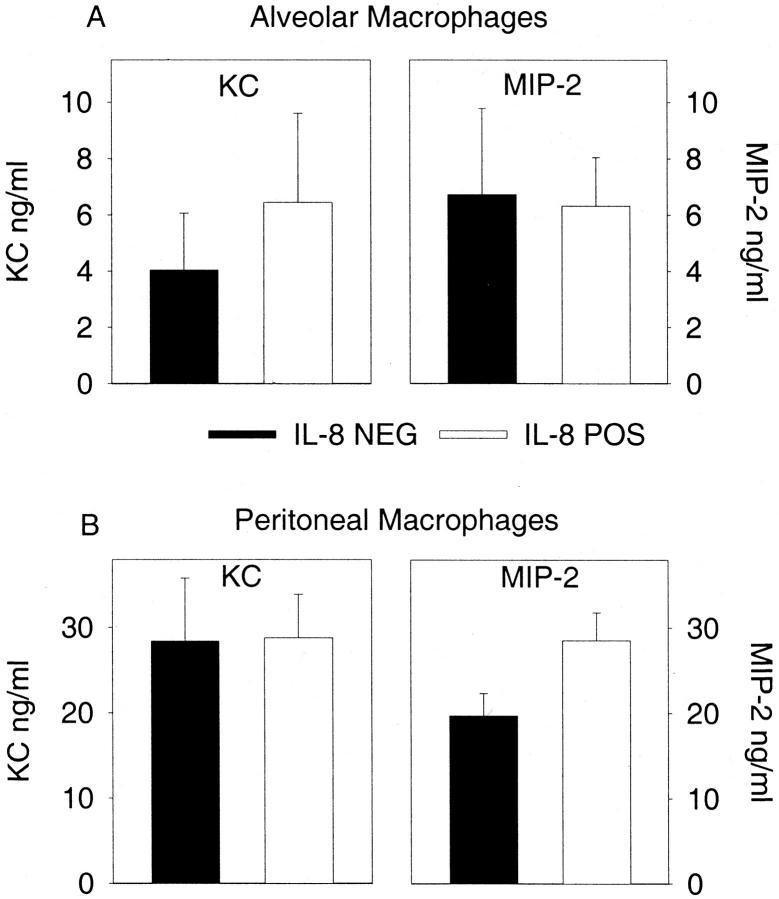
The data indicate that neutrophils will be recruited to the local site of inflammation even in the presence of elevated plasma levels of IL-8. This suggests the possibility that additional chemotactic factors were produced in the local compartments to elicit neutrophils. We tested the ability of alveolar and peritoneal macrophages from both IL-8-positive and IL-8-negative mice to produce the murine chemokines KC and MIP-2 after in vitro stimulation with 1 μg/ml of lipopolysaccharide. Figure 5 ▶ demonstrates that there was no significant difference in the inherent ability of IL-8-positive or IL-8-negative macrophages to produce the murine chemokines.
Figure 5.
Chemokine production by alveolar and peritoneal macrophages. Cells were isolated from IL-8-negative or IL-8-positive mice and stimulated in vitro with lipopolysaccharide and murine chemokines were measured in the supernatant after 24 hours. There are no differences between the IL-8-positive and IL-8-negative mice. A: Alveolar macrophages. B: Peritoneal macrophages. Each value is the mean ± SEM for cells isolated from six individual mice.
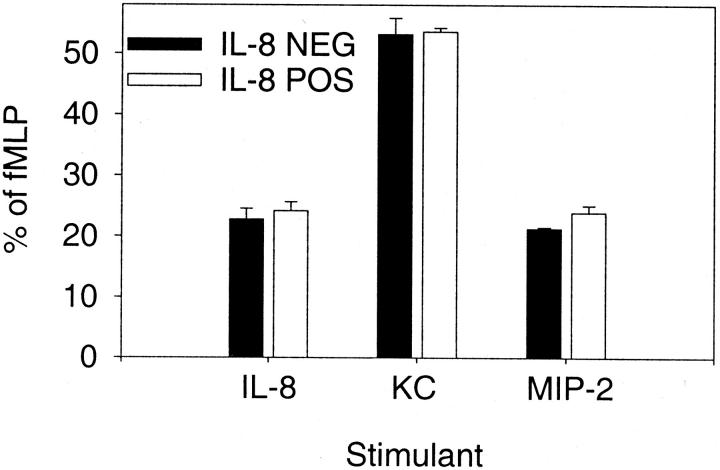
We next evaluated the neutrophils to determine whether they would still respond appropriately to a gradient of murine chemokines. Neutrophils were isolated from the peripheral blood of either IL-8-positive or IL-8-negative mice and their ability to undergo chemotaxis toward recombinant human IL-8, murine KC, or murine MIP-2 was determined using our previously described chemokine concentrations. 19 The PMN chemotactic response in both the IL-8-positive and IL-8-negative mice was the same (Figure 6) ▶ . Although KC was more potent than IL-8 or MIP-2, the response of the neutrophils from the IL-8-positive and IL-8-negative mice was equivalent. These data indicate that the peripheral blood neutrophils from both groups of mice have the capacity to move toward a chemotactic stimulus. Therefore, if there were alterations in the CXCR2 chemokine receptor these changes did not result in a decrease of neutrophil function as measured by the chemotactic response.
Figure 6.
Neutrophil chemotaxis in IL-8-positive and IL-8-negative mice. Peripheral blood neutrophils were isolated and subjected to chemotaxis toward IL-8 (10−5 mol/L), KC (10−6 mol/L), or MIP-2 (10−6 mol/L). Neutrophils from both IL-8-positive and IL-8-negative mice exhibit similar directed migration toward the chemotactic agent. Each value is the mean ± SEM for five replicate wells and is expressed as the percentage of chemotaxis toward 10−6 mol/L fMLP.
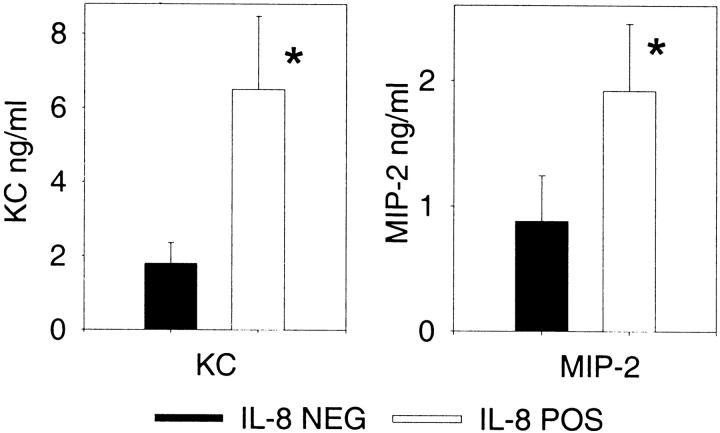
We next addressed the mechanism of how neutrophils would respond to the inflammatory challenge, because previous work had demonstrated that high plasma levels of IL-8 would effectively reduce local neutrophil recruitment. 8-10 We studied the thioglycollate model, because the kinetics of chemokine production in this model are rapid compared to CLP 30 or acid lung injury 25 and local cytokine concentrations are easily measured. This in vivo model was also less complex than either the CLP or acid lung injury model. Because peritoneal neutrophil recruitment in response to thioglycollate was nearly equal in both IL-8-positive and IL-8-negative mice, we measured murine chemokines in the peritoneal fluid to determine whether enhanced local concentrations could have been generated to offset the plasma IL-8 levels. Figure 7 ▶ shows that both groups of mice have striking up-regulation of chemokines within the peritoneal cavity. The IL-8-positive mice, however, had significantly higher concentrations of both KC and MIP-2 compared to the IL-8-negative mice, showing that enhanced local production of these murine chemokines occurred. The increased levels of these chemokines may represent the biological response to overcome the IL-8 chemotactic gradient and effectively recruit neutrophils to the sites of local inflammation.
Figure 7.
Peritoneal chemokines after thioglycollate injection. IL-8-positive and IL-8-negative mice were injected with thioglycollate and the peritoneal contents analyzed 4 hours later. Each value is the mean ± SEM for 12 to 13 mice for the chemokine measurement. *, P < 0.05 IL-8-positive compared to IL-8-negative.
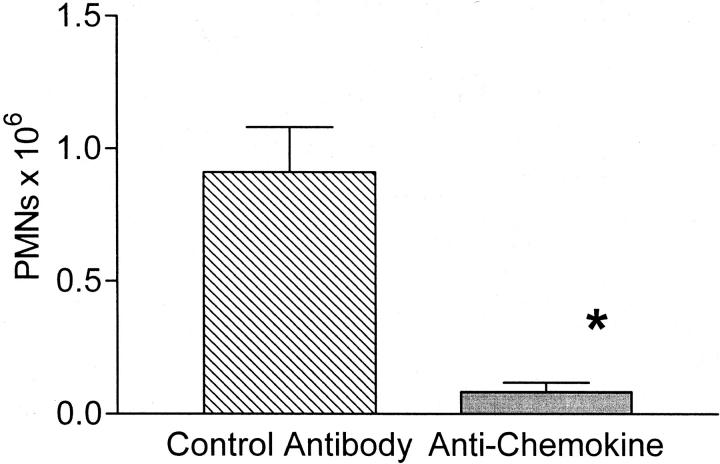
To test our hypothesis that the local up-regulation of endogenous murine chemokines was responsible for the recruitment of the neutrophils, we used antibody inhibition of the murine chemokines. For this experiment, mice were pretreated with a combination of antibodies directed against the murine chemokines KC and MIP-2 18,19,25,31 and IL-8-positive mice injected intraperitoneally with thioglycollate. These antibodies have previously been shown to significantly reduce peritoneal recruitment in response to thioglycollate injection in BALB/c mice. 19 Antibody inhibition of the murine chemokines resulted in near complete reduction in the number of recruited neutrophils (Figure 8) ▶ . The number of peritoneal neutrophils was equivalent to those observed in the mice injected with normal saline (thioglycollate + anti-chemokine = 42,000 total neutrophils versus saline = 45,000 total neutrophils). These data provide compelling evidence that the up-regulated murine chemokines were responsible for the neutrophil recruitment.
Figure 8.
Anti-chemokine inhibition of peritoneal neutrophil recruitment to thioglycollate. IL-8-positive mice were pretreated with a combination of antibodies to murine KC and MIP-2, followed 2 hours later by thioglycollate injection. Antibody treatment resulted in >90% reduction in the number of recruited neutrophils. Each value is the mean ± SEM for six control antibody-treated mice and seven anti-chemokine-treated mice. *, P < 0.005 control antibody versus anti-chemokine.
Discussion
There is extensive interest in the role of chemokines as mediators of neutrophil recruitment to sites of local inflammation. 32,33 Previous work has suggested that elevated levels of plasma chemokines, particularly IL-8, would reduce neutrophil recruitment to local sites of inflammation presumably by altering the chemokine gradient from the vascular space to the local tissue. Systemic injection of high doses of either a 77-amino acid form, or a 72-amino acid form of IL-8 reduced neutrophil recruitment. 8,9 Additionally, high-dose 77-amino acid IL-8 provided significant cardiac protection in a rabbit model of ischemia/reperfusion. 10 Previous work has demonstrated that rodent neutrophils will undergo chemotaxis to human IL-8. 12 Our investigations sought to determine whether high levels of circulating IL-8 would cause a reduction in the neutrophil recruitment in response to different inflammatory challenges. We hoped to extend previous observations concerning these mice and determine the mechanism of how neutrophil recruitment could occur even in the presence of a negative chemotactic gradient. The most severe insult occurs in the CLP model of peritonitis. After CLP, the presence of chronic high levels of IL-8 did not reduce lethality, cytokine production, or recruitment of neutrophils to the site of inflammation. Similar findings were observed in other models of acute inflammation in which the presence of high levels of IL-8 failed to reduce inflammation.
Neutrophils are the signature cell of acute inflammation, and by extension IL-8 should only be involved in acute inflammatory processes. Nevertheless, IL-8 may persist for prolonged periods in tissue culture or stimulated whole blood. 34 The persistence of IL-8 has also been documented in vivo in multiple studies. In chronic disease states, there are increased systemic levels of IL-8, including patients with HIV infections, 35 or β-thalassemia. 36 In longitudinal studies, peripheral blood levels of IL-8 persisted throughout several days in patients with malaria. 37 IL-8 stability has also been documented in local forms of inflammation. These data include finding IL-8 in lavage fluid for up to 7 days in patients who develop nosocomial pneumonia. 29 IL-8 has been identified within empyema fluid, 38 in patients with chronic lung diseases, 39 and has been recovered from the joint fluid of patients with arthritis. 40 High local levels of chemokines have been found in patients with lung inflammation such as acute respiratory distress syndrome 41 and asthma. 42 IL-8 has been found in the dialysate of patients on continuous peritoneal dialysis for up to 5 days, 43 and within the colonic mucosa of patients with chronic ulcerative colitis. 44 Ischemia/reperfusion injury to the heart will induce the formation of IL-8 mRNA in the myocardium and the mRNA will persist beyond 24 hours. 45 Given all of this information it is apparent that IL-8 has the potential to be long lasting and neutrophils may need to move into a local site of inflammation in the face of prolonged, elevated plasma levels of IL-8.
Our data show that neutrophil recruitment to local sites of inflammation will take place in the setting of chronically elevated systemic levels of IL-8. A possible explanation for the failure of high plasma levels of IL-8 to prevent neutrophil recruitment is desensitization of the chemokine receptors. Desensitization occurs between the CXC chemokines so that previous exposure to one chemokine renders a cell refractory to stimulation with a second chemokine. 46,47 Although this desensitization occurs in vitro with murine CXC chemokines, 48 the in vivo chemokine/receptor interaction is more dynamic. The IL-8 receptor (CXCR1) is rapidly internalized after cell surface binding and is then re-expressed on the cell surface within 10 minutes. 49 This chemokine receptor is now available for binding to the chemokine to exert its biological effect. Our data show that receptor desensitization has not occurred because neutrophils from IL-8-positive and IL-8-negative mice exhibit equal chemotaxis toward recombinant IL-8.
The failure of chronic elevated levels of IL-8 to reduce inflammation, and the specific failure to reduce neutrophil recruitment may be understood because of the complexity and redundancy of chemokines and their receptors. CXC chemokines represent important mediators for the influx of neutrophils to sites of acute inflammation. 50,51 The precise participation of the chemokines is also complicated by the presence of multiple chemokine receptors with relative specificity for the different chemokines. In general, CXC chemokines only bind the CXC chemokine receptors. 52 With the redundancy and overlap in chemokines, a complex in vivo focus of inflammation will involve multiple chemokines that would be available to recruit neutrophils. 53-55 The exact murine homolog of human IL-8 has not been identified, 56 but the two most probable candidates are either KC or MIP-2. Both of these chemokines will be rapidly up-regulated in a rodent model of acute lung injury. 4 Our data show that high levels of both of these chemokines are found within the peritoneum after thiolglycollate injection, and that IL-8-positive mice had significantly higher levels than the IL-8-negative mice. The peritoneal chemokine levels were higher, and the number of recruited neutrophils in multiple experiments was essentially equivalent in the IL-8-positive and IL-8-negative mice. This suggests that enhanced local production of chemokines was the appropriate biological response to generate a sufficient chemotactic gradient to induce neutrophils to migrate toward the peritoneum against the IL-8 chemotactic gradient. To provide additional support for this concept we performed antibody inhibition studies in the IL-8-positive mice. Antibody inhibition of the endogenous murine chemokines resulted in a >90% reduction in the number of peritoneal neutrophils. We believe our data support the concept that redundancy in the inflammatory response helps to ensure local neutrophil recruitment. 1,2
Footnotes
Address reprint requests to Dr. D. G. Remick, Department of Pathology, 1301 Catherine St., Ann Arbor, MI 48109-0602. E-mail: remickd@umich.edu.
Supported in part by National Institutes of Health grants GM 44918 and GM 50401.
References
- 1.Mantovani A: The chemokine system: redundancy for robust outputs. Immunol Today 1999, 20:254-257 [DOI] [PubMed] [Google Scholar]
- 2.Devalaraja MN, Richmond A: Multiple chemotactic factors: fine control or redundancy? Trends Pharmacol Sci 1999, 20:151-156 [DOI] [PubMed] [Google Scholar]
- 3.Haelens A, Wuyts A, Proost P, Struyf S, Opdenakker G, van Damme J: Leukocyte migration and activation by murine chemokines. Immunobiology 1996, 195:499-521 [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Paulauskis JD, Godleski JJ, Kobzik L: Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am J Pathol 1992, 141:981-988 [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard EJ, Skeel A, Yoshimura T, Noer K, Kutvirt S, Van Epps D: Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol 1990, 144:1323-1330 [PubMed] [Google Scholar]
- 6.Schroder JM, Mrowietz U, Morita E, Christophers E: Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol 1987, 139:3474-3483 [PubMed] [Google Scholar]
- 7.Rot A: Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol 1993, 23:303-306 [DOI] [PubMed] [Google Scholar]
- 8.Hechtman DH, Cybulsky MI, Fuchs HJ, Baker JB, Gimbrone MA, Jr: Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol 1991, 147:883-892 [PubMed] [Google Scholar]
- 9.Ley K, Baker JB, Cybulsky MI, Gimbrone MA, Jr, Luscinskas FW: Intravenous interleukin-8 inhibits granulocyte emigration from rabbit mesenteric venules without altering L-selectin expression or leukocyte rolling. J Immunol 1993, 151:6347-6357 [PubMed] [Google Scholar]
- 10.Lefer AM, Johnson G, III, Ma XL, Tsao PS, Thomas GR: Cardioprotective and endothelial protective effects of [Ala-IL8]77 in a rabbit model of myocardial ischaemia and reperfusion. Br J Pharmacol 1991, 103:1153-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonet WS, Hughes TM, Nguyen HQ, Trebasky LD, Danilenko DM, Medlock ES: Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J Clin Invest 1994, 94:1310-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rot A: Chemotactic potency of recombinant human neutrophil attractant/activation protein-1 (interleukin-8) for polymorphonuclear leukocytes of different species. Cytokine 1991, 3:21-27 [DOI] [PubMed] [Google Scholar]
- 13.DeForge LE, Remick DG: Sandwich ELISA for detection of picogram quantities of interleukin-8. Immunol Invest 1991, 20:89-97 [DOI] [PubMed] [Google Scholar]
- 14.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL: Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol 1990, 136:49-60 [PMC free article] [PubMed] [Google Scholar]
- 15.Remick D, Manohar P, Bolgos G, Rodriguez J, Moldawer L, Wollenberg G: Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 1995, 4:89-95 [DOI] [PubMed] [Google Scholar]
- 16.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG: Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 1992, 148:2724-2730 [PubMed] [Google Scholar]
- 17.Remick DG, Call DR, Ebong SJ, Newcomb DE, Nybom P, Nemzek JA, Bolgos GE: Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit Care Med 2001, 29:473-481 [DOI] [PubMed] [Google Scholar]
- 18.Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Wollenberg GK, Remick DG: Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock 2001, 15:278-284 [DOI] [PubMed] [Google Scholar]
- 19.Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Remick DG: Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am J Pathol 2001, 158:715-721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskandari MK, Nguyen DT, Kunkel SL, Remick DG: WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol Invest 1990, 19:69-79 [DOI] [PubMed] [Google Scholar]
- 21.Espevik T, Nissen Meyer J: A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods 1986, 95:99-105 [DOI] [PubMed] [Google Scholar]
- 22.Aarden LA, De Groot ER, Schaap OL, Lansdorp PM: Production of hybridoma growth factor by human monocytes. Eur J Immunol 1987, 17:1411-1416 [DOI] [PubMed] [Google Scholar]
- 23.Harvath L, Falk W, Leonard EJ: Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods 1980, 37:39-45 [DOI] [PubMed] [Google Scholar]
- 24.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC: Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 1995, 96:107-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemzek JA, Call DR, Ebong SJ, Newcomb DE, Bolgos GL, Remick DG: Immunopathology of a two-hit murine model of acid aspiration lung injury. Am J Physiol 2000, 278:L512-L520 [DOI] [PubMed] [Google Scholar]
- 26.Matthay MA, Rosen GD: Acid aspiration induced lung injury. New insights and therapeutic options. Am J Respir Crit Care Med 1996, 154:277-278 [DOI] [PubMed] [Google Scholar]
- 27.Baron EJ, Proctor RA: Elicitation of peritoneal polymorphonuclear neutrophils from mice. J Immunol Methods 1982, 49:305-313 [DOI] [PubMed] [Google Scholar]
- 28.Bagby GJ, Plessala KJ, Wilson LA, Thompson JJ, Nelson S: Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis 1991, 163:83-88 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez JL, Miller CG, DeForge LE, Kelty L, Shanley CJ, Bartlett RH, Remick DG: Local production of interleukin-8 is associated with nosocomial pneumonia. J Trauma 1992, 33:74-81 [DOI] [PubMed] [Google Scholar]
- 30.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D: Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 1999, 67:6603-6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer-Jones MA, Shrotri MS, Peyton JC, Remick DG, Cheadle WG: Neutrophil sequestration in liver and lung is differentially regulated by C-X-C chemokines during experimental peritonitis. Inflammation 1999, 23:305-319 [DOI] [PubMed] [Google Scholar]
- 32.Van Damme J, Van Beeumen J, Opdenakker G, Billiau A: A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med 1988, 167:1364-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rampart M, Van Damme J, Zonnekeyn L, Herman AG: Granulocyte chemotactic protein/interleukin-8 induces plasma leakage and neutrophil accumulation in rabbit skin. Am J Pathol 1989, 135:21-25 [PMC free article] [PubMed] [Google Scholar]
- 34.Villarete LH, Remick DG: Transcriptional and post-transcriptional regulation of interleukin-8. Am J Pathol 1996, 149:1685-1693 [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto T, Miike T, Nelson RP, Trudeau WL, Lockey RF, Yodoi J: Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol 1993, 93:149-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uguccioni M, Meliconi R, Nesci S, Lucarelli G, Ceska M, Gasbarrini G, Facchini A: Elevated interleukin-8 serum concentrations in beta-thalassemia and graft-versus-host disease. Blood 1993, 81:2252-2256 [PubMed] [Google Scholar]
- 37.Friedland JS, Ho M, Remick DG, Bunnag D, White NJ, Griffin GE: Interleukin-8 and Plasmodium falciparum malaria in Thailand. Trans R Soc Trop Med Hyg 1993, 87:54-55 [DOI] [PubMed] [Google Scholar]
- 38.Broaddus VC, Hebert CA, Vitangcol RV, Hoeffel JM, Bernstein MS, Boylan AM: Interleukin-8 is a major neutrophil chemotactic factor in pleural liquid of patients with empyema. Am Rev Respir Dis 1992, 146:825-830 [DOI] [PubMed] [Google Scholar]
- 39.Richman-Eisenstat JB, Jorens PG, Hebert CA, Ueki I, Nadel JA: Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol 1993, 264:L413-L418 [DOI] [PubMed] [Google Scholar]
- 40.Remick DG, DeForge LE, Sullivan JF, Showell HJ: Profile of cytokines in synovial fluid specimens from patients with arthritis. Interleukin 8 (IL-8) and IL-6 correlate with inflammatory arthritides. Immunol Invest 1992, 21:321-327 [DOI] [PubMed] [Google Scholar]
- 41.Jorens PG, Van Damme J, De Backer W, Bossaert L, De Jongh RF, Herman AG, Rampart M: Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine 1992, 4:592-597 [DOI] [PubMed] [Google Scholar]
- 42.Mattoli S, Marini M, Fasoli A: Expression of the potent inflammatory cytokines, GM-CSF, IL6, and IL8, in bronchial epithelial cells of asthmatic patients. Chest 1992, 101:S27-S29 [DOI] [PubMed] [Google Scholar]
- 43.Lin CY, Lin CC, Huang TP: Serial changes of interleukin-6 and interleukin-8 levels in drain dialysate of uremic patients with continuous ambulatory peritoneal dialysis during peritonitis. Nephron 1993, 63:404-408 [DOI] [PubMed] [Google Scholar]
- 44.Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C: Interleukin-8 and neutrophil markers in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol 1992, 87:1447-1452 [PubMed] [Google Scholar]
- 45.Kukielka GL, Smith CW, LaRosa GJ, Manning AM, Mendoza LH, Daly TJ, Hughes BJ, Youker KA, Hawkins HK, Michael LH, Rot A, Entman ML: Interleukin-8 gene induction in the myocardium after ischemia and reperfusion in vivo. J Clin Invest 1995, 95:89-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M: Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med 1990, 171:1797-1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser B, Schumacher C, Von Tscharner V, Clark-Lewis I, Baggiolini M: Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem 1991, 266:10666-10671 [PubMed] [Google Scholar]
- 48.Farber JM: Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997, 61:246-257 [PubMed] [Google Scholar]
- 49.Samanta AK, Oppenheim JJ, Matsushima K: Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem 1990, 265:183-189 [PubMed] [Google Scholar]
- 50.Rollins BJ: Chemokines. Blood 1997, 90:909-928 [PubMed] [Google Scholar]
- 51.Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 [DOI] [PubMed] [Google Scholar]
- 52.Raport CJ, Schweickart VL, Chantry D, Eddy RL, Jr, Shows TB, Godiska R, Gray PW: New members of the chemokine receptor gene family. J Leukoc Biol 1996, 59:18-23 [DOI] [PubMed] [Google Scholar]
- 53.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ: Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol 1998, 161:2435-2440 [PubMed] [Google Scholar]
- 54.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR: Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol 1997, 159:3595-3602 [PubMed] [Google Scholar]
- 55.Nakagawa H, Ando Y, Takano K, Sunada Y: Differential production of chemokines and their role in neutrophil infiltration in rat allergic inflammation. Int Arch Allergy Immunol 1998, 115:137-143 [DOI] [PubMed] [Google Scholar]
- 56.Widmer U, Manogue KR, Cerami A, Sherry B: Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol 1993, 150:4996-5012 [PubMed] [Google Scholar]