Abstract
Interactions of tumor cells with lymphatic vessels are of paramount importance for tumor progression, however, the underlying molecular mechanisms are poorly understood. Whereas enlarged lymphatic vessels are frequently observed at the periphery of malignant melanomas, it has remained unclear whether intratumoral lymphangiogenesis occurs within these tumors. Here, we demonstrate the presence of intratumoral lymphatics and enlargement of lymphatic vessels at the tumor periphery in vascular endothelial growth factor (VEGF)-C-overexpressing human melanomas transplanted onto nude mice. VEGF-C expression also resulted in enhanced tumor angiogenesis, indicating a coordinated regulation of lymphangiogenesis and angiogenesis in melanoma progression. The specific biological effects of VEGF-C were critically dependent on its proteolytic processing in vivo. Furthermore, VEGF-C induced chemotaxis of macrophages in vitro and in vivo, revealing a potential function of VEGF-C as an immunomodulator. Taken together, our results identify VEGF-C as multifunctional factor involved in regulating tumor lymphangiogenesis, angiogenesis, and immune response.
The spread of tumor cells via lymphatic vessels to the regional lymph nodes is one of the most important indicators of tumor aggressiveness, and the extent of lymph node involvement is a major determinant for the staging and the prognosis of most human malignancies. 1-3 Abundant, rearranged, and often dilated lymphatic vessels containing clusters of tumor cells are commonly observed at the periphery of many tumors, including malignant melanomas. 1,4-7 However, the presence of lymphatic vessels within tumors and the ability of tumor cells to induce lymphangiogenesis have remained controversial. 8,9
Whereas tumor angiogenesis involving the formation of new blood vessels has been studied extensively, the role of lymphatic vessels in tumor pathology and the molecules regulating lymphangiogenesis have remained mostly unknown. Recently, a novel member of the vascular endothelial growth factor (VEGF) family has been identified that is distinguished by its capacity to stimulate lymphangiogenesis. 10,11 Vascular endothelial growth factor-C (VEGF-C) stimulated lymphangiogenesis in the avian chorioallantoic membrane assay, 12 and transgenic mice overexpressing VEGF-C in the skin were characterized by specific hyperplasia of the lymphatic network without obvious effects on blood vessels. 13 In normal adult human tissues, the VEGF-C receptor VEGFR-3 (Flt-4) has been found predominantly expressed by lymphatic endothelium. 7,14 However, VEGF-C also binds VEGFR-2 (KDR), that is mainly expressed by activated endothelium of blood vessels, suggesting a potential function of VEGF-C in the induction of angiogenesis. 10,15 Indeed, VEGF-C stimulates the proliferation and migration of blood vascular endothelial cells in vitro and has been shown to increase vascular permeability in the Miles assay, 10,11,16,17 and to induce angiogenesis in the mouse corneal micropocket assay and in the rabbit ischemic hind limb model. 18,19
VEGF-C mRNA expression has been demonstrated in a large number of human tumors, including malignant melanomas. 20-32 Expression of its receptor VEGFR-3 has been detected in lymphatic vessels and occasionally also in blood vessels adjacent to cancer cells. 7,14,21 Most recently, we have shown that VEGF-C overexpression in experimental breast cancer selectively induced intratumoral lymphangiogenesis, leading to increased tumor metastasis, without obvious effects on angiogenesis. 33
In the present study we demonstrate the occurrence of intratumoral lymphangiogenesis in VEGF-C-overexpressing human melanomas transplanted onto nude mice. Moreover, VEGF-C also induced tumor angiogenesis and recruitment of macrophages. Our results indicate that the distinct biological effects of VEGF-C are critically dependent on its proteolytic processing in vivo.
Materials and Methods
Cell Culture
The human malignant melanoma cell lines MeWo, 34,35 WM9, and WM239 were provided by Dr. Robert S. Kerbel (Sunnybrook Health Science Center, Toronto, Canada). Human melanoma cell lines PM-WK, RPM-MC, RPM-EP, and MM-LH 36 were obtained from Dr. Randy Byers (Boston University Medical School, Boston, MA). The human melanoma cell lines SK-MEL-5, SK-MEL-25, SK-MEL-28, MelJuso, Colo 38, MML-1, HS695T, and MELKL-2 were obtained from the tumor bank of the German Cancer Research Center in Heidelberg, Germany. All melanoma cell lines were maintained in RPMI 1640 medium with 5% fetal bovine serum; human prostatic adenocarcinoma PC-3 cells (American Type Culture Collection, Rockville, MD) in Ham’s F12 medium with 5% fetal bovine serum. All media were purchased from Life Technologies, Inc., Grand Island, NY.
Cell Transfection and Selection
A human VEGF-C cDNA comprising the complete coding sequence (GenBank accession number X94216) 10 was cloned into a pcDNA3.1/Zeo expression vector (Invitrogen, San Diego, CA) that contains a CMV-enhancer promoter and a Zeocin selection cassette. The sequence and the orientation of the VEGF-C gene in the construct were verified by restriction mapping and direct sequencing. Human MeWo malignant melanoma cells were transfected either with the human VEGF-C cDNA cloned into a pcDNA3.1/Zeo vector or with the vector alone using the Superfect transfection reagent (Qiagen, Chatsworth, CA). Transfected cells were selected and maintained in growth medium containing 50 μg/ml Zeocin. Stably transfected cell clones were individually expanded and analyzed for VEGF-C mRNA expression and protein secretion.
RNA Isolation and Northern Analysis
Total cellular RNA was isolated from cultured cells and from tumors using the RNeasy kit (Qiagen). The isolated RNA was subjected to electrophoresis (15 μg per lane) and transferred to Hybond-N+ membranes (Amersham, Arlington Heights, IL). 32P-radiolabeled DNA probes were labeled by the random priming method (Multiprime labeling kit; Amersham, Arlington Heights, IL). The VEGF-C probe used was a fragment containing nucleotides 581 to 1634 of human VEGF-C cDNA. The probe for human VEGF hybridizes with a region of VEGF mRNA common to all known VEGF splice variants. 37 A human β-actin cDNA probe (Clontech, Palo Alto, CA) was used as a control for equal RNA loading. Blots were hybridized at 65°C for 24 hours, washed at high stringency, and exposed to X-OMAT MR film (Kodak, Rochester, NY).
Western Analysis
Cells grown to 80% confluency were lysed with Izuhara buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 1% Triton X-100, 30 mmol/L Na-pyrophosphate, 50 mmol/L Na-fluoride, 1 mmol/L Na-orthovanadate, pH 7.4) containing protease inhibitors (phenylmethylsulfonyl fluoride, 0.02 mol/L; leupeptin, 50 μg/ml; aprotinin, 50 μg/ml). The amount of protein was determined with the BioRad protein assay (BioRad, Hercules, CA), and 15 μg were analyzed on the gel. Conditioned media were obtained from subconfluent cells grown for 60 hours in serum-free media, concentrated 100-fold using Centricon-10 columns (Amicon, Beverly, MA), and 15 μg of protein were loaded on the gel. Tumors were snap-frozen in liquid nitrogen, lysed by homogenizing the tissue (0.5 g) in 2 ml of a buffer containing 2% sodium dodecyl sulfate, 50 mmol/L Tris (pH 7.4), and protease inhibitors as above, and 10 μl were loaded onto the gel. All samples were boiled in denaturing Laemmli sample buffer (BioRad), analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto polyvinylidene difluoride membranes (BioRad). Filters were blocked overnight with 5% nonfat milk in phosphate-buffered saline (PBS)/0.1% Tween 20 and were incubated with a rabbit antiserum against human VEGF-C (1:1000). 16 After washes, membranes were incubated with horseradish-peroxidase-conjugated anti-rabbit IgG (1:2000; Amersham), washed, and analyzed using the Amersham ECL+ chemiluminescence reagents. Receptor phosphorylation assays were performed as described, 33 using antibodies against mouse VEGFR-2 (Santa Cruz Biotechnology, Santa Cruz, California) and phosphotyrosine (PY-20; ICN Biomedicals, Aurora, Ohio).
Tumorigenicity Assay
Stably transfected MeWo cells (2 × 10 6 in 100 μl of serum-free culture medium) were injected intradermally on each side of the back of 8-week-old female Swiss/c (nu/nu) nude mice (five mice for each clone). Three clones were analyzed for each construct. Tumor growth was measured weekly using a digital caliper. Tumors were harvested when they reached a size of >1200 mm 3 or after 6 to 8 weeks, and were embedded in OCT compound and frozen in liquid nitrogen or were fixed in 4% paraformaldehyde/PBS and processed for routine histology. For RNA or protein extractions, tumors were snap-frozen in liquid nitrogen. Two independent experiments with five mice in each group were performed.
Generation of a Polyclonal Anti-Mouse VEGFR-3 Antibody
The 19-amino acid synthetic peptide CYPGKQAERAKWVPERRSQ, corresponding to amino acid residues 265 to 285 in the Ig-like domain 3 of the mouse VEGFR-3 extracellular domain, was used to immunize rabbits with standard techniques. The antisera were affinity purified and specific staining of lymphatic vessels was confirmed in adult mouse tissues (heart, lung, spleen, and liver).
Indirect Immunofluorescence
Cryosections were stained as previously described, 38 using antibodies against mouse CD31 (dilution 1/30; Pharmingen, San Diego, CA), LYVE-1 (1/300), 33,39 VEGFR-3 (1/50; rabbit polyclonal; 1/30; R&D Systems, Minneapolis, MN), CD11b/Mac-1 (1/200; Pharmingen), or F4/80 (1/200; Serotec, Raleigh, NC). The secondary antibodies, labeled with either Texas Red or fluorescein isothiocyanate (Jackson ImmunoResearch, West Grove, PA) were used at the dilution 1/50. Cell nuclei were counterstained with Hoechst bisbenzimide (Sigma, St. Louis, MO) at 20 μg/ml. Specimens were examined by using a Nikon E-600 microscope (Nikon, Melville, NY).
Computer-Assisted Morphometric Analysis
For analysis of tumor vascularization, immunohistochemical stainings were performed on 6-μm frozen sections of tumor xenografts using an antibody against mouse CD31. 40 Tissue sections were viewed using a Nikon E-600 microscope and images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Two MeWo/control and two MeWo/VEGF-C clones were analyzed, five tumors each. On each tumor section, three randomly chosen fields were evaluated at ×60 magnification. Morphometric analysis was performed using the IPLab software (Scanalytics, Fairfax, VA) to determine vessel density, size distribution, and the relative tumor area covered by vessels. 33 For analysis of macrophage densities, sections were stained with CD11b/Mac-1 antibody as described above. Three MeWo/control and three MeWo/VEGF-C clones were analyzed, three tumors each. For each tumor, the total area of the adjacent overlying skin was analyzed, and the relative area of the skin occupied by macrophages was determined using the IPLab software. The unpaired t-test was used for statistical analysis.
Computed Tomography
Computed tomography was performed to determine the clearance rate of a lymphographic contrast agent from the tumors. Mice were imaged 5 weeks after tumor cell inoculation. Mice bearing MeWo/control tumors were additionally analyzed when the tumor size equaled that of the MeWo/VEGF-C tumors at 5 weeks (∼1000 mm3). Three mice carrying two tumors each were analyzed in both groups. After anesthesia with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), mice were injected with an iodinated lymphographic contrast agent, 41 and imaging was performed by using a helical computed tomography scanner (TCT900S/xII; Toshiba, Tokyo, Japan). The contrast agent was administered very slowly by injection into the center of each tumor (20 μl per tumor) by using a 30-gauge needle attached to a Hamilton syringe. Mice were imaged before, immediately after, 6 hours, 24 hours, 48 hours, and 72 hours after the injection of the contrast agent. Each imaging study consisted of a helical data acquisition through the tumor and the axillary region for the assessment of axillary lymph nodes. The imaging parameters were 120-kV tube voltage, 150-mA tube current, 2-mm slice thickness, and a high-resolution imaging mode with an 80-mm field of view. Images were reconstructed onto a 512 × 512 image matrix yielding 0.05 mm 3 voxels. Data analysis was performed with the image analysis program DIP Station (HIPG, Boulder, CO) to obtain the average signal intensity value for the tumor volume. Signal intensity values in computed tomography are expressed as Hounsfield units that change linearly as a function of contrast agent concentration in the tissue. 42 A signal-time curve was created for each tumor and the rate constant was calculated using a mono-exponential model as a first approximation. The average clearance rate was then calculated for each group of animals.
Isolation of Mouse Macrophages, Flow Cytometry, and Chemotaxis Assay
Macrophage accumulation was induced in Swiss/c nu/nu mice by intraperitoneal injection of 1 ml of 3% Brewer thioglycollate medium and macrophages were harvested 5 days after injection by lavage with Hanks’ balanced salt solution. 43 For flow cytometry, macrophages were detached from the tissue culture dish by scraping, incubated for 15 minutes at 4°C with antibodies to CD11b or mouse VEGFR-3 and with fluorescein isothiocyanate-labeled corresponding secondary antibodies. Chemotaxis was examined using 24-well Transwell migration chambers (8 μm pore size; Costar). Inserts were coated on the underside with 10 μg/ml of collagen type I (Collagen Corp., Palo Alto, CA) and 4 × 10 5 cells were added to the upper compartment in 100 μl of serum-free Dulbecco’s modified Eagle’s medium. VEGF-C (1 to 100 ng/ml) or the macrophage chemoattractant N-formyl-met-leu-phe (fMLP; 10−6 mol/L) were added to 600 μl of serum-free media in the lower compartment only. After 3 hours, inserts were fixed and washed as described 44 and cell nuclei were stained with propidium iodide. The number of migrated cells was determined by using the IPLab software. For every insert, three fields were evaluated at ×120 magnification. All assays were performed in triplicate.
Cell Growth Assays
In vitro proliferation rates of transfected MeWo cell clones were determined by using the BrdU labeling and detection kit (Boehringer Mannheim, Mannheim, Germany). Cells were plated in 96-well plates (5 × 10 3 cells/well; 8 wells/cell clone) and were allowed to proliferate for 24 hours before addition of BrdU (10 μmol/L) for 6 hours. The absorbance was determined at 405 nm using a microtiter plate reader (Titertek, Huntsville, AL). To assess the effect of VEGF or VEGF-C on MeWo cell proliferation, MeWo/control clone 5 was seeded into a 96-well plate at a density of 2.5 × 10 3 cells/well, and cells were treated for 5 days with 20 ng/ml of recombinant human VEGF-C 16 or VEGF (R&D Systems), six wells each. Cell proliferation was determined by using the BrdU kit. In additional experiment, MeWo/control clones were plated in 12-well plate inserts (1 μm pore size; 1 × 10 4 cells/insert; four inserts each), and were co-cultured for 7 days with either MeWo/VEGF-C cl. 23 or MeWo/control cl. 5 (1 × 10 4 cells/well). Total cell nuclei were stained with the Hoechst bisbenzimide DNA stain and cells that had incorporated BrdU (10 μmol/L, 2 hours) were visualized by immunofluorescent staining using an anti-BrdU antibody 38 (Pharmingen, San Diego, CA). Inserts were mounted on slides and the percentage of proliferating cells was determined by using the IPLab software. For every insert, three randomly chosen fields were evaluated at ×120 magnification. All experiments were repeated twice. The unpaired t-test was used for statistical analysis.
Results
Overexpression of VEGF-C in the Human Melanoma Cell Line MeWo
We first examined the expression of VEGF-C in several human melanoma cell lines by Northern analysis and found that VEGF-C mRNA was expressed in most (10 of 15) melanoma cell lines studied, as a single band of 2.4 kb (Figure 1A) ▶ . In contrast, normal human melanocytes expressed little or no VEGF-C mRNA in vitro (data not shown). To examine the role of VEGF-C in tumorigenesis, we transfected the melanoma cell line MeWo, established from a lymph node metastasis of a nodular malignant melanoma, 34,35 devoid of VEGF-C expression, to constitutively overexpress VEGF-C. As determined by Northern analysis, the parental MeWo cell line and three vector-transfected control clones (MeWo/control) did not express any detectable amounts of VEGF-C mRNA in vitro or in vivo (Figure 1B) ▶ . Three VEGF-C-transfected cell clones (MeWo/VEGF-C) expressed high levels of VEGF-C mRNA in culture, as well as in tumors that reached the size of ∼1200 mm 3 (Figure 1B) ▶ . To exclude the possibility of altered production of VEGF or other angiogenic factors by MeWo/VEGF-C cells, we examined the expression of VEGF, basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF)-B in MeWo transfectants by Northern analysis. Parental, control, and VEGF-C-transfected MeWo cells expressed comparable, low levels of endogenous human VEGF mRNA in vitro and in vivo (Figure 1B) ▶ , and little or no bFGF or PDGF-B mRNA (data not shown).
Figure 1.
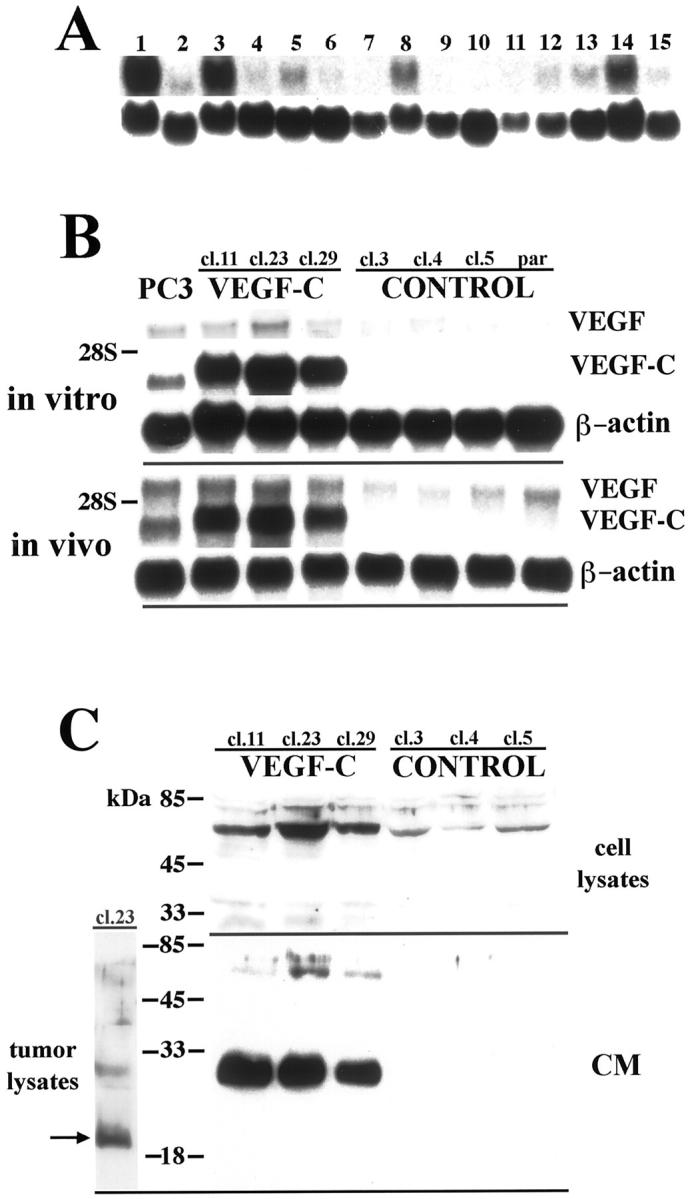
Expression of VEGF-C in melanoma cell lines and overexpression of VEGF-C in stably transfected MeWo cell clones. A: Northern blot analysis of the expression of VEGF-C mRNA (2.4 kb) in cultured melanoma cell lines. 1, positive control PC-3 mRNA; 2, SK-MEL-5; 3, SK-MEL-25; 4, SK-MEL-28; 5, WM9; 6, WM 239; 7, Colo; 8, MelJuso; 9, MML-1; 10, MS695T; 11, Melkl2; 12, PM-WK; 13, RPM-EP; 14, RPM-MC; 15, MM-LH. Hybridization with β-actin was performed as a loading control (bottom panel). B: Northern blot analysis of cultured MeWo cell clones and extracts of MeWo cell clones grown as tumors in vivo demonstrates strong overexpression of VEGF-C in VEGF-C-transfected clones. VEGF-C mRNA was not detectable in clones transfected with the control vector or in the parental cell line. The prostatic carcinoma cell line PC-3 served as a positive control. Note that VEGF-C expression did not alter VEGF mRNA expression in transfected clones. C: Western blot analysis of cultured cells demonstrates increased amounts of VEGF-C precursor protein (∼60 kd) in cell lysates and secretion of large amounts of the partially processed VEGF-C (∼31 kd) into the media by MeWo/VEGF-C cells, as compared to control transfectants. Western blot of VEGF-C-overexpressing tumor lysates shows that VEGF-C is fully processed in vivo into the mature, 21-kd form (arrow).
Western blot analyses confirmed that high VEGF-C mRNA levels correlated with high amounts of VEGF-C protein expression (Figure 1C) ▶ . We detected a strong band of ∼60 kd in cell lysates of VEGF-C transfectants, corresponding to VEGF-C precursor, 16 and only trace amounts in control cells. Large amounts of the secreted 31-kd form that activates VEGFR-3 16 were selectively found in culture supernatants of VEGF-C-transfected clones. None of the clones expressed any detectable levels of the 21-kd form in vitro. In VEGF-C-overexpressing tumors however, we predominantly detected the mature 21-kd VEGF-C form, a high-affinity ligand for both VEGFR-2 and VEGFR-3, demonstrating that complete processing of VEGF-C occurred in vivo (Figure 1C) ▶ .
VEGF-C Induces Tumor Lymphangiogenesis
The VEGF-C-overexpressing MeWo cell lines were injected intradermally into immunodeficient mice and tumors were harvested 6 weeks after the injection. To visualize tumor-associated lymphatic vessels we used an antibody against LYVE-1, a recently discovered specific marker of lymphatic vessels in normal tissues 39,45 and in tumors. 33 We detected lymphatic vessels with mostly compressed lumina in the skin surrounding control tumors (Figure 2A ▶ , arrowheads). In contrast, lymphatic vessels were frequently enlarged in the skin surrounding VEGF-C-overexpressing tumors (Figure 2B ▶ , arrowheads) and also within the peripheral areas of the tumors (Figure 2B ▶ , arrows). Importantly, numerous small lymphatic vessels were observed within VEGF-C-producing tumors (Figure 2, C and D ▶ ; arrows), but not in control tumors (Figure 2A) ▶ , demonstrating that VEGF-C induced tumor lymphangiogenesis. All LYVE-1-positive lymphatic vessels also expressed VEGFR-3 (Figure 2, E and F) ▶ . The effects of VEGF-C overexpression on lymphatic vessels were not associated with a modulation of the expression of the related lymphangiogenesis factor, VEGF-D, 46,47 because, as determined by Northern analyses, little or no VEGF-D mRNA was detected in cultured cells and in tumor extracts of control and VEGF-C-overexpressing cells (data not shown).
Figure 2.
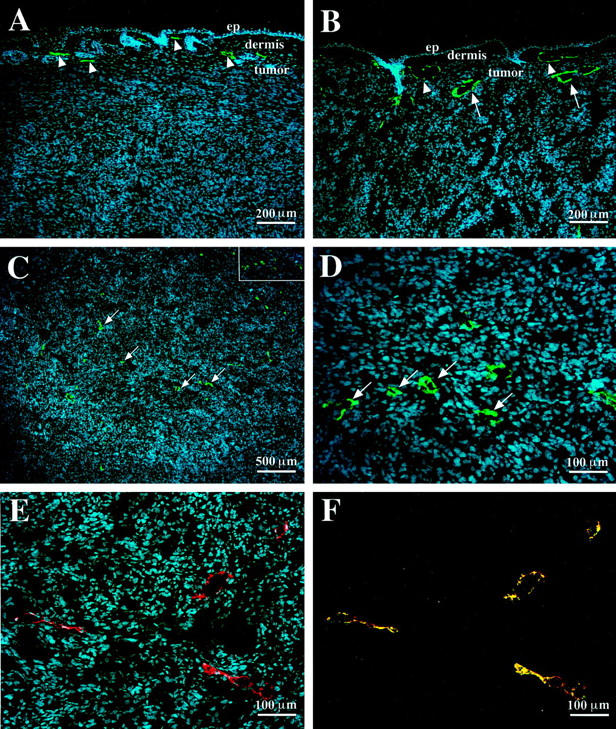
VEGF-C-overexpressing tumors are infiltrated with lymphatic vessels. A: Immunofluorescent staining for LYVE-1 (green) demonstrates compressed lymphatic vessels (arrowheads) in the dermis overlying MeWo/control tumor. Note the absence of lymphatic vessels within the tumor mass. B: Enlarged lymphatic vessels in the overlying dermis (arrowheads) and within the peripheral rim of the MeWo/VEGF-C tumor (arrows). C and D: Numerous lymphatic vessels in the central areas of the MeWo/VEGF-C tumor (arrows). Expression of VEGFR-3 (E) (red) and co-localization of VEGFR-3 with LYVE-1 (F) (green) on intratumoral lymphatic vessels. Cell nuclei are counterstained blue with Hoechst. ep, epidermis.
Using computed tomography we found that the clearance rate of a contrast agent from VEGF-C-overexpressing tumors to regional lymph nodes was considerably slower than from control tumors (Figure 3) ▶ . The clearance rate was independent of tumor size. Assessment of axillary lymph nodes confirmed that the contrast agent had been transported by the lymphatic vessels to the lymph nodes (data not shown).
Figure 3.
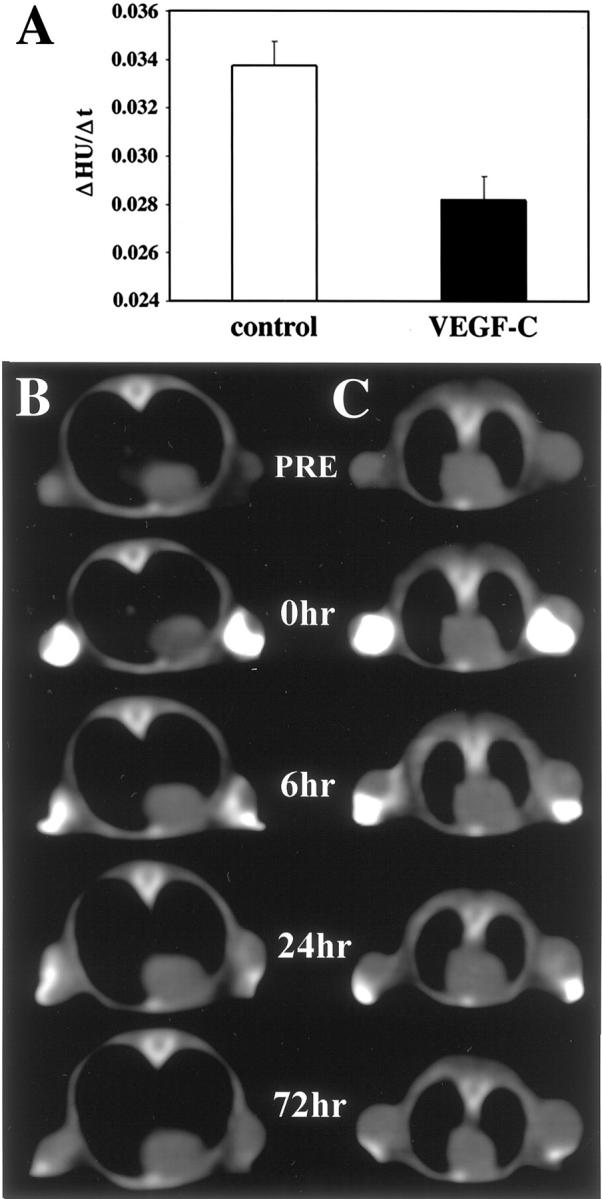
Impaired clearance of the lymphographic contrast agent from VEGF-C-overexpressing MeWo tumors. Contrast agent was injected into the tumors and the images of tumors were obtained with a computed tomography scanner for up to 72 hours after injection. A: Signal intensity values, expressed as Hounsfield units, were plotted against time and the clearance rate constant (ΔHounsfield units/Δt) was calculated for each tumor. The data are expressed as the mean values ± SD of six tumors for each tumor type. Representative images of control (B) and VEGF-C-overexpressing tumors of comparable size (C), obtained before (PRE) and after injection of the contrast agent (0 to 72 hours).
VEGF-C Increases Tumor Angiogenesis
To analyze intratumoral angiogenesis, tumor sections were stained with an antibody against mouse CD31. 48 In both experimental groups, vessels were distributed rather uniformly throughout the tumors (Figure 4, A and B) ▶ . Computer-assisted morphometric analysis revealed significantly increased vascular densities (Figure 4C) ▶ in 6-week-old tumors derived from VEGF-C-overexpressing clones (40 ± 12 vessels/mm2) as compared to control clones (23 ± 7 vessels/mm2; P < 0.0002). Furthermore, the relative tumor area covered by vessels was significantly increased in MeWo/VEGF-C tumors (Figure 5D) ▶ as compared to control tumors (2.65 ± 0.9% versus 1.48 ± 0.4%; P < 0.0001). The distribution of vessel sizes however, was not significantly different (data not shown). The majority of tumor vessels were smaller than 800 μm2, with approximately half of all vessels in both control and VEGF-C-overexpressing tumors smaller than 400 μm2.
Figure 4.
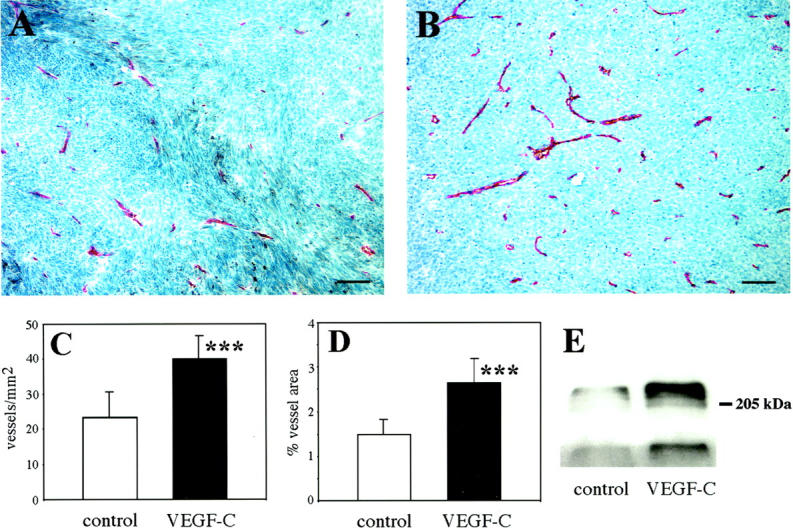
Increased vascularization of MeWo/VEGF-C-overexpressing tumors. A and B: Immunohistochemical staining with an anti-mouse CD31 antibody reveals increased vascularization in VEGF-C-overexpressing xenotransplants (B), as compared to control tumors (A). Scale bars, 50 μm. C: Markedly increased vascular densities in MeWo/VEGF-C-overexpressing tumors, expressed as number of vessels per mm 2 tumor area (P < 0.0002). D: Significant increase of the relative area covered by vessels in VEGF-C-overexpressing tumors (P < 0.0001). Data are expressed as mean values ± SD of two individual clones per tumor type (five tumors each). E: Immunoprecipitation analysis of tumor lysates revealed increased amounts of VEGFR-2 (top panel) and enhanced VEGFR-2 phosphorylation levels (bottom panel) in VEGF-C-expressing MeWo tumors.
Figure 5.
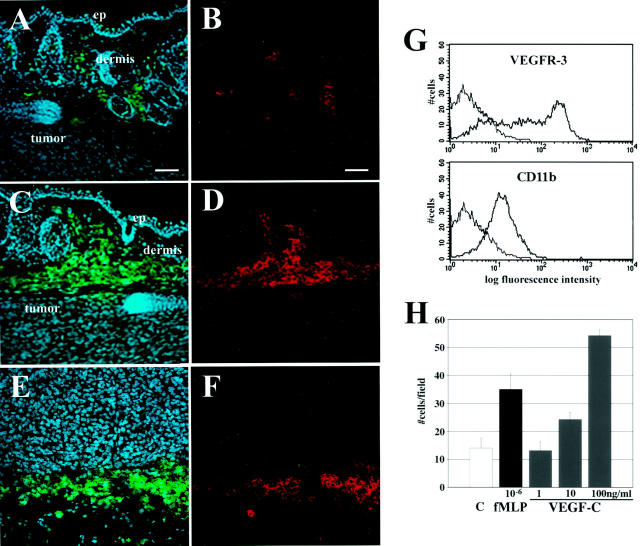
VEGF-C has chemotactic activity for macrophages. A–D: Double immunofluorescent staining for the macrophage marker CD11b (green, A and C) and mouse VEGFR-3 (red, B and D) in the skin overlying MeWo/control (A and B) and MeWo/VEGF-C (C and D) tumors, demonstrating expression of VEGFR-3 by macrophages. Note increased number of macrophages in the skin surrounding VEGF-C-overexpressing tumors. Macrophages surrounding MeWo/VEGF-C tumors also expressed another macrophage marker, F4/80 (E) (green), and VEGFR-3 (F) (red). Cell nuclei are counterstained blue with Hoechst in A, C, and E. Scale bars, 50 μm. G: Flow cytometry analysis reveals expression of VEGFR-3 on mouse peritoneal macrophages. The x axis values are displayed on a logarithmic scale as arbitrary fluorescence units and the y axis values are on a linear scale and represent number of cells. H: VEGF-C dose-dependently stimulates migration of mouse peritoneal macrophages in a chemotaxis assay. The macrophage chemoattractant N-formyl-met-leu-phe (fMLP) was used as a positive control. Data are expressed as the mean values ± SD for a representative experiment.
Immunoprecipitation studies of tumor lysates revealed increased amounts of VEGFR-2 and enhanced VEGFR-2 phosphorylation levels in VEGF-C-overexpressing tumors (Figure 4E) ▶ , which is in accordance with the observed production of the mature form of VEGF-C in these tumors. Taken together, these results identify the mature form of VEGF-C as a potent tumor angiogenesis factor.
VEGF-C Stimulates Recruitment of Macrophages
Histological analysis revealed an increased inflammatory response in the skin surrounding VEGF-C-transfected melanomas, as compared to control tumors. Using an antibody to CD11b that has broad reactivity with dermal macrophages, 49 we observed a markedly increased density of macrophages surrounding MeWo/VEGF-C tumors (Figure 5, A and C) ▶ . Immunofluorescent analysis demonstrated that CD11b-positive macrophages expressed VEGFR-3 (Figure 5, B and D) ▶ . The identity of VEGFR-3-positive cells as macrophages was further confirmed by staining with an additional macrophage marker, F4/80 50 (Figure 5, E and F) ▶ .
Flow cytometry analysis confirmed the expression of VEGFR-3 on cultured mouse peritoneal macrophages (Figure 5G) ▶ . Importantly, VEGF-C stimulated macrophage chemotaxis in vitro in a dose-dependent manner (Figure 5H) ▶ .
Melanoma Cell Growth in Vivo and in Vitro
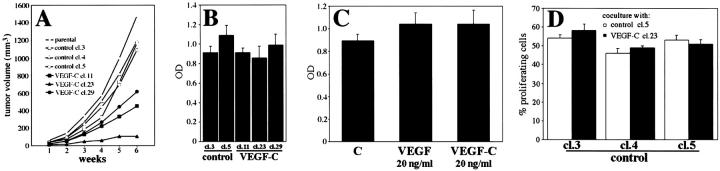
After orthotopic injection into immunodeficient mice, MeWo/control tumors reached a volume of ∼1200 mm 3 within 5 to 6 weeks after injection. Overexpression of VEGF-C did not increase the tumor growth rate, but rather resulted in growth reduction by 40 to 80% after 6 weeks, as compared to control tumors (Figure 6A) ▶ . The extent of tumor growth suppression was correlated with the macrophage densities in the peritumoral areas. In the skin surrounding control tumors, macrophages occupied 4.0 ± 0.8% of the total skin area examined. In contrast, tumors derived from MeWo/VEGF-C cl.29 cells that exhibited a growth suppression of up to 40% at 6 weeks showed an increase of peritumoral macrophage densities to 12 ± 1.5%. Tumors derived from MeWo/VEGF-C cl.23 cells that exhibited the most prominent growth suppression (up to 80%), showed further increase in macrophage densities to 23 ± 1.9%.
Figure 6.
Growth of VEGF-C-transfected MeWo cell clones in vivo and in vitro. A: Markedly decreased tumor growth of VEGF-C-transfected MeWo cells. Each point represents a mean value of 10 tumors. B: Control-transfected and VEGF-C-transfected cell clones exhibited similar proliferation rates in vitro, as determined by BrdU incorporation. C: Proliferation of control-transfected MeWo cells (clone 5) was not altered by addition of exogenous VEGF or VEGF-C (20 ng/ml), as determined by BrdU incorporation. D: MeWo/control cells showed comparable proliferation rates when co-cultured with MeWo/VEGF-C or MeWo/control cells, as determined by counting BrdU-labeled cells. Data are expressed as the mean values ± SD for a representative experiment.
To determine whether VEGF-C expression directly affected tumor cell proliferation, MeWo transfectants were tested in several in vitro assays. As measured by BrdU incorporation, no significant differences in cell proliferation rates were detected between MeWo/control and MeWo/VEGF-C clones (Figure 6B) ▶ . Moreover, the proliferation of control clones was not significantly altered on addition of 20 ng/ml recombinant human VEGF or VEGF-C to the culture medium (Figure 6C) ▶ . Proliferation of control cells was also not altered by secretion of VEGF-C by MeWo/VEGF-C cells into the culture medium in a co-culture experiment (Figure 6D) ▶ . In accordance with these findings, MeWo transfectants did not express any detectable levels of VEGFR-2 or VEGFR-3, as determined by reverse transcriptase-polymerase chain reaction, Northern analysis, and in situ hybridization (data not shown). The rate of spontaneous apoptosis in vitro was comparable between control and VEGF-C clones (1.3 ± 0.3% versus 1.1 ± 0.2%). Taken together, these results demonstrate that VEGF-C did not exert direct effects on the growth of MeWo cells.
Discussion
Whereas the significance of angiogenesis in tumors has been well documented, the ability of tumor cells to stimulate lymphangiogenesis and the existence of intratumoral lymphatic vessels have remained controversial. 8,9,51 Expression of the lymphangiogenic factor VEGF-C has been detected in many tumor types 20-32 including malignant melanomas, 20 and our results demonstrate expression of VEGF-C in several human melanoma cell lines in vitro. To directly investigate the biological role of VEGF-C in tumors, we studied the effects of VEGF-C overexpression in an orthotopic nude mouse tumor model using the human melanoma cell line MeWo 35 stably transfected with VEGF-C.
Our results demonstrate infiltration of VEGF-C-overexpressing tumors with lymphatic vessels, even in the most central tumor areas. It has been suggested previously that lymphatic vessels are absent from tumors. 8,52,53 However, most evidence in support of this concept has been indirect and has been based on the absence of detectable perfusion of lymphatic vessels after injection of contrast agent into tumors. 9,54,55 Moreover, the direct visualization of lymphatic vessels in tissue sections has been problematic because of the lack of molecular markers that reliably distinguish the lymphatic from the blood vasculature. 56 By using a recently derived antibody to the mouse hyaluronan receptor LYVE-1, that is selectively expressed on lymphatic vessels in normal tissues 39,45 and in tumors, 33 we were able to demonstrate intratumoral lymphatics in experimental melanomas overexpressing VEGF-C. All lymphatic vessels selectively expressed VEGFR-3. These results extend our recent findings that demonstrated, for the first time, the occurrence of intratumoral lymphangiogenesis in an orthotopical model of breast cancer. 33
VEGF-C overexpression also resulted in enlargement of peritumoral lymphatic vessels, whereas intratumoral lymphatics did not appear to be enlarged and were found either with an open lumen or collapsed. Under normal circumstances lymphatic capillaries are partially or fully collapsed, because of the lack of smooth muscle coverage and the low pressure within the lymphatic system. 57-59 Increased demand for fluid transport in tissue results in widening of their lumina; however, if overdistended, lymphatics become dysfunctional. 60 Hence, the functional state of lymphatic vessels cannot be deduced from their morphology, because an open lumen can indicate both vessels with dysfunction as well as normally functioning vessels with increased load. Functional studies of lymphatic vessels in mice have been extremely difficult to perform because of the low spatial resolution and the insufficient signal intensity imposed by most currently used methods. By using computed tomography and a recently developed lymphographic contrast agent with an increased concentration of iodine that results in image enhancement, 41 we have established a simple, minimally invasive procedure to measure the efficacy of lymphatic transport in mice. In comparison to control tumors, lymphatic transport from MeWo/VEGF-C tumors to regional lymph nodes was markedly retarded, indicating functional impairment of lymphatic vessels in terms of transport or uptake of fluid or functional overload because of an increase in VEGF-C-induced vascular permeability. The significance of delayed fluid clearance from tumors for the rate of tumor cell metastasis via the lymphatics remains to be established. At present, however, it is unclear how tumor cells are transported within lymphatic vessels, and the mechanisms of fluid and particle uptake into lymphatic vessels are most likely distinct from the mechanisms of tumor cell entry into the lymphatics.
VEGF-C has been reported to increase microvascular permeability in the Miles assay, with a fourfold to fivefold lower potency than VEGF. 16 VEGF-C was also 50- to 100-fold less potent than VEGF in inducing proliferation of bovine capillary endothelial cells, 10,16 human umbilical vein endothelial cells, or lung microvascular endothelial cells in vitro. 11,19 In contrast, VEGF-C promoted proliferation of human dermal microvascular endothelial cells with a similar potency as VEGF, 17 suggesting that microvascular endothelial cells derived from the skin blood vessels may be more responsive to VEGF-C than endothelial cells from other tissues. Moreover, VEGF-C acted synergistically with VEGF on in vitro angiogenesis, 17,61 suggesting that in VEGF-C-expressing melanomas VEGF-C may act in cooperation with VEGF to increase vascular permeability and angiogenesis. In fact, our results demonstrate that, in addition to its role in lymphangiogenesis, VEGF-C also acted as a tumor angiogenesis factor. We detected increased microvascular densities in VEGF-C-overexpressing melanomas by morphometric analyses of CD31-stained tumor sections. In accordance with these findings, computed tomography with an intravascular contrast agent revealed increased perfusion of MeWo/VEGF-C tumors (data not shown). This was not because of up-regulation of the major angiogenic factors VEGF, bFGF, and PDGF-B, because their expression remained comparable between VEGF-C-overexpressing and control tumors. The stimulatory effect of VEGF-C on tumor angiogenesis seemed to be mediated through VEGFR-2, because we observed marked induction of VEGFR-2 expression and phosphorylation in VEGF-C-overexpressing melanomas, as compared to control tumors.
VEGF-C was originally described as a specific growth factor for lymphatic vessels 12,13 but was later also found to induce angiogenesis of blood vessels, 18,19 raising a question about the mechanisms that determine the distinct effects of VEGF-C in vivo. As demonstrated in vitro, the secreted 31-kd VEGF-C protein predominantly activates VEGFR-3 whereas the mature, fully processed 21-kd form also activates VEGFR-2, suggesting that the biological functions of VEGF-C may be regulated by differential proteolytic processing. 16 The major VEGF-C form produced by MeWo/VEGF-C cells in vitro was the partially processed 31-kd form. In tumors however, the secreted VEGF-C was processed further to the mature 21-kd form, indicating an important role of tumor-host interactions for VEGF-C processing. As predicted by in vitro studies, the mature 21-kd form of VEGF-C stimulated both lymphangiogenesis and angiogenesis in VEGF-C-overexpressing melanomas. In contrast, overexpression of VEGF-C in breast cancer has been recently shown to selectively induce tumor lymphangiogenesis, but not tumor angiogenesis. 33 In the breast cancer model, however, the predominant VEGF-C form detected was the 31-kd form that selectively activated VEGFR-3 on the lymphatic vessels. Together, these results demonstrate that the biological effects of VEGF-C in tumors are critically dependent on the in vivo proteolytic processing and provide an explanation for the selective induction of lymphangiogenesis in certain physiological and pathological settings.
Lymphatic vessels, serving as a pathway for trafficking of antigen-presenting cells and macrophages, are important components of the immune system; therefore, activation of the lymphatic system by VEGF-C might have an impact on immune functions. In fact, we detected expression of VEGFR-3 on mouse macrophages in vitro and in vivo by flow cytometry and immunofluorescent analysis, and VEGF-C induced macrophage chemotaxis, indicating that VEGF-C can act as a direct immunomodulator. In agreement with our results, expression of VEGFR-3 has been reported in some hematopoietic and leukemia cells. 62,63 Furthermore, we observed increased densities of peritumoral macrophages in the skin surrounding VEGF-C-transfected melanomas. Enhanced host-tumor defense capabilities by increased recruitment of macrophages may explain the observed growth impediment of VEGF-C-overexpressing melanomas, because increased densities of peritumoral macrophages were correlated with the extent of tumor growth suppression. VEGF-C did not exert direct effects on tumor cells, because co-culture of VEGF-C-overexpressing cells with control cells did not result in altered proliferation of control cells, and addition of recombinant VEGF-C to control cells did not affect their growth rate in vitro. MeWo transfectants also did not express any detectable levels of VEGFR-2 or VEGFR-3, as determined by reverse transcriptase-polymerase chain reaction, Northern analysis, and in situ hybridization (data not shown). Our finding that VEGF-C did not increase the growth of melanomas is in agreement with a recent report showing that VEGF-C did not promote breast cancer growth, although it promoted tumor metastasis. 33
Taken together, our results provide evidence that the proteolytic processing of VEGF-C in vivo is critical for determining its biological function in tumors. In addition to its role as a tumor lymphangiogenesis factor, our findings identify novel functions of the mature form of VEGF-C in melanoma, acting as a tumor angiogenesis factor and as an immunomodulator.
Note Added in Proof
While this paper was being published, three other papers also reported increased tumor lymphangiogenesis in different VEGF-C or VEGF-D overexpressing mouse tumor models. 64-66
Acknowledgments
We thank Dr. David Jackson for the anti-LYVE-1 antibody, Dr. Robert Kerbel for providing the MeWo cells, Drs. Nicole Avitahl and Vladimir Joukov for helpful suggestions, and Diane Kirstead and Robert Fogle for assistance with computed tomography.
Footnotes
Address reprint requests to Michael Detmar, M.D., CBRC/Dept. of Dermatology, Massachusetts General Hospital, Building 149, 13th St., Charlestown, MA 02129. E-mail: michael.detmar@cbrc2.mgh.harvard.edu.
Supported by the Human Frontier Science Program (to M. S.), by the National Institutes of Health/National Cancer Institute (grants CA69184 and CA86410 to M. D.), by the Deutsche Forschungsgemeinschaft (to T. H.), by the Dermatology Foundation (to M. St.), and by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (to M. D.).
References
- 1.Witte MH, Way DL, Witte CL, Bernas M: Lymphangiogenesis: mechanisms, significance and clinical implications. Exs 1997, 79:65-112 [DOI] [PubMed] [Google Scholar]
- 2.Lauria R, Perrone F, Carlomagno C, De Laurentiis M, Morabito A, Gallo C, Varriale E, Pettinato G, Panico L, Petrella G, Bianco AR, De Placido S: The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer 1995, 76:1772-1778 [DOI] [PubMed] [Google Scholar]
- 3.Willis RA (Ed). The Spread of Tumors in the Human Body. St. Louis, C. V. Mosby Co., 1952, pp 18–35
- 4.Hartveit E: Attenuated cells in breast stroma: the missing lymphatic system of the breast. Histopathology 1990, 16:533-543 [DOI] [PubMed] [Google Scholar]
- 5.Cann SA, van Netten JP, Ashby TL, Ashwood-Smith MJ, van der Westhuizen NG: Role of lymphagenesis in neovascularisation. Lancet 1995, 346:903. [DOI] [PubMed] [Google Scholar]
- 6.Deutsch A, Lubach D, Nissen S, Neukam D: Ultrastructural studies on the invasion of melanomas in initial lymphatics of human skin. J Invest Dermatol 1992, 98:64-67 [DOI] [PubMed] [Google Scholar]
- 7.Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K: Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res 1998, 58:1599-1604 [PubMed] [Google Scholar]
- 8.Folkman J: Angiogenesis and tumor growth. N Engl J Med 1996, 334:921. [PubMed] [Google Scholar]
- 9.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK: Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 2000, 60:4324-4327 [PubMed] [Google Scholar]
- 10.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K: A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996, 15:290-298 [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Gray A, Yuan J, Luoh SM, Avraham H, Wood WI: Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci USA 1996, 93:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J: VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 1997, 188:96-109 [DOI] [PubMed] [Google Scholar]
- 13.Jeltsch M, Kaipainen A, Joukov V, Kukk E, Lymbousssaki AXM, Lakso M, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 14.Kaipainen A, Korhonen J, Mustonen T, van HV, Fang GH, Dumont D, Breitman M, Alitalo K: Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 1995, 92:3566-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 16.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson WL, Cao Y, Saksela O, Kalkkinen N, Alitalo K: Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 1997, 16:3898-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skobe M, Brown LF, Tognazzi K, Ganju RK, Dezube BJ, Alitalo K, Detmar M: Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi’s sarcoma. J Invest Dermatol 1999, 113:1047-1053 [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K: Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA 1998, 95:14389-14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu JS, Isner JM: Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol 1998, 153:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salven P, Lymboussaki A, Heikkila P, Jaaskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H: Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 1998, 153:103-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valtola R, Salven P, Heikkila P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K: VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol 1999, 154:1381-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurebayashi J, Otsuki T, Kunisue H, Mikami Y, Tanaka K, Yamamoto S, Sonoo H: Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res 1999, 90:977-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andre T, Kotelevets L, Vaillant JC, Coudray AM, Weber L, Prevot S, Parc R, Gespach C, Chastre E: Vegf, Vegf-B, Vegf-C and their receptors KDR, FLT-1 and FLT-4 during the neoplastic progression of human colonic mucosa. Int J Cancer 2000, 86:174-181 [DOI] [PubMed] [Google Scholar]
- 24.Akagi K, Ikeda Y, Miyazaki M, Abe T, Kinoshita J, Maehara Y, Sugimachi K: Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer 2000, 83:887-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S: Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res 2000, 6:2431-2439 [PubMed] [Google Scholar]
- 26.Ohta Y, Nozawa H, Tanaka Y, Oda M, Watanabe Y: Increased vascular endothelial growth factor and vascular endothelial growth factor-c and decreased nm23 expression associated with microdissemination in the lymph nodes in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2000, 119:804-813 [DOI] [PubMed] [Google Scholar]
- 27.Shushanov S, Bronstein M, Adelaide J, Jussila L, Tchipysheva T, Jacquemier J, Stavrovskaya A, Birnbaum D, Karamysheva A: VEGF-C and VEGFR3 expression in human thyroid pathologies. Int J Cancer 2000, 86:47-52 [DOI] [PubMed] [Google Scholar]
- 28.Bunone G, Vigneri P, Mariani L, Buto S, Collini P, Pilotti S, Pierotti MA, Bongarzone I: Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 1999, 155:l967-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellmer PT, Sato K, Tanaka R, Okamoto T, Kato Y, Kobayashi M, Shibuya M, Obara T: Vascular endothelial growth factor-C gene expression in papillary and follicular thyroid carcinomas. Surgery 1999, 126:1056-1061 [DOI] [PubMed] [Google Scholar]
- 30.Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, Bandou E, Taniguchi K, Miwa K, Ohoyama S, Sugiyama K, Sasaki T: Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 1999, 5:1823-1829 [PubMed] [Google Scholar]
- 31.Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, Carbone M, Watanabe Y, Pass HI: VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer 1999, 81:54-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP: High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 2000, 6:1900-1908 [PubMed] [Google Scholar]
- 33.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001, 7:192-198 [DOI] [PubMed] [Google Scholar]
- 34.Sordat BCM, Ueyama Y, Fogh J: The Nude Mouse in Experimental and Clinical Research. Edited by J Fogh J, BC Giovanella. New York, Academic Press, 1982, pp 95–147
- 35.Kerbel RS, Man MS, Dexter D: A model of human cancer metastasis: extensive spontaneous and artificial metastasis of a human pigmented melanoma and derived variant sublines in nude mice. J Natl Cancer Inst 1984, 72:93-108 [DOI] [PubMed] [Google Scholar]
- 36.Byers HR, Etoh T, Lee KW, Mihm MJ, Gattoni CS: Organ-specific metastases in immunodeficient mice injected with human melanoma cells: a quantitative pathological analysis. Melanoma Res 1993, 3:247-253 [PubMed] [Google Scholar]
- 37.Berse B, Brown LF, Van De Water L, Dvorak HF, Senger DR: Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 1992, 3:211-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE: Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 1997, 3:1222-1227 [DOI] [PubMed] [Google Scholar]
- 39.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG: Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001, 276:19420-19430 [DOI] [PubMed] [Google Scholar]
- 40.Detmar M, Brown LF, Schön MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK: Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol 1998, 111:1-6 [DOI] [PubMed] [Google Scholar]
- 41.Wolf GL, Shore MT, Bessin G, McIntire GL, Bacon ER, Illig KJ: Lymph node extraction of radiopaque nanoparticulates in the rabbit as measured in vivo with CT. Acad Radiol 1999, 6:55-60 [DOI] [PubMed] [Google Scholar]
- 42.Groothuis DR, Lapin GD, Vriesendorp FJ, Mikhael MA, Patlak CS: A method to quantitatively measure transcapillary transport of iodinated compounds in canine brain tumors with computed tomography. J Cereb Blood Flow Metab 1991, 11:939-948 [DOI] [PubMed] [Google Scholar]
- 43.Edelson PJ, Cohn ZA: In Vitro Methods in Cell Mediated Tumor Immunity 1. 1976:pp 333-340 Academic Press, New York
- 44.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Perruzzi CA, Detmar M: Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin, and thrombin. Am J Pathol 1996, 149:293-305 [PMC free article] [PubMed] [Google Scholar]
- 45.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG: LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999, 144:789-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA: Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (FLT-4). Proc Natl Acad Sci USA 1998, 95:548-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada Y, Nezu J, Shimane M, Hirata Y: Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics 1997, 42:483-488 [DOI] [PubMed] [Google Scholar]
- 48.Dejana E, Corada M, Lampugnani MG: Endothelial cell-to-cell junctions. FASEB J 1995, 9:910-918 [PubMed] [Google Scholar]
- 49.Weber-Matthiesen K, Sterry W: Organization of the monocyte/macrophage system of normal human skin. J Invest Dermatol 1990, 95:83-89 [DOI] [PubMed] [Google Scholar]
- 50.Austyn JM, Gordon S: F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981, 11:805-815 [DOI] [PubMed] [Google Scholar]
- 51.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 52.Jain R: Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst 1989, 81:570-576 [DOI] [PubMed] [Google Scholar]
- 53.Jain RK: The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation 1997, 4:1-23 [DOI] [PubMed] [Google Scholar]
- 54.Jain RK: Transport of molecules in the tumor interstitium: a review. Cancer Res 1987, 47:3039-3051 [PubMed] [Google Scholar]
- 55.Baxter LT, Jain RK: Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvascular Res 1990, 40:246-263 [DOI] [PubMed] [Google Scholar]
- 56.Skobe M, Detmar M: Structure, function and molecular control of the skin lymphatic system. Review. J Invest Dermatol Symp Proceed 2000, 5:14-19 [DOI] [PubMed] [Google Scholar]
- 57.Aukland K, Reed RK: Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 1993, 73:1-78 [DOI] [PubMed] [Google Scholar]
- 58.Schmid-Schönbein GW: Mechanisms causing initial lymphatics to expand and compress to promote lymph flow. Arch Histol Cytol 1990, 53:107-114 [DOI] [PubMed] [Google Scholar]
- 59.Ryan TJ: Structure and function of lymphatics. J Invest Dermatol 1989, 93:18S-24S [DOI] [PubMed] [Google Scholar]
- 60.Taylor AE, Gibson WH, Granger HJ, Guyton AC: The interaction between intracapillary and tissue forces in the overall regulation of interstitial fluid volume. Lymphology 1973, 6:192-208 [PubMed] [Google Scholar]
- 61.Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K: Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 1998, 177:439-452 [DOI] [PubMed] [Google Scholar]
- 62.Aprelikova O, Pajusola K, Partanen J, Armstrong E, Alitalo R, Bailey SK, McMahon J, Wasmuth J, Huebner K, Alitalo K: FLT4, a novel class III receptor tyrosine kinase in chromosome 5q33-qter. Cancer Res 1992, 52:746-748 [PubMed] [Google Scholar]
- 63.Fielder W, Graeven U, Ergun S, Verago S, Kilic N, Stockschlader M, Hossfeld DK: Expression of FLT4 and its ligand VEGF-C in acute myeloid leukemia. Leukemia 1997, 11:1234-1237 [DOI] [PubMed] [Google Scholar]
- 64.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS: Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001, 20:672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG: VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001, 7:186-191 [DOI] [PubMed] [Google Scholar]
- 66.Karpanen T, Egeblad M, Karkkainen MJ, Kibo H, Yla-Herttuala S, Jaattela M, Alitalo K: Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001, 61:1786-1790 [PubMed] [Google Scholar]