Abstract
Immune evasion in lung cancer results from both structural and functional alterations of human leukocyte antigen (HLA) class I molecules and the local release of immunosuppressive cytokines. Recent data suggest that HLA-G, a nonclassical class Ib molecule, is involved in immune evasion by tumor cells. We sought to determine whether HLA-G could contribute to immunescape in lung cancer. All of 19 tumor specimens examined demonstrated detectable membrane-bound (HLA-G1), as well as soluble (HLA-G5) isoform transcription. Nine of 34 (26%) tumors were positive by immunohistochemistry using monoclonal antibody (mAb) 4H84, recognizing all denatured HLA-G isoforms, of which six were positive using mAb 16G1, recognizing soluble HLA-G. HLA-G immunoreactivity correlated with high-grade histology, with HLA-G being preferentially expressed on large-cell carcinomas. In these patients, loss of classical HLA class I molecules was observed to associate with HLA-G protein up-regulation. Moreover, we found interleukin-10 expressed in 15 of 34 (44%) tumors, and in most of the HLA-G-positive cases (7 of 9), suggesting up-modulation of HLA-G by interleukin-10. It is conceivable that HLA-G expression in lung cancer might be one of the ways how the tumor down-regulates host immune response, in addition to interleukin-10 production and HLA class I loss.
Tumor escape from host immunosurveillance is frequently attributed to the release of immunosuppressive cytokines or the alteration of antigen-presenting molecules on neoplastic cells. 1-6 The complete loss of human leukocyte antigen (HLA) class I molecules results in a resistance to cytotoxic T lymphocyte (CTL)-mediated lysis, but it in turn renders tumor cells susceptible to natural killer (NK) cell-mediated killing. 7 However, a more selective lack, ie, locus-specific or allelic deletion, could allow immune evasion from effector cells, while retaining inhibitory effects on NK cells. 7,8
The nonclassical, HLA class Ib molecule, HLA-G is physiologically expressed in tissues harboring delicate immune interactions, such as the placenta and the thymus. 9 HLA-G is presumed to mediate immunological privilege for the semi-allogeneic fetus during pregnancy. 10,11 HLA-G transcripts are expressed at a low level in a variety of human adult tissues, including peripheral blood mononuclear cells and lung tissue. 12 Alternative splicing creates at least seven isoforms, of which four are membrane-bound and three coding for soluble proteins of HLA-G. 9,13,14 HLA-G is shown to protect HLA class I-deficient targets from NK-mediated lysis, through interactions with killer inhibitory receptors on NK cells. 13,15-17 The fact that lymphocytes, macrophages, and dendritic cells harbor HLA-G-specific inhibitory ligands, such as p49, ILT-2, and ILT-4, 18-20 elucidated HLA-G-mediated inhibition of cytotoxic T cell (CTL)-specific response. 21 To date, transcription of HLA-G has been reported in a variety of human malignancies, including lung cancer. 22-25 Although HLA-G protein expression was recently described on intratumoral macrophages and dendritic cells, 23,26 the HLA-G up-regulation on lung cancer cells remains controversial. 22,23
In lung cancer, the secretion of immunosuppressive Th2 type cytokines, dominated by interleukin-10, is a frequent event and contributes to the progression of disease. 1,27-29 By down-regulating HLA class I expression and selective HLA-G induction on tumor cells, 30 interleukin (IL)-10 could contribute to an impaired immune recognition of the tumor. 28,31
Using real-time quantitative polymerase chain reaction (PCR) (LightCyclerTM) we sought to determine HLA-G transcriptional levels in lung cancer. In addition, we assessed HLA-G protein expression by immunohistochemistry. Here we report that full-length membrane-bound (HLA-G1) and soluble (HLA-G5) message was detectable in all tumor specimens and controls. However, only a group of tumors displayed HLA-G immunoreactivity, of which some were as well positive for expression of the soluble isoform. HLA-G protein expression correlated with both histological tumor type and grade. Loss of classical HLA class I molecules coincided with HLA-G protein up-regulation. All HLA-G-expressing tumors demonstrated IL-10 immunoreactivity.
Materials and Methods
Tissue Samples and Cell Lines
A total of 34 lung cancer samples were collected after surgical resection, after obtaining a previous informed consent of the patient. The patients’ data, histological diagnoses, and assigned stage are summarized in Table 1 ▶ . Normal lung tissue as internal control was available from seven patients. The human choriocarcinoma HLA-G-positive cell line, JEG-3 (American Type Culture Collection, Manassas, VA) was cultured in Dulbecco’s modified Eagle’s medium (Biochrom KG, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum with antibiotics-antimycotic (GibcoBRL, Life Technologies AG, Basel, Switzerland), 1 mmol/L sodium-pyruvate (GibcoBRL, Life Technologies AG) and 2 mmol/L l-glutamine (Biochrom KG). First trimester trophoblast tissue was obtained from elective termination of pregnancy after informed consent of the patient, and used as an additional positive control.
Table 1.
Clinical, Histological, and Immunohistochemical Data from Lung Cancer Patients
| No. | Sex | Age | Tumor histology | Tumor grade | Clinical stage | HLA-G protein expression | sHLA-G protein expression | pan-HLA class I expression | IL-10 protein expression | Infiltrating NK cells |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | M | 53 | SCC | II | IIIb | R | − | FL | − | − |
| T2 | M | 66 | SCC | II | Ib | R | − | CL | − | + |
| T3 | M | 78 | LCC | III | IIIa | T | T | CL | + | + |
| T4 | M | 59 | LCC | III | Ib | T, R, S | T | CL | + | + |
| T5 | F | 62 | AC | I | Ia | R | − | No loss | + | − |
| T6 | M | 78 | LCC | III | Ib | T, R, S | T | CL | + | + |
| T7 | F | 76 | AC | II | Ia | − | − | CL | − | + |
| T8 | F | 52 | LCC | III | IIIa | T, R, S | T | CL | + | + |
| T9 | M | 48 | SCLC | − | extensive disease | T, R, S | T | FL | + | − |
| T10 | M | 69 | AC | III | Ib | R | − | FL | + | + |
| T11 | M | 70 | SCC | II | IIb | R, S | − | CL | + | − |
| T12 | M | 63 | SCC | III | IIb | R, S | − | CL | + | + |
| T13 | M | 76 | SCC | III | IIb | R | − | No loss | − | − |
| T14 | F | 54 | SCC | III | Ib | R | − | FL | − | − |
| T15 | M | 60 | AC | III | IIb | T, R | T | CL | + | + |
| T16 | F | 71 | AC | II | Ib | R, S | − | FL | − | + |
| T17 | F | 57 | LCC | III | Ib | T, S | − | CL | + | + |
| T18 | F | 56 | AC | III | IIb | T, S | − | FL | − | − |
| T19 | M | 72 | LCC | III | IIIb | − | − | CL | − | + |
| T20 | M | 48 | SCC | I | IIb | T | − | FL | − | + |
| T21 | M | 67 | AC | I | IIb | − | − | CL | + | − |
| T22 | M | 65 | AC | I | Ib | R | − | FL | − | − |
| T23 | M | 66 | AC | I | IIb | S | − | CL | − | − |
| T24 | M | 68 | AC | I | Ib | − | − | FL | − | − |
| T25 | M | 62 | AC | I | IIIA | R, S | − | FL | − | − |
| T26 | F | 74 | AC | I | IIa | − | − | FL | + | − |
| T27 | M | 68 | AC | I | IIIa | S | − | CL | + | + |
| T28 | M | 71 | AC | I | Ib | − | − | CL | − | − |
| T29 | M | 74 | SCC | II | Ia | R, S | − | FL | + | − |
| T30 | M | 61 | SCC | II | IIIa | S | − | FL | − | + |
| T31 | M | 66 | AC | II | IV | R | − | FL | − | + |
| T32 | M | 65 | AC | II | Ib | R, S | − | CL | − | + |
| T33 | M | 76 | AC | II | IIb | R | − | CL | − | − |
| T34 | M | 59 | SCC | II | Ia | R | − | CL | − | + |
M, male; F, female; SCC, squamous cell carcinoma; LCC, large cell carcinoma; AC, adenocarcinoma; SCLC, small cell lung cancer; R, immunoreactivity of residual bronchial structures; S, immunoreactivity of tumor-surrounding lung; T, tumor immunoreactivity; CL, complete loss of pan-class I expression (defined as less than 25% of the cells showing immunoreactivity with TP25.99 mAb); FL, focal loss of pan-class I expression (circumscribed area of TP25.99 immunonegative cells within the tumor); +, immunoreactivity; −, no immunoreactivity with anti-sHLA-G, anti-IL-10 or anti-CD56 mAb.
RNA Extraction and cDNA Synthesis
Total RNA was extracted from 19 frozen tumor and 7 control lung tissue samples using High Pure RNA tissue kit (Roche Molecular Biochemicals, GmbH, Mannheim, Germany) according to the manufacturer’s recommendations. Approximately 1 μg of RNA was reverse-transcribed using oligo-p(dT)15 priming and avian myeloblastosis virus (AMV) reverse transcriptase [First Strand cDNA synthesis kit for reverse transcriptase-PCR (AMV), Roche Molecular Biochemicals] at 42°C for 1 hour. cDNAs were then stored at −20°C until further use.
Real-Time Quantitative PCR
HLA-G-specific PCR amplifications were performed using the following primer sets: U522(exon 3) and L922 (exon 5) detecting full-length membrane-bound HLA-G1 isoform; G5U522 (exon 3) and G5L990 (intron 4) amplifying full-length soluble, HLA-G5 isoform (Table 2 ▶ .). Design of these two primer pairs and optimization for the use in LightCycler quantification system was done with the Oligo 5.0 primer analysis software (Molecular Biology Insights Inc., Cascade, CO). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and plasmids pCRII.GAPDH were kindly provided by John Gribben (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA). A Hot-Start PCR was performed using 2 μl of ready-to-use mastermix (LightCycler-Faststart DNA Master SYBR Green I, Roche Molecular Biochemicals, containing thermostable recombinant Taq polymerase, reaction buffer, dATP, dCTP, dGTP, dUTP), 0.5 μmol/L of each oligonucleotide primers (desalted PCR grade; Microsynth, Balgach, Switzerland), variable free MgCl2 concentrations (Table 2) ▶ , 2 μl of cDNA, and water to a final volume of 20 μl. After an initial denaturation of 7 minutes to activate the FastStart enzyme, amplification occurred as a three-step cycling procedure: denaturation at 95°C for 15 seconds, ramp rate 20°C/second; annealing (Table 2) ▶ , 10 seconds, ramp rate 20°C/second; and elongation at 72°C (Table 2) ▶ , ramp rate 2°C/second, for 40 cycles. The acquisition of fluorescence was done at 87°C to avoid contribution of nonspecific products to the overall signal. Finally, the temperature was raised gradually (0.2°C/second) starting from 70 to 99°C for the melting curve analysis.
Table 2.
Oligonucleotides and PCR Conditions Used
| Primers | Amplicon size, bp | Annealing °t, elongation time | Free MgCl2 | |
|---|---|---|---|---|
| U522 (exon 3) | 5′-CAA TGT GGC TGA ACA AAG GA-3′ | |||
| L922 (exon 5) | 5′-CCA GCA ACG ATA CCC ATG-3′ | 420 | 60°C; 25 s | 4 mmol/L |
| G5U522 (exon 3) | 5′-CAA TGT GGC TGA ACA AAG GAG AG-3′ | |||
| G5L990 (intron 4) | 5′-ACC GAC CCT GTT AAA GGT CTT-3′ | 450 | 58°C; 25 s | 3 mmol/L |
| GAP1U19 | 5′-GAA GGT GAA GGT CGG AGT C-3′ | |||
| GAP206L19 | 5′-GAA GAT GGT GAT GGG ATT T-3′ | 224 | 55°C; 10 s | 4 mmol/L |
External standards for the GAPDH quantification consisted of six serial 1:10 dilutions (5 × 10 2 to 5 × 10 7 molecules per reaction) of pCRII.GAPDH plasmids. Plasmids containing the full-length HLA-G insert, pLNCX.G1 32 were used as six external standards for HLA-G1 and HLA-G5 quantitative PCR, containing 10 2 to10 7 copies per reaction (a kind gift from D. E. Geraghty, Fred Hutchinson Cancer Research Center, Seattle, WA). External standards were run concomitantly with patient samples under identical conditions. The PCR reactions were run as triplicates, and run data were analyzed with the quantification program V3.39 (Roche Molecular Biochemicals). The fluorescence signal was plotted against the cycle number for all samples and external standards. The fit-points method option was used in the course of analyzing quantification data, allowing the definition of a noise band and subsequent background fluorescence subtraction, and resulting in the display of only log-linear and plateau amplification phases. The point when the signal rose above the background level, so-called “crossing point” was then determined for each standard dilution. The standard curve was then generated for each run, plotting the crossing point against the log concentration of the standards. The single HLA-G1, HLA-G5, and GAPDH standard curves as a result of triplicate runs were then used as references to calculate the number of target molecules in each sample. Results were expressed initially as the absolute copy number/μl. Normalization of the estimated HLA-G1 or HLA-G5 amount was achieved by calculating the ratios between HLA-G1 or HLA-G5 and GAPDH copy number in 1 μl of cDNA, to compensate the variations in quantity and quality of starting mRNA. The normalized values were then multiplied by the constant 104.
Each amplification product was analyzed for appropriate length by electrophoresis of on 1.6% agarose gel stained with ethidium bromide. The estimated size of the amplified fragments matched the calculated size. In addition product identity was confirmed by melting curve analysis, an application in the LightCycler analysis program. The specificity of the obtained PCR products was finally confirmed by sequencing.
Immunohistochemistry
In all 34 cases, formalin-fixed paraffin-embedded material was available for immunohistochemistry. After microwave antigen recovery an alkaline phosphatase-anti-alkaline phosphatase technique with the following primary antibodies (Abs) was performed: 4H84 (1:500 dilution), IgG1 mouse monoclonal (mAb) raised against denatured HLA-G α1-domain (kindly provided by M. McMaster, University of California, San Francisco, CA); 16G1 (1:250) IgG2a mouse mAb detecting soluble HLA-G (kindly provided by D. E. Geraghty, Fred Hutchinson Cancer Research Center, Seattle, WA); anti-MHC class I mAb TP25.99, reacting with HLA-A, -B, -C, but not HLA-G (kind gift from S. Ferrone, Roswell Park Cancer Institute, Buffalo, NY); anti-human IL-10 (E-10) mouse IgG2b mAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-human CD56 mouse IgG1 mAb (Novocastra Laboratories Ltd., Newcastle, UK); isotype-matched controls IgG1, IgG2a, and IgG2b (DAKO, Glostrup, Denmark).
Double stainings were performed for IL-10 and CD56 against the mAb 4H84 (1:300 dilution). Briefly, after deparaffinization and antigen retrieval, nonspecific binding sites were blocked by incubating slides with 20% AB serum/phosphate-buffered saline for at least 15 minutes at room temperature. Tissue sections were incubated with different primary antibodies or isotype-matched control and secondarily, always with 4H84 mAb for 60 minutes. Each antibody application was followed by two cycles of sequential incubations with rabbit anti-mouse IgG and alkaline phosphatase-anti-alkaline phosphatase-complexes (DAKO). The immunoreaction was visualized with developing solutions, containing blue-purple 5-bromo-4-chloro-3-indoxyl phosphate with nitro-blue-tetrazolium-chloride (BCIP/NBT, from DAKO), which labeled primary antibody and red neufuchsin (DAKO) marking HLA-G. Finally, sections were counterstained with hematoxylin. All of the incubations were performed at room temperature in a moist chamber.
Statistical Analysis
Statistical analysis was performed using a SPSS statistical software (version 10.0; SPSS, Inc.)
Results
Differential Expression of HLA-G mRNA Isoforms
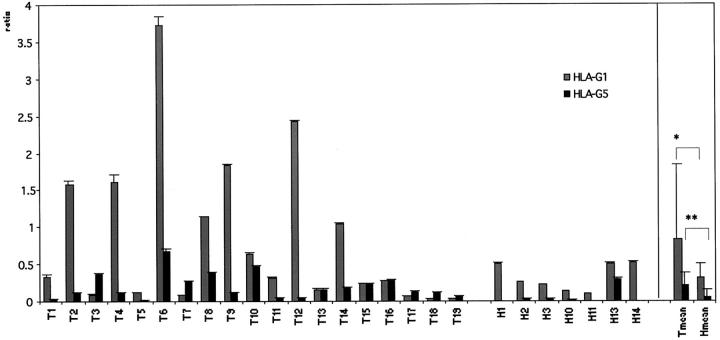
Quantitative reverse transcriptase (RT)-PCR analysis revealed that all tumor samples had detectable levels of both HLA-G1 and HLA-G5 transcripts (Figure 1) ▶ . However, uninvolved lung tissue specimens transcribed preferentially full-length membrane-bound isoform HLA-G1 (Figure 1) ▶ . Tumor tissue samples displayed an average of a 2.6-fold increase in HLA-G1 (t-test, P = 0.047) and a fourfold increase in HLA-G5 transcription (t-test, P = 0.016) when compared to unaffected lung samples (Figure 1) ▶ . The transcriptional level for soluble HLA-G isoforms was significantly lower than for the membrane bound (t-test, P = 0.01). Variable HLA-G transcription within the samples as determined by quantitative PCR however did not correlate to the tumor histology.
Figure 1.
Relative HLA-G1 and HLA-G5 transcriptional levels in lung cancer. Values are expressed as mean ± SD GAPDH-balanced ratios (as a result of triplicate runs), multiplied with 104. Pattern of HLA-G1 and HLA-G5 mRNA expression in tumor (n = 19) versus tumor-unaffected (n = 7) tissue. Student’s t-test was performed: *, P = 0.047; **, P = 0.016.
Tumors of High-Grade Histology Preferentially Express HLA-G Protein
Immunohistochemistry detected HLA-G in 9 of 34 tumors (26%), of which 6 (18%) were also positive for sHLA-G protein (Table 1 ▶ and Figure 2; A to C ▶ ). HLA-G positivity for membrane-bound isoform (mAb 4H84) and soluble HLA-G isoform (mAb 16G1) was heterogeneous, ranging from single cells to larger clusters of positive cells (Figure 2, A to D) ▶ .
Figure 2.
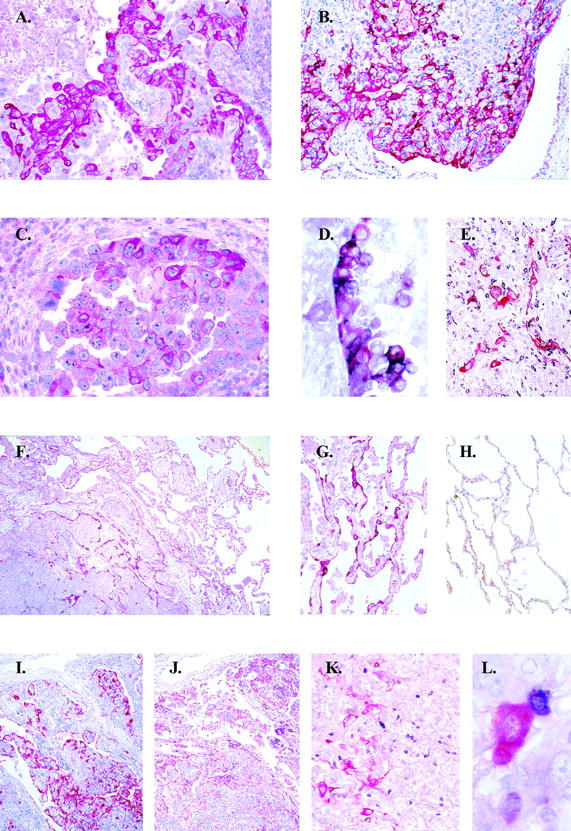
Immunohistochemistry of lung tumors and adjacent lung tissue. A: Expression of membrane-bound HLA-G (mAb 4H4) on a large-cell carcinoma, T3. B: Heterogeneous membrane-bound HLA-G expression on a large-cell carcinoma, T17. C: Expression of soluble HLA-G (mAb 16G1) on a large-cell carcinoma, T3. D: Double staining for IL-10 (black chromogen) and membrane-bound HLA-G (red chromogen) in a large-cell carcinoma showing HLA-G-positive tumor cells co-expressing IL-10 (violet chromogen), T3. E: HLA-G-expressing tumor cells associated with IL-10-positive infiltrating lymphocytes, T8. F: Induction of membrane-bound HLA-G on residual lung tissue infiltrated by the tumor, T6. G: Induction of membrane-bound HLA-G on lung tissue adjacent to the tumor. H: Negativity in lung tissue distant to the tumor. I and J: HLA-G expression (I) and partial loss of MHC class I expression (J) on tumor cells on corresponding tumor sections, T17. K and L: Double staining for CD56 (black chromogen) and membrane-bound HLA G (red chromogen) showing close association of NK cells with intact HLA-G-expressing tumor cells, T18. Original magnifications: ×200 (A, E, G, H, and K), ×100 (B), ×400 (C and D), ×50 (F, I, and J), ×960 (L).
In HLA-G-positive tumors, the loss of HLA class I immunoreactivity was a consistent finding (Figure 2, H and I) ▶ . In six of nine cases we detected complete loss of HLA class I molecules and focal loss in the remaining three cases (Table 1) ▶ . Conversely, tumor-unaffected lung displayed no detectable HLA-G protein expression, whereas positivity for HLA class I antigens was found as expected (Figure 2, G to I) ▶ . Immunoreactivity with 4H84 was mainly detected on large-cell carcinomas (Spearman’s rho = 0.529, P = 0.001) and on the tumors of high-grade histology (Spearman’s rho = 0.489, P = 0.004) (Figure 2, A and B) ▶ . Accordingly, the expression of sHLA-G was primarily encountered in large-cell carcinomas (Spearman’s rho = 0.582, P < 0.001) and in high-grade tumors (Spearman’s rho = 0.495, P = 0.003) (Figure 2C) ▶ . In addition, sHLA-G protein expression correlated with HLA-G5 transcriptional levels (Spearman’s rho = 0.455, P = 0.05).
NK Cell Infiltrate in the Absence of HLA Class I Expression
NK cells infiltrating the tumor margin were detected by immunohistochemistry in 18 (53%) of the tumor samples. In seven of the cases NK cells were found adjacent to HLA-G-positive tumor cells (Figure 2, J and K) ▶ . NK cell infiltration associated with focal or complete loss of HLA class I molecules on tumor cells (Spearman’s rho = −0.338, P = 0.05) (Table 1) ▶ .
HLA-G Protein Up-Regulation in Tumor-Associated Lung Tissue
Apart from the tumor cells positivity, two additional patterns of HLA-G expression could be observed. Within the tumor, residual alveolar structures displayed HLA-G immunoreactivity in the majority [20 (59%)] of the cases (Figure 2E) ▶ . The second observed pattern consisted of HLA-G expression in tumor surrounding the lung parenchyma in 16 of 34 cases (Figure 2, E and F) ▶ . In eight of these cases HLA-G positivity of the neighboring lung could be observed, although the tumor was HLA-G-negative (Figure 2E) ▶ . Additionally, tumor-infiltrating lymphocytes were found to be positive for HLA-G expression in 10 (29%) cases. Macrophages and dendritic cells displayed occasional HLA-G immunoreactivity, and positivity was independent of the tumor histological type (data not shown).
IL-10 Expression Coincides with HLA-G Protein Up-Regulation
Interleukin-10-producing cells were detected in 15 (44%) cases, where HLA-G was either expressed by the tumor in 7 of 9 cases (Spearman’s rho = 0.407, P = 0.017). The tumor cells and tumor-infiltrating lymphocytes were the source of IL-10 (Figure 2D) ▶ . IL-10-producing cells were often localized in the vicinity of the HLA-G-positive cells (Figure 2D) ▶ .
Discussion
Published data suggest that the expression of HLA-G interferes with the function of immunocompetent NK and T cells leading to immunological tolerance. 13,15 Besides beneficial effects on semigeneic and allogeneic graft acceptance, 33,34 the induction of the HLA-G molecule may be used by viruses 35 and tumors 23,36,37 to evade host immunosurveillance. We have investigated the expression of this nonclassical HLA class I molecule in lung cancer, addressing whether HLA-G could serve as an additional mechanism modulating anti-tumor immune response.
Here we demonstrate that HLA-G message is present in tumors as well as in uninvolved lung tissue. Although full-length membrane-bound and soluble HLA-G transcripts were detected in all tumor samples examined, HLA-G1 transcriptional levels were on average higher than those of HLA-G5. 24 However, normal uninvolved lung tissue preferentially transcribed membrane-bound, HLA-G1 isoform as previously reported. 12 We also show considerable intertumoral variability in HLA-G isoform transcription that might reflect differences in tumor biology. It is likely that the tumor environment, including tumor-infiltrating cells could contribute to the fact that HLA-G transcription in the average was significantly higher in tumors as in uninvolved lung tissue. 23,24,38
Immunohistochemistry detected HLA-G protein expression in approximately one third of the tumor specimens. A substantial proportion of these tumors was also positive for soluble HLA-G. Although HLA-G message was present in all tumors available for PCR analysis, protein expression could not be detected in some cases, suggesting strong posttranscriptional regulatory mechanisms. 39 In fact, HLA-G protein could not be detected in three cases despite elevated transcriptional levels. HLA-G was preferentially induced on large-cell carcinomas and on tumors of high histological grade, that are associated with a poor prognosis. 36 A frequent focal or complete loss of HLA class I molecules associated with HLA-G protein expression in our tumor samples indicating an additional factor for impaired immune recognition by effector cells. 8,36 With its co-expression on tumor cells, bearing intact HLA class I molecules, HLA-G might enhance class I-mediated NK lysis inhibition, by acting as a major inhibitory ligand. 40,41 Given its role in inhibition of allo- and antigen-specific CTL response, 21,41,42 tumors bearing HLA-G would accomplish escape from cell-mediated immunosurveillance.
Of note, the expression of soluble HLA-G on transcriptional and protein level seemed to be a phenomenon restricted to malignant tissue, because its induction could not be detected outside the tumor tissue. It remains speculative whether in poorly differentiated lung tumors, the release of soluble HLA-G into the blood stream may contribute to the systemic immunosuppression by inducing apoptosis in T cells. 42,43 In addition, HLA-G expression at the tumor-lung interface as well as in tumor-infiltrating lymphocytes, macrophages, and dendritic cells might promote the presentation of tumor derived- and self-peptides, 23,37,44,45 which in turn could provide a tolerant environment for the spread of disease.
In lung cancer, the impairment of anti-tumor immunity 46 is frequently associated with a shift toward a Th2 cytokine profile during progression of the disease, 27 resulting in unresponsiveness to the autologous tumor cells. 1,4,28,29 It has been demonstrated previously that within the Th2 cytokine profile, IL-10 has the capability to induce HLA-G expression. 30,47 Because HLA-G up-regulation associated with IL-10 secretion either from the tumors or subsets of tumor infiltrating cells, HLA-G expression might be one of the mechanisms how IL-10 exerts its immunosuppressive effects. Via nonclassical action modus, HLA-G could also mediate the induction of IL-10 and other Th2 type cytokines, 48-50 thereby forming a vicious autocrine circle of immune response abrogation in cancer. 30,42
In conclusion, HLA-G protein expression that localizes to both the lung tumor as well as the tumor-lung interface may participate in a dynamic immunoregulatory process enabling the tumor expansion. Our data suggest, that both inflammatory cells and tumor-derived cytokines could further facilitate immune response evasion through HLA-G up-regulation. Additional studies on the structural and functional alterations of HLA-G expression in lung tumors may help to identify those patients who could benefit from immunomodulatory treatment.
Footnotes
Address reprint requests to Dr. Andreas Trojan, Abteilung Onkologie, Department Innere Medizin, UniversitätsSpital Zürich, Rämistrasse 100, 8091 Zürich, Switzerland. E-mail: andreas.trojan@dim.usz.ch.
Supported by the Kanton Zürich Cancer League (to A. T.).
A part of this work was presented at the 92nd Annual Meeting of American Association for Cancer Research, March 24 to 28, 2001, New Orleans, LA, for which M. U. received the American Association for Cancer Research Scholar-in-Training Award.
References
- 1.Smith DR, Kunkel SL, Burdick MD, Wilke CA, Orringer MB, Whyte RI, Strieter RM: Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol 1994, 145:18-25 [PMC free article] [PubMed] [Google Scholar]
- 2.Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC: Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 1996, 73:148-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraki A, Kaneshige T, Kiura K, Ueoka H, Yamane H, Tanaka M, Harada M: Loss of HLA haplotype in lung cancer cell lines: implications for immunosurveillance of altered HLA class I/II phenotypes in lung cancer. Clin Cancer Res 1999, 5:933-936 [PubMed] [Google Scholar]
- 4.Yanagawa H, Takeuchi E, Suzuki Y, Hanibuchi M, Haku T, Ohmoto Y, Sone S: Production of interleukin-10 by alveolar macrophages from lung cancer patients. Respir Med 1999, 93:666-671 [DOI] [PubMed] [Google Scholar]
- 5.Gilboa E: How tumors escape immune destruction and what we can do about it. Cancer Immunol Immunother 1999, 48:382-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geertsen R, Hofbauer G, Kamarashev J, Yue FY, Dummer R: Immune escape mechanisms in malignant melanoma. Int J Mol Med 1999, 3:49-57 [DOI] [PubMed] [Google Scholar]
- 7.Rees RC, Mian S: Selective MHC expression in tumours modulates adaptive and innate antitumour responses. Cancer Immunol Immunother 1999, 48:374-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Marincola FM, Ferrone S: HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today 1999, 5:178-186 [DOI] [PubMed] [Google Scholar]
- 9.Carosella ED, Rouas Freiss N, Paul P, Dausset J: HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today 1999, 20:60-62 [DOI] [PubMed] [Google Scholar]
- 10.Rouas Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED: Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA 1997, 94:11520-11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biassoni R, Bottino C, Millo R, Moretta L, Moretta A: Natural killer cell-mediated recognition of human trophoblast. Semin Cancer Biol 1999, 9:13-18 [DOI] [PubMed] [Google Scholar]
- 12.Onno M, Guillaudeux T, Amiot L, Renard I, Drenou B, Hirel B, Girr M, Semana G, Le Bouteiller P, Fauchet R: The HLA-G gene is expressed at a low mRNA level in different human cells and tissues. Hum Immunol 1994, 41:79-86 [DOI] [PubMed] [Google Scholar]
- 13.Rouas Freiss N, Khalil-Daher I, Riteau B, Menier C, Paul P, Dausset J, Carosella ED: The immunotolerance role of HLA-G. Semin Cancer Biol 1999, 9:3-12 [DOI] [PubMed] [Google Scholar]
- 14.Paul P, Adrian Cabestre F, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Moya Quiles RM, Bermond F, Dausset J, Carosella ED: Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol 2000, 61:1138-1149 [DOI] [PubMed] [Google Scholar]
- 15.Khalil-Daher I, Rouas-Freiss N, Carosella ED, Dausset JB: Human leukocyte antigen-G: immunotolerant major histocompatibility complex molecule in transplantation. World J Surg 2000, 24:819-822 [DOI] [PubMed] [Google Scholar]
- 16.Pazmany L, Mandelboim O, Vales Gomez M, Davis DM, Becker TC, Reyburn HT, Seebach JD, Hill JA, Strominger JL: Human leucocyte antigen-G and its recognition by natural killer cells. J Reprod Immunol 1999, 43:127-137 [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Botet M, Navarro F, Llano M: How do NK cells sense the expression of HLA-G class Ib molecules? Semin Cancer Biol 1999, 9:19-26 [DOI] [PubMed] [Google Scholar]
- 18.Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez Botet M: A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 1997, 186:1809-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O’Callaghan CA, Dunbar R, Ogg GS, Cerundolo V, Rolink A: Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol 1998, 160:3096-3100 [PubMed] [Google Scholar]
- 20.Cantoni C, Verdiani S, Falco M, Pessino A, Cilli M, Conte R, Pende D, Ponte M, Mikaelsson MS, Moretta L, Biassoni R: p49, a putative HLA class I-specific inhibitory NK receptor belonging to the immunoglobulin superfamily. Eur J Immunol 1998, 28:1980-1990 [DOI] [PubMed] [Google Scholar]
- 21.Le Gal FA, Riteau B, Sedlik C, Khalil Daher I, Menier C, Dausset J, Guillet JG, Carosella ED, Rouas Freiss N: HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol 1999, 11:1351-1356 [DOI] [PubMed] [Google Scholar]
- 22.Real LM, Cabrera T, Collado A, Jimenez P, Garcia A, Ruiz Cabello F, Garrido F: Expression of HLA G in human tumors is not a frequent event. Int J Cancer 1999, 81:512-518 [DOI] [PubMed] [Google Scholar]
- 23.Pangault C, Amiot L, Caulet Maugendre S, Brasseur F, Burtin F, Guilloux V, Drenou B, Fauchet R, Onno M: HLA-G protein expression is not induced during malignant transformation. Tissue Antigens 1999, 53:335-346 [DOI] [PubMed] [Google Scholar]
- 24.Paul P, Cabestre FA, Le Gal FA, Khalil Daher I, Le Danff C, Schmid M, Mercier S, Avril MF, Dausset J, Guillet JG, Carosella ED: Heterogeneity of HLA-G gene transcription and protein expression in malignant melanoma biopsies. Cancer Res 1999, 59:1954-1960 [PubMed] [Google Scholar]
- 25.Paul P, Rouas-Freiss N, Khalil-Daher I, Moreau P, Riteau B, Le Gal FA, Avril MF, Dausset J, Guillet JG, Carosella ED: HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA 1998, 95:4510-4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onno M, Le Friec G, Pangault C, Amiot L, Guilloux V, Drenou B, Caulet-Maugendre S, Andre P, Fauchet R: Modulation of HLA-G antigens expression in myelomonocytic cells. Hum Immunol 2000, 61:1086-1094 [DOI] [PubMed] [Google Scholar]
- 27.Ito N, Nakamura H, Tanaka Y, Ohgi S: Lung carcinoma: analysis of T helper type 1 and 2 cells and T cytotoxic type 1 and 2 cells by intracellular cytokine detection with flow cytometry. Cancer 1999, 85:2359-2367 [PubMed] [Google Scholar]
- 28.Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, Dubinett SM: T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol 1999, 163:5020-5028 [PubMed] [Google Scholar]
- 29.De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, Catalano G: Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest 2000, 117:365-373 [DOI] [PubMed] [Google Scholar]
- 30.Moreau P, Adrian Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P: IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol 1999, 11:803-811 [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci MG, Kiessling R: Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med 1994, 180:2371-2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E: An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc Natl Acad Sci USA 1994, 91:4209-4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lila N, Carpentier A, Amrein C, Khalil-Daher I, Dausset J, Carosella ED: Implication of HLA-G molecule in heart-graft acceptance. Lancet 2000, 355:2138. [DOI] [PubMed] [Google Scholar]
- 34.Thellin O, Coumans B, Zorzi W, Igout A, Heinen E: Tolerance to the foeto-placental ’graft’: ten ways to support a child for nine months. Curr Opin Immunol 2000, 12:731-737 [DOI] [PubMed] [Google Scholar]
- 35.Onno M, Pangault C, Le Friec G, Guilloux V, Andre P, Fauchet R: Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J Immunol 2000, 164:6426-6434 [DOI] [PubMed] [Google Scholar]
- 36.Wagner SN, Rebmann V, Willers CP, Grosse-Wilde H, Goos M: Expression analysis of classic and non-classic HLA molecules before interferon alfa-2b treatment of melanoma. Lancet 2000, 356:220-221 [DOI] [PubMed] [Google Scholar]
- 37.Fukushima Y, Oshika Y, Nakamura M, Tokunaga T, Hatanaka H, Abe Y, Yamazaki H, Kijima H, Ueyama Y, Tamaoki N: Increased expression of human histocompatibility leukocyte antigen-G in colorectal cancer cells. Int J Mol Med 1998, 2:349-351 [DOI] [PubMed] [Google Scholar]
- 38.Amiot L, Onno M, Renard I, Drenou B, Guillaudeux T, Le Bouteiller P, Fauchet R: HLA-G transcription studies during the different stages of normal and malignant hematopoiesis. Tissue Antigens 1996, 48:609-614 [DOI] [PubMed] [Google Scholar]
- 39.Copeman J, Han RNN, Caniggia I, McMaster M, Fisher SJ, Cross JC: Posttranscriptional regulation of human leukocyte antigen G during human extravillous cytotrophoblast differentiation. Biol Reprod 2000, 62:1543-1550 [DOI] [PubMed] [Google Scholar]
- 40.Riteau B, Menier C, Khalil-Daher I, Martinozzi S, Pla M, Dausset J, Carosella E, Rouas-Freiss N: HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol 2001, 13:193-201 [DOI] [PubMed] [Google Scholar]
- 41.Riteau B, Rouas-Freiss N, Menier C, Paul P, Dausset J, Carosella ED: HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol 2001, 166:5018-5026 [DOI] [PubMed] [Google Scholar]
- 42.Kapasi K, Albert SE, Yie S, Zavazava N, Librach CL: HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology 2000, 101:191-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fournel S, Aguerre Girr M, Huc X, Lenfant F, Alam A, Toubert A, Bensussan A, Le Bouteiller P: Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 2000, 164:6100-6104 [DOI] [PubMed] [Google Scholar]
- 44.Lee N, Malacko AR, Ishitani A, Chen MC, Bajorath J, Marquardt H, Geraghty DE: The membrane-bound and soluble forms of HLA-G bind identical sets of endogenous peptides but differ with respect to TAP association. Immunity 1995, 3:591-600 [DOI] [PubMed] [Google Scholar]
- 45.Diehl M, Munz C, Keilholz W, Stevanovic S, Holmes N, Loke YW, Rammensee HG: Nonclassical HLA-G molecules are classical peptide presenters. Curr Biol 1996, 6:305-314 [DOI] [PubMed] [Google Scholar]
- 46.Ortegel JW, Staren ED, Faber LP, Warren WH, Braun DP: Cytokine biosynthesis by tumor-infiltrating T lymphocytes from human non-small-cell lung carcinoma. Cancer Immunol Immunother 2000, 48:627-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hennessy A, Pilmore HL, Simmons LA, Painter DM: A deficiency of placental IL-10 in preeclampsia. J Immunol 1999, 163:3491-3495 [PubMed] [Google Scholar]
- 48.Maejima M, Fujii T, Kozuma S, Okai T, Shibata Y, Taketani Y: Presence of HLA-G-expressing cells modulates the ability of peripheral blood mononuclear cells to release cytokines. Am J Reprod Immunol 1997, 38:79-82 [DOI] [PubMed] [Google Scholar]
- 49.Hamai Y, Fujii T, Yamashita T, Miki A, Kozuma S, Geraghty DE, Taketani Y: Peripheral blood mononuclear cells from women with recurrent abortion exhibit an aberrant reaction to release cytokines upon the direct contact of human leukocyte antigen-G-expressing cells. Am J Reprod Immunol 1998, 40:408-413 [DOI] [PubMed] [Google Scholar]
- 50.Kanai T, Fujii T, Unno N, Yamashita T, Hyodo H, Miki A, Hamai Y, Kozuma S, Taketani Y: Human leukocyte antigen-G-expressing cells differently modulate the release of cytokines from mononuclear cells present in the decidua versus peripheral blood. Am J Reprod Immunol 2001, 45:94-99 [DOI] [PubMed] [Google Scholar]