Abstract
The numbers of functional olfactory receptor (OR) genes in humans and mice are about 400 and 1,000 respectively. In both humans and mice, these genes exist as genomic clusters and are scattered over almost all chromosomes. The difference in the number of genes between the two species is apparently caused by massive inactivation of OR genes in the human lineage and a substantial increase of OR genes in the mouse lineage after the human–mouse divergence. Compared with mammals, fishes have a much smaller number of OR genes. However, the OR gene family in fishes is much more divergent than that in mammals. Fishes have many different groups of genes that are absent in mammals, suggesting that the mammalian OR gene family is characterized by the loss of many group genes that existed in the ancestor of vertebrates and the subsequent expansion of specific groups of genes. Therefore, this gene family apparently changed dynamically depending on the evolutionary lineage and evolved under the birth-and-death model of evolution. Study of the evolutionary changes of two gene families for vomeronasal receptors and two gene families for taste receptors, which are structurally similar, but remotely related to OR genes, showed that some of the gene families evolved in the same fashion as the OR gene family. It appears that the number and types of genes in chemosensory receptor gene families have evolved in response to environmental needs, but they are also affected by fortuitous factors.
Keywords: Olfactory receptors, Multigene family, Birth-and-death evolution, Vomeronasal receptors, Taste receptors, Phylogenetic analysis, Vertebrate evolution
Introduction
Olfaction, the sense of smell, is essential for the survival of animals. Olfactory signals are used to find foods, identify mates and offspring, recognize territories, and avoid danger. The proteins for detecting odor molecules are called olfactory receptors (ORs). In order to distinguish among a wide variety of odor molecules in the environment, there are a huge number of OR genes in animal genomes. In the case of mice, the number of OR genes is as large as 1,000, which comprises about 4% of the entire proteome. OR genes form the largest multi-gene family in mammals.
Olfactory receptor genes were discovered by Buck and Axel (1991), and this discovery opened the door for molecular studies of the olfactory system. For this achievement, they were awarded the 2004 Nobel Prize in Physiology or Medicine (Firestein 2004). OR genes are mainly expressed in sensory neurons of main olfactory epithelia (MOEs) in the nasal cavity. It is generally believed that each olfactory neuron expresses only one of the hundreds of functional OR genes (Chess et al. 1994; Malnic et al. 1999; Serizawa et al. 2000), but this one neuron–one receptor hypothesis is still controversial (Mombaerts 2004a). OR genes are also expressed in testis, and it has been suggested that they mediate sperm chemotaxis (Parmentier et al. 1992; Spehr et al. 2003). ORs are G-protein coupled receptors (GPCRs) that contain seven transmembrane α-helical regions. It has been suggested that an odor molecule binds to a pocket formed by the third, fifth, and sixth α-helices (Hall et al. 2004; Katada et al. 2005). Binding of an odorous ligand to an OR activates a G protein and subsequent signaling cascades. OR genes do not have any introns in their coding regions, as in many other GPCR genes. This makes it easy to identify OR genes from genomic sequences.
Since the genomic sequence is now available from a number of species in vertebrates, many authors have attempted to determine the total number of OR genes in each model organism (e.g., humans or mice). Although the number of genes identified in this way is not necessarily accurate, this information has contributed greatly to our understanding of the patterns and mechanisms of evolution of OR genes (reviewed by Firestein 2001; Laurent 2002; Mombaerts 2004b; Ache and Young 2005). In the past few years, we have also studied this problem using the DNA sequences of functional OR genes and pseudogenes that were determined as accurately as possible (Niimura and Nei 2003, 2005a, 2005b, 2005c).
In this paper we present a brief review of recent progress in the study of evolution of OR genes in vertebrates. However, we are not intending to cover all the papers concerning this subject. Rather, we are interested in discussing the general principles of evolution of OR genes. We are also going to include a substantial amount of our own work to illustrate our major arguments, partly because it has been done recently and partly because we are familiar with detailed aspects of the research conducted. Furthermore, we will consider the evolution of vomeronasal and taste receptor genes to have a better understanding of chemosensory receptor genes.
OR genes in the human genome
Number of functional genes and pseudogenes
The number of OR genes in humans has been studied by a number of authors (Glusman et al. 2001; Zozulya et al. 2001), but these early studies were conducted by using draft human genome sequences. In view of this situation, we decided to use the final (or semi-final) human genome sequence data and identified all the functional OR genes and pseudogenes by using bioinformatic techniques (Niimura and Nei 2003). Here, any gene with a complete open reading frame was defined as a functional gene (intact gene), and a gene with interrupting stop codons, frameshift mutations or long deletions was defined as a pseudogene. The numbers of functional genes and pseudogenes identified in this way were 388 and 414 respectively. The number of functional genes was considerably greater than the previous estimates (Glusman et al. 2001; Zozulya et al. 2001).
We also determined the genomic location of all of these genes. This study showed that OR genes exist as genomic clusters (clusters of genes in specific genomic locations; Fig. 1), and these clusters are scattered all over the chromosomes except chromosomes 20 and Y, as was previously observed (Glusman et al. 2001). Here, a genomic cluster was defined as a cluster of OR genes that are located within 500 kb or less between any pair of neighboring OR genes in a given genomic location. Examining all chromosomes, we identified 95 genomic clusters. Here, an OR gene that exists as a singleton is also regarded as a cluster.
Fig. 1.
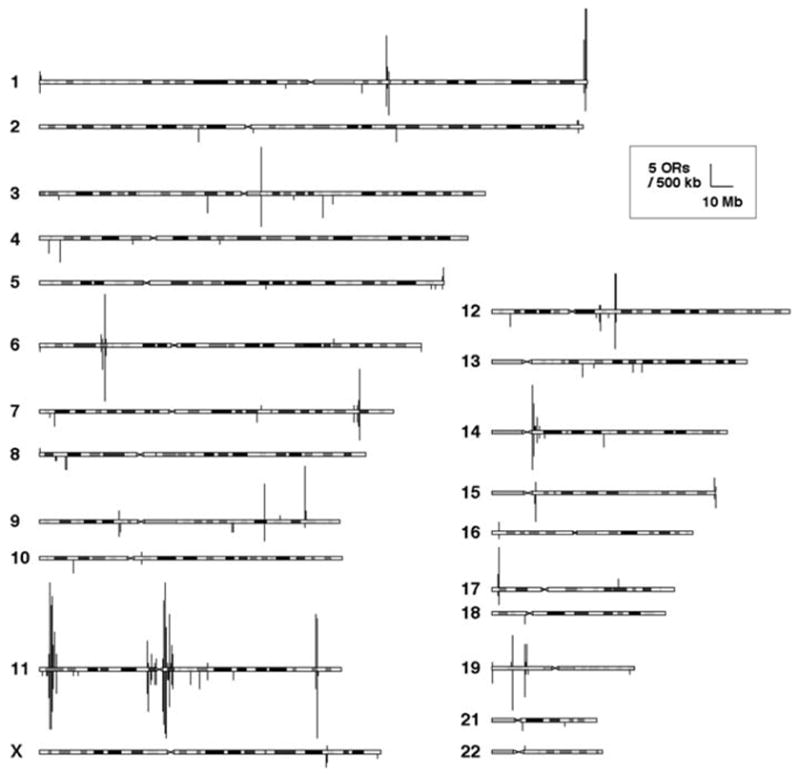
Distribution of olfactory receptor (OR) genes in human chromosomes. Vertical bars above and below the chromosomes indicate locations of OR functional genes and pseudogenes respectively. The height of each bar represents the number of OR genes in a non-overlapping 500-kb window at the position. Chromosomes 20 and Y are omitted, because OR genes were not found in these chromosomes. Modified from Niimura and Nei (2003)
To understand the evolutionary relationships of the functional OR genes, we constructed a phylogenetic tree using the neighbor-joining (NJ) method (Saitou and Nei 1987). This tree (Fig. 2a) showed that OR genes can be classified into class I and II genes, as was previously noted (Glusman 2000). Class II genes can further be divided into 19 monophyletic clades (A~S) that are supported by high bootstrap values (>90%) and contain five or more member genes. The genes showing unclear phylogenetic relationships were called ‘‘unclassified genes.’’
Fig. 2.
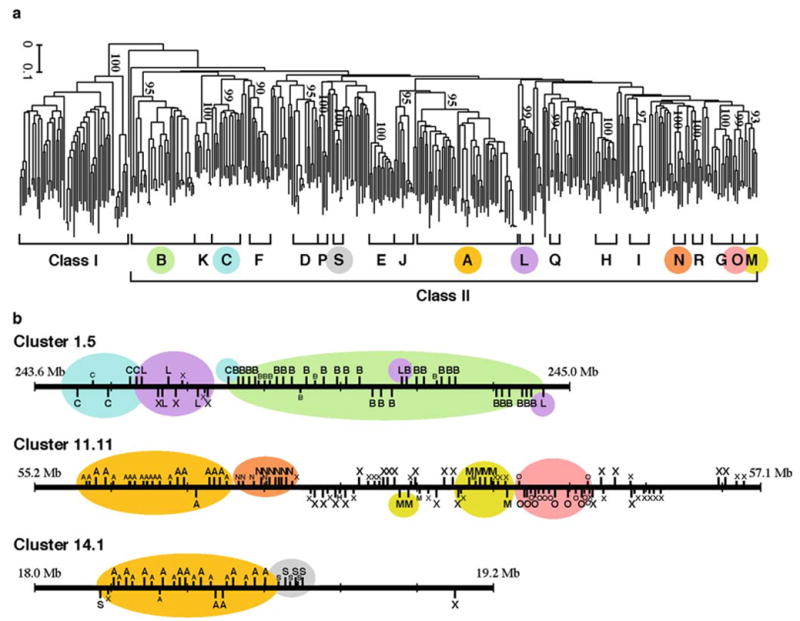
a Neighbor-joining (NJ) phylogenetic tree of 388 human functional OR genes constructed by using Poisson correction distances (Nei and Kumar 2000) for all pairs of genes. b Arrangement of OR genes in three genomic clusters. The position of each OR gene is shown by a vertical bar above or below a horizontal line, the latter indicating the opposite transcriptional direction to the former. The phylogenetic clades are defined by the tree in a. A pseudogene is represented by a shorter bar. X indicates an unclassified gene. Modified from Niimura and Nei (2003)
Tandem gene duplication and chromosomal rearrangement
When we examined the relationships between the phylogenetic clades and genomic clusters, we found that many genes belonging to a phylogenetic clade were located in the same genomic cluster. In particular, all 57 genes belonging to phylogenetic clade class I are located in the genomic cluster 11.3. In our study genomic clusters were designated according to the chromosome number and its location in the chromosome. For example, the cluster 11.3 represents the third cluster in chromosome 11. Figure 2b shows the chromosomal maps of genomic clusters 1.5, 11.11, and 14.1. Cluster 14.1 contains 21 clade A genes (including pseudogenes), 7 clade S genes, and 2 unclassified genes (X). All of the clade A genes are tandemly arranged, and so are most of the clade S genes. Similarly, clade B, C, and L genes in cluster 1.5 each form a tandem gene cluster, though a few clade L genes are included in the clade B gene cluster. The same pattern of gene arrangement was observed in most genomic clusters examined here. This suggests that the majority of human OR genes are generated by tandem gene duplication.
However, one genomic cluster often contained the genes belonging to different phylogenetic clades. For example, cluster 1.5 contains the genes belonging to the clades B, C, and L, which are distantly related to one another as shown in Fig. 2a. Moreover, the genes belonging to the same phylogenetic clade can be located in several different genomic clusters. For example, clade A genes are located in clusters 11.11 and 14.1 (and many other clusters). This kind of relationship between phylogenetic clades and genomic clusters was often observed. This is apparently caused by chromosomal rearrangements or translocations that have occurred in the regions of OR genomic clusters in the past.
Positive Darwinian selection
Gilad et al. (2000, 2003) reported that the human olfactory genes are under positive selection, whereas Gimelbrant et al. (2004) could not find any such evidence. As will be mentioned later, the number of OR genes varies extensively among vertebrate species, and the number of genes rapidly increased in some species, whereas massive pseudogenization occurred in other species. This suggests that the ability of olfaction is largely determined by the number of genes. If this is the case, a small degree of positive selection would not be important even if it occurs.
Pseudogenes
Earlier we mentioned that the human genome contains a larger number of pseudogenes (52%) in comparison to the number of functional genes. Why are there so many pseudogenes? An answer to this question is that humans do not need so many OR genes compared with other mammals, possibly because olfaction is less important in humans in the presence of well-developed visual sense (Gilad et al. 2004). This problem will be considered later in some detail.
Evolutionary relationships of human and mouse OR genes
Mouse OR genes
The OR genes in mice have also been studied extensively because of the availability of the genome sequences (Zhang and Firestein 2002; Young et al. 2002; Zhang et al. 2004; Godfrey et al. 2004). Niimura and Nei (2005a) conducted a detailed study of functional OR genes and pseudogenes in mice. They identified 1,037 functional genes and 354 pseudogenes. The numbers of functional genes is ~2.7 times greater than that of humans, whereas the number of pseudogenes is slightly smaller in mice than in humans. Therefore, the proportion of functional genes in mice is 75% and is much higher than that (48%) in humans. These results are essentially the same as those obtained by previous studies (Zhang and Firestein 2002; Young et al. 2002; Zhang et al. 2004). The genomic locations of all OR genes were determined, and the results showed that all chromosomes except chromosomes 18 and Y contained OR genes. These genes were distributed in 69 genomic clusters, and this number is smaller than that in humans. However, many OR gene clusters in the human genome contain a small number of member genes. Actually, the numbers of clusters containing five or more genes are the same (34) in humans as in mice.
Comparison of genomic clusters between humans and mice
Figure 3a shows the relationships of OR genes in several homologous genomic regions between humans and mice. In general, the gene order and the transcriptional orientations of orthologous OR genes are well conserved. Apparently, the gene arrangement of OR gene clusters did not substantially change from the most recent common ancestor (MRCA) of the two species to the present humans or mice. However, there were a few cases in which the organization of OR genes is quite different between the two species. For example, cluster 1.5 in humans (Hs1.5) contains the genes that are orthologous to the mouse genes located in four different chromosomes. This is apparently due to the translocation of chromosomes that occurred in the mouse lineage.
Fig. 3.
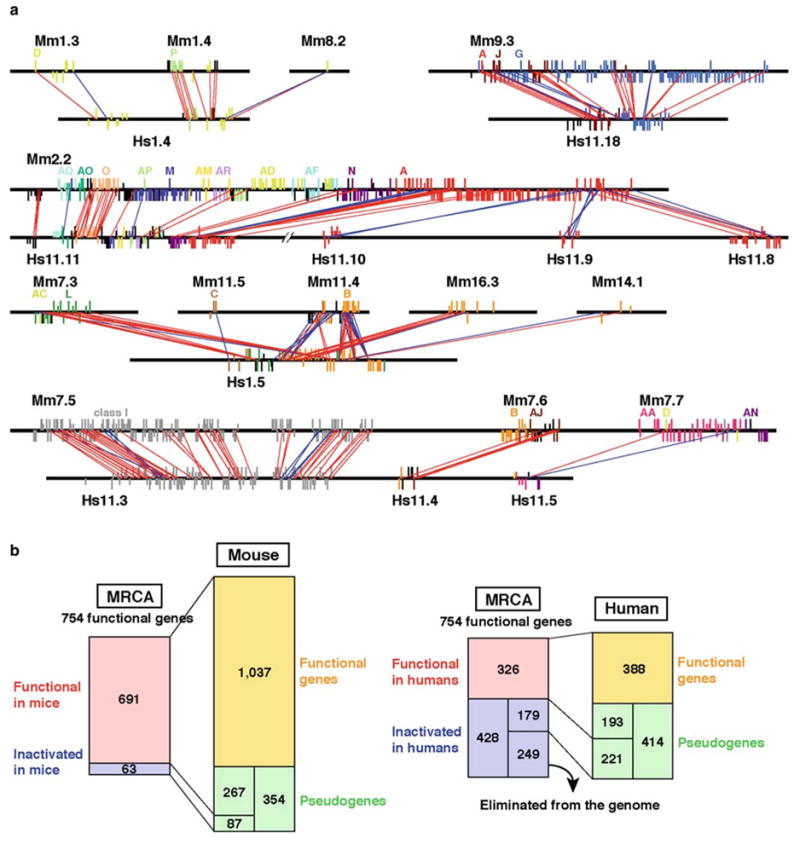
a Orthologous relationships of OR genes between mouse (Mm) and human (Hs) genomic clusters. Long and short vertical bars show the locations of functional and nonfunctional OR genes respectively. A vertical bar above a horizontal line indicates the opposite transcriptional direction to that below a horizontal line. Different colors represent different phylogenetic clades. Unclassified class II OR genes are shown in black. Red and blue lines connecting mouse and human OR genes represent orthologous gene pairs. Red lines indicate that transcriptional directions of orthologous genes are conserved between mice and humans, whereas blue lines indicate that they are inverted. b Evolutionary changes in the numbers of OR genes in the human lineage (right) and in the mouse lineage (left). Right: it was estimated that 691 functional genes in the most recent common ancestor (MRCA; red) have generated 1,037 functional genes (orange) and 267 pseudogenes out of 354 (green) in mice. The other 87 pseudogenes in mice were estimated to have originated from 63 functional genes in the MRCA (blue). From these numbers, the number of functional genes in the MRCA is estimated to be 754. Left: it was estimated that 326 functional genes in the MRCA (red) have generated 388 functional genes and 193 pseudogenes in humans. Out of the 428 functional genes in the MRCA that were inactivated in the human lineage, 179 genes were estimated to have become 221 pseudogenes. The other 249 genes appear to have been eliminated from the genome. Modified from Niimura and Nei (2005b)
The orthologous relationships of OR genes are often one copy to many copies or many copies to many copies rather than one to one. This indicates that human or mouse genes are frequently duplicated after the separation of the two species. Figure 3a also indicates that the number of OR genes in a mouse cluster is usually larger than that in the corresponding human cluster, as expected from the OR gene repertoire, which is larger in mice than in humans. Therefore, it appears that the difference in OR gene numbers between the two species was generated primarily by tandem gene duplications within each genomic cluster.
Evolutionary changes in the number of OR genes in the human and mouse lineages
Gilad et al. (2004) reported that the fraction of OR pseudogenes is significantly higher in humans, apes, and Old World monkeys (OWMs) than in most New World monkeys (NWMs) or mice. From this observation, they suggested that a large fraction of pseudogenes in higher primates is caused by the acquisition of a full trichromatic vision. Their reasoning was that since full trichromatic vision helps higher primates to find food, mates, territory, etc., there is no strong demand for olfaction and consequently many functional OR genes have become inactivated. Therefore, the smaller number of functional OR genes in humans than in mice can be explained by the hypothesis that the number of OR genes has decreased in the human lineage. However, it can also be explained by the hypothesis that the number of OR genes has increased in the rodent lineage because rodents probably need a higher level of olfaction to survive in heterogeneous environments.
Which hypothesis is more likely to be correct, the first or the second? To gain some insight into this question, we have developed a method to estimate the number of functional OR genes in the MRCA between humans and mice (Niimura and Nei 2005b). We first constructed a phylogenetic tree for all the functional genes from humans and mice. We then constructed a linearized tree (Nei and Kumar 2000) and counted the number of gene duplications that occurred in either human or mouse lineage after the human–mouse divergence. In this way, we estimated that the human and mouse lineages acquired about 60 and 350 new OR genes after the divergence respectively. We also estimated that the numbers of functional genes in the MRCA that were inactivated in the human and mouse lineage were about 430 and 60 respectively (see Niimura and Nei 2005b for details). From these numbers, the number of functional genes in the MRCA was calculated to be 754 (Fig. 3b). This number is close to the mean of the numbers of functional genes in current humans and mice. Therefore, it appears that the gene loss in the human lineage and the gene gain in the mouse lineage have nearly equally contributed to the generation of the difference in the number of OR genes between the two species. This result suggests that both of the above hypotheses are partially correct.
However, it is interesting to note that dogs, which are believed to have a good sense of smell, have a smaller number of functional OR genes than mice or rats (Table 1). This suggests that there are factors determining the ability of olfaction other than the number of OR genes. In fact, the relationship between the number of OR genes and the sense of smell may not be straightforward. Several studies have suggested that humans have an equal or even better sense of smell for detecting some odors than rats or dogs (Laska et al. 2000; Shepherd 2004), though rats and dogs have about 1,200 (Quignon et al. 2005) and 900 functional OR genes (Quignon et al. 2003, 2005; Olender et al. 2004) respectively (Table 1). Shepherd (2004) pointed out that many different regions in the brain are actually involved in olfactory perception. For this reason, he hypothesized that higher brain mechanisms such as good memory may give humans better olfactory ability than expected from the small number of genes, particularly in detecting fine differences in food flavors. Nevertheless, the number of OR genes should be an important factor for determining the sense of smell. We therefore consider this factor only in this paper.
Table 1.
Numbers of functional genes and pseudogenes for olfactory and other chemosensory receptor genes in vertebrate species. ND not determined
| OR | V1R | V2R | T1R | T2R | |
|---|---|---|---|---|---|
| Human | 388 (414)a | 2 (115)f | 0i | 3l | 25 (11)l |
| Mouse | 1,037 (354)b | 165 (165)f | 61 (148)j | 3l | 35 (6)l |
| Rat | 1,201 (292)c | 106 (110)f | 57 (111)j | 3l | 37 (5)l |
| Dog | 872 (222)c | 8 (22)g | ND | 3l | 15 (5)l |
| Cow | ND | 32 (41)g | ND | ND | 12 (15)l |
| Opossum | ND | 49 (53)g | ND | 3l | 26 (5)l |
| Chicken | 82 (476d)e | ND | ND | 2l | 3 (0)l |
| Frog | 410 (478d)e | ND | ND | 0l | 49 (12)l |
| Pufferfish | 44 (54d)e | 1h | ND | 4l | 4 (0)l |
| Zebrafish | 102 (35d)e | 1h | 70 (18)k | 1l | 4 (0)l |
Number of pseudogenes is shown in parentheses. If the number of pseudogenes has not been determined, it is not shown
Because the draft genome sequence used for the analysis contained many short fragments, this number may include a considerable number of partial sequences of functional genes
From Young et al. (2005)
From Grus et al. (2005)
From Yang et al. (2005)
From Shi and Zhang (2006)
Evolutionary dynamics of OR genes in vertebrates
OR genes in different vertebrate species
Olfactory receptor genes are present in all vertebrate species (Table 2). Fishes recognize water-soluble odorants such as amino acids, bile acids, sex steroids, and prostaglandins. Ngai et al. (1993) reported that the OR gene family in fishes is much smaller than that in mammals and estimated the number of OR genes in fishes to be about 100. Later on, several OR genes have been identified from lampreys, one of the most primitive vertebrate species (Berghard and Dryer 1998; Freitag et al. 1999). Lampreys possess two different groups of genes for odor detection. One group of genes are members of the vertebrate OR multigene family, whereas the other group of genes shows a higher similarity to amine receptors than to OR genes in jawed vertebrates. Therefore, it has been speculated that the latter group of genes have acquired the ability of detecting odors independently in the lamprey lineage and that their ligands might be lamprey-specific odors such as bile acids released from ammocoete larvae (Dryer 2000). For this reason, the latter group of lamprey OR genes were not included in our phylogenetic analysis (Fig. 4a). Recently, Satoh (2005) has identified a putative GPCR gene as having significant similarity to known vertebrate OR genes from amphioxus, the closest relative to vertebrates. However, it appears that Ciona intestinalis, a basal chordate, does not have any genes that are orthologous to vertebrate ORs (Satoh 2005), though its whole genome draft sequence is known (Dehal et al. 2002).
Table 2.
Numbers of functional OR genes belonging to different groups in six vertebrate species. A airborne odorants, W water-soluble odorants
| Type | Groupa | Zebrafish | Pufferfish | Frog | Chicken | Mouse | Human | Putative ligand |
|---|---|---|---|---|---|---|---|---|
| 1 | α (I) | 0 (0) | 0 (0) | 2 (0) | 9 (11) | 112b (11) | 57 (15) | A |
| β (I) | 1 (1) | 1 (2) | 5 (1) | 0 (0) | 3b(0) | 0 (0) | ? | |
| γ (II) | 1 (1) | 0 (0) | 370 (90) | 72 (88) | 922 (89) | 331 (85) | A | |
| δ (I) | 44 (43) | 28 (64) | 22 (5) | 0 (0) | 0 (0) | 0 (0) | W | |
| ε (I) | 11 (11) | 2 (5) | 6 (1) | 0 (0) | 0 (0) | 0 (0) | W | |
| ζ (I) | 27 (26) | 6 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | W | |
| 2 | η | 16 (16) | 5 (11) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | W |
| θ | 1 (1) | 1 (2) | 1 (0) | 1 (1) | 0 (0) | 1?c (0) | ? | |
| κ | 1 (1) | 1 (2) | 1 (0) | 0 (0) | >1c (0) | 0 (0) | ? | |
| Total | 102 (100) | 44 (100) | 410 (100) | 82 (100) | 1,037 (100) | 388 (100) |
Numbers in parentheses represent the percentage of the genes belonging to each group
(I) and (II) indicate class I and class II genes respectively
These numbers are different from Niimura and Nei (2005c), because we have recently found that three class I genes in mice should be classified into group β rather than group α
We have recently found that humans and mice have some group θ or κ genes that were not described in Niimura and Nei (2005c). They were not included in the total number of OR genes in humans or mice. These genes will be described in detail elsewhere
Fig. 4.
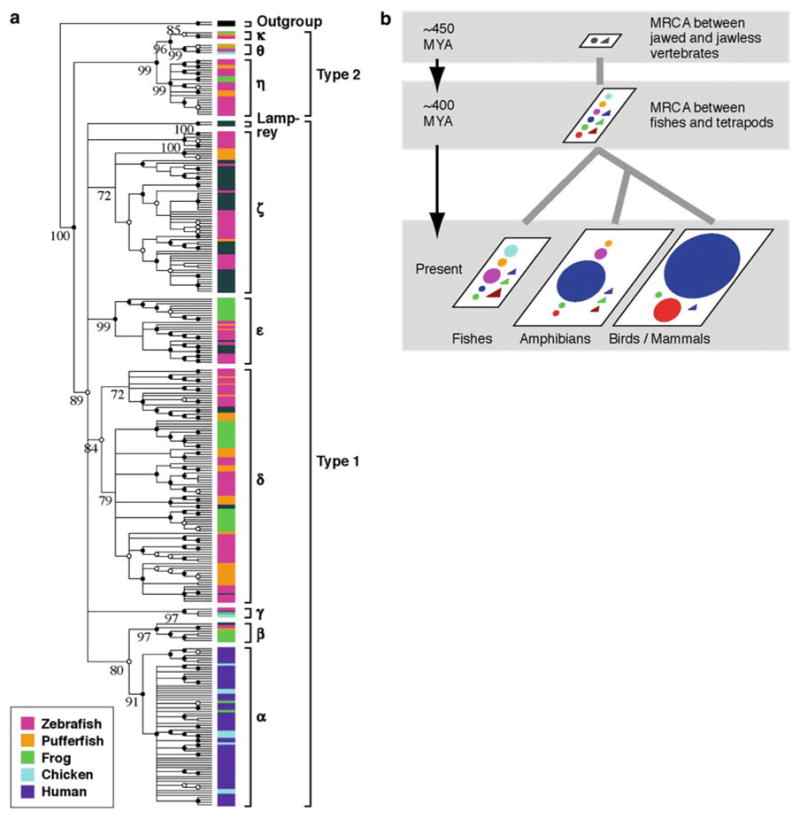
a Condensed phylogenetic tree (Nei and Kumar 2000) at the 70% bootstrap value level for 310 functional OR genes and two outgroup non-OR G-protein coupled receptor (GPCR) genes (bovine adenosine A1 receptor and rat α2B-adrenergic receptor). This condensed tree was produced from the NJ tree by assuming that all the interior branches showing <70% bootstrap values had a branch length of 0. Note that this tree represents the topology only and the branch lengths do not reflect their evolutionary distances. Gray bars are fish genes from the species other than zebrafish or pufferfish that are available from databases. Black and white dots at nodes indicate the branches supported by >90% and >80% bootstrap values respectively. b Evolutionary dynamics of vertebrate OR genes. The MRCA between jawed and jawless vertebrates and that between fishes and tetrapods were estimated to have had at least two and nine OR genes respectively. Fishes currently retain eight out of nine group genes that were present in the MRCA between fishes and tetrapods, probably because their environment has not changed substantially compared with that of the MRCA. In the tetrapod lineage, group α and γ genes seem to have acquired the ability to detect airborne odorants at the time of terrestrial adaptation. It appears that the importance of olfactory information is greater in terrestrial organisms than in marine organisms, and therefore the OR genes, especially group γ genes, have expanded enormously in the former. In mammals and birds, the genes that are specific to water-soluble odorants have apparently been eliminated from the genome because they are useless for terrestrial life. On the other hand, amphibians still keep the genes for water-soluble odorants, reflecting that they have adapted to both aquatic and terrestrial environments. Modified from Niimura and Nei (2005c)
Here we should note that insects also have genes called ‘‘olfactory receptors’’ or ‘‘ORs’’. Their function is similar to that of vertebrate OR genes and they are also GPCRs with seven transmembrane regions (for reviews, see Matsunami and Amrein 2003; Dahanukar et al. 2005). However, insect OR genes do not show any significant sequence similarity to vertebrate OR genes and they have introns in the coding regions unlike vertebrate ORs. Moreover, they are not evolutionarily related to vomeronasal or taste receptor genes in vertebrates (see below). In this review, we would like to confine our consideration only to vertebrate chemosensory receptor genes.
Evolution of vertebrate OR genes
In order to examine the long-term evolution of vertebrate OR multigene families, we conducted an extensive homology search for OR genes against the draft genome sequences of zebrafish, pufferfish, western clawed frogs (Xenopus tropicalis), and chickens (Niimura and Nei 2005c). Table 1 summarizes the numbers of OR genes (or OR-like genes; see below) identified from these and some other species. Note that the numbers of functional genes represent the lower bounds, because genome sequencing for some species is still incomplete. For example, the chicken genome sequence used for this analysis (version 2) contained many short contigs that are a few kilobases long, and thus the number shown in parentheses (476) is thought to include a considerable number of partial sequences of functional OR genes. For this reason, we cannot directly compare the fractions of OR pseudogenes among the species in Table 1.
We constructed an NJ phylogenetic tree for all the functional genes from the four species mentioned above and humans. The result showed that about 90% of frog and chicken genes form a tight phylogenetic clade with human class II genes. We called the genes belonging to this clade the group γ genes (see below). To examine the evolutionary relationships among vertebrate OR genes in more detail, we then constructed a phylogenetic tree using all the non-group γ genes and only four representative group γ genes to reduce the size of the tree. The result (Fig. 4a) suggested that the vertebrate OR genes can be classified into two groups; types 1 and 2 genes. The clade of type 1 genes contained lamprey genes, suggesting that the divergence between types 1 and 2 genes predates the divergence between jawed vertebrates and jawless vertebrates. Figure 4a also indicates that the MRCA between fishes and tetrapods had at least nine ancestral OR genes and that all OR genes identified were classified into nine groups (α~κ) each of which originated from one ancestral gene. For example, the group ε clade was supported by a high bootstrap value (99%) and consisted of two subclades, the tetrapod-specific subclade and the fish-specific subclade. This indicates that the MRCA between fishes and tetrapods is likely to have had one ancestral group ε gene. The group α clade was specific to tetrapods. However, clade β, the sister clade of clade α, contained both tetrapod and fish genes, implying that the divergence between clade α nd clade β occurred before the divergence between fishes and tetrapods. Therefore, the MRCA between fishes and tetrapods should have had at least one ancestral group α gene. Group α genes were not observed from the fish species examined, apparently because they were lost in the fish lineage or because the availability of fish genome sequence data is limited. Using this logic, we estimated that the number of ancestral OR genes in the MRCA was at least nine.
Table 2 showed the number of functional OR genes belonging to each group. The results can be summarized in the following way:
Eight out of the nine group genes were observed in current fish species, while only two group (α and γ ) genes were found from mammalian or avian genomes with a few exceptions. Therefore, although the sizes of these OR gene families in fishes are smaller than those in mammals or birds, the diversity of the OR gene family in fishes is much larger than that in mammals or birds.
Group α and γ genes, which are abundant in mammals or birds, are virtually absent in fishes. On the other hand, four major group (δ, ε, ζ, η) genes present in fishes are completely absent in mammals or birds.
In mammals, birds, and frogs, about 90% of OR genes belong to group γ. At the same time, frogs have group δ, ε, and η genes, which are abundant in fishes. Therefore, the frog OR gene family has both mammal-like and fish-like genes.
These observations suggest that group α and γ genes are specific for detecting airborne odorants, whereas group δ, ε, ζ and η genes are for detecting water-soluble odorants. The functions of group β, θ, and κ genes are unclear at this stage. It is likely that group θ and κ genes are not odorant receptors and have other functions, because they are highly divergent from other documented OR genes, but are conserved from fishes to mammals. Therefore, it would be appropriate to call group θ and κ genes ‘‘OR-like’’ genes. These groups of genes will be discussed in more detail elsewhere.
The evolutionary dynamics of vertebrate OR genes that were inferred from the above observations are presented in Fig. 4b. The difference in OR gene repertoire among vertebrate species is probably due to the environmental changes that organisms have experienced after the divergence between fishes and tetrapods. In the tetrapod lineage, repeated gene duplications and massive gene losses appear to have occurred to adapt in the terrestrial environment, whereas the change in the OR gene repertoire in the fish lineage seems to be small, possibly because the environmental change has been small in the fish lineage. These findings indicate that the OR gene family is subject to an extreme form of birth-and-death evolution (Nei et al. 1997 ; Nei and Rooney 2005 and the references therein).
Classification of vertebrate OR genes
As shown in Fig. 2a, mammalian OR genes are clearly separated into two groups called class I and class II genes (Glusman et al. 2000). Note that this class I/II distinction is different from the type 1/type 2 distinction mentioned above. Mammalian class I genes correspond to our group α genes and a few group β genes (Table 2), while class II genes are equivalent to our group γ genes. Therefore, both class I and class II genes belong to type 1 genes. Previously, it was generally believed that all jawed vertebrate OR genes can be classified into class I and class II genes and that all fish genes belong to class I (Glusman et al. 2000). The distinction between class I and class II genes was first proposed by Freitag et al. (1995) on the basis of Xenopus laevis OR genes. X. laevis has two anatomically different nasal cavities, water-filled lateral diverticulum and air-filled medial diverticulum. They found that class I genes were exclusively expressed in the lateral diverticulum and were similar in amino acid sequence to fish OR genes that were known at that time, whereas class II genes were exclusively expressed in the medial diverticulum and were similar to known mammalian OR genes. For this reason, they called class I and class II genes ‘‘fish-like’’ and ‘‘mammalian-like’’ OR genes respectively. In Freitag et al. (1998), they extended this view and proposed that fishes have only class I genes, mammals have only class II genes, and amphibians have both of them. For this reason, class I genes were assumed to be specialized for water-soluble odorants and class II genes were for airborne odorants.
Later Glusman et al. (2000) collected more than 800 OR genes from various species that were available in the database and conducted a phylogenetic analysis. On the basis of this analysis, they suggested that all vertebrate OR genes were classified into class I and class II genes with several unclassified genes and that all fish genes and some non-fish genes including human genes were categorized into class I. However, their phylogenetic analysis appears to be unreliable, because many partial sequences were included and therefore the number of informative sites used for phylogenetic analysis was small, and because the bootstrap value supporting the class I clade was not shown. At this stage, a small number of mammalian class I genes were known, and they were assumed to be evolutionary relics. However, Glusman et al. (2001) found that the human genome sequences actually contain more than 100 putatively functional class I genes. Zhang and Firestein (2002) reached the same conclusion for the mouse OR gene family. Because class I genes were believed to be for water-soluble odorants, the function of class I genes in mammals has become enigmatic. However, this diffculty can be resolved if mammalian class I genes are not ‘‘fish-like’’ genes. Our results showed that mammalian class I genes are not necessarily closely related to fish genes, and therefore the division of vertebrate OR genes into class I (‘‘fish-like’’) and class II (‘‘mammalian-like’’) genes should be abandoned. Yet, the classification of mammalian genes into class I and class II genes is convenient and is well established with a few exceptions (Table 2). Therefore, we propose that this terminology should be used only for mammalian genes.
Evolution of other chemosensory receptor genes
Vomeronasal receptors
In addition to OR genes, four more GPCR gene families are involved in vertebrate chemosensation: two families of vomeronasal receptors (V1Rs and V2Rs) and two families of taste receptors (T1Rs and T2Rs; Table 1). In mice, V1Rs and V2Rs are expressed in the vomeronasal organ (VNO), which is located at the base of the nasal cavity and is separated from the MOEs expressing ORs (reviewed by Dulac and Torello 2003; Brennan and Keverne 2004; Mombaerts 2004b). Previously, the VNO was regarded as an organ specialized for pheromone detection. However, it now appears that the olfactory and vomeronasal systems have some overlapping functions (Baxi et al. 2006). Although the chemical structures of insect pheromones have been well characterized, the molecular identity of mammalian pheromones is still poorly understood with a few exceptions (Dulac and Torello 2003; Brennan and Keverne 2004; Stowers and Marton 2005, and the references therein). Recently, Kimoto et al. (2005) identified a sex pheromone in mice, which is a male-specific peptide secreted from the eyes and is encoded by a multigene family closely linked to the major histocompatibility complex (MHC) class I region.
The family of V1Rs were first identified in rats (Dulac and Axel 1995). Like OR genes, they are GPCRs and do not have introns in the coding regions. V2Rs were reported in 1997 as the second GPCR family that is found in the VNO (Herrada and Dulac 1997; Matsunami and Buck 1997; Ryba and Tirindelli 1997). They have a large N-terminal extracellular domain encoded by multiple exons. Recently, it was reported that non-classical MHC class I genes are co-expressed with specific V2Rs (Ishii et al. 2003; Loconto et al. 2003). ORs, V1Rs, and V2Rs show virtually no sequence similarity to one another despite their similar tertiary structure.
The entire sets of V1R genes in humans, mice, and rats have been identified by Rodriguez et al. (2002), Grus and Zhang (2004), Zhang et al. (2004), Shi et al. (2005), and Young et al. (2005; Table 1). Mice and rats have more than 100 intact V1R genes. In contrast, only two or five intact V1R genes were found from the human genome sequence, though there are more than 100 pseudogenes (Rodriguez and Mombaerts 2002; Young et al. 2005). Among them, at least one V1R gene is expressed at the mRNA level in the human olfactory mucosa (Rodriguez et al. 2000). However, although the VNO develops in the human fetus, it degenerates before birth. Anatomical evidence suggests that any VNO-like structures in human adults are vestigial (Trotier et al. 2000; Meredith 2001). Moreover, the gene encoding an ion channel named TPR2 (Liman et al. 1999), which is essential for VNO function in mice, is a pseudogene in humans, apes, and OWMs (Zhang and Webb 2003; Liman and Innan 2003). Therefore, it appears that the deterioration of the vomeronasal system occurred in parallel to the inactivation of OR genes mentioned above. Grus et al. (2005) and Young et al. (2005) reported a dramatic variation in the number of V1R genes among different mammalian orders. While rodents have more than 100 functional genes, dogs have only eight intact V1R genes, although they have a functional VNO (Table 1). Fishes seems to have only one functional V1R gene (see below).
Because of the complex structure of V2R genes, their identification from the genomic sequences is more difficult than that for OR or V1R genes. More than 50 intact V2R genes were identified from the mouse and rat genomes (Yang et al. 2005), whereas no intact V2R genes were found from the human genome (Bjarnadottir et al. 2005) and all the V2R genes identified from goats were pseudogenes (Wakabayashi et al. 2002). It is therefore possible that the numbers of functional V2R genes in non-rodent placental mammals are quite small.
Discrete VNOs do not exist in fishes. It is generally believed that the first animals that had separate olfactory and vomeronasal organs were early tetrapods (Eisthen 2004; Baxi et al. 2006). However, V2R genes are known to exist in goldfish (Cao et al. 1998) and pufferfish (Naito et al. 1998). V2R genes are actually expressed in olfactory epithelia in fishes. Therefore, it might be more appropriate to call them ‘‘V2R-like’’ genes. Hashiguchi and Nishida (2005) identified 70 intact V2R genes in the zebrafish genome. The presence of V2R genes in X. laevis has also been reported, and they are predominantly expressed in the VNO (Hagino-Yamagishi et al. 2004). Recently, Pfister and Rodriguez (2005) reported the existence of a single V1R gene in the genomes of several fish species. This V1R gene is expressed in olfactory neurons.
Taste receptors
Mammals can perceive five major types of taste: sweet, sour, bitter, salty, and umami (taste of L-glutamate). Of these five modalities, salty and sour tastes are detected by ion channels, whereas sweet, bitter, and umami tastes are perceived by two distinct families of GPCR genes: T1R and T2R genes (reviewed by Lindemann 2001; Montmayeur and Matsunami 2002; Mombaerts 2004b). T1Rs are associated with sweet and umami tastes (Nelson et al. 2001, 2002; Li et al. 2002), and T2Rs are for detecting bitter tastes (Adler et al. 2000; Chandrashekar et al. 2000; Matsunami et al. 2000). T1R and T2R genes exhibit no significant sequence similarity to each other. Interestingly, however, T1Rs and T2Rs are closely related to V2Rs and V1Rs respectively. T1R genes contain multiple introns and have long N-terminal domains like V2R genes, whereas T2R genes are intronless like V1R or OR genes. Mammals have only three T1R genes, which are named T1R1, T1R2, and T1R3. They form heterodimers. T1R1 and T1R3 are combined to function as an umami taste receptor. Interestingly, when T1R3 is combined with T1R2, it functions as a sweet taste receptor. In contrast to T1R genes, humans and mice have about 30 T2R genes (Conte et al. 2003; Shi et al. 2003). This might be due to the importance of the ability of bitter taste perception, which enables animals to avoid ingesting potentially toxic and harmful substances. As shown in Table 1, the number of T1R genes repertoire is virtually the same for all mammals, whereas the number of T2R genes shows a large variation (Shi and Zhang 2006). Wang et al. (2004) and Go et al. (2005) suggested that the selective constraint for T2R genes has been relaxed in humans in comparison with those in non-human primates or rodents. Therefore, it appears that the capability of all the three chemosensory systems involved in olfactory, pheromone, and taste perception has declined in the human lineage in comparison with other mammals. Chickens and fishes also tend to have a smaller number of genes in the three chemosensory systems. These species are known to have generally small-sized multigene families.
Conclusions
In this paper we examined the pattern of evolutionary change of OR genes, which form a large gene family composed of about 1,000 genes in mammals. In humans and mice these genes are located in almost all chromosomes. Comparison of the human and mouse OR genes indicated that the increase in gene number in this gene family occurred primarily by tandem gene duplications, and these genes were dispersed on different chromosomal locations by chromosomal translocation or rearrangement. The number of genes in this family expanded enormously from a small number in fishes to a large number in mammals. However, most mammals have many pseudogenes, the proportion of pseudogenes being as high as 52% in humans. Phylogenetic analysis of these genes suggested that this gene family has been subjected to an extreme form of birth-and-death evolution (Nei et al. 1997; Nei and Rooney 2005). The expansion and contraction of the gene family can be explained by natural selection due to environmental changes, but some parts of the changes seem to be fortuitous. We also examined the evolutionary dynamics of gene families for pheromone and taste receptors. Some of the gene families showed an evolutionary pattern similar to that of OR genes, but in other gene families almost the same number of genes was maintained for the entire evolutionary process from fishes to mammals. At the present time, however, the number of species studied is too small to draw a general conclusion.
Acknowledgments
This study was supported by the National Institute of Health grant GM020293 to M.N. and by grant 17710162 from the Ministry of Education, Culture, Sports, Science and Technology, Japan to Y.N.
References
- Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006;29:1–7. doi: 10.1016/j.tins.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Berghard A, Dryer L. A novel family of ancient vertebrate odorant receptors. J Neurobiol. 1998;37:383–392. [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Schiöth HB. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene. 2005;362:70–84. doi: 10.1016/j.gene.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Something in the air? New insights into mammalian pheromones. Curr Biol. 2004;14:R81–R89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cao Y, Oh BC, Stryer L. Cloning and localization of two multigene receptor families in goldfish olfactory epithelium. Proc Natl Acad Sci USA. 1998;95:11987–11992. doi: 10.1073/pnas.95.20.11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Conte C, Ebeling M, Marcuz A, Nef P, Andres-Barquin PJ. Evolutionary relationships of the Tas2r receptor gene families in mouse and human. Physiol Genomics. 2003;14:73–82. doi: 10.1152/physiolgenomics.00060.2003. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr Opin Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Dehal P, Satou Y, Campbell RK, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Dryer L. Evolution of odorant receptors. Bioessays. 2000;22:803–810. doi: 10.1002/1521-1878(200009)22:9<803::AID-BIES5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Eisthen HL. The goldfish knows: olfactory receptor cell morphology predicts receptor gene expression. J Comp Neurol. 2004;477:341–346. doi: 10.1002/cne.20258. [DOI] [PubMed] [Google Scholar]
- Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- Firestein S. A nobel nose: the 2004 Nobel Prize in Physiology and Medicine. Neuron. 2004;45:333–338. doi: 10.1016/j.neuron.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Freitag J, Krieger J, Strotmann J, Breer H. Two classes of olfactory receptors in Xenopus laevis. Neuron. 1995;15:1383–1392. doi: 10.1016/0896-6273(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Freitag J, Ludwig G, Andreini I, Rossler P, Breer H. Olfactory receptors in aquatic and terrestrial vertebrates. J Comp Physiol [A] 1998;183:635–650. doi: 10.1007/s003590050287. [DOI] [PubMed] [Google Scholar]
- Freitag J, Beck A, Ludwig G, von Buchholtz L, Breer H. On the origin of the olfactory receptor family: receptor genes of the jawless fish (Lampetra fluviatilis) Gene. 1999;226:165–174. doi: 10.1016/s0378-1119(98)00575-7. [DOI] [PubMed] [Google Scholar]
- Gilad Y, Segre D, Skorecki K, Nachman MW, Lancet D, Sharon D. Dichotomy of single-nucleotide polymorphism haplotypes in olfactory receptor genes and pseudogenes. Nat Genet. 2000;26:221–224. doi: 10.1038/79957. [DOI] [PubMed] [Google Scholar]
- Gilad Y, Bustamante CD, Lancet D, Pääbo S. Natural selection on the olfactory receptor gene family in humans and chimpanzees. Am J Hum Genet. 2003;73:489–501. doi: 10.1086/378132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Wiebe V, Przeworski M, Lancet D, Pä ä bo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;2:E5. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimelbrant AA, Skaletsky H, Chess A. Selective pressures on the olfactory receptor repertoire since the human-chimpanzee divergence. Proc Natl Acad Sci USA. 2004;101:9019–9022. doi: 10.1073/pnas.0401566101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glusman G, Bahar A, Sharon D, Pilpel Y, White J, Lancet D. The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mamm Genome. 2000;11:1016–1023. doi: 10.1007/s003350010196. [DOI] [PubMed] [Google Scholar]
- Glusman G, Yanai I, Rubin I, Lancet D. The complete human olfactory subgenome. Genome Res. 2001;11:685–702. doi: 10.1101/gr.171001. [DOI] [PubMed] [Google Scholar]
- Go Y, Satta Y, Takenaka O, Takahata N. Lineage-specific loss of function of bitter taste receptor genes in humans and nonhuman primates. Genetics. 2005;170:313–326. doi: 10.1534/genetics.104.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey PA, Malnic B, Buck LB. The mouse olfactory receptor gene family. Proc Natl Acad Sci USA. 2004;101:2156–2161. doi: 10.1073/pnas.0308051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grus WE, Zhang J. Rapid turnover and species-specificity of vomeronasal pheromone receptor genes in mice and rats. Gene. 2004;340:303–312. doi: 10.1016/j.gene.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Grus WE, Shi P, Zhang YP, Zhang J. Dramatic variation of the vomeronasal pheromone receptor gene repertoire among five orders of placental and marsupial mammals. Proc Natl Acad Sci USA. 2005;102:5767–5772. doi: 10.1073/pnas.0501589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino-Yamagishi K, Moriya K, Kubo H, Wakabayashi Y, Isobe N, Saito S, Ichikawa M, Yazaki K. Expression of vomeronasal receptor genes in Xenopus laevis. J Comp Neurol. 2004;472:246–256. doi: 10.1002/cne.20073. [DOI] [PubMed] [Google Scholar]
- Hall SE, Floriano WB, Vaidehi N, Goddard WA., III Predicted 3-D structures for mouse I7 and rat I7 olfactory receptors and comparison of predicted odor recognition profiles with experiment. Chem Senses. 2004;29:595–616. doi: 10.1093/chemse/bjh063. [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Nishida M. Evolution of vomeronasal-type odorant receptor genes in the zebrafish genome. Gene. 2005;362:19–28. doi: 10.1016/j.gene.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Ishii T, Hirota J, Mombaerts P. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Curr Biol. 2003;13:394–400. doi: 10.1016/s0960-9822(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Laska M, Seibt A, Weber A. ‘Microsmatic’ primates revisited: olfactory sensitivity in the squirrel monkey. Chem Sense. 2000;25:47–53. doi: 10.1093/chemse/25.1.47. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- Loconto J, Papes F, Chang E, Stowers L, Jones EP, Takada T, Kumanovics A, Fischer Lindahl K, Dulac C. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Amrein H. Taste and pheromone perception in mammals and flies. Genome Biol. 2003;4:220. doi: 10.1186/gb-2003-4-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433–445. doi: 10.1093/chemse/26.4.433. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Curr Opin Neurobiol. 2004a;14:31–36. doi: 10.1016/j.conb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004b;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol. 2002;12:366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- Naito T, Saito Y, Yamamoto J, Nozaki Y, Tomura K, Hazama M, Nakanishi S, Brenner S. Putative pheromone receptors related to the Ca2+-sensing receptor in Fugu. Proc Natl Acad Sci USA. 1998;95:5178–5181. doi: 10.1073/pnas.95.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular evolution and phylogenetics. Oxford University Press; New York: 2000. [Google Scholar]
- Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet. 2005;39:121–152. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci USA. 1997;94:7799–7806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Ngai J, Dowling MM, Buck L, Axel R, Chess A. The family of genes encoding odorant receptors in the channel catfish. Cell. 1993;72:657–666. doi: 10.1016/0092-8674(93)90395-7. [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nei M. Evolution of olfactory receptor genes in the human genome. Proc Natl Acad Sci USA. 2003;100:12235–12240. doi: 10.1073/pnas.1635157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y, Nei M. Comparative evolutionary analysis of olfactory receptor gene clusters between humans and mice. Gene. 2005a;346:13–21. doi: 10.1016/j.gene.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nei M. Evolutionary changes of the number of olfactory receptor genes in the human and mouse lineages. Gene. 2005b;346:23–28. doi: 10.1016/j.gene.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nei M. Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proc Natl Acad Sci USA. 2005c;102:6039–6044. doi: 10.1073/pnas.0501922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olender T, Fuchs T, Linhart C, Shamir R, Adams M, Kalush F, Khen M, Lancet D. The canine olfactory subgenome. Genomics. 2004;83:361–372. doi: 10.1016/j.ygeno.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Parmentier M, Libert F, Schurmans S, Schiffann S, Lefort A, Eggerickx D, Ledent C, Mollereau C, Gerard C, Perret J, Grootegoed A, Vassart G. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. 1992;355:453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- Pfister P, Rodriguez I. Olfactory expression of a single and highly variable V1r pheromone receptor-like gene in fish species. Proc Natl Acad Sci USA. 2005;102:5489–5494. doi: 10.1073/pnas.0402581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignon P, Kirkness E, Cadieu E, Touleimat N, Guyon R, Renier C, Hitte C, Andre C, Fraser C, Galibert F. Comparison of the canine and human olfactory receptor gene repertoires. Genome Biol. 2003;4:R80. doi: 10.1186/gb-2003-4-12-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignon P, Giraud M, Rimbault M, Lavigne P, Tacher S, Morin E, Retout E, Valin AS, Lindblad-Toh K, Nicolas J, Galibert F. The dog and rat olfactory receptor repertoires. Genome Biol. 2005;6:R83. doi: 10.1186/gb-2005-6-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez I, Mombaerts P. Novel human vomeronasal receptor-like genes reveal species-specific families. Curr Biol. 2002;12:R409–R411. doi: 10.1016/s0960-9822(02)00909-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Greer CA, Mok MY, Mombaerts P. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet. 2000;26:18–19. doi: 10.1038/79124. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Del Punta K, Rothman A, Ishii T, Mombaerts P. Multiple new and isolated families within the mouse superfamily of V1r vomeronasal receptors. Nat Neurosci. 2002;5:134–140. doi: 10.1038/nn795. [DOI] [PubMed] [Google Scholar]
- Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Satoh G. Characterization of novel GPCR gene coding locus in amphioxus genome: gene structure, expression, and phylogenetic analysis with implications for its involvement in chemoreception. Genesis. 2005;41:47–57. doi: 10.1002/gene.20082. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Ishii T, Nakatani H, Tsuboi A, Nagawa F, Asano M, Sudo K, Sakagami J, Sakano H, Ijiri T, Matsuda Y, Suzuki M, Yamamori T, Iwakura Y, Sakano H. Mutually exclusive expression of odorant receptor transgenes. Nat Neurosci. 2000;3:687–693. doi: 10.1038/76641. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The human sense of smell: are we better than we think? PLoS Biol. 2004;2:E146. doi: 10.1371/journal.pbio.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol Biol Evol. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J, Yang H, Zhang YP. Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol Biol Evol. 2003;20:805–814. doi: 10.1093/molbev/msg083. [DOI] [PubMed] [Google Scholar]
- Shi P, Bielawski JP, Yang H, Zhang YP. Adaptive diversification of vomeronasal receptor 1 genes in rodents. J Mol Evol. 2005;60:566–576. doi: 10.1007/s00239-004-0172-y. [DOI] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Stowers L, Marton TF. What is a pheromone? Mammalian pheromones reconsidered. Neuron. 2005;46:699–702. doi: 10.1016/j.neuron.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Trotier D, Eloit C, Wassef M, Talmain G, Bensimon JL, Doving KB, Ferrand J. The vomeronasal cavity in adult humans. Chem Senses. 2000;25:369–380. doi: 10.1093/chemse/25.4.369. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Mori Y, Ichikawa M, Yazaki K, Hagino-Yamagishi K. A putative pheromone receptor gene is expressed in two distinct olfactory organs in goats. Chem Senses. 2002;27:207–213. doi: 10.1093/chemse/27.3.207. [DOI] [PubMed] [Google Scholar]
- Wang X, Thomas SD, Zhang J. Relaxation of selective constraint and loss of function in the evolution of human bitter taste receptor genes. Hum Mol Genet. 2004;13:2671–2678. doi: 10.1093/hmg/ddh289. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi P, Zhang YP, Zhang J. Composition and evolution of the V2r vomeronasal receptor gene repertoire in mice and rats. Genomics. 2005;86:306–315. doi: 10.1016/j.ygeno.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Young JM, Friedman C, Williams EM, Ross JA, Tonnes-Priddy L, Trask BJ. Different evolutionary processes shaped the mouse and human olfactory receptor gene families. Hum Mol Genet. 2002;11:535–546. doi: 10.1093/hmg/11.5.535. [DOI] [PubMed] [Google Scholar]
- Young JM, Kambere M, Trask BJ, Lane RP. Divergent V1R repertoires in five species: amplification in rodents, decimation in primates, and a surprisingly small repertoire in dogs. Genome Res. 2005;15:231–240. doi: 10.1101/gr.3339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Webb DM. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci USA. 2003;100:8337–8341. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rodriguez I, Mombaerts P, Firestein S. Odorant and vomeronasal receptor genes in two mouse genome assemblies. Genomics. 2004;83:802–811. doi: 10.1016/j.ygeno.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Zozulya S, Echeverri F, Nguyen T. The human olfactory receptor repertoire. Genome Biol. 2001;2:0018.1–0018.12. doi: 10.1186/gb-2001-2-6-research0018. RESEARCH. [DOI] [PMC free article] [PubMed] [Google Scholar]


