Abstract
To clarify the role of human papillomavirus (HPV) in penile cancer we evaluated the prevalence of HPV DNA in different histological subtypes of penile carcinoma, dysplasia, and condyloma using a novel, sensitive SPF10 HPV polymerase chain reaction assay and a novel genotyping line probe assay, allowing simultaneous identification of 25 different HPV types. Formalin-fixed, paraffin-embedded tissue samples were collected from the United States and Paraguay. HPV DNA was detected in 42% cases of penile carcinoma, 90% cases of dysplasia, and 100% cases of condyloma. There were significant differences in HPV prevalence in different histological cancer subtypes. Although keratinizing squamous cell carcinoma and verrucous carcinoma were positive for HPV DNA in only 34.9 and 33.3% of cases, respectively, HPV DNA was detected in 80% of basaloid and 100% of warty tumor subtypes. There was no significant difference in HPV prevalence between cases from Paraguay and the United States. In conclusion, the overall prevalence of HPV DNA in penile carcinoma (42%) is lower than that in cervical carcinoma (∼100%) and similar to vulvar carcinoma (∼50%). In addition, specific histological subtypes of penile cancer—basaloid and warty—are consistently associated with HPV, however, only a subset of keratinizing and verrucous penile carcinomas is positive for HPV DNA, and thus these two tumor groups seem to develop along different pathogenetic pathways.
Penile cancer (PC) is an uncommon disease in the United States and in Europe and has a yearly incidence of 0.29 per 100,000 among whites in the United States. 1 The incidence is an order of magnitude higher in some of the African and South American countries, such as Uganda (incidence of 4.4 per 100,000) or Paraguay (incidence 4.2 per 100,000). 2,3 The etiology of PC is not well understood. Traditionally, lack of neonatal circumcision was considered to be the most significant risk factor for PC, however, the causal relationship has never been established. In the most recent detailed epidemiological study, the highest risk for PC was associated with a history of penile rash lasting more than a month (Bowen’s disease?) [relative risk (RR) = 9.4] and a history of genital warts (RR = 5.9). 4 As compared with these two high-risk factors, the risk of cancer in men uncircumcised or circumcised after the neonatal period was lower: RR = 3.2, and RR = 3.0, respectively. Other risk factors identified in the study included: penile tear (RR = 3.9), difficulty of foreskin retraction (RR = 3.5), more than 30 lifetime sexual partners (RR = 3.4), smoking (RR = 2.8), and smegma (RR = 2.1). 4 The results suggest a strong association between human papillomavirus (HPV) infection and development of PC.
High oncogenic risk HPVs have been detected in virtually 100% of carcinomas of the uterine cervix and the role of HPV in malignant transformation of the cervical epithelium has been well established. 5,6 In contrast to the consistent finding of HPV in the cervical tumors, the reported prevalence of HPV in PC is highly variable, from 15 to 71%, depending on the sensitivity of the detection method and the selection of the tumor type. 4,7 Penile carcinomas include several different histological subtypes. The majority of tumors are well-differentiated, keratinizing squamous cell carcinomas (SCCs) (Figure 1A) ▶ that resemble SCCs arising in nongenital skin. The second most common tumor subtype, verrucous carcinoma (Figure 1B) ▶ , and the less prevalent variants, namely, basaloid carcinoma (Figure 1C) ▶ and warty carcinoma (Figure 1D) ▶ arise most frequently on the mucosal surfaces of the anogenital and oropharyngeal regions, and on the penis these tumors most often involve the glans. 8-10 The histological subtypes of PC are identical to those described in the vulva and it is plausible that penile carcinogenesis parallels the pathogenetic mechanisms of malignant transformation of the vulvar epithelium. The current concept of vulvar carcinogenesis includes at least two independent pathways: one, HPV-related and the other, not associated with HPV infection. 11 Tumors associated with HPV tend to occur in younger women with past history of genital warts or cervical dysplasia and arise from the in situ lesions similar to those found in the cervix. The carcinomas are frequently of basaloid or warty type. 12 The prevalence of HPV in these tumors ranges from 75 to 100%. 13,14 Tumors not associated with HPV occur in older women and are typically well-differentiated keratinizing SCCs arising in a background of differentiated vulvar intraepithelial neoplasia and/or lichen sclerosus. 13 The risk factors are unknown.
Figure 1.
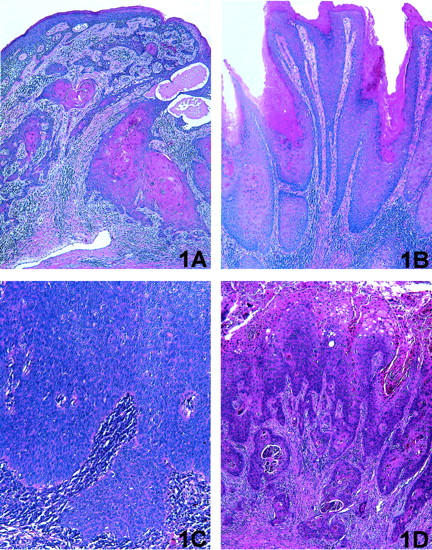
Main histological subtypes of penile carcinoma analyzed in the study. A: Keratinizing SCC: infiltrating tumor characterized by nests and tongues of malignant squamous epithelium with prominent central keratin pearls. B: Verrucous carcinoma: exophytic tumor characterized by papillary architecture and pushing invasive border; malignant squamous epithelium is well differentiated and no koilocytic atypia is present. C: Basaloid carcinoma: infiltrating tumor characterized by nests and cords of immature malignant squamous epithelium with areas of central necrosis; foci of keratin pearls may be also present. D: Warty carcinoma: exophytic/infiltrating tumor characterized by papillary architecture and marked cytological atypia with prominent koilocytic features.
Few studies of PC have analyzed the different histological subtypes separately. This lack of separation of histological types may have confounded both the results of the HPV studies and the epidemiological findings. The goal of this study was to further investigate the relationship between HPV and PC, by examining the prevalence of HPV DNA in a large series of tumors encompassing a spectrum of histological differentiation. In addition we wanted to analyze the prevalence and the distribution of HPV types in the premalignant penile lesions (penile dysplasia). Benign penile condylomas were included in the study as the HPV-positive control group.
The specific questions that we wanted to answer in this study were: 1) are there one or many pathways of penile carcinogenesis?; 2) are the various histological subtypes of penile carcinoma etiologically related?; 3) are there differences in frequency of various histological tumor subtypes between the high-risk and low-risk geographical regions (Paraguay versus the United States)?; 4) are there differences in HPV prevalence in the tumors from the high-risk and low-risk geographical regions (Paraguay versus the United States)?
HPV DNA amplification was performed using a novel, sensitive, broad-spectrum HPV polymerase chain reaction (PCR) assay (SPF 10 PCR), which permits general HPV DNA amplification of at least 43 known HPV types. 15,16 HPV genotyping was performed using a novel line probe assay (LiPA). LiPA assay enables simultaneous identification of 25 individual HPV genotypes, allowing efficient detection of single and/or multiple HPV infection. 16 High sensitivity of the assays used in this study was confirmed in a previous investigation in which HPV DNA was detected in 100% of cases of cervical carcinoma. 6
Materials and Methods
Clinical Specimens
Cases of penile condyloma, dysplasia (including Bowenoid papulosis and Bowen’s disease), and carcinoma were retrieved from the archives of the Pathology Departments at University of Michigan (Ann Arbor, MI), Baylor College of Medicine (Houston, TX), Yale University (New Haven, CT), Universidad Nacional de Asuncion, (Asuncion, Paraguay), and the Weill Medical College of Cornell University (New York, NY). Twelve cases of condyloma, 30 cases of dysplasia, and 155 cases of PC were collected. All cases were reviewed and diagnostic groups were assigned and graded according to standard histological criteria. 17 A representative tissue block from each case was selected for HPV analysis. Clinicopathological parameters were obtained from the pathology reports.
DNA Extraction and β-Globin Amplification
Three, 5-μm thick sections of formalin-fixed, paraffin-embedded tissue were placed on glass slides after cutting deep into the block. The microtome blade was changed after each case. The tissue sections were deparaffinized in xylene and stained with hematoxylin. Tumor tissue was dissected from the adjacent squamous epithelium and stroma using a sterile scalpel blade. The samples were digested with proteinase K (1 mg/ml) in a volume of 0.25 ml at 56°C for 18 hours. Proteinase K was heat inactivated at 95°C for 10 minutes and 10 μl of DNA aliquot was used directly for PCR. To ensure adequate DNA quality, PCR amplification of the β-globin gene was performed in a separate reaction using primers PC03 and PC04, resulting in a 96-bp product. 18 In 13 of 155 (8%) cancer cases no β-globin amplification was achieved. Repeated digestion was ineffective in these cases. These results indicated marked DNA degradation and the cases were excluded from further analysis (11 keratinizing, 1 basaloid, and 1 verrucous carcinoma).
HPV DNA Detection and Typing
Broad-spectrum HPV DNA amplification was performed using the short PCR fragment (SPF10) primer set. 15 The SPF10 primers amplify a 65-bp fragment from the L1 region of the HPV genome. 16 The primer sequences and exact HPV PCR conditions were described previously. 15 The PCR products were run on a 3% agarose gel and the 65-bp product was visualized with ethidium bromide staining. Additional confirmation of the presence of amplified HPV-specific sequences was performed using HPV DNA enzyme immunoassay, a microtiter plate-based hybridization assay. The exact HPV DNA enzyme immunoassay conditions were described previously. 15 All HPV-negative cases were confirmed by the second PCR assay using standard DNA concentration as well as 10× diluted DNA sample to exclude the presence of PCR inhibitors. Appropriate positive and negative PCR controls were run with all reactions.
Samples identified as positive for HPV DNA were genotyped with the INNO-LiPA HPV prototype research assay (LiPA). 16 Twenty-five individual HPV genotypes (high-risk HPV: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 70, and low-risk HPV: 6, 11, 34, 40, 42–44, 53, 54, 74) can be identified simultaneously in a single assay. In this assay, 10 μl of denatured HPV PCR product was hybridized to the genotype-specific probes immobilized as parallel lines on a nitrocellulose strip. After the washing step, the products of hybridization were detected by a color reaction with alkaline phosphatase-streptavidin conjugate and substrate (5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium), which results in a purple precipitate. The exact assay conditions were described previously. 16 The results of hybridization were assessed visually by comparing to the standard grid (Figure 2) ▶ .
Figure 2.
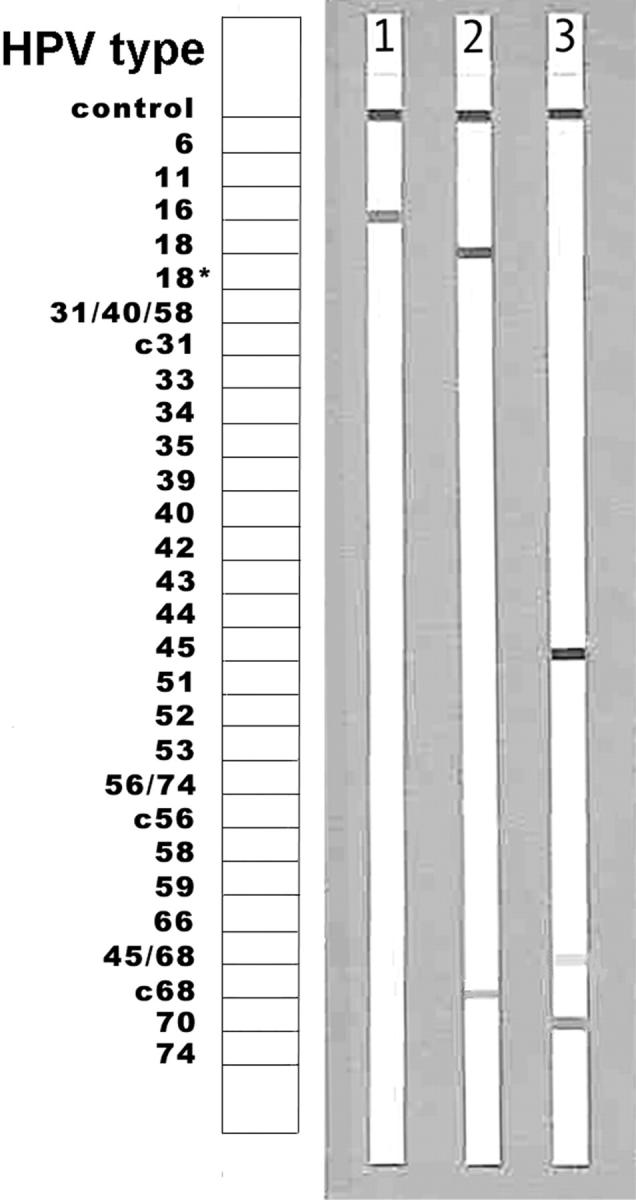
Identification of HPV genotypes using LiPA. LiPA strips with hybridization bands indicating a single HPV type infection: lane 1 = HPV 16; lane 2 = HPV 18; and a multiple HPV type infection: lane 3 = HPV 45 and 70. Note: HPV 18 is reactive with two probes, 18 and c68, and HPV 45 with probes 45 and 45/68.
Results
Clinicopathological Data
The study group included 106 cases of keratinizing SCC (33% grade 1 tumors, 48% grade 2 tumors, and 18% grade 3 tumors), 12 cases of verrucous SCC, 15 cases of basaloid SCC, 5 cases of warty SCC, 2 cases of clear cell SCC, 1 case of sarcomatoid SCC, and 1 case of a metastatic penile SCC in which the primary lesion was not available for a review. Benign cases consisted of a group of penile dysplasia (n = 30) and penile condylomas (n = 12). The average age of patients with cancer was 61.2 years (range, 31 to 94 years), with dysplasia was 58.1 years (range, 27 to 85 years), and with condyloma was 50.6 years (range, 34 to 82 years).
There was no significant difference in the distribution of tumor subtypes between the cases from Paraguay and the United States (Table 1) ▶ .
Table 1.
Histologic Subtypes of Penile Carcinoma by Country of Origin
| Diagnosis | n | Paraguay | United States | Chi-square*P | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Keratinizing scc | 106 | 41 | 75.9 | 65 | 73.8 | >0.05† |
| Basaloid scc | 15 | 3 | 5.5 | 12 | 13.6 | >0.05† |
| Verrucous scc | 12 | 7 | 12.9 | 5 | 5.6 | >0.05† |
| Warty scc | 5 | 2 | 3.7 | 3 | 3.4 | >0.05† |
| Clear cell scc | 2 | 1 | 1.8 | 1 | 1.1 | >0.05† |
| Sarcomatoid scc | 1 | 0 | 0.0 | 1 | 1.1 | >0.05† |
| Metastatic scc | 1 | 0 | 0.0 | 1 | 1.1 | >0.05† |
| Total | 142 | 54 | 100.0 | 88 | 100.0 | |
*Chi-square test for the difference in the frequency of the tumor subtype between the series from Paraguay and the United States.
†No statistically significant difference.
scc, Squamous cell carcinoma.
HPV DNA Detection and Typing
Only the cases with positive β-globin DNA amplification were included in the study. HPV DNA was amplified in 99 of 182 benign and malignant cases, some of which were stored in the paraffin blocks for as long as 20 years. HPV DNA amplification was confirmed with the HPV DNA enzyme immunoassay and the individual HPV genotypes were subsequently identified with the LiPA. The cases, in which HPV DNA was not detected, were of various storage ages.
HPV DNA was detected in 100% of condylomas, 90% cases of dysplasia, and 42% of penile carcinomas. The results of HPV DNA detection in the different histological categories are summarized in Tables 2 and 3 ▶ ▶ . All but one case of condyloma were associated with the low-risk viral types, with HPV 6 being the most prevalent type. In contrast, penile dysplasia was associated with high-risk HPVs in 81.5% of cases, and HPV 16 was most frequently detected. In PC, there were significant differences in HPV prevalence among the different histological tumor subtypes. Keratinizing and verrucous carcinoma were positive for HPV DNA in 34.9 and 33.3% of the cases, respectively. Basaloid and warty PCs were positive for HPV DNA in 80 and 100% of the cases, respectively. The difference in HPV prevalence between these two groups was statistically significant (chi-square test, P < 0.05).
Table 2.
HPV DNA Detection in Penile Condyloma, Dysplasia, and Carcinoma
| Diagnosis | n | HPV-positive | Single HPV type low risk | Single HPV type high risk | Multiple HPV types high risk | Multiple HPV types low and high risk | X types | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | %* | n | %* | n | %* | n | %* | n | %* | ||
| Condyloma | 12 | 12 | 100.0 | 11 | 91.7 | 1 | 8.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysplasia | 30 | 27 | 90.0 | 5 | 18.5 | 16 | 59.3 | 4 | 14.8 | 2 | 7.4 | 0 | 0 |
| All benign cases | 42 | 39 | 92.8 | 16 | 41.0 | 17 | 43.6 | 4 | 10.3 | 2 | 5.1 | 0 | 0 |
| Keratinizing scc | 106 | 37 | 34.9 | 0 | 0 | 23 | 62.1 | 4 | 10.8 | 4 | 10.8 | 6 | 16.2 |
| Verrucous scc | 12 | 4 | 33.3 | 1 | 25.0 | 2 | 50.0 | 0 | 0 | 0 | 0 | 1 | 25.0 |
| Basaloid scc | 15 | 12 | 80.0 | 0 | 0 | 11 | 91.7 | 1 | 8.3 | 0 | 0 | 0 | 0 |
| Warty scc | 5 | 5 | 100.0 | 0 | 0 | 4 | 80.0 | 0 | 0 | 1 | 20.0 | 0 | 0 |
| Clear cell scc | 2 | 1 | 50.0 | 0 | 0 | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sarcomatoid scc | 1 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metastatic scc | 1 | 1 | 100.0 | 0 | 0 | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 |
| All cancer cases | 142 | 60 | 42.2 | 1 | 1.6 | 42 | 70.0 | 5 | 8.3 | 5 | 8.3 | 7 | 11.6 |
*, Percent of HPV-positive cases.
scc, Squamous cell carcinoma.
Low risk HPVs detected: 6,11,74; High risk HPVs detected: 16,18,31,33,35,39,44,45,51,52,53,54,58,66,68,70; X types: unknown HPV types.
Table 3.
HPV Types Identified in Penile Condyloma, Dysplasia, and Carcinoma
| Diagnosis | HPV+ n | HPV 6 | HPV 11 | HPV 16 | HPV 18 | HPV 35 | HPV 45 | HPV 52 | HPV 68 | Other HPVs | HPV X | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %* | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Condyloma | 12 | 9 | 75.0 | 2 | 16.7 | 1 | 8.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysplasia | 27 | 6 | 22.2 | 1 | 3.7 | 11 | 40.7 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 14.8 | 1 | 3.7 | 9A | 33.3 | 0 | 0 |
| All HPV-positive benign cases | 39 | 15 | 38.5 | 3 | 7.7 | 12 | 30.8 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 10.3 | 1 | 2.6 | 9 | 23.1 | 0 | 0 |
| Keratinizing scc | 37 | 4 | 10.8 | 0 | 0 | 19 | 51.3 | 1 | 2.7 | 2 | 5.4 | 4 | 10.8 | 4 | 10.8 | 3 | 8.1 | 3B | 8.1 | 6 | 16.2 |
| Verrucous scc | 4 | 1 | 25.0 | 0 | 0 | 1 | 25.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1C | 25.0 | 1 | 25.0 |
| Basaloid scc | 12 | 0 | 0 | 0 | 0 | 10 | 83.3 | 0 | 0 | 1 | 8.3 | 0 | 0 | 0 | 0 | 0 | 0 | 3D | 25.0 | 0 | 0 |
| Warty scc | 5 | 0 | 0 | 0 | 0 | 5 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clear cell scc | 1 | 0 | 0 | 0 | 0 | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metastatic scc | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| All HPV-positive cancer cases | 60 | 5 | 8.3 | 0 | 0 | 36 | 60.0 | 2 | 3.3 | 3 | 5.0 | 4 | 6.6 | 4 | 6.6 | 3 | 5.0 | 7 | 11.6 | 7 | 11.6 |
*The percentages of all HPV types detected in a histological subtype may add to more than 100% because of multiple infections.
scc, Squamous cell carcinoma.
Other HPV types: A = HPV 31, 33, 39, 44, 51, 54, 58, 66; B = HPV 51, 70, 74; C = HPV 53; D = HPV 31, 54, 70. X types: unknown HPV types.
HPV 16 was the most common viral type identified in PC (60% of HPV+ cases) and was detected in 29 tumors as a single HPV type (48.3%) and as multiple-type infection in 7 tumors (11.6%) (Table 3 ▶ and Figure 2 ▶ ). Other HPV types were relatively less common. The types that were detected as a single viral infection included: HPV 45 (n = 4), HPV 35 (n = 3), HPV 18 (n = 2), HPV 52 (n = 2), HPV 68 (n = 2), HPV 31 (n = 1), HPV 53 (n = 1), and HPV 6 (n = 1). Other high-risk HPVs (51, 54, and 70) were detected in multiple-type infections with HPV 16. Undetermined HPVs (X types) were present in 7.8% of the cases. Multiple viral types were detected in 16.6% of HPV-positive tumors (Table 2) ▶ . There was no statistically significant difference in HPV prevalence/type distribution between the cases from Paraguay and the United States (Table 4) ▶ .
Table 4.
Prevalence of Different HPV Types in Penile Cancers from Paraguay and the United States
| HPV phylogenetic groups* detected in penile carcinomas | Paraguay | United States | Chi-square†P | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Any HPV positive | 20 | 37.0 | 40 | 45.4 | >0.05‡ |
| A10: HPV 6,11,74 | 3 | 13.0 | 4 | 8.5 | >0.05‡ |
| A9: HPV 16,31,33,35,52 | 12 | 52.1 | 32 | 68.0 | >0.05‡ |
| A7: HPV 18,39,45,68,70 | 4 | 17.3 | 6 | 12.7 | >0.05‡ |
| A5: HPV 51,69 | 1 | 4.3 | 1 | 2.1 | >0.05‡ |
| Other HPVs | 3 | 13.0 | 4 | 8.5 | >0.05‡ |
*Phylogenetic viral groups as classified in Mayers and colleagues. 34
†Chi-square test for the difference between the frequency of HPV types in the cases from Paraguay and the United States.
‡No statistically significant difference.
The average age of the patients with HPV DNA-positive versus HPV DNA-negative tumors (58.3 years versus 61.2 years) was not significantly different (Student’s t-test, P > 0.05). No association between the tumor grade and HPV positivity was identified.
Discussion
The present report is the largest multicenter study of HPV DNA prevalence in PC. In this study, HPV DNA amplification was performed using a novel, currently the most sensitive, broad-spectrum HPV PCR assay (SPF 10), which allows for the detection of at least 43 known HPV types. The SPF 10 assay significantly diminishes the problems of HPV detection in formalin-fixed tissue by amplifying only a 65-bp fragment located within the L1 region of the HPV genome. The amplification product is much shorter than the products obtained with other frequently used general primer sets such as My11/09 (450 bp) or GP 5+/6+ (150 bp). 19,20 The kinetics of the PCR reaction favors amplification of shorter DNA sequences and consequently, the SPF assay has been shown to be more sensitive than amplification systems using My11/09 or GP 5+/6+ primers. 15 In addition, a short target sequence is statistically less likely to be affected by either DNA fragmentation or loss during viral integration. High sensitivity of this technique was confirmed in the previous investigation in which HPV DNA was detected in 100% of cases of cervical carcinoma. 6
The overall prevalence of HPV DNA in PC detected in this study was 42.2%. This result is similar to the results of previously published studies (Table 5) ▶ . However, we have observed great differences in HPV prevalence depending on the histological subtype of PC. And although basaloid and warty carcinomas were found to be consistently associated with HPV presence, only a subset of keratinizing and verrucous PCs was positive for HPV DNA. The most common viral type identified in PC was HPV 16, which was detected in 60% of HPV+ cancers. This result is similar to those reported by other investigators in which HPV 16 was found in 65 to 74% of HPV-positive tumors. 4,21,22 In this study we did not find a significant difference between HPV prevalence in tumors from Paraguay and the United States; further, no significant difference in the distribution of the histological tumor subtypes was found. We therefore conclude, that pathogenetic pathways of penile carcinogenesis are likely to be similar in the high-risk and the low-risk geographical regions.
Table 5.
Reported Prevalence of HPV DNA in Penile Carcinoma in the Largest Published Studies
| Reference | Detection method | n | HPV-positive, % | Country |
|---|---|---|---|---|
| Rubin et al 2001 current study | PCR | 142 | 42 | United States and Paraguay |
| Picconi et al 2000 35 | PCR | 38 | 71 | Argentina |
| Gregoire et al 1995 10 | PCR | 117 | 22 | United States and Paraguay |
| Cupp et al 1995 21 | PCR | 42 | 55 | United States |
| Chan et al 1994 36 | PCR | 41 | 15 | Hong-Kong |
| Maden et al 1993 4 | PCR | 67 | 49 | United States and Canada |
| Iwasawa et al 1993 37 | PCR | 111 | 63 | Japan |
| McCance et al 1986 7 | Southern blot | 53 | 51 | Brasil |
In the cervix, all main histological subtypes of carcinoma: SCC, adenocarcinoma, and adenosquamous carcinoma are associated with HPV infection. 6,23 In contrast, SCCs of the vulva seem to have multiple pathogenetic pathways. Basaloid and warty carcinomas are consistently associated with high-risk HPVs. 12-14 Well-differentiated keratinizing SCCs and verrucous carcinomas have a low prevalence of HPV DNA. 12-14 Rare cases of verrucous carcinoma of the vulva have been described to be associated with low oncogenic risk HPVs. 24,25 The results of our current study and previously published reports indicate that the etiology and the pathogenetic pathways of PC may parallel the pathogenetic pathways of vulvar, but not cervical carcinoma. First, the overall prevalence of HPV DNA in PC (42.2%) is lower than that in cervical carcinoma (100%) 5,6 and similar to that reported for vulvar carcinoma (50%). 14 Second, the correlation between HPV DNA detection and histological tumor subtypes is similar in vulvar and PC. In our study two histological subtypes of PC: basaloid and warty were found to be positive for HPV DNA in almost 100% of cases and associated with HPV 16. This result is consistent with previously published reports. Cubilla and colleagues 8 reported detection of HPV 16 in 9 of 11 (81%) cases of basaloid and 3 of 5 (60%) cases of warty SCC of the penis. 9 Interestingly, basaloid carcinomas of the anus and the head and neck mucosa were also described as the specific histological tumor subtypes with high prevalence of HPV. 26-28 Verrucous carcinomas of the penis analyzed in this study were most commonly HPV-negative and of a total of 26 cases reported in the multiple publications only 3 were found to be positive for HPV DNA (12%), and all were positive for low-risk HPVs. 10,21,29-32
The reported prevalence of HPV DNA in penile dysplasia or penile intraepithelial neoplasia ranges from 75 to 100%. 33 Results of our study (90% HPV +) are consistent with the previous reports. High-risk HPVs were detected in 81.5% of these cases, with HPV 16 being the most prevalent viral type. These findings suggest that penile intraepithelial neoplasia or penile dysplasia, as defined currently, is a precursor lesion to only a subset of tumors, which include basaloid and warty carcinomas. The precursor lesion for keratinizing SCC or verrucous carcinoma is not well established. Squamous cell hyperplasia and/or lichen sclerosus frequently coexist with keratinizing SCC and verrucous carcinoma, however the significance of this association remains to be determined. 17 Table 6 ▶ summarizes the hypothetical pathways of penile carcinogenesis after combining the results of the current and previous investigations. There are still multiple unknowns that await further research.
Table 6.
Hypothetical Model of Multiple Pathways of Penile Carcinogenesis
| Etiologic factor | Cancer precursor | Cancer | ||
|---|---|---|---|---|
| (?) and/or HPV | ➞ | (?) | ➞ | Keratinizing squamous cell carcinoma ∼30% HPV+ |
| (?) and/or HPV | ➞ | (?) | ➞ | Verrucous carcinoma ∼30% HPV+ |
| HPV and (?) | ➞ | Penile dysplasia = penile intraepithelial neoplasia | ➞ | Basaloid carcinoma and warty carcinoma 80–100% HPV+ |
Numerous molecular genetic studies have provided strong evidence that HPV is an oncogenic virus. HPV was found to inactivate some of the mechanisms regulating the cellular mitotic cycle. By doing this, the virus launches a cascade of uncontrolled genetic events that may lead to malignant transformation of the host cell. Specifically, HPV has been shown to interfere with the functions of retinoblastoma protein and p53 tumor suppressor protein. Inactivation of retinoblastoma protein keeps the cell in a perpetual proliferative state. The fidelity of cellular DNA replication is maintained by p53. Alteration of p53 expression by HPV renders cellular DNA susceptible to carcinogenic effects of mutagens (eg, cigarette carcinogens). In time, the unchecked replication of damaged DNA may result in malignant transformation of the cell because of accumulation and propagation of DNA errors. However, as the cell cycle is maintained by redundant, multilevel mechanisms, it is thought that more than five different alterations of the major regulatory proteins are required before the cell acquires full malignant potential. And thus, although HPV has been firmly established as a causative factor of many cancers, HPV infection alone is insufficient to cause malignancy. The results of the epidemiological studies clearly indicate that numerous factors, including lack of neonatal circumcision, foreskin injuries, and cigarette smoking contribute to penile carcinogenesis. The exact molecular mechanisms by which these factors increase the risk of PC remain to be determined.
Footnotes
Address reprint requests to Dr. Edyta C. Pirog, Department of Pathology, Weill Medical College of Cornell University, 525 E 68th St., F-766, New York, NY 10021. E-mail: ecpirog@mail.med.cornell.edu.
References
- 1.Frisch M, Goodman MT: Human papillomavirus-associated carcinomas in Hawaii and the mainland U.S. Cancer 2000, 88:1464-1469 [DOI] [PubMed] [Google Scholar]
- 2.: International Agency for Research on Cancer, World Health Organization: Cancer Incidence in Five Continents. Age-standardized incidence rates, four-digit rubrics, and age-standardized and cumulative incidence rates, three-digit rubrics. IARC Sci Publ 1992, 120:871-1011 [PubMed] [Google Scholar]
- 3.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S: Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer 2000, 82:1585-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maden C, Sherman KJ, Beckmann AM, Hislop TG, Teh CZ, Ashley RL, Daling JR: History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 1993, 85:19-24 [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV: Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst 1995, 87:796-802 [DOI] [PubMed] [Google Scholar]
- 6.van Muyden RC, ter Harmsel BW, Smedts FM, Hermans J, Kuijpers JC, Raikhlin NT, Petrov S, Lebedev A, Ramaekers FC, Trimbos JB, Kleter B, Quint WG: Detection and typing of human papillomavirus in cervical carcinomas in Russian women: a prognostic study. Cancer 1999, 85:2011-2016 [PubMed] [Google Scholar]
- 7.McCance DJ, Kalache A, Ashdown K, Andrade L, Menezes F, Smith P, Doll R: Human papillomavirus types 16 and 18 in carcinomas of the penis from Brazil. Int J Cancer 1986, 37:55-59 [DOI] [PubMed] [Google Scholar]
- 8.Cubilla AL, Reuter VE, Gregoire L, Ayala G, Ocampos S, Lancaster WD, Fair W: Basaloid squamous cell carcinoma: a distinctive human papilloma virus-related penile neoplasm: a report of 20 cases. Am J Surg Pathol 1998, 22:755-761 [DOI] [PubMed] [Google Scholar]
- 9.Cubilla AL, Velazques EF, Reuter VE, Oliva E, Mihm MC, Young RH: Warty (condylomatous) squamous cell carcinoma of the penis: a report of 11 cases and proposed classification of ’verruciform’ penile tumors. Am J Surg Pathol 2000, 24:505-512 [DOI] [PubMed] [Google Scholar]
- 10.Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD: Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst 1995, 87:1705-1709 [DOI] [PubMed] [Google Scholar]
- 11.Crum CP: Carcinoma of the vulva: epidemiology and pathogenesis. Obstet Gynecol 1992, 79:448-454 [DOI] [PubMed] [Google Scholar]
- 12.Kurman RJ, Toki T, Schiffman MH: Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol 1993, 17:133-145 [DOI] [PubMed] [Google Scholar]
- 13.Toki T, Kurman RJ, Park JS, Kessis T, Daniel RW, Shah KV: Probable nonpapillomavirus etiology of squamous cell carcinoma of the vulva in older women: a clinicopathologic study using in situ hybridization and polymerase chain reaction. Int J Gynecol Pathol 1991, 10:107-125 [DOI] [PubMed] [Google Scholar]
- 14.Bloss JD, Liao SY, Wilczynski SP, Macri C, Walker J, Peake M, Berman ML: Clinical and histologic features of vulvar carcinomas analyzed for human papillomavirus status: evidence that squamous cell carcinoma of the vulva has more than one etiology. Hum Pathol 1991, 22:711-718 [DOI] [PubMed] [Google Scholar]
- 15.Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W: Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 1998, 153:1731-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W: Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 1999, 37:2508-2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternberg SS, Antonioli DA: Diagnostic Surgical Pathology, ed 3. Philadelphia, Lippincott Williams & Wilkins, 1999
- 18.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N: Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230:1350-1354 [DOI] [PubMed] [Google Scholar]
- 19.Manos M, Ting Y, Wright D, Lewis A, Broker T, Wolinksy S: Use of polymerase chain reaction amplification for detection of genital human papillomavirus. Cancer Cells 1989, 7:209-214 [Google Scholar]
- 20.de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ: The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol 1995, 76:1057-1062 [DOI] [PubMed] [Google Scholar]
- 21.Cupp MR, Malek RS, Goellner JR, Smith TF, Espy MJ: The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol 1995, 154:1024-1029 [PubMed] [Google Scholar]
- 22.Varma VA, Sanchez-Lanier M, Unger ER, Clark C, Tickman R, Hewan-Lowe K, Chenggis ML, Swan DC: Association of human papillomavirus with penile carcinoma: a study using polymerase chain reaction and in situ hybridization. Hum Pathol 1991, 22:908-913 [DOI] [PubMed] [Google Scholar]
- 23.Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C: Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 2000, 157:1055-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djurdjevic S, Devaja O, Hadzic B: Malignant potential of gigantic condylomatous lesions of the vulva. Eur J Gynaecol Oncol 1999, 20:63-66 [PubMed] [Google Scholar]
- 25.Kondi-Paphitis A, Deligeorgi-Politi H, Liapis A, Plemenou-Frangou M: Human papilloma virus in verrucus carcinoma of the vulva: an immunopathological study of three cases. Eur J Gynaecol Oncol 1998, 19:319-320 [PubMed] [Google Scholar]
- 26.Frisch M, Fenger C, van den Brule AJ, Sorensen P, Meijer CJ, Walboomers JM, Adami HO, Melbye M, Glimelius B: Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res 1999, 59:753-757 [PubMed] [Google Scholar]
- 27.Vincent-Salomon A, de la Rochefordiere A, Salmon R, Validire P, Zafrani B, Sastre-Garau X: Frequent association of human papillomavirus 16 and 18 DNA with anal squamous cell and basaloid carcinoma. Mod Pathol 1996, 9:614-620 [PubMed] [Google Scholar]
- 28.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D: Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000, 92:709-720 [DOI] [PubMed] [Google Scholar]
- 29.Noel JC, Vandenbossche M, Peny MO, Sassine A, de Dobbeleer G, Schulman CC, Verhest A: Verrucous carcinoma of the penis: importance of human papillomavirus typing for diagnosis and therapeutic decision. Eur Urol 1992, 22:83-85 [DOI] [PubMed] [Google Scholar]
- 30.Masih AS, Stoler MH, Farrow GM, Wooldridge TN, Johansson SL: Penile verrucous carcinoma: a clinicopathologic, human papillomavirus typing and flow cytometric analysis. Mod Pathol 1992, 5:48-55 [PubMed] [Google Scholar]
- 31.Masih AS, Stoler MH, Farrow GM, Johansson SL: Human papillomavirus in penile squamous cell lesions. A comparison of an isotopic RNA and two commercial nonisotopic DNA in situ hybridization methods. Arch Pathol Lab Med 1993, 117:302-307 [PubMed] [Google Scholar]
- 32.Dianzani C, Bucci M, Pierangeli A, Calvieri S, Degener AM: Association of human papillomavirus type 11 with carcinoma of the penis. Urology 1998, 51:1046-1048 [DOI] [PubMed] [Google Scholar]
- 33.Aynaud O, Ionesco M, Barrasso R: Penile intraepithelial neoplasia. Specific clinical features correlate with histologic and virologic findings. Cancer 1994, 74:1762-1767 [DOI] [PubMed] [Google Scholar]
- 34.Mayers G, Baker C, Munger C, Sverdrup F, McBride A, Bernard H: Human Papillomaviruses 1997. A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. 1997, Theoretical Biology and Biophysics Group T-10, Los Alamos
- 35.Picconi MA, Eijan AM, Distefano AL, Pueyo S, Alonio LV, Gorostidi S, Teyssie AR, Casabe A: Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol 2000, 61:65-69 [DOI] [PubMed] [Google Scholar]
- 36.Chan KW, Lam KY, Chan AC, Lau P, Srivastava G: Prevalence of human papillomavirus types 16 and 18 in penile carcinoma: a study of 41 cases using PCR. J Clin Pathol 1994, 47:823-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwasawa A, Kumamoto Y, Fujinaga K: Detection of human papillomavirus deoxyribonucleic acid in penile carcinoma by polymerase chain reaction and in situ hybridization. J Urol 1993, 149:59-63 [DOI] [PubMed] [Google Scholar]


