Abstract
The potential of embryonal day (ED) 14 fetal liver epithelial progenitor (FLEP) cells from Fischer (F)344 rats to repopulate the normal and retrorsine-treated liver was studied throughout a 6-month period in syngeneic dipeptidyl peptidase IV (DPPIV−) mutant F344 rats. In normal liver, FLEP cells formed: 1) hepatocytic clusters ranging in size up to ∼800 to 1000 cells; 2) bile duct structures connected to pre-existing host bile ducts; and 3) mixed clusters containing both hepatocytes and bile duct epithelial cells. Liver repopulation after 6 months was moderate (5 to 10%). In retrorsine-treated liver, transplanted cells formed large multilobular structures containing both parenchymal and bile duct cells and liver repopulation was extensive (60 to 80%). When the repopulating capacity of ED 14 FLEP cells transplanted into normal liver was compared to adult hepatocytes, three important differences were noted: 1) FLEP cells continued to proliferate at 6 months after transplantation, whereas adult hepatocytes ceased proliferation within the first month; 2) both the number and size of clusters derived from FLEP cells gradually increased throughout time but decreased throughout time with transplanted mature hepatocytes; and 3) FLEP cells differentiated into hepatocytes when engrafted into the liver parenchyma and into bile epithelial cells when engrafted in the vicinity of the host bile ducts, whereas adult hepatocytes did not form bile duct structures. Finally, after transplantation of ED 14 FLEP cells, new clusters of DPPIV+ cells appeared after 4 to 6 months, suggesting reseeding of the liver by transplanted cells. This study represents the first report with an isolated fetal liver epithelial cell fraction in which the cells exhibit properties of tissue-determined stem cells after their transplantation into normal adult liver; namely, bipotency and continued proliferation long after their transplantation.
The existence and possible function of progenitor or stem cells in the adult liver has been controversial for many years. 1,2 The existence of such cells, postulated more than 40 years ago, was based on studies in rodents in which it seemed that cells in the distal cholangioles of the bile ducts seemed to be responsible for restoration of liver mass after dietary injury. 3 At that time, it was also established that bile duct epithelial cells and hepatocytes are of common embryological origin, derived from hepatoblasts emanating from the foregut endoderm. 4-7 Therefore, a potential precursor/product relationship between cells of the distal cholangioles and hepatocytes seemed reasonable. However, specific identification of these potential precursor cells has been problematic, because unique markers for liver stem/progenitor cells have not yet been identified.
Studies conducted in the 1960’s also established that the proliferative activity of adult hepatocytes is sufficient to repopulate the liver after two-thirds partial hepatectomy (PH) and participation by stem/progenitor cells is not required. 8 However, under conditions in which the proliferative capacity of hepatocytes is impaired, progenitor or facultative stem cells are activated to proliferate and differentiate into mature hepatocytes. 9-12 Epithelial cell lines have also been established from neonatal and adult liver. 13 Some of these cell lines express liver-specific genes under induced experimental conditions 13-15 and can also differentiate into mature hepatocytes after transplantation into the adult liver, 16,17 suggesting that they retain some stem cell-like properties. 8
Several recent studies have used immunoselection with specific antibodies to separate and begin to define the properties of liver stem/progenitor cells. 18-20 However, the ultimate test to determine whether a particular cell has stem cell properties is to follow its proliferation and phenotypic differentiation in vivo after transplantation. Fortunately, in rodents, several excellent models have been developed to study liver repopulation after transplantation of hepatic cells, the urokinase plasminogen activator (uPA) transgenic mouse, 21,22 the fumaryl acetoacetate hydrolase null mouse, 23 the retrorsine (Rs)-treated rat 24 and the liver x-irradiated rat. 25 In the former two models, selective repopulation of the liver by transplanted cells is based on continuous destruction of host hepatocytes by toxic 21 or metabolic injury, 23 and in the latter two models on compromised proliferative activity of endogenous hepatocytes resulting from DNA damage, 24,25 possibly coupled with augmented apoptosis. 26
In the rat, an excellent model has been developed to follow the fate of transplanted liver cells, the syngeneic dipeptidyl peptidase IV (DPPIV−) mutant Fischer (F) 344 rat. 27 DPPIV is an exopeptidase that is highly expressed in epithelial cells of many organs and is also weakly expressed in endothelial cells. In the liver, it is expressed in both hepatocytes and bile duct epithelial cells, in the former in a characteristic bile canalicular distribution and in the latter in a diffuse cytoplasmic expression pattern. 27-30 Both of these cellular phenotypes can be readily detected and distinguished from each other by DPPIV enzyme histochemistry. 31
In the DPPIV− mutant F344 rat, we previously reported that mature hepatocytes can fully repopulate the liver. 24 This required pretreatment of the animals with Rs, a DNA-alkylating agent that disrupts cell cycle progression in hepatocytes, so that these cells cannot proliferate. Therefore, when wild-type hepatocytes are transplanted in conjunction with two-thirds PH in Rs-treated rats, transplanted cells selectively proliferate and repopulate the liver. However, in the absence of Rs treatment, transplanted hepatocytes have no proliferative advantage over endogenous hepatocytes and selective liver repopulation does not occur. 24
Because the fetal liver contains highly proliferative epithelial cells 32 that are progenitors of both hepatocytes and cholangiocytes, 4-7 we reasoned that these cells should have a higher proliferative capacity than mature hepatocytes and might be able to selectively repopulate the liver under normal experimental conditions. Previously, we determined that embryonal day (ED) 14 rat liver contains a subpopulation of bipotent epithelial cells that can differentiate into hepatocytes or bile duct epithelial cells, depending on their engraftment site in the liver parenchyma. 33 In the present study, we have followed the properties of transplanted ED 14 fetal liver epithelial progenitor (FLEP) cells and the ability of these cells to repopulate the liver throughout a period of 6 months. We have found that ED 14 FLEP cells expand very rapidly in Rs-treated animals with extensive liver repopulation (up to 60 to 80%) and form complete new liver lobules containing both hepatocytes and bile ducts. However, significant liver repopulation by transplanted ED 14 FLEP cells (5 to 10%) also occurs in the normal liver. Most interestingly, the number and size of DPPIV+ cell clusters increases progressively throughout time, clusters of both unipotent (hepatocytic or bile ductular) and bipotent (mixed hepatocytic and bile ductular) phenotype are observed, some transplanted cells are still proliferating 6 months after transplantation, and there is progressive reseeding of the liver with transplanted cells throughout time. Thus, in contrast to adult hepatocytes, which can repopulate the liver only when there is substantial selection pressure, early fetal liver epithelial cells can repopulate the liver in a normal hepatocellular environment.
Materials and Methods
Materials
Rs and diaminobenzidine, glycine-proline-4-methoxy-β-naphtylamide, 4-methoxy-β-naphtylamide, glycine-proline p-nitroaniline, sodium nitrite, ammonium sulfamate, N-1(-naphthyl)ethylenediamine dihydrochloride were purchased from Sigma Chemical Co (St. Louis, MO). Rat endothelial cell antigen, RECA-1, clone HIS 52, was from Accurate Chemical and Scientific Corporation (Westbury, NY). DPPIV monoclonal antibody (monoclonal mouse anti-rat CD26), clone OX61, was from Harlan Sera-Lab Limited (Loughborough, England). Vectastain Elite ABC kit was from Vector Laboratories, Burlingame, CA. Rabbit anti-rat red blood cell IgG was from Rockland (Gilbertsville, PA). Radioactive 35S-UTP (SJ603) and cytokeratin (CK)-19 antibody (RPN 1165) were obtained from Amersham Life Science Products (Arlington Heights, IL). Autoradiographic emulsion, type NBT2, was purchased from Eastman Kodak Company (New Haven, CT).
Animals and Animal Treatment
Timed, pregnant Fischer (F) 344 rats were purchased from Taconic Farms (Germantown, NY). Mutant DPPIV-deficient (DPPIV−) F344 rats were obtained from the Special Animal Core of the Liver Research Center, Albert Einstein College of Medicine. All studies with animals were conducted under protocols approved by the Animal Care Use Committee of the Albert Einstein College of Medicine and were in accordance with National Institutes of Health Guidelines. Rs treatment of the animals was as described previously. 24 For studies in normal rats, animals of 150 to 180 g were used as cell transplantation recipients.
Isolation and Transplantation of FLEP Cells
FLEP cells were isolated on ED 14, 16, or 18 from normal DPPIV+ pregnant rats by a modification of the procedure of Sigal and colleagues, 34 as described previously. 33 Freshly isolated FLEP (4.0 × 105) cells were transplanted through the portal vein immediately after two-thirds PH. FLEP cells were transplanted into normal and Rs-treated, male or female DPPIV− F344 rats. The livers were removed and the tissue analyzed 1, 2, 4, and 6 months after cell transplantation. In most experiments, four animals were used for each time point. To compare the extent of liver repopulation by adult hepatocytes and fetal hepatoblasts, equal numbers of cells (4.0 × 105) were injected into the portal vein of normal animals in conjunction with PH and livers were removed 1, 2, 4, and 6 months after cell transplantation.
Histochemical Detection and Enzyme Assay for DPPIV
The histochemical detection of DPPIV-positive transplanted cells in the liver of mutant DPPIV− F344 rats was performed on frozen liver sections, as described previously. 31 DPPIV enzyme activity was determined in homogenates of liver tissue by a modification of the procedure of Nagatsu and colleagues. 35 The substrate used for the enzyme assay was glycyl-proline p-nitroanilide (Sigma), which is cleaved by DPPIV, releasing p-nitroaniline. The latter is subjected to diazotization and converted into an azo dye with maximum optical density at 540 nm. The readings were taken against a control sample processed in the presence of 10 mmol/L Diprotin A (Sigma), a specific inhibitor of DPPIV. A standard linear curve was prepared with 0.05 to 1.0 μm p-nitroaniline, dissolved in 2% methanol. In brief, 0.1 ml of 3 mmol/L substrate solution (prepared in 1% Triton X-100) was added to 0.1 ml of 0.1 to 1% liver homogenate, prepared in 0.1 mol/L glycine buffer, pH 8.7, containing 1% Triton X-100. The samples were incubated for 30 minutes at 37°C. The reaction was stopped by adding 0.8 ml of 5% HClO4 and centrifuged for 10 minutes at 4000 rpm in the cold. One half ml of clear supernatant was transferred to a new tube and 0.5 ml of 0.2% sodium nitrite was added for diazotization. After 10 minutes incubation in the cold, 0.5 ml of 0.5% of ammonium sulfamate was added to decompose excess sodium nitrite and the samples were incubated for an additional 2 minutes at room temperature. One ml of a 0.05% solution of N-(1-naphthyl)ethylenediamine (Sigma) in 95% ethanol was then added and the samples were incubated for 30 minutes at 37°C to form the azo dye salt. Readings were taken at 540 nm against a control processed the same way, but to which 10 mmol/L Dipronin A was added.
Dual in Situ Hybridization and Immunohistochemistry Labeling for DPPIV and Histone 3 mRNA Expression
Dual in situ hybridization/immunohistochemistry labeling for DPPIV and histone 3 mRNA was performed on frozen sections, as described previously. 36 First, the sections were processed with a monoclonal antibody against DPPIV (anti-rat CD26) and peroxidase activity was revealed by diaminobenzidine. In situ hybridization was then performed, using a 35S-labeled histone-3 antisense riboprobe to detect cells in S phase, as previously reported. 36
Determination of Liver Repopulation by Transplanted FLEP Cells
Analysis of liver repopulation was conducted by two independent methods: 1) scanning slides histochemically stained for DPPIV enzyme activity with a high-resolution Polaroid CS-600 scanner (Polaroid Corp., Cambridge, MA) and measuring the red stained areas (DPPIV+) versus the total area of the liver section, using Adobe Photoshop; and 2) biochemical determination of DPPIV enzyme activity in liver homogenate, as described above. DPPIV enzyme activity of the livers after transplantation versus the enzyme activity of normal liver, taken as 100, was used to calculate the percent liver repopulation.
Results
Repopulation of the Liver by ED 14 FLEP Cells in Rs-Treated Rats
In our previous studies of liver repopulation with adult hepatocytes, pretreatment of recipients with Rs was used to augment proliferation of transplanted cells. Therefore, we first transplanted ED 14 FLEP cells under the Rs/PH protocol to gauge their proliferative potential under maximally induced host conditions. As shown in Figure 1 ▶ , there was substantial proliferation of transplanted ED 14 FLEP cells within 1 month, as evidenced by circular clusters containing 50 to 100 DPPIV+ cells in 5-μm sections (Figure 1A) ▶ , increasing to >250 DPPIV+ cells at 2 months (Figure 1B) ▶ , 500 to 1000 DPPIV+ cells at 4 months (Figure 1C) ▶ and >1000 DPPIV+ cells at 6 months (Figure 1D) ▶ . At 4 and 6 months, transplanted cell clusters became confluent (Figure 1, C and D) ▶ , encompassing multiple lobules. We also observed DPPIV+ cholangiocytes that formed mature DPPIV+ bile ducts. DPPIV+ bile duct structures were evident as early as 1 month after ED 14 FLEP cell transplantation, but became more numerous and fully developed at later time points (Figure 1, C and D ▶ , arrows).
Figure 1.
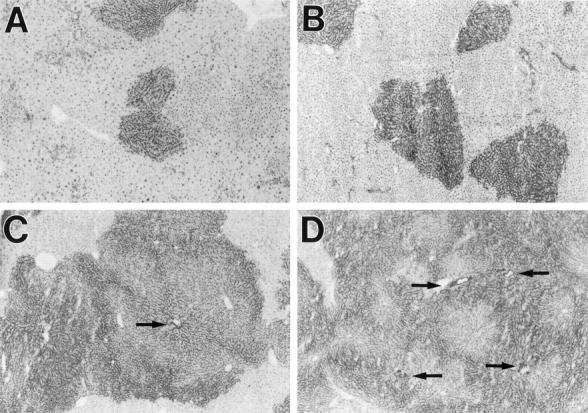
Repopulation of the liver by ED 14 FLEP cells in Rs-treated rats. Rats treated with Rs, as noted in Materials and Methods, were transplanted with an enriched cell fraction containing 4 × 10 5 ED 14 FLEP cells in conjunction with two-thirds PH. Animals were sacrificed after 1, 2, 4, or 6 months, and the presence of transplanted cells was detected by DPPIV enzyme histochemistry: 1 month (A), 2 months (B), 4 months (C), and 6 months (D). Bile duct structures containing transplanted cells are highlighted by arrows. Original magnifications, ×40.
Repopulation of the Liver by ED 14 FLEP Cells in Normal Rats
In the absence of Rs pretreatment, clusters of DPPIV+ cells were still observed at 1 month after ED 14 FLEP cell transplantation but were less numerous and contained fewer cells than observed in Rs-treated animals (Figure 2A) ▶ . However, the number and size of DPPIV+ cell clusters increased throughout time (Figure 2; B, C, and D ▶ ) and at 4 to 6 months after ED 14 FLEP cell transplantation, mature bile duct structures represented a prominent feature (Figure 2D) ▶ . In both Rs-treated and normal rats, PH was required for proliferation of transplanted ED 14 FLEP cells.
Figure 2.
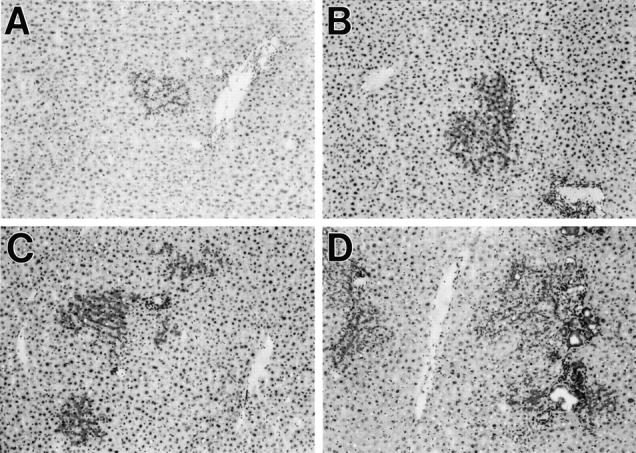
Repopulation of the liver by ED 14 FLEP cells in normal rats. Untreated DPPIV− rats were transplanted with an enriched cell fraction containing 4 × 10 5 ED 14 FLEP cells in conjunction with two-thirds PH. Animals were sacrificed after 1, 2, 4, or 6 months, and the presence of transplanted cells was detected by DPPIV enzyme histochemistry: 1 month (A), 2 months (B), 4 months (C), and 6 months (D). Extensive, multilobulated bile duct structures, linked to pre-existing host bile ducts, were observed in many of the proliferating cell clusters (D). Original magnifications, ×100.
Comparison of Repopulation in Normal Liver by ED 14 FLEP Cells Versus Adult Hepatocytes
Liver repopulation by ED 14 FLEP cells versus adult hepatocytes transplanted into normal liver showed vastly different results, because there was a progressive increase in the number and size of DPPIV+ clusters throughout time with ED 14 FLEP cells, but no increase in the number or size of clusters after the first month with adult hepatocytes. In addition, DPPIV+ bile duct structures were observed only very rarely after transplantation of adult hepatocytes.
To quantitate liver repopulation in the normal rat using FLEP cells compared with adult hepatocytes, we determined the number of clusters/cm2, the number of cells/cluster, the estimated number of cell divisions/cluster, and the percentage of liver repopulation. As shown in Table 1 ▶ , with ED 14 FLEP cells, there was a progressive increase in the number of clusters/cm 2 throughout time, with a threefold increase between 1 and 2 months, a threefold increase between 2 and 4 months, and a fivefold increase between 4 and 6 months. The number of cells/cluster also increased, as did the calculated average number of cell divisions/cluster (assuming that each cluster was derived from a single cell, that there was no loss of cells from these clusters, and that the general shape of the clusters is spherical). It should be noted, however, that in the 6-month specimens, small clusters were not included in the calculation of the number of cells/cluster. These small clusters were often multiple and located in the vicinity of large clusters (see below). The percentage of liver repopulation by ED 14 FLEP cells also increased throughout time, with the greatest increase occurring between 4 to 6 months, reaching a maximum of 5 to 10%. In selected areas, liver repopulation was as high as 20 to 30%.
Table 1.
Kinetics of Liver Repopulation by Transplanted ED 14 FLEP Cells versus Adult Hepatocytes in Normal Rats
| Months after cell transplantation | ||||
|---|---|---|---|---|
| 1 | 2 | 4 | 6 | |
| ED-14 fetal liver epithelial cells | ||||
| Clusters/cm2 | 1.7 | 5.0 | 15 | 82 |
| Cells/cluster | 50 | 192 | 485 | 920 |
| Divisions/cluster | 5–6 | 7–8 | 9 | 10 |
| % Repopulation | 1.4 | 1.8 | 2.4 | 6.6 |
| Adult hepatocytes | ||||
| Clusters/cm2 | 70 | 30 | — | 40 |
| Cells/cluster | 16 | 14 | — | 10 |
| Divisions/cluster | 4 | 4 | — | 3 |
| % Repopulation* | 0.6 | 0.07 | — | 0.06 |
*Due to a technical limitation of the computerized scanning method to detect very small clusters of DPPIV+ cells, the percent repopulation by adult hepatocytes is underestimated. This discrepancy is most evident at the 1-month time point; however, it does not affect the overall interpretation of the data.
The above findings were in marked contrast to results obtained with adult hepatocytes (Table 1) ▶ . The number of clusters/cm 2 at 1 month after cell transplantation was much higher with adult hepatocytes compared to ED 14 FLEP cells (70 versus 1.7). However, the average size of clusters produced by hepatocytes at 1 month after cell transplantation was much smaller than with ED 14 FLEP cells, the number of clusters/cm 2 did not increase throughout time (they actually decreased by ∼40%), the number of cell divisions per cluster (∼3 to 4) also did not increase and the overall percentage of liver repopulation was nil after the first month.
Phenotypic Characterization of DPPIV+ Cell Clusters at 6 Months after ED 14 FLEP Cell Transplantation
To further characterize individual clusters produced from ED 14 FLEP cells, 50 serial sections were prepared from a normal rat 6 months after cell transplantation. Individual clusters were mapped by color digital photography of sequential fields of the 25th section, preparation of a large composite print from the sequential photomicrographs and assignment of a number to each cluster. One hundred and five clusters were examined through the complete series of serial sections. In most instances, the entire cluster could be viewed from top to bottom, although some clusters spanned beyond the 50 sections.
Table 2 ▶ shows the proportions of unipotent (hepatocytic or bile ductular) and bipotent (hepatocytic/bile ductular) cells in the fraction used for ED 14 FLEP cell transplantation, as determined by α-fetoprotein (AFP) and CK-19 expression, 33 and the proportions of hepatocytic, bile ductular and mixed clusters (containing both hepatocytes and mature bile ducts) at 6 months after cell transplantation, as determined by histological analysis. In addition to the three types of hepatic epithelial cell clusters, we also observed clusters of transplanted endothelial cells. Examples of each cell cluster type are shown in Figure 3 ▶ ; A, hepatocytic; B, bile ductular; C, mixed, and D, endothelial. The vast majority of cells in the ED 14 FLEP cell fraction used for transplantation were unipotent, 88% hepatocytic and 9% bile ductular; only 3% were bipotential (Table 2) ▶ . However, at 6 months after cell transplantation, the majority of clusters were of mixed phenotype (56%), comprised of large numbers of hepatocytes together with mature, multilobulated bile ducts in one or more regions (Figure 3C) ▶ . By analysis of serial sections, most new bile ducts could be traced back to portal regions. In some instances, duct prolifes were present at one or both extreme ends of a large hepatocytic cluster. Virtually all of the large clusters (ie, those containing >500 cells and spanning >30 sections) were of mixed phenotype. Pure hepatocytic clusters (31%, example shown in Figure 3A ▶ ) and bile duct cell clusters (5%, example shown in Figure 3B ▶ ) generally spanned 10 to 25 serial sections for hepatocytes and 6 to 12 serial sections for bile ducts. The former were generally located in the parenchyma and the latter in conjunction with portal regions. Endothelial cell clusters (7% of total, example shown in Figure 3D ▶ ) were initially thought to represent FLEP cells that had proliferated but remained undifferentiated. However, dual immunohistochemistry revealed that they were positive for both DPPIV and anti-rat RECA-1 antibody, which is specific for a surface protein of endothelial cells 37 (data not shown).
Table 2.
Distribution Profile of Unipotent and Bipotent ED 14 FLEP Cells before Transplantation and Cell Clusters in the Liver 6 Months after Transplantation
| Phenotypic expression of isolated ED 14 FLEP cells before transplantation | ||
|---|---|---|
| Hepatocytic (AFP+) | Ductular (CK-19+) | Bipotential (AFP+/CK-19+) |
| 88% | 9% | 3% |
| Morphologic appearance of cell clusters derived from ED 14 FLEP cells six months after transplantation | |||
|---|---|---|---|
| Hepatocytic | Ductular | Mixed | Endothelial |
| 31%* | 5% | 56% | 7% |
*Approximately one-half of these clusters were very small and in the vicinity immediately adjacent to large clusters of mixed phenotype containing both hepatocytes and small epithelial cells in mature duct structures and appeared to result from reseeding of the liver from proliferating cells in these large mixed clusters.
Figure 3.
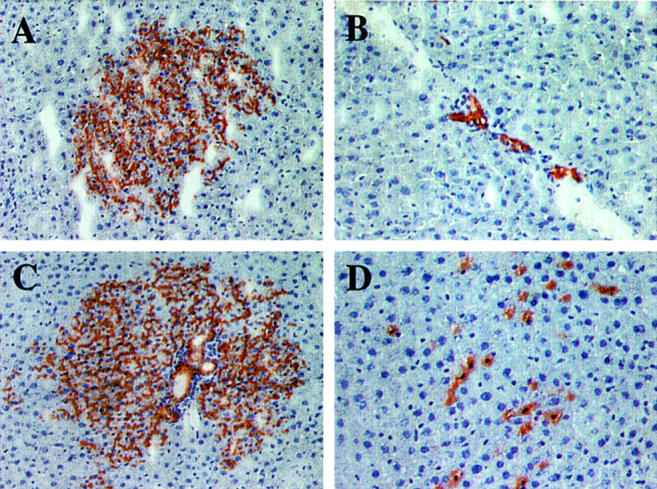
Morphological appearance of proliferated cell clusters at 6 months after transplantation of ED 14 FLEP cells. A: Moderately large hepatocytic cluster. B: Cluster of mature bile duct cells that have proliferated within a host bile duct next to a venous channel. C: Large mixed cluster of transplanted cells containing both hepatocytes and well-differentiated bile ducts. At the periphery of the cluster, cells with a hepatocytic phenotype appear to be extending into the surrounding parenchyma. D: Endothelial cell cluster. Cells with a small nucleus and extensive DPPIV+ cytoplasm are loosely interspersed between hepatocytes and appear to be within the liver sinusoids. By immunohistochemistry, these cells co-stain for rat endothelial cell antigen, RECA-1 (data not shown). Original magnifications: ×100 (A and C); ×200 (B and D).
Bipotency of Transplanted ED 14 FLEP Cells
From the above analysis, it was not possible to distinguish whether the mixed clusters are derived from bipotent progenitor cells or from small aggregates of transplanted cells containing both unipotent hepatocytic and bile ductular progenitors. However, two observations with serial sections suggested that a substantial number are truly bipotent. In some areas, we observed multiple small to mid-sized clusters of hepatocytes close to a portal region (Figure 4) ▶ . On following these clusters through the serial sections, initially they were negative for DPPIV+ bile duct cells (Figure 4, A and B) ▶ . However, when some came into contact with the portal space, DPPIV+ bile duct cells were noted (Figure 4C) ▶ . Continuing further through the sections, when the transplanted hepatocytic clusters were no longer in contact with the portal region (which was still present in the section), DPPIV+ bile duct cells were no longer present (Figure 4, D and E) ▶ . We interpret these results as evidence that some cells in proliferated clusters within the hepatic parenchyma seem to have the ability to cross the limiting plate and differentiate into bile duct epithelial cells. In other instances, we observed mature DPPIV+ bile duct structures in portal regions in which a few cells in the adjacent parenchyma in continuity with the duct exhibited a differentiated hepatocytic morphology (Figure 5) ▶ . These clusters, which are primarily of biliary phenotype, also seem to be derived from bipotent cells.
Figure 4.
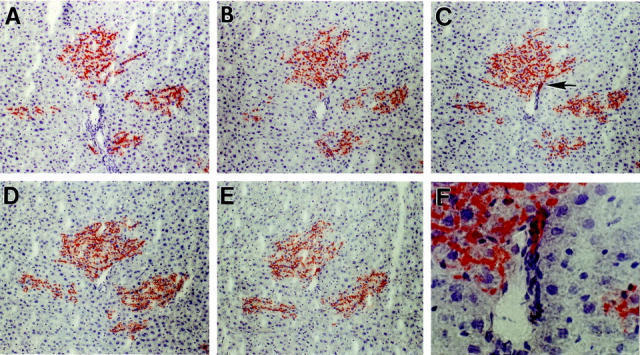
Cluster of transplanted cells with a hepatocytic morphology forming mature bile duct cells when it transiently contacts a pre-existing bile duct. The cluster of interest spanned sections 12 to 50. When this cluster came into contact with a host bile duct, transplanted cells (DPPIV+) with a mature bile duct phenotype (highlighted by an arrow in C) were observed in three serial sections, 35, 36, and 37. The specific sections illustrated in this figure are: 40 (A), 38 (B), 36 (C), 34 (D), 32 (E), and 36 (F) at higher magnification. Original magnifications: ×100 (A–E); ×400 (F).
Figure 5.
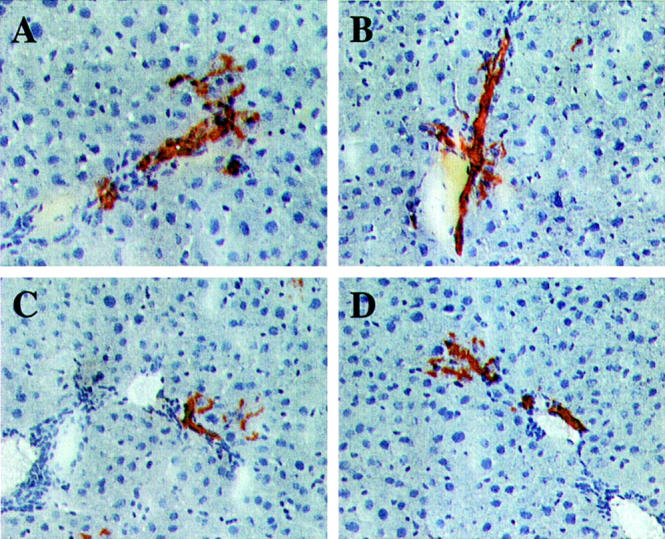
Clusters with a bile duct phenotype exhibiting a few cells with a hepatocytic morphology in one area of the immediately adjacent parenchyma. A–D: Four examples taken from five clusters in the serial sections exhibiting this phenotype. Original magnifications: ×200.
Reseeding of the Liver by ED 14 FLEP Cells
A most interesting observation during the 6-month period after transplantation of FLEP cells into the normal adult rat liver was a 50-fold increase in the number of clusters/cm 2 (Table 1) ▶ . This increase was most accentuated between 4 to 6 months. On analysis of serial sections, most of the new clusters were located in regions adjacent to large mixed clusters and were of either hepatocytic, mixed, or ductal phenotype (Figure 6) ▶ . These secondary clusters were often multiple and occasionally showed bile ductular elements when they came into contact with pre-existing portal ducts, again suggesting bipotency.
Figure 6.
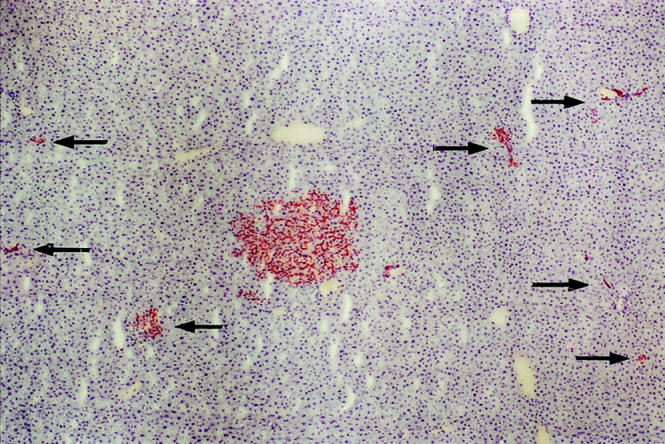
Small satellite clusters adjacent to a large cluster with a mixed phenotype. Small cell clusters with a hepatocytic, mixed, or ductal morphology are seen in the vicinity of a large cluster of mixed phenotype. On analysis of serial sections, these small clusters (denoted by arrows) remained physically separate from the large cluster. Several small clusters very close to the large cluster are not highlighted by arrows, because they were shown to be part of the large cluster on serial sections. Original magnification, ×40.
Reduced Repopulation Potential of ED 16–18 FLEP Cells
To further evaluate the repopulation potential and phenotypic properties of FLEP cells at different times during liver development, studies were conducted in normal animals with FLEP cells isolated from ED 16 and ED 18 fetal rat liver. With ED 16 FLEP cells, ∼2 clusters/cm 2 were observed at 1 or 2 months after cell transplantation and ∼3 to 4 clusters/cm 2 were present at 4 months. The clusters observed were of small to medium size and were predominantly hepatocytic (Figure 7, A and B) ▶ . However, several medium-sized clusters with a mixed phenotype were also identified (Figure 7C) ▶ . The ductular elements in these mixed clusters were smaller and less well developed than those observed with ED 14 FLEP cells. A significant number of pure bile duct clusters was also observed (Figure 7D) ▶ . With ED 18 FLEP cells, sparsely scattered, very small hepatocytic clusters were observed at 1 month after cell transplantation (Figure 7E) ▶ , comparable in size with clusters obtained after transplantation of adult hepatocytes. At 4 months after transplantation of ED 18 FLEP cells, DPPIV+ transplanted cell clusters were still present but were not increased significantly in size or number (Figure 7F) ▶ .
Figure 7.
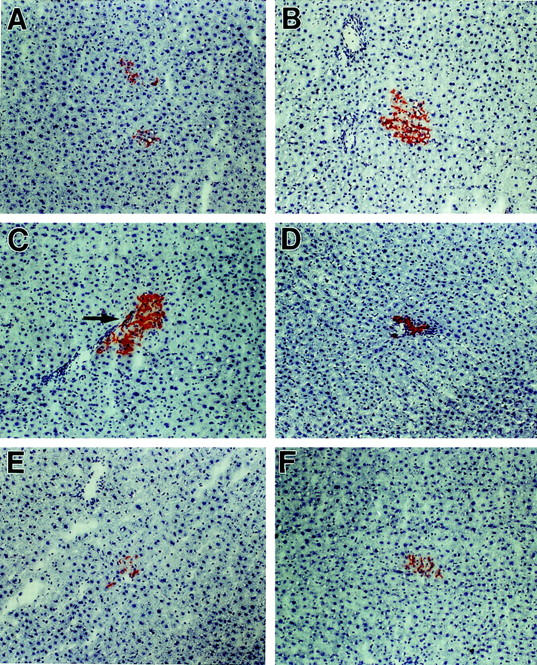
Repopulation of the liver by ED 16–18 FLEP cells. FLEP cells (4 × 105) isolated from wt F344 rats at ED 16 and ED 18 were transplanted to the liver of DPPIV− F344 rats as noted in Materials and Methods. Transplanted animals were sacrificed at 1, 2, and 4 months after cell transplantation and tissue sections were stained histochemically for DPPIV. A and B: DPPIV+ cell clusters at 1 and 4 months, respectively, after transplantation of ED 16 FLEP cells. C: Cluster derived from ED 16 FLEP cells exhibiting a mixed phenotype. D: Cluster derived from ED 16 FLEP cells exhibiting a bile duct phenotype. E and F: 1 and 4 months, respectively, after transplantation of ED 18 FLEP cells, showing small hepatocytic clusters that did not increase significantly in size after the first month. Original magnifications, ×100.
Long-Term Proliferative Capacity of FLEP Cells
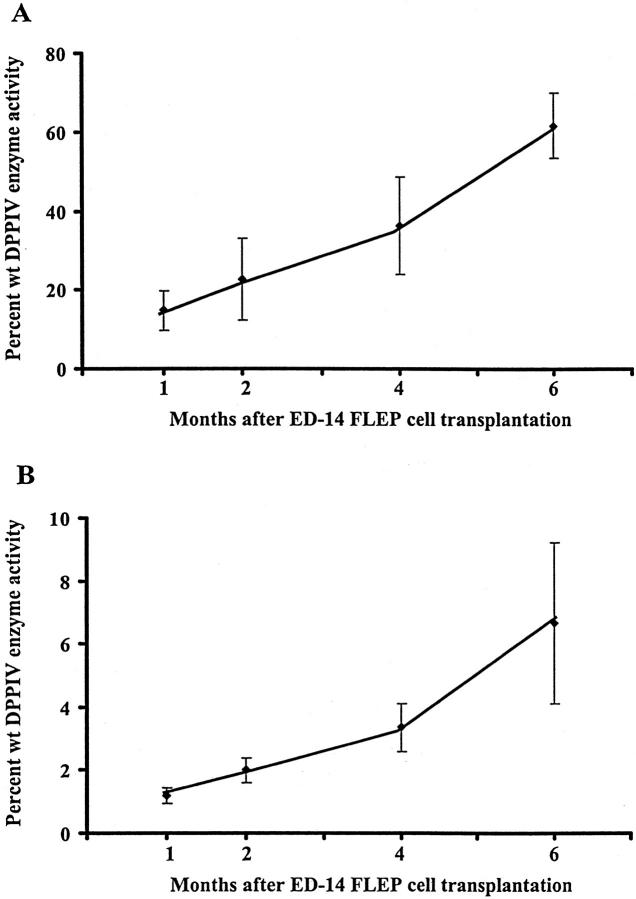
Histochemical analysis of DPPIV expression in DPPIV− rats transplanted with DPPIV+ ED 14 FLEP cells showed a progressive increase in the size and number of DPPIV+ cell clusters in both Rs-treated and normal rats in the 6-month period after cell transplantation. To quantitate liver repopulation in the whole liver, we developed an assay for DPPIV enzyme activity in liver homogenate. As shown in Figure 8 ▶ , DPPIV enzyme activity produced by transplanted ED 14 FLEP cells increased progressively throughout time in both Rs-treated (Figure 8A) ▶ and normal rats (Figure 8B) ▶ . These results were consistent with the percentage of liver repopulation determined by histochemical analysis (see Table 1 ▶ ). By both methods, the greatest increase in liver repopulation in normal rats transplanted with ED 14 FLEP cells occurred between 4 and 6 months, which most likely reflects reseeding of the liver by transplanted ED 14 FLEP cells.
Figure 8.
Liver repopulation by ED 14 FLEP cells as determined by DPPIV enzyme activity. Tissue homogenates were prepared from multiple regions in each liver and DPPIV enzyme activity was determined as noted in Materials and Methods. A: Rs-treated rats; B: Untreated rats, all transplanted with 4 × 10 5 ED 14 FLEP cells. Four to six animals were used for each time point (with one exception in which three animals were used). Each time point represents the mean ± SD for DPPIV enzyme activity as a percentage of DPPIV enzyme activity obtained with tissue homogenates from wt F344 rats.
To directly demonstrate the proliferative activity of ED 14 FLEP cells and their progeny, combined immunohistochemistry for DPPIV to identify transplanted cells (brown color at the canalicular surface of hepatocytes) and in situ hybridization for histone-3 mRNA (autoradiographic grains) was performed to identify actively proliferating cells (S phase of the cell cycle). As shown in Figure 9 ▶ , in both Rs-treated and normal liver at 4 and 6 months after ED 14 FLEP cell transplantation, DPPIV+ cells in S phase were readily identified. These results demonstrate the long-term proliferative capacity of transplanted rat ED 14 FLEP cells and support our other evidence for continuous repopulation of the liver by these cells.
Figure 9.
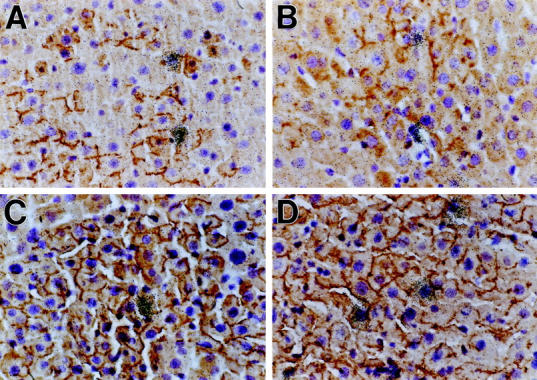
Detection of proliferating DPPIV+ hepatic cells 6 months after transplantation of ED 14 FLEP cells by dual-label in situ hybridization/immunohistochemistry. Normal rats (A and B) or Rs-treated rats (C and D) were transplanted with 4 × 10 5 ED 14 FLEP cells in conjunction with two-thirds PH. Proliferating transplanted cells were detected by in situ hybridization with a 35S-histone 3 mRNA riboprobe and immunohistochemistry for DPPIV, using a monoclonal antibody, as previously reported. 36 A and C are from liver taken 4 months after cell transplantation and B and D are from liver taken 6 months after cell transplantation. Original magnifications, ×400.
Discussion
The main objectives of this study were to characterize the phenotypes of clusters produced by ED 14 fetal liver epithelial cells after their transplantation into the liver and to derive conclusions regarding their stem cell properties; namely, bipotency, enhanced proliferative activity, and long-term repopulation capacity in a normal liver environment, using the DPPIV− mutant F344 rat cell transplantation model system. 24,27 Previously, we identified three subpopulations of epithelial progenitor cells in ED 14 FLEP cells, one that is bipotent (AFP+/Alb+/CK-19+) and two that are unipotent (AFP+/Alb+/CK-19− and AFP−/Alb−/CK-19+). 33 We also determined that bipotent ED 14 FLEP cells proliferated rapidly in Rs-treated rats and that cells engrafted into the bile duct region differentiated into bile duct cells, whereas cells engrafted into the parenchyma exhibited a hepatocytic phenotype. 33
In the present study, there was extensive proliferation and liver repopulation with ED 14 cells in Rs-treated rats (60 to 80% in 6 months), but more importantly, significant repopulation also occurred in normal rats (5 to 10% in 6 months). In both Rs-treated and normal rats, PH was required to obtain significant repopulation by transplanted ED 14 FLEP cells, probably related to increased seeding or engraftment efficiency after PH. Although the seeding efficiency (determined by the number of clusters observed at 1 month) was 5- to 10-fold higher with adult hepatocytes compared to ED 14 FLEP cells, long-term repopulation did not occur with adult hepatocytes in normal rats. From these studies, we conclude that ED 14 FLEP cells have a clear proliferative advantage after transplantation to the liver compared to mature hepatocytes.
Stem Cell Properties of ED 14 FLEP Cells
Stem cells are generally considered to exhibit the following properties: 1) self replication or renewal, 2) differentiation into two or more specific cell phenotypes, and 3) long-term repopulation of the host under appropriate circumstances. 38 Because there are no specific markers for liver stem cells, it was not possible to demonstrate self-renewal of undifferentiated liver stem cells in our model system. However, we have obtained considerable evidence for bipotency of transplanted ED 14 FLEP cells and long-term liver repopulation. 1) Although only 3% of cells in the transplanted fraction were dually marked for AFP and CK-19, >50% of the clusters observed after 6 months contained both hepatocytes and mature bile ducts (termed “mixed” clusters), suggesting that bipotent progenitor cells preferentially engrafted compared to unipotent progenitors. 2) Mixed clusters were the largest, indicating that they have greater proliferative activity than clusters containing only hepatocytes or bile duct cells. 3) Many mixed clusters also contained mature bile ducts in different regions, often separated by large distances. In other instances, newly synthesized bile ducts were located in a single portal region at one edge of a very large cluster. In both cases, it would seem that the original transplanted cells engrafted at or near a junction between the portal space and parenchymal cords (ie, the limiting plate) and then began to proliferate in both directions. Thus, ED 14 FLEP cells have the flexibility to differentiate into both hepatic epithelial cell types and cues from the lobular zone in which they are engrafted seem to direct their differentiation.
Other observations on serial sections further support the bipotency of transplanted ED 14 FLEP cells. First, when some small to moderately sized cell clusters with an apparent hepatocytic phenotype contacted a specific portal region, a few transplanted cells differentiated into mature bile duct cells. Secondly, we observed very well-differentiated bile ducts with a small number of mature hepatocytes emanating from one region of the duct. These findings are consistent with a recent report by Paku and colleagues 39 in the rat 2-AAF/PH model in which proliferating epithelial progenitor cells within the biliary compartment have been shown to migrate across the ductular basement membrane into the surrounding hepatic parenchyma. Therefore, it would seem that bidirectional flow of hepatic epithelial cells occurs across the ductular basement membrane and that these cells may then differentiate into hepatocytes or bile duct cells depending on local factors in the cellular compartment in which they reside.
A third observation that became apparent at 4 and 6 months after cell transplantation was that many large clusters of mixed phenotype contained small secondary clusters in their immediate vicinity of hepatocytic, mixed, or ductal phenotype. This suggested that transplanted cells separated from their original clusters and continued to proliferate and differentiate along both the hepatocytic and ductal lineages. Nonetheless, although the above evidence seems compelling, formal proof that large mixed clusters are derived from bipotent cells will require viral genetic marking studies.
Long-term liver repopulation has been achieved previously with mature hepatocytes in both rats and mice. 22-26 However, in all of these studies, strong selection pressure was needed to favor the proliferation and/or survival of transplanted hepatocytes, and mature bile ducts were not produced, although this could reflect selective injury to host hepatocytes in these models. In addition, serial transplantation and repopulation has been achieved with mature hepatocytes in the fumaryl acetoacetate hydrolase null mouse, indicating that these highly differentiated cells exhibit far more proliferative capacity than previously imagined. 40 Although the level of repopulation with ED 14 FLEP cells was much less in the present study, it occurred in a nonselective environment, under conditions in which mature hepatocytes do not repopulate the liver. We also noted a reduced proliferative capacity and repopulation by ED 16 and ED 18 FLEP cells. The property of forming secondary clusters or reseeding of the liver also appears to be unique to ED 14 FLEP cells. The number of clusters increased 50-fold during the 6-month period after cell transplantation, and from serial section analysis, we estimate that the entire liver contained ∼10,000 to 20,000 clusters. Finally, direct evidence for long-term proliferative activity of repopulating FLEP cells was obtained by demonstrating histone 3 mRNA expression in DPPIV+ cells 6 months after their transplantation into the normal liver.
Factors Driving Liver Repopulation by ED 14 FLEP Cells
The mechanism and factors leading to preferential accumulation and repopulation of the liver by ED 14 FLEP cells are not known. Acute reduction in liver mass stimulates an immediate proliferative response in the remaining liver, first in hepatocytes and then in nonparenchymal cells. 8 During this period, both endogenous hepatocytes and transplanted cells are stimulated to proliferate. However, once the liver mass returns to normal (within 10 to 14 days), there is no apparent stimulus for continued proliferation of transplanted (or endogenous) cells. Consistent with this notion, the size of transplanted cell clusters derived from adult hepatocytes did not increase after 1 month. However, with transplanted ED 14 FLEP cells, proliferation continued progressively during the entire 6-month experimental period. Why ED 14 FLEP cells continued to proliferate in the host liver after the apparent stimulus for liver growth had ceased remains unclear. The mechanism by which PH enhances engraftment of ED 14 FLEP cells is also not known, but this is probably related to the induction of specific hormones, cytokines, and growth factors, as well as other changes in the liver microenvironment and extracellular matrix that occur during liver regeneration. 41-43
In the field of hematopoietic cell transplantation, making space for transplanted cells to repopulate the host has been a long-held concept; however, recent studies have suggested that this may not be necessary under some circumstances. 44,45 Our present studies in the liver suggest that making space for transplanted cells is not necessary for expansion of ED 14 FLEP cells once they have engrafted. Whether transplanted ED 14 FLEP cells release signals that cause a dropout of neighboring hepatocytes and their replacement by newly proliferated transplanted cells is an intriguing question that needs to be explored.
Plasticity of Transplanted Cells
Recent studies have shown that crude bone marrow cells or purified hematopoietic stem cells can differentiate into hepatocytes on engraftment into the liver 46-50 and into brain or muscle phenotypes when transplanted into these respective tissues. 51-53 An interesting question is whether hematopoietic cells in our transplanted cell fraction might contribute to liver repopulation. It is also possible that some transplanted hematopoietic or fetal liver stem/progenitor cells initially remain dormant within the liver and become activated throughout time. However, we consider this to be unlikely, because we did not observe the late appearance of DPPIV+ cells in the liver in the absence of PH or activation of dormant ED 14 FLEP cells if PH was performed weeks to months after cell transplantation.
Other Models for Normal Liver Repopulation
Most previous attempts to repopulate the normal rat liver have transplanted large numbers of adult hepatocytes (up to 1 × 10 8 cells) into the spleen or have used repeated transplantation of cells. 54-56 Other studies have used ED 18 and older fetal liver tissue transplanted to the spleen or on solid support matrices implanted intraperitoneally, 57-60 as well as mature hepatocytes attached to microcarrier beads. 61 In addition, isolated fetal hepatocytes from late gestation when transplanted intraportally into Nagase analbuminemic rats gave partial correction of serum albumin when atrophy of nontransplanted lobes was subsequently induced by portal branch ligation. 62 All of these approaches, however, have limited practical application.
Utility of ED 14 FLEP Cells
The advantages of using early fetal liver stem/progenitor cells for cell transplantation therapy are the small size of the cells (10 to 12 mμ), so that they might disperse more broadly throughout the liver parenchyma, their lesser tendency to obstruct the liver sinusoids and produce portal hypertension, and their ability to repopulate an essentially normal liver. These cells also replace parenchymal hepatocytes and form new bile ducts for an extended period after their transplantation. The present study demonstrates, in vivo, the tissue-determined stem cell potential of early rat FLEP cells and suggests that such cells may be useful for therapeutic liver repopulation, as well as to serve as a vehicle for ex vivo gene therapy.
Acknowledgments
We thank Dr. Konstatin Dobrenis, Department of Neuroscience, Albert Einstein College of Medicine, for assistance with computerized digital photography of DPPIV-stained microscopic slides; and Ms. Anna Caponigro for secretarial assistance.
Footnotes
Address reprint requests to David A. Shafritz, M.D., Liver Research Center, Albert Einstein College of Medicine, 1300 Morris Park Ave., Bronx, NY 10461. E-mail: shafritz@aecom.yu.edu.
Supported in part by National Institutes of Health grants RO1 DK17609, RO1 DK56496, and P30 DK41296 (all to D. A. S.) and the Gail I. Zuckerman for Research in Chronic Liver Diseases of Children (to M. D. D.).
References
- 1.Sell S: Is there a liver stem cell? Cancer Res 1990, 50:3811-3815 [PubMed] [Google Scholar]
- 2.Thorgeirsson SS: Hepatic stem cells. Am J Pathol 1993, 142:1331-1333 [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson JW, Leduc EH: Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol 1958, 76:441-449 [DOI] [PubMed] [Google Scholar]
- 4.DuBois AM: The embryonic liver. Rouiller CH eds. The Liver. 1963, : Academic Press, New York, [Google Scholar]
- 5.Wilson JW, Groat CS, Leduc EH: Histogenesis of the liver. Ann NY Acad Sci 1963, 111:8-22 [DOI] [PubMed] [Google Scholar]
- 6.Le Douarin NM: An experimental analysis of liver development. Med Biol 1975, 53:427-455 [PubMed] [Google Scholar]
- 7.Houssaint E: Differentiation of mouse hepatic primordium: an analysis of tissue interactions in hepatocytes differentiation. Cell Growth Differ 1980, 9:269-279 [DOI] [PubMed] [Google Scholar]
- 8.Grisham JW, Thorgeirsson SS: Liver stem cells. Potten CS eds. Stem Cells, ch 8. 1997, :pp 233-282 Academic Press, New York [Google Scholar]
- 9.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS: A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 1987, 8:1737-1740 [DOI] [PubMed] [Google Scholar]
- 10.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS: In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res 1989, 49:1541-1547 [PubMed] [Google Scholar]
- 11.Lemire JM, Shiojiri N, Fausto N: Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol 1991, 139:535-552 [PMC free article] [PubMed] [Google Scholar]
- 12.Dabeva MD, Shafritz DA: Activation, proliferation and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol 1993, 143:1606-1620 [PMC free article] [PubMed] [Google Scholar]
- 13.Grisham JW: Cell types in long-term propagable cultures of rat liver. Ann NY Acad Sci 1980, 349:128-137 [DOI] [PubMed] [Google Scholar]
- 14.Coleman WB, Smith GJ, Grisham JW: J Cell Physiol 1994, 161:464–469 [DOI] [PubMed]
- 15.Nagy P, Evarts RP, McMahon JB, Thorgeirsson SS: Role of TGF-β in normal differentiation and oncogenesis in rat liver. Mol Carcinogenesis 1989, 2:345-354 [DOI] [PubMed] [Google Scholar]
- 16.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW: Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stem-like) cells by the hepatic microenvironment. Am J Pathol 1993, 142:1373-1382 [PMC free article] [PubMed] [Google Scholar]
- 17.Grisham JW, Coleman WB, Smith GJ: Isolation, culture, and transplantation of rat hepatocytic precursor (stem-like) cells. Proc Soc Exp Biol Med 1993, 204:270-279 [DOI] [PubMed] [Google Scholar]
- 18.Kubota H, Reid LM: Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineage are lacking classical major histocompatibility complex class I antigen. Proc Natl Acad Sci USA 2000, 97:12132-12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki A, Zheng YW, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H: Flow cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 2000, 32:1230-1239 [DOI] [PubMed] [Google Scholar]
- 20.Crosby HA, Kelly DA, Strain AJ: Human hepatic stem-like cells isolated using c-kit or CD34 can differentiate into biliary epithelium. Gastroenterology 2001, 120:534-544 [DOI] [PubMed] [Google Scholar]
- 21.Sangren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degan JL: Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991, 66:245-256 [DOI] [PubMed] [Google Scholar]
- 22.Rhim J, Sangren EP, Degen JL, Palmiter RD, Brinster RL: Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263:1149-1152 [DOI] [PubMed] [Google Scholar]
- 23.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M: Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet 1996, 12:266-273 [DOI] [PubMed] [Google Scholar]
- 24.Laconi E, Oren R, Mukhopadhyay D, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA: Long term, near total liver replacement by transplantation of isolated hepatocytes. Am J Pathol 1998, 153:319-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, Sokhi R, Roy Chowdhury N, Tanaka KE, Vikram B, Roy Chowdhury J: Amelioration of radiation-induced liver damage in partially hepatectomied rats by hepatocytes transplantation. Cancer Res 1999, 59:5871-5874 [PubMed] [Google Scholar]
- 26.Oren R, Dabeva MD, Karnezis AN, Petkov PM, Rosencrantz R, Sandhu JP, Moss SF, Wang S, Hurston E, Laconi E, Holt PR, Thung SN, Zhu L, Shafritz DA: Role of thyroid hormone in stimulating liver repopulation by transplanted hepatocytes. Hepatology 1999, 30:903-913 [DOI] [PubMed] [Google Scholar]
- 27.Thompson NL, Hixson DC, Callanan H, Panzica M, Flanagan D, Faris RA, Hong WJ, Hartel-Schenk S, Doyle D: A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein: use as a liver-cell transplantation model. Biochem J 1991, 273:497-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gossrau R: Peptidasen II. Zur localization der dipeptidylpeptidase IV (DPPIV). Histochemistry 1979, 60:231-248 [DOI] [PubMed] [Google Scholar]
- 29.Hubbard AL, Bartles JR, Braiterman LT: Identification of rat hepatocytes plasma membrane proteins using monoclonal antibodies. J Cell Biol 1985, 100:1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walborg EF, Jr, Tsuchida S, Weeden DS, Thomas MW, Barrick M, McEntire KD, Allison JP, Hixson DC: Identification of dipeptidylpeptidase IV as a protein shared by the plasma membrane of hepatocytes and liver biomatrix. Exp Cell Res 1985, 158:509-518 [DOI] [PubMed] [Google Scholar]
- 31.Dabeva MD, Hwang SG, Vasa SRG, Hurston E, Novikoff PM, Hixson DC, Gupta S, Shafritz DA: Differentiation of pancreatic epithelial progenitor cells into hepatocytes following transplantation into rat liver. Proc Natl Acad Sci USA 1997, 94:7356-7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greengard O, Federman M, Knox WE: Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol 1972, 52:261-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA: Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol 2000, 156:2017-2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigal SH, Rajvanshi P, Reid LM, Gupta S: Demonstration of differentiation in hepatocyte progenitor cells using dipeptidyl peptidase IV deficient mutant rats. Cell Mol Biol Res 1995, 41:39-47 [PubMed] [Google Scholar]
- 35.Nagatsu T, Hino M, Fuyamada H, Hayakawa T, Sakakibara S, Nakagawa Y, Takemoto T: New chromogenic substrates for X-prolyl dipeptidyl-aminopeptidase. Anal Biochem 1976, 74:466-476 [DOI] [PubMed] [Google Scholar]
- 36.Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA: Liver regeneration and α-fetoprotein mRNA expression in the retrorsine model for hepatocytes transplantation. Cancer Res 1998, 58:5825-5834 [PubMed] [Google Scholar]
- 37.Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ: Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest 1992, 66:459-466 [PubMed] [Google Scholar]
- 38.Potten CS, Loeffler M: Stem cells: attributes, cycles, spirals, pitfalls and uncertainties: lessons for and from the crypt. Development 1990, 110:1101-1120 [DOI] [PubMed] [Google Scholar]
- 39.Paku S, Schnur J, Nagy P, Thorgeirsson SS: Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol 2001, 158:1313-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overturf K, Al-Dhalimy M, Ou CN, Finegold M, Grompe M: Serial transplantation reveals the stem-cell like regenerative potential of adult mouse hepatocytes. Am J Pathol 1997, 151:1273-1280 [PMC free article] [PubMed] [Google Scholar]
- 41.Michalopoulos GK, DeFrances MC: Liver regeneration. Science 1997, 276:60-66 [DOI] [PubMed] [Google Scholar]
- 42.Taub R, Greenbaum LE, Peng Y: Transcriptional regulatory signals define cytokine-dependent and independent pathways in liver regeneration. Semin Liver Dis 1999, 19:117-127 [DOI] [PubMed] [Google Scholar]
- 43.Fausto N: Liver regeneration. J Hepatol 2000, 32(Suppl 1):S19-S31 [DOI] [PubMed] [Google Scholar]
- 44.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R: Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic disorders. Blood 1998, 91:756-763 [PubMed] [Google Scholar]
- 45.Cradock C: Hematopoietic stem-cell transplantation: recent progress and future promise. Lancet Oncol 2000, 1:227-234 [DOI] [PubMed] [Google Scholar]
- 46.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP: Bone marrow as a potential source of hepatic oval cells. Science 1999, 284:1168-1170 [DOI] [PubMed] [Google Scholar]
- 47.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS: Derivation of hepatocytes from bone marrow cells in mice after radiation induced myeloablation. Hepatology 2000, 31:235-240 [DOI] [PubMed] [Google Scholar]
- 48.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS: Liver from bone marrow in humans. Hepatology 2000, 32:11-16 [DOI] [PubMed] [Google Scholar]
- 49.Alison MR, Poulson R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA: Hepatocytes from non-hepatic adult stem cells. Nature 2000, 406:257. [DOI] [PubMed] [Google Scholar]
- 50.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman JL, Grompe M: Purified hematopoietic stem cells can differentiate to hepatocytes in vivo. Nat Medicine 2000, 6:1229-1234 [DOI] [PubMed] [Google Scholar]
- 51.Kopen GC, Prockop DJ, Phinney DG: Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999, 96:10711-10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrari G, Cusella-DeAngelis G, Coletta M, Paolucci E, Stomaiuolo A, Cossu G, Mavilio F: Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279:1528-1530 [DOI] [PubMed] [Google Scholar]
- 53.Fuchs E, Segre JA: Stem cells: a new lease on life. Cell 2000, 100:143-155 [DOI] [PubMed] [Google Scholar]
- 54.Rajvanshi PA, Kerr A, Bhargava KK, Burk RD, Gupta S: Studies on liver repopulation using the dipeptidyl peptidase IV deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocytes mass in the liver lobule. Hepatology 1996, 23:482-496 [DOI] [PubMed] [Google Scholar]
- 55.Rozga J, Holzman M, Moscioni AD, Fujioka H, Morsiani E, Demetriou AA: Repeated intraportal hepatocytes transplantation in analbuminemic rats. Cell Transplant 1995, 4:237-243 [DOI] [PubMed] [Google Scholar]
- 56.Rajvanshi P, Kerr A, Bhargava KK, Burk RD, Gupta S: Efficacy and safety of repeated hepatocytes transplantation for significant liver repopulation in rodents. Gastroenterology 1996, 111:1092-1102 [DOI] [PubMed] [Google Scholar]
- 57.Kuasano M, Sawa M, Jiang B, Kino S, Itoh K, Sakata H, Katoh K, Mito M: Proliferation and differentiation of fetal liver cells transplanted into rat spleen. Transplant Proc 1992, 24:2960-2961 [PubMed] [Google Scholar]
- 58.Borel-Rinkes IHM, Bijma AM, Kappers WA, Sinaasappel M, Hoek FJ, Jansen PLM, Valerio D, Terpstra OT: Evidence of metabolic activity of adult and fetal rat hepatocytes transplanted into solid supports. Transplantation 1992, 54:210-214 [DOI] [PubMed] [Google Scholar]
- 59.Kokudo N, Otsu I, Okazaki T, Takahashi S, Sanjo K, Adachi Y, Makino S, Nozawa M: Long-term effect of intrasplenically transplanted adult hepatocytes and fetal liver in hyperbilirubinemic Gunn rats. Transplant Int 1995, 8:262-267 [DOI] [PubMed] [Google Scholar]
- 60.Kato K, Kato J, Hodgson WJ, Abraham NG, Onodera K, Imai M, Kasai S, Mito M: Enzymatic activity and expression of cytochrome P450 LA omega within intrasplenically transplanted fetal hepatocytes in spontaneously hypertensive rats. Cell Transplant 1997, 6:531-534 [DOI] [PubMed] [Google Scholar]
- 61.Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR: Replacement of liver function in rats by transplantation of microcarrier attached hepatocytes. Science 1986, 233:1190-1192 [DOI] [PubMed] [Google Scholar]
- 62.Lilja H, Arkadopoulos N, Blanc P, Susumu E, Middleton Y, Meurlling S, Demetriou AA, Rozga J: Fetal rat hepatocytes. Isolation, characterization and transplantation in the Nagase analbuminemic rats. Transplantation 1997, 64:1240-1248 [DOI] [PubMed] [Google Scholar]