Abstract
Oncostatin M (OSM), a member of the IL-6 family has been postulated to be a potent recruiter of leukocytes, however information regarding the molecular mechanism(s) underlying this event is extremely limited. Therefore, the aim of this study was to investigate the role of OSM-mediated leukocyte recruitment in a human system in vitro under flow conditions. A parallel-plate flow chamber assay was used to examine leukocyte recruitment from whole blood by human umbilical vein endothelium treated for 24 hours with OSM. OSM in a dose-response manner revealed very significant leukocyte rolling and adhesion reaching optimal levels at a very low concentration of OSM (10 ng/ml). The OSM-induced leukocyte rolling and adhesion was comparable to levels seen with tumor necrosis factor. OSM was extremely selective for neutrophil recruitment (96%) with <3% lymphocyte recruitment. By contrast, tumor necrosis factor-α revealed no such selectivity, recruiting 70% neutrophils and at least 25% lymphocytes and detectable levels of eosinophils at 24 hours. The molecular mechanism underlying the leukocyte recruitment seemed to be entirely dependent on P-selectin as leukocyte recruitment could be completely blocked by the addition of a P-selectin-blocking antibody. An elevation in both P-selectin message and protein was observed with 24 hours of OSM stimulation of endothelium. By contrast, E-selectin and VCAM-1 were not detectable after OSM stimulation. Similar results were seen with passaged dermal microvascular endothelium that does not have a prestored pool of P-selectin. Based on these results, we conclude that OSM may be a very selective potent recruiter of neutrophils in more prolonged inflammatory conditions, an event exclusively dependent on P-selectin.
Leukocytes are recruited to sites of inflammation through a well-defined series of interactions with activated endothelium. Cytokines induce endothelium to express various adhesion molecules that mediate this cascade of events. 1-3 Initially, selectins on endothelium mediate transient interactions with their leukocyte ligands, resulting in leukocyte tethering and rolling along the vessel wall. Activation of leukocytes by chemoattractants leads to functional up-regulation of integrins, resulting in firm adhesion to their immunoglobulin superfamily ligands on endothelium and permitting extravasion into the surrounding tissue. 1-3
Very early neutrophil recruitment to inflammatory sites is thought to be almost entirely dependent on P-selectin, an adhesion molecule prestored in Weibel-Palade bodies of endothelial cells. This molecule is rapidly translocated to the endothelial surface in response to a variety of inflammatory mediators including histamine, oxidants, and thrombin. However, as quickly as it is expressed, it is also reinternalized 4-6 and other molecules including E-selectin and VCAM-1 are synthesized to begin to recruit monocytes, lymphocytes, and eosinophils. To date, there is limited evidence in human systems that the role of P-selectin extends past this time frame, even though neutrophil recruitment can often persist for days or even weeks. Although, interleukin (IL)-4, 7 IL-3, 8 and oncostatin M (OSM) 7 have been shown to stimulate synthesis of P-selectin, IL-3 and IL-4 are not thought to be involved in neutrophil recruitment. The OSM recruitment profile remains unknown.
OSM is a member of the IL-6 family and, like all members of its family, generates a signal through a heterodimer receptor containing gp130. 9 Human endothelial cells express two receptors for OSM: a high-affinity receptor that has an α-chain specific for OSM, 10 and a low-affinity receptor shared with leukemia inhibitory factor. 11 OSM is expressed by activated T cells and monocytes late in the immune response 12-14 but neutrophils are themselves a source of OSM 15 and release the cytokine from preformed stores within 1 hour of treatment with lipopolysaccharide, tumor necrosis factor (TNF)-α, or granulocyte/monocyte colony-stimulating factor. The fact that neutrophils are usually the most abundant cell type in early inflammation, together with the knowledge that neutrophils are a source of OSM, raises the very interesting possibility that the early infiltration of activated, OSM-releasing neutrophils could be an important mechanism for subsequent mononuclear cell recruitment. Alternatively, if OSM is a recruiter of neutrophils rather than mononuclear cells, neutrophils could release OSM in a vicious cycle of neutrophils recruiting neutrophils. Although no one to date has demonstrated that OSM can stimulate neutrophil recruitment per se, Modur and colleagues 16 demonstrated that OSM could induce isolated neutrophils to adhere to human umbilical vein endothelial cells (HUVECs) after 4 hours of treatment with this cytokine.
Using a novel whole-blood flow chamber approach, we report that treatment of human endothelium (microvascular and HUVEC), throughout a prolonged period of time (24 hours) with low, physiologically relevant levels of OSM results in a unique and very selective neutrophil recruitment. The molecular mechanism seems to be dependent on de novo synthesis of P-selectin without any effect on other adhesive mechanisms including E-selectin or VCAM-1.
Materials and Methods
Antibodies and Cytokines
The blocking anti-P-selectin antibody (G1), the nonblocking anti-P-selectin antibody (S12), and anti-E-selectin antibody (7A9) were generously provided by Dr. R. P. McEver (University of Oklahoma). GA6, another blocking anti-P-selectin antibody was purchased from Becton Dickinson (Mississauga, ON, Canada). Because both P-selectin antibodies were effective inhibiting antibodies, they were used based on availability. The anti-VCAM-1 antibody (4B9) was a gift from Dr. Roy Lobb (Biogen, MA). The anti-CD18 antibody (IB4) was generously provided by Dr. Paul Naccache (Laval University, Quebec City, Quebec, Canada). Human OSM was purchased from R&D Systems Inc. (Minneapolis, MN) and human TNF-α was purchased from Collaborative Biochemical Products (Chicago, IL). Human thrombin was purchased from Sigma Chemical Co. (St. Louis, MO).
HUVEC Isolation
HUVECs were harvested from freshly obtained umbilical cords as previously described. 17-19 Briefly, umbilical cord veins were rinsed of formed blood products with warm phosphate-buffered saline (PBS), after which the vein was filled with collagenase (320 U/ml in PBS; Worthington Biochemical Corporation, Lakewood, NJ). After a 20-minute incubation period in warm PBS, the cords were gently massaged to ensure detachment of endothelial cells from the vessel wall. The digest was collected into centrifuge tubes containing heat-inactivated fetal bovine serum to inactivate the collagenase, and centrifuged (400 × g for 8 minutes). The pellet was resuspended in Medium 199 (Life Technologies, Inc., Grand Island, NY) supplemented with 2.4 μg/ml of thymidine (Sigma Chemical Co., Oakville, ON, Canada), 10 U/ml heparin, 20% fetal bovine serum, and an antibiotic cocktail. Cells were cultured until confluent (5 days). The cells were then seeded heavily onto glass coverslips or 6-well polystyrene tissue culture plates (Corning Costar, Cambridge, MA) coated with fibronectin and allowed to settle and become confluent overnight. Treatments with OSM or TNF-α were started 24 hours before experiments.
Human Dermal Microvascular Endothelial Cell (HDMEC) Isolation
HDMECs were isolated as previously described. 20 Briefly, foreskins were collected and processed within 2 hours of collection. After subcutaneous tissues were removed, skins were dissected into 2- to 3-mm 2 segments. Segments from two skins were pooled and incubated for 16 hours at 4°C in Medium 199 containing 200 U/ml of penicillin, 200 μg/ml of streptomycin, and 0.5 mg/ml of collagenase type 1A (Boehringer Mannheim Biochemicals, Indianapolis, IN). Digested segments were then washed three times in Hanks’ balanced salt solution (HBSS) and gently compressed with a spatula to release microvessels. To remove large debris, vessel preparations were passed through a 100-μm nylon mesh (Becton Dickinson, Mountain View, CA). The cells were centrifuged and pellets resuspended in endothelial basal medium-2 (Clonetics, San Diego, CA) supplemented with human fibroblast factor-8, human recombinant epidermal growth factor, recombinant human vascular endothelial growth factor, hydrocortisone, long R insulin-like growth factor-1, gentamicin sulfate, amphotericin-B, ascorbic acid, and 5% fetal bovine serum. Cells were seeded onto gelatin-treated culture dishes. Only cell preparations that were >95% positive for von Willebrand factor and PECAM-1 expression by immunohistochemistry were used in flow experiments. HDMEC was used from passage 1 to 7 during which adhesion molecule expression at rest and stimulation was shown to be stable.
Flow Chamber Assay
A flow chamber assay was used as previously described 17 to study leukocyte recruitment from whole blood by cytokine-treated HUVECs (OSM, 1 to 10 ng/ml, or TNF-α, 25 ng/ml, for 24 hours; thrombin, 0.5 U/ml for 10 minutes). Glass coverslips with confluent monolayers of HUVECs or HDMECs were mounted into a polycarbonate chamber with parallel plate geometry. The flow chamber was placed onto an inverted microscope stage, which was enclosed in a warm air cabinet, and the temperature maintained at 37°C. Whole blood was collected from healthy donors, and a syringe pump (Harvard Apparatus, Saint Laurent, Quebec, Canada) was used to draw the whole blood or diluted whole blood over monolayers at a shear force of 10 dynes/cm 2 for whole blood or 2 dynes/cm 2 for diluted blood. Leukocyte interactions were visualized and recorded (×10 objective, ×10 eyepiece) using phase contrast microscopy. Two anti-P-selectin antibodies were used. The P-selectin blocking antibody G1 (2 μg/ml) or a second P-selectin antibody GA6 (10 μg/ml) were added before perfusion across endothelial coverslips when indicated. Incubation of endothelium with P-selectin antibody for 30 minutes at 37°C before perfusion worked as effectively as adding antibodies directly to blood suggesting that endothelial P-selectin was inhibited in these experiments. The monoclonal antibody IB4 was used to block CD18-mediated adhesion. Whole blood was incubated with 20 μg/ml of the antibody for 10 minutes before perfusion across endothelium. After flow chamber experiments, coverslips were recovered and stained with Geimsa-Wright stains (Hemacolor Stain Set, EM Science, Gibbstown, NJ). Microscopy was used to differentiate cell types based on their morphology.
Under-Agarose Gel Migration Assay
The under-agarose gel assay was preformed as previously described. 21 Briefly, 3 ml of an agarose/RPMI solution (50:50 HBSS/RPMI-1640 (both Life Technologies, Inc., Burlington, ON, Canada), 10% heat-inactivated fetal bovine serum, and 1.2% agarose (Life Technologies, Inc.) was poured into 35-mm-diameter Falcon tissue culture dishes (Becton Dickinson, Franklin Lakes, NJ). After the gel had hardened, 3.5-mm holes (guided by a template) were cut into each gel, with a single central well surrounded by 4 wells 2.2 mm from the central well. Ten μl of either 0.1 μmol/L fMetLeuPhe (fMLP) or various concentrations of OSM ranging from 0.1 to 100 ng/ml was loaded into the center well and 10 μl of a neutrophil suspension (1 × 107) was loaded into each surrounding well. Gels were incubated at 37°C and 5% CO2 for 2 hours before leukocyte migration was measured by microscopy.
Neutrophils were prepared for this assay from blood collected from healthy volunteers. A standard 6% Dextran sedimentation followed by lysis with ddH2O (and hypertonic rescue with 1/3 volumes of 0.6 mol/L KCl) was used to clear erythrocytes. Granulocytes were isolated using a Ficoll density gradient centrifugation (Histopaque-1077; Sigma Chemical Co., Oakville, ON, Canada) and resuspended in PBS for loading into gels.
RT-PCR for P-Selectin Gene Expression
RNA from control or cytokine-treated HUVECs was extracted with TRIzol (Life Technologies, Inc.) according to product instructions. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using one-step RT-PCR method with OneStep RT-PCR kit (Qiagen, Valencia, CA). The primer pairs were as follows: P-selectin (forward) TGAAGAAAAAGCACGCATTG and (reverse) AGCGGCTCACACGAAATAG (714 bp); and β-actin (forward) CATGGATGATGATATCGCCG and (reverse) ACAGCCTGGATAGCAACGTA (417 bp). The RT-PCR condition was optimized so that both P-selectin and β-actin were expanding linearly, as follows: 10 ng total RNA, 0.4 μmol/L of each P-selectin primers, 0.2 μmol/L of each β-actin primers, and 30 cycles PCR. PCR products were electrophoresed through a 2% agarose gel containing 0.5 μg/ml of ethidium bromide. Bands were visualized and analyzed using a Fluor-S MAX MultiImager and Quantity One software (Bio-Rad Laboratories, Richmond, CA). To semiquantify results, volume-controlled arbitrary densitometry units for P-selectin were compared to the internal control β-actin and are expressed as a ratio.
Western Blot for P-Selectin Protein Expression
Cells were lysed with RIPA buffer (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40, 1 mmol/L phenyl methyl sulfonyl fluoride, 0.05% Aprotinin) on ice for 10 minutes. Insoluble protein was discarded by high-speed centrifugation for 20 minutes at 4°C. Total protein concentration in the supernatant was measured using a protein assay (Pierce Chemical Company, Rockford, IL). Equal amounts of total protein were resuspended in Laemmli buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein was transferred onto polyvinylidene difluoride membrane (Millipore, Etobicoke, ON) and immunoblotted with a monoclonal anti-P-selectin monoclonal antibody (S12). To visualize the protein bands, chemiluminescent substrate (Pierce) was used.
Enzyme-Linked Immunosorbent Assay for Cell Surface Adhesion Molecule Expression
Briefly, HUVECs were seeded at confluence into fibronectin-coated 48-well tissue culture plates (Costar, Cambridge, MA). Endothelium was treated for 24 hours with cytokine before being assayed for adhesion molecule expression. Monolayers were fixed with a 1% formalin solution and blocked with 1% bovine serum albumin in PBS. The endothelial cells were then labeled with 10 μg/ml of 7A9 (an anti-E-selectin antibody) or 2 μg/ml of 4B9 (an anti-VCAM-1 antibody). Cells were then washed and labeled with a peroxidase-labeled goat anti-mouse IgG2o antibody (1 μg/ml; DAKO, Carpinteria, CA), washed a final time, and color developed with a 3,3′ 5,5′ tetramethyl-benzidine base (TMB) one-step substrate system (DAKO). The color reaction was stopped with 0.18 mol/L of H2SO4, and color was read on a plate reader at 450 nm.
Whole Cell Enzyme-Linked Immunosorbent Assay for Total P-Selectin Expression by HDMECs
Briefly, HDMECs were brought to confluence in 48-well tissue culture plates (Costar, Cambridge, MA). After 48 hours of treatment with OSM, cells were lysed in 300 μl of lysis buffer (0.1 mol/L sodium phosphate, pH 8.0, 1% Triton X-100, 1.5 μg/ml aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml pepstatin A, 21 μmol/L leupeptin). Lysates were centrifuged 10,000 × g for 10 minutes at 4°C and supernatants loaded into 96-well plates coated with S12. After 90 minutes of incubation, S12-bound P-selectin was detected with a polyclonal rabbit anti-human P-selectin 1o antibody (Pharmingen, La Jolla, CA) and an HRP-conjugated goat anti-rabbit 2o antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). TMB substrate was added (TMB one-step substrate system, DAKO, Carpinteria, CA) for color development. The color reaction was stopped with 0.18 mol/L of H2SO4, and color was read on a plate reader at 450 nm.
Statistics
Data in graphs is shown as mean ± SEM unless indicated otherwise. A Student’s t-test with Bonferroni correction was used for multiple comparisons. Statistical significance was set at P < 0.05.
Results
OSM Mediates the Specific Recruitment of Neutrophils from Whole Blood Perfused over HUVECs
We used the parallel-plate flow chamber assay to investigate leukocyte recruitment from whole blood by cytokine-treated HUVECs. Whole blood was perfused across HUVECs at a shear force of 10 dynes/cm 2 for 5 minutes. Whole blood perfusion was followed by perfusion with HBSS buffer, permitting observations of leukocyte-endothelial interactions. Figure 1 ▶ shows leukocyte rolling (Figure 1A) ▶ and adhesion (Figure 1B) ▶ on either control, 24-hour OSM-treated, or 24-hour TNF-α-treated HUVECs at physiological shear conditions. Both we (data not shown) and others 22 have shown that 24-hour stimulation with OSM is required for optimal up-regulation of P-selectin transcription in HUVECs. For this reason, and because our interest was in the role of OSM in an established inflammatory response, the 24-hour time point was chosen for all experiments. We did not observe P-selectin expression after 10 hours of treatment with OSM. Leukocyte adhesion to OSM-treated HUVECs was dose-dependent with significantly greater adhesion at 10 ng/ml of OSM than 1 ng/ml of OSM (Figure 1B) ▶ . Rolling was less dependent on dose because no significant difference in rolling was observed between the two concentrations of OSM (Figure 1A) ▶ . Leukocyte recruitment by OSM was comparable to TNF-α-mediated recruitment at 25 ng/ml, a dose that we have previously found to be optimal (our unpublished data).
Figure 1.
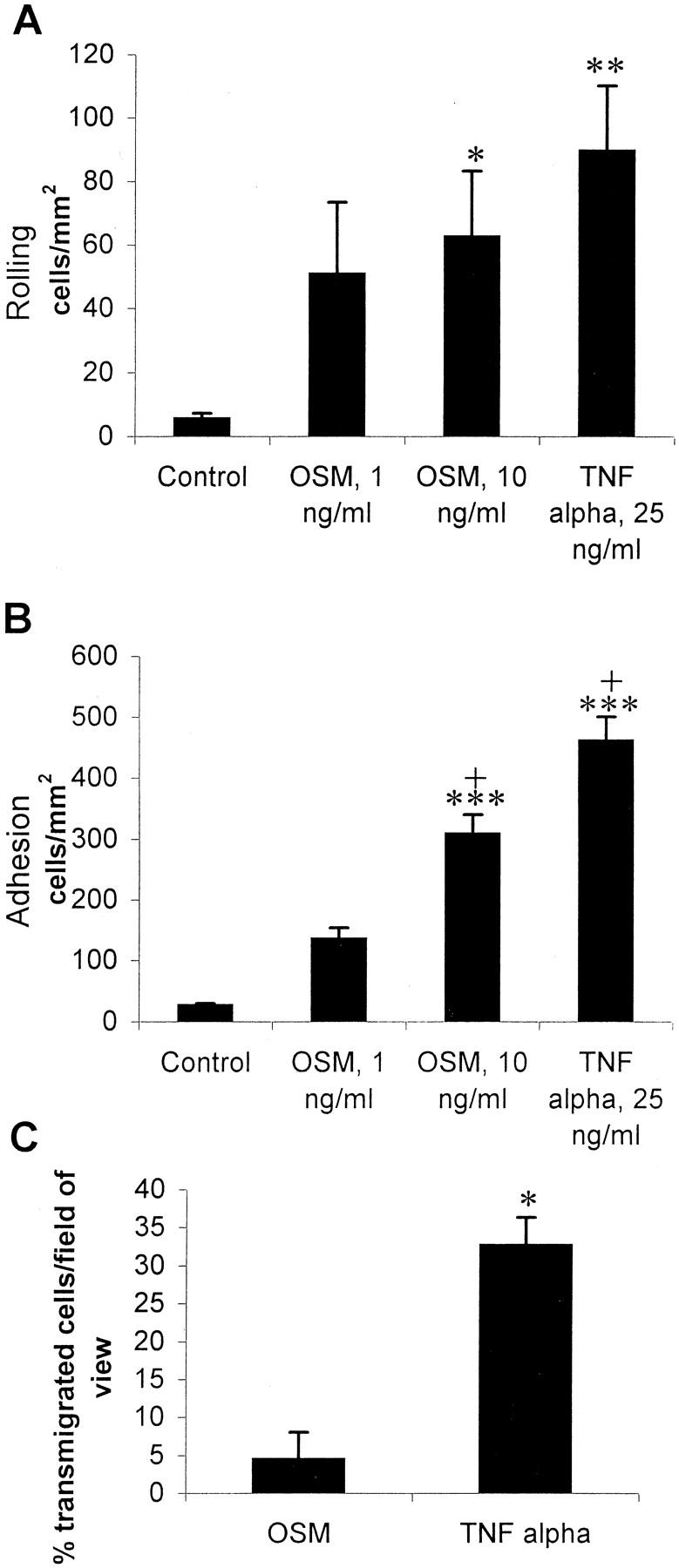
Leukocyte rolling (A) and adhesion (B) on control endothelium or endothelium treated with either 1 ng/ml or 10 ng/ml of OSM, or 25 ng/ml of TNF-α. Whole blood was perfused across cytokine-treated HUVECs at a shear force of 10 dynes/cm2, cleared with HBSS, and interacting leukocytes were counted. Rolling cells were defined as those interacting with the endothelium and traveling at a rate less than free-flowing cells. Adherent cells were defined as those remaining stationary on the endothelium for at least 10 seconds. Results are shown as mean ± SEM. *, P < 0.05 versus control; **, P < 0.01 versus control; ***, P < 0.001 versus control; +, P < 0.01 versus OSM 1 ng/ml. n = 8 for control and OSM 10 ng/ml, n = 3 for OSM 1 ng/ml, and n = 5 for TNF-α 25 ng/ml. C: Leukocyte transmigration across HUVECs treated with 10 ng/ml of OSM or 25 ng/ml of TNF-α. Data (mean ± SEM) is shown as percent transmigration of total leukocytes in a field of view. *, P = 0.0015 as determined by a two-tailed t-test. n = 4.
Interestingly, OSM was a very poor inducer of leukocyte transmigration across HUVEC monolayers (<5%). By comparison, TNF-α induced >35% of cells to emigrate (Figure 1C) ▶ . To ensure that OSM was not chemotactic for neutrophils and therefore keeping cells from migrating below the monolayer, we used an under-agarose chemotaxis assay. Isolated neutrophils were loaded into wells punched into an agarose gel and migration toward a central well containing either fMLP or OSM was measured. OSM did not induce significant neutrophil migration at any concentration tested (Figure 2) ▶ . Subtle levels of chemokinesis of neutrophils in gels containing OSM was noted, particularly at 1 ng/ml. However, the cells moved equally in all directions giving the impression of some migration in Figure 2 ▶ . In contrast, neutrophil migration toward fMLP was directional.
Figure 2.
Neutrophil migration to fMLP and OSM in the under gel migration assay. Migration of the neutrophil front from four wells toward a central well containing chemoattractant was measured by microscopy and averaged. Data are shown as mean ± SEM of three separate experiments. *, P > 0.001.
Figure 3 ▶ shows the absolute numbers of leukocyte subsets recruited after treatment of HUVECs with OSM or TNF-α. OSM (10 ng/ml) recruited almost exclusively neutrophils from whole blood to HUVECs. Absolute numbers of neutrophils recruited by OSM were comparable to neutrophil recruitment by TNF-α (Figure 3A) ▶ . Unlike TNF-α, OSM did not recruit any lymphocytes compared to control values (Figure 3B) ▶ . Monocyte recruitment was not observed for either cytokine at the 24-hour time point (data not shown). To date, no other cytokine has recruited neutrophils with this level of selectivity. Indeed, the percentage of leukocyte populations recruited by 25 ng/ml of TNF-α reflected leukocyte percentages in blood; neutrophils (65 to 70%), lymphocytes (20 to 25%), and eosinophils (∼5%) (Figure 3D) ▶ . In contrast, OSM recruitment was 96% neutrophils.
Figure 3.
Leukocyte recruitment by subset from whole blood by HUVECs treated with 10 ng/ml of OSM or 25 ng/ml of TNF-α. A: Neutrophils. B: Lymphocytes. C: Eosinophils. Coverslips from flow chamber experiments were saved for Geimsa-Wright staining and identification of leukocytes recruited onto cytokine-treated HUVECs. To get the absolute number of interacting cells for each subset the fraction of each subset was multiplied by the total number of interactions observed in flow chamber experiments. Results are shown as mean ± SEM. *, P < 0.001 versus control. n = 8 for control and OSM, n = 4 for TNF-α. D: Leukocyte subset recruitment by percent. *, P < 0.0051 versus OSM; **, P < 0.0028 versus OSM as determined by a two-tailed t-test. n = 8 for OSMS, n = 4 for TNF-α.
Others have reported anti-inflammatory effects of OSM. 12,23 However, in our system when we co-incubated HUVECs with OSM (1 or 10 ng/ml) and TNF-α (2.5 or 25 ng/ml) in various combinations, neither synergistic nor inhibitory alterations in the pattern of leukocyte recruitment could be discerned (data not shown).
OSM-Mediated Leukocyte Recruitment onto Human Endothelium Is Because of Exclusive P-Selectin Up-Regulation
To determine the mechanism of leukocyte recruitment we investigated the expression of adhesion molecules by OSM-treated endothelium. First, we used RT-PCR to examine whether P-selectin expression was up-regulated at the level of mRNA. This revealed an increase in P-selectin mRNA over control in response to 24 hours of OSM (Figure 4A) ▶ . A corresponding increase in P-selectin protein was detected by Western blot (Figure 4B) ▶ . In agreement with previous reports, 7,8,22 TNF-α had no effect on P-selectin expression (data not shown). For completeness, we also examined levels of E-selectin and VCAM-1 protein on the surface of endothelium (Figure 5) ▶ . The results clearly demonstrate that no E-selectin (Figure 5A) ▶ or VCAM-1 (Figure 5B) ▶ was detectable on OSM-treated endothelium. TNF-α was used as a positive control and revealed very significant E-selectin and VCAM-1 expression.
Figure 4.
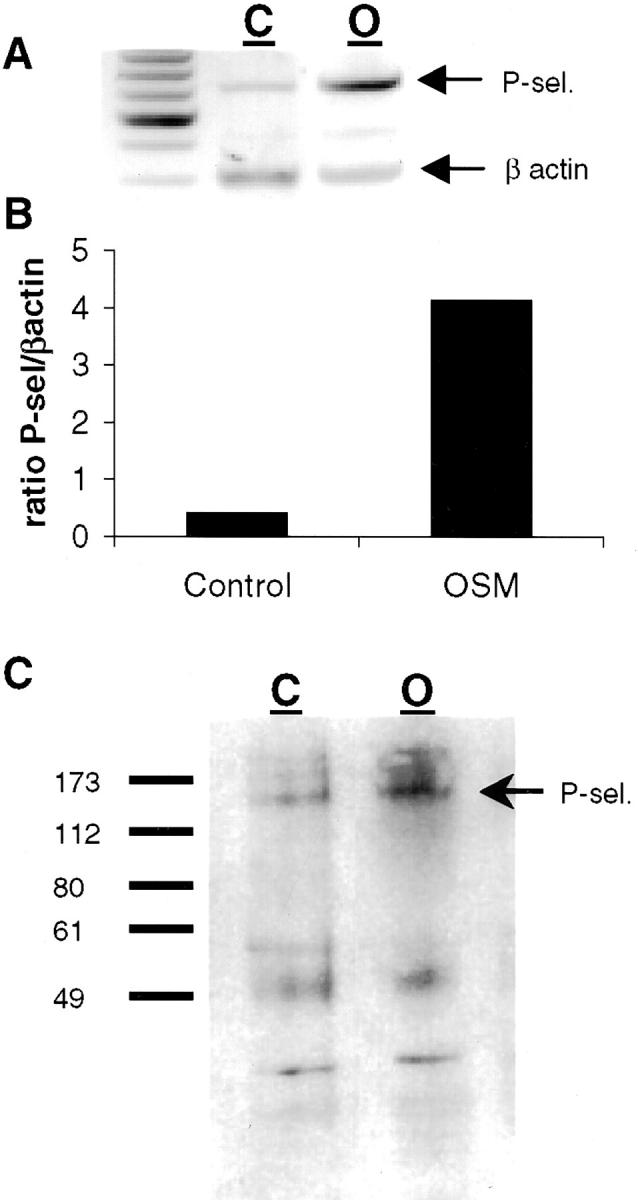
P-selectin is expressed by HUVECs in response to 24-hour treatment with OSM. A: P-selectin expression by HUVECs treated for 24 hours with 10 ng/ml of OSM as determined by RT-PCR. C, control; O, OSM, 10 ng/ml. B: Densitometry analysis of RT-PCR. P-selectin expression was standardized to β-actin and results are expressed as a ratio. C: Western blot demonstrating P-selectin protein expression after 24-hour treatment with 10 ng/ml of OSM.
Figure 5.

Enzyme-linked immunosorbent assays demonstrating E-selectin (A) and VCAM-1 (B) surface expression by control HUVECs or HUVECs treated with either 10 ng/ml of OSM or 25 ng/ml of TNF-α. One typical representative of six (E-selectin) or five (VCAM-1) experiments is shown.
Treatment of OSM-stimulated HUVECs with G1, an anti-P-selectin antibody, reduced both OSM-induced leukocyte rolling (Figure 6A) ▶ and adhesion (Figure 6B) ▶ to control levels. The nonblocking anti-P-selectin antibody S12 did not have any effect on leukocyte rolling or adhesion (data not shown). To further support the view that the OSM response was because of P-selectin synthesis we used microvascular endothelium from human dermis (HDMEC) that lacks prestored P-selectin (because of passaging). In addition, microvascular endothelium can differ from HUVECs in response to some inflammatory stimuli, so we also looked at whether HDMECs had exclusive P-selectin-dependent leukocyte recruitment. Indeed, OSM (24 hours) induced a very dramatic increase in leukocyte rolling and adhesion on HDMECs. Similar to HUVECs, anti-P-selectin antibody also returned OSM-induced leukocyte recruitment on HDMECs to control levels (Figure 7A) ▶ . Neither E-selectin nor VCAM-1 were up-regulated on OSM-treated HDMECs (data not shown) whereas total P-selectin levels were dramatically elevated with OSM (Figure 7B) ▶ .
Figure 6.

Inhibition of leukocyte rolling (A) and adhesion (B) by a P-selectin-blocking antibody. G1 was included with the whole blood perfused across HUVECs treated with 10 ng/ml of OSM. Data shown as mean ± SEM. *, P < 0.05 versus control; n = 3.
Figure 7.

A: Inhibition of leukocyte interactions with HDMECs by the P-selectin blocking antibody GA6. Whole blood diluted 1:10 in HBSS was perfused across either control or HDMECs treated for 48 hours with 10 ng/ml of OSM at 2 dynes/cm2. HDMEC monolayers were incubated with antibody for 30 minutes at 37°C at a final concentration of 10 μg/ml. Data are shown as mean ± SEM. *, P < 0.05 versus control. n = 4 for control, n = 7 for OSM, n = 2 for OSM + GA6. B: P-selectin protein expression by HDMECs. Whole-cell enzyme-linked immunosorbent assays were performed on cell lysates generated from controls and HDMECs treated for 48 hours with 10 ng/ml of OSM. One typical representative of three is shown.
To further investigate the selective mechanism(s) of OSM-mediated recruitment, we treated HUVECs with 0.5 U/ml thrombin for 10 minutes to induce rapid mobilization of P-selectin. This system, like the 24-hour treatment with OSM, results in leukocyte recruitment that is entirely dependent on P-selectin. 24 Using the parallel plate flow chamber we found that although the leukocyte profile recruited by 10 minutes of thrombin consisted mostly of neutrophils, thrombin was not as selective for these cells as OSM. Indeed, compared to the recruitment profile of OSM a significantly lower proportion of cells recruited by thrombin were neutrophils and a greater proportion were lymphocytes (Figure 8A) ▶ . Similarly, although monocytes and eosinophils made up only a very minor component of the profile recruited by thrombin (5.7% and 2.1%, respectively), they still represented a significantly greater portion of the total population than that recruited by OSM (0% and 0.8%, data not shown). Because both recruitment profiles were selectin-dependent the results suggest the thrombin induced a different expression (distribution pattern or amount) of P-selectin such that other leukocyte types could also be recruited. Alternatively, the mechanism could involve the preferential retention of neutrophils through selective CD18-dependent adhesion. Addition of CD18 antibody very significantly inhibited the number of adherent cells (data not shown). This resulted in a slight, significant reduction in the specificity for neutrophils because the proportion of neutrophils recruited by OSM was significantly reduced whereas the corresponding proportion of lymphocytes significantly increased (Figure 8B) ▶ . Nevertheless, CD18 binding could not account for the majority of the selectivity noted on OSM-treated endothelium. Neither monocytes nor eosinophils were observed to be recruited (data not shown).
Figure 8.
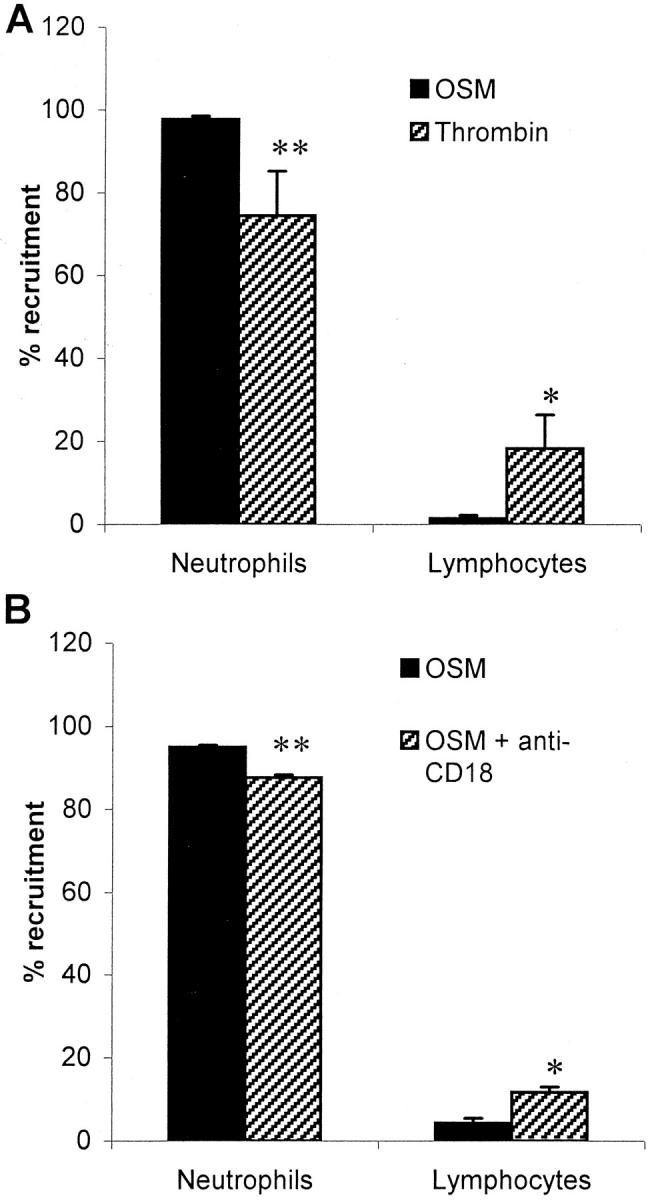
Mechanisms of OSM specificity for neutrophils. A: Comparison of recruitment profiles of two different P-selectin-dependent systems. HUVECs were treated for 24 hours with 10 ng/ml of OSM or for 10 minutes with 0.5 U/ml of thrombin. Whole blood was perfused across at 10 dynes/cm 2 and Geimsa-Wright staining was used to identify leukocyte subsets recruited to cytokine-treated HUVECs. The neutrophil and lymphocyte contributions to the recruitment profile are presented as a percent. Data are shown as mean ± SEM. *, P < 0.0026 versus OSM; **, P < 0.0022 versus OSM as determined by a two-tailed t-test. n = 5 for OSM, n = 3 for thrombin. B: Anti-CD18 treatment reduces the specificity of OSM-mediated recruitment. Whole blood was pretreated with 20 μg/ml of IB4 for 10 minutes before perfusion across endothelium. The neutrophil and lymphocyte contributions to the recruitment profile are presented as a percent. Data are shown as mean ± SEM. *, P < 0.0141 versus OSM; **, P < 0.0011 versus OSM as determined by a two-tailed t-test. n = 3 for OSM both groups.
Discussion
In this study we demonstrate that treatment of HUVECs with OSM for 24 hours resulted in both leukocyte rolling and adhesion at levels comparable to optimal TNF-α-mediated recruitment. Surprisingly, all of the recruitment was neutrophilic in nature. This OSM-dependent recruitment was entirely mediated through the up-regulation of P-selectin as demonstrated by the selective up-regulation of P-selectin but not E-selectin or VCAM-1 and by the almost complete abrogation of HUVEC-neutrophil interactions by a P-selectin-blocking antibody. The exclusive up-regulation of P-selectin by OSM was not restricted to the umbilical vein, but extended to human microvascular endothelium that, when passaged, lacks a prestored pool of P-selectin.
Clearly OSM has a very unique recruitment profile relative to other cytokines. OSM recruited 96% neutrophils, suggesting that adhesion molecule profiles on the endothelium selected for neutrophils and discriminated against other cell types. We have previously shown that immobilized P-selectin displays a similar (90%) selective recruitment for neutrophils from whole blood, 25 suggesting that the exclusive expression of this adhesion molecule is an important selective mechanism in OSM-mediated neutrophil recruitment. However, expression of other molecules in addition to P-selectin results in a dramatic change in the leukocyte recruitment profile. For example, although IL-4 has previously been shown to share with OSM the ability to up-regulate P-selectin mRNA and protein in the human, 7 VCAM-1 is also involved 26 and a very different leukocyte profile dominated by lymphocytes and eosinophils (with some neutrophils) is seen.
To further probe the exclusivity of P-selectin for neutrophils, we used another P-selectin-dependent leukocyte recruitment system (thrombin-induced mobilization of preformed pools) and interestingly noted less selectivity for neutrophils than was seen with OSM-treated endothelium. One possible explanation is that if neutrophils are more adhesive for P-selectin then perhaps greater amounts of P-selectin or altered distribution (more clustered) is required to recruit other cell types. Whether thrombin induces a particular distribution of P-selectin remains unknown. Clearly additional as-yet unidentified mechanisms are also likely to play a role.
The leukocytes found on the endothelial surface in this assay system likely reflect both rolling and adherent cells with the adherent cells slowly accumulating throughout the time period and reflecting a permanent population and the rolling cells being a more transient population of cells attaching and then letting go. Therefore, once the cells tether to the endothelium via P-selectin, they subsequently detach or rapidly adhere. It is probable that OSM remodels the endothelial surface (expression of certain chemokines) to preferentially accumulate neutrophils through adhesion. Indeed, addition of CD18 antibody prevented firm adhesion and this resulted in a small reduction in specificity for neutrophils (a slight increase in lymphocytes) on the endothelial surfaces. CD18 is critically important for neutrophil adhesion 3 whereas lymphocytes have other mechanisms of adhesion, such as α4-integrin. 26 It is important to note that even inhibition of adhesion still resulted in neutrophils comprising 90% of the tethered and rolling cells. Clearly, a preferential recruitment of neutrophils exists even at the very first (tethering) stage of leukocyte recruitment from the main stream of blood.
The role of OSM in leukocyte recruitment is poorly defined. In fact, there has been controversy in the literature as to whether OSM plays a proinflammatory role or is instead involved in the resolution of the inflammatory response. The evidence for an anti-inflammatory role for OSM is its ability to down-regulate the expression of TNF-α 12 and other proinflammatory cytokines involved in leukocyte migration to sites of inflammation. 23 Furthermore, treatment of some models of chronic inflammation with OSM may prevent induction of disease or lessen symptoms. 12 It is important to note that in a number of these studies, human OSM was injected into rodent systems and therefore unexpected cross-species complications could have resulted. Indeed, recent evidence suggests that human OSM does not interact with the murine OSM receptor 27 suggesting nonspecific effects of this human cytokine in rodent systems. Our own work with murine OSM injected into mice revealed a proinflammatory role (our unpublished data). Finally, although others have suggested that the proinflammatory effects of OSM may be because of endotoxin contamination, this is also not likely as lipopolysaccharide induces E-selectin, VCAM-1, and not P-selectin on endothelium, 28,29 a profile very different to OSM’s exclusive P-selectin adhesion profile. Moreover, the OSM used in this study had minimal levels of lipopolysaccharide (<0.1 ng per 1 μg, according to the manufacturer).
It is interesting that cytokines such as TNF-α induce significant cellular transmigration, whereas OSM failed to induce this response. Although one possible explanation is that OSM is a chemotactic agent and prevented migration away from its source on the apical surface of the endothelial monolayer, our in vitro chemotaxis assay did not support this view. With OSM the cells stay at the endothelial interface, which could indicate that the importance of this molecule is to strictly induce neutrophil adhesion. OSM has previously been shown to elicit very limited chemokine expression from endothelial cells, 16,23 consistent with the lack of transmigration across OSM-treated HUVECs without extravascular cells.
Although P-selectin is most commonly associated with early leukocyte recruitment in an inflammatory response, a role for P-selectin has also been identified in more delayed forms of inflammation. Studies in rodents have implicated P-selectin up-regulation in rheumatoid arthritis, 30 atherosclerosis, 31,32 and allergy, 33 however the mediator responsible remains unknown. This is intriguing in light of the fact that in these chronic human diseases, bursts of neutrophil recruitment are a common feature. In this regard, OSM may represent an important mechanism to elicit P-selectin up-regulation in a dysregulated inflammatory response thereby inducing ongoing recruitment of neutrophils. The low concentrations of OSM used in this study have been previously demonstrated to act through the high, but not the low-affinity OSM receptor. 16,22 This suggests that low concentrations of OSM produced at sites of inflammation may act to maintain the recruitment of neutrophils to the inflammatory site throughout an extended period of time and may contribute to the inappropriate recurrent neutrophil recruitment observed in relapsing inflammatory bowel disease, arthritis, and other chronic inflammatory diseases.
Acknowledgments
We thank Dr. Rodger McEver, Dr. Roy Lobb, and Dr. Paul Naccache for their generous gifts of reagents; the nurses of the Labor and Delivery Unit at the Foothills Hospital, Calgary, for their assistance in providing umbilical cords; Dr. C. Lane and her colleagues at the Valley View Family Practice Clinic, Calgary, for providing foreskins; and Evelyn Brawn for technical aspects associated with transmission electron microscopy.
Footnotes
Address reprint requests to Dr. Paul Kubes, Immunology Research Group, Department of Physiology and Biophysics, University of Calgary, 3330 Hospital Dr., NW, Calgary, Alberta, Canada T2N 4N1. E-mail: pkubes@ucalgary.ca.
Supported by a Heart and Stroke Foundation of Canada grant, an Alberta Foundation for Medical Research and Medical Research Council of Canada Scientist grant (to P. K. ), a Multiple Sclerosis Society of Canada Studentship grant (to S. K.), and the National Institutes of Health (grant HL-42550) and the American Lung Association (grant RG-068-N) (to A. R. B.).
K.P. is an and Medical Research Council of Canada Scholar and M. H. is an Alberta Foundation for Medical Research Scholar.
References
- 1.Springer TA: Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Annu Rev Physiol 1995, 57:827-872 [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman GA, McIntyre TM, Prescott SM: Adhesion and signaling in vascular cell-cell interactions. J Clin Invest 1996, 98:1699-1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albelda SM, Smith CW, Ward PA: Adhesion molecules and inflammatory injury. FASEB J 1994, 8:504-512 [PubMed] [Google Scholar]
- 4.Subramaniam M, Koedam JA, Wagner DD: Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell 1993, 4:791-801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattori R, Hamilton KK, Fugate RD, McEver RP, Sims PJ: Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem 1989, 264:7768-7771 [PubMed] [Google Scholar]
- 6.Green SA, Setiadi H, McEver RP, Kelly RB: The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol 1994, 124:435-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao L, Pan J, Setiadi H, Patel KD, McEver RP: Interleukin 4 or oncostatin M induces a prolonged increase in P-selectin mRNA and protein in human endothelial cells. J Exp Med 1996, 184:81-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khew-Goodall Y, Butcher CM, Litwin MS, Newlands S, Korpelainen EI, Noack LM, Berndt MC, Lopez AF, Gamble JR, Vadas MA: Chronic expression of P-selectin on endothelial cells stimulated by the T-cell cytokine, interleukin-3. Blood 1996, 87:1432-1438 [PubMed] [Google Scholar]
- 9.Taga T, Kishimoto T: Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997, 15:797-819 [DOI] [PubMed] [Google Scholar]
- 10.Murakami-Mori K, Taga T, Kishimoto T, Nakamura S: AIDS-associated Kaposi’s sarcoma (KS) cells express oncostatin M (OM)-specific receptor but not leukemia inhibitory factor/OM receptor or interleukin-6 receptor. Complete block of OM-induced KS cell growth and OM binding by anti-gp130 antibodies. J Clin Invest 1995, 96:1319-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B: The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science 1992, 255:1434-1437 [DOI] [PubMed] [Google Scholar]
- 12.Wallace PM, Macmaster JF, Rouleau KA, Brown TJ, Loy JK, Donaldson KL, Wahl AF: Regulation of inflammatory responses by oncostatin M. J Immunol 1999, 162:5547-5555 [PubMed] [Google Scholar]
- 13.Guillet C, Fourcin M, Chevalier S, Pouplard A, Gascan H: ELISA detection of circulating levels of LIF, OSM, and CNTF in septic shock. Ann NY Acad Sci 1995, 762:407-409 [DOI] [PubMed] [Google Scholar]
- 14.Malik N, Kallestad JC, Gunderson NL, Austin SD, Neubauer MG, Ochs V, Marquardt H, Zarling JM, Shoyab M, Wei CM: Molecular cloning, sequence analysis, and functional expression of a novel growth regulator, oncostatin M. Mol Cell Biol 1989, 9:2847-2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grenier A, Dehoux M, Boutten A, Arce-Vicioso M, Durand G, Gougerot-Pocidalo MA, Chollet-Martin S: Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 1999, 93:1413-1421 [PubMed] [Google Scholar]
- 16.Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, McIntyre TM: Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J Clin Invest 1997, 100:158-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrovsky L, Woodman RC, Payne D, Teoh D, Kubes P: Antithrombin III prevents and rapidly reverses leukocyte recruitment in ischemia/reperfusion. Circulation 1997, 96:2302-2310 [DOI] [PubMed] [Google Scholar]
- 18.Kanwar S, Woodman RC, Poon MC, Murohara T, Lefer AM, Davenpeck KL, Kubes P: Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules. Blood 1995, 86:2760-2766 [PubMed] [Google Scholar]
- 19.Niu XF, Smith CW, Kubes P: Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res 1994, 74:1133-1140 [DOI] [PubMed] [Google Scholar]
- 20.McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR: Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest 1997, 100:2521-2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foxman EF, Campbell JJ, Butcher EC: Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol 1997, 139:1349-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP: Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood 1999, 94:3820-3828 [PubMed] [Google Scholar]
- 23.Richards CD, Langdon C, Botelho F, Brown TJ, Agro A: Oncostatin M inhibits IL-1-induced expression of IL-8 and granulocyte-macrophage colony-stimulating factor by synovial and lung fibroblasts. J Immunol 1996, 156:343-349 [PubMed] [Google Scholar]
- 24.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA: Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol 1991, 115:223-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhardt PH, Kubes P: Differential leukocyte recruitment from whole blood via endothelial adhesion molecules under shear conditions. Blood 1998, 92:4691-4699 [PubMed] [Google Scholar]
- 26.Patel KD: Mechanisms of selective leukocyte recruitment from whole blood on cytokine-activated endothelial cells under flow conditions. J Immunol 1999, 162:6209-6216 [PubMed] [Google Scholar]
- 27.Lindberg RA, Juan TS, Welcher AA, Sun Y, Cupples R, Guthrie B, Fletcher FA: Cloning and characterization of a specific receptor for mouse oncostatin M. Mol Cell Biol 1998, 18:3357-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galdiero M, Folgore A, Molitierno M, Greco R: Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro. Clin Exp Immunol 1999, 116:453-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalogeris TJ, Kevil CG, Laroux FS, Coe LL, Phifer TJ, Alexander JS: Differential monocyte adhesion and adhesion molecule expression in venous and arterial endothelial cells. Am J Physiol 1999, 276:L9-L19 [DOI] [PubMed] [Google Scholar]
- 30.Birner U, Issekutz TB, Issekutz AC: The role of selectins in VLA-4 and CD18-independent neutrophil migration to joints of rats with adjuvant arthritis. Eur J Immunol 1999, 29:1094-1100 [DOI] [PubMed] [Google Scholar]
- 31.Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD: Absence of P-selectin delays fatty streak formation in mice. J Clin Invest 1997, 99:1037-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K: Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res 1999, 84:1237-1244 [DOI] [PubMed] [Google Scholar]
- 33.Kanwar S, Bullard DC, Hickey MJ, Smith CW, Beaudet AL, Wolitzky BA, Kubes P: The association between alpha4-integrin, P-selectin, and E-selectin in an allergic model of inflammation. J Exp Med 1997, 185:1077-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]




