Abstract
The aim of our study was to address the question of whether muscle fibers express major histocompatibility complex (MHC) class II in inflammatory myopathies. For this purpose we performed a systematic study of MHC class II antigen expression on muscle fiber membranes in muscle tissue from polymyositis and dermatomyositis patients in various stages of disease activity. Thirty-two patients with classical clinical signs of myositis were divided into subgroups depending on duration of clinical signs of myositis and presence or absence of inflammatory infiltrates in muscle tissue. Immunohistochemistry as well as double-immunofluorescence stainings were used to identify the presence of MHC class II in muscle tissue. MHC class I was included for comparison. Quantification of positive staining was performed using an image analysis system in addition to evaluation by manual microscopic scoring and laser confocal microscopy. It was demonstrated that a significant proportion of skeletal muscle fibers in inflammatory myopathies express MHC class II as well as MHC class I and that MHC antigen expression is independent of the inflammatory cell infiltration. Furthermore, there were no differences in staining pattern between polymyositis and dermatomyositis patients. Our results indicate that MHC class II and MHC class I molecules may be involved in initiating and maintaining the pathological condition in myositis rather than only being a consequence of a preceding local inflammation.
Idiopathic inflammatory myopathies are muscle disorders of unknown origin that are classically characterized by clinical signs of proximal muscle weakness and by histopathological demonstration of inflammatory infiltrates in the clinically affected muscles. 1,2 Based on clinical as well as histopathological criteria such as localization and distribution of inflammatory cells, idiopathic inflammatory myopathies have been classified into polymyositis (PM), dermatomyositis (DM), and inclusion body myositis. 3 Etiology and pathogenesis of the idiopathic inflammatory myopathies are primarily unknown, as is the cause of muscle weakness. 2 It has been implied that the inflammatory infiltrates in muscle tissue cause the clinical symptoms in PM and DM. However, several studies have described patients with myositis with pronounced muscle weakness and fatigue but without detectable infiltration of inflammatory cells in muscle tissues. 4,5 In a recent study of patients with inactive, chronic PM and DM other more sensitive markers of an ongoing pathological process were identified in muscle tissue, such as endothelial cell expression of interleukin-1α (IL-1α) and expression of major histocompatibility complex (MHC) class I on muscle fiber membranes. 4 Such endothelial and muscle fiber phenotypes may therefore be more closely associated with the clinical symptoms than presence of inflammatory cells.
It should therefore be of interest to study the phenotypes of the muscle fibers in further detail, with focus on their expression of immunologically important molecules at different phases of myositis. In this context, the expression of MHC class I and MHC class II molecules is of special interest because MHC class I and II expression on muscle fibers may be associated with a drastically changed potential to participate in immunological reactions related to T cell activation and anergization. Neither MHC class I nor class II molecules have been detected on muscle fibers from normal muscle tissues with currently used methods, 6,7 but both myoblasts and myotubes constitutively express MHC class I antigen when grown in vitro and expression of MHC class II antigens can be induced in the same cells by interferon-γ stimulation. 8-10
It is now well established that MHC class I molecules are expressed on muscle fibers in inflammatory myopathies as well as in other types of myopathies. 4,6,7,11-20 To what extent muscle fibers from patients with inflammatory myopathies express MHC class II molecules has remained more controversial, however. For instance, several immunohistochemical studies have reported the presence of MHC class II molecules on the surface of as well as intracellularly in muscle fibers in tissues with inflammatory infiltrates from myositis patients. 14-17,22-24 Other studies, using similar methodologies have failed to detect MHC class II molecules on muscle fibers and/or indicated that stainings for MHC class II on muscle fibers might represent an artificial staining resulting from shedding of MHC class II from adjacent active inflammatory infiltrates. 6,11,20-21
Considering the potentially important pathophysiological role in myositis of MHC class II expression on muscle cells, we wanted to readdress the question of whether muscle fibers in myositis express MHC class II molecules. Two new approaches were used for this purpose. One approach was to study MHC class II expression in various populations of myositis patients, including patients with clinical signs of myositis both with and without inflammatory infiltrates in muscle tissue. To determine the tissue distribution of MHC class II molecules in a wider context, we also included muscle tissue from normal healthy volunteers and disease controls consisting of patients with various forms of muscle dystrophies or neuropathies. Another approach was to use laser confocal microscopy in parallel with conventional immunohistology to study the MHC class II expression in detail in various compartments of the muscle cell. For comparison, we also included investigation of MHC class I in our study.
Materials and Methods
Patients
A total of 32 patients with regular check-ups at the Department of Rheumatology at the Karolinska Hospital were included in the present investigation and they all fulfilled the diagnosis of PM and DM based on the Bohan and Peter classification. 25,26 As control samples we included muscle biopsies from 11 healthy individuals and from 10 patients with muscle dystrophies or neuropathies. Muscle biopsies were taken from vastus lateralis or tibialis anterior muscle under local anesthesia by a semiopen technique using a conchotome. 27 In cases when a repeated muscle biopsy was conducted it was performed on the contralateral side. Muscle pathology was assessed by routine examination by a neuropathologist (IN) at the Division of Pathology, Huddinge Hospital. For immunohistochemical analyses and immunofluorescence studies the biopsies were frozen and stored at −70°C until the staining procedure was performed. Biopsies collected were from different time points of disease activity and therefore the patients were further divided into three groups according to their disease duration and inflammatory activity in muscle biopsy at time of muscle sampling (Table 1) ▶ .
Table 1.
Overview of Patient Information
| Patient | Sex/age at time for biopsy | Diagnosis | Symptom duration at time of muscle sampling (months)* | Inflammatory infiltrates in biopsy used in present study | Immunosuppressive treatment |
|---|---|---|---|---|---|
| 1 | F /49 | PM | 4 | no | no |
| 2 | F /26 | PM, SLE | 9 | no | no |
| 3 | M /45 | PM, MCTD | 2 | no | no |
| 4 | M /56 | PM | 1 | no | no |
| 5 | F /39 | PM | 3 | no | pred, aza |
| 6 | F /45 | PM | 4 | no | pred, cyclo |
| 7 | M /64 | DM | 1 | no | no |
| 8 | F /30 | DM, RA, SLE | 1 | no | pred |
| 9 | F /61 | DM | 2 | no | aza |
| 10 | F /48 | DM | 2 | no | pred |
| 11 | M /38 | DM | 1 | no | no |
| 12 | F /23 | PM | 4 | yes | no |
| 13 | F /49 | PM | 24 | yes | no |
| 14 | F /64 | PM | 6 | yes | no |
| 15 | F /43 | PM | 2 | yes | no |
| 16 | F /61 | PM | 12 | yes | no |
| 17 | F /45 | PM, SS | 12 | yes | pred |
| 18 | M /56 | PM | 2 | yes | no |
| 19 | F /26 | DM, SLE | 9 | yes | pred |
| 20 | F /80 | DM, RA | 7 | yes | MTX |
| 21 | M /40 | DM | 5 | yes | no |
| 22 | F /54 | PM, SS, systemic sclerosis | 56 | pred, aza | |
| 23 | F /56 | PM | 102 | no | aza |
| 24 | F /60 | PM | 38 | no | no |
| 25 | F /56 | PM | 18 | no | pred |
| 26 | F /37 | PM | 44 | no | pred, aza |
| 27 | F /27 | PM | 83 | no | pred |
| 28 | M /47 | PM | 18 | no | pred |
| 29 | F /52 | DM | 44 | no | no |
| 30 | F /59 | DM | 40 | no | pred, aza |
| 31 | F /50 | DM | 34 | no | pred |
| 32 | M /44 | DM, SS | 49 | no | MTX |
Patients were divided into three groups according to presence or absence of inflammatory infiltrates in muscle biopsy and according to their symptom duration at time for muscle sampling.
Patients 1–11 belong to a group of patients with clinical active myositis, but without inflammatory infiltrates in muscle tissue used for this study. These patients have a short duration of muscle symptoms and no infiltrates as yet typical for myositis in biopsy. Patients 12–21 belong to a classical group of myositis patients with clinical signs of muscle weakness and typical infiltrates in muscle tissue. Patients 22–32 belong to a group of patients with a long disease duration and persisting muscle weakness despite effective therapy in diminution of inflammatory cell infiltration. F, female; M, male; PM, polymyositis; DM, dermatomyositis; SLE, systemic lupus erythematosis; MCTD, mixed connective tissue disease; RA, rheumatoid arthritis; SS, Sjögren’s syndrome; pred, prednisolone; aza, azathioprine; cyclo, cyclophosphamide; MTX, methotrexate.
*For patients 1 to 21 symptom duration is counted from start of clinical signs, for patients 22 to 32 symptom duration is counted from time point of diagnosis.
Group 1: Patients with Clinical Signs of Myositis but without Detectable Infiltration of Clusters of Inflammatory Cells in Muscle Tissues
The first group consisted of 11 patients with a short duration of clinical symptoms of myositis but without detectable infiltration in their muscle biopsies. Eight patients were biopsied because of suspicion of myositis and three patients because of possible relapse. The median age at time of muscle biopsy was 45 years (range, 26 to 64 years). Symptom duration varied between 1 and 9 months with a median duration of 2 months. Despite clinical signs of myositis the histopathological evaluation of the muscle biopsies revealed no inflammatory infiltrates typical for myositis in these patients at this time point. Diagnosis was either later (eight patients) or earlier (three patients) confirmed by a muscle biopsy containing inflammatory infiltrates or by clinical and laboratory findings. Six patients were diagnosed as PM (four females and two males) and five were diagnosed as DM (three females and two males). Three patients also fulfilled the criteria for other rheumatic diseases, one for mixed connective tissue disease, one for rheumatoid arthritis as well as systemic lupus erythematosus, and a third patient fulfilled the criteria for systemic lupus erythematosus. Five patients, the three relapse patients and two patients with suspicion of myositis, received immunosuppressive therapy, prednisolone on its own or in combination with azathioprine or cyclophosphamine or azathioprine on its own, at time of muscle biopsy.
Group 2: Patients in an Active Stage of Disease with Clinical Signs of Myositis and Typical Clusters of Inflammatory Cells in Muscle Tissue
The second group was defined as patients fulfilling both clinical and histopathological criteria for myositis according to the Peter and Bohan’s 25,26 classification and they were further characterized histopathologically as PM or DM according to the Arahata and Engel classification. 3 Symptom duration varied between 2 to 24 months with a median duration of 7 months. All patients had detectable infiltrates that included T lymphocytes as identified with a CD3 antibody in tissue sections. Seven patients had active PM (six females and one male) and three patients had active DM (two females and one male). The median age at diagnosis was 52 years (range, 23 to 80 years). These 10 patients have been described in a previous study. 28 Apart from fulfilling the diagnostic criteria for PM or DM, one patient also fulfilled the criteria for systemic lupus erythematosus, one the criteria for rheumatoid arthritis, and one the criteria for Sjögren’s syndrome. The criteria for these other diseases were fulfilled before the diagnosis of myositis was made and these patients received immunosuppressive treatment at the time of biopsy. The systemic lupus erythematosus and Sjögren’s syndrome patients were on low-dose prednisolone and the rheumatoid arthritis patient was on methotrexate treatment. A muscle biopsy was in all other cases performed before immunosuppressive treatment was initiated. A repeated muscle biopsy was performed after 3 to 6 months of therapy.
Group 3: Patients in a Chronic, Inactive Phase of Myositis without Detectable Clusters of Inflammatory Cells
This group consisted of 11 patients with a long disease duration and persisting muscle weakness despite effective therapy that had resulted in diminution of inflammatory cell infiltration. This patient group has previously been described. 4 Seven patients were classified as PM (six females and one male) and four patients as DM (three females and one male). Disease duration from diagnosis to muscle biopsy sampling ranged from 18 to 102 months with a median duration of 40 months. Inactive disease was defined as absence of inflammatory infiltrates in muscle biopsy and absence of signs of inflammation by magnetic resonance imaging. Two patients had other rheumatic diseases, one Sjögren’s syndrome and one Sjögren’s syndrome plus systemic sclerosis. At time of biopsy seven patients received prednisolone, four patients were treated with azathioprine, and one with a low dose of methotrexate. None of the patients had had a change of immunosuppressive therapy for the last 3 months before muscle biopsy.
Control Biopsies
Eleven healthy volunteers (seven males and four females) were included as normal controls, their median age at time for muscle biopsy being 43 years (range, 22 to 50 years). Ten patients with other muscle disorders were also included as controls. Five patients (two females and three males) with a median age of 63 years (range, 48 to 68 years) had groups of atrophic fibers in skeletal muscle, indicating neurological damage. This patient population represents a control group with structural changes without signs of inflammation in muscle tissues. Five patients with muscle dystrophies including limb-girdle, facio-scapulo-humeral, and dystrophinopati (two females and three males) with a median age of 26 years (range, 2 to 39 years), constituted a control group with structural changes as well as inflammatory infiltrates in muscle tissues.
Immunohistochemistry and Immunofluorescence Studies
Seven-μm thick cryostat sections were mounted on gelatin-coated glass slides (Celline Associates, Newfield, NJ) and air-dried for 30 minutes at room temperature. The first and the last sections were stained with hematoxylin and eosin to confirm that the histopathology of the biopsy was unchanged in the consecutive series of sections collected for immunostainings.
A standard immunohistochemistry protocol was applied to identify expression of MHC class I and MHC class II. 29 All antibodies used were carefully evaluated by titration on lymphoid tissue. Lymphoid tissue was also used for positive controls at time of staining the patient material. MHC class I was detected with a mouse anti-human leukocyte antigen (HLA)-ABC IgG2a antibody clone W6/32 (DAKO A/S, Glostrup, Denmark) diluted 1/3500. MHC class II was detected with a mouse anti-human HLA-DR IgG2a antibody clone L243 (Becton Dickinson, San Jose, CA) diluted 1/640. Anti-HLA-DR reacts with a nonpolymorphic HLA-DR epitope and does not cross-react with HLA-DQ or HLA-DP molecules. 30 We also included a mouse anti-human HLA-DQ IgG1 antibody clone SK10 (Becton Dickinson, San Jose, CA) diluted 1/80 to detect MHC class II.
In parallel, double-immunofluorescence labeling was performed for MHC class II detected with the same antibody as above but diluted 1/50 and for laminin detected with rabbit anti-rat laminin IgG antibody diluted 1/2000 (DAKO A/S). Antibody concentrations were optimized for double-immunofluorescence staining. Phosphate-buffered saline (PBS) was used as a buffer throughout the protocol and sections were rinsed in PBS between the different incubations. Sections were blocked for 15 minutes with 2% normal horse serum (Vector Laboratories, Burlingame, CA). Thereafter, endogenous biotin reactivity was blocked using an avidin-biotin blocking kit (Vector Laboratories), undiluted avidin being applied for 15 minutes and subsequently undiluted biotin was applied for 15 minutes. The tissue sections were incubated for 60 minutes with primary antibodies in a cocktail diluted in PBS supplemented with 1% bovine serum albumin (Sigma Chemical Co., St. Louis, MO). Thereafter, sections were incubated for 30 minutes with appropriate secondary antibody diluted in PBS supplemented with 1% normal horse serum (Cy3-conjugated donkey anti-mouse, diluted 1/50, biotinylated donkey anti-rabbit, diluted 1/500, both from Jackson ImmunoResearch Laboratories, West Grove, PA). Finally, sections were incubated for 30 minutes with Oregon Green coupled to avidin at a concentration of 2 μg/ml diluted in PBS (Molecular Probes, Eugene, OR). Irrelevant isotype-matched control antibodies, in the same concentrations as respective specific antibody, were used as negative controls for all experiments, mouse IgG1, mouse IgG2a (DAKO), and rabbit IgG (Jackson, ImmunoResearch Laboratories).
Evaluation of Staining
Conventional Microscopic Evaluation
The immunostained tissue sections were analyzed using a Reichert-Jung Polyvar microscope equipped with a SONY-3CCD color video camera (Model OXC-930P, Sony Corporation, Japan) connected to a SONY-color video printer (Model Mavigraph, Sony Corporation, Japan). Expression of HLA-ABC and HLA-DR was assessed as follows: 0, negative staining of muscle fibers, positively stained endothelial cells only; 1+, positive staining of endothelial cells and 1 to 10% positively stained fibers; 2+, positive staining of endothelial cells and 11 to 25% positively stained fibers; 3+, positive staining of endothelial cells and 26 to 50% positively stained fibers; and 4+, positive staining of endothelial cells and 51 to 100% positively stained fibers. The expression of HLA-DQ was limited and only qualitative assessment with description of staining pattern was performed.
Image Analysis
The immunostained sections were also analyzed using a Quantimet 600 (Q600) image analyzer (Leica Cambridge Ltd., Cambridge, UK). The image processor was directed by a PC computer. Special software was written in high-level language, QUIPS, for this application. Image analysis was used to quantify the total positively stained area (mm2) for HLA-ABC and HLA-DR as well as the total tissue area of the respective sections from each biopsy and percent HLA-ABC and percent HLA-DR expression were calculated for each individual. The results are presented as median (M) values for each patient group with respective interquartile range (IQR) values. The HLA-ABC and HLA-DR were chosen for evaluation by image analysis because of a large number of positive fibers, whereas HLA-DQ was only expressed on a few fibers and in few muscle tissue sections making microscopic measurement more reliable for this molecule.
Laser Confocal Microscopy
The laser confocal system consisted of a BioRad MRC 1024 unit attached to a Nikon Optiphot-2 microscope. The objective used was Nikon Plan Apo ×40 (oil, N.A 1.3). Excitation at 568 nm was used for Cy3 (HLA-DR)-stained structures with an emission filter at 585 nm and excitation at 488 nm for fibers stained with Oregon Green (Laminin) with an emission filter at 522 nm. Images were converted from BioRad’s file format to fit format using the Confocal Assistant software version 4.02 and photomontage was arranged in Adobe Illustrator version 8.0 for Macintosh.
Statistical Analysis
Data were analyzed using Stat View 4.5 statistical software for Macintosh. Owing to small sample size and nonnormality of the data in this study exact nonparametric tests were used to test for significance. To compare the HLA-ABC and the HLA-DR expression between the different patient groups and the control groups we used the Mann-Whitney U test. To compare the HLA-ABC and the HLA-DR expression in the clinically and inflammatory active myositis group before and after treatment the Wilcoxon signed rank test was used.
Results
Normal Control Tissue
MHC Class II Immunoreactivity
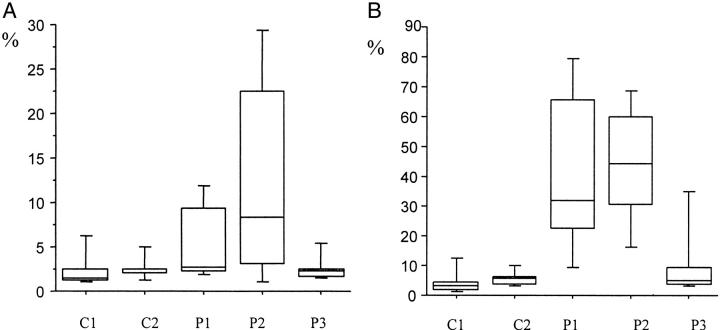
HLA-DR expression in muscle tissue from 11 healthy controls was located to endothelial cells of arterioles, venules, and capillaries (Figure 1A) ▶ . In two cases occasional fibers expressed the DR antigen. Image analysis values were M, 1.5%; IQR, 1.2% (Figure 2A) ▶ . HLA-DQ expression was noted in scattered endothelial cells of arterioles, venules, and capillaries. No muscle fibers expressed the DQ antigen.
Figure 1.
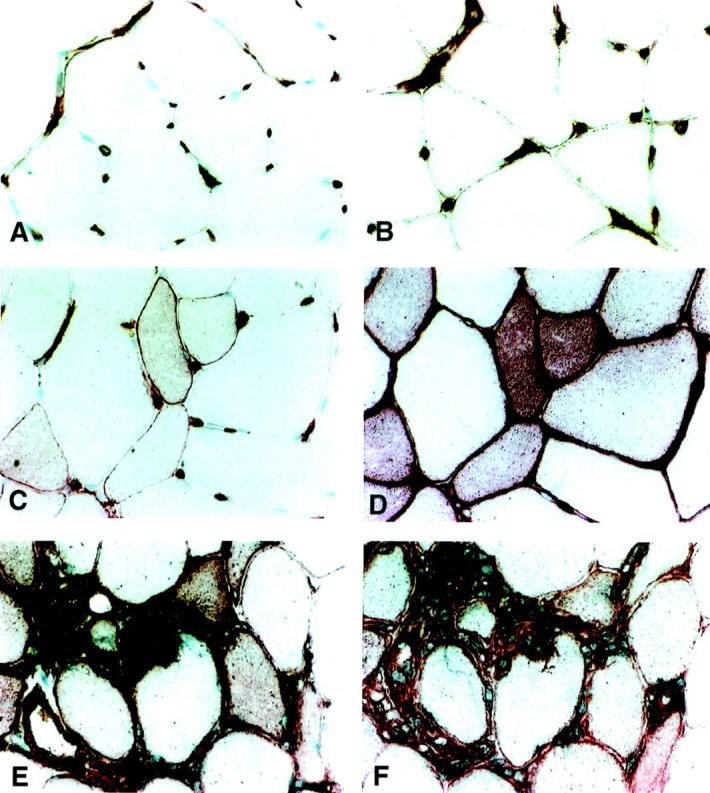
Immunohistochemical localization of HLA-DR and HLA-ABC in muscle tissue. Dark red staining demonstrates a positive staining. Original magnifications, ×340. A, C, and E depict HLA-DR staining and B, D, and F depict HLA-ABC staining. A and B are representative stainings for healthy control tissue with MHC reactivity in endothelial cells. C and D illustrate immunostaining in tissue from a PM patient with clinical symptoms of muscle weakness but without detectable infiltration of inflammatory cells in muscle tissue. Distinct HLA-DR and HLA-ABC fiber reactivity is present despite absence of infiltration of inflammatory cells. E and F represent stainings of tissue from a PM patient with clinical symptoms of muscle weakness and heavy infiltration of inflammatory cells in muscle tissue. Strong immunoreactivity of HLA-DR and HLA-ABC on muscle fibers, inflammatory cells, and endothelial cells.
Figure 2.
Image analysis values of immunostainings for HLA-DR and HLA-ABC in muscle biopsies from control and patient groups. C1, control group 1, includes 11 healthy individuals; C2, control group 2, includes 10 patients with other muscle disorders; P1, patient population 1, includes 11 patients with clinical signs of myositis but without detectable infiltration of inflammatory cells in muscle tissue; P2, patient population 2, includes 10 patients in an active stage of disease with clinical signs of myositis and typical inflammation in tissue; P3, patient population 3, includes 11 patients in a chronic, inactive phase of myositis with minimal infiltration of inflammatory cells. A: Percentage of HLA-DR expression in muscle tissue from myositis patients in various stages of disease activity and from controls. A statistically significant difference was observed between, C1 and P1 (P = 0.02), C1 and P2 (P = 0.008), and between C2 and P2 (P = 0.006). B: Percentage of HLA-ABC expression in muscle tissue from myositis patients in various stages of disease activity and from controls. A statistically significant difference was observed between, C1 and all three patient populations, P1, P2, and P3 (P = 0.0002, P = 0.0001, P = 0.03), C2 and P1 (P = 0.0007), and between C2 and P2 (P = 0.0002).
MHC Class I Immunoreactivity
HLA-ABC expression in muscle tissue was detected in endothelial cells of arterioles, venules, and capillaries (Figure 1B) ▶ . In two cases the same fibers that expressed DR antigens also expressed the ABC antigens. Image analysis values were M, 3.0%; IQR, 2.7% (Figure 2B) ▶ .
Group 1: Patients with Clinical Signs of Myositis but without Detectable Infiltration of Clusters of Inflammatory Cells in Muscle Tissues
MHC Class II Immunoreactivity
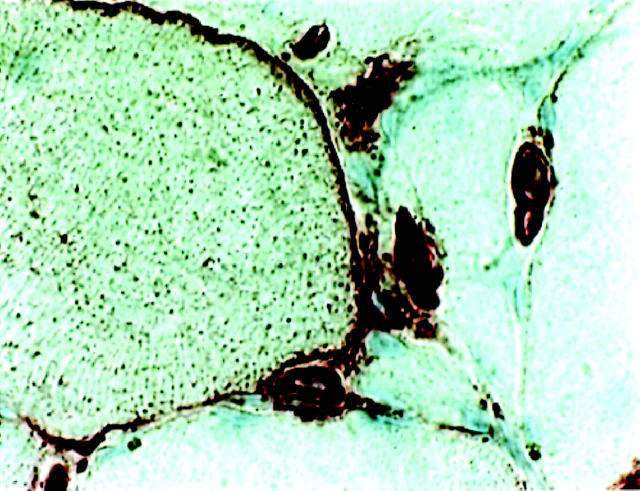
In five of six PM patients and five of five DM patients without infiltration of mononuclear leukocytes in tissue, muscle fibers were positive for the DR antigen (Figure 1C) ▶ , with muscle fiber staining ranging from 1+ to 2+. Quantitative image analysis gave a median value of 2.7% and interquartile range of 7.1% (Figure 2A) ▶ . We observed HLA-DR expression on the surface membranes of the muscle fibers (Figure 3) ▶ and in some cases the fibers also displayed a reticular pattern of intracellular staining. In many cases HLA-DR expression was seen in the same areas as those where HLA-ABC antigens were expressed (Figure 1, C and D) ▶ . The PM patient who lacked HLA-DR reactivity in muscle fibers also lacked ABC expression on fiber membranes. No difference in staining pattern was observed between PM and DM patients. In all 11 patients the DR antigen was expressed on endothelial cells and on scattered mononuclear inflammatory cells (Figure 1C) ▶ . HLA-DQ expression was observed in scattered inflammatory cells and in some endothelial cells. In two cases occasional muscle fibers expressed the DQ antigen.
Figure 3.
Immunohistochemical staining for HLA-DR on a muscle fiber membrane from a PM patient. Original magnification, ×680.
MHC Class I Immunoreactivity
In 10 of 11 patients strong immunoreactivity for the HLA-ABC antigens on muscle fibers was observed (five of six PM and five of five DM). The positive muscle fibers displayed staining of the sarcolemma and in some cases of the cytoplasm (Figure 1D) ▶ . The staining varied between 2+ to 4+. Quantitative investigation gave a median of 32.1% with an interquartile range of 43.4% (Figure 2B) ▶ . We observed HLA-ABC expression on either isolated muscle fibers, or on scattered muscle fibers and in some cases on almost all muscle fibers. In some cases the positively stained fibers were accumulated near perimysial areas of the biopsy and this was apparent in both PM and DM patients. No difference in staining intensity or distribution of staining was observed between PM and DM patients. In all 11 patients the ABC antigens were expressed on endothelial cells of all types of blood vessel (Figure 1D) ▶ .
Group 2: Patients in an Active Stage of Disease with Clinical Signs of Myositis and Typical Clusters of Inflammatory Cells in Muscle Tissue
MHC Class II Immunoreactivity
The HLA-DR antigens were expressed on muscle fiber membranes in nine patients (seven of seven PM, two of three DM) (Figure 1E) ▶ . Staining on muscle fibers ranged from 1+ to 3+ with manual scoring and image analysis measurement gave a median value of 8.6% with an IQR of 19.2% (Figure 2A) ▶ . HLA-DR reactivity was sometimes noted in the cytoplasm (Figure 1E) ▶ . Both normal and morphologically changed fibers expressed DR antigens (Figure 1E) ▶ . Fibers invaded by inflammatory cells expressed a strong HLA-DR reactivity (Figure 1E) ▶ . Inflammatory cells and endothelial cells also expressed the DR antigen (Figure 1E) ▶ . No difference in staining pattern between PM and DM patients was recorded.
In this group with active myositis patients, biopsies were repeated after 3 months of treatment with corticosteroids. Five out of nine patients still expressed HLA-DR after treatment (four of seven PM, one of three DM). Staining intensities ranged from 1+ to 3+. The median value for HLA-DR was 2.1% with an IQR of 1.6%. In these biopsies the HLA-DR-stained area was significantly smaller compared to that in the first set of biopsies performed before treatment (P = 0.03).
HLA-DQ reactivity was detected in 8 out of 10 biopsies, located to inflammatory cells, endothelial cells of capillaries, arterioles, and venules. In two sections immunoreactivity for HLA-DQ was present on fiber membranes, in both cases in very few fibers. After 3 months of treatment 6 out of 10 biopsies expressed HLA-DQ reactivity. Staining was located to inflammatory cells and endothelial cells, no positive staining being observed on muscle fiber membranes.
MHC Class I Immunoreactivity
All 10 patients investigated had a very strong and intense expression of HLA-ABC on almost all muscle fiber membranes and also in the cytoplasm of the muscle fibers (Figure 1F) ▶ , data previously presented. 28 The muscle tissue was heavily infiltrated with inflammatory cells and these were strongly positive for HLA-ABC (Figure 1F) ▶ . The staining intensity on muscle fibers varied between 2+ to 4+. The quantitative examination gave M, 47.5%; IQR, 26.4% (Figure 2B) ▶ . HLA-ABC expression was lower after treatment, M, 28.8%; IQR, 36.9%, but not statistically significant, as with HLA-DR.
Group 3: Patients in a Chronic, Inactive Phase of Myositis without Detectable Clusters of Inflammatory Cells
MHC Class II Immunoreactivity
In this group anti-HLA-DR reactivity with muscle fibers was noted in one of seven patients with PM and in one of four patients with DM, both scored 2+. Median value of HLA-DR staining assessed by image analysis was 2.2% with an interquartile range of 0.9% (Figure 2A) ▶ . HLA-DR reactivity was preferentially noted in areas that also stained for HLA-ABC. Endothelial cells and scattered inflammatory cells expressed HLA-DR antigens in all 11 cases.
HLA-DQ antigens were expressed in three patients with PM and two patients with DM, restricted to scattered inflammatory cells, endothelial cells of capillaries, arterioles, and venules but were not evident in any muscle fibers.
MHC Class I Immunoreactivity
Nine of 11 patients exhibited immunoreactivity for HLA-ABC on muscle fiber membranes (five of seven PM and four of four DM) as previously reported. 4 The positive muscle fibers displayed staining of sarcolemma and in some cases in the cytoplasm. A varying pattern and intensity of staining was observed, biopsies being scored between 1+ to 4+. Image analysis gave M = 4.7%, IQR = 5.5% (Figure 2B) ▶ . In some biopsies HLA-ABC was present in clusters of fibers and in others almost every fiber was positive. HLA-ABC-positive fibers were apparent in all parts of the biopsy and were not confined to areas of structural changes. Staining for HLA-ABC was also present in endothelial cells of capillaries, arterioles, and venules in all biopsies. No dissimilarities in HLA-ABC expression were observed between PM and DM patients.
Patients with Dystrophies and Neuropathies
MHC Class II Immunoreactivity
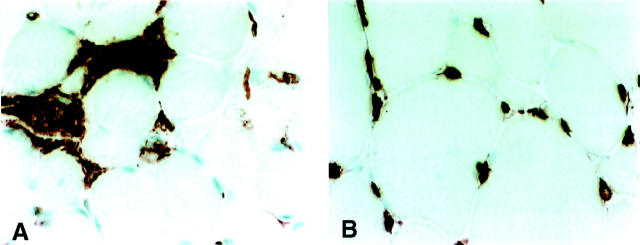
As control tissue we also included biopsies from 10 patients with other muscle disorders, five patients with neuropathies and five with muscle dystrophies. The staining pattern for DR and DQ antigens on muscle fibers from patients with neuropathies and dystrophies was similar to the expression in control biopsies from healthy individuals, no expression of HLA-DR being seen on the muscle fibers (Figure 4, A and B) ▶ . Quantification of HLA-DR by image analysis gave a median value of 2.4% and IQR of 0.6% for patients with neuropathies and dystrophies.
Figure 4.
Immunolocalisation of HLA-DR in muscle tissue from a patient with muscle dystrophy (A) and a patient with muscle neuropathy (B). Original magnifications, ×340. A: Despite the presence of intense mononuclear infiltrates similar to those of active myositis no HLA-DR expression was evident on muscle fiber membranes. B: No HLA-DR staining on muscle fiber despite morphologically changed muscle fibers.
MHC Class I Immunoreactivity
The endothelial expression of HLA-ABC in tissue was similar to the healthy control tissue but a larger number of biopsies also expressed the ABC antigens on muscle fiber surfaces. Three out of five patients with neurological disturbances and three out of five patients with muscle dystrophies exhibited fiber reactivity for HLA-ABC with intensities ranging from 1+ to 2+. Median value of image analysis for patients with neuropathies and dystrophies was 5.5% with an IQR of 2.4%.
Statistical Analysis
MHC Class II Immunoreactivity
A statistically significant difference in percentage of HLA-DR expression in the respective biopsies was observed between healthy controls and patients with clinical symptoms of myositis but without inflammatory infiltrates (P = 0.02), as well as between healthy controls and patients in an active phase of disease with inflammatory infiltrates (P = 0.008). No statistically significant difference was observed between normal controls and patients in a chronic, inactive phase of disease. When comparing patients with other muscle disorders with the different myositis groups a statistically significant difference in stained area for HLA-DR was only noted for patients in an active phase of disease (P = 0.006).
MHC Class I Immunoreactivity
The positively stained areas for HLA-ABC were significantly larger in the biopsies from myositis patients in all three groups compared to biopsies from healthy controls (P = 0.0002, P = 0.0001, P = 0.03, respectively). When comparing HLA-ABC expression from the different myositis groups with patients with other muscle disorders, a significantly larger area was stained in biopsies from myositis patients with clinical symptoms but without clusters of inflammatory cells in tissue (P = 0.0007) and in biopsies from active myositis patients (P = 0.0002), whereas no significant difference was observed with patients in a chronic, inactive phase of disease.
Laser Confocal Microscopy
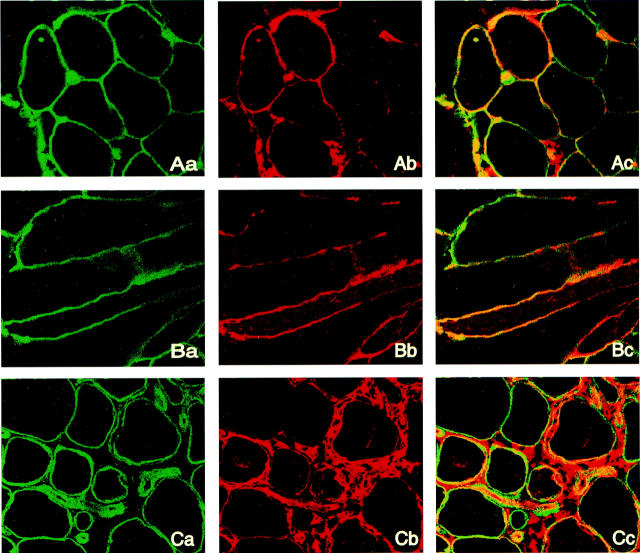
Double-immunofluorescent labeling with antibodies directed toward both HLA-DR and laminin (a membrane protein) gave a staining pattern for HLA-DR similar to the immunohistochemistry stainings. All muscle fiber membranes and vessel membranes were positive for laminin staining. When images were analyzed by laser confocal microscopy co-localization of HLA-DR and laminin expression was evident (Figure 5) ▶ . This co-localization was obvious in muscle tissue from patients in both histopathologically normal and morphologically changed muscle fibers such as atrophic or degenerating fibers. Double-stained fibers were present in areas both with and without inflammatory infiltrates. HLA-DR staining was not evenly distributed on the muscle fiber surface, as was the case with laminin, but rather a patchy staining pattern with accumulations in some areas of the membrane was observed (Figure 5) ▶ . This was particularly obvious using double staining as co-localization of HLA-DR and laminin was only detected on parts of the muscle fiber membrane.
Figure 5.
Double-immunofluorescent staining for HLA-DR and laminin, analyzed by laser confocal microscopy. Original magnifications, ×200. Lane a, sections positive for laminin, green fluorescence; lane b, sections stained for HLA-DR, red fluorescence; and lane c, co-localization of the two molecules to the muscle fiber membrane. The yellow color indicates co-localization whereas the green and red fluorescence indicates positive staining of laminin and HLA-DR respectively. A: A section from a PM patient, with histopathological normal fibers and no infiltration of inflammatory cells. B: Longitudinally sectioned biopsy from a PM patient showing laminin and HLA-DR staining along the fiber membrane. C: A section from a PM patient, with morphological changed fibers and infiltration of inflammatory cells.
Discussion
In this study we demonstrate that a significant proportion of skeletal muscle fibers express MHC class II as well as MHC class I in inflammatory myopathies and that MHC antigen expression can occur independently of the presence of inflammatory cell infiltration. Furthermore, there was no difference in MHC class II or MHC class I staining patterns between PM and DM patients.
Our finding of MHC class II antigens on muscle fibers in patients with active idiopathic inflammatory myopathy is in accordance with some previous studies, 14-17,22-24 but in contrast to other reports in which no MHC class II expression on muscle fibers was detected. 6,7,11,20,21,31 Considering the potentially important role that MHC class II antigens may have in the pathogenesis of inflammatory diseases, such as myositis, it should be essential to determine to which extent these previous divergent results are because of methodological differences or may also be explained by different patient groups analyzed.
Conventional indirect immunohistochemistry is a standard method for demonstration of MHC class II and I molecule expression in tissues and cell cultures. We used carefully selected monoclonal antibodies specific for MHC class II and for MHC class I, respectively. Optimal concentrations for primary antibodies as well as optimal dilutions for secondary reagents were determined by stepwise titration on normal human tonsils. In all experiments human tonsils were included as positive controls, a procedure that was not always included in some earlier studies that reported negative MHC class II staining. To exclude nonspecific staining in our experiments isotype-matched irrelevant control antibodies were included. For evaluation of staining all sections were read coded both for the descriptive evaluation with conventional microscopic enumeration and for the quantitative measurement by image analysis. In addition we were able to demonstrate, by double-immunofluorescent staining evaluated by laser confocal microscopy, that MHC class II is expressed on the muscle fiber membrane and is not an artifact caused by shedding of molecules from the contacting inflammatory cells.
Another critical point to consider is the selection of the patient population. In this study we have a unique patient material encompassing PM and DM patients in both acute and chronic phases of the disease and those with acute PM and DM includes patients both with and without inflammatory infiltrates present in muscle tissues. Interestingly, a common denominator for all three groups was the expression of both MHC class II and class I on muscle fiber membranes. The observed expression of MHC class II on muscle fibers in tissues without inflammatory infiltrates supports a distinct staining of the muscle fiber membrane that could in these cases not be because of an artifact caused by diffusion of stained molecules from adjacent activated inflammatory cells.
Both MHC class II and MHC class I expression on muscle fibers can be considered as induced because a significantly higher expression was observed in PM and DM patients compared to healthy controls. MHC class II antigen expression on muscle cells also seems to be restricted to the inflammatory myopathies because none of the cases with dystrophies or neuropathies, representing muscle tissues with inflammatory infiltrates or muscle fiber changes, exhibited this feature. Similar observations in tissue from patients with dystrophies or neuropathies, with absence of MHC class II antigens expressed on muscle fibers, have been reported by others. 14,23,24 Conversely, HLA-ABC antigen expression differs from the restricted HLA-DR expression because increased muscle fiber expression of HLA-ABC was detected in a variety of muscle disorders in both our study and in those of others. 6,7,12,14
Our finding of induced MHC class II and class I expression in inflammatory myopathies at various stages of disease activity is supportive evidence for a possible role played by these glycoproteins in the pathogenesis of these diseases. Expression of MHC class II and MHC class I on muscle fibers indicates that the muscle fibers are immunologically activated, but the mechanism underlying this expression as well as the consequences are as yet unknown. This is especially interesting when considering activated and phenotypically changed fibers in the absence of infiltration of inflammatory cells. It is therefore not likely that induction of MHC expression in these situations occurs in response to local lymphocyte products such as interferon-γ, as has been previously suggested. 8-10,13,32,33 Neither is a nonspecific induction of MHC class II expression in diseased skeletal muscle likely because no expression was recorded in biopsy specimens from muscular dystrophies or neuropathies.
Another interesting molecule with potential to up-regulate MHC class II and I molecules that has been detected in muscle tissue is IL-1α. 34,35 IL-1α is mainly expressed by endothelial cells of capillaries, arterioles, and venules in inflammatory myopathies 36 and IL-1α has also been observed in muscle tissue without any infiltration of inflammatory cells. 4 Thus one possibility is that MHC class II and class I expression is regulated in response to IL-1α. Proinflammatory cytokines, among them IL-1α, have recently been observed to influence MHC expression on human myoblasts and myotubes in in vitro experiments. 37
It is tempting to speculate that muscle cells that acquire MHC class II and MHC class I expression may in some way contribute to antigen presentation. Neuronal cells, another cell type that does not under normal conditions express MHC molecules, can be induced to express MHC class I and MHC class II. It has been hypothesized that there is a suppressed inducibility of MHC in functionally active neurons but with eg, viral damage, loss of bioelectric activity of cells could up-regulate MHC molecules for recognition by T cells and thus clearance of abnormal cells. 38 Whether differentiation of muscle fibers, with the expression of MHC on the fiber membrane, contributes to activation or to development of anergy in T lymphocytes is probably dependent on presence of classical co-stimulatory molecules. Such molecules are those within the B7 family, and these have not been demonstrated on muscle fibers to date. 39,40
The present study clearly demonstrates the presence of MHC class II and MHC class I expression on muscle fiber membranes in muscle tissue from PM and DM patients, both when inflammatory infiltrates are present and absent in muscle tissue. Our results indicate an important role for the muscle fibers in the pathogenesis of inflammatory myopathies. The expression of MHC class II and MHC class I does not seem to be a consequence of the infiltrating inflammatory cells. Rather, MHC class II and MHC class I might be an even earlier event than leukocyte infiltration in initiating and maintaining pathological events in myositis.
Acknowledgments
We thank Prof. Jan Lännergren and Joseph Bruton from the Department of Physiology and Pharmacology at Karolinska Institutet for use of their MRC 1024 laser confocal microscope and Assoc. Prof. Robert Harris from the Department of Medicine at Karolinska Institutet for linguistic advice.
Footnotes
Address reprint requests to Pernilla (Nyberg) Englund, Rheumatology Research Unit, CMM L8:04, Karolinska Hospital, 171 76 Stockholm, Sweden. E-mail: pernilla.englund@cmm.ki.se.
Supported by grants from Swedish Rheumatism Foundation, The King Gustaf V 80 Year Foundation, Börje Dahlin Foundation, Åke Wiberg Foundation, Professor Nanna Svartz-Foundation, Alex and Eva Wallström Foundation, Karolinska Institutet Research Foundation, and The Vardal Foundation for Health and Sciences and Allergy Research.
References
- 1.Mantegazza R, Bernasconi P, Confalonieri P, Cornelio F: Inflammatory myopathies and systemic disorders: a review of immunopathogenetic mechanisms and clinical features. J Neurol 1997, 244:277-287 [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC: Immunopathogenesis of inflammatory myopathies. Ann Neurol 1995, 37:S74-S86 [DOI] [PubMed] [Google Scholar]
- 3.Arahata K, Engel AG: Monoclonal antibody analysis of mononuclear cells in myopathies. I: Quantitation of subsets according to diagnosis and sites of accumulation and demonstration and counts of muscle fibers invaded by T cells. Ann Neurol 1984, 16:193-208 [DOI] [PubMed] [Google Scholar]
- 4.Nyberg P, Wikman A-L, Nennessmo I, Lundberg I: Increased expression of interleukin-1 alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol 2000, 27:940-948 [PubMed] [Google Scholar]
- 5.Olsen NJ, Park JH: Inflammatory myopathies: issues in diagnosis and management. Arthritis Care Res 1997, 10:200-207 [DOI] [PubMed] [Google Scholar]
- 6.Karpati G, Pouliot Y, Carpenter S: Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 1988, 23:64-72 [DOI] [PubMed] [Google Scholar]
- 7.Emslie-Smith AM, Arahata K, Engel AG: Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol 1989, 20:224-231 [DOI] [PubMed] [Google Scholar]
- 8.Michaelis D, Goebels N, Hohlfeld R: Constitutive and cytokine-induced expression of human leukocyte antigens and cell adhesion molecules by human myotubes. Am J Pathol 1993, 143:1142-1149 [PMC free article] [PubMed] [Google Scholar]
- 9.Hohlfeld R, Engel AG: Induction of HLA-DR expression on human myoblasts with interferon-gamma. Am J Pathol 1990, 136:503-508 [PMC free article] [PubMed] [Google Scholar]
- 10.Mantegazza R, Hughes SM, Mitchell D, Travis M, Blau HM, Steinman L: Modulation of MHC class II antigen expression in human myoblasts after treatment with IFN-gamma. Neurol 1991, 41:1128-1132 [DOI] [PubMed] [Google Scholar]
- 11.Rowe D, Isenberg DA, Beverley PC: Monoclonal antibodies to human leucocyte antigens in polymyositis and muscular dystrophy. Clin Exp Immunol 1983, 54:327-336 [PMC free article] [PubMed] [Google Scholar]
- 12.Appleyard ST, Dunn MJ, Dubowitz V, Rose ML: Increased expression of HLA ABC class I antigens by muscle fibres in Duchenne muscular dystrophy, inflammatory myopathy, and other neuromuscular disorders. Lancet 1985, 1:361-363 [DOI] [PubMed] [Google Scholar]
- 13.Isenberg DA, Rowe D, Shearer M, Novick D, Beverley PC: Localization of interferons and interleukin 2 in polymyositis and muscular dystrophy. Clin Exp Immunol 1986, 63:450-458 [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi I, Nerenberg M, Ijichi T, Fukunaga H, Arimura K, Usuki F, Kuriyama M, Osame M: Vacuolar myositis with expression of both MHC class I and class II antigens on skeletal muscle fibers. J Neurol Sci 1991, 106:60-66 [DOI] [PubMed] [Google Scholar]
- 15.Bartoccioni E, Gallucci S, Scuderi F, Ricci E, Servidei S, Broccolini A, Tonali P: MHC class I, MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression in inflammatory myopathies. Clin Exp Immunol 1994, 95:166-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima Y, Moriwaka F, Tashiro K: Temporal alterations of immunohistochemical findings in polymyositis. Int Med 1994, 33:263-270 [DOI] [PubMed] [Google Scholar]
- 17.Lindberg C, Oldfors A, Tarkowski A: Local T-cell proliferation and differentiation in inflammatory myopathies. Scand J Immunol 1995, 41:421-426 [DOI] [PubMed] [Google Scholar]
- 18.Fladby T, Kampman MT, Loseth S, Lindal S, Mellgren SI: Human leukocyte antigen class I in polymyositis: leukocyte infiltrates, regeneration, and impulse block. Muscle Nerve 1997, 20:1534-1540 [DOI] [PubMed] [Google Scholar]
- 19.Topaloglu H, Muntoni F, Dubowitz V, Sewry C: Expression of HLA class I antigens in skeletal muscle is a diagnostic marker in juvenile dermatomyositis. J Child Neurol 1997, 12:60-63 [DOI] [PubMed] [Google Scholar]
- 20.Figarella-Branger D, Pellissier JF, Bianco N, Devictor B, Toga M: Inflammatory and non-inflammatory inclusion body myositis. Characterization of the mononuclear cells and expression of the immunoreactive class I major histocompatibility complex product. Acta Neuropathol 1990, 79:528-536 [DOI] [PubMed] [Google Scholar]
- 21.Rowe DJ, Isenberg DA, McDougall J, Beverley PC: Characterization of polymyositis infiltrates using monoclonal antibodies to human leucocyte antigens. Clin Exp Immunol 1981, 45:290-298 [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson T, Henriksson KG, Klareskog L, Forsum U: HLA-DR expression, T lymphocyte phenotypes, OKM1 and OKT9 reactive cells in inflammatory myopathy. Muscle Nerve 1985, 8:419-425 [DOI] [PubMed] [Google Scholar]
- 23.Zuk JA, Fletcher A: Skeletal muscle expression of class II histocompatibility antigens (HLA-DR) in polymyositis and other muscle disorders with an inflammatory infiltrate. J Clin Pathol 1988, 41:410-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inukai A, Kuru S, Liang Y, Takano A, Kobayashi Y, Sakai M, Doyu M, Sobue G: Expression of HLA-DR and its enhancing molecules in muscle fibers in polymyositis. Muscle Nerve 2000, 23:385-392 [DOI] [PubMed] [Google Scholar]
- 25.Bohan A, Peter JB: Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975, 292:344-347 [DOI] [PubMed] [Google Scholar]
- 26.Bohan A, Peter JB: Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975, 292:403-407 [DOI] [PubMed] [Google Scholar]
- 27.Henriksson KG: “Semi-open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 1979, 59:317-323 [PubMed] [Google Scholar]
- 28.Lundberg I, Kratz AK, Alexanderson H, Patarroyo M: Decreased expression of interleukin-1alpha, interleukin-1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum 2000, 43:336-348 [DOI] [PubMed] [Google Scholar]
- 29.Frostegård JU, Ulfgren A-K, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK: Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999, 145:33-43 [DOI] [PubMed] [Google Scholar]
- 30.Robbins PA, Evans EL, Ding AH, Warner NL, Brodsky FM: Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Hum Immunol 1987, 18:301-313 [DOI] [PubMed] [Google Scholar]
- 31.Matsubara S, Hirai S, Sawa Y: Pulsed intravenous methylprednisolone therapy for inflammatory myopathies: evaluation of the effect by comparing two consecutive biopsies from the same muscle. J Neurol 1997, 76:75-80 [DOI] [PubMed] [Google Scholar]
- 32.Kalovidouris AE: The role of cytokines in polymyositis: interferon-gamma induces class II and enhances class I major histocompatibility complex antigen expression on cultured human muscle cells. J Lab Clin Med 1992, 120:244-251 [PubMed] [Google Scholar]
- 33.Bao SS, King NJ, dos Remedios CG: Elevated MHC class I and II antigens in cultured human embryonic myoblasts following stimulation with gamma-interferon. Immunol Cell Biol 1990, 68:235-241 [DOI] [PubMed] [Google Scholar]
- 34.Speiser P, Zeillinger R, Wiltschke C, Sedlak J, Chorvath B: IL-1 alpha induced, TNF alpha mediated HLA class II (DR) antigen up-regulation in a human ductal breast carcinoma cell line ZR-75–1. Neoplasma 1993, 40:137-140 [PubMed] [Google Scholar]
- 35.Wicks IP, Leizer T, Wawryk SO, Novotny JR, Hamilton J, Vitti G, Boyd AW: The effect of cytokines on the expression of MHC antigens and ICAM-1 by normal and transformed synoviocytes. Autoimmunity 1992, 12:13-19 [DOI] [PubMed] [Google Scholar]
- 36.Lundberg I, Ulfgren AK, Nyberg P, Andersson U, Klareskog L: Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum 1997, 40:865-874 [DOI] [PubMed] [Google Scholar]
- 37.Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P: A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol 1998, 113:407-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann H, Cavalié A, Jenne DE, Wekerle H: Induction of MHC class I genes in neurons. Science 1995, 269:549-552 [DOI] [PubMed] [Google Scholar]
- 39.Bernasconi P, Confalonieri P, Andreetta F, Baggi F, Cornelio F, Mantegazza R: The expression of co-stimulatory and accessory molecules on cultured human muscle cells is not dependent on stimulus by pro-inflammatory cytokines: relevance for the pathogenesis of inflammatory myopathy. J Neuroimmunol 1998, 85:52-58 [DOI] [PubMed] [Google Scholar]
- 40.Curnow SJ, Willcox N, Vincent A: Induction of primary immune responses by allogeneic human myoblasts: dissection of the cell types required for proliferation, IFNgamma secretion and cytotoxicity. J Neuroimmunol 1998, 86:53-62 [DOI] [PubMed] [Google Scholar]