Abstract
Angiogenesis is an important aspect of the vasculoproliferation found in the rheumatoid arthritic (RA) pannus. We have previously implicated members of the CXC chemokine family as potent angiogenic mediators in RA. We investigated the possibility that the sole member of the CX3C chemokine family, fractalkine (fkn), induces angiogenesis and that fkn might mediate angiogenesis in RA. Recombinant human fkn significantly induced migration of human dermal microvascular endothelial cells (HMVECs), a facet of the angiogenic response, in the pmol/L range in a concentration-dependent manner (P < 0.05). Fkn also induced the formation of significantly more endothelial tubes on Matrigel than did a negative control (P < 0.05). Fkn significantly induced 2.3-fold more blood vessel growth than control in the in vivo Matrigel plug assays (P < 0.05). We identified HMVEC expression of the fkn receptor, CX3CR1. Next, we determined if RA synovial fluid (SF)-induced angiogenesis was fkn-dependent. SFs from six RA patients immunodepleted of soluble fkn induced 56% less migration of HMVECs than did sham-depleted RA SFs (P < 0.05). In vivo, immunodepletion of fkn from six RA SFs significantly inhibited their angiogenic activity in Matrigel plug assays (P < 0.05). Immunodepletion of fkn from five RA synovial tissue homogenates inhibited their ability to induce angiogenesis in in vivo Matrigel plug assays (P < 0.05). These results establish a new function for fkn as an angiogenic mediator and suggest that it may mediate angiogenesis in RA.
Angiogenesis, the growth and proliferation of new blood vessels, is an important aspect of the vasculoproliferation found in tumor growth, wound repair, and inflammatory states such as rheumatoid arthritis (RA). 1-3 A number of mediators orchestrate the angiogenic process. These include members of the adhesion molecule superfamily, such as vascular cell adhesion molecule-1 (VCAM-1) or E-selectin 4,5 and chemokines such as interleukin (IL)-8. 6
The chemokines are mainly homologous 8- to 10-kd proteins that are subdivided into four families (C, CC, CXC, and CX3C) on the basis of the relative position of the cysteine residues in the mature protein. 7,8 Although the chemokines are generally thought to function as leukocyte attractants, we have previously identified the CXC chemokine, IL-8, as a mediator of angiogenesis. 6 Further studies have shown that the CXC chemokines containing the ELR motif, consisting of glutamic acid-leucine-arginine preceding the CXC sequence are not only chemotactic for neutrophils but are angiogenic. 9 The CC chemokine monocyte chemotactic protein-1 (MCP-1) has recently been identified as an inducer of endothelial cell (EC) chemotaxis in vitro 10 and as a mediator of inflammatory angiogenesis in vivo. 11 Viral CC chemokine-like proteins vMIP-I and vMIP-II, but not their cellular counterpart macrophage inflammatory protein-1α (MIP-1α), have also been shown to induce angiogenesis in vivo. 12
We have previously described fractalkine (fkn), the sole member of the CX3C chemokine family, as a mediator of inflammation in RA. 13 As many inflammatory mediators are also angiogenic mediators in RA synovial tissues (STs) and synovial fluids (SFs) we investigated the angiogenic properties of fkn. In this report we define fkn as a potent angiogenic mediator in RA. Fkn was named for its fractal geometry and is distinct from other chemokines in that it contains the CX3C motif with three amino acids between the two terminal cysteines. 14 Fkn also is unique in that it is a transmembrane protein displaying its chemokine domain perched on a long (241 amino acid) negatively charged mucin-like stalk that extends away from the cell surface. In addition, fkn is much larger than any other chemokine consisting of 373 amino acids and can be cleaved via a syndecan-like cleavage motif proximal to the membrane resulting in a soluble 95-kd glycoprotein. 14,15
Soluble fkn is a monomer that like other chemokines functions as a chemoattractant for natural killer cells, T lymphocytes, and monocytes. 14,16-19 Unlike other chemokines, membrane-bound fkn can directly mediate firm cell adhesion, and initiate leukocyte capture. 14,17,20 Specifically, membrane-bound fkn has been shown to be involved in adhesion of monocytes, T lymphocytes, and natural killer cells to ECs in vitro. 14,20,21 Fkn expression on human ECs is induced by the inflammatory cytokines IL-1 or tumor necrosis factor-α. 14 The mouse homologue of fkn, neurotactin, is up-regulated on ECs in inflamed brain in allergic encephalomyelitis. 22 Thus, in inflammation fkn can function both as a chemoattractant for leukocytes and as an EC adhesion molecule.
We show fkn to induce both EC migration and tube formation in vitro, to induce angiogenesis in vivo and to function as an angiogenic mediator present in RA SF and ST. Thus, in addition to being an adhesion molecule and chemoattractant for leukocytes, fkn functions as an angiogenic mediator.
Materials and Methods
Reagents
Human recombinant fkn, IL-8, epithelial neutrophil-activating protein-78 (ENA-78), basic fibroblast growth factor (bFGF), bovine acidic fibroblast growth factor (aFGF), goat polyclonal antibody (pAb) anti-human fkn, and control goat IgG were purchased from R&D Systems (Minneapolis, MN). Rabbit purified pAb anti-CX3CR1 was purchased from Imgenex (San Diego, CA). Dimethyl sulfoxide and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma (St. Louis, MO). Matrigel was purchased from Becton Dickinson (Bedford, MA). Enhanced chemiluminescence Western blotting detection reagents and goat horseradish peroxidase-conjugated antibody were obtained from Amersham Life Sciences (Arlington Heights, IL).
Cells
Human dermal microvascular endothelial cells (HMVECs) were purchased from BioWhittaker (San Diego, CA) and grown in EC growth medium (BioWhittaker) with 10% fetal bovine serum (FBS). Cell assays were performed in endothelial basal medium (BioWhittaker) supplemented with the appropriate amount of FBS for the assay and 0.1% gentamicin. THP-1 cells were purchased from the American Type Culture Collection (Manassas, VA) and grown in RPMI with 10% FBS.
EC Chemotaxis
HMVECs were cultured in endothelial cell growth medium containing 10% FBS. Chemotaxis was performed in 48-well blind-well chemotaxis chambers using gelatin-coated polycarbonate membranes with an 8-μm pore size (Neuroprobe, Cabin John, MD). 4,23 HMVECs (2.5 × 10 4 cells in 25 μl of endothelial basal medium containing 0.1% FBS) were added to the bottom wells. The chambers were inverted and incubated for 2 hours at 37°C allowing HMVEC attachment to the membrane. Fkn (10−12 to 10 2 nmol/L), phosphate-buffered saline (PBS), or positive control bFGF (60 nmol/L) were added to the top wells and the chambers incubated for 2 hours at 37°C. The membranes were removed, fixed in methanol, and stained with Diff-Quik (Baxter Scientific, Deerfield, IL). The number of cells that had migrated through the pores in the filter was counted per three high-power fields and each test group was assayed in quadruplicate. Checkerboard analyses were performed in a similar manner to chemotaxis assays except that the concentrations of fkn were varied in the upper and lower chambers.
Immunodepletion of Fkn in HMVEC Chemotaxis Assays
Fkn (10 1 nmol/L and 10−3 nmol/L) or PMA (60 nmol/L) were incubated with 10 to 25 μg/ml of either pAb anti-fkn or control goat IgG for 1 hour at 37°C. On completion of this neutralization period, the fkn/Ab and PMA/Ab combinations were assayed in the HMVEC chemotaxis assay as described above.
Immunodepletion of Fkn in RA SFs for HMVEC Chemotaxis Assays
SFs were isolated from six patients with RA during therapeutic arthrocentesis with Institutional Review Board approval. RA SF was diluted 1 to 50 with PBS and preincubated with 25 μg/ml of pAb anti-fkn or goat IgG control for 1 hour at 37°C. On completion of this neutralization period, the RA SF/Ab combination was assayed in the HMVEC chemotaxis assay as described above.
Formation of EC Tubes on Matrigel in Vitro
Matrigel was thawed on ice to prevent premature polymerization; 125 μl were plated into individual wells of eight-well chamber slides (Falcon, Bedford, MA) and allowed to polymerize at 37°C for 30 to 60 minutes. HMVECs were removed from culture by trypsinization and resuspended at 4 × 10 4 cells/ml in Medium 199 (Life Technologies, Inc., Grand Island, NY) containing 2% FBS and 200 μg/ml EC growth supplement. 24 Four hundred μl of cell suspension containing fkn, 50 nmol/L PMA, or vehicle control (PBS for fkn, PBS and dimethyl sulfoxide for PMA) were plated in each well and plates incubated for 16 to 18 hours at 37°C in a 5% CO2 humidified atmosphere. 25 Culture medium was aspirated off and cells were fixed with Diff-Quik Fixative and stained with Diff-Quik Solution II. Each chamber was photographed using a Polaroid Microcam camera at a final magnification of ×22. The number of tube branches was quantitated by a blinded observer. 26 Each concentration of control or test substance was assayed in triplicate.
HMVEC Proliferation Assay
HMVEC proliferation was quantified using a CellTiter 96 Aqueous assay (Promega, Madison, WI). 4,23 HMVECs in endothelial basal medium, 2% FBS, and 0.1% gentamicin were plated in 96-well plates (2500 cells/well) for 4 hours, allowing cells to adhere to the plates. The test substances, diluted in medium, were added to the appropriate wells and incubated according to the manufacturer’s suggested conditions of 37°C and 5% CO2 for 72 hours. After the incubation, viable cells were detected by their reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) into a formazan. The number of living cells in culture is directly proportional to the quantity of formazan product as measured at a wavelength of 490 nm. These absorbance values were compared to a positive control, bFGF, and a negative control, medium alone.
Reverse Transcriptase-Polymerase Chain Reaction (PCR) Amplification of HMVEC CX3CR1
HMVECs were cultured in endothelial cell growth medium (BioWhittaker) containing 10% FBS. Total RNA (1 μg) was prepared from HMVECs and first-strand cDNAs were synthesized using an oligo dT primer and AMV RT (Promega, Madison, WI). Subsequent amplification of CX3CR1 from HMVEC cDNA was performed using specific 5′ and 3′ primers: forward primer 5′CTCTATGACTTCTTTCCCAGTTGT3′; reverse primer 5′AGACACAAGGCTTTGGGATTC3′. 27 PCR cycling conditions were 95°C for 5 minutes followed by 30 cycles of 95°C for 1 minute, 52°C for 1 minute, and 72°C for 1 minute, and ended by 10 minutes at 72°C. Amplification products were characterized by size fractionation on 1% agarose gels.
Western Blot Analysis
HMVECs were cultured in endothelial cell growth medium (BioWhittaker) containing 10% FBS. THP-1 cells were cultured in RPMI containing 10% FBS. Cells were lysed in extraction buffer containing 10 mmol/L Tris, pH 7.4, 100 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L ethyleneglycoltetraacetic acid, 1 mmol/L NaF, 20 mmol/L NaP2O4, 2 mmol/L Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% sodium dodecyl sulfate, 0.5% deoxycholate, 1 mmol/L phenylmethyl sulfonyl fluoride, and protease inhibitors (1 tablet/10 ml, Proteinase inhibitor cocktail tablets; Boehringer Mannheim, Mannheim, Germany). Cell lysates were mixed 1:1 with Laemmli’s sample buffer and boiled for 5 minutes. Equal amounts of sample were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred from the gel onto nitrocellulose membranes using a Tris-glycine buffer. To block nonspecific binding, membranes were incubated with 5% milk in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 hour at room temperature. The blots were incubated with anti-human CX3CR1 Ab (Imgenex) diluted 1:500 in TBST and 5% milk at 4°C overnight. After washing with TBST, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (diluted 1:10,000) for 45 minutes at room temperature. An enhanced chemiluminescence detection system (ECL+, Amersham) was used to detect the CX3CR1 band.
Matrigel Plug Assay for Angiogenesis in Vivo
Female 8- to 12-week-old C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were each injected subcutaneously near their abdominal midline using a 30-gauge needle with 0.5 ml of Matrigel combined with either PBS, fkn (100 nmol/L), IL-8 (100 nmol/L), ENA-78 (100 nmol/L), or positive control bovine aFGF (63 pmol/L). 28,29 Seven to 10 days later, the mice were sacrificed and the Matrigel plugs were removed, weighed, and processed for histology or hemoglobin concentration determination. For histological analysis plugs were formalin-fixed, paraffin-embedded, cut into 4-μm sections, and Masson trichrome-stained. For hemoglobin determination, which correlates with the number of blood vessels, plugs were homogenized in 1 ml of distilled water. Hemoglobin concentration was determined either by the Drabkin method using a Drabkin’s reagent kit (Sigma) or using 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma).
Immunodepletion of Fkn in RA SFs for Matrigel Plug Angiogenesis Assays
SFs were isolated from six patients with RA during therapeutic arthrocentesis with Institutional Review Board approval. RA SFs were pooled and diluted 1 to 10 with PBS and preincubated with 25 μg/ml of pAb anti-fkn or goat IgG control for 1 hour at 37°C. On completion of this neutralization period, the RA SF/Ab combination was diluted again 1 to 10 with Matrigel and assayed in the in vivo Matrigel plug angiogenesis assay as described above.
Immunodepletion of Fkn in RA STs for Matrigel Plug Angiogenesis Assays
STs were obtained from five patients undergoing total joint replacement who met the American College of Rheumatology criteria for RA. 30-32 RA STs were homogenized in 1 ml of an anti-protease buffer as described. 33 Samples were sonicated, centrifuged at 900 × g for 15 minutes and filtered through a 1.2-μm pore size sterile Acrodisk (Gelman Sciences, Ann Arbor, MI), and frozen at −80°C until thawed for assay. ST homogenates were thawed, normalized, pooled, and preincubated with 25 μg/ml of pAb anti-fkn or goat IgG control for 1 hour at 37°C. On completion of this neutralization period, the RA ST homogenates/Ab combination was diluted 1 to 25 with Matrigel and assayed in the in vivo Matrigel plug angiogenesis assay as described above.
Statistical Analysis
Data were analyzed using Student’s t-test. P values <0.05 were considered significant.
Results
Fkn Induces HMVEC Migration (Chemotaxis and Chemokinesis) in Vitro
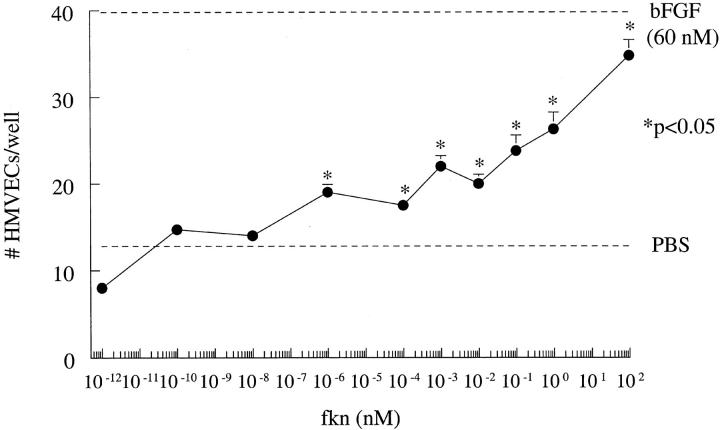
Fkn was assayed for its ability to induce HMVEC chemotaxis in vitro. Results of a representative experiment of four are shown in Figure 1 ▶ . Fkn induced chemotaxis in a concentration-dependent manner in the pmol/L and nmol/L concentration range. Fkn (10−1 pmol/L to 10 2 nmol/L) significantly increased EC chemotaxis over negative control PBS (P < 0.05). Checkerboard analysis was performed to determine whether fkn was chemotactic and/or chemokinetic for ECs. Representative results of four checkerboard assays showing fkn as both chemotactic and chemokinetic for HMVECs are shown in Table 1 ▶ .
Figure 1.
Fkn induces HMVEC migration. Results represent the mean number of cells/well ± SE of one representative assay of four. *, P < 0.05, significantly different from PBS control.
Table 1.
Checkerboard Analysis of Fkn-Mediated HMVEC Migration
| Lower chamber fkn* | Upper chamber fkn* | |||
|---|---|---|---|---|
| 0 nmol/L | 10−5 nmol/L | 10−3 nmol/L | 10−1 nmol/L | |
| 0 nmol/L | 8.5 ± 0.5 | 16.3 ± 0.3 | 16.0 ± 1.8 | 22.0 ± 0.9 |
| 10−5 nmol/L | 17.8 ± 2.6 | 15.0 ± 0.7 | 14.3 ± 1.4 | 18.3 ± 1.0 |
| 10−3 nmol/L | 16.8 ± 2.8 | 15.5 ± 0.7 | 13.3 ± 0.8 | 22.0 ± 1.6 |
| 10−1 nmol/L | 18.0 ± 0.7 | 18.5 ± 1.9 | 13.0 ± 1.6 | 18.0 ± 0.5 |
*fkn (10−5 to 10−1 nmol/L) was assayed for EC migration. The results represent 12 high-power fields (×400) per sample and are expressed as the number of cells ± SE per replicate well. This results represents one of four experiments. Positive control migration in response to bFGF (60 nmol/L) was a mean of 26 cells/well. Negative control migration in response to PBS was nine cells/well.
Fkn-Induced Chemotactic Activity for HMVECs Is Decreased by Immunodepletion of Fkn
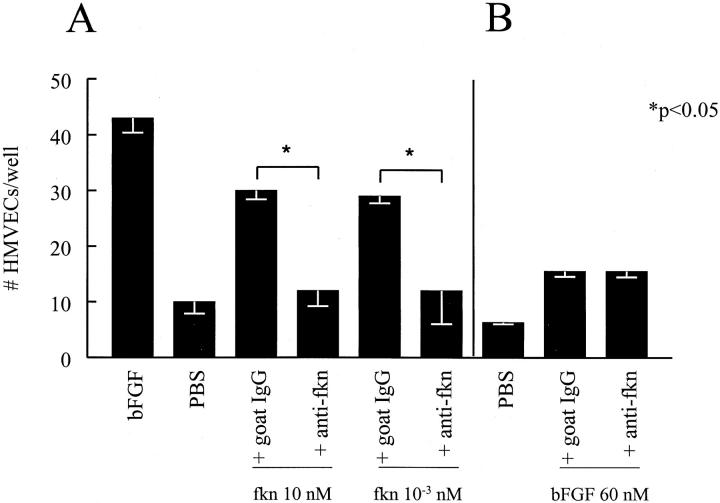
We next determined whether the chemokine domain of fkn was responsible for the EC chemotactic ability of fkn. Fkn was incubated with 25 μg/ml of an antibody specific for the CX3C chemokine domain of fkn and then assayed for HMVEC chemotaxis ability. Figure 2A ▶ shows that at concentrations from 1 pmol/L to 10 nmol/L of fkn, the anti-CX3C domain antibody completely inhibited fkn-induced HMVEC migration (P < 0.05). This inhibition of migration by anti-CX3C domain antibody was specific for fkn-induced HMVEC migration as bFGF-induced migration was not affected by incubation with this antibody (Figure 2B) ▶ .
Figure 2.
Anti-fkn inhibits fkn-induced, but not bFGF-induced HMVEC migration. A: Anti-fkn inhibited fkn-induced HMVEC migration. B: Anti-fkn did not inhibit bFGF-induced HMVEC migration. Results represent the mean number of cells/well ± SE of one of three similar assays. *, P < 0.05, significantly different from goat IgG control.
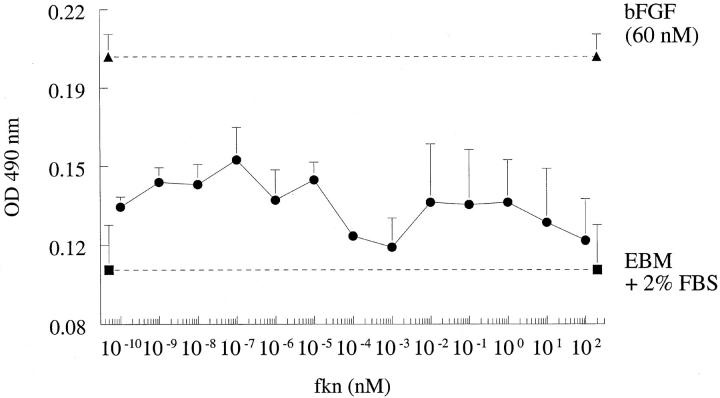
Fkn Does Not Induce HMVEC Proliferation in Vitro
We next assessed the ability of fkn to act as a mitogen for ECs in vitro. When assayed in concentrations of 10−10 to 10 2 nmol/L, fkn did not induce a mitogenic response, in contrast to 60 nmol/L of bFGF that induced potent EC proliferation. We have shown previously that angiogenic soluble adhesion molecules such as soluble VCAM-1 (sVCAM-1) or sE-selectin did not induce EC mitogenesis in vitro although they were potently angiogenic in vivo. 4 Results of a representative experiment of four experiments is shown in Figure 3 ▶ .
Figure 3.
Fkn does not induce HMVEC proliferation. Results represent the mean absorbance of quadruplicate wells ± SE of one representative assay of four assays. No values were significantly different (P < 0.05) from media alone.
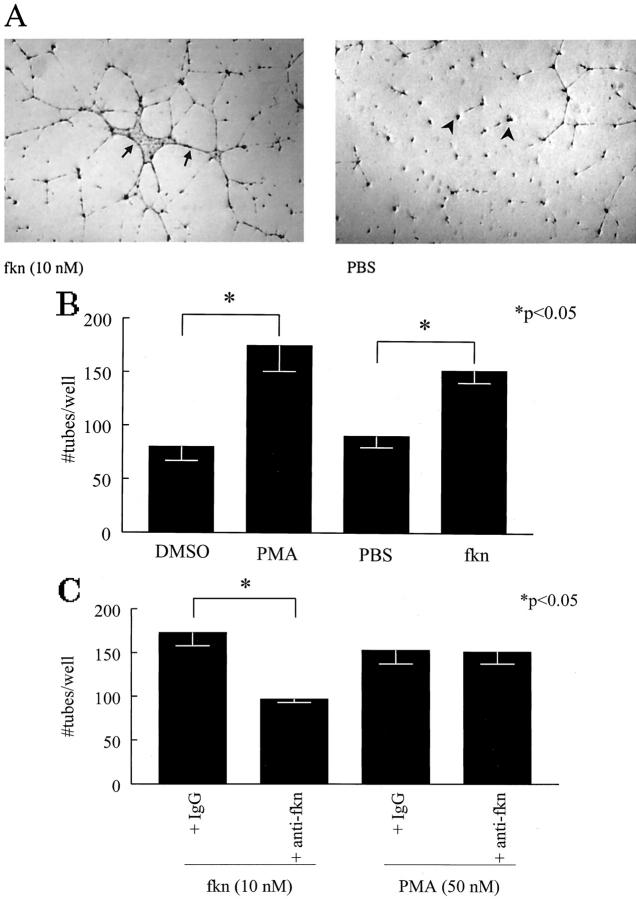
Fkn-Induced HMVEC Tube Formation on Matrigel in Vitro
Tube formation, one facet of the angiogenic response, can be assayed for in vitro by testing the ability of HMVECs plated on Matrigel to form tubes. We investigated the ability of fkn to induce tube formation on Matrigel in eight-well chamber slides. The results of a representative experiment of four experiments is shown in Figure 4 ▶ . Figure 4A ▶ shows a photomicrograph of tube formation induced by fkn. In contrast, PBS did not induce EC tube formation. To quantify tube formation in the Matrigel matrices, a blinded observer counted EC tubes in each experimental well. Figure 4B ▶ shows EC tube counts for fkn-induced tube formation along with tube counts induced by positive control PMA and the vehicle controls dimethyl sulfoxide and PBS. Fkn induced significantly more EC tube formation than negative control PBS (152 ± 11.7 versus 90 ± 10.7 tubes/well; P < 0.05, n = 4). We have also used this technique to test the ability of the angiogenic chemokines, IL-8 and ENA-78, to induce EC tube formation. Both IL-8 and ENA-78 induced EC tube formation greater than PBS controls (data not shown). Next, to help discern whether fkn-induced tube formation was because of the chemokine domain or to the mucin stalk, we incubated fkn with an anti-CX3C domain antibody. Anti-fkn significantly inhibited fkn-induced tube formation over isotype control antibody (P < 0.05, n = 3) (Figure 4C) ▶ . This inhibition of tube formation by anti-fkn was specific to fkn-induced formation as PMA-induced tube formation was not affected by incubation with the antibody (Figure 4C) ▶ .
Figure 4.
Fkn induces EC tube formation in vitro. A: Representative assay showing fractalkine-induced HMVEC tube formation and PBS control (original magnification, ×22). Individual tubes are shown in fractalkine-treated well (arrows) and non-tube-forming ECs are identified in a PBS-treated well (arrowheads). B: Fkn and PMA both induce HMVEC tube formation relative to their negative controls. C: Anti-fkn inhibits fkn-induced, but not PMA-induced HMVEC tube formation. Values represent the mean number of HMVEC tube branches/well ± SE for three or four assays. *, P < 0.05, significantly different from vehicle control.
HMVECs Express CX3CR1 in Vitro
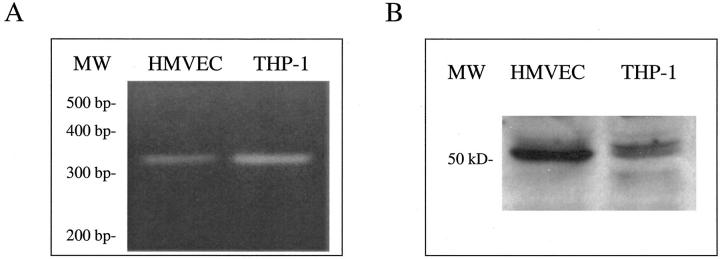
To better understand how fkn may interact with ECs to induce migration (chemotaxis and chemokinesis) and tube formation, we tested whether the only known fkn receptor, CX3CR1, was expressed by ECs. Previous reports have identified endothelial expression of fkn but did not examine expression of its receptor CX3CR1. Reverse transcriptase-PCR was performed on HMVEC cDNAs along with cDNAs from THP-1 cells, a myeloid cell line previously reported to express high amounts of CX3CR1 mRNA. 34 PCR products were synthesized using specific human CX3CR1 primers that amplify a 320-bp fragment. As shown in Figure 5A, a ▶ 320-bp PCR product was amplified from both HMVEC cDNAs as well as the positive control, THP-1 cDNAs, indicating EC expression of CX3CR1. Next, Western blot analysis was performed to demonstrate endothelial CX3CR1 protein expression. Cell lysates were prepared from both HMVECs and THP-1 cells and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Western blotting, performed with a IgG-purified pAb specific for human CX3CR1, revealed a band of the correct size (50 kd) in both the EC and positive control, THP-1 cell, lanes (Figure 5B) ▶ . 35
Figure 5.
HMVECs express CX3CR1. A: Agarose gel showing 320-bp CX3CR1 reverse transcriptase-PCR products from HMVECs and THP-1 cells. B: Western blot showing 50-kd CX3CR1 band in both HMVECs and THP-1 cells. MW, molecular weight markers.
Fkn-Induced Angiogenesis in Matrigel Plugs in Vivo
To determine whether fkn functions as an angiogenic mediator in vivo, we used the mouse Matrigel plug assay. Matrigel plugs containing negative control PBS, fkn, angiogenic chemokines IL-8 and ENA-78, or positive control aFGF were implanted subcutaneously into the abdomen of mice. A representative photomicrograph of a plug fixed and Masson trichrome-stained is shown in Figure 6A ▶ . In fkn-containing plugs marked new blood vessel growth can be seen. In contrast, minimal blood vessel growth was induced by negative control PBS. Figure 6B ▶ shows the hemoglobin content normalized to the weight of the Matrigel plugs. The hemoglobin content correlates with the number of blood vessels in the plugs. By this method, fkn induced significantly more blood vessels in the Matrigel plugs than did negative control PBS (0.77 ± 0.15 versus 0.33 ± 0.08 g/dl of hemoglobin/mg of plug weight, respectively; n = 18, P < 0.05). To compare the relative angiogenic potency of fkn to other known angiogenic chemokines, IL-8 and ENA-78 were also tested in the Matrigel plug model. The relative angiogenic potencies for fkn, IL-8, and ENA-78 as a percentage of the angiogenic potency of the positive control, aFGF, are shown in Figure 6C ▶ . Fkn exhibited 78% of the angiogenic potency of aFGF, whereas IL-8 and ENA-78 exhibited 65% and 44%, respectively (n = 7 to 9).
Figure 6.
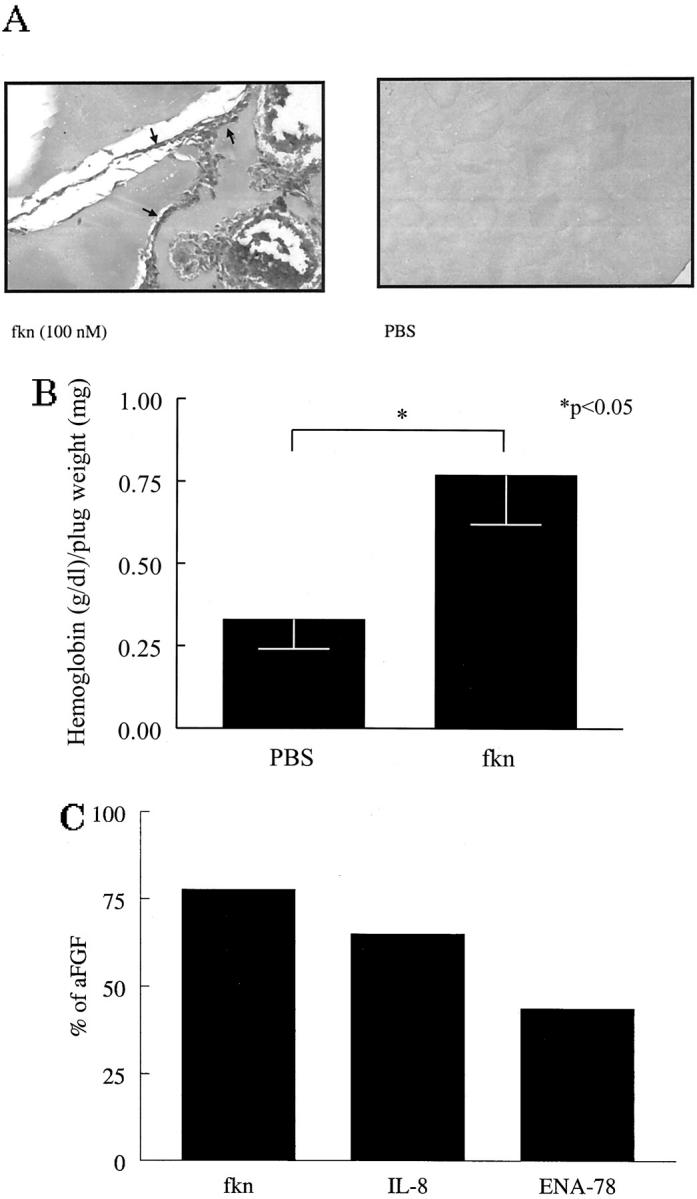
Fkn induces angiogenesis in Matrigel plugs in vivo. A: Representative assay showing Masson trichrome staining of blood vessels in Matrigel plugs. Fkn-induced blood vessel formation compared to PBS control (original magnification, ×66). Blood vessels are shown in fractalkine-treated well (arrows). B: Values represent the concentration of hemoglobin (g/dl)/Matrigel plug weight (mg) ± SE for 18 assays. C: Values represent the concentration of hemoglobin (g/dl)/Matrigel plug weight (mg) as a percentage of the positive control aFGF-induced hemoglobin (g/dl)/Matrigel plug weight (mg) for between seven to nine assays. *, P < 0.05 significantly different from vehicle control.
RA SF Chemotactic Activity for HMVECs Is Decreased by Immunodepletion of Fkn
To determine whether fkn has biological relevance in a disease characterized by angiogenesis, SFs from six patients with RA were immunodepleted of fkn and assayed for their HMVEC chemotactic activity. Results of immunodepletion experiments are shown in Table 2 ▶ . Although RA SF was potently chemotactic for HMVECs, immunodepletion of fkn resulted in significantly decreased (56.1 ± 2.4%, mean ± SE) chemotactic ability for HMVECs relative to immunodepletion with isotype control antibody (P < 0.05).
Table 2.
Migration of HMVECs in Response to RA SF Incubated in the Presence and Absence of Anti-Fkn
| Patient | Mean cells/well* | % Suppression† | |
|---|---|---|---|
| Goat IgG1 | Anti-fkn | ||
| 1 | 35 | 17 | 51.4 |
| 2 | 31 | 15 | 51.6 |
| 3 | 28 | 11 | 60.7 |
| 4 | 25 | 9 | 64.0 |
| 5 | 32 | 16 | 50.0 |
| 6 | 29 | 12 | 58.6 |
| Mean± SE | 56.1± 2.4 | ||
*RA SFs were assayed for their ability to induce migration of HMVECs. The results represent mean number of cells per well as measured in three high-power fields (×400). Each sample was tested in two to four wells. The ability of anti-fkn antibodies (25 μg/ml) to neutralize the migratory properties of RA SF was determined as percent suppression of migration compared with that of isotype control antibody. Positive control migration in response to bFGF (60 nmol/L) was a mean of 44 cells/well. Negative control migration in response to PBS was 12 cells/well.
†P value for percent suppression compared to isotype control matched IgG in all patient samples assayed was <0.05.
RA SF Angiogenic Activity Is Decreased by Immunodepletion of Fkn
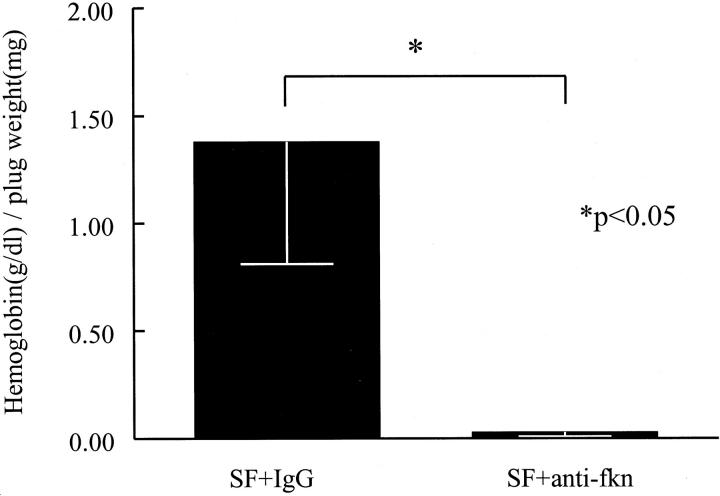
To determine whether fkn is responsible for a portion of the angiogenic properties of RA SF, SFs from six RA patients were pooled, immunodepleted of fkn, and assayed for angiogenic activity in vivo. Fkn-immunodepleted SFs were diluted in Matrigel and injected subcutaneously into mice. Results of these immunodepletion experiments are shown in Figure 7 ▶ . The angiogenesis induced by the pooled SFs was significantly decreased by immunodepleting fkn compared to sham immunodepletion (0.028 ± 0.02 versus 1.38 ± 0.57 g/dl of hemoglobin/mg of plug weight, n = 12, respectively, P < 0.05).
Figure 7.
RA SF angiogenic activity is decreased by immunodepletion of fkn in vivo. SFs from six RA patients were pooled, immunodepleted of fkn, and assayed for their angiogenic ability in Matrigel plugs implanted in mice. Results represent the mean concentration of hemoglobin (g/dl)/Matrigel plug weight (mg) ± SE. *, P < 0.05, significantly different from IgG control.
RA ST Angiogenic Activity Is Decreased by Immunodepletion of Fkn
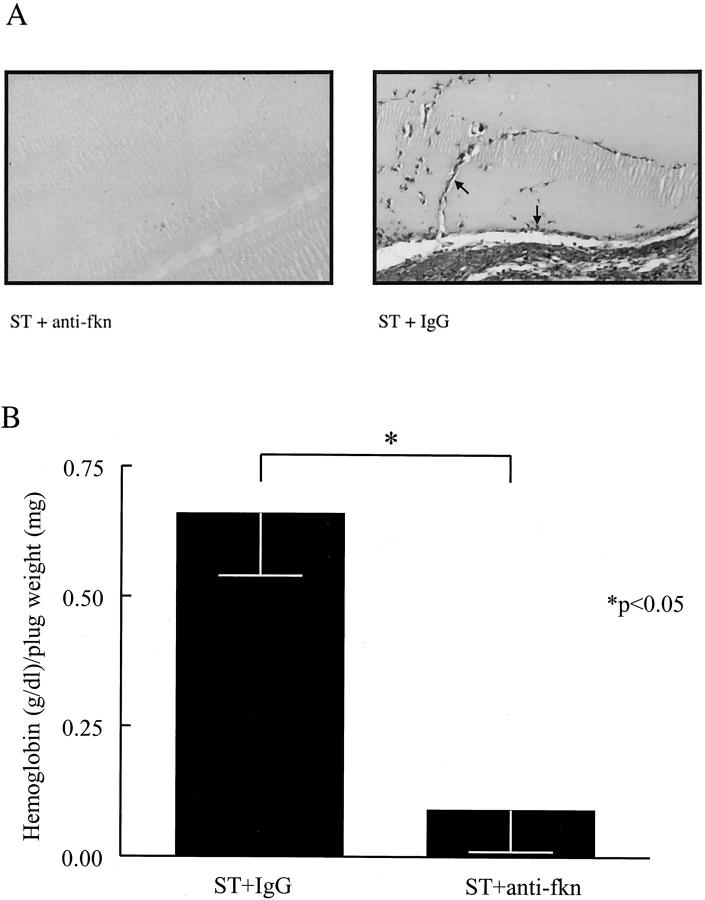
To determine whether fkn is responsible for a portion of the angiogenic properties of RA ST homogenates, ST homogenates from five RA patients were pooled, immunodepleted of fkn, and assayed for in vivo angiogenic activity. Representative photomicrographs of these Matrigel plugs fixed and Masson trichrome-stained are shown in Figure 8A ▶ . Matrigel plugs containing pooled RA ST homogenates have significant new blood vessel growth, whereas Matrigel plugs containing RA ST homogenate immunodepleted with anti-fkn have minimal blood vessel growth. Figure 8B ▶ shows the hemoglobin content normalized to the weight of the Matrigel plugs. The angiogenesis induced by the pooled ST homogenates was significantly decreased by immunodepleting fkn compared to sham immunodepletion (0.09 ± 0.08 versus 0.66 ± 0.12 g/dl of hemoglobin/mg of plug weight, n = 12, respectively, P < 0.05).
Figure 8.
RA ST angiogenic activity is decreased by immunodepletion of fkn in vivo. ST homogenates prepared from five RA patients were pooled, immunodepleted of fkn, and assayed for their angiogenic ability in Matrigel plugs implanted in mice. A: Representative assay showing Masson trichrome staining of blood vessels in Matrigel plugs. Lack of RA ST-induced blood vessel formation after immunodepletion of fkn with anti-fkn compared to IgG control (original magnification, ×66). Blood vessels are indicated by arrows. B: Results represent the mean concentration of hemoglobin (g/dl)/Matrigel plug weight (mg) ± SE. *, P < 0.05, significantly different from IgG control.
Discussion
Chemokines are divided into four families CXC, CC, C, and CX3C. Members of the CXC chemokine family containing the amino acid sequence Glu-Leu-Arg (the ELR motif) and the CC chemokines MCP-1, vMIP-I, and vMIP-II have been shown to be angiogenic. 9-12 Here we show fkn inducing HMVEC migration in a concentration-dependent manner from as low as 10−6 nmol/L upwards to 10 2 nmol/L where it had similar activity to the potent EC chemoattractant bFGF (60 nmol/L) (Figure 1) ▶ . In Table 1 ▶ , a checkerboard assay shows fkn to induce EC migration both when it is added directly to the cells and when it is added to the chamber on the other side of the membrane, indicating that in addition to inducing directional migration (chemotaxis), fkn stimulates ECs to randomly migrate (chemokinesis). Fkn (100 nmol/L) induced ECs to form tubes in Matrigel in vitro at the same rate as the angiogenic chemokines, IL-8 and ENA-78 (10 μmol/L) (data not shown), and PMA (50 μmol/L), a strong inducer of EC differentiation and EC tube formation (Figure 4B) ▶ . 25 We also show the angiogenic properties of fkn in vivo, as fkn induced angiogenesis in Matrigel plugs inserted in mice comparable in potency to the known angiogenic chemokines IL-8 and ENA-78 (Figure 6) ▶ . Thus, fkn is the first CX3C chemokine shown to function as an inducer of EC migration and angiogenesis.
Because fkn contains both a chemokine domain and a mucin stalk resembling an adhesion molecule, we questioned which domain was responsible for its EC migration and tube-forming properties. We found that the chemokine domain of fkn is necessary and that the mucin domain is not sufficient for inducing EC migration and tube formation on Matrigel, as an antibody specific for the chemokine domain completely inhibited fkn-induced HMVEC migration and tube formation (Figure 2 ▶ and Figure 4C ▶ ). Because fkn does not contain an ELR motif, the mechanism by which fkn induces EC migration seems unique from that of the other angiogenic chemokines.
Chemokines have been shown to induce EC chemotaxis through binding their EC chemokine receptors. Moore and co-workers 36,37 showed that angiogenesis induced by the CXC chemokines, IL-8, KC, MIP-2, and ENA-78 was mediated through the EC chemokine receptor CXCR2. Fiel and Augustin 38 showed that SDF-1-CXCR4 interactions are involved in bovine aortic EC chemotaxis. Weber and co-workers 10 inhibited MCP-1-induced EC chemotaxis with a CCR2, the MCP-1 receptor, antagonist. Here we demonstrate CX3CR1 mRNA and protein expression in HMVECs in culture (Figure 5) ▶ . We have also demonstrated by immunohistochemistry EC expression of CX3CR1 in ST in adjuvant-induced arthritic rats. 13 Thus, it is possible that fkn-induced EC migration is mediated through interaction of fkn with its EC receptor CX3CR1.
The angiogenic properties of fkn are similar in potency to other angiogenic mediators. Fkn induced a doubling in the amount of EC migration, a technical indicator of potent migration, at 1 nmol/L and reached statistical significance at concentrations as low as 10−6 nmol/L. Fkn induced angiogenesis in vivo at 100 nmol/L. These concentrations of fkn are comparable to concentrations of the CXC chemokines, IL-8, ENA-78, and growth-related oncogene-α (GRO-α), shown to induce EC chemotaxis and angiogenesis. We previously showed IL-8 to induce a doubling in human umbilical vein EC chemotaxis at 1.25 nmol/L and to induce angiogenesis at 10 nmol/L. 6 ENA-78 induced bovine adrenal gland capillary EC chemotaxis at as low as 5 nmol/L and ENA-78 and GRO-α induced angiogenesis in the rat cornea neovascularization assay at 10 nmol/L. 9 Thus, fkn is a powerful chemoattractant for ECs and is angiogenic in vivo in the nmol/L range, similar to other angiogenic CXC chemokines.
Angiogenic factors function to form intact microvessels by inducing EC migration, proliferation, elongation, orientation, and differentiation resulting in lumen formation, re-establishment of the basement membrane and anastomosis with other vessels. We and others have reported ELR motif-containing CXC chemokines such as IL-8 and GRO-α to induce both EC chemotaxis and proliferation. 6,39 We also have recently shown the cytokine IL-13 to be chemotactic for ECs but not to induce EC proliferation. 23 Here we show that fkn induces EC migration (chemotaxis and chemokinesis), but not proliferation. In this way, fkn acts in the same manner as other angiogenic mediators by inducing some facets of the angiogenic process while having no effect on others. In this work, we also demonstrated that fkn can induce ECs to form tubes on Matrigel in vitro and to form functional blood vessels in Matrigel plugs in vivo, thus establishing its angiogenic properties.
RA ST is replete with newly formed blood vessels in response to the increased demand for nutrients and oxygen by the proliferating pannus tissue. 1-3 The level of RA ST vascularity correlates with more severe clinical and inflammatory scores and is greater than degrees of vascularity seen in osteoarthritis ST. 1,40,41 RA SF and ST homogenates are potent EC chemotactic agents and contain several mediators that are chemotactic for ECs including the chemokines, IL-8, ENA-78, and GRO-α. 4,42-47 In another report, we have shown RA SF and ST contain greater levels of antigenic fkn than SF and ST from patients with osteoarthritis or other forms of arthritis. 13 We report here that RA SF immunodepleted of fkn has significantly reduced chemotactic activity for ECs and that RA SF and ST homogenates immunodepleted of fkn have significantly reduced angiogenic activity. The complete nature of the reduction in RA SF- and ST-induced migration and angiogenesis with anti-fkn treatment is possibly because of the complex sharing of chemokine receptors and signaling molecules between different chemokines or possibly synergy between the different angiogenic mediators. In this manner, immunodepletion of an individual factor may have a profound impact on the total angiogenic response, because of the unique dynamics of stimulating cells with intricate biological tissues. Our findings suggest an important role for fkn in inducing EC migration (chemotaxis and chemokinesis) and angiogenesis in RA and identify a new potential target for treating the disease.
In summary, fkn, the sole member of the CX3C chemokine family, induces EC migration (chemotaxis and chemokinesis), EC tube formation, and blood vessel formation in vivo. RA SF and ST homogenates’ angiogenic activities are in part because of fkn. We hypothesize that in a disease state such as RA, fkn may act in an autocrine manner. Specifically, two prominent proinflammatory cytokines in RA, IL-1β, and tumor necrosis factor-α, activate ECs to produce fkn on their surface. Next, EC surface fkn is released by enzymatic cleavage and the resulting soluble fkn binds EC CX3CR1 inducing EC migration and synovial angiogenesis.
Acknowledgments
We thank our colleague Dr. David Stulberg and the Cooperative Human Tissue Network for supplying the synovial tissues, Dr. Richard Pope for supplying the synovial fluids, and Vanessa Jones for her technical expertise.
Footnotes
Address reprint requests to Dr. Alisa E. Koch, 303 East Chicago Ave., Ward 3-315, Chicago, IL 60611. E-mail: ae-koch@nwu.edu.
Supported by the National Institute of Health (grants AR30692, HL-58695, AI40987), the Chicago Chapter of the Arthritis Foundation, the Gallagher Professorship for Arthritis Research, and funds from the Veteran’s Administration Research Service.
M. V. V. and J. M. W. both contributed equally to the work reported in this article.
References
- 1.Colville-Nash PR, Scott DL: Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann Rheum Dis 1992, 51:919-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szekanecz Z, Szegedi G, Koch AE: Angiogenesis in rheumatoid arthritis: pathogenic and clinical significance. J Invest Med 1998, 46:27-41 [PubMed] [Google Scholar]
- 3.Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995, 1:27-31 [DOI] [PubMed] [Google Scholar]
- 4.Koch AE, Halloran MM, Haskell CJ, Shah MR, Polverini PJ: Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature 1995, 376:517-519 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen M, Corless CL, Kraling BM, Tran C, Atha T, Bischoff J, Barsky SH: Vascular expression of E-selectin is increased in estrogen-receptor-negative breast cancer: a role for tumor-cell-secreted interleukin-1 alpha. Am J Pathol 1997, 150:1307-1314 [PMC free article] [PubMed] [Google Scholar]
- 6.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM: Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258:1798-1801 [DOI] [PubMed] [Google Scholar]
- 7.Luster AD: Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 338:436-445 [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D: The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 1995, 270:27348-27357 [DOI] [PubMed] [Google Scholar]
- 10.Weber KS, Nelson PJ, Grone H-J, Weber C: Expression of CCR2 by endothelial cells: implications for MCP-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol 1999, 19:2085-2093 [DOI] [PubMed] [Google Scholar]
- 11.Goede V, Brogelli L, Ziche M, Augustin HG: Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer 1999, 82:765-770 [DOI] [PubMed] [Google Scholar]
- 12.Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, Weiss RA: Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 1997, 278:290-294 [DOI] [PubMed] [Google Scholar]
- 13.Ruth JH, Volin MV, Haines GK, III, Woodruff DC, Katschke KJ, Jr, Woods JM, Park CC, Morel JCM, Koch AE: Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant induced arthritis. Arthritis Rheum 2001, 44:1568-1581 [DOI] [PubMed] [Google Scholar]
- 14.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385:640-644 [DOI] [PubMed] [Google Scholar]
- 15.Schall T: Fractalkine—a strange attractor in the chemokine landscape. Immunol Today 1997, 18:147. [DOI] [PubMed] [Google Scholar]
- 16.Mizoue LS, Bazan JF, Johnson EC, Handel TM: Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry 1999, 38:1402-1414 [DOI] [PubMed] [Google Scholar]
- 17.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O: Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91:521-530 [DOI] [PubMed] [Google Scholar]
- 18.Al-Aoukaty A, Rolstad B, Giaid A, Maghazachi AA: MIP-3alpha, MIP-3-beta and fractalkine induce the locomotion and the mobilization of intracellular calcium, and activate the heterotrimeric G proteins in human natural killer cells. Immunology 1998, 95:618-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanazawa N, Nakamura T, Tashiro K, Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S, Honjo T: Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur J Immunol 1999, 29:1925-1932 [DOI] [PubMed] [Google Scholar]
- 20.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, Patel DD: Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med 1998, 188:1413-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haskell CA, Cleary MD, Charo IF: Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem 1999, 274:10053-10058 [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D: Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387:611-617 [DOI] [PubMed] [Google Scholar]
- 23.Halloran MM, Haskell CJ, Woods JM, Hosaka S, Koch AE: Interleukin-13 is an endothelial chemotaxin. Pathobiology 1997, 65:287-292 [DOI] [PubMed] [Google Scholar]
- 24.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D’Orazi G, Murphy AN, Bird RE, Hoythya M, Fuerst TR, French DL, Quigley JP, Kleinman HK: Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol 1993, 156:235-246 [DOI] [PubMed] [Google Scholar]
- 25.Kinsella JL, Grant DS, Weeks BS, Kleinman HK: Protein kinase C regulates endothelial cell tube formation on basement membrane matrix, Matrigel. Exp Cell Res 1992, 199:56-62 [DOI] [PubMed] [Google Scholar]
- 26.Gately S, Twardowski P, Stack MS, Patrick M, Boggio L, Cundiff DL, Schnaper HW, Madison L, Volpert O, Bouck N, Enghild J, Kwaan HC, Soff GA: Human prostate carcinoma cells express enzymatic activity that converts human plasminogen to the angiogenesis inhibitor, angiostatin. Cancer Res 1996, 56:4887-4890 [PubMed] [Google Scholar]
- 27.Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P, Theodorou I, Combadiere C: Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science 2000, 287:2274-2277 [DOI] [PubMed] [Google Scholar]
- 28.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR: A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 1992, 67:519-528 [PubMed] [Google Scholar]
- 29.Johns A, Freay AD, Fraser W, Korach KS, Rubanyi GM: Disruption of estrogen receptor gene prevents 17 beta estradiol-induced angiogenesis in transgenic mice. Endocrinology 1996, 137:4511-4513 [DOI] [PubMed] [Google Scholar]
- 30.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, III, Mankin H, McShane DJ, Medsger TJ, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F: Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 1986, 29:1039-1049 [DOI] [PubMed] [Google Scholar]
- 31.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Feldman D, Greenwald R, Hochberg M, Howell D, Ike R, Kapila P, Kaplan D, Koopman W, Marino C, McDonald E, McShane DJ, Medsger TJ, Michel B, Murphy WA, Osial T, Ramsey-Goldman R, Rothschild B, Wolfe F: The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 1991, 34:505-514 [DOI] [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TAJ, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG: The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988, 31:315-324 [DOI] [PubMed] [Google Scholar]
- 33.Keane MP, Arenberg DA, Lynch JP, III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM: The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997, 159:1437-1443 [PubMed] [Google Scholar]
- 34.Raport CJ, Schweickart VL, Eddy RL, Jr, Shows TB, Gray PW: The orphan G-protein-coupled receptor-encoding gene V28 is closely related to genes for chemokine receptors and is expressed in lymphoid and neural tissues. Gene 1995, 163:295-299 [DOI] [PubMed] [Google Scholar]
- 35.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H: CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol 2000, 164:4313-4320 [DOI] [PubMed] [Google Scholar]
- 36.Moore BB, Arenberg DA, Addison CL, Keane MP, Strieter RM: Tumor angiogenesis is regulated by CXC chemokines. J Lab Clin Med 1998, 132:97-103 [DOI] [PubMed] [Google Scholar]
- 37.Moore BB, Arenberg DA, Addison CL, Keane MP, Polverini PJ, Strieter RM: CXC chemokines mechanism of action in regulating tumor angiogenesis. Angiogen 1998, 2:123-124 [DOI] [PubMed] [Google Scholar]
- 38.Feil C, Augustin HG: Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun 1998, 247:38-45 [DOI] [PubMed] [Google Scholar]
- 39.Fujisawa N, Hayashi S, Kurdowska A, Carr FK, Miller EJ: Inhibition of GROalpha-induced human endothelial cell proliferation by the alpha-chemokine inhibitor antileukinate. Cytokine 1999, 11:231-238 [DOI] [PubMed] [Google Scholar]
- 40.Szekanecz Z, Haines GK, Lin TR, Harlow LA, Goerdt S, Rayan G, Koch AE: Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum 1994, 37:221-231 [DOI] [PubMed] [Google Scholar]
- 41.Johnson BA, Haines GK, Harlow LA, Koch AE: Adhesion molecule expression in human synovial tissue. Arthritis Rheum 1993, 36:137-146 [DOI] [PubMed] [Google Scholar]
- 42.Koch AE, Volin MV, Woods JM, Kunkel SL, Connors MA, Harlow LA, Woodruff DC, Burdick MD, Strieter RM: Regulation of angiogenesis by the C-X-C chemokines interleukin-8 and epithelial neutrophil acti-vating peptide 78 in the rheumatoid joint. Arthritis Rheum 2001, 44:31-40 [DOI] [PubMed] [Google Scholar]
- 43.Koch AE, Harlow LA, Haines GK, Amento EP, Unemori EN, Wong WL, Pope RM, Ferrara N: Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol 1994, 152:4149-4156 [PubMed] [Google Scholar]
- 44.Lupia E, Montrucchio G, Battaglia E, Modena V, Camussi G: Role of tumor necrosis factor-alpha and platelet-activating factor in neoangiogenesis induced by synovial fluids of patients with rheumatoid arthritis. Eur J Immunol 1996, 26:1690-1694 [DOI] [PubMed] [Google Scholar]
- 45.Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM: Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol 1991, 147:2187-2195 [PubMed] [Google Scholar]
- 46.Koch AE, Kunkel SL, Shah MR, Hosaka S, Halloran MM, Haines GK, Burdick MD, Pope RM, Strieter RM: Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol 1995, 155:3660-3666 [PubMed] [Google Scholar]
- 47.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Walz A, Strieter RM: Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Invest 1994, 94:1012-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]