Interstitial fibrosis is characteristic of many clinical entities including diabetes, ureteral obstruction, transplant rejection, and glomerulonephritis. 1 The interstitial fibrosis that accompanies renal disease is a complex process involving derangements in both the synthesis and degradation of collagen and other extracellular matrix proteins. Cellular sources of the extracellular matrix laid down during the development of interstitial fibrosis include fibroblasts and infiltrating macrophages. 1 The fibroblasts may be resident renal fibroblasts, fibroblasts that migrate into the kidney from external sources, or a specialized population of fibroblasts known as myofibroblasts. 2 Furthermore, recent evidence suggests that during renal injury, renal epithelial cells may transform into fibroblasts in a process known as epithelial-mesenchymal transdifferentiation (EMT). 3,4 The synthesis and processing of extracellular matrix that malfunction in fibrosis are under the complex control of many cytokines and other factors. 1 One cytokine in particular that has been implicated in fibrotic disease, both in the kidney and in other tissues, is transforming growth factor-β (TGF-β).
In the accompanying article “Renal Fibrosis: Collagen Composition and Assembly Regulates Epithelial-Mesenchymal Transdifferentiation,” by Zeisberg and colleagues 5 in this issue of The American Journal of Pathology, Zeisberg and co-workers use murine renal cell lines in culture to demonstrate that the integrity of basement membrane collagen has a significant effect on EMT in vitro. They further demonstrate that when basement membrane assembly is disrupted, the production of TGF-β is up-regulated. In this article we will discuss the emerging role of EMT in fibrosis and its regulation, both in vitro and in vivo, with emphasis on effects of TGF-β and collagen IV.
TGF-β
TGF-β is a multifunctional cytokine that has been extensively studied in fibrotic disease. 6 It is found in three isoforms (TGF-β1, TGF-β2, and TGF-β3) in most mammalian tissues, which are encoded by three separate genes. TGF-β acts by binding to three high-affinity receptors known as types I, II, and III. 7 TGF-β either binds directly to the type II receptor, or binds to the type III receptor, which in turn binds to the type II receptor. TGF-β complexed to the type II receptor then binds to the type I receptor. TGF-β bound to the type I receptor then phosphorylates transcription factors known as Smads, which translocate to the nucleus and result in multiple effects. 8
In addition to its role in renal fibrosis, TGF-β has a myriad of biological effects including cell proliferation and differentiation, immune regulation, and effects on inflammation. A description of these activities is beyond the scope of this commentary, but they have been covered recently in a symposium 6,8,9 and in other reviews. 10,11
The Role of TGF-β in EMT
Transdifferentiation is the process by which cells lose one phenotype and acquire a new one. 3,12 Cells may transform from epithelium to mesenchyme (EMT) or from mesenchyme to epithelium. These transformations are active in many tissues and are a part of both normal development and disease processes.
In the primitive chordate, the major tissue type is epithelium. By adopting a mesenchymal phenotype, cells can invade the extracellular matrix which is between epithelia, and can thereby increase their distribution to different parts of the body. 3 Subsequently, both mesenchymal to epithelial transdifferentiation and EMT are active in many tissues.
There are many cytokines, growth factors, and adhesion molecules that are involved in these processes. 3,12 TGF-β has been implicated in EMT in several different tissues. The approaches taken to understanding TGF-β’s role in these systems may be useful in studies on EMT in fibrosis.
In the heart, the endothelial cells that line the lumen of the heart are transformed to mesenchymal cells, which then invade the underlying extracellular matrix leading to subsequent cardiac valve formation. TGF-β-induced EMT is blocked by antibodies to TGF-β type II and type III receptors, as well by antisense oligonucleotides to TGF-β3. 13-15
In palatal fusion, median edge epithelial cells transform to mesenchyme. 16 In knockout experiments it has been shown that TGF-β3 is essential to this process. 17 Furthermore, it was shown that all palatal epithelia express TGF-β3 and the TGF-β type I and II receptors, yet only the median edge cells undergo EMT. Recent studies 18 demonstrated that the TGF-β type III receptor was temporally and spatially restricted only to those cells that undergo EMT. Not only does this provide an explanation for the selectivity of the EMT process, but it also suggests an important role for the TGF-β type III receptor that is usually described as a nonsignaling receptor.
EMT is also involved in tumorigenesis. 3,19 The ability of mature epithelial cells to acquire a mesenchymal phenotype can increase their ability to invade the extracellular matrix. This contributes to their ability to metastasize throughout the body. The invasiveness of several tumor lines from different organs has been correlated with the loss of epithelial markers. 20
TGF-β’s effects on cell growth and differentiation, as well as its immunosuppressive actions, contribute to its tumorigenicity. 10,11 Moreover, its ability to induce EMT adds to this effect. Two examples are given here. In murine skin carcinoma, TGF-β is essential to the transformation of keratinocytes into a spindle shape. 21 In mammary epithelial cells it has been shown that TGF-β stimulation results in EMT, 22 as shown in Figure 1 ▶ . Recent studies have begun to more specifically detail this process. In NMuMG breast epithelial cells, various constructs have been used to demonstrate that TGF-β type I receptor/ALK-5 is essential to TGF-β-induced EMT and that Smad 2 or Smad3 mediates the TGF-β response. 23
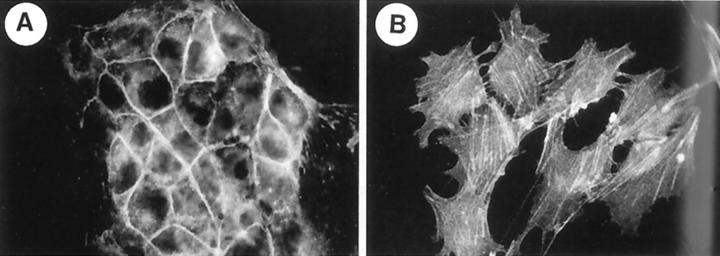
Figure 1.
Organization of the actin cytoskeleton in normal and transdifferentiated NmuMG cells. Cells were stimulated for 36 hours with vehicle (A) or 100 pmol/L TGF-β1 (B). Reprinted from J Cell Sci 1999, 112:4557–4568 with permission from Company of Biologists Ltd.
The Role of TGF-β in Renal Tubular EMT in Vitro
In contrast to the detailed description of the role of TGF-β’s isoforms and its receptor subtypes in EMT in development and carcinogenesis, the role of TGF-β in the EMT of renal fibrosis is not as well defined.
In vitro, it has been shown that tubule cells can be transformed into cells with a myofibroblastic phenotype. Fan and colleagues 24 have demonstrated that incubation of NRK-52E cells, a normal rat kidney epithelial cell line that is commercially available, with varying doses of TGF-β caused the following changes: 1) the typical cobblestone appearance of cells in culture was replaced by the elongated spindle shape associated with fibroblasts. 2) Using scanning electron microscopy, the cells were shown to lose their apical-basal polarity and cell surface microvilli. The apical-basal polarity was replaced by a front end-back end fibroblast-like polarity with cytoplasmic projections at the front end. 3) Under transmission electron microscopy, large bundles of actin microfilaments and dense bodies were seen. 4) The presence of α-smooth muscle actin in the TGF-β-transformed cells was confirmed by immunohistochemistry, Western blot, and flow cytometry. 5) There was a loss of the epithelial antigen, E-cadherin. Moreover, Fan and colleagues 24 illustrated that these effects were blocked by a neutralizing antibody to TGF-β, suggesting that they are indeed TGF-β-dependent.
Similar studies 25 using interleukin-1 have shown that it, too, can transform NRK-52E cells into cells with a fibroblastic appearance. These transdifferentiated cells express α-smooth muscle actin and have diminished expression of E-cadherin. Addition of neutralizing antibody to TGF-β blocked the effects of interleukin-1 on EMT, once again suggesting TGF-β dependence.
Fibroblast-Specific Protein as a Fibroblast Marker
Although the previously described combinations of antigen expression and cell shape changes have been used to document EMT, there is a lack of specificity in the antigens used to characterize the transformed cells as fibroblasts. Strutz and colleagues 4 sought to find a marker that would be specific for fibroblasts. Using subtractive and differential hybridization techniques, they compared murine epithelial and fibroblast cell lines. A differentially expressed protein was subsequently characterized and named fibroblast-specific protein-1 (FSP-1). By Northern blot analysis, FSP-1 was found to be expressed in fibroblast cell lines, but not expressed in other cell lines such as renal proximal tubular cells, mesangial cells, endothelial cells, or hepatocytes. In addition, when a polyclonal antibody was directed against FSP-1, FSP could be detected in fibroblast cell lines, but not in renal tubular cell lines.
When epithelial cells were transfected with FSP-1, there was a change in morphology to a stellate and elongated shape, characteristic of fibroblasts. In addition, when epithelial cells were grown in collagen gels, they began to express vimentin ( a mesenchymal marker), and FSP-1, and lost the ability to express keratin. 4
Further evidence of FSP-1 as a specific fibroblast marker was demonstrated when Okada and colleagues 26 showed in vitro that MCT cells (a renal proximal tubular cell line) incubated with TGF-β or with a combination of TGF-β and epidermal growth factor showed morphological changes consistent with EMT. In addition, cytokeratin and Z-01 (a marker for epithelial cell tight junctions) were lost, and vimentin was expressed. Along with transdifferentiation, FSP-1 was expressed in these cells. Treatment of cells with FSP-1 antisense oligomers suppressed de novo expression of FSP-1 and concomitant changes in morphology, collagen synthesis, and decreased cytokeratin expression. FSP-1 is now being used to examine EMT in vivo in renal fibrosis models.
Effects of TGF-β on Renal Tubular EMT in Vivo
The demonstration of EMT in vivo in fibrosis has been more challenging than its in vitro characterization. However, Okada and colleagues 27 have attempted to demonstrate in vivo EMT using an immunohistochemical approach. In their studies they examined expression of the epithelial marker cytokeratin, FSP-1, and HSP47, a marker for collagen synthesis. They used DBA/2-pcy mice that exhibit progressive renal cyst formation, and which may serve as a model for polycystic kidney disease. In control DBA/2-pcy mice, cytokeratin was visualized in normal tubules. As the disease progressed, epithelial cells in remnant tubules, which were trapped within fibrotic septa around adjacent cysts, expressed FSP1 and HSP47, and lost expression of cytokeratin. Because FSP1-positive cells were in remnant tubules, it was inferred that EMT had taken place. The demonstration of HSP47 positivity in these cells further suggested the fibroblastic function of collagen synthesis. Of additional interest was their finding that α-smooth muscle actin was not co-expressed with FSP-1 or HSP. Thus contrary to others 2,28 who have shown an up-regulation of α-smooth muscle actin in fibrotic states, Okada and colleagues 27 were able to demonstrate a transdifferentiation of epithelium to fibroblasts, but not a transdifferentiation of epithelium to myofibroblasts.
In another approach to demonstrating EMT in vivo, Dautheville and colleagues 29 presented a study in which transgenic mice were produced with a lacZ reporter gene downstream of a large segment of pro-α2 collagen promoter. Thus collagen synthesis was coupled to lacZ expression. In unobstructed murine kidneys, the relative absence of LacZ expression indicated minimal levels of collagen synthesis. However, kidneys subjected to 2 weeks of unilateral ureteral obstruction exhibited two lacZ-positive cell populations. One population was in the interstitium, and some of those cells had the shape of fibroblasts. The second lacZ-positive population was comprised of tubular epithelial cells. These results suggest that epithelial cells in unilateral ureteral obstruction can synthesize collagen, which would indicate some degree of EMT.
In a fibrotic model produced by 5/6 nephrectomy, Ng and colleagues 30 have used morphological and antigen markers to assess EMT. At 3 weeks after nephrectomy, α-smooth muscle actin was expressed in renal tubular epithelial cells. Ultrastructurally, actin filaments and dense bodies were present in the epithelial cells, corroborating the EMT (although in this case it seems to be an epithelial to myofibroblast transdifferentiation). As the experiment progressed, the epithelial cells lost their apical-basal polarity and became elongated. Of interest to the concurrently published article by Zeisberg and colleagues, 5 expression of α-smooth muscle actin was associated with disruption of the tubular basement membrane. Furthermore, the transformed cells appeared to migrate into the peritubular interstitium through the damaged basement membrane.
Indeed, Zeisberg and colleagues 5 suggest that basement membrane integrity regulates EMT via a TGF-β-dependent mechanism. We now turn our attention to basement membranes, with a focus on collagen IV. We shall briefly explore the physiological roles and molecular compositions of these structures in an effort to better understand their roles in EMT and renal interstitial fibrosis.
Basement Membranes
Basement membranes are specializations of extracellular matrix that separate epithelia, endothelia, peripheral nerves, muscle cells, and adipose cells from the supporting stroma. As such, basement membranes comprise the immediate microenvironment in which parenchyma exists. They are composed of a complex network of collagen IV, laminin, entactin/nidogen, and sulfate proteoglycans. 31 Although the role and importance of basement membranes as a scaffolding are well understood, their roles in cellular signaling and EMT are only recently beginning to be elucidated.
The Role of Collagen IV in Basement Membranes
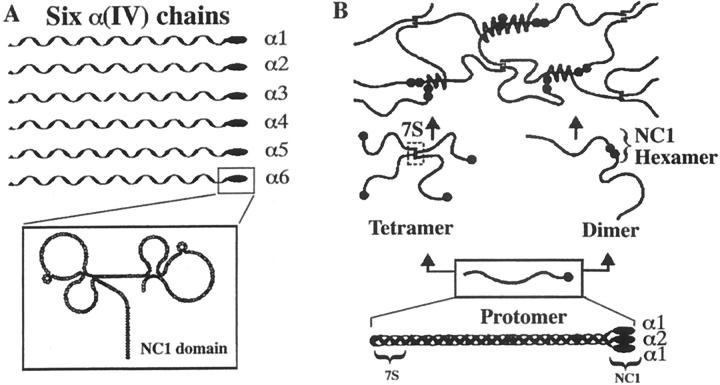
Collagen IV is the main structural protein found in basement membranes. Six homologous peptides, designated α1(IV) through α6(IV), have been identified as the chains that assemble to form the collagen IV protomer. The genes for α1(IV) and α2(IV) exist pairwise in a head-to-head manner on chromosome 13 in humans. 32 The α3(IV) and α4(IV) gene loci are positioned similarly on chromosome 2, with the α5(IV) and α6(IV) genes located on the X chromosome. 33 Each α chain is ∼1400 residues in length. The collagenous portion of the polypeptide is comprised of a series of Gly-X-Y triplets, and is interrupted by ∼20 short noncollagenous sequences. The carboxyl terminus is a 230 residue globular domain designated NC1 (noncollagenous 1) that has been demonstrated to direct protomer assembly. 34
Three α chains associate via NC1 interactions, after which their collagenous domains fold into triple helices through covalent and noncovalent interactions to yield the trimeric collagen IV protomer. 33,35-37 The collagen IV protomer is divided into three regions: an amino terminus 7S domain, a middle triple-helical domain, and a carboxyl terminus NC1 domain 33,36,37 that is globular, noncollagenous, and comprised of the individual NC1 domains of the component α chains. Protomers dimerize via head-to-head interactions of their NC1 carboxy-terminal domains to yield hexamers and/or tetramerize via their 7S regions to yield a complex meshwork (Figure 2) ▶ . 38 The six α(IV) chains enable assembly of 56 distinct protomers. The diversity of protomer phenotype enables tissue-specific expression of unique basement membrane networks. 31
Figure 2.
Schematic representation of collagen type IV chains and supramolecular networks. Type IV collagen comprises a family of six homologous chains. Each chain has a 7S domain at the amino terminus, a long collagenous domain (∼1400 residues), and a noncollagenous domain (NC1) of ∼230 residues at the carboxyl terminus (A). Three α chains assemble into a triple helical protomer, as exemplified by the (α1)2 α2 molecule. Protomers interact head-to-head, end-to-end, and by lateral associations, forming networks of distinct chain compositions. The α1/α2 network is common to all basement membranes (B) Reprinted from J Biol Chem 2000, 275:8051–8061, with permission from the American Society for Biochemistry and Molecular Biology.
Basement membranes in the kidney exhibit specialized collagen IV expression. Collagen IV networks containing α3(IV) through α6(IV) have been identified in human distal tubular basement membranes, in contrast to proximal tubule that contains only α1(IV)/α2(IV) protomers. 31 Bowman’s capsular basement membrane is composed of protomers containing α1(IV), α2(IV), α5(IV), and α6(IV). 31 The glomerular basement membrane is composed of two specific networks. The major protomeric subtype present in the mature glomerulus is a thick subepithelial network composed of α3(IV)/α4(IV)/α5(IV) protomers. The minor network, thin and located subendothelially, is an embryological remnant of the glomerular precursor network composed of α1(IV)/α2(IV) protomers. 31,39
Segment-specific heterogeneity of collagen IV protomer expression in the nephron hints at dynamic and complex function in renal cellular physiology. In the accompanying article, Zeisberg and colleagues 5 clearly demonstrate that collagen IV plays a regulatory role in EMT. By incubating mouse proximal tubular epithelial cells (MCT) with recombinant α1NC1, Zeisberg and colleagues effected the transdifferentiation of MCT cells to cells of fibroblast-like morphology. The transdifferentiated cells exhibited increased expression of FSP-1 and vimentin, showed decreased cytokeratin expression, and became spindle-shaped. Northern blot assay revealed that the transdifferentiation coincided with increased expression of mRNA for TGF-β1 and that the transdifferentiation could be blocked by anti-TGF-β1 antibodies. 5
The Interplay between TGF-β and Basement Membrane Integrity
In addition to its other functions discussed in this review, TGF-β1 regulates basement membrane integrity. Ultrastructural analysis of the effect of TGF-β1 on basement membrane formation by immortalized type II alveolar epithelial cells in vitro has revealed that basement membrane formation depends on TGF-β1. 40 However, increasing the level of TGF-β1 beyond a threshold level causes discontinuous basement membrane formation, despite increased expression of the basement membrane component molecules. Excessive production of basement membrane components is thought to obstruct their integration into a continuous basement membrane. 40
Furthermore, TGF-β up-regulates expression of the 92- and 72-kd type IV collagenases, MMP-9 and MMP-2. 41 MMP-9 and MMP-2 therefore likely play a role in TGF-β-mediated basement membrane disruption. Indeed, MMP-2 activity has been demonstrated to be crucial for EMT in avian cardiogenesis. By antagonizing MMP-2, Song and colleagues 42 were able to impede the transdifferentiation of endocardial cells to mesenchyme. Furthermore, they demonstrated that MMP-2-mediated proteolytic alteration of type IV collagen is important for the migration of transdifferentiated cells. Moreover, TGF-β1 up-regulates expression of collagen I, which induces MMP-9 synthesis 43 and stabilizes the mesenchymal phenotype. 5 Disruption of basement membrane integrity and increased TGF-β1 expression thus seem to interact in an autocrine, positive feedback loop that drives EMT (Figure 3) ▶ . 5
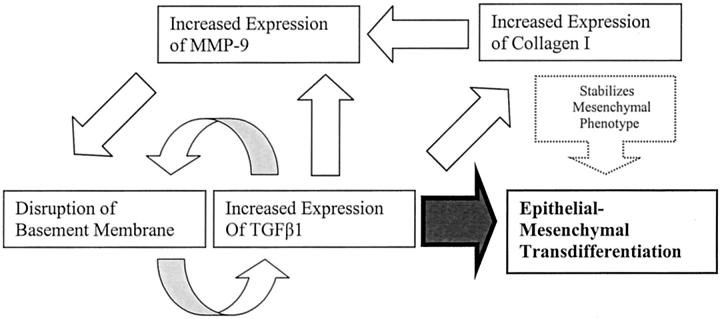
Figure 3.
EMT is a target of TGF-β. TGF-β induces EMT directly, and also participates in a proposed positive feedback loop via up-regulation of MMP-9 and disruption of basement membrane. In addition, it increases the synthesis of collagen I, which stabilizes the mesenchymal phenotype and increases MMP-9.
The molecular mechanism by which recombinant α1NC1 domain effects an in vitro increase in levels of TGF-β1 is unknown. Zeisberg and colleagues 5 hypothesize that without a collagenous domain, recombinant α1NC1 exerts a dominant-negative effect by disrupting helix formation in any protomer or protomeric dimer in which it is incorporated. They use FLAG-tagging to demonstrate that the recombinant α1NC1 becomes incorporated into protomers. The resultant incomplete collagen IV molecules are presumably degraded. This insult to basement membrane integrity is thought to trigger a cascade that up-regulates expression of TGF-β1. The notion that recombinant α1NC1 induces TGF-β1 indirectly via disruption of basement membrane integrity is supported by an increase in collagen IV degradation products in the supernatants taken from α1NC1-treated cell cultures.
In addition to the elegant experiments presented in the accompanying paper by Zeisberg and colleagues, 5 recent studies have demonstrated other novel actions of NC1 that may be relevant to EMT. Collagen IV NC1 domains have been shown to be integrin ligands. 38 The integrins are a family of transmembrane heterodimeric receptors capable of initiating signaling cascades via the inositol phospholipid pathway or tyrosine phosphorylation. 44 Petitclerc and colleagues’ 38 work with monoclonal antibodies and human endothelial cells reveals that α2NC1 ligates with αvβ3 integrin, as well as with integrin αvβ5 and the β1 integrins. α3NC1 and α6NC1 interact with αvβ3, but not with the β1 integrins. Systemic administration of α2NC1, α3NC1, and α6NC1 inhibit cytokine-induced angiogenesis and tumor growth in CAMs of chick embryos. The underlying mechanism by which α1NC1 induces TGF-β1 is likely complex and warrants further investigation.
EMT, TGF-β, and Basement Membranes: Implications for Renal Fibrosis
The final outcome of many types of injury to the kidney is interstitial fibrosis. It is already clear that the fibrotic process is accompanied by changes in the cellular composition of the kidney, as well as the cytokine milieu. Evidence has accumulated throughout the last several years that TGF-β is a key cytokine in this process. Experimental overexpression of TGF-β results in fibrosis, and overexpression of TGF-β in clinical and experimental renal disease has been documented. Both antisense and antibodies to TGF-β have been shown to ameliorate fibrosis in experimental renal models of unilateral ureteral obstruction, diabetes, and glomerulonephritis. 45-51
The interplay between basement membrane integrity, TGF-β1, and EMT and resulting renal fibrosis has yet to be fully elucidated. Of interest, however, are the findings in Alport syndrome, which is characterized by progressive glomerulonephritis that culminates in fibrosis and renal failure. 52,53 This loss of renal function results from the absence of a functional α3(IV), α4(IV), or α5(IV) gene. Alport patients cannot synthesize the α3(IV)/α4(IV)/α5(IV) protomeric network of the mature glomerulus. They therefore retain the embryonic α1/α2(IV) network phenotype, which appears normal at the onset of life but deteriorates throughout time, explaining the delayed onset, progressive course of Alport syndrome. 31 Although the mechanism of damage to the glomerular basement membrane is not fully understood, TGF-β1 has been implicated. Alport renal disease progression has been shown to induce TGF-β1 in humans and mice. 53 Furthermore, in the murine model of Alport disease, damage to the glomerular basement membrane is ameliorated by the administration of TGF-β soluble receptor. 52 Given that Alport syndrome involves TGF-β1-dependent fibrosis and disruption of the glomerular basement membrane, it is possible that EMT contributes to its pathogenesis.
Conclusion
The current studies suggest a broader role for TGF-β in fibrosis. In addition to its previously documented effects on collagen synthesis and degradation, TGF-β’s effects on EMT and basement membrane integrity likely contribute to its profibrotic actions. Although this review has focused on TGF-β, the molecular mechanisms behind EMT are not yet clear enough to rule out TGF-β-independent transdifferentiation pathways. Regardless, EMT has emerged as a very likely contributor to renal interstitial fibrosis. Furthermore, Zeisberg and colleagues 5 have clearly demonstrated that disruption of basement membrane integrity up-regulates EMT in a TGF-β-dependent mechanism.
Although our understanding of the pathogenesis of renal fibrosis is incomplete, the important experimental work reviewed herein suggests novel anti-fibrotic therapeutic approaches. In addition to targeting TGF-β directly, strategies focused on basement membrane stabilization, inhibition of type IV collagenases, or direct suppression of EMT may well prove effective in combating renal interstitial fibrosis.
Footnotes
Address reprint requests to Dr. Diane Felsen, Weill Medical College of Cornell University, Dept. of Urology, 1300 York Ave., New York, NY 10021-4896. E-mail: dfelsen@med.cornell.edu.
References
- 1.Eddy AA: Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 1996, 7:2495-2508 [DOI] [PubMed] [Google Scholar]
- 2.Powell DW, Mifflin R, Valentich J, Croew S, Saada J, West A: Myofibroblasts. I: paracrine cells important in health and disease. Am J Physiol 1999, 277:C1-C19 [DOI] [PubMed] [Google Scholar]
- 3.Hay ED, Zuk A: Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis 1995, 26:678-690 [DOI] [PubMed] [Google Scholar]
- 4.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995, 130:393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R: Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 2001, 159:1313-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branton MH, Kopp JB: TGF-beta and fibrosis. Microbes Infect 1999, 1:1349-1365 [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Feng XH: TGF-beta receptor signaling. Biochim Biophys Acta 1997, 1333:F105-F150 [DOI] [PubMed] [Google Scholar]
- 8.Roberts AB: TGF-beta signaling from receptors to the nucleus. Microbes Infect 1999, 1:1265-1273 [DOI] [PubMed] [Google Scholar]
- 9.Wahl SM: TGF-beta in the evolution and resolution of inflammatory and immune processes. Introduction. Microbes Infect 1999, 1:1247-1249 [DOI] [PubMed] [Google Scholar]
- 10.Blobe GC, Schiemann WP, Lodish HF: Role of transforming growth factor beta in human disease. N Engl J Med 2000, 342:1350-1358 [DOI] [PubMed] [Google Scholar]
- 11.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 12.Ekblom P, Weller A: Ontogeny of tubulointerstitial cells. Kidney Int 1991, 39:394-400 [DOI] [PubMed] [Google Scholar]
- 13.Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB: Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci USA 1991, 88:1516-1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CB, Boyer AS, Runyan RB, Barnett JV: Antibodies to the type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol 1996, 174:248-257 [DOI] [PubMed] [Google Scholar]
- 15.Brown CB, Boyer AS, Runyan RB, Barnett JV: Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science 1999, 283:2080-2082 [DOI] [PubMed] [Google Scholar]
- 16.Shuler CF: Programmed cell death and cell transformation in craniofacial development. Crit Rev Oral Biol Med 1995, 6:202-217 [DOI] [PubMed] [Google Scholar]
- 17.Kaartinen V, Cui X, Heisterkamp N, Groffen J, Shuler C: Transforming growth factor-beta3 regulates transdifferentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev Dyn 1997, 209:255-260 [DOI] [PubMed] [Google Scholar]
- 18.Cui XM, Shuler CF: The TGF-beta type III receptor is localized to the medial edge epithelium during palatal fusion. Int J Dev Biol 2000, 44:397-402 [PubMed] [Google Scholar]
- 19.Birchmeier W, Birchmeier C: Epithelial-mesenchymal transitions in development and tumor progression. EXS 1995, 74:1-15 [DOI] [PubMed] [Google Scholar]
- 20.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W: E-Cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portella G, Cumming SA, Liddell J, Cui W, Ireland H, Akhurst RJ, Balmain A: Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ 1998, 9:393-404 [PubMed] [Google Scholar]
- 22.Miettinen PJ, Ebner R, Lopez AR, Derynck R: TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 1994, 127:2021-2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P: TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 1999, 112:4557-4568 [DOI] [PubMed] [Google Scholar]
- 24.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999, 56:1455-1467 [DOI] [PubMed] [Google Scholar]
- 25.Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis 2001, 37:820-831 [DOI] [PubMed] [Google Scholar]
- 26.Okada H, Danoff TM, Kalluri R, Neilson EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol 1997, 273:F563-F574 [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG: Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int 2000, 58:587-597 [DOI] [PubMed] [Google Scholar]
- 28.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP: Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 1994, 72:283-292 [DOI] [PubMed] [Google Scholar]
- 29.Dautheville S, Fischer E, Mougenot B, Delauche M, Ronco P, Rossert J: Identification of type I collagen-producing cells during interstitial fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol 1998, 9:514A [Google Scholar]
- 30.Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY: Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int 1998, 54:864-876 [DOI] [PubMed] [Google Scholar]
- 31.Miner JH: Renal basement membrane components. Kidney Int 1999, 56:2016-2024 [DOI] [PubMed] [Google Scholar]
- 32.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993, 268:26033-26036 [PubMed] [Google Scholar]
- 33.Hudson B, Wisdom B, Gunwar S, Noelken M: Pathobiochemistry. Edited by A Kang. Boca Raton, CRC Press, 1991, pp 17–30
- 34.Kalluri R, Cosgrove D: Assembly of type IV collagen. Insights from alpha3(IV) collagen-deficient mice. J Biol Chem 2000, 275:12719-12724 [DOI] [PubMed] [Google Scholar]
- 35.Paulsson M: Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 1992, 27:93-127 [DOI] [PubMed] [Google Scholar]
- 36.Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K: A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem 1981, 120:203-211 [DOI] [PubMed] [Google Scholar]
- 37.Timpl R: Structure and biological activity of basement membrane proteins. Eur J Biochem 1989, 180:487-502 [DOI] [PubMed] [Google Scholar]
- 38.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC: New functions for non-collagenous domains of human collagen type IV: novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem 2000, 275:8051-8061 [DOI] [PubMed] [Google Scholar]
- 39.Desjardins M, Bendayan M: Ontogenesis of glomerular basement membrane: structural and functional properties. J Cell Biol 1991, 113:689-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furuyama A, Iwata M, Hayashi T, Mochitate K: Transforming growth factor-beta1 regulates basement membrane formation by alveolar epithelial cells in vitro. Eur J Cell Biol 1999, 78:867-875 [DOI] [PubMed] [Google Scholar]
- 41.Wahl SM, Allen JB, Weeks BS, Wong HL, Klotman PE: Transforming growth factor beta enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci USA 1993, 90:4577-4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song W, Jackson K, McGuire PG: Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev Biol 2000, 227:606-617 [DOI] [PubMed] [Google Scholar]
- 43.Sarret Y, Woodley DT, Goldberg GS, Kronberger A, Wynn KC: Constitutive synthesis of a 92-kDa keratinocyte-derived type IV collagenase is enhanced by type I collagen and decreased by type IV collagen matrices. J Invest Dermatol 1992, 99:836-841 [DOI] [PubMed] [Google Scholar]
- 44.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD: Molecular Biology of the Cell. 1994:pp 995-999 Garland Publishing, Inc., New York
- 45.Bottinger EP, Letterio JJ, Roberts AB: Biology of TGF-beta in knockout and transgenic mouse models. Kidney Int 1997, 51:1355-1360 [DOI] [PubMed] [Google Scholar]
- 46.Border WA, Noble NA: Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int 1998, 54:1390-1391 [DOI] [PubMed] [Google Scholar]
- 47.Isaka Y, Akagi Y, Ando Y, Tsujie M, Sudo T, Ohno N, Border WA, Noble NA, Kaneda Y, Hori M, Imai E: Gene therapy by transforming growth factor-beta receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 1999, 55:465-475 [DOI] [PubMed] [Google Scholar]
- 48.Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M: Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 2000, 58:1885-1892 [DOI] [PubMed] [Google Scholar]
- 49.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D: Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 2000, 58:2301-2313 [DOI] [PubMed] [Google Scholar]
- 50.Wright EJ, McCaffrey TA, Robertson AP, Vaughan ED, Felsen D: Chronic unilateral ureteral obstruction is associated with interstitial fibrosis and tubular expression of transforming growth factor-beta. Lab Invest 1996, 74:528-537 [PubMed] [Google Scholar]
- 51.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 2000, 97:8015-8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R: Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol 2000, 157:1649-1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sayers R, Kalluri R, Rodgers KD, Shield CF, Meehan DT, Cosgrove D: Role for transforming growth factor-beta1 in Alport renal disease progression. Kidney Int 1999, 56:1662-1673 [DOI] [PubMed] [Google Scholar]