Abstract
The most common cause of failure of retinal reattachment surgery is formation of fibrocellular contractile membranes on both surfaces of the neuroretina. This intraocular fibrosis, known as proliferative vitreoretinopathy, results in a blinding tractional retinal detachment because of the contractile nature of the membrane. Contractility is a cell-mediated event that is thought to be dependent on locomotion and adhesion to the extracellular matrix. Interactions between cells and the extracellular matrix can be influenced by matrix metalloproteinases (MMPs) and we investigated the role of MMPs in two in vitro models (two- and three-dimensional) of human retinal pigment epithelial (RPE) cell-mediated contraction. MMP activity was detected using enzyme-linked immunosorbent assays and zymography techniques that revealed MMP-1, -2, -3, and -9 positivity during the collagen matrix contraction assays. RPE-populated collagen matrix contraction (three-dimensional) was inhibited using a cocktail of anti-MMP antibodies and with Galardin (a broad-spectrum MMP inhibitor). Galardin inhibition was dose-dependent, reversible, and dependent on cell number. MMP inhibitors had no effect on contraction when RPEs were seeded on two-dimensional collagen matrices or on cellular adhesion to collagen type I. Our results suggest that MMP activity may be required for three-dimensional but not two-dimensional RPE-collagen matrix contraction.
In the eye, retinal function is severely impaired when the neuroretina becomes detached from its normal location. Retinal detachment may become permanent if fibrocellular membranes form on the surfaces of the neuroretina and within the vitreous cavity [a process known as proliferative vitreoretinopathy (PVR)]. 1,2 The periretinal membranes keep the retina detached by virtue of their contractile nature. Periretinal membrane contraction is cell mediated and the major cell component of the membrane typically is retinal pigment epithelial cells (RPE cells). These RPE cells are dedifferentiated and exhibit a mixed phenotype (including fibroblastic) in the membranes, which often constitute layers on the membrane surfaces and a dedifferentiated fibroblastic phenotype within the tissue, 3-9 RPE cells are particularly implicated in this process. 10,11
Cell-mediated contraction is a complex process that may involve a number of cellular activities including migration and reorganization of the extracellular matrix. Both of these cell activities are dependent on cell-matrix interactions 12 and the matrix of developing PVR membranes is replete with extracellular matrix, including collagen types I and III. 8 In general, interactions between these interstitial collagens and cells are thought to involve matrix metalloproteinases (MMPs). RPE cells have been shown to synthesize MMP-1, -2, -3, and -9 in vitro. 13,14 Indeed these MMPs have also been implicated in periretinal membranes of eyes with PVR 15 and moreover elevated levels of MMP-2 and -9 have been found in the vitreous of PVR eyes. 16 Therefore we hypothesized that MMPs may play a critical role in RPE-matrix interactions and specifically, in PVR membrane contraction. To further investigate this concept we examined 1) the expression of MMPs by RPE cells interacting with collagen in an in vitro model of this disease in which both RPE phenotype and collagen type is similar to that of PVR membranes, 17 and 2) studied the effects of modulating MMP expression in the in vitro model.
Materials and Methods
Primary Culture of Human RPE Cells
Human postmortem eyes up to, but not exceeding, 48 hours postmortem were obtained from the Manchester Royal Hospital Eye bank and complied with the Declaration of Helsinki. Human RPEs (HRPEs) were isolated following the procedure outlined by Edwards 18 and modified by Boulton, Marshall, and Mellerio. 19 The anterior segment and vitreous were removed after soaking the eyes for 10 minutes in phosphate-buffered saline (PBS) containing fungizone, 100 U of penicillin, and streptomycin. The neural retina was teased away from the RPE and the exposed RPE was washed three times in PBS to remove adherent photoreceptor debris. The posterior eyecup was divided into three segments and areas of the RPE monolayer were isolated with brass cloning rings. The wells formed by the cloning rings were filled with a mixture of 0.25% trypsin, 0.02% ethylenediaminetetraacetic acid, and PBS, and incubated at 37°C for 30 minutes. Dissociated RPE cells were aspirated from Bruch’s membrane and seeded into 25-cm 2 tissue-culture flasks coated with 1 ml of newborn calf serum (NCS) each. The RPEs were fed with Ham’s F-10 culture medium (50 ml ×10 Ham’s F10, 425 ml distilled water, 5 ml glutamine, 5 ml fungizone, 5 ml penicillin/streptomycin, 8 ml of 7.5% sodium bicarbonate, and 1 ml of 1 mol/L NaOH; all Gibco, Paisley, UK), supplemented with 15% NCS and 3 mg/ml glucose. The cultures were maintained at 37°C in 5% CO2 and air. Primary cultures reached confluence within 2 to 3 weeks and were passaged on. The purity of the cultures was confirmed by immunohistochemical labeling with a wide-spectrum anti-cytokeratin monoclonal antibody (clone K8.13; ICN Biomedicals Ltd., High Wycombe, UK) known to stain the HRPE cell population. 20 HRPE cells between the 3rd and 10th passage were used in this study and before collagen gel contraction studies cells were grown in flasks until they were preconfluent. Cells were removed from their flasks by using 0.1% trypsin and 0.04% ethylenediaminetetraacetic acid, then mixed with complete medium and centrifuged for 10 minutes at 800 rpm. After the supernatant was discarded, the cell pellet was resuspended in NCS. Cell numbers and viability were determined by trypan blue exclusion in a hemocytometer.
Preparation of Media
NCS was depleted of exogenous MMPs by following the method described by Azzam and Thompson 21 in which gelatin-coated Sepharose beads (Pharmacia) were incubated with NCS at a 1:10 dilution for 2 hours (4°C) on a rotamix. The beads were then removed by passing the mixture through a sterile Millipore filter (0.2 mm) and the resultant NCS was used in subsequent collagen gel formation and in complete tissue culture media (F10 and 15% NCS).
Preparation of Cell-Populated Three-Dimensional Collagen Matrices
The formation collagen matrices were prepared with rat tail type I collagen (Sigma, Dorset, UK) at 5 mg/ml in 0.1% acetic acid stock solution. To prepare a collagen matrix at a final concentration of 1.5 mg/ml for each assay, 2.1 ml of concentrated culture medium (15 ml of 10× MEM, 35 ml distilled water, 1.5 ml penicillin/streptomycin, 1.5 ml glutamine, 1.5 ml fungizone, and 3 ml of 7.5% sodium bicarbonate) was added with 3.6 ml of collagen solution at 4°C. To this mixture, 0.9 ml of serum containing the appropriate amount of cells was added. Gel contraction studies were performed in either 6-or 24-well plates in which each well received either 1 ml (6 well) or 0.25 ml (24 well) of this final mixture (Sterilin, Stone, UK) and then was transferred to a humidified 37°C, 5% CO2 incubator where the matrix set within 1 minute. After 10 minutes the matrices were overlaid with either 6 ml (6 well) or 1.5 ml (24 well) of complete medium, detached from the base using a pipette tip and floated.
Cells Seeded on Two-Dimensional Collagen Matrices
Collagen matrix formation followed the above protocol except 0.9 ml of cell-free NCS was used in the matrix formula. After matrix formation, cells were seeded onto the matrix in complete media and allowed to settle for 30 minutes in the incubator. Matrices were then detached, floated, and returned to the 37°C incubator.
MMP Inhibitors and Antibodies
Mouse monoclonal antibodies against either α2 or β1 integrins (Serotec) and mouse monoclonal and rabbit polyclonal antibodies to MMP-1, -2, -3, and -9 (Biogenesis Ltd., Poole, UK) and were diluted 1:10 (v/v) in complete media. A control solution containing 0.1% w/v sodium azide and 0.14% v/v mouse or rabbit IgG was diluted 1:10 in complete media. We also used a broad-spectrum, potent hydroxamic acid-derived MMP inhibitor developed by Galardy and associates 22,23 known as Galardin. This compound was dissolved in complete media at logarithmic concentrations ranging from 100 mmol/L to 0.1 nmol/L. Eqimolar hyroxamic acid (100 mmol/L) was dissolved in complete media as a control solution.
Gel Contraction Experiments
To assess the effect of MMP inhibitors, triplicate wells were used. The overlying media of the gels contained varying concentrations of the inhibitors (see above) or the relevant controls. The gel area was measured at days 1, 3, and 7 after seeding and represented as percentage size ±SEM. Cell morphology within the collagen matrices was followed by phase contrast microscopy throughout the duration of the experiments. To determine whether the effects of the MMP inhibitor Galardin were reversible, gels were incubated with complete media containing 10 mmol/L Galardin for 5 days, which was then removed and replaced with complete media containing 100 mmol/L hyroxamic acid. The gel contraction was measured at days 1, 3, 5, 7, and 10 after seeding.
Morphological Assessment of in Vitro Preparations
A number of different methods were used to study the morphological changes of tissue culture preparations (monolayer cultures and matrices) throughout the duration of the experiments. Living tissue cells were visualized using phase-contrast and time-lapse video microscopy, which allowed experiments to run continuously and to be viewed at numerous time points throughout the experiment. Examination at a higher resolution (at the electron microscopy level) and a more detailed analysis expression using immunohistochemical techniques required fixation of specimens at fixed time points.
Phase-Contrast and Time-Lapse Video Microscopy
Routine observations and photographs of living tissue-culture cells were performed using an inverted phase-contrast microscope (Diaphot, Nikon). In addition to phase-contrast microscopy the movement of cells within collagen matrices was monitored throughout 72 hours using time-lapse video microscopy. Collagen matrices containing cells were prepared as previously described but were floated in a minimal amount of tissue culture media. The matrices were then transferred to a humidified chamber maintained at 37°C and 5% CO2 in air, which was mounted onto an inverted phase-contrast microscope (Nikon). The microscope was connected to a video camera (JVC KS300) and recorder system (JVC-BR906OE) that allowed the experiments to be recorded for up to 72 hours.
Light Microscopy
A number of different methods were used to study the cellular changes visible at the light microscopy level. These methods require different fixation and processing protocols and are presented below individually. All collagen matrices were rinsed in PBS three times, each for 5 minutes, before fixation.
Whole Mount Preparations
Whole collagen matrices were fixed in 10% neutral-buffered formalin overnight. Matrices were either then stained for histological examination with hematoxylin or by a fluorescent immunohistochemical method.
Indirect Immunofluorescence of Whole Collagen Matrices
The matrices were fixed in 10% neutral-buffered formalin as described earlier and were initially rinsed by immersion in Eppendorfs, which contained PBS (10 minutes). Nonspecific binding was blocked by exposure to 5% normal goat serum (Sigma) in PBS (v/v) for 120 minutes at room temperature on a rotamix. Serum was removed and replaced with the primary antibody to cytokeratins (clones CK8.13 and CK18; Sigma) at the relevant dilution for 24 hours at +4°C on a rotamix. Extensive washing of matrices was performed by immersing the matrices in rotating 7-ml tubes containing PBS (four 30-minute washes). The matrix was then returned to a new Eppendorf and the goat anti-mouse fluorescein isothiocyanate conjugate (1:40; Sigma) secondary antibody was added via a Millipore filter and left overnight on a rotamix at +4°C. The specimens were further washed (as earlier described) three times on the rotamix in PBS (30 minutes each) and mounted onto a glass slide under a glass coverslip, using fluorostab mounting medium (Euro-Path Ltd.). The specimens were then viewed at an excitation/emission wavelength of 490/525 nm. Photographs were taken on both black and white (Tmax 400) and color slide film (Agfa chrome RS1000).
Scanning Electron Microscopy (SEM)
Collagen matrices were initially rinsed in PBS (3 × 5 minutes) and subsequently fixed in 2.5% glutaraldehyde (EM grade vacuum-distilled Fisons Polaron, Loughborough, UK) in Sorensen’s phosphate buffer, pH 7.4, for 1 hour. After washing in Sorensen’s phosphate buffer (three changes of buffer) for 15 minutes, the specimens were postfixed for 1 hour in 1% (w/v) osmium tetroxide in the same buffer, followed by a further 15-minute wash in buffer alone. The specimens were then washed in distilled water, with one change at 30 minutes, followed by three changes at 10 minutes. The specimens were then dehydrated through a series of graded alcohols, each for 15 minutes (25%, 50%, 70%, 80%, 90%, and three times at 100%; AnaLar grade, BDH).
The specimens were then critical point-dried (Polaron, Hemel Hampstead, UK), mounted on metal stubs with double-sided adhesive tape, and gold coated (∼12 to 16 nm thickness) using a Polaron sputter coater. The three-dimensional matrices were then examined in a Hitachi S-520 scanning electron microscope, using secondary electron detection.
Preparation and Collection of Media for Zymography and Enzyme-Linked Immunosorbent Assay (ELISA)
Collagen matrices were prepared as above containing 1 × 10 5 cells/ml in 6-well plates. Media overlying the collagen lattices was removed at days 1, 3, and 7 after seeding and stored at −70°C until required. Samples were then lyophilized and reconstituted in 2 ml of PBS before analysis by gelatin zymography or ELISA.
Gelatin Zymography
Samples were denatured with an equal volume (15 ml) of dissociating buffer (125 mmol/L Tris-HCl, pH 6.8, 20% (v/v) glycerol, 4% (w/v) sodium dodecyl sulfate, 0.005% (v/v) bromophenol blue; Novex, R&D Systems Ltd.) for 10 minutes at room temperature. The samples were then run on a 10% (v/v) Tris-glycine polyacrylamide gel (Novex, R&D Systems Ltd.) containing 0.1% (w/v) gelatin for 90 minutes with constant 125 V voltage and 40 mA current, within running buffer (25 mmol/L Tris base, 192 mmol/L glycine, 0.1% (w/v) sodium dodecyl sulfate, pH 8.3; Novex, R&D Systems Ltd.). Prestained molecular weight markers (Mr 7200 to 208,000; Bio-Rad) were also ran with the samples. The gels were then placed in renaturing buffer with gentle agitation for 30 minutes. The renaturing buffer was removed and replaced with developing buffer (2.5% (v/v) Triton X-100; Novex, R&D Systems Ltd.) for 30 minutes, which was then replaced with fresh developing buffer and incubated overnight at 37°C. The gel was then stained with 0.5% (w/v) Coomassie blue (Bio-Rad) in 45% (v/v) methanol/45% (v/v) distilled water/5% (v/v) glacial acetic acid for 2 hours. The gel was destained in 45% (v/v) methanol/45% (v/v) distilled water/5% (v/v) glacial acetic acid to visualize the clear bands of protease activity against the blue background.
To detect whether the MMPs were in a proenzyme or activated smaller molecular weight form of the enzyme, samples were also incubated with 2 mmol/L of aminophenylmercuric acetate (APMA; Sigma) for 2 hours at 37°C before zymography. APMA causes autocatalytic cleavage of the inactive proenzyme form to its lower molecular weight active form therefore allowing an assessment of the form of MMPs secreted.
Quantification of MMPs by ELISA
Samples of conditioned media (days 1, 3, and 7) from collagen lattices were quantified using specific ELISA kits (Amersham, UK) to determine the levels of total MMP-1, -2, -3, and -9 and TIMP-1 protein produced. All of the assays were based on a two-site ELISA sandwich format using two antibodies directed against different epitopes of the relevant MMP. The MMP-1 ELISA differed slightly from those for MMP-2, -3, and -9 because these used a horseradish peroxidase-conjugated rabbit-raised polyclonal antibody, whereas the rabbit polyclonal antibody directed to MMP-1 was unconjugated. This procedure therefore required an additional step in the protocol in which a horseradish peroxidase-conjugated donkey and rabbit antibody was added.
All samples of media from the collagen gel experiments were assayed at dilutions 1:1, 1:5, and 1:10 to find readings that fell within those of the standard curves of each assay. Comparisons of MMP quantities within each assay were only made with samples run at the same dilution.
The double-sandwich ELISA method according to manufacturer’s instructions was used for quantifying MMP-1 protein. Briefly MMP-1 standards that were prepared at 0, 6.25, 12.5, 25, 50, and 100 ng/ml and conditioned media were aliquoted into duplicate wells (100 μl/well) coated with monoclonal antibody to MMP-1 and incubated at 25°C for 2 hours. The wells were subsequently washed and emptied four times with wash buffer (350 μl/well; 0.0067 mol/L phosphate buffer, pH 7.5, containing 0.033% Tween 20) before the addition of 100 μl/well of polyclonal antibody (rabbit anti-MMP-1) for 2 hours at room temperature. The wells were then washed four times (350 μl/well) with wash buffer and 100 μl/well of horseradish peroxidase-conjugated donkey anti-rabbit antibody added for 1 hour at room temperature. The wells were washed again as described previously and 100 μl/well of 3,3′,5,5′-tetramethylbenzidine/hydrogen peroxide in dimethylformamide (20%, v/v) was added and incubated at 25°C for 30 minutes. The visualization reaction was then stopped with 100 μl/well of 1 mol/L sulfuric acid and the optical densities read on a microplate reader at 450 nm.
MTT Assay
The MTT (3-{4.5-dimethylthiazol-2-yl}-2,5-diphenyltetrazolium bromide; Sigma) assay was used to indirectly assess cellular proliferation as it reflects the cytosolic dehydrogenase activity in cells. This activity has been shown previously to increase in proliferating RPE cells 24 and so was used to determine whether the MMP inhibitors had any effect on cellular proliferation in vitro. HRPE cells were harvested as previously described and resuspended in complete media. HRPE cells were aliquoted into 96-well culture plates at seeding densities of 0.625, 1.25, 2.5, 5, and 10 × 10 4 cells per ml (180 μl per well) in media containing varying concentrations of the inhibitors, antibodies, or controls. Cells were incubated at 37°C for 3 days to allow adequate settlement and growth. The media from each well was carefully removed and replaced with 150 μl of fresh serum-free media. Each well immediately received 20 μl of MTT (5 mg/ml) and was incubated for 4 hours at 37°C.
The media was discarded and replaced with 150 μl of dimethyl sulfoxide and once the MTT crystals dissolved, the wells read at 540 nm on a microtiter plate reader (Dynatech Laboratories). A minimum of six replicates was used in all experiments and results were expressed in terms of percentage survival taking the absorbance of the control wells to be 100% survival.
Adhesion Assay to Collagen Type I
After the effects on matrix contraction, the influence of MMP inhibitors and integrins on HRPE adhesion to collagen I was assessed using a semiquantitative colorimetric assay (Cytomatrix; Chemicon). Briefly, 96-well plates precoated with collagen type I, were washed with 100 μl of 1%(w/v) bovine serum albumin in PBS for 30 minutes at 37°C to block nonspecific binding sites. Preconfluent HRPE cells were detached enzymatically (0.1% trypsin and 0.04% ethylenediaminetetraacetic acid), then mixed with complete medium and centrifuged for 10 minutes at 800 rpm and resuspended in 1 ml of complete media and counted using a hemocytometer. Cells were resuspended at cell concentrations of 1 × 105/ml and 5 × 104/ml in F10 containing different concentrations of the MMP inhibitors [Galardin (100 nmol/L to 100 μmol/L) and MMP antibodies (1:10 to 1:100) or antibodies directed against integrin subunits α2 and β1). One hundred μl of each sample was added to triplicate wells and incubated either for 1 or 4 hours at 37°C in a CO2 incubator. After these incubations the wells were washed gently four times with 200 μl of PBS before the addition of 100 μl/well of 0.2% crystal violet in 10% ethanol for 5 minutes at room temperature. The wells were subsequently washed with 200 μl of PBS (repeated four times) and the adherent-stained cells lysed with 100 μl/well of 1% sodium dodecyl sulfate (v/v) in PBS added for 5 minutes at room temperature. The absorbance of the stained wells was read at 540 nmol/L on a microtiter plate reader (Dynatech Laboratories).
Statistical Evaluations
Statistical analysis of data were performed using the statistics package Unistat (Unistat Ltd., London, UK). Comparisons of data samples from contraction and adhesion assays were by analysis of variance that allowed multiple comparisons between data sets of two or more. Data were presented as mean ±SEM unless otherwise stated and significance was expressed as P < 0.05.
Results
In Vitro Models of RPE-Dependent Collagen Matrix Contraction
Because PVR membranes characteristically involve RPE cells both at membrane surfaces and (as fibroblastic cells) within the avascular collagenous fibrous tissue, 7-11 we mimicked this arrangement in vitro by either seeding cells on two-dimensional collagen lattices (corresponding to cells on a membrane) or cells within (three-dimensional) collagen matrices (cells located within the epiretinal membrane). Matrix contraction occurred in both models in a cell number-dependent manner and no contraction occurred in cell-free matrices. Optimal contraction occurred at 4 × 104/ml (two-dimensional) and 4 × 105/ml (three-dimensional) and thus were used in all subsequent experiments unless otherwise stated.
The Production and Distribution of MMPs during RPE-Dependant Collagen Matrix Contraction
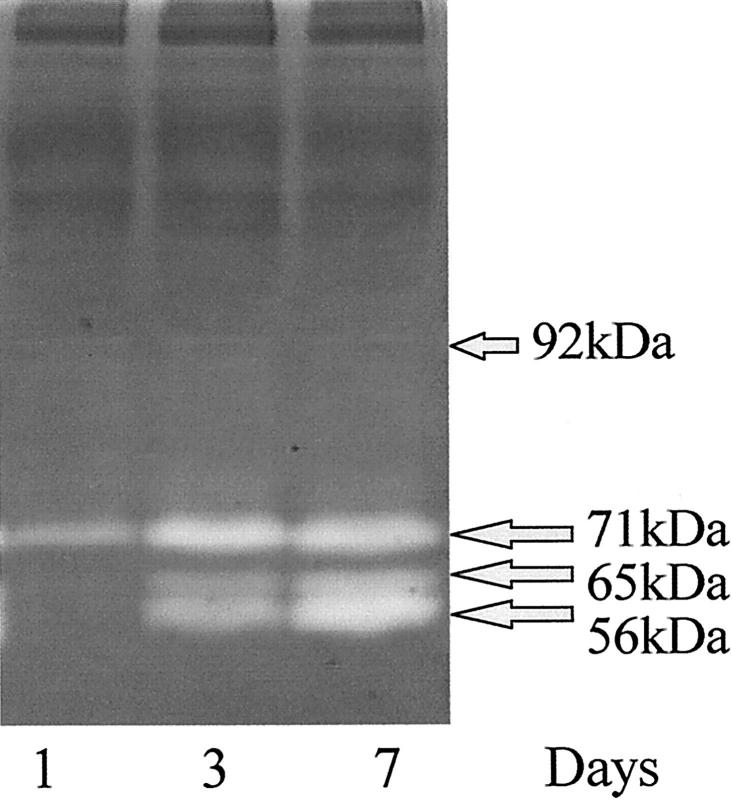
Analysis of the matrices and surrounding media for the presence of MMPs using gelatin zymography revealed both for HRPE seeded in three-dimensional matrices and HRPE seeded on two-dimensional matrices, the presence of three major bands at molecular weights of 71, 65, and 56 kd. These bands increased in intensity through 7 days. The molecular weights of two of these bands corresponded to MMP-2 in both its latent (71 kd) and active form (65 kd). The 56-kd band corresponds to MMP-1 or MMP-3 (Figure 1) ▶ . In addition, two faint minor bands were seen at similar intensities on all days studied. These two minor bands were at 100 kd and 92 kd and corresponded to MMP-9. A similar profile for gelanolytic activity also was observed from the media of HRPE seeded on the surface of collagen matrices.
Figure 1.
A zymogram gel in which media collected from contracting three-dimensional collagen matrices at time points of days 1, 3, and 7 after seeding. A similar profile was observed for both cells on and in the matrices. The molecular weights of the major bands indicated the presence of MMP-2 in both its latent (71 kd) and active (65 kd) form and MMP-1 or MMP-3 at 56 kd. All three bands were seen to increase in intensity during of contraction of the gels. Two very faint bands could just be seen at similar intensities on all days studied at molecular weights corresponding to the presence of MMP-9 at 100 and 92 kd.
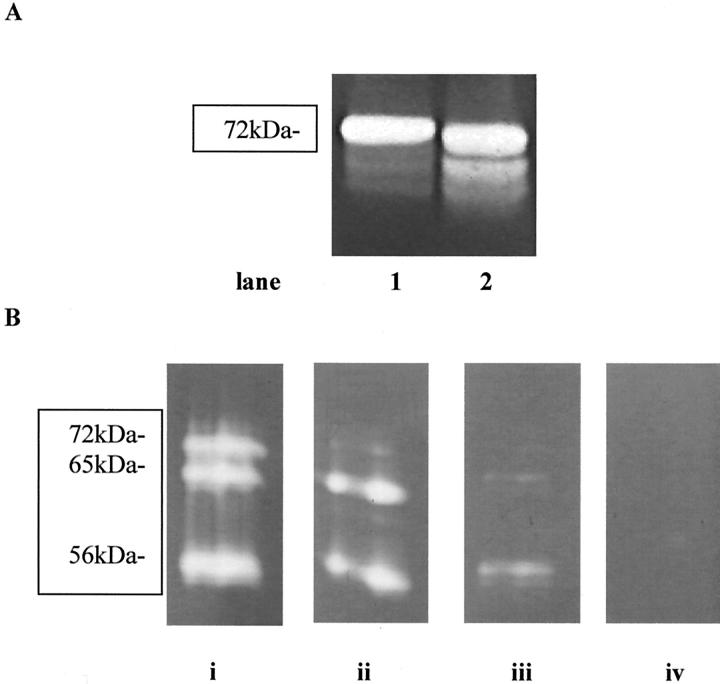
Treatment with APMA resulted in band 71 kd decreasing in intensity that indicated that at least some of the MMP-2 was secreted in a latent form. The remaining bands (65 kd and 56 kd) showed no decrease in intensity, indicating the presence of active forms of enzymes (Figure 2A) ▶ . Controls of pure proenzyme standards for MMP-2 and MMP-9 were also run both with and without APMA pretreatment. The MMP-2 protein profile showed bands at 71 and 69 kd that, after activation with APMA, gave an extra band at 65 kd indicating an active form of the enzyme. The MMP-9 protein showed a major band at 92 kd that on APMA activation gave a lower molecular weight at 80 kd corresponding to active MMPs (not shown).
Figure 2.
A: A zymogram in which samples have been run after incubation with (lane 2) and without (lane 1) APMA. After activation with APMA there is a small reduction of the proenzyme (arrow) at ∼ 72 kd and an increase in the smaller active 65-kd band. B: Zymograms in which samples of media (day 7) were incubated with increasing concentrations of phenanthroline within the developing buffer. A reduction in the amount of gelatinolytic activity produced during collagen gel contraction is observed with increasing concentrations of phenanthroline (i = developing buffer only, ii (200 nmol/L), iii (200 μm), and iv (20 mmol/L) show the final concentrations of phenanthroline within the developing buffer).
To confirm whether the bands seen were a result of MMP activity, samples of media (day 7) were incubated with an inhibitor of MMP activity, 1,10-phenanthroline. 25 Dose-dependent inhibition of MMP activity was seen with no MMP activity detected with a concentration of 20 mmol/L and above (Figure 2B) ▶ .
As gelatin zymography only allows semiquantification, samples of conditioned media from the matrices were also quantified for the levels of MMPs-1, -2, -3, and -9 and TIMP 1 using specific ELISAs. The findings demonstrate a time-dependent increase in each of these enzymes with MMP-2 showing the most significant increase in protein level detected (Table 1) ▶ .
Table 1.
Summarizes the Mean Protein Content Found (ng/ml) for TIMP-1 and MMPs -1, -2, -3, and -9 during HRPE Cell-Populated Collagen Matrix Contraction
| Day 1 | Day 3 | Day 7 | |
|---|---|---|---|
| MMP-1 | 2.4 (0.07) | 4.3 (1.1) | 6.8 (0.2) |
| MMP-2 | 12.4 (1.2) | 58.3 (3.2) | 125.9 (3.5) |
| MMP-3 | 1.9 (0.05) | 4.4 (0.04) | 6.7 (1.4) |
| MMP-9 | 1.2 (0.05) | 1.4 (0.1) | 1.9 (0.02) |
| TIMP-1 | 5.6 (0.2) | 15.9 (2.5) | 22.4 (3.7) |
All the proteins studied increase in quantity throughout the 7-day period. Data is represented as mean (SE).
Manipulation of Collagen Matrix Contraction by MMP Inhibition
Attempts to inhibit MMP activity during HRPE-mediated collagen matrix contraction used antibodies and chemical inhibitors (see below). These experiments were performed to ascertain whether inhibition of these molecules could have a direct effect on the contractile process. Because the combined zymography and ELISA results indicated the presence of MMP-1, -2, -3, and -9 in the matrices, antibodies specific to each of these enzymes were used to evaluate whether contraction of the cell-mediated contraction of the collagen matrices was MMP-dependent. Controls included the use of inhibitory antibodies to integrins α2 and β1 subunits, which have been shown to inhibit RPE-mediated contraction.
The Effect of MMP Inhibition on Contraction of HRPE-Populated Three-Dimensional Matrices
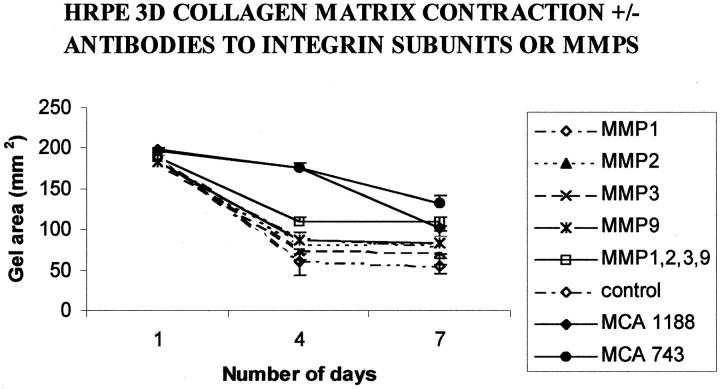
Antibodies directed against MMP-1, -2, -3, and -9 individually had only a small individual effect on the contraction rate of HRPE-populated matrix contraction when compared to control matrices. Although all of the antibodies tested seemed to decrease the rate of contraction, this reduction was not significant at any time point tested (analysis of variance, P > 0.05; Figure 3 ▶ ). Phase-contrast microscopy showed there was no apparent difference in the settlement and spreading of treated and control cells within the matrix. Antibodies directed against the subunits of the α2β1 integrin significantly reduced matrix contraction from the fourth day onwards (P < 0.01).
Figure 3.
Graph representing the mean collagen matrix area (±SD) for HRPE-populated collagen matrices (three-dimensional) either in the presence or absence of individual antibodies directed against either β-1 integrin (MCA1188); α-2 integrin (MCA743); MMP-1, -2, -3, and -9; or a cocktail of antibodies directed against all four of the above MMPs. A significant (P < 0.001) inhibition of contraction was observed in matrices incubated with antibody (10 μg/ml) directed against both the integrin subunits on day 4 and the MCA743 antibody maintained this inhibition on the seventh day. At no time point was there any significant difference between single MMP antibody-treated and control groups (P > 0.05). A significant inhibition of contraction occurred on days 4 and 7 with the cocktail of antibodies (P < 0.05).
As individual antibodies did not significantly inhibit three-dimensional-matrix contraction, combinations of antibodies were used. A cocktail of antibodies directed against MMP-1, -2, -3, and -9 inhibited contraction (compared to controls) that, from day 4 onwards, reached significance (P < 0.05; analysis of variance). By day 7 the inhibition was highly significant when matrices treated with a cocktail of antibodies had contracted to 50.4 ± 2.9% of the original area compared to controls at 29.1 ± 7.4% (mean ± SD, P < 0.01; Figure 3 ▶ )
The Effect of MMP Inhibition on Contraction of Matrices Overlain with HRPE (Two-Dimensional)
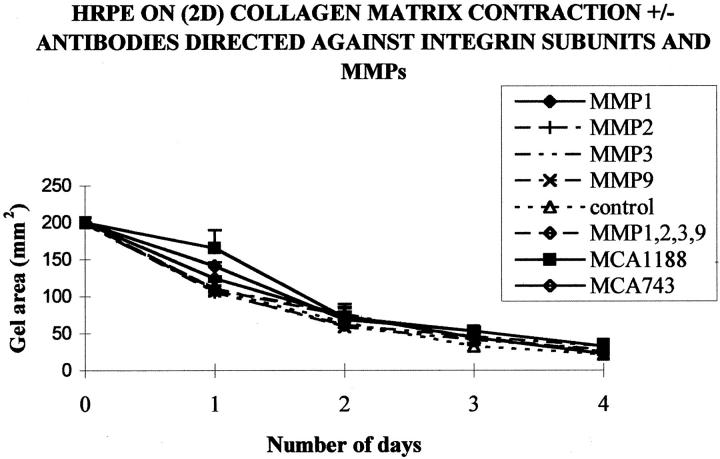
Antibodies directed alone or in combination against MMP-1, -2, -3, and -9 had no significant effect on the contraction of collagen matrices on which HRPE were seeded (two-dimensional). No significant inhibition of contraction was achieved with any of the antibodies used at any dilution (Figure 4) ▶ . The contraction of the matrices tended to be reduced by the fourth day in the presence of antibodies against MMP-1 (44.3 ± 10.97), MMP-2 (45.67 ± 12.89), MMP-3 (41 ± 8.19), and MMP-9 (41 ± 8.19) compared to controls (32 ± 4.58), but these reductions were not significant. A cocktail of antibodies directed against MMP-1, -2, -3, and -9 also did not inhibit contraction, whereas the antibody directed against the β1 subunit of the α2β1 integrin, significantly reduced matrix contraction on the first day after seeding (P < 0.01).
Figure 4.
Graph showing the mean collagen matrix area for HRPE seeded on two-dimensional collagen matrices either in the presence or absence of antibodies directed against MMP-1, -2, -3, or -9. At no time point was there a significant difference (P > 0.05) between control and any MMP antibody-treated groups. Significant inhibition of contraction occurred at day 1 in matrices containing the antibody (10 μg/ml) directed against the β-1 integrin subunit (MCA 1188; P < 0.05; n = 6).
Manipulation of HRPE-Mediated Two-Dimensional and Three-Dimensional Matrix Contraction by a Broad Spectrum MMP Inhibitor
As combinations of antibodies inhibited cell-populated contraction, further studies were undertaken to evaluate the role of MMPs in matrix contraction using the broad-spectrum MMP inhibitor, hydroxamic derivative Galardin that is known to inhibit the activity of all MMPs.
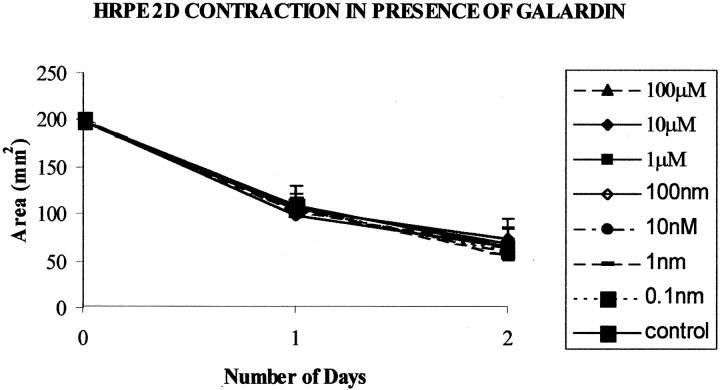
No significant inhibition of contraction occurred with HRPE seeded on collagen matrices when two-dimensional matrices were exposed to Galardin diluted in normal culture media at any Galardin concentration (0.1 nmol/L to 100 μmol/L) or time point tested (Figure 5) ▶ . No noticeable morphological differences between the inhibitor-treated cells and control cells were observed.
Figure 5.
Graph illustrates HRPE cell-mediated contraction for cells seeded on collagen matrices (two-dimensional) in the presence of MMP inhibitor Galardin. The presence of Galardin did not significantly alter the rate or extent of matrix contraction at any concentration. Data are represented as means ±SD.
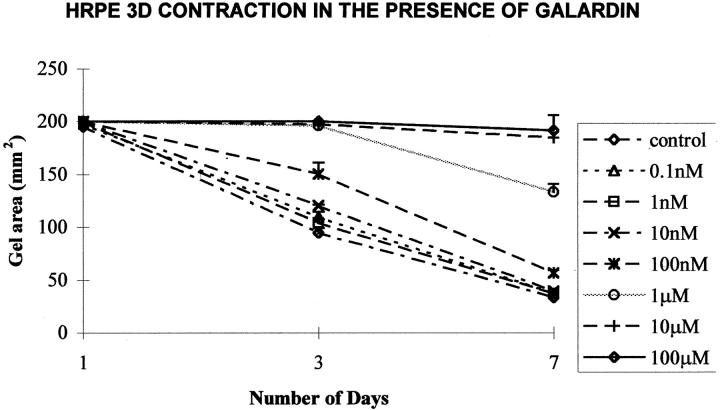
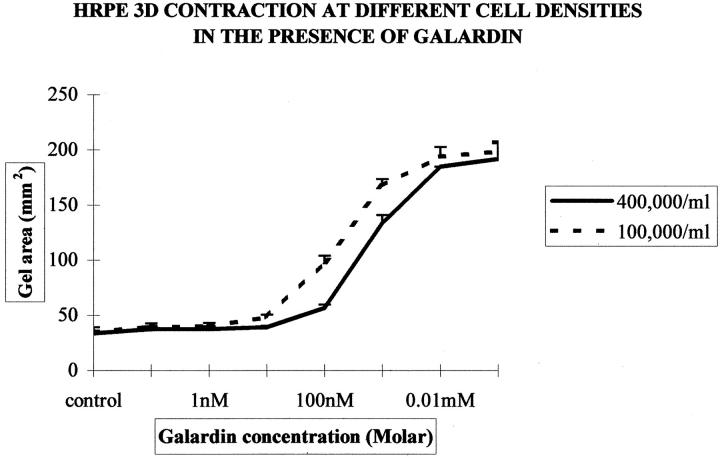
Exposure to Galardin diluted in normal culture media significantly inhibited contraction of HRPE-populated (three-dimensional) collagen matrices in a dose-dependent manner (Figure 6) ▶ . At day 7, this inhibition was obtained with concentrations of Galardin of 100 nmol/L and above for 4 × 10 5 cells (Figure 6) ▶ . The inhibition of contraction also seemed to be cell number-dependent as matrices seeded with fewer cells required a reduced concentration of Galardin to achieve the same level of inhibition. Thus matrices seeded at a cell density of 1 × 10 5 required 10 nmol/L Galardin to significantly (P < 0.05) reduce contraction (Figure 7) ▶ .
Figure 6.
Graph illustrating the mean gel area (SD) against time for HRPE-populated collagen gels (three-dimensional) surrounded with media containing different concentrations of Galardin. From day 3 onwards, 10 nmol/L of Galardin and above significantly (P < 0.05) inhibited collagen gel contraction compared to controls.
Figure 7.
Graph showing the mean gel area (mm2) at day 7 for HRPE-populated matrices containing either 4 × 10 5 or 1 × 10 5 cells/ml that have undergone contraction with different concentrations of Galardin (0.1 nmol/L to 100 μmol/L). High-populated matrices require a greater concentration of inhibitor to exert anti-contractile effects. The inhibition of contraction appears in a cell number- and dose-dependant manner.
To determine whether Galardin inhibited MMP activity, samples of media from contracting matrices (day 7) were assayed by gelatin zymography. Inhibition of MMP activity was clearly seen with Galardin as evidenced by reduced bands.
Cellular Morphology in Collagen Matrices
During collagen matrix contraction the cells were initially round within the matrix and most cells progressed from the initial development of small processes (day 1), through to a stellate and spindle-shaped appearance by day 7 (Figure 8, A and B) ▶ . No differences in morphology between any of the MMP antibody-treated groups and control cells could be distinguished. Whole matrix preparations also showed no differences in cellular appearance and distribution during contraction. At day 7, cells within all matrices appeared equally spread and dedifferentiated and reorganization of surrounding collagen was seen in all matrices.
Figure 8.
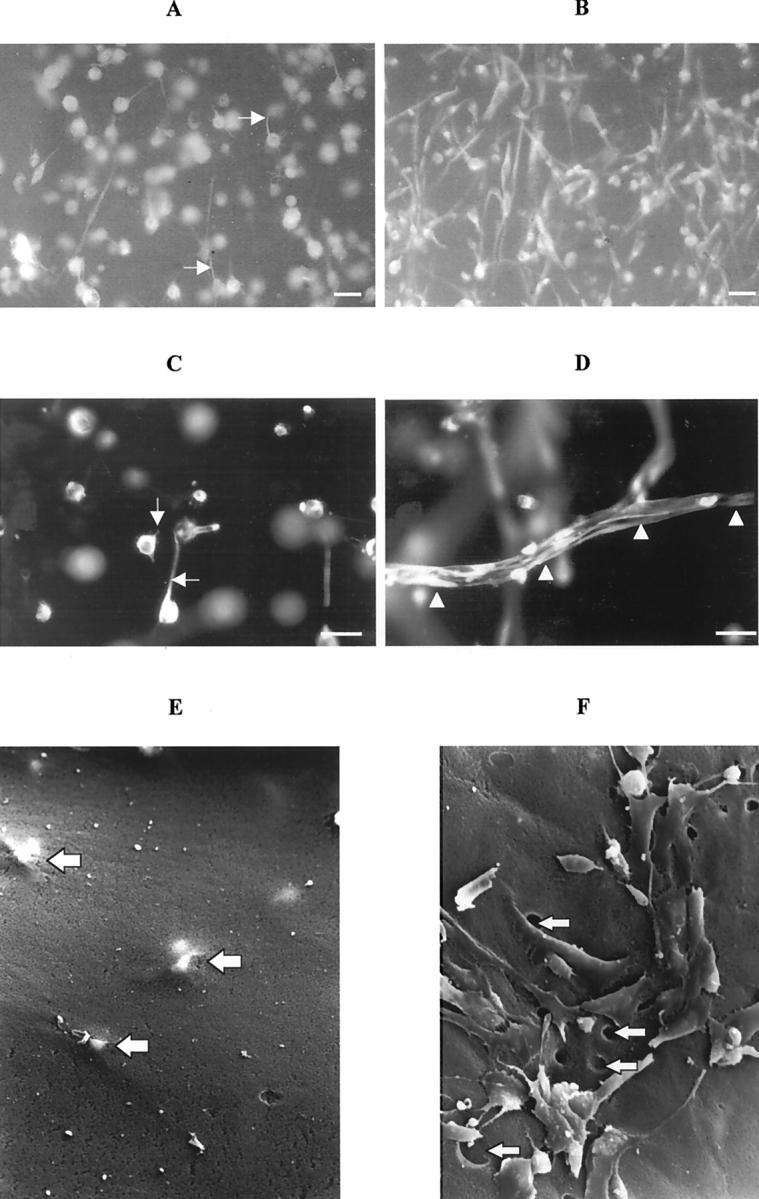
Photomicrographs of whole matrices fluorescently immunostained for CK18 (A–D). During collagen matrix contraction the cells were initially round with small processes extending within the matrix (A; day 1) and were later seen to adopt a stellate and spindle-shaped appearance by day 7 (B). The cells in matrices incubated with Galardin only extended out small processes into the surrounding matrix (C, arrows; day 1) whereas in control cell-populated matrices, the cells characteristically were stellate in appearance and even formed tunnels within the matrix (D, arrowheads; scale bars, 10 μm). Electron micrographs of matrices (E and F) illustrating that no holes or cells are present on the matrix surface, early in the contractile process (day 1; F). RPE cells can still be visualized just beneath the matrix surface (F, large arrows). By the seventh day (E), cells within untreated matrices had reached the matrix surface and numerous empty holes could be seen (E, arrows; original magnification, ×1150).
Galardin-treated, matrix-embedded cells did not progress beyond extending small cytoplasmic processes into the surrounding matrix. Whole matrix immunohistochemistry illustrated that most cells in the 10 μmol/L- and 100 μmol/L-treated matrices still only extended small processes into the surrounding matrix by the seventh day after seeding (Figure 8C) ▶ . Histological and SEM examination of matrices demonstrated the presence of holes or tunnels in control matrices that were not observed in matrices significantly inhibited with Galardin (Figure 8; D to F ▶ ). It was further observed that cells failed to appear on the matrix surface in Galardin-treated matrices even after 7 days, whereas control matrices showed the presence of cells (and holes) on the surface from 24 hours (Figure 8F) ▶ . Time-lapse video microscopy of contracting matrices also revealed that cells were stationary within the Galardin treated matrices, whereas control matrices showed cells acquired a stellate/spindle shape and migrated through the surrounding matrix (Figure 9, A and B) ▶ .
Figure 9.
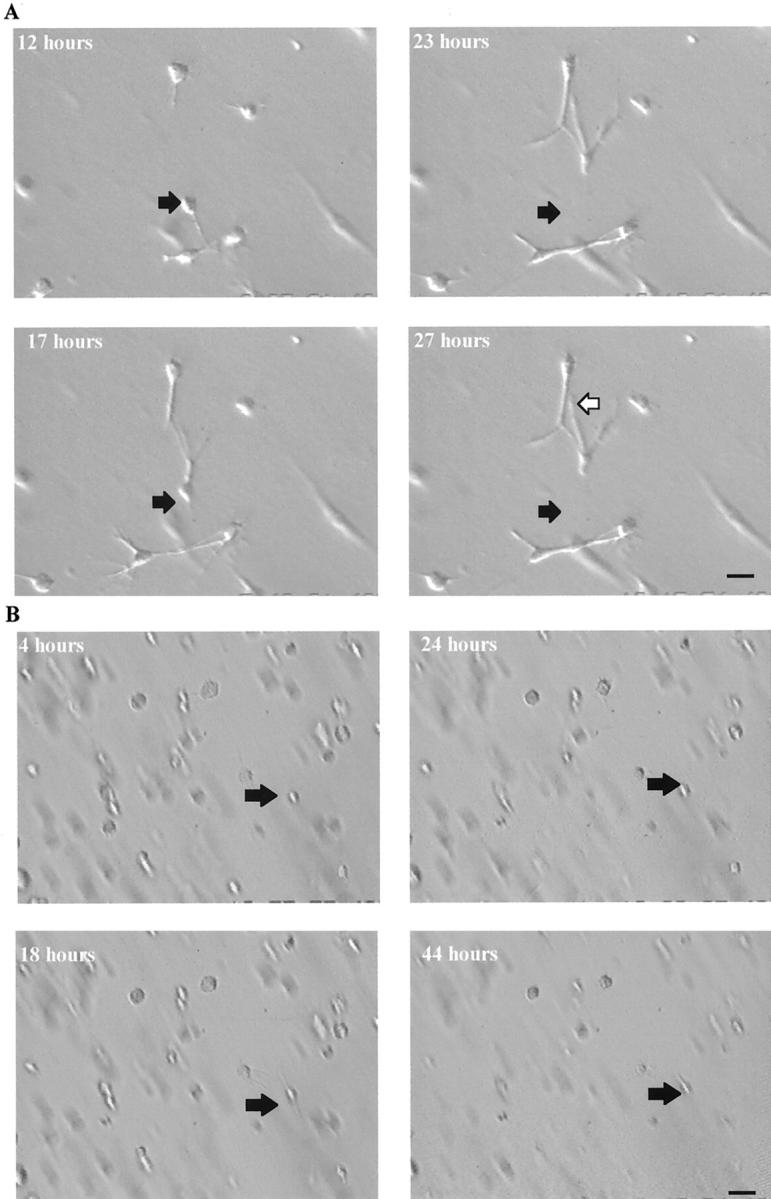
Snapshots from a recording by time-lapse video-microscopy of HRPE-populated matrices, which illustrates the movement of a HRPE cell within a control collagen matrix (A). The black arrow represents the initial position of a cell and the white arrow its different position after 27 hours (scale bar, 5 μm). Little or no movement of HRPE cells within a collagen matrix after incubation with 10 μmol/L of Galardin was seen (B) compared to controls (A). The black arrow represents the initial position of a cell up to and including 44 hours after seeding illustrating the cell has not migrated during the experiment (scale bar, 3 μm).
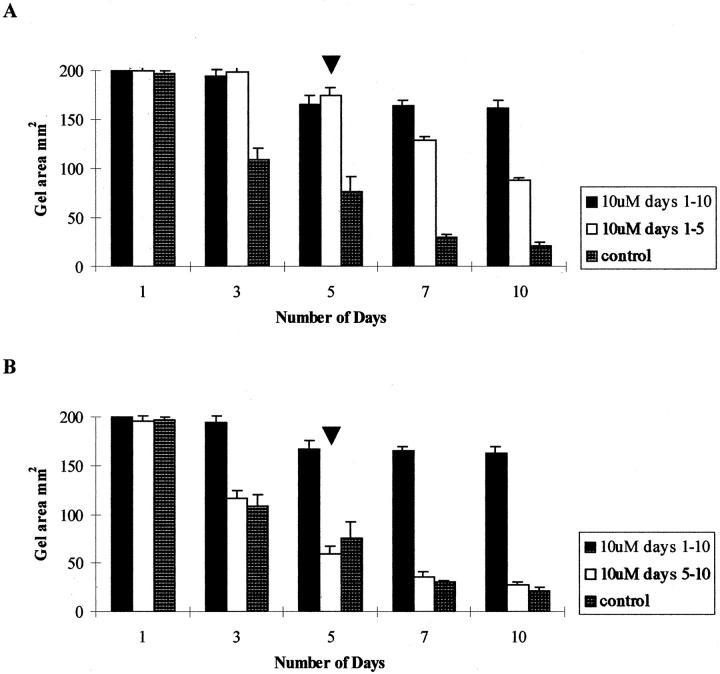
Reversibility of Contraction
To assess whether the effects of Galardin were reversible, cell-populated matrices were incubated with complete media containing 10 mmol/L Galardin for 5 days (by which time inhibition of contraction had occurred). The medium was then removed and replaced with complete media containing 10 mmol/L of hydroxamic acid (control). The matrix contraction was measured after seeding as before. Whereas matrices treated with complete media containing 10 mmol/L Galardin for the 10-day duration of the experiment exhibited inhibition of contraction when compared to untreated controls (P < 0.01). However, when Galardin was removed from the incubating media at day 5 (exchange experiment) there was an increased rate of contraction (Figure 10A) ▶ . The difference in contracted matrix area between exchanged and nonexchanged experiments reached significance by the tenth day after seeding (P < 0.01). However, if Galardin was added (at day 5) to the media of matrices that were already contracting (ie, incubated with control media for days 1 to 5) then no change (compared to controls) in the rate of contraction was observed (Figure 10B) ▶ . Morphological examination of exchange experiment matrices revealed that after removal, of the Galardin, the cells progressed to stellate and spindle shapes as in the control untreated matrices.
Figure 10.
Histogram showing that the effects of Galardin on HRPE-populated collagen matrix contraction after removal of the inhibitor. A reduction in matrix area can be seen after removal of the inhibitor at day 5 (A) whereas the effects of Galardin on HRPE-populated collagen matrix contraction could not be reversed after addition of the inhibitor at day 5 (B).
Cytotoxic and Adhesion Assays of MMP Inhibitor-Treated RPE
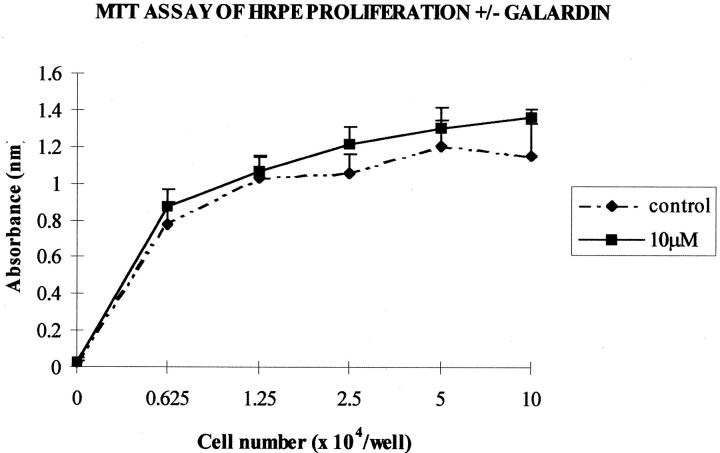
Trypan blue exclusion studies showed no cytotoxic effects at any Galardin concentration studied (not shown). The MTT assay of activity in HRPE seeded at different cell densities in the presence or absence of Galardin showed no difference (P > 0.05) in cytosolic dehydrogenase activity (absorbance) after 4 days (Figure 11) ▶ . No difference was seen between any groups of controls or cells grown in media containing Galardin at concentrations from 0.1 nmol/L to 10 μmol/L. This confirmed the lack of toxicity observed for HRPE seeded on matrices (two-dimensional) as well as the reversibility experiments performed with HRPE seeded within the matrix.
Figure 11.
A graphical illustration of HRPE at different cell densities grown in media in the presence or absence of Galardin for 4 days. No difference (P > 0.05) in cystolic dehydrogenase activity (absorbance) was seen between controls or cells grown in media containing up to 10 μmol/L of Galardin.
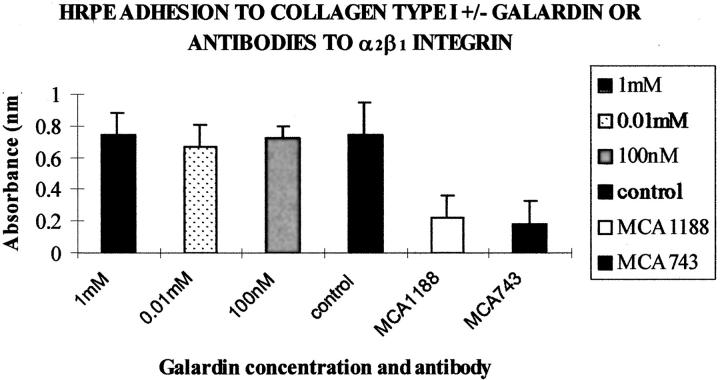
To determine whether MMP-inhibitors were exerting their effect of three-dimensional matrix contraction by inhibiting adhesion between cells and matrices, adhesion of HRPE to collagen type I was studied at various concentrations of Galardin, including those that had been shown to inhibit matrix contraction. The results were compared to controls using inhibition induced by antibodies to integrins α2 and β1 (10 μg/ml). The integrin antibodies significantly reduced adhesion to collagen type I (P > 0.05; Figure 12 ▶ ). However, no statistical difference was seen between adhesion of cells seeded in the absence of Galardin and cells seeded with Galardin at any of the concentrations of the agent tested (Figure 12) ▶ . Furthermore no difference was seen in cell morphology or cytoskeletal staining in either the controls or the inhibitor treated groups (Figure 13) ▶ . This data supports the two-dimensional model findings, ie, HRPE adhesion to and subsequent contraction of the underlying collagen matrix was not significantly affected in the presence of MMP inhibitors.
Figure 12.
Histogram representation of adhesion of HRPE to collagen type I in the presence or absence of Galardin at varying concentrations and with antibodies directed against the α2β1 integrin subunits (MCA743 and MCA1188). No statistical difference in was seen (P > 0.05) on cellular adhesion to type I collagen at any concentration of Galardin studied. However the antibodies directed against the α2β1 integrin subunits at 10 μg/ml significantly reduced adhesion of HRPE cells to collagen type I (P < 0.01, n = 12).
Figure 13.
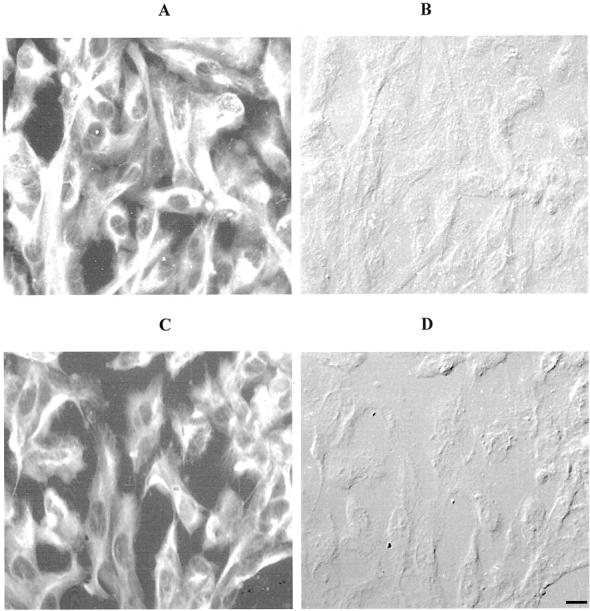
Photomicrographs illustrating positive immunoreactivity (fluorescein isothiocyanate; A and C) and corresponding DIC (B and D) for cells stained with a broad-spectrum cytokeratin antibody (CK8.13). No differences in cytokeratin immunoreactivity were observed for HRPE in either the presence (A and B) or absence (C and D) or Galardin (scale bar, 4 μm).
Discussion
MMPs have been shown to be present in almost all PVR membranes but their role in PVR contraction is unclear. 15 Cells of RPE origin (both in differentiated or fibroblastic form) are typically the principal cells in PVR membranes and can be either located within this extracellular fibrosis or as layers or a foci of epithelial cells on the membrane surface. 11 Therefore, in vitro models of membranes have been developed using RPE seeded in or on collagen matrices. 17,26 We examined the morphology of RPE placed either in a three-dimensional or on two-dimensional collagen matrix and found that RPE within the matrix (three-dimensional) become fibroblastic whereas cells seeded on the surface (two-dimensional) adopt an epithelial appearance. The similarity to the morphology of RPE in PVR membranes, together with the propensity of both two-dimensional and three-dimensional models to contract, led us to use both models to study MMP expression by RPE in and on collagen matrices. Analysis of the matrices and surrounding media for the presence of MMPs using gelatin zymography revealed the presence of three major bands, which increased in intensity from days 1 through to 7, at molecular weights of 71, 66, and 56 kd. The molecular weights of these bands indicate the presence of MMP-2 in both its latent (71 kd) and active form (66 kd), and MMP-1 (56 kd). In addition, two minor bands are seen (at similar intensities on all days studied) at molecular weights corresponding to the presence of MMP-9 at 100 and 92 kd. ELISA data confirmed the increase in quantity of these enzymes throughout the period of matrix contraction.
The MMP profile in both three-dimensional and two-dimensional matrices is similar to that the MMP profile observed in PVR membranes by Webster and colleagues 15 that collectively demonstrate the presence of MMP-1, -2, -3, and -9 in the scars. These observations prompted us to use the RPE-collagen matrices to further investigate the role of MMPs in the contraction process, using selective and general MMP-neutralizing agents. For this part of the study, we used neutralization of α2β1 integrins as a control because neutralization of these receptors previously has been shown to inhibit contraction of two-dimensional matrices by RPE. 27,28 The results of our investigation revealed fundamental differences between the mechanisms involved in the contraction of two-dimensional and three-dimensional matrices.
Specific individual antibodies against individual MMPs did not significantly alter cell-mediated contraction of the collagen matrices in either the two-dimensional or three-dimensional models, whether polyclonal or monoclonal antibodies were used. However, a combination of all of the MMP antibodies did exert an effect, albeit only with cells seeded within the matrix (three-dimensional). As the combination of MMP antibodies showed some significant inhibition of cell-populated matrices, a potent broad-spectrum MMP inhibitor was used to further investigate the role of MMPs in RPE-populated collagen matrix contraction. The hydroxamic derivative known as Galardin inhibits the activity of all MMPs. 29,30 Therefore, this compound was used to determine whether MMP production in the two models is essential for, rather than incidental to, matrix contraction. The results show that, whereas the adhesive integrins α2β1 are required for both systems, MMPs are only needed for three-dimensional matrix contraction. Indeed, exposure of RPE seeded within a three-dimensional, but not on a two-dimensional, collagen matrix to Galardin within normal culture media inhibits contraction of collagen matrices in a dose-dependent manner. Moreover, the inhibition of contraction induced by Galardin in the three-dimensional model was far greater than observed using the combinations of antibodies directed against MMPs. It is possible that MMP antibodies may not be as efficient as Galardin at MMP blockade or that Galardin may block MMPs in addition to those detected by us in the matrices.
Because Galardin has no effect on RPE adhesion and the anti-contractile effects in three-dimensional matrices are reversed by exchanging the Galardin containing media with normal culture media, the mechanism of action seems to be directly because of inhibition of MMPs rather than Galardin-induced RPE cytotoxicity or interference with RPE-matrix adhesion.
Although, collagen degradation has been shown to be minimal during three-dimensional matrix contraction by fibroblasts, 31 only minimal degradation of the total matrix collagen by cells would be expected in such a system. Moreover, in vivo studies on tight-skin mice wounds, which characteristically have delayed wound contraction of full-thickness wounds, have shown a marked decrease in collagenase content compared to control wounds, 32 an observation consistent with our finding that MMPs are required for contraction of three-dimensional matrices. However it is not clear why MMPs are important for three-dimensional but not two-dimensional matrix contraction.
It is not clear why MMPs are important for three-dimensional but not two-dimensional matrix contraction. The morphological and time-lapse studies of RPE-populated matrices reported here show cells in control matrices initially form small processes into the surrounding matrix (day 1). From day 2 they show longer processes penetrating deeper into the matrix and finally a stellate or a dedifferentiated spindle morphology is seen by day 7. In contrast, the cells in Galardin-treated matrices at day 7 exhibit small processes into their surrounding matrix and not a stellate or spindle shape. Although the precise function of MMPs in three-dimensional matrix contraction is unclear, it seems plausible that MMPs permit the penetration of cellular processes into adjacent matrix and enable reorganization of this matrix. Indeed, previous ultrastructural studies of RPE-populated matrices have revealed bundling or clumping of collagen fibers around RPE cells. 17 Moreover, we did not observe cell processes penetrating the underlying matrix in two-dimensional matrices. Therefore, we are undertaking ultrastructural studies of MMP inhibitor-treated matrices, to determine whether the collagen-bundling process is MMP-dependent. Our findings indicate that adhesive and collagenolytic cell-matrix interactions are required for three-dimensional matrix contraction but that MMP activity may not be necessary for two-dimensional matrix contraction. However adhesive interactions are still needed for contraction of the underlying collagen matrix. If collagen reorganization is key to matrix contraction, it might be expected that agents such as Galardin would inhibit two-dimensional contraction. However, the finding that MMP-blockade only inhibits three-dimensional contraction suggests that contraction is dependent on cell-matrix interactions other than collagen reorganization. Such mechanism purported to generate contractile forces is that of cell migration: it is known that migratory cells impart a force to their substrate as they move and it is suggested that this force compacts and contracts loose matrices. Cell migration both in and on a collagen matrix (ie, three-dimensional and two-dimensional systems) depends on α2β1 integrins and thus our finding that blockade of adhesive integrins in both systems is consistent with the cell migration theory of matrix contraction. In the two-dimensional system, cells may not require a path to be cleaved to permit locomotion whereas, in the three-dimensional system RPE may require such a path and hence MMPs to create such a path. One analogy that may explain the differences is between a person walking on a path (two-dimensional) system and a person walking through a jungle: only the latter would need to clear a pathway whereas both need focal contact between their feet and the ground to propel themselves forward.
Footnotes
Address reprint requests to Dr. C. M. Sheridan, Department of Medicine, University Clinical Departments, Royal Liverpool University Hospital, Duncan Building, Daulby Street, Liverpool, United Kingdom. L69 3GA. E-mail: carlos@liverpool.ac.uk.
Supported in part by the Foundation for the Prevention of Blindness, Guide Dogs for the Blind Association, Dunhill Medical Trust, International Glaucoma Association and Medical Research Council (grant G9330070).
References
- 1.: The Retina Society Terminology Committee: The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 1983, 90:121-125 [DOI] [PubMed] [Google Scholar]
- 2.Machemer R, Aaberg TM, MacKenzie Freeman H, Irvine AR, Lean JS, Michels RM: An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 1991, 112:159-165 [DOI] [PubMed] [Google Scholar]
- 3.Clarkson JG, Green WR, Massof D: A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol 1977, 84:1-17 [PubMed] [Google Scholar]
- 4.Machemer R, Van Horn DL, Aaberg TM: Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol 1978, 85:181-191 [DOI] [PubMed] [Google Scholar]
- 5.Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC: Epiretinal and vitreous membranes; comparative study of 56 cases. Arch Ophthalmol 1981, 99:1445-1454 [DOI] [PubMed] [Google Scholar]
- 6.Hiscott PS, Grierson I, Trombetta CJ, Rahi AHS, Marshall J, McLeod D: Retinal and epiretinal glia-an immunohistochemical study. Br J Ophthalmol 1984, 68:698-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerdan JA, Pepose JS, Michels RG, Hayashi H, de Bustros S, Sebag M, Glaser BM: Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 1989, 96:801-810 [DOI] [PubMed] [Google Scholar]
- 8.Morino I, Hiscott P, McKechnie N, Grierson I: Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol 1990, 74:393-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidenkummer HP, Kampik A: Comparative immunohistochemical studies of epiretinal membranes in proliferative vitreoretinal diseases. Fortschr Ophthalmol 1991, 88:219-224 [PubMed] [Google Scholar]
- 10.Grierson I, Mazure A, Hogg P, Hiscott P, Sheridan C, Wong D: Non-vascular vitreoretinopathy: the cells and the cellular basis of contraction. Eye 1996, 10:671-684 [DOI] [PubMed] [Google Scholar]
- 11.Hiscott P, Sheridan CM, Magee RM, Grierson I: Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Ret Res 1999, 18:167-190 [DOI] [PubMed] [Google Scholar]
- 12.Harris AK, Stopak D, Wild P: Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981, 290:249-251 [DOI] [PubMed] [Google Scholar]
- 13.Padgett LC, Lui GM, Werb Z, LaVail MM: Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in the retinal pigment epithelium and interphotoreceptor matrix: vectorial secretion and regulation. Exp Eye Res 1997, 64:927-938 [DOI] [PubMed] [Google Scholar]
- 14.Hunt RC, Fox A, Pakalnis VA, Sigel M, Kosnosky W, Choudury P, Black EP: Cytokines cause cultured retinal pigment epithelial cells to secrete metalloproteinases and to contract collagen cells. Invest Ophthalmol Vis Sci 1993, 34:3179-3186 [PubMed] [Google Scholar]
- 15.Webster L, Chignell AH, Limb GA: Predominance of MMP-1 and MMP-2 in epiretinal and subretinal membranes of proliferative vitreoretinopathy. Exp Eye Res 1999, 68:91-98 [DOI] [PubMed] [Google Scholar]
- 16.Kon CH, Occleston NL, Charteris D, Daniels J, Aylward GW, Khaw PT: A prospective study of matrix metalloproteinases in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1998, 39:1524-1529 [PubMed] [Google Scholar]
- 17.Mazure A, Grierson I: In vitro studies of the contractility of cell types involved in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1992, 33:3407-3416 [PubMed] [Google Scholar]
- 18.Edwards RB: Culture of mammalian retinal pigment epithelium and neural retina. Methods Enzymol 1982, 81:39-43 [DOI] [PubMed] [Google Scholar]
- 19.Boulton ME, Marshall J, Mellerio J: Human retinal pigment epithelial cells in tissue culture: a means of studying inherited retinal diseases. Birth Defects 1982, 18:101-118 [PubMed] [Google Scholar]
- 20.McKechnie NM, Boulton M, Robey HL, Savage FJ, Grierson I: The cytoskeletal elements of the human retinal pigment epithelium: in vitro and in vivo. J Cell Sci 1988, 91:303-312 [DOI] [PubMed] [Google Scholar]
- 21.Azzam HS, Thompson EW: Collagen-induced activation of the M(r) 72,000 type IV collagenase in normal and malignant human fibroblastoid cells. Cancer Res 1992, 52:4540-4544 [PubMed] [Google Scholar]
- 22.Schultz GS, Strelow S, Stern GA, Chegini N, Grant MB, Galardy RE, Grobelny D, Rowsey JJ, Stonecipher K, Parmley V: Treatment of alkali-injured rabbit corneas with a synthetic inhibitor of matrix metalloproteinases. Invest Ophthalmol Vis Sci 1992, 33:3325-3331 [PubMed] [Google Scholar]
- 23.Barletta JP, Angella G, Balch KC, Dimova HG, Stern GA, Moser MT, van Setten GB, Schultz GS: Inhibition of pseudomonal ulceration in rabbit corneas by a synthetic matrix metalloproteinase inhibitor. Invest Ophthalmol Vis Sci 1996, 37:20-28 [PubMed] [Google Scholar]
- 24.Wagner M, Benson MT, Rennie IG, MacNeil S: Effects of pharmacological modulation of intracellular signaling systems on retinal pigment epithelial cell attachment to extracellular matrix proteins. Curr Eye Res 1995, 14:373-384 [DOI] [PubMed] [Google Scholar]
- 25.Le Q, Shah S, Nguyen H, Cortez S, Baricos W: A novel metalloproteinase present in freshly isolated rat glomeruli. Am J Physiol 1991, 260:F555-F561 [DOI] [PubMed] [Google Scholar]
- 26.Hiscott P, Sheridan C: The retinal pigment epithelium, epiretinal membranes and proliferative vitreoretinopathy. Marmor MF Wolfensberger TJ eds. Retinal Pigment Epithelium—Current Aspects of Function and Disease. 1998, :pp 478-491 Harvard University Press, Cambridge [Google Scholar]
- 27.Kupper TS, Ferguson TA: A potential pathophysiologic role for alpha 2 beta 1 integrin in human eye diseases involving vitreoretinal traction. FASEB J 1993, 7:1401-1406 [DOI] [PubMed] [Google Scholar]
- 28.Hunt RC, Pakalnis VA, Choudhury P, Black EP: Cytokines and serum cause alpha 2 beta 1 integrin-mediated contraction of collagen gels by cultured retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1994, 35:955-963 [PubMed] [Google Scholar]
- 29.Grobelny D, Poncz L, Galardy RE: Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 1992, 11:7152-7154 [DOI] [PubMed] [Google Scholar]
- 30.Levy DE, Lapierre F, Liang W, Ye W, Lange CW, Li X, Grobelny D, Casabonne M, Tyrrell D, Holme K, Nadzan A: Matrix metalloproteinase inhibitors: a structure-activity study. J Med Chem 1998, 41:199-223 [DOI] [PubMed] [Google Scholar]
- 31.Guidry C, Grinnell F: Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 1985, 79:67-81 [DOI] [PubMed] [Google Scholar]
- 32.Agren MS, Mertz PM: Are excessive granulation tissue formation and retarded wound contraction due to decreased collagenase activity in wounds in tight-skin mice? Br J Dermatol 1994, 131:337-340 [DOI] [PubMed] [Google Scholar]