Abstract
Peritonitis causes mesothelial detachment that may result in persistent peritoneal denudation and fibrosis. We investigated whether hepatocyte growth factor (HGF), a scatter factor that induces detachment from substrate and fibroblastic transformation of several cell types, is produced during peritonitis and is active on mesothelial cells. We studied 18 patients on peritoneal dialysis, 9 uncomplicated, 9 with peritonitis. HGF was measured in serum, peritoneal fluid, and supernatant of peripheral blood mononuclear cells and peritoneal mononuclear cells. Primary culture of human peritoneal mesothelial cells and the human mesothelial cell line MeT-5A were conditioned with recombinant HGF, serum, and peritoneal fluid. HGF levels were significantly higher in serum and peritoneal fluid of peritonitic than uncomplicated patients. Mononuclear cells of peritonitic patients produced more HGF than cells of uncomplicated patients. Recombinant HGF, serum, and peritoneal fluid of peritonitic patients caused mesothelial cell growth, detachment, transformation from epithelial to fibroblast-like shape, overexpression of vimentin, and synthesis of type I and III collagen. In conclusion, HGF released during peritonitis causes a change in mesothelial cell phenotype and function. HGF may affect the healing process facilitating repair through mesothelial cell growth, but may contribute to peritoneal fibrosis inducing cell detachment with mesothelial denudation and collagen synthesis.
In patients on continuous ambulatory peritoneal dialysis (CAPD) recurrent episodes of peritonitis may result in peritoneal fibrosis and consequently in dialysis failure. 1,2 The pathogenic mechanisms accounting for the phenomenon are poorly understood, but loss of mesothelial cell adhesion with separation of cells from one another and detachment from the basal membrane, extracellular matrix deposition, and angiogenesis in the submesothelial layer are characteristic features of the process. 3 Although mesothelial cells are the victim of peritoneal inflammation, recent studies suggest that they participate as culprit in the fibrotic transformation of the peritoneal membrane. In fact, mesothelial cells can release inflammatory cytokines 4-7 and when exposed to peritoneal effluents obtained from patients on CAPD they can up-regulate transcription and expression of collagen. 8 However, the factor(s) that induces such collagenogenic activity is unknown.
Hepatocyte growth factor (HGF)/scatter factor was first identified as a mitogen for hepatocytes. It was then shown that the receptor for HGF is the product of met proto-oncogene, 9 that HGF stimulates the proliferation of other cell types (renal tubular cells, endothelial cells, some tumor cell lines) and that its biological effects include loss of cell adhesion from substrate, cell migration, transformation of cell into a fibroblast-like phenotype, angiogenesis, and morphogenesis. 10-12 Experimental and human studies have shown that HGF is released in organs acutely injured by toxic, inflammatory, or mechanical insults and works as a paracrine effector of tissue repair. 13,14 In addition, circulating substances released from the damaged tissue induce the production of HGF in distant intact organs, so that HGF secreted in an endocrine manner participates in tissue regeneration. 15 Cells of mesenchymal origin, such as Kupffer cells and pulmonary fibroblasts were first identified as the producers of HGF. 13,15 We have shown that glomerular mesangial cells 14 and peripheral blood mononuclear cells (PBMCs) activated by cytokines also are a source of HGF. 16
Peritonitis is an acute inflammatory injury that may stimulate both local and distant HGF production. HGF released during peritoneal inflammation may act on the peritoneum in several ways: it may induce proliferation of mesothelial cells, thus favoring remesothelization, or it may account for phenomena that forerun peritoneal fibrosis, i.e., mesothelial cells detachment with peritoneal denudation and submesothelial angiogenesis. Furthermore, mesothelial cells under the effect of HGF may assume a fibroblast-like phenotype and synthesize collagen. Based on these premises, our study was performed with two objectives: 1) to investigate whether HGF is released during peritonitis, and 2) to demonstrate that HGF produced during peritonitis causes phenotypic and functional changes in mesothelial cells.
Materials and Methods
Patients
The study was performed in 18 patients on CAPD, 9 of whom were studied during uncomplicated treatment (CAPD) and 9 during acute peritonitis (CAPDP). The diagnosis of peritonitis was based on clinical signs (cloudy peritoneal fluid, abdominal pain), leukocyte count >100/mm 3 and positive culture in peritoneal fluid. The two groups of patients were matched for age, time on CAPD, number of peritonitic episodes, and index of dialysis adequacy (KT/V). All underwent four exchanges of dialysate daily with a glucose concentration of 1.36 to 3.86 g/dl, according to the need of body fluid balance. Six normal volunteers (N) and six uremic patients on conservative treatment undergoing peritoneal catheter implantation (uremic/non-CAPD) were used as controls. Patients and controls had normal liver biochemical indexes, negative tests for hepatitis B and C virus infection, and denied alcohol abuse.
Experimental Design
The study was designed to investigate whether peritonitis causes release of HGF in blood and peritoneum, and stimulates PBMCs and peritoneal mononuclear cells (PtMCs) to produce HGF. In addition, we studied the effects of recombinant HGF (rHGF), serum, and peritoneal fluid on mesothelial cells in culture (growth, immunophenotype, morphology, collagen production, see later). Because commercial rHGF (R&D Systems, Minneapolis, MN) is available only in monomeric, biologically inactive form, we tested whether it is transformed into its dimeric active form after being added to mesothelial cell culture. To discriminate the specific effects of HGF contained in serum and peritoneal fluid, we designed experiments in which cell cultures were preincubated with anti-HGF antibody at neutralizing concentration, using mouse anti-human IgG antibody (DAKO, Glostrup Denmark) as control.
Peritoneal effluent was collected from patients on CAPD after overnight dwell. As control of peritoneal fluid collected from patients on CAPD we used the fluid (2 L) introduced into the peritoneal cavity just after catheter implantation in uremic patients on conservative treatment starting a CAPD program (uremic/non-CAPD peritoneal fluid). The uremic/non-CAPD peritoneal fluid was drained after 10 to 15 minutes dwell. Blood and peritoneal fluid samples were collected from peritonitic patients within 36 hours of appearance of clinical signs of peritonitis (turbid peritoneal fluid, abdominal tenderness, fever).
Part of the blood (5 ml) was centrifuged and the serum was stored at −80°C, part (20 ml) was used to isolate PBMCs. Similarly, an aliquot of cell-free peritoneal fluid (10 ml) was stored at −80°C and the rest was used to isolate PtMCs.
For conditioning mesothelial cells in culture, 2-ml aliquots of serum and 4-ml aliquots of peritoneal fluid sampled from single patients were mixed to form four final pools (normal, uremic/non-CAPD, uncomplicated CAPD, CAPD with peritonitis).
Mesothelial Cell Culture
Primary Culture of Human Peritoneal Mesothelial Cells (HPMCs)
Specimens of human omentum were obtained during elective abdominal surgery. After several washings with sterile phosphate-buffered saline (PBS) (Sigma) 4-to 5-cm 2 tissue segments were incubated with 10 ml of PBS and 10 ml of trypsin/ethylenediaminetetraacetic acid 1× (Sigma) at 37°C for 30 minutes on a shaker. The pieces of tissue were then discarded and the solution containing cells in suspension was centrifuged at 1500 rpm for 10 minutes. The pellet was washed twice in RPMI 1640 (Sigma). After final resuspension the cells were planted in 75-cm 2 flasks (Corning, Cambridge, MA) with 20 ml of RPMI 1640, 20% fetal calf serum (FCS), 1% Insulin Transferrin Selenium-S (ITS) (Sigma), 1% penicillin/streptomycin. After 48 hours cells in suspension and microscopic tissue fragments were removed with culture medium. The adhering cells were cultured and characterized at passage 3 using the following monoclonal antibodies: mouse anti-human cytokeratin [diluted 1:2 in PBS/bovine serum albumin (BSA) 1%; Becton-Dickinson, Franklin Lakes, NJ, USA], mouse anti-human factor VIII (diluted 1:40 in PBS/BSA 1%; DAKO), mouse anti-human antigen mesothelial cell HMBE1 (diluted 1:1000 in PBS/BSA 1%; DAKO). HPMCs were positive for cytokeratin and HMBE1 and negative for factor VIII. The cells were used for experiments at early passages 4-5 to minimize dedifferentiation and modification of original phenotype.
Human Mesothelial Cell Line MeT-5A
Human mesothelial cell line MeT-5A (ATCC, Rockville, MD) was cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (1:1) medium (Sigma) supplemented with 10% FCS at 37°C in a 5% CO2 humidified incubator (Celbio, Milan, Italy).
In the various experiments designed (see later) the culture medium of HPMCs and MeT-5A was supplemented with rHGF at scalar concentrations of 0, 3, 7, 10, and 50 ng/ml and pooled serum or pooled peritoneal fluid (20%). Fifty μg/ml of anti-HGF antibody (Sigma) were added in neutralization experiments.
PtMCs Preparation and Culture
PtMCs were isolated from peritoneal fluid collected after overnight dwell in patients on CAPD, and from uremic/non-CAPD peritoneal fluid. Peritoneal fluid was filtered through sterile gauze and centrifuged at 1800 rpm for 30 minutes at 4°C. The cell pellet was washed three times with PBS, centrifuged at 1500 rpm for 8 minutes at 4°C, layered on lymphocyte separation medium (Hystopaque, Sigma) and centrifuged again at 1500 rpm for 35 minutes at 4°C. Cell subpopulations were studied by hemocytometer and yielded the following cell types: uremic/non-CAPD, lymphocytes 49 to 53%, monocytes 45 to 51%, neutrophils 1 to 6%; uncomplicated CAPD, lymphocytes 46 to 52%, monocytes 45 to 52%, neutrophils 2 to 5%; CAPD with peritonitis, lymphocytes 46 to 51%, monocytes 42 to 50%, neutrophils 4 to 6%. Cell viability was tested by trypan blue (Sigma) exclusion test and yielded 95 to 98% viable cells. The mononuclear cell layer was harvested and washed with calcium- and magnesium-free PBS, finally the cells were resuspended at a concentration of 1 × 106/ml in Iscove’s medium (Gibco, Milan, Italy), containing 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5% heat-inactivated FCS.
PtMC cultures were incubated for 48 hours at 37°C in 5% CO2 and the supernatant was collected and stored at −80°C.
PBMC Isolation and Culture
We have described methods of PBMC culture in detail previously. 16 Briefly, PBMCs were separated by Hystopaque gradient and cultured in suspension (1 × 106/ml) for 48 hours in Iscove’s containing 100 IU/ml penicillin and 100 μg/ml streptomycin to which 5% decomplemented FCS was added. Hemocytometry yielded 80 to 87% lymphocytes, 11 to 17% monocytes, and 3 to 5% neutrophils. Cell viability was tested by trypan blue exclusion test and yielded 96 to 98% viable cells.
Cell-Free Peritoneal Fluid Preparation
After collection, peritoneal fluid was centrifuged at 1500 rpm for 15 minutes at 4°C. Supernatant was filtered through 0.2-μm cellulose acetate filters (Corning) to eliminate cells and cell debris.
HGF Measurement
HGF concentration was measured in serum, peritoneal fluid, and cell (PtMC and PBMC) culture supernatant using a commercial Enzymatic Immuno Assay (EIA) (R&D Systems), as described previously. 14,16 The EIA detects 0.1 ng/ml of purified HGF and displays no cross-reactivity with a wide variety of cytokines.
Mesothelial Cell Studies
Cell Growth Assay
HPMC (1 × 104/cm2) and MeT-5A (3 × 103/cm2) were planted in 96-well dishes (Corning) and stimulated with rHGF at scalar concentrations (0, 3, 7, 10, 50 ng/ml), pooled serum, and pooled peritoneal fluid. After 48 hours cell growth was evaluated with Cell Titer 96 assay (Promega Biotec, Madison, WI). The assay is based on a colorimetric method determining the number of viable cells. 17 The [3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenil)-2-C4-sulfophenyl)-2H] (MTS) tetrazolium compound (Owen’s reagent) is bioreduced by metabolic active cells into a colored formazan product that is soluble in culture medium. Color intensity recorded at 570 nm is proportional to number of viable cells. Experiments were repeated eight times with each dose of rHGF and each pool of serum and peritoneal fluid.
Morphology
HPMCs (1 × 104/cm2) at passage 5 and MeT-5A (3 × 103/cm2) were planted in 6-well dishes and stimulated with scalar concentrations of rHGF, and the pools of serum and peritoneal fluid. After 48 hours the cells were studied at inverted microscopy and photographed.
Immunocytochemistry
HPMCs (1 × 104/cm2) and MeT-5A (3 × 103/cm2) were planted on chamber slides (Nunc, Roskilde, Denmark) and stimulated for 48 hours with scalar concentrations of rHGF, and the pools of serum and peritoneal fluid, with and without the addition of anti-HGF antibody at neutralizing concentration. HPMCs were subsequently fixed in formalin-acetate 6% for 10 minutes, washed in PBS, and incubated in the dark-humid chamber at room temperature for 60 minutes with mouse monoclonal antibody against vimentin (DAKO) 1:20 v/v in PBS/BSA 1%, and pan-cytokeratin 1:20 v/v in PBS/BSA 1%. After three further washings in PBS, the cells were treated with the secondary antibody and the complex streptavidin-biotin-peroxidase according to the manufacturers of the LSAB+ kit (DAKO). Visualization was in 3,3 diaminobenzidine. Harris hematoxylin was used to counterstain the nuclei lightly. Finally the cells were dehydrated in increasing alcohol scale (95°C to 100°C, xylol) and the coverslip was mounted with synthetic nonaqueous mounting media (DAKO) for analysis with a Zeiss microscope. All experiments were quadruplicated.
Collagen Synthesis
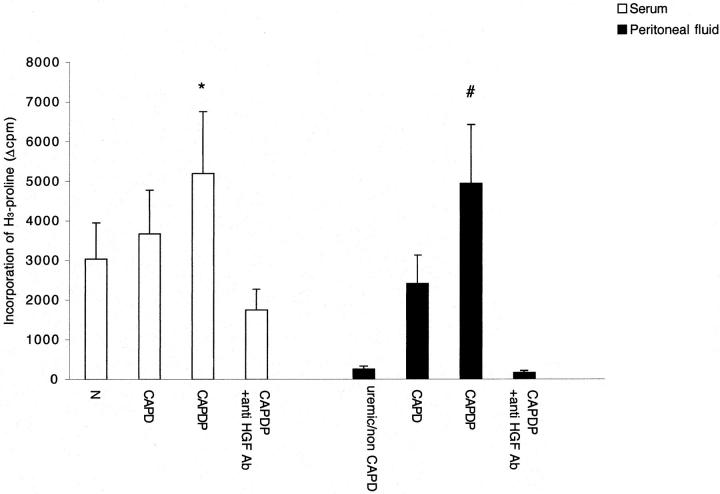
MeT-5A grown for 35 days in proline-free DMEM (Sigma) were planted (3 × 105/dish) in 35-mm dishes (Falcon, Oxnard, CA) and cultured for 48 hours in ascorbate-supplemented (80 μg/ml) medium containing scalar concentrations of rHGF, the pools of serum, and peritoneal fluid. Then, the medium in each dish was substituted for a fresh one containing marked solution 0.7 ml made of 0.21 ml of 3H-proline (1 mCi; (Amersham Corp., Arlington Heights, IL) and 4.2 ml of DMEM, and 0.1 ml of vitamin C (80 μg/ml) and 1.2 ml of DMEM. After incubation for 24 hours at 37°C 70 μl of a commercial mixture 10× of protease inhibitors (benzamidine, ethylenediaminetetraacetic acid, phenylmethyl sulfonyl fluoride, N-ethylmaleimide, Sigma) were added in each dish. Then, the supernatant was collected, 20 μl of collagen carrier (native collagen used to facilitate protein precipitation, 1 mg/ml; Sigma) were immediately added to supernatant and proteins were precipitated by adding ethanol (415 μl) for 1 hour at 4°C. After centrifugation at 13,000 rpm for 10 minutes, the pellet containing precipitated proteins was collected and resuspended in 600 μl of 0.5 mol/L acetic acid solution containing 100 μg/ml pepsin (Sigma), i.e., an enzyme that digests all proteins but collagen ones. After overnight incubation at 4°C, the digestion was stopped by adding 600 μl of 4 mol/L NaCl solution in 1 mol/L acetic acid. Samples were then centrifuged at 13,000 rpm at 4°C and supernatant was discarded and substituted for 100 μl of sample buffer. Samples were warmed at 80°C for 5 minutes to achieve denaturation, then allowed to return to room temperature and centrifuged again (10 minutes at 13,000 rpm) to achieve the final samples. Five μl of each final sample were added to 5 ml of scintillation solution (Ecolume; ICN Pharmaceutical Inc., Irvine, CA) and radioactivity was counted (10 minutes, double reading) in a Packard 1500 Tri-Carb liquid scintillation analyzer. A corresponding reagent blank was prepared for each counting condition and subtracted as background. Experiments were repeated eight times with each pool of serum and peritoneal fluid.
Collagen Protein Electrophoresis
Aliquots of the final samples containing identical levels of radioactivity were used to analyze the types of procollagen by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Samples were run at 15 mA for 30 minutes in glycine sodium dodecyl sulfate-Tris buffer. The gel was washed with dimethyl sulfoxide two times for 30 minutes and then treated for 3 hours with 2,5 diphenyloxazole solution (15% in dimethyl sulfoxide). After washing with water for 1 hour and drying, autoradiography was performed on a Kodak Safety film (Eastman Kodak, Rochester, NY). The photograph plate was exposed for 8 days. The control consisted of supernatant of cultured human skin fibroblasts containing procollagen type I and type III, kindly provided by Dr. G. Cetta, Department of Biochemistry, University of Pavia. The methods by which these fibroblasts are grown and their collagen production is characterized have been described in detail. 18
Expression of HGF Receptor
The expression of the HGF receptor c-met was investigated in HPMCs by Western blot analysis. Cells (2 × 106) were lysed in lysis buffer containing 20% glycerol, 30% sodium dodecyl sulfate 10×, 12.5% Upper Tris, 0.25 TIU/ml aprotinin, and 10 mg/ml leupeptin. Aliquots of lysate (40 μl) were size-fractionated by sodium dodecyl sulfate-gel electrophoresis (3 to 10% gradient gels) and the proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were blocked with 5% skim milk and incubated overnight at 4°C with mouse anti-c-met monoclonal antibody diluted 1:20 in PBS/BSA 1% (Novocastra, Newcastle on Tyne, UK). After three washings with PBS, the membranes were incubated at room temperature with the secondary antibody and the complex streptavidin-biotin-peroxidase according to the manufacturers of the LSAB+ Kit (DAKO). Visualization was in 3,3 diaminobenzidine.
Statistical Analysis
Analysis of variance and Tukey Kramer test were used for comparison of the means.
Results
After being added to mesothelial cell culture, rHGF is transformed into its active form. The transformation is complete at 48 hours as shown by Western blot analysis performed on supernatant of HPMC cultures (Figure 1) ▶ .
Figure 1.
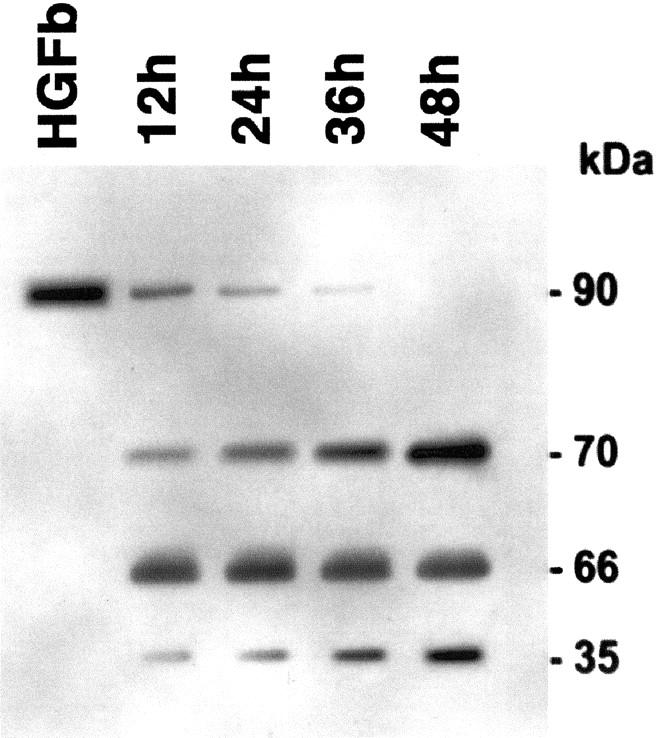
Western blot analysis of commercial recombinant HGF before (HGF b) and after being added to supernatant of HPMC culture for 12 hours, 24 hours, 36 hours, and 48 hours. The 90-kD band represents monomeric pro-HGF (inactive form); the 70- and 35-kD bands are the two chains of the dimeric active form of HGF; the 66-kD band is BSA.
Effects of Peritonitis on HGF Release
The levels of HGF in serum, peritoneal fluid and supernatants of PBMCs and PtMCs are shown in Table 1 ▶ . CAPD per se did not raise HGF levels either in serum or in peritoneal fluid, whereas HGF concentration increased significantly in serum and peritoneal fluid of patients on CAPD complicated with peritonitis. Similarly, PBMCs and PtMCs collected from patients on uncomplicated CAPD did not produce amounts of HGF different from their respective controls, whereas the amounts of HGF released by PBMCs and PtMCs collected during peritonitis were significantly increased.
Table 1.
HGF Levels (ng/ml) in Serum, Peritoneal Fluid, and Supernatant of PBMCs and PtMCs
| Non-CAPD | CAPD | CAPDP | |
|---|---|---|---|
| Serum | 0.94 ± 0.12 | 1.10 ± 0.15 | 2.90 ± 0.12* |
| Peritoneal fluid | 0.13 ± 0.04 | 0.25 ± 0.06 | 2.5 ± 0.90* |
| PBMC supernatant | 0.12 ± 0.05 | 0.12 ± 0.05 | 0.40 ± 0.08* |
| PtMC supernatant | 0.29 ± 0.04 | 0.40 ± 0.06 | 0.92 ± 0.06* |
Non-CAPD are samples from controls not treated with peritoneal dialysis (normal volunteers for serum and PBMC supernatant; uremic patients on conservative treatment for peritoneal fluid and PtMC supernatant; see Methods for details); CAPD are samples from patients on peritoneal dialysis without peritonitis; CAPDP are samples from patients on peritoneal dialysis with peritonitis. Numbers are means ± SD. *P < 0.001 versus CAPD and non-CAPD.
Effect of rHGF, Serum, and Peritoneal Fluid on Mesothelial Cells
Cell Growth
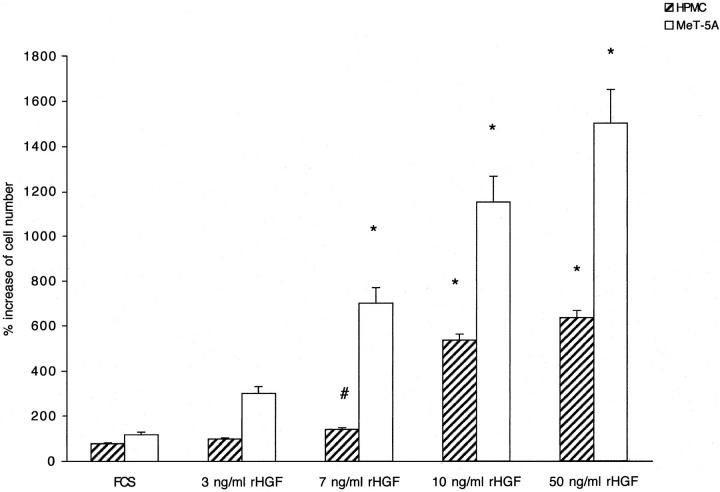
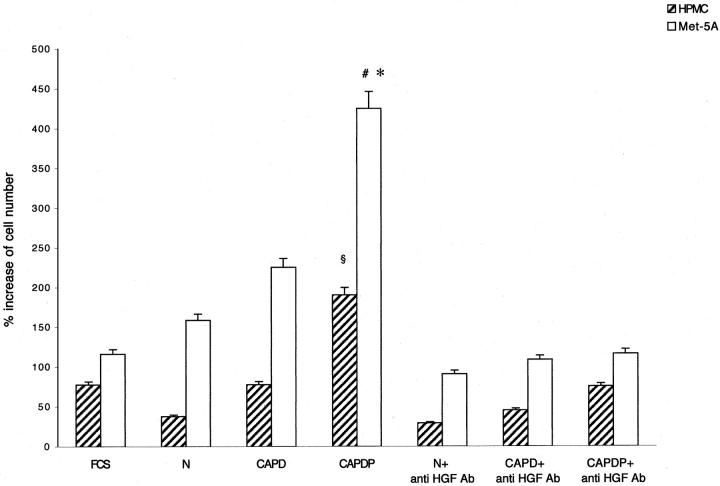
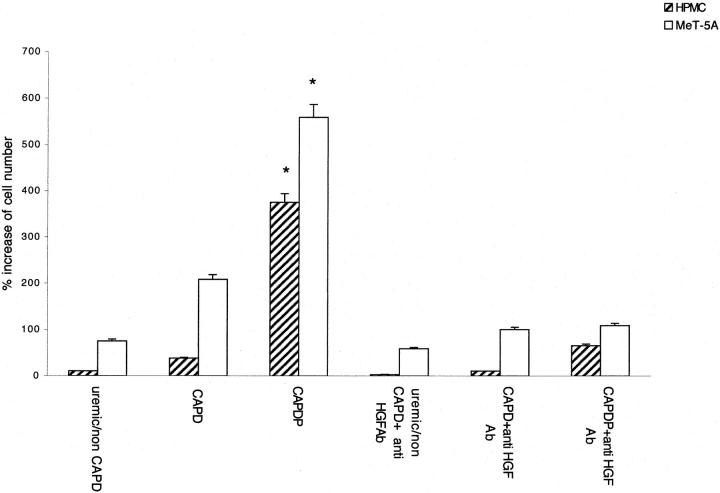
The addition of rHGF to culture medium of both HPMCs and MeT-5A caused a growth of mesothelial cells in dose-dependent manner (Figure 2) ▶ . The cell growth was completely abolished by anti-HGF antibody (not shown). Incubation for 48 hours with serum of patients affected by peritonitis, but not with normal serum or serum of patients on CAPD without peritonitis, was associated with a significant growth of HPMCs and MeT-5A. The growth was abolished by anti-HGF antibody (Figure 3) ▶ . Similarly, incubation for 48 hours with peritoneal fluid collected from patients during peritonitis stimulated HGF-dependent cell growth (the effect was abolished by anti-HGF antibody), whereas no growth was caused by uremic/non-CAPD fluid and fluid collected from patients on uncomplicated CAPD (Figure 4) ▶ .
Figure 2.
Effect of recombinant HGF (rHGF) on growth of mesothelial cells. Hatched columns, HPMC; open columns, MeT-5A. Columns are means of eight repeated experiments; bars, SD. *, P < 0.001; #, P < 0.005 versus FCS.
Figure 3.
Effect of serum on growth of mesothelial cells. Hatched columns, HPMC, open columns, MeT-5A. Columns are means of eight repeated experiments; bars, SD. N, serum of normal volunteers; CAPD, serum of patients on peritoneal dialysis without peritonitis; CAPDP, serum of patients on peritoneal dialysis with peritonitis. §, P < 0.001 versus all other HPMC. *, P < 0.001 versus MeT-5A FCS, N, and CAPDP + anti-HGF antibody. #, P < 0.01 versus MeT-5A CAPD.
Figure 4.
Effect of peritoneal fluid on growth of mesothelial cells. Hatched columns, HPMC; open columns, MeT-5A cells. Columns are means of eight repeated experiments; bars, SD. Uremic/non-CAPD, uremic patients on conservative treatment; CAPD, patients on peritoneal dialysis without peritonitis; CAPDP, patients on peritoneal dialysis with peritonitis. *, P < 0.001 versus uremic/non-CAPD, CAPD, and CAPDP + anti-HGF antibody.
Morphology
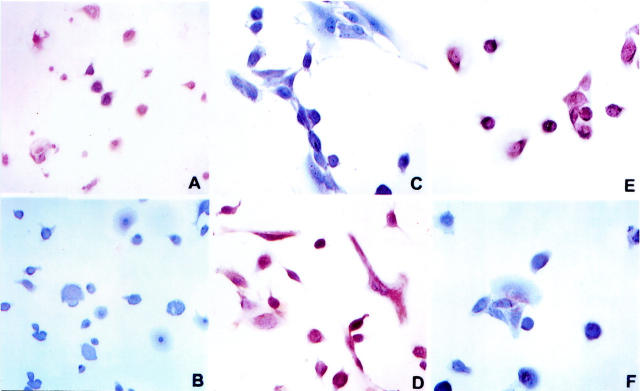
Confluent MeT-5A cells cultured in control medium were polygonal, adherent to the dish, and formed a cobblestone, epithelium-like sheet (Figure 5A) ▶ . The addition of rHGF caused the loss of adherence in several cells that became floating in the medium. The cells that remained attached spread and lost their normal polygonal form, becoming elongated, stellate, or spindle-like. These effects were dose-dependent and were maximal at rHGF concentrations of 50 ng/ml (Figure 5B) ▶ . A similar transformation into fibroblast-like cells was observed in cells cultured for 48 hours with serum and peritoneal fluid collected from CAPD patients during peritonitis (Figure 5, C and D) ▶ and these changes were abolished by anti-HGF antibody (Figure 5, E and F) ▶ . Recombinant HGF, serum, and peritoneal fluid of patients with peritonitis caused transformation into a fibroblast-like phenotype also of HPMCs and the transformation was abolished by anti-HGF antibody (Figure 6 ▶ ; A to F). Incubation of cells with serum of normal patients, serum of patients on uncomplicated CAPD, uremic/non-CAPD fluid, and peritoneal fluid from patients on uncomplicated CAPD did not affect Met-5A or HPMC morphology.
Figure 5.
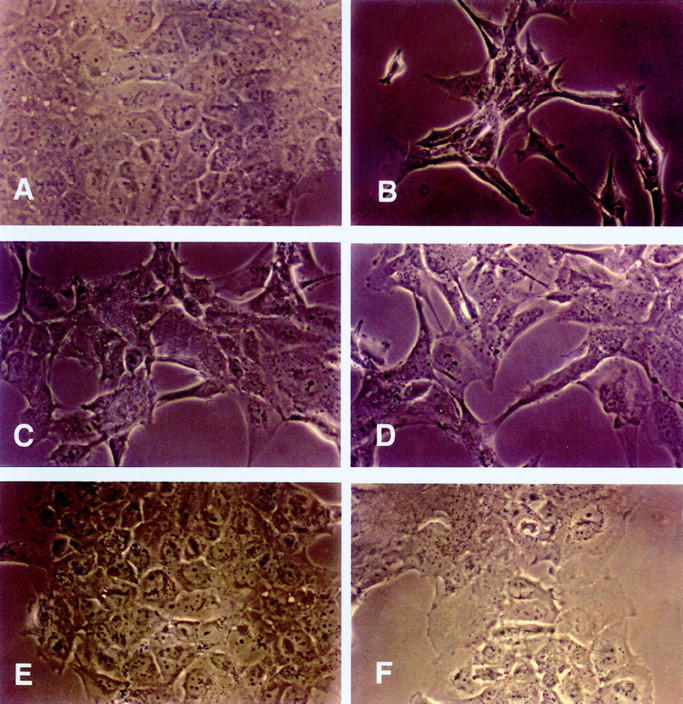
Light micrograph of cultured MeT-5A cells conditioned with FCS (A), recombinant HGF (50 ng/ml) (B), serum (C) and peritoneal fluid (D) of patients with peritonitis, serum (E) and peritoneal fluid (F) of patients with peritonitis + anti-HGF antibody. Original magnifications: ×20 (A, E, F) and ×40 (B, C, D).
Figure 6.
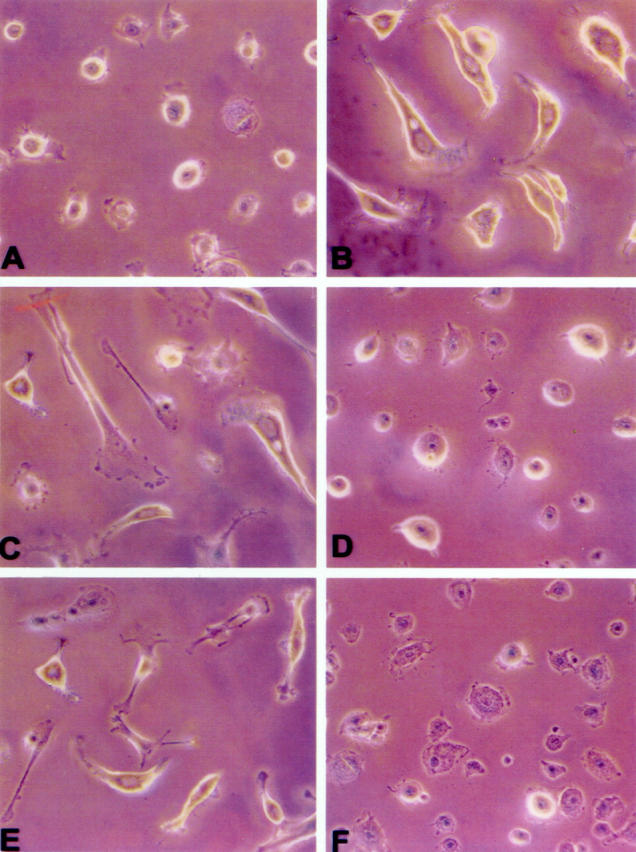
Light micrograph of HPMC conditioned with FCS (A), recombinant HGF (50 ng/ml) (B), serum and peritoneal fluid of patients with peritonitis (C and E, respectively), serum and peritoneal fluid of patients with peritonitis + anti-HGF antibody (D and F, respectively). Original magnifications, ×20.
Expression of Intermediate-Type Filaments
HPMCs expressed constitutively cytokeratin (Figure 7A) ▶ but not vimentin (Figure 7B) ▶ . Peritoneal fluid of peritonitic patients added to HPMC culture abolished the constitutive expression of cytokeratin (Figure 7C) ▶ and caused de novo expression of vimentin (Figure 7D) ▶ ; the phenomenon was prevented by neutralizing anti-HGF antibody (Figure 7, E and F) ▶ . Serum of peritonitic patients caused in HPMCs the same modification in the expression of intermediate-type filaments as their peritoneal fluid (not shown). In contrast, serum or peritoneal fluid of patients on CAPD without peritonitis caused no modification.
Figure 7.
Micrograph showing the effect of peritoneal fluid of peritonitic patients on expression of intermediate-type filaments in HPMC. Original magnifications, ×20. Cells were incubated with anti-cytokeratin (A, C, E) or anti-vimentin (B, D, F) antibody, followed by secondary biotinylated antibody and the streptavidin-diaminobenzidine reaction that stains positive cells in brown. Cells cultured with uremic/non-CAPD peritoneal fluid express cytokeratin (A), but not vimentin (B). Addition in the cell cultures of peritoneal fluid of peritonitic patients suppresses the constitutive expression of cytokeratin (C) and causes de novo expression of vimentin (D). Anti-HGF antibody prevents the suppression of cytokeratin (E) and the expression of vimentin (F) induced by peritoneal fluid of peritonitic patients. Note the fibroblastic transformation of cells treated with peritoneal fluid of peritonitic patients.
In confluent MeT-5A constitutive expression of vimentin was low whereas that of cytokeratin was high. Incubation with rHGF, serum, and peritoneal fluid of patients with peritonitis caused a marked increase of expression of vimentin (not shown).
Collagen Synthesis
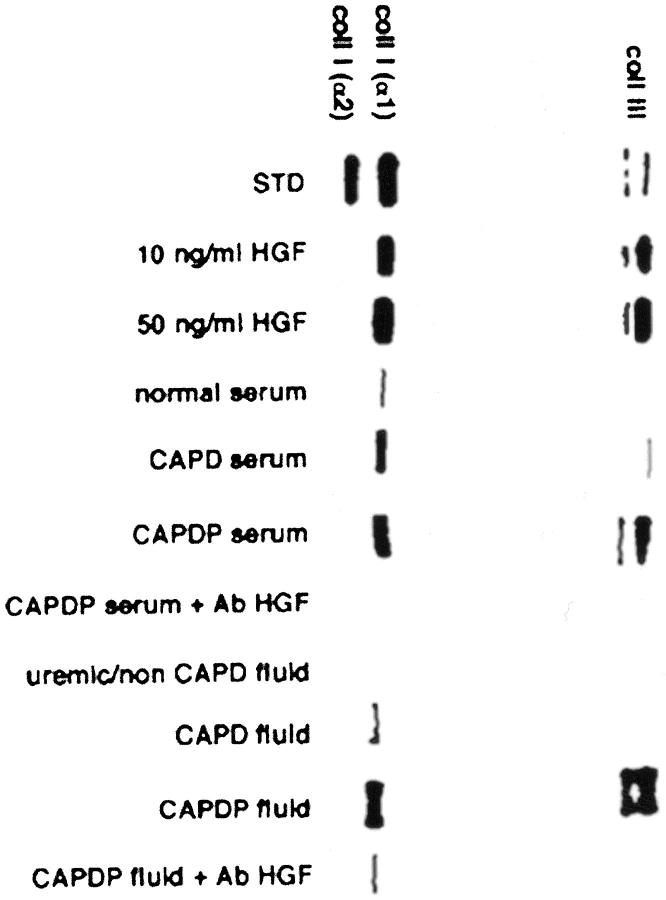
Collagen synthesis was evaluated by tritiated proline incorporation and collagen protein electrophoresis. rHGF stimulated collagen synthesis in a dose-dependent manner (3 ng/ml increased proline incorporation by 2.7 times, 10 ng/ml by 8.9 times and 50 ng/ml by 20.8 times, respectively). As shown in Figure 8 ▶ , serum of patients with peritonitis induced more collagen synthesis than serum of normal patients and serum of patients on uncomplicated CAPD. Similarly, peritoneal fluid collected from patients with peritonitis stimulated collagen synthesis more that uremic/non-CAPD fluid and peritoneal fluid collected from patients on uncomplicated CAPD. The stimulation of collagen synthesis caused by serum and peritoneal fluid of peritonitic patients was abolished by anti-HGF antibody. Mesothelial cells stimulated by rHGF, serum, and peritoneal fluid of patients with peritonitis synthesized and secreted collagen type III and type I, the latter in a homotrimeric α1(I)3 form (Figure 9) ▶ .
Figure 8.
Effect of serum (open columns) and peritoneal fluid (filled columns) on collagen production in supernatant of cultured MeT-5A cells. Columns are means of eight repeated experiments; bars, SD. N, normal volunteers; CAPD, patients on peritoneal dialysis without peritonitis; CAPDP, patients on peritoneal dialysis with peritonitis; uremic/non-CAPD, uremic patients on conservative treatment. *, P < 0.05 versus N, CAPD, and CAPDP + anti-HGF antibody; #, P < 0.001 versus uremic/non-CAPD, CAPD, and CAPDP + anti-HGF antibody.
Figure 9.
Electrophoresis of collagen proteins in MeT-5A cell culture supernatant. Supernatant of cultured fibroblasts was used as standard (STD) for the bands of types I and III collagen. Recombinant HGF (10 and 50 ng/ml), serum, and peritoneal fluid of patients with peritonitis (CAPDP serum, CAPDP fluid) increased the production of collagen types I and III, and the phenomenon was abolished by neutralizing anti-HGF antibody (Ab HGF). Note that type I collagen produced by mesothelial cells consists only of α1 chain, i.e., is a homotrimeric (α1)3 form.
Expression of HGF Receptor
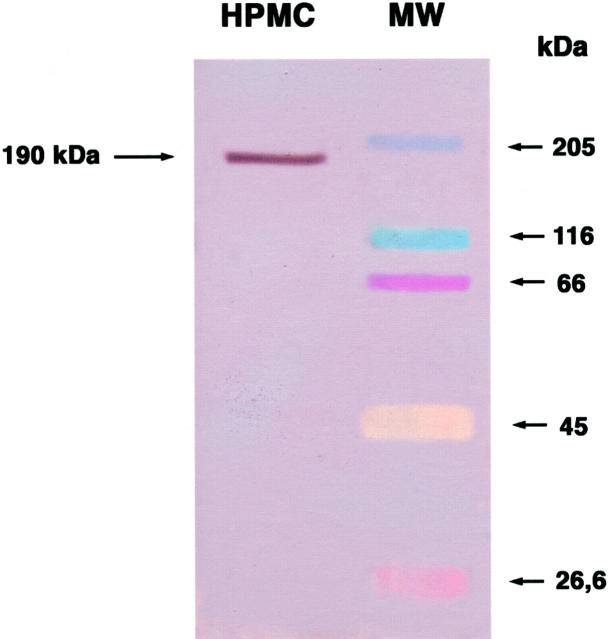
Western blot analysis of HPMC lysate with monoclonal anti-c-met antibody showed that HPMCs constitutively express HGF receptor (Figure 10) ▶ .
Figure 10.
Western blot analysis of HPMC lysate. Incubation of lysate with anti-c-met monoclonal antibody showed a distinct band of 190 kd (brown color, left lane) corresponding to c-met. The colored bands in the right lane are molecular weight markers.
Discussion
Our study shows that inflammatory injury of the peritoneum stimulates the release of HGF both locally and in blood. In fact, HGF levels rise significantly in peritoneal fluid and in serum within 36 hours of the onset of peritonitis. We reported previously that PBMCs can produce HGF. 16 Here we give evidence that both peripheral blood and peritoneal mononuclear cells (PtMCs) participate in HGF production associated with peritoneal inflammation.
Understanding the mediators that stimulate HGF production during peritonitis was not the aim of the present study. Actually, the nature of the factors that cause HGF release is still quite obscure. A substance with molecular mass of 10 to 20 kDa inducing expression of the gene for HGF has been purified in rat serum. 19 We have reported that cytokines, i.e., interleukin (IL)-1, IL-6, tumor necrosis factor-α, and IL-8 are circulating HGF inducers in man. 20 Because the production of these cytokines is stimulated by peritonitis, 21-24 we suggest that cytokines are responsible for HGF release during peritonitis.
To understand whether the release of HGF occurring during peritonitis is a biologically relevant phenomenon, we studied the effects of rHGF on mesothelial cell phenotype and function and investigated whether the changes induced by rHGF are reproduced by HGF released in serum and peritoneal fluid. HGF is known to act as a mitogen for hepatocytes, renal tubular cells, keratinocytes, melanocytes, endothelial cells, and several epithelial cell lines in culture 11,13,19,25,26 . Recently, it has been shown that HGF induces proliferation of pleural mesothelial cells. 27 Here we demonstrate that HPMCs express the receptor for HGF c-met, and that rHGF stimulates the growth of peritoneal mesothelial cells in a dose- and time-dependent manner. In addition, we show that serum and peritoneal fluid of patients with peritonitis induce mesothelial cell growth and that HGF accounts for the phenomenon, because cell growth is abrogated by anti-HGF antibody.
HGF promotes the acquisition of a fibroblast-like phenotype in epithelial cells, i.e., induces the epithelial-mesenchymal transition 28 and is named “scatter factor” for its ability to cause loss of cell adhesion and cell motility. 11,12 Rounding up, loss of adhesion, and detachment from substrate was observed in a mesothelial cell line exposed to peritoneal fibroblast-conditioned media, and it was suggested that HGF accounted for these effects because they were partly inhibited by anti-HGF neutralizing antibody. 29 We demonstrate that rHGF causes morphological changes and scattering of mesothelial cells, both in a cell line and in primary culture of peritoneal cells. In fact, incubation with rHGF causes transformation of mesothelial cells to a stellate or spindle-like shape, similar to that of fibroblasts. In addition, rHGF causes in confluent cultures loss of cell adhesion, cell detachment, and floating. The same morphological changes and loss of adhesion are induced by serum and peritoneal fluid obtained from patients on CAPD with peritonitis, but not by their respective controls and are prevented by neutralization of HGF with anti-HGF antibody.
Expression of cytokeratin is a distinctive feature of mesothelial cells that allows their differentiation from other cells of mesodermal origin. 30 Expression of vimentin in mesothelial cells is not univocal, because vimentin is not detectable in normal intact mesothelial tissue, but may be found in primary cultures of mesothelial cells and mesothelial cell lines. 31 Because in epithelial cells the transition to the mesenchymal phenotype induced by HGF is associated with the progressive replacement of the cytokeratin network with vimentin, 32 we have investigated whether HGF induces a similar rearrangement of intermediate filament proteins in mesothelial cells. In primary culture of human mesothelial cells, cytokeratin was expressed and vimentin was absent in constitutive conditions, whereas cytokeratin was suppressed and vimentin was induced by HGF present in serum and peritoneal fluid of peritonitic patients. The combined observations that mesothelial cells treated with rHGF or biological fluids containing high HGF levels assume a spindle-like or stellate conformation and overexpress vimentin, i.e., a characteristic fibroblastic marker, indicate that HGF induces in mesothelial cells a process of transformation to mesenchymal/fibroblast phenotype.
To evaluate whether the new cell phenotype acquired by HGF-conditioned cells is also associated with specific mesenchymal functions, we investigated the capacity of HGF to induce collagen synthesis in mesothelial cells. Previous indirect evidence suggested that HGF may increase the synthesis of collagen proteins and induce fibrosis. In fact, autocrine release of HGF induces overexpression of fibronectin mRNA in renal tubular cells transfected with human HGF cDNA, 33 and transgenic mice overexpressing HGF undergo histopathological and ultrastructural changes in the kidney consistent with an increase in mesangial matrix and glomerulosclerosis. 34 In contrast, it has been shown that HGF prevents fibrosis in liver and pulmonary injury models 35-37 and mediates in part the antifibrotic effect of angiotensin II blockade in a model of cardiomyopathy. 38 Our study demonstrates that rHGF induces a dose-dependent synthesis of collagen in mesothelial cells. The production of collagen is stimulated also by serum and peritoneal fluid of patients with peritonitis and is prevented by anti-HGF antibody. The collagen proteins produced by mesothelial cells are type III and type I (α1)3, i.e., the two types, especially type III, that are assembled as fibrotic tissue in organs exposed to chronic inflammation. 39
The release of HGF during peritonitis and the evidence that HGF acts on mesothelial cells suggest that HGF influences in several ways the process of healing of the peritoneum, especially in patients undergoing peritoneal dialysis. In fact, mesothelial cell growth stimulated by HGF may facilitate the replacement of damaged cells and account for the prompt recovery of mesothelial integrity that usually occurs after single, not severe episodes of peritonitis. However, other effects of HGF may contrast, rather than facilitate, the restoration of peritoneal normality in patients on CAPD. In fact, collagen production by mesothelial cells transformed into fibroblast-like cells may contribute to the submesothelial deposition of new collagen. In addition, in cases of severe or repeated episodes of peritonitis, that cause large gaps in the continuity of mesothelial layer, remesothelization by contiguous vital cells may be hampered by HGF. In fact, whereas in solid organs the loss of cell adhesion induced by HGF facilitates tissue repair, by allowing spreading of regenerating cells, the loss of adherence and detachment of vital mesothelial cells, followed by their removal with drained dialytic fluid may result in delayed healing and persistent mesothelial denudation. The failure of remesothelization, in turn, seems to play a pivotal role in the pathogenesis of peritoneal tanning and fibrosis. 2
In summary, our study gives evidence that the HGF repair system is activated during peritonitis and demonstrates that HGF has relevant effects on the phenotype and function of mesothelial cells. Although our results suggest that HGF may interfere in several ways with the process of peritoneal healing, further studies are to be designed to understand the clinicopathological impact of its pleiotropic actions in the wide panorama of clinical settings depicted as peritonitis.
Acknowledgments
We thank Prof. Giuseppe Cetta and Dr. Antonio Rossi, Department of Biochemistry, University of Pavia, who provided cultured fibroblasts and helped characterization of type of collagen in mesothelial cell culture supernatant.
Footnotes
Address reprint requests to Prof. Antonio Dal Canton, M.D., Unità di Nefrologia Dialisi e Trapianto, Policlinico San Matteo, 27100 Pavia, Italy. E-mail: dalcanton@mbox.medit.it.
This study was presented as a poster at the XVth International Congress of Nephrology, Buenos Aires, Argentina, May 2 to 9, 1999.
References
- 1.Di Paolo N, Sacchi G, De Mia M, Gaggiotti E, Capotondo L, Rossi P, Bernini M, Pucci AM, Ibba L, Sabatelli F, Alessandrini C: Morphology of the peritoneal membrane during continuous ambulatory peritoneal dialysis. Nephron 1986, 44:204-211 [DOI] [PubMed] [Google Scholar]
- 2.Dobbie JW, Anderson JD, Hind C: Long-term effects of peritoneal dialysis on peritoneal morphology. Perit Dial Int 1994, 14(Suppl 3):S16-S20 [PubMed] [Google Scholar]
- 3.Verger C, Luger A, Moore HL, Nolph KD: Acute changes in peritoneal morphology and transport properties with infectious peritonitis and mechanical injury. Kidney Int 1983, 23:823-831 [DOI] [PubMed] [Google Scholar]
- 4.Topley N, Jörres A, Luttmann W, Petersen MM, Lang MJ, Thierauch K-H, Müller C, Coles GA, Davies M, Williams JD: Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1β and TNF α. Kidney Int 1993, 43:226-233 [DOI] [PubMed] [Google Scholar]
- 5.Jonjic N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A: Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med 1992, 176:1165-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topley N, Brown Z, Jörres A, Westwick J, Davies M, Coles GA, Williams JD: Human peritoneal mesothelial cells synthesize interleukin-8: synergistic induction by interleukin 1-β and tumor necrosis factor-α. Am J Pathol 1993, 142:1876-1886 [PMC free article] [PubMed] [Google Scholar]
- 7.Lanfrancone L, Boraschi D, Ghiara P, Falini B, Grignani F, Peri G, Mantovani A, Pelicci PG: Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage-CSF, IL-1[IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood 1992, 80:2835-2842 [PubMed] [Google Scholar]
- 8.Perfumo F, Altieri P, Degl’Innocenti ML, Ghiggeri GM, Caridi G, Trivelli A, Gusmano R: Effects of peritoneal effluents on mesothelial cells in culture: cell proliferation and extracellular matrix regulation. Nephrol Dial Transplant 1996, 11:1803-1809 [PubMed] [Google Scholar]
- 9.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM: Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene 1991, 6:501-504 [PubMed] [Google Scholar]
- 10.Furlong RA, Takehara T, Taylor WG, Nakamura T, Rubin JS: Comparison of biological and immunochemical properties indicates that scatter factor and hepatocyte growth factor are indistinguishable. J Cell Sci 1991, 100:173-177 [DOI] [PubMed] [Google Scholar]
- 11.Bussolino F, Di Renzo MF, Ziche L, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM: Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 1992, 119:629-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantley LG, Barros EJ, Gandhi M, Rauchman M, Nigam SK: Regulation of mitogenesis, motogenesis and tubulogenesis by hepatocyte growth factor in renal collecting duct cells. Am J Physiol 1994, 267:F271-F280 [DOI] [PubMed] [Google Scholar]
- 13.Boros P, Miller CM: Hepatocyte growth factor: a multifunctional cytokine. Lancet 1995, 345:293-295 [DOI] [PubMed] [Google Scholar]
- 14.Libetta C, Rampino T, Esposito C, Fornoni A, Semeraro L, Dal Canton A: Stimulation of hepatocyte growth factor in human acute renal failure. Nephron 1998, 80:41-45 [DOI] [PubMed] [Google Scholar]
- 15.Yanagita K, Nagaike M, Ishibashi H, Niho Y, Matsumoto K, Nakamura T: Lung may have an endocrine function producing hepatocyte growth factor in response to injury of distal organs. Biochem Biophys Res Commun 1992, 182:802-809 [DOI] [PubMed] [Google Scholar]
- 16.Rampino T, Libetta C, De Simone W, Ranghino A, Soccio G, Gregorini M, Guallini P, Tamagnone L, Dal Canton A: Hemodialysis stimulates hepatocyte growth factor release. Kidney Int 1998, 53:1-7 [DOI] [PubMed] [Google Scholar]
- 17.Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A: In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14:359-364 [DOI] [PubMed] [Google Scholar]
- 18.Tenni R, Cetta G, Dyne K, Rossi A, Quacci D, Lenzi L, Castellani AA: Type I procollagen in the severe non-lethal form of osteogenesis imperfecta. Hum Genet 1988, 79:245-250 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K, Tajima H, Hamanoue M, Kohno S, Kinoshita T, Nakamura N: Identification and characterization of “injurin”, an inducer of expression of the gene for hepatocyte growth factor. Proc Natl Acad Sci USA 1992, 89:3800-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rampino T, Libetta C, Guallini P, Gregorini M, Soccio G, Maggio M, Evangelisti G, Dal Canton A: Cytokines are “injurins” and cause hepatocyte growth factor release during dialysis. Haematologica 1998, 83(Suppl):S10-S12 [Google Scholar]
- 21.Lin CY, Lin CC, Huang TP: Serial changes of interleukin-6 and interleukin-8 levels in drain dialysate of uremic patients with continuous ambulatory peritoneal dialysis during peritonitis. Nephron 1993, 63:404-408 [DOI] [PubMed] [Google Scholar]
- 22.Goldam M, Vandenabeele P, Moulart J, Amraoui Z, Abramowicz D, Nortier J, Vanherweghem JL, Fiers W: Intraperitoneal secretion of interleukin-6 during continuous ambulatory peritoneal dialysis. Nephron 1990, 56:277-280 [DOI] [PubMed] [Google Scholar]
- 23.Ko YC, Mukaida N, Kasahara T, Muto S, Matsushima K, Kusano E, Asano Y, Itoh Y, Yamagishi Y, Kawai T: Specific increase in interleukin-8 concentrations in dialysis fluid of patients with peritonitis receiving continuous ambulatory peritoneal dialysis. J Clin Pathol 1995, 48:115-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT: Appearance of tumor necrosis factor-alfa and soluble TNF-receptor I and II in peritoneal effluent of CAPD. Kidney Int 1994, 46:1422-1430 [DOI] [PubMed] [Google Scholar]
- 25.Rosen EM, Meromsky L, Setter E, Vinter DW, Goldberg ID: Purified scatter factor stimulates epithelial and vascular endothelial cell migration. Proc Soc Exp Biol Med 1990, 195:34-43 [DOI] [PubMed] [Google Scholar]
- 26.Ratajczac MZ, Marlicz W, Ratajczac J, Wasik M, Machalinski B, Carter A, Gewirtz AM: Effect of hepatocyte growth factor on early human haemopoietic cell development. Br J Haematol 1997, 99:228-236 [DOI] [PubMed] [Google Scholar]
- 27.Adamson IY, Bakowska J, Prieditis H: Proliferation of rat pleural mesothelial cells in response to hepatocyte and keratinocyte growth factors. Am J Respir Cell Mol Biol 2000, 23:345-349 [DOI] [PubMed] [Google Scholar]
- 28.Camussi G, Montrucchio G, Lupia E, Soldi R, Comoglio PM, Bussolino F: Angiogenesis induced in vivo by hepatocyte growth factor is mediated by platelet-activating factor synthesis from macrophages. J Immunol 1997, 158:1302-1309 [PubMed] [Google Scholar]
- 29.Yashiro M, Chung YS, Inoue T, Nishimura S, Matsuoka T, Fujihara T, Sowa M: Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts may affect mesothelial cell morphology and promote peritoneal dissemination. Int J Cancer 1996, 67:289-293 [DOI] [PubMed] [Google Scholar]
- 30.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R: The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31:11-24 [DOI] [PubMed] [Google Scholar]
- 31.Stylianou E, Jenner LA, Davies M, Coles GA, Williams JD: Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int 1990, 37:1563-1570 [DOI] [PubMed] [Google Scholar]
- 32.Tsarfaty I, Rong S, Resau JH, Rulong S, da Silva PP, Vande Woude GF: The Met proto-oncogene mesenchymal to epithelial cell conversion. Science 1994, 263:98-101 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Centracchio JN, Lin L, Sun AM, Dworkin LD: Constitutive expression of HGF modulates renal epithelial cell phenotype and induces c-met and fibronectin expression. Exp Cell Res 1998, 242:174-185 [DOI] [PubMed] [Google Scholar]
- 34.Takayama H, LaRochelle WJ, Sabnis SG, Otsuka T, Merlino G: Renal tubular hyperplasia, polycystic disease, and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth factor/scatter factor. Lab Invest 1997, 77:131-138 [PubMed] [Google Scholar]
- 35.Matsuda Y, Matsumoto K, Ichida T, Nakamura T: Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J Biochem (Tokyo) 1995, 118:643-649 [DOI] [PubMed] [Google Scholar]
- 36.Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, Matsumoto K, Nakamura T, Takahashi T, Nukiwa T: Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murin lung injury induced by bleomycin: a morphologic study. Am J Respir Crit Care Med 1997, 156:1937-1944 [DOI] [PubMed] [Google Scholar]
- 37.Matsuda Y, Matsumoto K, Yamada A, Ichda T, Asakura H, Komoriya Y, Nishiyama E, Nakamura T: Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology 1997, 26:81-89 [DOI] [PubMed] [Google Scholar]
- 38.Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei-Kim, Matsumoto K, Nakamura T, Higaki J, Ogihara T: Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation 2000, 102:246-252 [DOI] [PubMed] [Google Scholar]
- 39.Bacchella L, Tinelli P, Gile LS, Peona V, Aprile C, Gorrini M, Pasturenzi L, Cetta G, Luisetti M: Serum type I and type III procollagen peptide levels in sarcoidosis. Eur Respir J 1996, 9:1648-1651 [DOI] [PubMed] [Google Scholar]