Abstract
Dramatic activation of the coagulation cascade has been extensively documented for pulmonary fibrosis associated with acute and chronic lung injury. In addition to its role in hemostasis, thrombin exerts profibrotic effects via activation of the major thrombin receptor, protease-activated receptor-1. In this study, we examined the effect of the direct thrombin inhibitor, UK-156406 on fibroblast responses in vitro and on bleomycin-induced pulmonary fibrosis in rats. UK-156406 significantly inhibited thrombin-induced fibroblast proliferation, procollagen production, and connective tissue growth factor (CTGF) mRNA levels when used at equimolar concentration to the protease. Thrombin levels in bronchoalveolar lavage fluid and expression of thrombin and protease-activated receptor-1 in lung tissue were increased after intratracheal instillation of bleomycin. The characteristic doubling in lung collagen in bleomycin-treated animals (38.4 ± 2.0 mg versus 17.1 ± 1.4 mg, P < 0.01) was preceded by significant elevations in α1(I) procollagen and CTGF mRNA levels (3.0 ± 0.4-fold and 6.3 ± 0.4-fold respectively, (P < 0.01), and total inflammatory cell number. UK-156406, administered at an anticoagulant dose, attenuated lung collagen accumulation in response to bleomycin by 35 ± 12% (P < 0.05), inhibited α1(I) procollagen and CTGF mRNA levels by 50% and 35%, respectively (P < 0.05), but had no effect on inflammatory cell recruitment. This is the first report showing that direct thrombin inhibition abrogates lung collagen accumulation in bleomycin-induced pulmonary fibrosis.
Pulmonary fibrosis is the end stage of a heterogeneous group of disorders characterized by the excessive deposition of extracellular matrix proteins within the pulmonary interstitium. In addition to increased levels of classical growth factors and cytokines, such as transforming growth factor-β1, platelet-derived growth factor (PDGF), and insulin growth factor-1, 1 activation of coagulation cascade with the resultant generation of coagulation proteases has been implicated in the pathogenesis of this disease. Consistent with this observation, intra-alveolar accumulation of fibrin has been described for patients with pulmonary fibrosis, 2-4 acute lung injury (ALI), and the acute respiratory distress syndrome, 5,6 a condition in which rapid fibroproliferation and matrix synthesis can lead to the development of extensive fibrotic lesions. 7 Excessive procoagulant activity in the lung is thought to arise from an imbalance in pro- and anti-coagulant factors. For example, bronchoalveolar lavage fluid (BALF) from patients with acute respiratory distress syndrome, has been shown to contain tissue factor/factor VII/VIIa complexes, which can activate factor X, and trigger activation of the coagulation cascade. 6 Increased tissue factor expression on alveolar macrophages and type II pneumocytes, in close association with fibrin deposits, has also been described in the fibrotic lung. 8 We and others have also shown that active thrombin levels are increased in BALF obtained from patients with pulmonary fibrosis associated with systemic sclerosis, 9,10 whereas others have shown that thrombin levels in BALF are elevated in bleomycin-induced pulmonary fibrosis in rats. 11,12 In terms of anticoagulant factors, serum levels of antithrombin III (AT III), which binds and irreversibly inactivates a number of activated serine proteases, including thrombin, are reduced in patients with acute respiratory distress syndrome. 13 In addition, decreased activation of protein C, which is the main modulator of coagulation activation, has been shown to be associated with abnormal collagen turnover in the intra-alveolar space of patients with interstitial lung disease. 14
A number of reports have examined the effects of modulating the coagulation cascade in ALI. For example, exogenous delivery of the highly specific direct thrombin inhibitor hirudin and AT III, have been shown to be protective in animal models of ALI. 15-17 In addition, heparin, which inhibits serine proteases by potentiating the formation of AT-III/serine protease complexes, but also has anti-inflammatory properties, lead to improved gas exchange in an animal model of ALI. 18 Finally, heparin has also been shown to attenuate bleomycin-induced pulmonary fibrosis in mice, 19 although it was uncertain in this study whether heparin was delivered at an anticoagulant dose and whether the protective effects were because of the its direct anti-proliferative effects.
Thrombin plays a central role in blood coagulation by converting soluble plasma fibrinogen into an insoluble fibrin clot and promoting platelet aggregation and degranulation, but also mediates a number of biological responses that may play important roles in subsequent inflammatory and tissue repair responses. Thrombin activates endothelial cells, 20 acts as a chemoattractant for inflammatory cells 21-23 and fibroblasts, 24 and stimulates fibroblast proliferation 24-26 and procollagen production. 27 Most of the cellular effects of thrombin are mediated by a unique family of ubiquitously expressed cell-surface receptors called protease-activated receptors (PARs), which are activated by limited proteolysis rather than direct ligand binding. 28 To date, four PARs have been characterized, of which three, (PAR-1, -3, and -4), are activated by thrombin, although PAR-1 has been shown to be the major receptor involved in mediating thrombin’s mitogenic, profibrotic, and proinflammatory effects in vitro. 27,29,30 After the interaction of thrombin with its receptors, most of its cellular effects are thought to be mediated via the induction and release of a host of secondary mediators. 31 For example, there is good evidence that the mitogenic effects of thrombin for lung fibroblasts in vitro are mediated, at least in part via the autocrine production of PDGF-AA and up-regulation of the PDGF-α receptor. 10 More recently, we have shown that thrombin is also a potent inducer of connective tissue growth factor (CTGF) production by human lung fibroblasts via direct proteolytic activation of PAR-1. 32 CTGF is a fibroblast mitogen, chemoattractant, and promoter of procollagen and fibronectin production in vitro. 33 It has therefore been proposed that some of the profibrotic effects of thrombin in vitro may also be mediated, at least in part, by increased production of this growth factor. CTGF is also induced in response to transforming growth factor-β1 and is thought to be responsible for mediating some of its downstream fibrogenic effects. 34 Further evidence that CTGF may be important in tissue repair and fibrosis has been provided by studies showing that repeated subcutaneous injection of CTGF into newborn mice leads to increased connective tissue deposition, 33 and that CTGF expression is dramatically increased in a number of fibrotic and fibroproliferative disorders, including pulmonary fibrosis 35 and in animal models of this disease. 36
Despite the evidence that thrombin may play an important role in the pathogenesis of pulmonary fibrosis, there have been no previous reports that have specifically addressed whether direct thrombin inhibition affects collagen accumulation in this disorder. The aim of this study was therefore to examine the effect of a potent and highly selective direct thrombin inhibitor, UK-156406 (Pfizer Central Research, Sandwich, Kent, UK) on thrombin-induced fibroblast responses in vitro and in bleomycin-induced pulmonary fibrosis in rats. Our data show that UK-156406 blocked the profibrotic effects of thrombin in vitro when used at equimolar concentration to the protease and attenuated lung collagen accumulation after bleomycin-induced lung injury. We further show that the protective effect of direct thrombin inhibition on lung collagen accumulation in this model was preceded by significant reductions in both α1(I) procollagen and CTGF mRNA levels, but not by changes in inflammatory cell recruitment. This is, to our knowledge, the first report to show that direct thrombin inhibition is associated with attenuation of both α1(Ι) procollagen and CTGF mRNA levels, and ultimately an abrogation in lung collagen accumulation in an animal model of pulmonary fibrosis.
Materials and Methods
Materials
Purified human α-thrombin (catalog no.T4393) was obtained from Sigma Chemical Co. Ltd., (Poole, Dorset, UK). The direct thrombin inhibitor, UK-156406 was a generous gift from Dr. Andrew Gray (Pfizer Central Research, Sandwich, Kent, UK). The cDNA probe for human CTGF was inserted into the EcoRI and NotI sites of pBluescript and kindly provided by Dr. Raj Beri (AstraZeneca R&D Charnwood, Loughborough, UK). The cDNA probe for FISP12 (the murine orthologue of CTGF), encompassing nucleotides 1663 to 2930 was generated from a plasmid (pBluescriptfisp12del) kindly provided by Dr. Joseph A. Lasky (Tulane University, New Orleans, LA). The pBluescriptfisp12del plasmid was subcloned from a plasmid (A12/pMexNeo I) originally obtained from Dr. Rolf-Peter Ryseck (Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, NJ). The cDNA probe for α1(I) procollagen (probe Hf677) was kindly provided by Dr. M. L. Chu (Thomas Jefferson University, Philadelphia, PA). Rat-specific PAR-1 antibodies were generated by immunizing rabbits with the agonist sequence SFFLRNPSENTFELVPL-NH2 and purified by affinity chromatography 37 and were a generous gift from Professor Eleanor Mackie (University of Melbourne, Melbourne, Australia).
Fibroblast Culture
Human fetal lung fibroblasts (HFL-1; American Type Culture Collection, Rockville, MD), were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with penicillin, streptomycin, and 10% (v/v) newborn calf serum (NCS), (Imperial Laboratories, Andover, Hampshire, UK). Cells were routinely passaged, tested for mycoplasma infection, and used for experiments between passages l5 to 20. In all in vitro experiments, UK-156406 was preincubated with human α-thrombin (10 nmol/L to 1 μmol/L) in serum-free DMEM for 60 minutes at 37°C. To examine the effects of thrombin alone, the protease was similarly preincubated in serum-free DMEM under the same conditions.
Animal Model of Pulmonary Fibrosis
Male Lewis rats, aged 6 weeks and weighing 140 to 170 g, were anesthetized by intramuscular injection of 0.75 to 1.0 ml/kg Hypnorm (fentanyl citrate, 0.315 mg/ml, and fluanisone, 10 mg/ml; Janssen Pharmaceutical, High Wycombe, UK). Bleomycin sulfate (Lundbeck, Luton, UK) was administered by a single intratracheal injection (l.5 mg/kg body weight in 0.3 ml of sterile saline) as described previously. 38 Control animals received 0.3 ml of saline alone. In initial experiments, groups of six rats were killed by pentobarbitone overdose after 6 days to allow assessment of thrombin levels in BALF, as described previously. 39 Separate groups of two rats were sacrificed 1, 3, 6, and 14 days after bleomycin instillation for immunohistochemical assessment of thrombin and PAR-1. Lungs were fixed by intratracheal instillation of 4% paraformaldehyde, the trachea ligated, and the inflated lungs and heart removed en bloc. Tissues were fixed and transferred to 15% sucrose in phosphate-buffered saline (PBS), before alcohol dehydration and embedding in paraffin wax. For assessment of the effect of UK-156406 on lung collagen accumulation after bleomycin-induced lung injury at 14 days, UK-156406 (0.5 mg/kg/hour in 0.9% sterile saline) was administered continuously via osmotic minipumps (Alzet, Palo Alto, CA) implanted subcutaneously, 24 hours before bleomycin instillation, to groups of six animals. Drug control animals received minipumps containing saline alone. An additional series of animals was killed 6 days after bleomycin or saline instillation for measurement of blood coagulation parameters, total and differential cell counts in BALF, and for Northern analysis of lung tissue CTGF and α1(Ι) procollagen mRNA levels. For measurement of coagulation parameters, blood was collected from the inferior vena cava of animals after laparotomy, and was immediately mixed, 10:1 with a solution of 3.8% trisodium citrate (w/v). For measurement of total lung collagen and CTGF and procollagen mRNA levels, the vasculature was perfused with 5 ml of sterile saline containing 100 U/ml heparin. The lungs were removed, weighed, and immediately snap-frozen in liquid N2 after removing the trachea and major airways.
Fibroblast Proliferation Assay
Fibroblast proliferation was assessed using a colorimetric assay based on the uptake and subsequent elution of the dye methylene blue as previously described. 40 Briefly, cells were seeded at 5 × 10 3 cells/well into 96-well plates (Nunc, Life Technologies, Paisley, Scotland, UK) in DMEM and 5% NCS. Control medium, thrombin, or thrombin plus UK-156406 (10 nmol/L to 1 μmol/L) were added to cell cultures for 48 hours. Cells were rinsed with PBS, fixed with formol-saline, and stained with a solution of methylene blue for 30 minutes. Plates were rinsed with borate buffer, bound dye was eluted from the cell monolayer by addition of acidified alcohol, and the absorbance was measured at 650 nmol/L using a microplate spectrophotometer. In some experiments, changes in fibroblast cell number were confirmed by direct cell counting with a standard hemocytometer (British Drug House/Merck, UK).
Determination of Fibroblast Procollagen Production in Vitro and Total Lung Collagen in Vivo
Fibroblast procollagen production in vitro and total lung collagen in vivo were assessed by quantitating hydroxyproline by reverse-phase HPLC as previously described. 38,41,42 Briefly, for in vitro studies, cells were seeded at 10 5 cells/ml in 2.4-cm diameter wells in DMEM and 5% NCS, grown to visual confluence and exposed to either control medium, thrombin, or thrombin plus with UK-156406 (10 nmol/L to 1 μmol/L) for 48 hours. At the end of the incubation period, proteins in the media and cell layer were ethanol precipitated and separated from free amino acids by filtration (0.45 μmol/L). Filters were hydrolyzed in HCl and hydrolysates prepared for quantitation of hydroxyproline by HPLC analysis (Beckman System Gold, Beckman, High Wycombe, UK), after derivatization with 7-chloro-4-nitrobenzofuran (Sigma) as previously described. 42 For measurement of total lung collagen, powdered lung tissue was weighed and was similarly hydrolyzed in HCl and prepared for HPLC analysis after diluting hydrolysate aliquots (1:100). The total amount of collagen in each lung was calculated, assuming that lung collagen contains 12.2% w/w hydroxyproline 43 and results were expressed as total lung collagen (mg).
Northern Analysis of CTGF and α1(Ι) Procollagen mRNA Levels
For Northern analysis of CTGF mRNA levels in vitro, fibroblasts were seeded at 2 × 10 5 cells/ml in 6-cm diameter dishes in DMEM and 5% NCS as described previously. 32 Briefly, on reaching visual confluence, cells were quiesced for 24 hours and exposed to control media, thrombin, or thrombin plus with UK-156406, (10 nmol/L to 100 nmol/L) in serum-free conditions. After 90 minutes, total RNA was isolated with TRIzol reagent according to the manufacturer’s instructions (Gibco BRL, Paisley, UK). For Northern analysis of CTGF and α1(Ι) procollagen mRNA levels in vivo, total RNA was isolated with TRIzol reagent, from powdered lung tissue, kept frozen. Total RNA from fibroblast cultures (5 μg), and lung tissue (10 μg) were mixed with RNA loading buffer (Sigma), and electrophoresed on a formaldehyde 1% (w/v) agarose gel. RNA loading and integrity was visualized and quantitated by fluorescent scanning of the gel (Fuji, FLA 3000) before transfer to nylon membranes (Hybond N; Amersham International, High Wycombe, UK) and fixation by UV crosslinking. Membranes were hybridized overnight in Denhardt’s-based standard hybridization solution at 65°C in the presence of the [32P]-dCTP-labeled cDNA probes for human CTGF (fibroblast cultures), or α1(Ι) procollagen and FISP12 (lung tissue). At the end of the hybridization, membranes probed for CTGF and FISP12 were rinsed at low stringency [2× standard saline citrate (SSC), 0.1% sodium dodecyl sulfate (SDS), for 5 minutes at room temperature], once at medium stringency (0.5× SSC, 0.1% SDS, for 25 minutes at 65°C) and once at high stringency (0.1× SSC, 0.1% SDS, for 5 minutes at 65°C). Membranes probed for α1(Ι) procollagen were rinsed at low stringency (2× SSC, 0.1% SDS, 5 minutes at room temperature, followed by 20 minutes at 65°C) and high stringency (0.1× SSC, 0.1% SDS for 20 minutes at 65°C). All membranes were exposed to phosphorimage storage screens (Fuji) for 2 to 4 hours and mRNA levels were quantitated by phosphorimage analysis (Fuji FLA 3000).
Estimation of Active Thrombin Levels in Rat Bronchoalveolar Lavage
BALF was centrifuged at 2000 × g for 10 minutes at 4°C and thrombin levels in the supernatant were estimated using a previously described spectrophotometric assay. 6 Briefly a 100-μl aliquot of the supernatant was mixed with 50 μl of 0.05 mol/L Tris, 0.1 mol/L NaCl (pH 7.35) buffer solution and 50 μl of the chromphore S2238 (1 mmol/L; H-d-phenylalanyl-l-pipecolyl-l-arginine-p-nitroaniline dihydrochloride; Chromogenix, Quadratech, Surrey, UK) at 37°C. Absorbance was read on a spectrophotometer at 405 nmol/L at regular intervals up to 60 minutes and BALF thrombin levels were derived by extrapolation from a thrombin standard curve.
Immunohistochemical Localization of Thrombin and PAR-1
Lung tissue sections (5 μm) were cut and mounted on glass slides before dewaxing in xylene and rehydration in ethanol according to standard histological procedures. Tissue endogenous peroxidase activity was blocked by incubating sections with 3% hydrogen peroxide (Sigma) before washing in PBS and incubation with normal goat serum (DAKO, High Wycombe, UK) (1:40 dilution). Active thrombin in lung sections was localized as previously described. 44 Briefly, sections were incubated with recombinant hirudin (1:50 dilution, Sigma), followed by a purified rabbit anti-hirudin antibody (1:1200 dilution; American Diagnostica, Greenwich, CT) for 1 hour each at 37°C. For immunohistochemical localization of PAR-1, sections were incubated with rabbit anti-rat PAR-1 primary antibodies 37 for 1 hour at room temperature. All sections were washed in PBS and incubated with a biotinylated goat anti-rabbit secondary antibody (1:300 dilution, DAKO) for 60 minutes and similarly washed in PBS. Sections were incubated with a streptavidin/peroxidase complex (1:300 dilution, DAKO) for a further 60 minutes, followed by a solution of 600 μg/ml of 3,3′-diaminobenzidine (Sigma) and 0.03% hydrogen peroxide. Sections were washed, counterstained with hematoxylin, dehydrated, and mounted with DPX mountant (Merck, Poole, UK). Control sections were incubated with normal goat serum or an isotype-specific, nonimmune rabbit IgG primary antibody (DAKO) instead of primary antibodies.
Assessment of Blood Clotting Parameters in Rat Plasma
Plasma was prepared by centrifugation of citrated blood samples at 2000 × g for 15 minutes. The plasma mean activated partial thromboplastin time (APTT) and the mean prothrombin time (PT) were assessed using Actin FS and Thromboplastin IS (Dade Behring, Marburg, Germany), respectively. Parameters were measured in duplicate using a mechanical KC-4A coagulometer (Amelung, Lemgo, Germany).
BALF Total Cell Number and Differential Cell Counts
BALF total cell number was assessed as previously described. 39 Briefly, BALF cells were pelleted by centrifugation at 300 × g for 10 minutes at 4°C. Cell pellets were re-suspended in DMEM (1 ml) and cells were counted with a standard hemocytometer. Cytospins were prepared by centrifuging 100-μl aliquots of the cell suspension for 3 minutes at 450 rpm (Cytospin 2; Shandon, Southern Products Ltd., Cheshire, UK). Slides were air-dried and stained with Diffquik Stain (Dade Behring, Dûdingen, Switzerland) and differential cell counts were performed by two investigators by counting a minimum of 500 cells per slide. Cells were identified as macrophages/monocytes, neutrophils, or lymphocytes and data were expressed as a percentage of the total number of lavageable cells.
Statistical Analysis
All data are presented as means ± SEM for six replicates, unless otherwise indicated. Statistical evaluation was performed using an unpaired Student’s t-test for single, and analysis of variance for multiple-group comparisons. A P value <0.05 was considered significant.
Results
Thrombin and PAR-1 in Bleomycin-Induced Pulmonary Fibrosis
To confirm the previous observation that BALF thrombin levels are elevated after bleomycin-induced lung injury, we assessed these levels in our model at a time point where they would be expected to be maximal (6 days). We found that BALF thrombin levels were indeed significantly increased from 0.30 ± 0.02 ng/ml in saline control animals to 2.97 ± 0.68 ng/ml in animals given bleomycin (n = 6, P < 0.01). We also performed experiments to determine the immunohistochemical localization of active thrombin and the major thrombin receptor, PAR-1, within the lung after bleomycin-induced lung injury at 1, 3, 6, and 14 days (Figure 1) ▶ . In saline-instilled rats, the appearance of the lung under light microscopy appeared normal with only weak positive staining for active thrombin that was associated with resident macrophages (Figure 1, a and b) ▶ . In contrast, in bleomycin-instilled rats, there was evidence of a diffuse inflammatory-cell influx at days 1 and 3, which was replaced by interstitial fibrosis with multiple inflammatory foci by day 6, and eventually mature regional fibrotic areas by day 14. 38 In the lungs of these animals there was intense and widespread staining for active thrombin, which was predominately associated with macrophages within inflammatory and fibroproliferative foci (Figure 1, c and d) ▶ , and also fibroblast-like interstitial cells (Figure 1d ▶ , arrows). These changes were evident throughout the time course but appeared maximal 6 days after bleomycin instillation. PAR-1 was consistently expressed on the bronchial epithelium of lung sections from both bleomycin- and saline-treated animals. However, in contrast to the parenchyma that was negative in saline-treated animals (Figure 1, e and f) ▶ , there was a dramatic increase in the expression of PAR-1, which again was associated with macrophages in inflammatory and fibroproliferative foci and was also maximal on day 6, in bleomycin-treated animals (Figure 1, g and h) ▶ .
Figure 1.
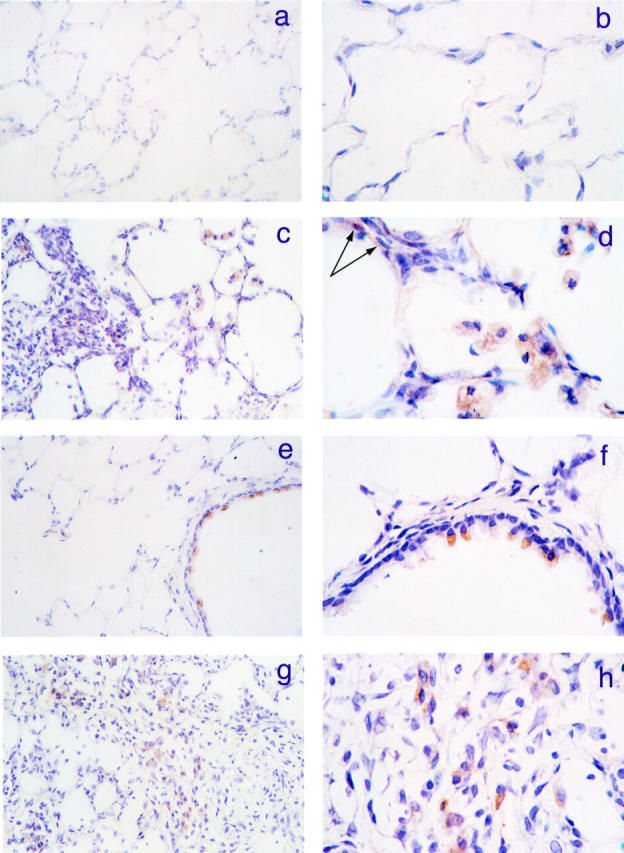
Thrombin and PAR-1 expression is increased in bleomycin-induced pulmonary fibrosis. a and b: A section of rat lung, 6 days after intratracheal instillation of saline. There is only weak staining for active thrombin on resident macrophages. c and d: A corresponding lung section, after intratracheal instillation of bleomycin. There is extensive staining for active thrombin, which is predominantly associated with macrophages in inflammatory and fibroproliferative foci, but is also localized to fibroblast-like interstitial cells (arrows in d). e and f: The immunohistochemical localization of PAR-1, 6 days after intratracheal saline instillation. PAR-1 was consistently expressed on the bronchial epithelium but the lung parenchyma was negative. g and h: A corresponding lung section from a bleomycin-treated animal at the same time point. The expression of PAR-1 was dramatically increased in the lung parenchyma of these animals and was again predominantly associated with macrophages in inflammatory and fibroproliferative foci. Original magnifications: panels a, c, e, and g ×400, panels b, d, f, and h ×1000; counterstained with Mayers hematoxylin.
Effect of UK-156406 on the Profibrotic Effects of Thrombin in Vitro
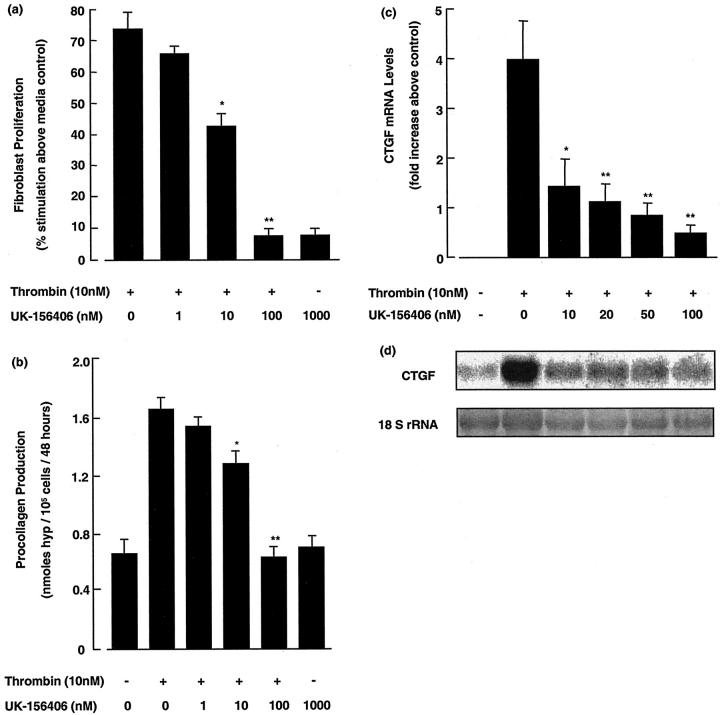
Before performing in vivo experiments with UK-156406, we assessed the efficacy of this compound to block fibroblast responses to thrombin in vitro. Figure 2 ▶ shows a representative experiment for the effect of UK-156406 on human fetal lung fibroblast (HFL-1) proliferation (Figure 2a) ▶ and procollagen production (Figure 2b) ▶ in response to an optimal concentration of thrombin (10 nmol/L). Thrombin stimulated fibroblast proliferation and procollagen production, assessed after 48 hours, by 72 ± 5% and 121 ± 6%, respectively, relative to media control cells (both P < 0.01). These fibroblast responses to thrombin were inhibited by UK-156406 in a dose-dependent manner from equimolar concentrations onwards (P < 0.05), and were completely blocked when UK-156406 was added in 10-fold excess (P < 0.01). In addition, UK-156406 had no effect on basal fibroblast proliferation or fibroblast procollagen production at all concentrations examined up to 1 μmol/L.
Figure 2.
UK-156406 blocks thrombin-induced fibroblast proliferation, procollagen production, and CTGF mRNA levels in vitro. a and b: The effect of UK-156406 on thrombin-induced fibroblast proliferation and procollagen production at the end of a 48-hour incubation period. For fibroproliferation and procollagen assays, data are expressed as mean ± SEM of six replicates from a representative experiment (n = 3). For comparison, optimal serum (10% NCS) stimulated fibroblast proliferation by 344 ± 2%. Procollagen production is based on hydroxyproline measured in the media and cell layer and values are corrected for hydroxyproline associated with the cell layer at the onset of the experiment and expressed as nmol hydroxyproline/10 5 cells/48 hours. c: A graph of the mean fold increases in CTGF mRNA levels at 90 minutes, normalized for RNA loading based on densitometric quantitation of rRNA, greater than control levels for four replicate cultures. d: A phosphorimage of a representative Northern blot of the 2.4-kb CTGF transcript and a laser scan of the corresponding ethidium bromide-stained 18 S rRNA bands. The P values denote the statistical significance of the indicated data compared to cells treated with thrombin alone (10 nmol/L): *, P < 0.05; **, P < 0.01. The data shown is representative of three separate experiments performed.
We have recently shown that thrombin increases fibroblast CTGF gene expression in vitro, 32 raising the possibility that thrombin exerts its effects on fibroblast function via the autocrine production of this mediator. We therefore also examined the effect of UK-156406 on thrombin-induced fibroblast CTGF mRNA levels by Northern analysis of total cellular RNA. The effect of UK-156406 on thrombin-induced CTGF mRNA levels, expressed as a fold increase greater than media controls for four replicate cultures and a representative Northern blot are shown in Figure 2, c and d ▶ , respectively. The characteristic increase in thrombin-stimulated CTGF mRNA levels at 90 minutes was decreased by 65% for fibroblasts exposed to thrombin in the presence of equimolar concentrations of UK-156406 (P < 0.05), and by 88% when UK-156406 was added in 10-fold excess (P < 0.01). In addition, at these concentrations, UK-156406 had no effect on basal CTGF mRNA levels.
Effect of UK-156406 on Blood Clotting Parameters in Vivo
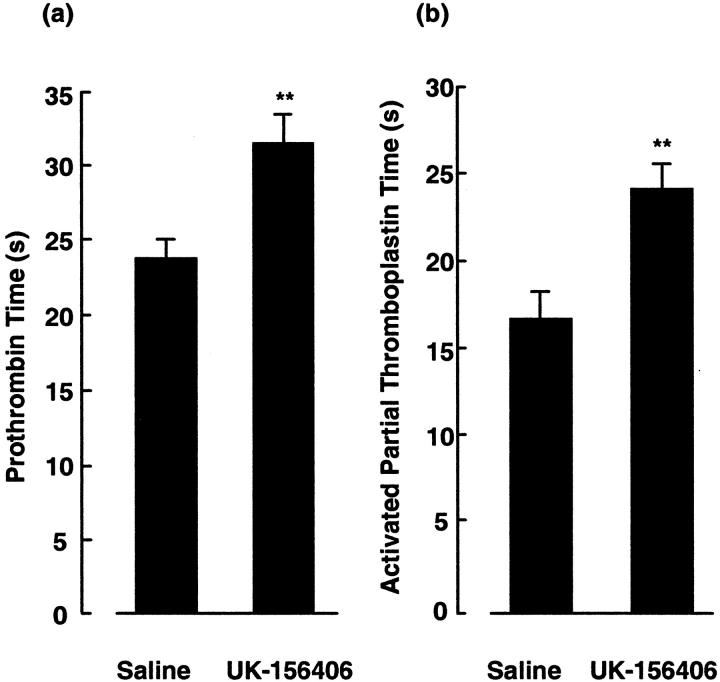
To ensure that UK-156406 was administered to animals at an anticoagulant dose, the compound was delivered by continuous subcutaneous infusion via an osmotic minipump and two ex vivo assays of thrombin-dependent coagulation, the mean APTT and PT, were measured when serum concentration of the drug would have reached steady-state levels, based on available pharmacokinetic data from Pfizer. Figure 3 ▶ shows that the mean PT of UK-156406-treated animals (Figure 3a) ▶ was prolonged from 24.5 ± 1.1 seconds to 31.4 ± 1.6 seconds (P < 0.01), whereas the mean APTT (Figure 3b) ▶ was increased from 17.0 ± 0.8 seconds to 24.5 ± 1.1 seconds (P < 0.01) compared to animals receiving drug vehicle alone. In addition, intratracheal bleomycin alone had no effect on these clotting parameters and the prolongation of APTT and PT in animals given UK-156406 was the same in all animals, whether they had previously received intratracheal bleomycin or saline (data not shown).
Figure 3.
UK-156406 prolongs rat blood coagulation parameters. Figure ▶ shows the effect of a continuous subcutaneous infusion of UK-156406 on the mean plasma PT and APTT (a and b), respectively. The P values denote the significance of the indicated data compared to animals treated with a continuous infusion of saline: **, P < 0.01; n = 6.
Effect of UK-156406 on Lung Collagen Accumulation in Bleomycin-Induced Pulmonary Fibrosis
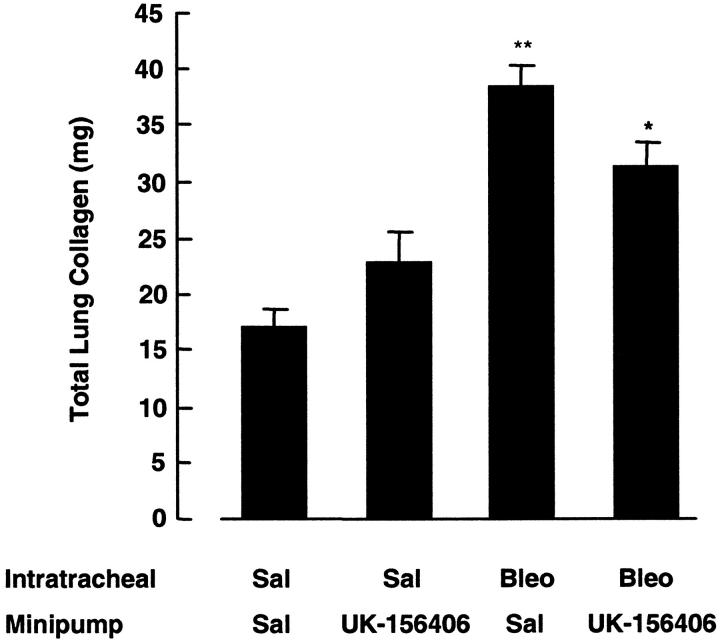
Figure 4 ▶ shows the effect of UK-156406 on total lung collagen accumulation in response to bleomycin, assessed by quantitating hydroxyproline in lung hydrolysates at 14 days. In bleomycin-instilled animals, total lung collagen was increased by 124% compared with animals given intratracheal saline (P < 0.01, n = 6). This increase in lung collagen accumulation was reduced by 35% (P < 0.05, n = 6) in bleomycin-treated rats given UK-156406 compared with animals given bleomycin and drug vehicle alone. In addition, UK-156406 had no effect on basal lung collagen levels in animals given intratracheal saline. The results shown are representative of three separate experiments performed, in which statistically significant differences in total lung collagen between drug-treated animals and those given bleomycin and drug vehicle alone were always obtained.
Figure 4.
UK-156406 attenuates lung collagen accumulation in bleomycin-induced pulmonary fibrosis. ▶ shows the effect of a continuous subcutaneous infusion of UK-156406 on lung collagen accumulation at 14 days, after a single intratracheal instillation of bleomycin (1.5 mg/kg). **, P < 0.01, denotes the statistical increase in total lung collagen between bleomycin-treated animals and saline-treated controls. *, P < 0.05, indicates the reduction in lung collagen accumulation in bleomycin-treated animals given UK-156406, n = 6. The results obtained are representative of three separate experiments.
Effect of UK-156406 on Lung CTGF and α1(I) Procollagen mRNA Levels in Vivo
To begin to examine the mechanism by which direct thrombin inhibition attenuates bleomycin-induced pulmonary fibrosis, we assessed whether the changes in lung collagen at the protein level were preceded by a reduction in rat CTGF and α1(Ι) procollagen mRNA levels. Figure 5 ▶ shows the data expressed as fold increases in CTGF and α1(Ι) procollagen mRNA levels greater than control levels assessed on day 6, for six animals per group, (Figure 5a) ▶ , as well as representative Northern blots. In lung tissue from bleomycin-treated animals, CTGF and α1(I) procollagen mRNA levels were increased 6.3 ± 0.4-fold and 3.0 ± 0.4-fold, respectively (P < 0.01). These mRNA levels were reduced by 35% for CTGF and by 50% for α1(I) procollagen in bleomycin-treated animals given UK-156406, compared to bleomycin-treated animals given drug vehicle alone (n = 6, P < 0.05). Finally, UK-156406 had no effect on CTGF and α1(I) procollagen mRNA levels in saline-treated animals.
Figure 5.
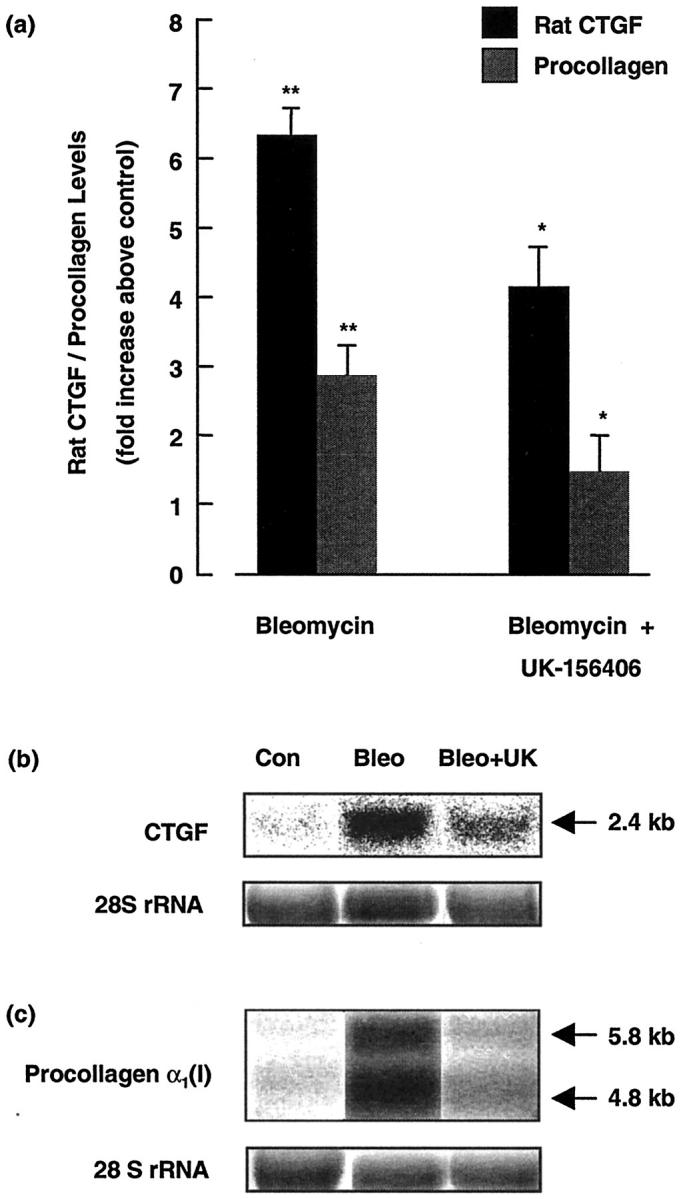
UK-156406 attenuates CTGF and α1(I) procollagen mRNA levels in bleomycin-induced pulmonary fibrosis. a: Data for bleomycin-treated animals expressed as fold increases in lung CTGF and α1(I) procollagen mRNA levels above saline control animals, normalized for RNA loading based on densitometric quantitation of rRNA (mean ± SEM, n = 6). **, P < 0.01, denotes the statistical increase in CTGF and α1(I) procollagen mRNA levels in bleomycin-treated animals compared to control animals. *, P < 0.05, indicates the reduction in these levels in bleomycin-treated animals given UK-156406, n = 6. b and c: Phosphorimages of representative Northern blots for the 2.4-kb CTGF mRNA transcript and the 5.8-kb and 4.8-kb α1(I) procollagen transcripts. The corresponding ethidium bromide-stained 28 rRNA S bands are also shown.
Effect of UK-156406 on Inflammatory Cell Recruitment in Bleomycin-Induced Pulmonary Fibrosis
Thrombin is known to exert potent effects on inflammatory cell migration in vitro. 21-23 To assess whether direct thrombin inhibition affected inflammatory cell recruitment in this model, we examined the effect of UK-156406 on total inflammatory cell number and the relative proportions of inflammatory cells in BALF, 6 days after bleomycin instillation (Table 1) ▶ . As expected, total inflammatory cell number in BALF was dramatically increased in bleomycin-treated animals compared with saline controls (P < 0.01) and there was a characteristic significant increase in the proportion of both neutrophils and lymphocytes and a corresponding reduction in the proportion of macrophages, compared to saline-treated controls (P < 0.01). However, UK-156406 had no effect on both total cell number and the relative proportion of inflammatory cells in bleomycin or in saline-treated control animals.
Table 1.
UK-156406 Does Not Affect Inflammatory Cell Recruitment in Bleomycin-Induced Pulmonary Fibrosis
| Day 6 intratracheal | Minipump | Total cell count (×10−4) | Macrophages, % | Lymphocytes, % | Neutrophils, % |
|---|---|---|---|---|---|
| Saline | Saline | 33 ± 3 | 97.7 ± 1.0 | 0.6 ± 0.3 | 1.7 ± 0.8 |
| Saline | UK-156406 | 28 ± 7 | 98.9 ± 0.4 | 0.2 ± 0.2 | 1.0 ± 0.5 |
| Bleomycin | Saline | 219 ± 228* | 63.8 ± 1.8* | 2.2 ± 0.2* | 34.0 ± 1.8* |
| Bleomycin | UK-156406 | 181 ± 108* | 62.3 ± 1.9* | 4.1 ± 0.8* | 33.7 ± 2.3* |
This table shows the effect of UK-156406 on total inflammatory cell number and the relative number of lavageable inflammatory cells in BALF from bleomycin- and saline-treated animals at day 6 (mean ± SEM, n = 6). The P values represent the significance of the indicated data compared to respective control groups: *P < 0.01.
Discussion
There is compelling clinical and experimental evidence that the coagulation cascade is activated in the lungs of patients with pulmonary fibrosis, but the contribution of the coagulation cascade to lung collagen accumulation in this disease remains uncertain. In this study, we show for the first time that direct thrombin inhibition attenuates lung collagen accumulation in the bleomycin model of pulmonary fibrosis. We further show that that the reduction in lung collagen accumulation was preceded by an abrogation in α1(I) procollagen and CTGF mRNA levels, but not inflammatory cell recruitment.
Active Thrombin and PAR-1 Are Increased in Bleomycin-Induced Pulmonary Fibrosis
Thrombin levels in BALF had previously been reported to be increased in bleomycin-treated animals compared to saline controls, 11,12 but the localization of thrombin in the lung after bleomycin injury had not been described. In this study we confirmed that BALF thrombin levels were elevated in bleomycin-treated animals. The specificity of this assay for thrombin was further confirmed by showing that thrombin activity in BALF could be abolished in the presence of the highly selective thrombin inhibitor, hirudin (data not shown). Immunohistochemical studies performed to localize thrombin in lung after bleomycin instillation revealed that thrombin was predominantly localized to macrophages in inflammatory and fibroproliferative foci and also fibroblast-like interstitial cells, and that immunoreactivity of thrombin was maximal on day 6. These findings are in accord with previous reports of peak thrombin levels 11,12 and procoagulant activity associated with alveolar macrophages in BALF of bleomycin-treated rats. 45
There are a number of potential mechanisms that may lead to increased expression of thrombin on macrophages after bleomycin injury. For example, the active protease may leak into the alveolar space from the vascular compartment as a result of chronic activation of the coagulation cascade, after the extensive and continued endothelial injury caused by bleomycin. However, macrophages have been shown to express both tissue factor, the primary cell-surface initiator of the extrinsic coagulation cascade in vitro 46 as well as membrane assembly sites for generation of the prothrombinase complex, 47 responsible for activating the intrinsic coagulation cascade. Macrophages are therefore thought to be able to accelerate membrane-dependent coagulation reactions and facilitate the local extravascular generation of thrombin, independent of classical blood coagulation. Indirect support that these effects may be important mechanisms occurring in the human lung has come from studies showing that thrombin is present on the surface of normal human alveolar macrophages 44 and that expression of tissue factor on these cells is dramatically increased in patients with pulmonary fibrosis. 8
As well as assessing the immunohistochemical localization of thrombin, we also examined expression of the major cellular receptor for thrombin, PAR-1. Although PAR-1 expression had never been described in the lung, PAR-1 mRNA had previously been detected in cultured alveolar macrophages. 48 Furthermore, specific agonists of this receptor have been shown to cause bronchoconstrictor responses in experimental animals, 49 suggesting that the lung does indeed express functional PAR-1 receptors. Our studies showed that PAR-1 was expressed on the bronchial epithelium in the lungs of all experimental animals, with no evidence of altered expression after bleomycin instillation. However, expression of PAR-1 in the lung parenchyma was dramatically different in bleomycin-treated animals compared to controls, where similar to thrombin, positive staining was predominately associated with macrophages in inflammatory and fibroproliferative foci and was also maximal 6 days after bleomycin instillation.
UK-156406 Blocks the Profibrotic Effects of Thrombin in Vitro
Before examining the effects of direct thrombin inhibition in the bleomycin model of pulmonary fibrosis, and as thrombin has a very high affinity for its receptor, 50 we first determined whether UK-156406, could block thrombin-induced fibroblast responses in vitro. UK-156406 is a hydrophilic, small peptide-based reversible inhibitor of thrombin proteolytic activity. It is very potent with a dissociation constant (Ki) of 0.39 nmol/L and displays good selectivity over other serine proteases, including factor Xa, plasmin, and factor VIIa (data provided by Pfizer, patent no. WO9513274). We showed that UK-156406 inhibited thrombin-induced fibroblast proliferation, procollagen production, and CTGF mRNA levels in a dose-dependent manner from equimolar concentrations of the protease onwards. These effects were comparable to those obtained with hirudin in previous reports, 27,32 so that this compound was deemed suitable for further evaluation in vivo.
Steady-State Levels of UK-156406 Prolong Clotting Parameters in Rat Plasma
UK-156406 has an in vivo half-life of ∼1 hour. Because thrombin is generated chronically after bleomycin-induced lung injury, we decided to deliver the compound continuously via osmotic minipumps, inserted into rats subcutaneously. Initial pilot studies revealed that at higher doses (up to 1.5 mg/kg/hour), UK-156406 caused local subcutaneous bleeding around the pump insertion site. In addition, two ex vivo assays of thrombin-dependent coagulation, the APTT and PT became unrecordably high (>180 seconds) at this dose (data not shown). In final experiments, a dose of 0.5 mg/kg/hour proved optimal, as it caused significant prolongation of the APTT and PT, without noticeable hemostatic complications. In addition, at this dose, subcutaneous delivery of UK-156406 was as effective at prolonging the APTT and PT as previously reported in in vivo studies, in which hirudin was administered as a continuous intravenous infusion, 15,16 or twice daily by subcutaneous injection. 30 We further calculated that this dose of UK-156406 would generate a plasma steady-state concentration approaching 1 μmol/L and that the concentration of thrombin detected in BALF after bleomycin instillation would be in the range of 50 to 100 nmol/L. Although this concentration is greater than that of thrombin used in the in vitro experiments of this study (10 nmol/L), fibroblast responses to thrombin in vitro are exerted over a range of concentrations between 10 pmol/L to 200 nmol/L, 27,32 with a plateau concentration for thrombin-induced fibroblast proliferation and procollagen production of 10 nmol/L. 27 In addition, the concentration of BALF thrombin after bleomycin instillation is similar to the concentration of thrombin detected in BALF from patients with pulmonary fibrosis 9 and also in blood clotting. 51 Because microvascular damage after bleomycin injury is widespread and associated with extensive vascular leak, 52 and given the small molecular weight of UK-156406 (611.7) it is theoretically at least feasible that the concentration of UK-156406 within the pulmonary interstitium may approach that of plasma, and therefore be sufficiently high to inhibit thrombin-mediated responses in the lung.
UK-156406 Reduces Lung Collagen Accumulation in Bleomycin-Induced Pulmonary Fibrosis
Evaluation of the effect of UK-156406 on bleomycin-induced lung injury showed that direct thrombin inhibition attenuated lung collagen accumulation in this model. Given thrombin’s pluripotent effects, there are a number of potential mechanisms by which direct thrombin inhibition may have afforded protection after bleomycin instillation, including blocking thrombin’s procoagulant effects (ie, fibrin deposition). Intra-alveolar accumulation of fibrin has been extensively documented in bleomycin-induced pulmonary fibrosis and in human studies of fibrotic lung disease, 2-4,53 and fibrin is thought to influence the fibrotic response by acting as a provisional matrix on which fibroblasts can proliferate and produce collagen in combination with fibronectin. 54 There is also evidence that fibrin can act as a reservoir of fibrogenic growth factors and cytokines that are released during fibrinolysis. 55 Fibrin has further been shown to protect active thrombin from inhibition from its physiological inhibitors so that it can remain available to exert its biological effects when bound to the provisional matrix. 56 Support for a role of fibrin in bleomycin-induced pulmonary fibrosis has come from studies using genetically modified mice that either overexpress or are completely deficient in plasminogen-activator inhibitor-1 (PAI-1), an endogenous inhibitor of fibrinolysis. In mice overexpressing PAI-1 (favoring fibrin persistence), collagen deposition was increased after bleomycin instillation, whereas in PAI-1-deficient mice (favoring fibrin clearance), collagen levels were similar to wild-type controls. 57 However, the role of fibrin in this model remains controversial as fibrinogen knockout mice are not protected from developing pulmonary fibrosis in response to bleomycin. 58,59
Modulation of thrombin’s numerous cellular effects may provide another important mechanism by which UK-156406 may have reduced lung collagen accumulation in this model, particularly in view of the fact that we have been able to demonstrate extensive expression of PAR-1 in the lung after bleomycin injury. PAR-1 has been reported to be expressed by a number of different cell types in vitro, including fibroblasts, 31 but in this study, PAR-1 seemed to be predominantly localized to both bronchial epithelial cells as well as macrophages in inflammatory and fibroproliferative foci. Although we were unable to demonstrate specific staining for PAR-1 on fibroblasts in the rat lung in vivo using conventional light microscopy, this may not be surprising given the elongated morphology of the interstitial fibroblast within the pulmonary parenchyma. However, we have able to show that primary rat lung fibroblasts isolated from male Lewis rats express PAR-1 at both the mRNA and protein level at the earliest passage number and that these cells further exhibit normal fibroblast responses to PAR-1 agonists and thrombin in vitro (data not shown).
In support of the hypothesis that UK-156046 may have exerted its protective effects in this model by directly interfering with thrombin-mediated fibroblast responses, we show that thrombin-induced fibroblast proliferation, procollagen, and CTGF mRNA levels were almost completely abolished in in vitro studies, when the inhibitor was used at concentrations that are likely to be attained at the dose used in this study in vivo. We also show that the previously reported increases in both CTGF and α1(I) procollagen mRNA levels in response to bleomycin-induced lung injury 36,60 were dramatically reduced in animals given UK-156406. This is, to our knowledge, the first report that a reduction in CTGF mRNA levels correlates with reduced α1(I) procollagen mRNA levels and ultimately lung collagen accumulation in an animal model of tissue fibrosis.
There is increasing in vitro evidence that thrombin exerts its profibrotic effects via the up-regulation of secondary mediators, including PDGF. 10 We recently reported that thrombin also induces fibroblast CTGF production, 32 but the role of CTGF in mediating the profibrotic effects of thrombin, as well as the contribution of CTGF to lung collagen accumulation in this model remain unclear. Although our in vivo experiments do not test causality directly, the coordinate down-regulation of both CTGF and procollagen gene expression by direct thrombin inhibition makes it tempting to speculate that the effects of thrombin on lung collagen deposition, may be mediated, at least in part, via a CTGF-dependent mechanism.
A final mechanism by which direct thrombin inhibition may influence the fibrotic response in this model may involve modulation of thrombin’s effects on inflammatory cell recruitment and activation. Thrombin is a potent chemoattractant for inflammatory cells 61 and stimulates the release of a number of proinflammatory cytokines, including monocyte chemotactic factor-1, interleukin-6, and interleukin-8, 62,63 predominantly via PAR-1-dependent mechanisms. Thrombin further influences inflammatory cell trafficking by inducing the expression of cell surface adhesion molecules, such as P-selectin and intercellular adhesion molecule-1. 64 Our results showed that inflammatory cell profiles in BALF after bleomycin instillation were unaffected by UK-156406, suggesting that inflammatory cell recruitment was not affected by direct thrombin inhibition. However, as thrombin is also known to stimulate the production of a number of fibrogenic cytokines, including PDGF from alveolar macrophages in vitro, 65 preventing thrombin-mediated PAR-1 activation of resident and recruited inflammatory cells may be another important mechanism by which direct thrombin inhibition may have attenuated lung collagen accumulation in this model. Future studies using PAR-1 knockout mice will be critical in further elucidating the contribution of the cellular versus the procoagulant effects of thrombin in promoting lung collagen deposition after bleomycin instillation. However, the recent finding that fibrinogen-null mice are not protected from bleomycin-induced lung injury 58,59 combined with our data, point to the possibility that the cellular effects of thrombin may play a key role in the fibrotic response after bleomycin injury.
Therapeutic Implications of This Study
Activation of the coagulation cascade is a feature of a number of lung disorders associated with inflammation and excessive deposition of extracellular matrix proteins including idiopathic pulmonary fibrosis, 2-4 pulmonary fibrosis associated with systemic sclerosis, 9,10 ALI/acute respiratory distress syndrome 5 and more recently, airway remodeling in asthma. 66 Our findings suggest that modulation of the coagulation cascade, and more specifically the profibrotic effects of coagulation proteases, may therefore warrant further evaluation as potential therapeutic strategies for treatment of these disorders. PAR-1 antagonists, blocking antibodies, and antisense oligonucleotides that are currently being developed as potential anti-thrombotic agents 67,68 may in the future provide an opportunity for selectively interfering with the profibrotic effects of thrombin, while avoiding potential hemostatic complications associated with direct proteolytic inhibition of coagulation proteases.
Acknowledgments
We thank Dr. Ian Mackie (Hemostasis Research Unit, UCL) for helpful discussions and in whose laboratory blood coagulation parameters were measured and Dr Andy Gray (Pfizer Central Research) for providing us with the direct thrombin inhibitor, UK-156406.
Footnotes
Address reprint requests to Rachel C. Chambers, Centre for Cardiopulmonary Biochemistry and Respiratory Medicine, Royal Free and University College London Medical School, The Rayne Institute, 5 University St., London WC1E 6JJ, UK. E-mail: r.chambers@ucl.ac.uk.
Supported by the Medical Research Council, U.K (Clinical Training Fellowship to D.C.J.H), The Wellcome Trust (program grant 051154), and The Middlesex Hospital Special Trustees.
D. C. J. H. and N. R. G. contributed equally to this work.
References
- 1.McAnulty RJ, Laurent GJ: Pathogenesis of lung fibrosis and potential new therapeutic strategies. Exp Nephrol 1995, 3:96-107 [PubMed] [Google Scholar]
- 2.Chapman HA, Allen CL, Stone OL: Abnormalities in pathways of alveolar fibrin turnover among patients with interstitial lung disease. Am Rev Respir Dis 1986, 133:437-443 [DOI] [PubMed] [Google Scholar]
- 3.Ikeda T, Hirose N, Koto H, Hirano H, Shigematsu N: Fibrin deposition and fibrinolysis in the pathogenesis of pulmonary fibrosis. Nippon Kyobu Shikkan Gakkai Zasshi 1989, 27:448-451 [PubMed] [Google Scholar]
- 4.Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, Takada A: Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res 1995, 77:493-504 [DOI] [PubMed] [Google Scholar]
- 5.Bachofen M, Weibel ER: Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982, 3:35-56 [PubMed] [Google Scholar]
- 6.Idell S, Gonzalez K, Bradford H, MacArthur CK, Fein AM, Maunder RJ, Garcia JG, Griffith DE, Weiland J, Martin TR: Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis 1987, 136:1466-1474 [DOI] [PubMed] [Google Scholar]
- 7.Marshall RP, Bellingan G, Laurent GJ: The acute respiratory distress syndrome: fibrosis in the fast lane. Thorax 1998, 53:815-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A: Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med 1997, 156:631-636 [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Rodriguez NA, Cambrey AD, Harrison NK, Chambers RC, Gray AJ, Southcott AM, duBois RM, Black CM, Scully MF, McAnulty RJ, Laurent GJ: Role of thrombin in pulmonary fibrosis. Lancet 1995, 346:1071-1073 [DOI] [PubMed] [Google Scholar]
- 10.Ohba T, McDonald JK, Silver RM, Strange C, LeRoy EC, Ludwicka A: Scleroderma bronchoalveolar lavage fluid contains thrombin, a mediator of human lung fibroblast proliferation via induction of platelet-derived growth factor alpha-receptor. Am J Respir Cell Mol Biol 1994, 10:405-412 [DOI] [PubMed] [Google Scholar]
- 11.Tani K, Yasuoka S, Ogushi F, Asada K, Fujisawa K, Ozaki T, Ogura T, Suzuki K: The role of thrombin on lung fibroblast growth and fibrosis in bleomycin-induced lung disorder. Nihon Kyobu Shikkan Gakkai Zasshi 1991, 29:211-219 [PubMed] [Google Scholar]
- 12.Tani K, Yasuoka S, Ogushi F, Asada K, Fujisawa K, Ozaki T, Sano N, Ogura T: Thrombin enhances lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 1991, 5:34-40 [DOI] [PubMed] [Google Scholar]
- 13.Kirschstein W, Heene DL: Fibrinolysis inhibition in acute respiratory distress syndrome Scand J Clin Lab Invest 1985, 178(Suppl):S87-S94 [PubMed] [Google Scholar]
- 14.Yasui H, Gabazza EC, Taguchi O, Risteli J, Risteli L, Wada H, Yuda H, Kobayashi T, Kobayashi H, Suzuki K, Adachi Y: Decreased protein C activation is associated with abnormal collagen turnover in the intra-alveolar space of patients with interstitial lung disease. Clin Appl Thromb Hemost 2000, 6:202-205 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann H, Siebeck M, Spannagl M, Weis M, Geiger R, Jochum M, Fritz H: Effect of recombinant hirudin, a specific inhibitor of thrombin, on endotoxin-induced intravascular coagulation and acute lung injury in pigs. Am Rev Respir Dis 1990, 142:782-788 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt B, Davis P, La-Pointe H, Monkman S, Coates G, deSa D: Thrombin inhibitors reduce intrapulmonary accumulation of fibrinogen and procoagulant activity of bronchoalveolar lavage fluid during acute lung injury induced by pulmonary overdistention in newborn piglets. Pediatr Res 1996, 39:798-804 [DOI] [PubMed] [Google Scholar]
- 17.Uchiba M, Okajima K: Antithrombin III (AT III) prevents LPS-induced pulmonary vascular injury: novel biological activity of AT III. Semin Thromb Hemost 1997, 23:583-590 [DOI] [PubMed] [Google Scholar]
- 18.Abubakar K, Schmidt B, Monkman S, Webber C, deSA D, Roberts R: Heparin improves gas exchange during experimental acute lung injury in newborn piglets. Am J Respir Crit Care Med 1998, 158:1620-1625 [DOI] [PubMed] [Google Scholar]
- 19.Piguet PF, Van GY, Guo J: Heparin attenuates bleomycin but not silica-induced pulmonary fibrosis in mice: possible relationship with involvement of myofibroblasts in bleomycin, and fibroblasts in silica-induced fibrosis. Int J Exp Pathol 1996, 77:155-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe EA, Grulich J, Weksler BB, Hampel G, Watanabe K: Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem 1987, 262:8557-8565 [PubMed] [Google Scholar]
- 21.Bar-Shavit R, Kahn A, Fenton JW, II, Wilner GD: Receptor-mediated chemotactic response of a macrophage cell line (J774) to thrombin. Lab Invest 1983, 49:702-707 [PubMed] [Google Scholar]
- 22.Bar-Shavit R, Kahn A, Fenton JW, II, Wilner GD: Chemotactic response of monocytes to thrombin. J Cell Biol 1983, 96:282-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bizios R, Lai L, Fenton JW, II, Malik AB: Thrombin-induced chemotaxis and aggregation of neutrophils. J Cell Physiol 1986, 128:485-490 [DOI] [PubMed] [Google Scholar]
- 24.Dawes KE, Gray AJ, Laurent GJ: Thrombin stimulates fibroblast chemotaxis and replication. Eur J Cell Sci 1993, 61:126-130 [PubMed] [Google Scholar]
- 25.Chen LB, Buchanan JM: Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci USA 1975, 72:131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carney DH, Cunningham DD: Cell surface action of thrombin is sufficient to initiate division of chick cells. Cell 1978, 14:811-823 [DOI] [PubMed] [Google Scholar]
- 27.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ: Thrombin stimulates fibroblast pro-collagen production via proteolytic activation of protease-activated receptor 1. Biochem J 1998, 333:121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu TK, Hung DT, Wheaton VI, Coughlin SR: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 [DOI] [PubMed] [Google Scholar]
- 29.Trejo J, Connolly AJ, Coughlin SR: The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J Biol Chem 1996, 271:21536-21541 [DOI] [PubMed] [Google Scholar]
- 30.Cunningham MA, Rondeau E, Chen X, Coughlin SR, Holdsworth SR, Tipping PG: Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J Exp Med 2000, 191:455-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dery O, Corvera C, Steinhoff M, Bunnet N: Proteinase-activated receptors: novel mechanisms of signalling by serine proteases. Am J Physiol , 274:C1429-C1452 [DOI] [PubMed] [Google Scholar]
- 32.Chambers R, Leoni P, Blanc-Brude O, Wembridge D, Laurent G: Thrombin is a potent inducer of connective tissue growth factor production via proteolytic activation of protease-activated receptor-1. J Biol Chem 2000, 275:35584-35591 [DOI] [PubMed] [Google Scholar]
- 33.Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst GR: Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 1996, 107:404-411 [DOI] [PubMed] [Google Scholar]
- 34.Grotendorst GR: Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev 1997, 8:171-179 [DOI] [PubMed] [Google Scholar]
- 35.Allen JT, Knight RA, Bloor CA, Spiteri MA: Enhanced insulin-like growth factor binding protein-related protein 2 (connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol 1999, 21:693-700 [DOI] [PubMed] [Google Scholar]
- 36.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M: Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol 1998, 275:L365-L371 [DOI] [PubMed] [Google Scholar]
- 37.Jenkins AL, Bootman MD, Taylor CW, Mackie EJ, Stone SR: Characterization of the receptor responsible for thrombin-induced intracellular calcium responses in osteoblast-like cells. J Biol Chem 1993, 268:21432-21437 [PubMed] [Google Scholar]
- 38.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ: Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol 1998, 18:611-619 [DOI] [PubMed] [Google Scholar]
- 39.McAnulty RJ, Guerreiro D, Cambrey AD, Laurent GJ: Growth factor activity in the lung during compensatory growth after pneumonectomy: evidence of a role for IGF-1. Eur Respir J 1992, 5:739-747 [PubMed] [Google Scholar]
- 40.Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ: A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors. J Cell Sci 1989, 92:513-518 [DOI] [PubMed] [Google Scholar]
- 41.Campa JS, McAnulty RJ, Laurent GJ: Application of high-pressure liquid chromatography to studies of collagen production by isolated cells in culture. Anal Biochem 1990, 186:257-263 [DOI] [PubMed] [Google Scholar]
- 42.Chambers RC, McAnulty RJ, Shock A, Campa JS, Newman Taylor AJ, Laurent GJ: Cadmium selectively inhibits fibroblast procollagen production and proliferation. Am J Physiol 1994, 267:L300-L308 [DOI] [PubMed] [Google Scholar]
- 43.Laurent GJ, Cockerill P, McAnulty RJ, Hastings JR: A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem 1981, 113:301-312 [DOI] [PubMed] [Google Scholar]
- 44.Zacharski LR, Memoli VA, Morain WD, Schlaeppi JM, Rousseau SM: Cellular localization of enzymatically active thrombin in intact human tissues by hirudin binding. Thromb Haemost 1995, 73:793-797 [PubMed] [Google Scholar]
- 45.Kobayashi N, Suko M, Sugiyama H, Dohi M, Okudaira H, Miyamoto T: Procoagulant activity of alveolar macrophages in bleomycin-induced lung fibrosis in rats. Nihon Kyobu Shikkan Gakkai Zasshi 1990, 28:867-874 [PubMed] [Google Scholar]
- 46.McGee MP, Devlin R, Saluta G, Koren H: Tissue factor and factor VII messenger RNAs in human alveolar macrophages: effects of breathing ozone. Blood 1990, 75:122-127 [PubMed] [Google Scholar]
- 47.McGee MP, Rothberger H: Assembly of the prothrombin activator complex on rabbit alveolar macrophage high-affinity factor Xa receptors. A kinetic study. J Exp Med 1986, 164:1902-1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelken NA, Soifer SJ, O’Keefe J, Vu TK, Charo IF, Coughlin SR: Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest 1992, 90:1614-1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cicala C, Bucci M, Dominicis G, Harriot P, Sorrentino L, Cirino G: Bronchoconstrictor effect of thrombin and thrombin receptor activating peptide in guinea-pigs in vivo. Br J Pharmacol 1999, 126:478-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuliopulos K, Covic L, Seeley S, Sheridan P, Helin J, Costello C: Plasmin desensitisation of the PAR-1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry 199, 38:4572–4585 [DOI] [PubMed]
- 51.Walz DA, Anderson GF, Ciaglowski RE, Aiken M, Fenton JW, II: Thrombin-elicited contractile responses of aortic smooth muscle. Proc Soc Exp Biol Med 1985, 180:518-526 [DOI] [PubMed] [Google Scholar]
- 52.Hay J, Shahzeidi S, Laurent G: Mechanisms of bleomycin-induced lung damage. Arch Toxicol 1991, 65:81-94 [DOI] [PubMed] [Google Scholar]
- 53.Idell S, James KK, Gillies C, Fair DS, Thrall RS: Abnormalities of pathways of fibrin turnover in lung lavage of rats with oleic acid and bleomycin-induced lung injury support alveolar fibrin deposition. Am J Pathol 1989, 135:387-399 [PMC free article] [PubMed] [Google Scholar]
- 54.Pohl J, Bruhn HD, Christophers E: Thrombin and fibrin-induced growth of fibroblasts: role in would repair and thrombus organisation. Klin Wochenschr 1979, 15:273-277 [DOI] [PubMed] [Google Scholar]
- 55.Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC: Release and activation of platelet latent TGF-beta in blood clots during dissolution with plasmin. Nat Med 1995, 1:932-937 [DOI] [PubMed] [Google Scholar]
- 56.Bar-Shavit R, Benezra A, Eldor A, Hy-Am E, Fenton JW, II, Wilner GD, Vlodavsky I: Thrombin immobilised to extracellular matrix is a potent mitogen for vascular smooth muscle cells: nonenzymatic mode of action. Cell Regul 1990, 1:453-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH: Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator-1 gene. J Clin Invest 1996, 97:233-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ploplis VA, Wilberding J, McLennan L, Liang Z, Cornelissen I, DeFord ME, Rosen ED, Castellino F: A total fibrinogen deficiency is compatible with the development of pulmonary fibrosis in mice. Am J Pathol 2000, 157:703-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF: Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 2000, 106:1341-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raghow R, Lurie S, Seyer JM, Kang AH: Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest 1985, 76:1733-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar-Shavit R, Kahn A, Wilner GD, Fenton JW, II: Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science 1983, 220:728-731 [DOI] [PubMed] [Google Scholar]
- 62.Sower LE, Froelich CJ, Carney DH, Fenton JW, Klimpel GR: Evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol 1995, 155:895-901 [PubMed] [Google Scholar]
- 63.Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T: Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology 1996, 88:76-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugama Y, Tiruppathi C, Offakidevi K, Andersen TT, Fenton JW, II, Malik AB: Thrombin-induced expression of endothelial P-selectin and intercellular adhesion molecule-1. J Cell Biol 1992, 119:935-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tani K, Ogushi F, Takahashi H, Kawano T, Endo T, Sone S: Thrombin stimulates platelet-derived growth factor release by alveolar macrophages-significance in bleomycin-induced pulmonary fibrosis. J Med Invest 1997, 44:59-65 [PubMed] [Google Scholar]
- 66.Gabazza EC, Taguchi O, Tamaki S, Takeya H, Kobayashi H, Yasui H, Kobayashi T, Hataji, Urano H, Zhou H, Suzuki K, Adachi Y: Thrombin in the airways of asthmatic patients. Lung 1999, 177:253-262 [DOI] [PubMed] [Google Scholar]
- 67.Brass LF: Thrombin receptor antagonists: a work in progress. Coron Artery Dis 1997, 8:49-58 [DOI] [PubMed] [Google Scholar]
- 68.Bernatowicz MS, Klimas CE, Hartl KS, Peluso M, Allegretto NJ, Seiler SM: Development of potent thrombin receptor antagonist peptides. J Med Chem 1996, 39:4879-4887 [DOI] [PubMed] [Google Scholar]