Abstract
Endothelin (ET) has been implicated in the regulation of hepatic microcirculation and development of portal hypertension. This study examined the localization of ETA receptor (ETAR) and ETB receptor (ETBR) in cirrhotic liver tissues from patients with hepatocellular carcinoma with hepatitis C-related cirrhosis, and normal liver samples from patients with metastatic liver carcinoma. Anti-ETAR and ETBR antibodies were used for immunohistochemistry and Western blot. Immunoelectron microscopy was conducted using immunoglobulin-gold and silver staining. For in situ hybridization (ISH), human ETAR and ETBR peptide nucleic acid probes were used with the catalyzed signal amplification system. In normal liver tissue, immunohistochemistry revealed that ETBR was predominantly expressed on hepatic sinusoidal lining cells, particularly on sinusoidal endothelial (SECs) and hepatic stellate cells (HSCs), and ETAR was scantily expressed. These findings were confirmed by Western blot and ISH. In cirrhotic liver tissue, overexpression of ETBR was demonstrated by Western blot and ISH. Morphometric analysis showed significant increase of ETBR expression on HSCs and SECs in cirrhotic liver, particularly on HSCs. ETAR expression was increased but remained low. Enhanced ETBR expression in cirrhosis may intensify the effect of endothelin on HSCs and increase hepatic microvascular tone.
A number of vasoactive substances have been implicated as potential mediators of intrahepatic portal hypertension. 1 The precise sites of action of these compounds remain controversial but in theory could be at any level within the liver; presinusoidal, sinusoidal, or postsinusoidal. 2-4 Endothelin (ET)-1 is a potent vasoconstrictive peptide composed of 21 amino residues, originally isolated from the supernatant of cultured porcine endothelial cell. 5 Two other endothelins, ET-2 and ET-3 6 were subsequently isolated. The mature peptides, each 21 amino acid residues in length, are cleavage products of larger precursor proteins. 7 Their major function appears to be control of local vascular tone, 8 but broad effects on growth and development have also been suggested. 9
Two types of ET receptors have been identified and studied; endothelin A (ETAR) and B receptors (ETBR), both signal via GTP-binding proteins. 10 Another type of endothelin receptor, ETCR, has been found but its function and distribution remains unknown. ETAR is found predominantly on vascular smooth muscle cells and the affinities for endothelins are in the order ET-1 > ET-2 > ET-3; the affinity for ET-1 is more than 100-fold that of ET-3. 11 The primary response mediated by ETAR is vasoconstriction. 11 ETBR stimulation leads to diverse responses depending, in part, on cell type. A study has proposed that ETBR-dependent relaxation and smooth muscle vasoconstriction are mediated by distinct ETBRs, termed ETB1 and ETB2, respectively. 11 While ET receptors are detectable on all cell types in rat liver, they are far more numerous on hepatic stellate cells (HSCs) than on other hepatic cells such as sinusoidal endothelial cells (SECs), Kupffer cells, and hepatocytes. 12,13 When ET-1 is perfused into the rat liver, its localization is consistent with binding to HSCs and SECs. 14 Rat HSCs express both ETAR and ETBR, as established by mRNA analysis and competitive binding assays. 13 Functional studies indicate that both receptors mediate biological effects. 15-17 The ETBR receptor appears to be involved additionally in regulating growth of human myofibroblast-like stellate cells. 16
While the data of ETR have largely been obtained using rat liver, there is relatively little information on human liver. No study so far has localized ETAR and ETBR in intact human liver tissue. Our present report is the first to investigate the expression and distribution of ETR subtypes in human normal liver and cirrhotic liver tissues at protein (immunohistochemistry, Western blot, immunoelectron microscopic study) and mRNA (in situ hybridization) levels.
Materials and Methods
Materials
As controls, wedge biopsy specimens from normal portions of the liver were obtained from 5 patients (4 males and 1 female; aged from 44 to 73 years with a mean of 57.3 years) who underwent surgical resection for metastatic liver carcinoma (4 colonic carcinomas and 1 gastric carcinoma). Cirrhotic liver specimens were obtained from gross cirrhotic portions surgically resected from 5 patients (5 males; aged from 62 to 75 years with a mean of 67.4 years) who had hepatocellular carcinoma combined with hepatitis C-related cirrhosis.
Immunoperoxidase Staining
Liver tissues (approximately 5 × 5 × 5 mm) were fixed in periodate-lysine-paraformaldehyde (PLP), rinsed in 0.01 mol/L phosphate buffer (pH 7.4) 18 containing 15 to 30% sucrose, embedded in Tissue-Tek OCT-compound (Sakura Finetek Inc., Torrance, CA), and frozen at −80°C until use. Semithin sections 5 μm in thickness were cut using a cryostat, and incubated two days at 4°C with 1:50 dilution of anti-ETAR or anti-ETBR rabbit polyclonal antibodies (Immunobiological Lab., Fujioka, Japan). 19 Then the sections were incubated with the EnVision system (DAKO, Glostrup, Denmark) 20 at room temperature for 120 minutes. After repeated washes with phosphate-buffered saline (PBS), the sections were reacted with diaminobenzidine containing 0.01% H202, and counterstained with hematoxylin for light microscopic study.
Immunogold-Silver Staining Method for Light and Electron Microscopy
For light microscopy, the sections were immersed in three changes of 0.01% PBS (pH 7.4) for 15 minutes and then incubated for two days at 4°C in a moist chamber with anti-ETAR or anti-ETBR rabbit polyclonal antibodies diluted 1:50 with 0.01 mol/L PBS containing 1% bovine serum albumin (BSA). After treating with PBS for 15 minutes three times, the sections were incubated for 40 minutes with 10-nm colloidal gold-conjugated anti-rabbit IgG antibody (Cosmo Bio Co., Tokyo, Japan) diluted 1:100. The slides were developed with a developing solution (described below) for 50 minutes at 20°C in a dark room, washed in running tap water, briefly counterstained with 0.1% nuclear fast red in 5% aluminum sulfate aqueous solution, dehydrated, cleared and mounted in Biolet. The developing solution had two components. Solution A contained 45 ml of 20% gum arabic aqueous solution (Kanto Chemical Co., Tokyo, Japan) and 1 ml of 10% silver nitrate solution. The gum arabic solution was prepared by centrifugation a 20% solution at 18,000 rpm for 30 minutes at 0°C and separation of the supernatant for use. Solution B contained 200 mg of hydroquinone (Kanto Chemical Co., Tokyo, Japan) and 300 mg of citric acid monohydrate (Kanto Chemical Co., Tokyo, Japan) in 10 ml of distilled water. The working developing solution was prepared by mixing solution A and B in a dark room under illumination of a photographic safety lamp. 21
For electron microscopy, the tissue specimens were treated with PBS for 15 minutes three times, fixed in 1.2% glutaraldehyde buffered with 0.01% phosphate buffer (pH 7.4) for 1 hour at 4°C, treated with graded series of ethanol solutions, and post-fixed with 1% osmium tetroxide in 0.01% phosphate buffer (pH 7.4). The liver tissues were embedded in Epon (Polyscience, Inc. Warrington, PA). Ultrathin sections cut with a diamond knife on a LKB ultramicrotome (Bromma, Sweden) were stained with uranyl acetate and observed under a transmission electron microscope (JEM-1200 EX, Tokyo, Japan) operated at an acceleration voltage of 80 kV.
Western Blotting
Western blotting was conducted using fresh control and cirrhotic liver tissues. Briefly, liver tissues were homogenized in 10 volumes of homogenization buffer (20 mmol/L Tris-HCl, pH 7.5, 5 mmol/L MgCl2, 0.1 mmol/L PMSF, 20 μmol/L pepstatin A, and 20 μmol/L leupeptin) using a polytron homogenizer at setting 7 for 90 seconds. The homogenates were centrifuged at 100,000 × g for 45 minutes. The membranes were washed three times, resuspended in 10 volumes of homogenization buffer, homogenized using a Teflon/glass homogenizer, and centrifuged. The membrane proteins thus obtained were used for immunoblotting. Proteins were separated on SDS/PAGE (4 to 20% gel) (Daiichi-Ikagaku, Tokyo, Japan) and transferred onto vinylidene difluoride membranes (Millipore, Bedford, MA). The blots were blocked with 5% (w/v) dried milk in PBS for 30 minutes, incubated with 5 μg/ml anti-ETAR or anti-ETBR antibody, washed in 0.1% Tween 20 in PBS, and incubated with 5000-fold diluted anti-rabbit goat IgG conjugated with horseradish peroxidase (Amersham-Pharmacia, Bioteck, Buckinghamshire, UK). Both primary and secondary antibodies were diluted with 0.1% Tween 20 in PBS, and incubation was at room temperature for 1 hour. The immunoreactive bands were visualized with the ECL Plus detection system (Amersham-Pharmacia).
In Situ Hybridization Technique
mRNA of ETR subtypes was detected in formalin-fixed, paraffin-embedded sections by in situ hybridization using peptide nucleic acid (PNA) probes 22 and the catalyzed signal amplification (CSA) technique. Liver tissues were cut into 4 μm-thick sections and adhered to silanated, RNase-free glass slides (prepared by heating in an oven at 60°C for 30 minutes). The sections were dewaxed in xylene (twice for 15 minutes each), followed by a graded ethanol series, rehydrated in RNase-free distilled water, and incubated for 30 minutes in Target Retrieval Buffer (DAKO JAPAN, Kyoto, Japan) preheated and maintained at 95°C. The slides were allowed to cool at room temperature for 20 minutes. The sections were then digested with 20 μg/ml proteinase K (DAKO) at room temperature for 30 minutes. The slides were rinsed in distilled water and rapidly air-dried. After pretreatment, air-dried sections were covered with approximately 15 ml of hybridization solution containing 10% (w/v) dextran sulfate, 10 mmol/L NaCl, 30% (v/v) formamide, 0.1% (w/v) sodium pyrophosphate, 0.2% (w/v) polyvinylpyrrolidone, 0.2% (w/v) Ficoll, 5 mmol/L Na2-EDTA, 50 mmol/L Tris-HCl, pH 7.5, and 1 μg/ml PNA probe. ETBR antisense (FITC-ACC/TGT/CAA/CAC/TTA), ETAR sense (FITC-TTA/GTG/TTG/ACA/GGT), ETBR antisense (FITC-ACA/CAA/GGC/AGG/ACA), or ETBR sense (FITC-TGT/CCT/GCC/TTG/TGT) probe was used. 23 The slides were evenly covered with the hybridization solution and incubated in a moist chamber at 43°C for 90 minutes. Following hybridization, the coverslips were removed, and the slides were transferred to pre-warmed TBS in a water bath at 49°C and washed for 30 minutes with gentle shaking (PNA hybridization kit; DAKO JAPAN, Kyoto, Japan). A non-isotopic, colorimetric signal amplification system (GenPoint Kit, DAKO JAPAN, Kyoto, Japan) was used to visualize specific hybridization signals. Briefly, tissue sections were incubated with a FITC-horseradish peroxidase reagent for 15 minutes, washed three times with TBST (150 mmol/L NaCl, 10 mmol/L Tris. pH 7.5, 1.1% v/v Tween 20), incubated with a solution containing H202 and biotinyl tyramide for 15 minutes, and washed three times with TBST. 24 This step resulted in catalyzed signal amplification by additional deposition of biotin at the site of probe hybridization. The sections were then incubated in streptavidin-horseradish peroxidase for 15 minutes and washed three times in TBST. Colorimetric signals were localized after incubation in diaminobenzidine solution containing 0.01% H2O2, and counterstained with hematoxylin for light microscopic examination.
Semiquantitative Analysis
The immunogold labeling in the ultra-thin sections of peri-sinusoidal SECs and HSCs was quantitated using the Mac Measure program, version 1.61. SECs and HSCs around sinusoids were selected randomly, and the numbers of gold particles per unit length of membrane were counted. Statistical significance of the difference between control and cirrhotic samples was assessed by Student’s t-test, and P < 0.05 was regarded as indicating a significant difference. Data are expressed as means ± SEM.
Results
Immunohistochemical Study
In control liver tissue, immunohistochemical study demonstrated that ETBR was moderately expressed on the hepatic sinusoidal lining cells, particularly on the SECs and HSCs, whereas ETAR was only rarely detected on the sinusoidal lining cells although it is found on portal vessels (Figure 1) ▶ . In cirrhotic liver tissue, immunohistochemical study demonstrated overexpression of ETBR on hepatic sinusoidal lining cells compared to control liver tissue, while ETAR showed an increase but remained low in level (Figure 2) ▶ . There was a patchy distribution of ET receptors.
Figure 1.
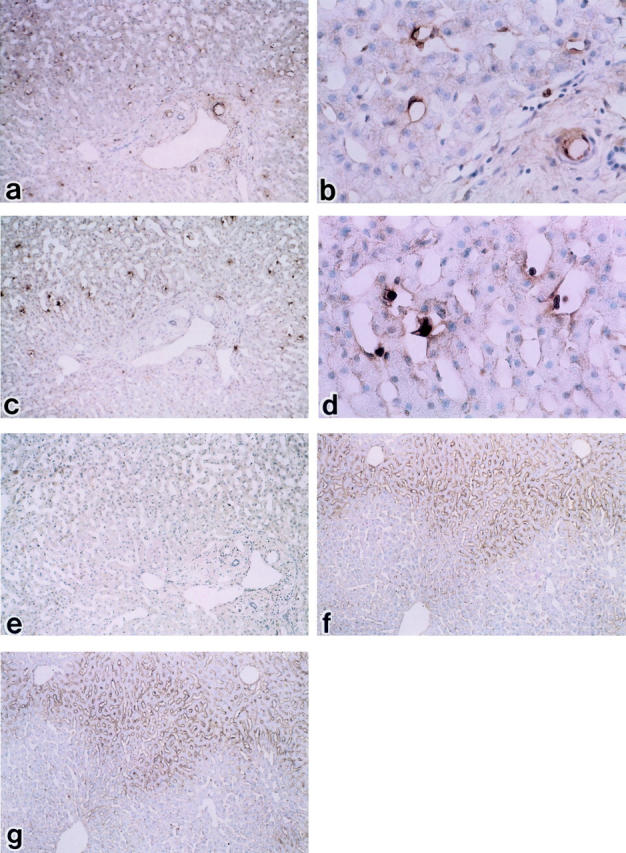
Light microscopic distributions of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) in human control liver using immunoperoxidase staining. a and b: Immunoperoxidase-positive substances showing the presence of ETAR are found on portal ducts and vessels, but rarely observed on hepatic lining cells (a: ×100; b: ×400). c and d: Reaction products showing ETBR are moderately localized on hepatic sinusoidal lining cells, particularly on sinusoidal endothelial cells and hepatic stellate cells (c: ×100; d: ×400). e: The section as a negative control with EnVision system fractions. f and g: Positive control: Immunoperoxidase-positive substances showing the presence of ETAR (F: ×100) and ETBR (G: ×100) and localized homogeneously on hepatic sinusoidal lining cells in rat control liver. Hematoxylin counterstain.
Figure 2.
Light microscopic distributions of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) in human cirrhotic liver using immunoperoxidase staining. a and b: Immunoperoxidase-positive substance for ETAR remains weakly detectable compared to control liver but remains low in level (a: ×100; b: ×400). c and d: Reaction products of ETBR are markedly increased on sinusoidal endothelial cells and hepatic stellate cells compared to control human liver (c: ×100; d: ×400). Hematoxylin counterstain.
To verify the methodology and performance of the antibodies, we included rat liver tissue in the staining as a positive control. In rat liver, immunoperoxidase-positive substances showing the presence of ETA receptor and ETB receptor were localized on hepatic sinusoidal lining cells, especially around portal venules. They showed a typical homogeneous distribution (Figure 1, f and g) ▶ .
Western Blot
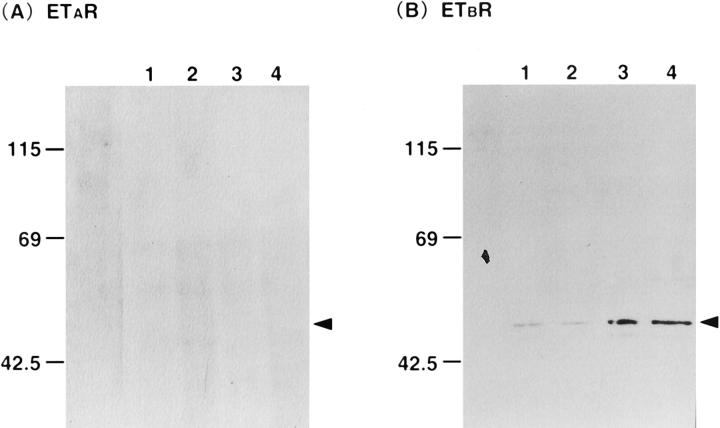
To confirm the immunohistochemical results, we investigated receptor protein expression by Western blotting. Samples containing 15 μg of protein were subjected to SDS/PAGE (4 to 20% gels) and analyzed by blotting. ETBR was found in abundance in cirrhotic liver, but ETAR was not detected either in control or cirrhotic liver (Figure 3) ▶ .
Figure 3.
Western blot analysis of expression of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) proteins in human control and cirrhotic liver tissues. Samples containing 15 μg protein were subjected to SDS/PAGE (4 to 20% gels) and analyzed by blotting. ETBR is found in abundance in cirrhotic liver, but ETAR is not detected either in control or cirrhotic liver. Lanes 1 and 2 denote control liver. Lanes 3 and 4 denote cirrhotic liver. Left: ETAR immunoblots. Right: ETBR immunoblots. Positions of molecular mass markers are shown in kilodaltons.
Ultrastructural Localization of ETR
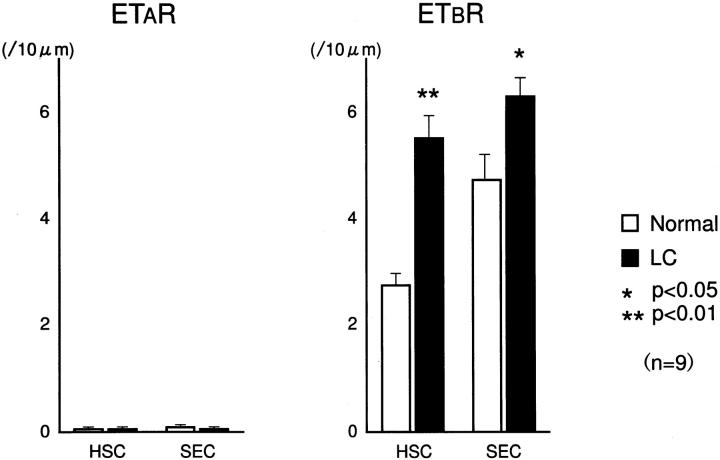
We examined the ultrastructural localization of ETRs on sinusoidal lining cells by immunogold electron microscopy. In control liver tissue, electron-dense gold particles showing the presence of ETAR were found scantily on hepatic sinusoidal lining cells, while ETBR labeling was evident on HSCs and SECs, mainly localized on the luminal side (Figure 4) ▶ . In cirrhotic liver tissue, ETBR was significantly increased not only on HSCs but also on SECs compared to control liver tissue, while ETAR labeling remained scanty (Figure 5) ▶ . In morphometric analysis of immunogold particle labeling for ETAR and ETBR (Figure 6) ▶ , ETAR labeling was extremely low on HSCs (control; 0.2 ± 0.1/10 μm, cirrhosis; 0.1 ± 0.1, not significant) and SECs (control; 0.1 ± 0.1/10 μm, cirrhosis; 0.1 ± 0.1, not significant) both in control and cirrhotic liver tissues. On the other hand, ETBR labeling was considerably high in HSCs and SECs in control liver, and was significantly increased on HSCs (control; 2.2 ± 0.6, cirrhosis; 5.8 ± 1.6. P < 0.01) and SECs (control; 4.8 ± 0.3, cirrhosis; 6.8 ± 1.8, P < 0.05) in cirrhotic liver. The increase in ETbR expression was particularly marked in HSCs. Therefore, HSCs and SECs in normal human liver tissue expressed predominantly ETBRs, and the expression of ETBRs was strongly enhanced on HSCs and slightly enhanced on SECs in cirrhotic liver.
Figure 4.
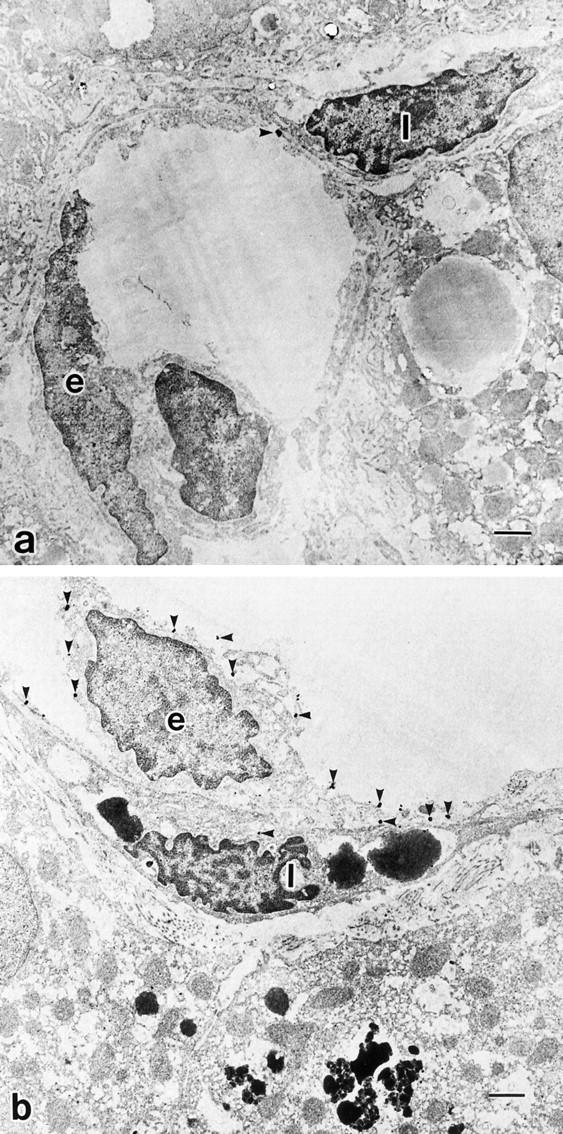
Ultrastructural localization of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) in control liver, by immunogold electron microscopy. a: Electron-dense immunoreaction product of ETAR is hardly detectable on sinusoidal endothelial cells (SECs) and hepatic stellate cells (HSCs) in control liver. b: ETBR is evident on the luminal side of HSCs as well as SECs in control liver. e: Sinusoidal endothelial cell. I: Ito cell (hepatic stellate cell). Scale bar, 1 μm. Uranyl acetate stain.
Figure 5.
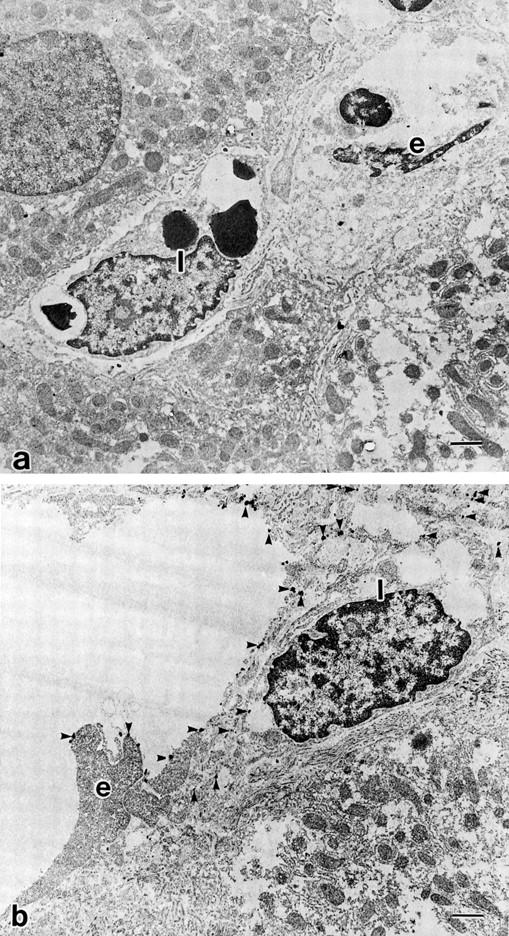
Ultrastructural localizations of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) in cirrhotic liver, by immunogold electron microscopy. a: Immunogold particles showing the presence of ETAR are hardly seen on sinusoidal endothelial cells (SECs) and hepatic stellate cells (HSCs). b: ETBRs are strongly expressed on the luminal sites of HSCs as well as SECs. e: sinusoidal endothelial cell. I: Ito cell (hepatic stellate cell). Scale bar, 1 μm. Uranyl acetate stain.
Figure 6.
Morphometric analysis of immunogold particle labeling for endothelin A receptor (ETAR) and endothelin B receptor (ETBR) around hepatic sinusoids in human control and cirrhotic livers from nine electronmicrographs. In cirrhotic human liver, ETbR is significantly increased on HSCs and SECs.
In Situ Hybridization
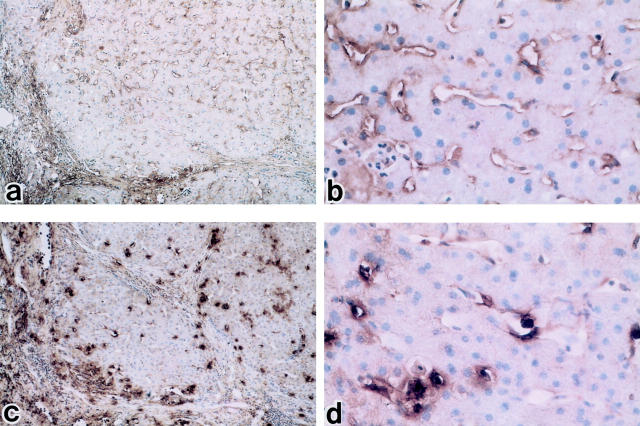
Next, we investigated the expression of endothelin receptors at mRNA level by in situ hybridization using peptide nucleic acid probe. In control liver tissue, ETAR mRNA was almost undetectable, but ETBR mRNA was detected on hepatic sinusoidal lining cells (Figure 7) ▶ . In cirrhotic liver tissue, ETBR mRNA expression was enhanced on hepatic sinusoidal lining cells, particularly on HSCs, compared with control liver tissue, while ETAR mRNA remained almost undetectable (Figure 8) ▶ . Similar results were obtained in all control (n = 5) and cirrhotic (n = 5) liver tissues.
Figure 7.
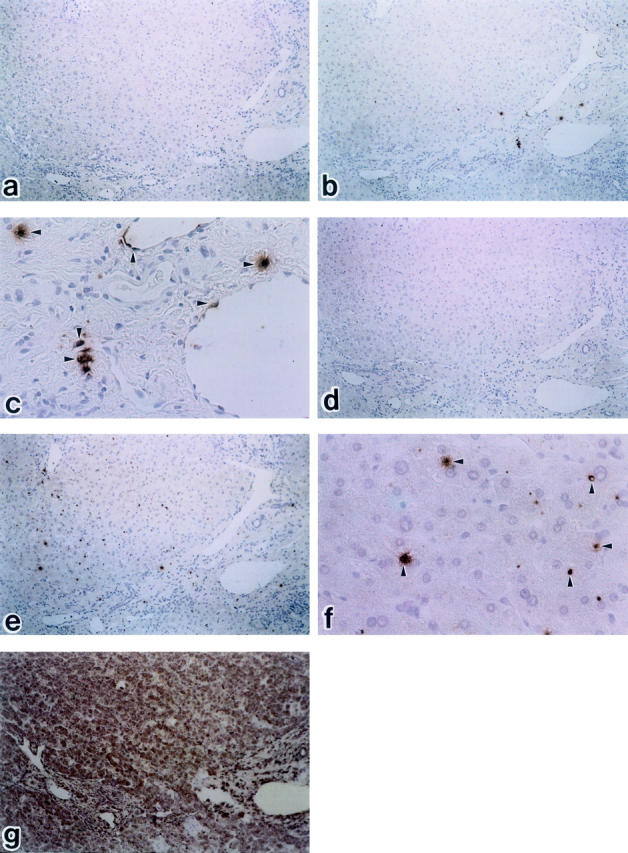
In situ hybridization for the detection of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) mRNA in normal liver tissue. b: Immunoperoxidase-positive substances showing the presence of ETAR are located on portal vein, but hardly observed on hepatic lining cells (b: ×100; c: ×400) e and f: A small number of sinusoidal mesenchymal cells show signals with the ETBR antisense probe (e: ×100; f: ×400). Arrowheads denote signal. Peptide nucleic acid probes were used with FITC labeling. a: ETAR sense probe (×100), b: ETAR antisense probe (×100), c: ETAR antisense probe (×400), d: ETBR sense probe (×100), e: ETBR antisense (×100), f: ETBR antisense probe (×400). g: positive control; glyceraldehide-3-phosphate dehydrogenase antisense probe (PNA hybridization kit; DAKO JAPAN, Kyoto, Japan). Hematoxylin counterstain.
Figure 8.
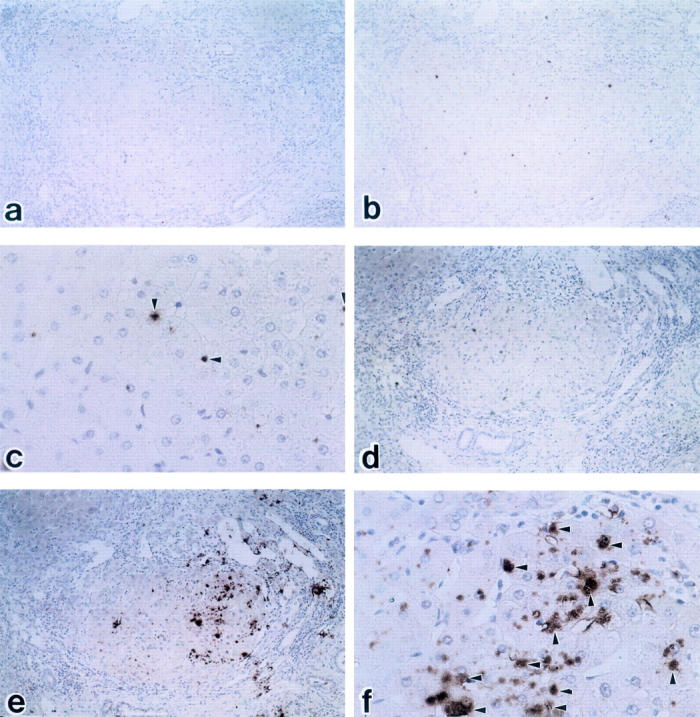
In situ hybridization for the detection of endothelin A receptor (ETAR) and endothelin B receptor (ETBR) mRNA in cirrhotic liver tissue. b: ETAR are partially observed on vessels, but are hardly observed on hepatic lining cells (b: ×100; c: ×400). Arrowheads denote signal. e and f: A large number of sinusoidal mesenchymal cells show signals with the ETBR antisense probe (c: ×100; d: ×400). Peptide nucleic acid probes were used with FITC labeling. a: ETAR sense probe (×100), b: ETAR antisense probe (×100), c: ETAR antisense probe (×400), d: ETBR sense probe (×100), e: ETBR antisense (×100), f: ETBR antisense probe (×400).
Discussion
In the present study, we demonstrated that HSCs and SECs in normal human liver tissue expressed predominantly ETBR and less ETAR, and the expression of ETBR was strongly enhanced in cirrhotic liver tissue while ETAR remained low. These results were consistent in immunoperoxidase study, Western blot, immunogold electron microscopic study, and in situ hybridization. Immunogold electron microscopy clearly localized ETBR on HSCs and SECs, and morphometric analysis showed significant increases in ETBR expression on HSCs and SECs in cirrhotic liver, with marked enhancement on HSCs. In situ hybridization using PNA probes demonstrated overexpression of only ETBR mRNA in cirrhotic liver compared to the control. To our knowledge, this is the first report of in vivo expression of ETAR and ETBR protein and mRNA in normal and cirrhotic livers.
Endothelin receptors have been studied by various techniques such as endothelin binding assay and mRNA measurement. However, no study so far has localized endothelin subtypes directly in human liver. The commercial antibodies against ETAR and ETBR we used were recently developed in Japan; they were rabbit antibodies against the C-terminal of human ETA and ETB receptors. Some of the methods used in this paper (immunohistochemistry, immunoelectron microscopy) have been established using normal and cirrhotic rat liver 25 using these antibodies and using the newly developed EnVision system, we were able to obtain rapid immunostaining of ETAR and ETBR in frozen liver tissue. We have verified these antibodies in rat liver tissues and clearly localized both ETAR and ETBR in rat liver by light and electron microscopy. 25 The EnVision system uses a polymeric conjugate consisting of a large number of peroxidase and secondary antibody molecules bound directly to an activated dextran backbone. The polymeric conjugates hold up to 100 enzyme molecules and up to 20 antibody molecules per backbone, 20 thus greatly increasing the sensitivity.
Immunogold-silver staining procedure not only allows ultrastructural localization of endothelin receptors but also easy quantitation of the immunogold-silver labeling in ultrathin sections of sinusoidal cells. 21 In control liver, ultrastructural localization of ETAR was hardly detectable on HSCs and SECs, while ETBR was evident on HSCs as well SECs. In cirrhotic liver, ETBRs were strongly expressed on HSCs. A previous autoradiographic endothelin binding study showed rare or few grains on the portal vein but abundant grains in HSCs and SECs, 14 which is consistent with our immunohistochemical results. ETBR was localized along the apical plasma membrane of endothelial cells. 26 In the present study, ETBR was mainly localized at the luminal side of SECs and HSCs.
We used PNA probes in in situ hybridization. PNAs are pseudo-peptides with DNA-binding capability first reported as nucleotide analogs capable of binding, in a sequence-specific fashion, to DNA and RNA. 27 In PNA, the sugar phosphate backbone found in DNA/RNA is replaced by a polyamide backbone, keeping the distance between the nucleotide bases the same as in DNA/RNA. Hybridization of PNA to DNA has been shown to obey the Watson-Crick rules, and the compounds have also been found to hybridize to their nucleic acid counterparts. 28 The relatively hydrophobic PNA probes penetrate tissues more easily than the corresponding DNA/RNA oligonucleotide-based probes, thereby allowing development of fast protocols for in situ hybridization. 22 While conventional non-radioactive ISH method is relatively insensitive, target amplification by PCR or signal amplification by CSA allows sensitive detection of few copies of nucleic acid sequences. Compared with PCR in situ amplification, CSA is simpler and faster and does not require thermal cycling or the specialized equipment. 29 In a preliminary experiment, we failed to obtain any signal using RNA probes to detect ETBR mRNA (data not shown), but we obtained satisfactory signals with PNA probes using the CSA method. The results of our trial clearly demonstrate the advantages of PNA probes and the CSA method. In cirrhotic liver, ETBR mRNA was overexpressed on sinusoidal lining cells.
Only a few studies have characterized ETRs in human liver cultured cells using binding or PCR studies. The detailed binding kinetic studies of Pinzanni et al 17 demonstrated that the progressive activation in HSCs in culture is associated with a progressive shift from a relative predominance of ETAR to relative predominance of ETBR, but the relative densities changed on serial subculture. A predominance of ETBR was observed when the cells had undergone complete transition to myofibroblast-like phenotype that resembles the in vivo condition of HSCs. Mallat et al 16 reported 20% ETAR and 80% ETBR binding sites in HSCs with myofibroblastic phenotype. ET-1 binding to ETBRs causes a potent growth inhibition of human myoblastic HSCs. Their results support our in vivo studies that ETBR is expressed predominantly in HSCs and SECs. An immunohistochemical study showed increase of ETR in human cirrhotic liver associated with decrease in intralobular innervation, but detailed information on subtypes was not provided. 30 A quantitative PCR in human liver tissue showed that both ETAR and ETBR mRNA levels were significantly increased in cirrhosis, correlating with increased portal pressure. 31 Their result indicated a higher level of ETBR mRNA than ETAR mRNA, and the authors stated that their method only provided an estimate of the number of copies. Therefore, our results and previous findings generally agree that ETBR is the predominant endothelin receptor in sinusoidal lining cells, especially in HSC, although our in vivo localization studies demonstrated an extremely low level of ETAR. Using the same antibodies in light and electron microscopic studies, we have localized similar densities of ETAR and ETBR in HSCs and SECs of rat liver. 25 There may be interspecies differences in ETR distribution. Results obtained from rat models may have to be interpreted with caution.
During liver injury, stellate cells undergo a process characterized by loss of retinoid droplets, enhanced collagen production, and expression of smooth muscle actin, which has been termed “activation.” 32 Following liver injury, activated HSCs are associated with increased proliferation and synthesis of the major components of liver fibrosis. 33 Activated HSCs display a large number of ET binding sites that mediate at least two biological effects of ET-1; contraction 15 and growth inhibition. 16 ET-1 inhibits proliferation of activated HSCs and the growth inhibition is mediated by ETBR. 16 ETBRs may be subdivided into two putative receptors, ETB1 and ETB2. 11,34,35 ETB1 is considered to induce NO release from endothelial cells and to promote vasodilation. 34 ETB2 may induce the contraction of vascular smooth muscles. 34
In a simultaneously stained rat section, there is a homogeneous distribution of ET receptors in rat liver (Figure 1, f and g) ▶ . In comparison, human liver tissue expresses ET receptors in a patchy pattern, and the sites of positive staining are fewer than the number of stellate cells (Figures 1 and 2) ▶ ▶ . Again, these findings validate the difference in ET distribution in human and rat livers. Our findings suggest a highly heterogeneous population of cells in human liver with only a minority being responsive to endothelin based on receptor expression. We speculate that the baseline ET receptor levels are lower in human liver than in rat liver, as confirmed by a low level of protein (Western blot) and mRNA (in situ hybridization) expression, and that the ET receptors are up-regulated in selected cells during liver injury. This is shown by the demonstration of ETB in areas of strong inflammation and at the edge of nodules and fibrotic septa in cirrhotic specimens.
Overexpression of ETbR expression in sinusoidal lining cells, especially on HSCs, in cirrhotic tissue may have several implications. It may amplify the growth inhibitory effect of ET-1 and play a role in negative control of liver fibrogenesis. On the other hand, Kaneda et al demonstrated that the parenchyma of ET-1 perfused liver in rat was distinguished histologically into two areas; one consisted of loosely woven plate of vacuolated hepatocytes with widened sinusoids, and the other with closely packed hepatic cell plates intervened by narrowed, collapsed sinusoids concomitant with constriction of preterminal portal venules. 36 Increased ETBR may mediate cell contraction and increase hepatic sinusoidal microvascular tone, and contribute to portal hypertension.
The therapeutic potential of ET blockade for portal hypertension remains a controversial topic. In experimental liver injury in rat, blockage of ETAR has been shown to disturb hepatic microcirculation and worsen endotoxin-induced liver injury, 37 while chronic blockade with a mixed antagonist Bosentan had no hemodynamic effects but increased the connective tissue content of cirrhotic liver. 38 Thus, precise elucidation of ETR, especially ETBR-mediated paracrine/autocrine functions of ET is required to understand the role of ET and its antagonists or agonists. Morphological combined with functional studies are essential. Our fast protocols for demonstrating ETR protein and mRNA may be useful assets in these investigations.
Acknowledgments
We thank Koichiro Kitagawa (DAKO, Japan) for technical assistance.
Footnotes
Address reprint requests to Hiroaki Yokomori, M.D., Kitasato Institute Medical Center Hospital, 121–1 Arai, Kitamoto-shi, Saitama 364-8501, Japan. E-mail: yokomori-hr@kitasako.or.jp.
References
- 1.Garcia-Pagan JC, Bosch J, Rodes J: The role of vasoactive mediators in portal hypertension. Semin Gastrointest Dis 1995, 6:140-147 [PubMed] [Google Scholar]
- 2.Lautt WW, Greenway CV, Legare DJ: Localization of intrahepatic portal vascular resistance. Am J Physiol 1986, 251:G375-G381 [DOI] [PubMed] [Google Scholar]
- 3.Zang JX, Bauer M, Clemens MG: Vessel- and target-cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am J Physiol 1995, 269:G269-G277 [DOI] [PubMed] [Google Scholar]
- 4.McCuskey RS: A dynamic and static study of hepatic arterioles and hepatic sphincter. Am J Anat 1966, 119:455-478 [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Kurihara H, Kumura S, Tomobe Y, Kobayashi M, Mitsui Y, Yasaki Y, Goto Y, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332:411-415 [DOI] [PubMed] [Google Scholar]
- 6.Yanagisawa M, Masaki T: Biochemistry and molecular biology of the endothelins. Trends Pharmacol Sci 1989, 10:374-378 [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M: ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of pig endothelin-1. Cell 1994, 78:473-485 [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa M: The endothelin system. A new target for therapeutic intervention. Circulation 1994, 89:1320-1322 [DOI] [PubMed] [Google Scholar]
- 9.Kurihara Y, Kurihara H, Suzuki H, Komada T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, Jishage K, Ouchi Y, Azuma S, Toyoda Y, Ishikawa T, Kumada M, Yazaki Y: Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 1994, 363:703-710 [DOI] [PubMed] [Google Scholar]
- 10.Sakurai T, Yanagisawa M, Masaki T: Molecular characterization of endothelin receptors. Trends Pharmacol Sci 1992, 13:103-108 [DOI] [PubMed] [Google Scholar]
- 11.Clozel M, Gray GA, Breu V, Loffler BM, Osterwalder R: The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun 1992, 186:867-873 [DOI] [PubMed] [Google Scholar]
- 12.Stephenson K, Harvey SA, Mustafa SB, Eakes AT, Olson MS: Endothelin association with the cultured rat Kupffer cell: characterization and regulation. Hepatology 1995, 22:896-905 [PubMed] [Google Scholar]
- 13.Housset C, Rockey DC, Bissell DM: Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci USA 1993, 90:9266-9270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya S, Naruse S, Nakayama T, Nokihara K: Binding of 125I-endothelin-1 to fat-storing cells in rat liver revealed by electron microscopic radioautography. Anat Embryol (Berl) 1992, 185:97-100 [DOI] [PubMed] [Google Scholar]
- 15.Rockey DC: Characterization of endothelin receptors mediating rat hepatic stellate cell contraction. Biochem Biophys Res Commun 1995, 207:725-731 [DOI] [PubMed] [Google Scholar]
- 16.Mallat A, Fouassier L, Preaux AM, Gal CS, Raufaste D, Rosenbaum J, Dhumeaux D, Jouneaux C, Mavier P, Lotersztajn S: Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic Ito cells: an endothelin B receptor-mediated pathway. J Clin Invest 1995, 96:42-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinzani M, Stefano M, de Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P: Endothelin is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology 1996, 110:534-548 [DOI] [PubMed] [Google Scholar]
- 18.Hsu SM, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 19.Hiraki H, Hoshi N, Hasegawa H, Tanigawa T, Emura I, Seito T, Yamaki T, Fukuda T, Watanabe K, Suzuki T: Regular immunohistochemical localization of endothelin-1 and endothelin B in normal, hyperplastic and neoplastic human adrenocortical cells. Pathol Int 1997, 47:117-125 [DOI] [PubMed] [Google Scholar]
- 20.Vyberg M, Nielsen S: Dextran polymer conjugate two-step visualization system for immunohistochemistry: a comparison of Envision+ with two three-step avidin-biotin techniques. Applied Immunohistochemistry 1998, 6:3-10 [Google Scholar]
- 21.Fujimori O, Nakamura M: Protein A gold-silver staining methods for light microscopic immunohistochemistry. Arch Histol Jpn 1985, 48:449-452 [DOI] [PubMed] [Google Scholar]
- 22.Thisted M, Just T, Pluzek KJ, Petersen KH, Hyldig-Nielsen JJ, Godtfredsen SE: Detection of immunoglobulin kappa light chain mRNA in paraffin sections by in situ hybridization using peptide nucleic acid probes. Cell Vision 1996, 3:358-363 [Google Scholar]
- 23.Nakamura M, Takayanagi R, Sakai Y, Sakamoto S, Hagiwara H, Mizuno T, Saito Y, Hirose S, Yamamoto M, Nawata H: Cloning of sequence analysis of a cDNA encoding human non-selective type of endothelin receptor. Biochem Biophys Res Commun 1991, 177:34-39 [DOI] [PubMed] [Google Scholar]
- 24.Kertens HM, Poddighe PJ, Hanselaar AG: A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem 1995, 43:347-352 [DOI] [PubMed] [Google Scholar]
- 25.Yokomori H, Oda M, Kamegaya Y, Ogi M, Tsukada N, Ishii H: Enhanced expressions of endothelin receptor subtypes in cirrhotic liver. Liver 2001, 21:114-122 [DOI] [PubMed] [Google Scholar]
- 26.Kashima K, Doi Y, Kudo H, Kiyonaga H, Fujimoto S: Effects of endothelin-1 on vasoactivity and its synthesis, storage, and acting sites in the rat superior mesenteric vasculature: an ultrastructural and immunocytochemical study. Med Electron Microsc 1999, 32:36-42 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen PE, Egoholm M, Roif HB, Buchardt O: Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254:1497-1500 [DOI] [PubMed] [Google Scholar]
- 28.Egoholm M, Buchardt O, Christensen L, Behrens C, Freiser SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE: PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365:566-568 [DOI] [PubMed] [Google Scholar]
- 29.Tani Y: PCR in situ amplification and catalyzed signal amplification: approaches of higher sensitive, non-radioactive in situ hybridization. Acta Histochem Cytochem 1999, 32:261-270 [Google Scholar]
- 30.Ueno T, Sata M, Sakata R, Torimura T, Tanikawa K: Hepatic stellate cells and intralobular innervation in human liver cirrhosis. Hum Pathol 1997, 28:1414-1417 [DOI] [PubMed] [Google Scholar]
- 31.Leivas A, Jimenez W, Bruix J, Boix L, Arroyo V, Rivera F, Rodes J: Gene expression of endothelin-1 and ETA and ETB receptors in human cirrhosis: relationship with hepatic hemodynamics. J Vasc Res 1998, 35:186-193 [DOI] [PubMed] [Google Scholar]
- 32.Friedman SL: The cellular basis of hepatic fibrosis. N Engl J Med 1993, 328:1828-1835 [DOI] [PubMed] [Google Scholar]
- 33.Friedman SL: Molecular regulation of hepatic fibrosis; an integrated cellular response to tissue injury. J Biol Chem 2000, 275:2247-2250 [DOI] [PubMed] [Google Scholar]
- 34.Mallat A, Lotersztajn S: Multiple hepatic functions of endothelin-1: physiopathosocial relevance. J Hepatol 1996, 25:405-413 [DOI] [PubMed] [Google Scholar]
- 35.Battistini B, D’Orleans-Juste P, Sirois P: Biology of disease. Endothelin: circulating plasma levels and presence in other biologic fluids. Lab Invest 1993, 68:600-628 [PubMed] [Google Scholar]
- 36.Kaneda K, Ekataksin W, Sogaya M, Mastumura A, Cho A, Kawada N: Endothelin-1 induced vasoconstriction causes a significant increase in portal pressure of rat liver: localized constrictive effect on the distal segment of preterminal portal venules as revealed by light and electron microscopy and serial reconstriction. Hepatology 1998, 27:735-747 [DOI] [PubMed] [Google Scholar]
- 37.Nishida T, Huang TP, Seiyama A, Ueshima S, Hamada E, Kamiike W, Ueshima S, Kazuo H, Matsuda H: Endothelin A-receptor blockage worsens endotoxin-induced hepatic microcirculatory changes and necrosis. Gastroenterology 1998, 115:412-420 [DOI] [PubMed] [Google Scholar]
- 38.Poo JL, Jimenez W, Munoz RM, Bosch-Marce M, Bordas N, Morales-Ruiz M, Perez M, Deulofeu R, Sole M, Arroyo V, Rodes J: Chronic blockade of endothelin receptors in cirrhotic rats: hepatic and hemodynamic effects. Gastroenterology 1999, 116:161-167 [DOI] [PubMed] [Google Scholar]