Abstract
Activated macrophages (Mφ) isolated from inflamed glomeruli or generated by interferon-γ and lipopolysaccharide treatment in vitro induce glomerular mesangial cell apoptosis by hitherto incompletely understood mechanisms. In this report we demonstrate that nitric oxide-independent killing of co-cultured mesangial cells by interferon-γ/lipopolysaccharide-activated Mφ is suppressed by binding/ingestion of apoptotic cells and is mediated by tumor necrosis factor (TNF). Thus, soluble TNF receptor-1 significantly inhibited induction of mesangial cell apoptosis by 1) rodent Mφ in the presence of nitric oxide synthase inhibitors or 2) human Mφ, both situations in which nitric oxide release was minimal. Furthermore, murine TNF knockout Mφ were completely unable to induce mesangial cell apoptosis in the presence of nitric oxide synthase inhibitors. We conclude that TNF-restricted Mφ-directed apoptosis of glomerular mesangial cells can be down-regulated by Mφ binding/ingestion of apoptotic cells, suggesting a new mechanism for negative feedback regulation of Mφ controls on resident cell number at inflamed sites.
Proliferation of resident cells is a prominent feature of inflammatory responses. In glomerular inflammation there is typically an increase in number of resident glomerular mesangial cells that adopt a myofibroblast-like phenotype, lay down excess abnormal matrix, and thereby threaten progression to scarring. 1 However, in self-limited nephritis excess mesangial cells are deleted by apoptosis and the glomerular cell complement returns to normal. 2,3 Until recently the mechanisms mediating deletion of myofibroblast-like mesangial cells have been obscure. However, we showed that activated macrophages (Mφ) can direct apoptosis of such cells, 4 mesangial cell killing being mediated by nitric oxide (in a rodent cell system) and another unknown factor. Because inflammatory Mφ can delete neutrophils 5 and a range of tumor cells 6-8 by tumor necrosis factor (TNF)-mediated mechanisms, there was a strong possibility that activated Mφ might use TNF to induce mesangial cell apoptosis, particularly in human cell systems in which Mφ production of nitric oxide is notoriously difficult to detect. 9
Furthermore, the demonstration that activated/inflammatory Mφ can kill resident glomerular cells immediately begs the question as to how the killing capacity of Mφ might be regulated. Importantly, work from Reiter and colleagues 10 demonstrated that the capacity of rodent bone marrow-derived Mφ stimulated with interferon (IFN)-γ and lipopolysaccharide (LPS) to induce tumor cell apoptosis was diminished to ∼30% of control by ingestion of apoptotic cells. However, although this was associated with a modest reduction in nitric oxide production to ∼75% of control, the Mφ killing mechanism(s) suppressed by ingestion of apoptotic cells was/were not characterized further. 10
In this study we set out to determine whether activated Mφ induction of glomerular mesangial cell apoptosis was suppressed by Mφ ingestion of apoptotic cells and to determine which Mφ mechanisms for triggering apoptosis in neighboring mesangial cells were subject to such control.
Materials and Methods
Materials
Media and fetal calf serum (FCS) were purchased from Life Technologies (Paisley, UK). Tissue culture plastic was from Falcon (Becton Dickinson, Mountainview, CA) and Costar (Cambridge, MA) as stated in the text. Cytokines were purchased from R&D Systems (Minneapolis, MN) and all other reagents from Sigma (St. Louis, MO) unless otherwise stated.
Cell Isolation and Preparation
Human mesangial cells were obtained from the cortex of fresh nephrectomy specimens. Mesangial cells were purified from outgrowths of whole, purified glomeruli and passaged according to standard techniques in full Dulbecco’s modified Eagle’s medium (DMEM)/F12 (with 10% FCS, and supplemented with penicillin and streptomycin (Life Technologies). 2 Rat mesangial cells were derived from outgrowths of whole glomeruli as described. 4 They were passaged according to standard techniques in full DMEM/F12, and were used between passage 6 and 14. Rodent macrophages were derived from bone marrow taken from the femur of Wistar rats or from murine strains (TNFα/β−/−) and wild-type littermate controls (C57BL6 × 129sv). In the TNFR1-Fc blockade studies, they were all C57BL6). Marrow was prepared and cultured in Teflon bags with murine M-CSF as previously described. 4 Cells were used after 7 to 10 days, then were plated into wells 16 hours before experimentation to ensure adequate adhesion and correct cell number. Human macrophages were derived from peripheral blood monocytes. Briefly, peripheral blood mononuclear cells were obtained from the buffy coat of fresh peripheral blood from healthy donors. Red cells were removed by dextran sedimentation and granulocytes were separated by centrifugation through a discontinuous Percoll gradient. 11 The monocytes were then purified using the MACS monocyte cell isolation system (Miltenyi Biotech, Cologne, Germany). Purity of monocytes (>95%) was established by flow cytometric characteristics. Monocytes were then cultured in Teflon wells (2 × 106/ml) in Iscove’s-modified DMEM with 10% autologous serum. Medium was changed on day 2 and day 4. Differentiation was confirmed by cytology on cytospins and CD14 immunofluorescence (not shown).
Generation of Apoptotic Cells
Rat mesangial cells were induced into apoptosis by ultraviolet irradiation. Subconfluent monolayers in T75 flasks (Costar) were exposed to UV irradiation (312 nm, 8 W, 3 minutes) followed by incubation for 16 hours. Nonadherent (apoptotic) cells were removed by agitation and collected in the supernatant. After centrifugation, apoptotic cells were further purified by washing with phosphate-buffered saline (PBS) 1× and centrifugation (190 × g, 5 minutes). Apoptosis was confirmed by histology, selective uptake of Hoechst 33342 (1 μg/ml), but exclusion of propidium iodide (PI) (1 μg/ml). Typically, <10% of cells were PI-positive and <5% did not exclude trypan blue (0.2%).
Mouse thymocytes were prepared from 20 g C57/BL6 mice by pressing the thymus through a 50-μm sieve. The single cell supernatant was resuspended (2 × 10 6 cells/ml) in RPMI supplemented with glutamine and 2-mercaptoethanol in addition to 10% FCS and antibiotics. Cells were either exposed to a 5-minute burst of UV irradiation (312 nm, 8 W) followed by 2.5 hours of culture, or dexamethasone (1 μmol/L) followed by culture for 6 hours. Typically >50% of induced cells were apoptotic (Annexin V binding; Boehringer Mannheim. Mannheim, Germany) and permeable to Hoescht 33342 (1 μg/ml)) whereas <5% of those were positive for the uptake of PI (flow cytometric analysis, data not shown).
Co-Culture of Mφ and Mesangial Cells
For a detailed description, see our earlier work. 4 Matured rodent bone marrow-derived macrophages were plated in 96-well plates initially at a density to cover 60 to 70% of the well surface; typically this required 2 × 104cells per well. Rat mesangial cells were prelabeled with CellTracker Green 5-chloromethylfluorescein diacetate CMFDA (Molecular Probes, Eugene, OR): cell cultures, 70 to 80% confluent, were washed with medium lacking serum and then incubated for 1 hour in serum-free medium containing CMFDA at 5 ng/ml. Cells were washed in medium containing 10% FCS to remove any unbound CMFDA, then trypsinized and added to cultured rodent Mφ in a 1.5 Mφ:1.0 mesangial cell ratio, previously shown to be optimal for demonstration of macrophage-directed mesangial cell apoptosis. 4 Experiments were performed in DMEM/F12 medium containing 10% FCS. Once cells had become adherent, typically 2 to 4 hours, wells were washed to remove nonadherent cells. In our earlier work 4 mixing unlabeled and labeled cells showed no evidence of transfer of CMFDA from labeled to unlabeled cells. Rodent co-cultures were activated with IFN-γ (100 U/ml) plus LPS (1 μg/ml).
In some experiments, unlabeled apoptotic cells (1 × 10 5 per well of a 96-well plate) were added to the established co-culture and to the control wells of mesangial cells alone, at the same time as activating cytokines. As a control for apoptotic cells, aliquots of 10-μm diameter sterile latex beads (Polysciences, UK) were added to adjacent co-culture. When thymocytes were used as apoptotic cells, aliquots were added to co-culture for 6 hours, then noningested cells gently washed away, before activation. In separate wells containing Mφ alone, apoptotic cells or latex beads were also incubated for 6 hours, then washed off, and wells were fixed for quantification of percentage Mφ that had phagocytosed apoptotic cells. This was assessed by phase contrast microscopy. Mφ (34 ± 5.6%) phagocytosed at least one apoptotic thymocyte and 30 ± 6.7% phagocytosed apoptotic mesangial cells. Mφ (67 ± 8.4%) ingested latex beads. By contrast only 1 ± 1% of Mφ phagocytosed the population of live thymocytes in this assay.
For human co-culture, human monocyte-derived Mφ were plated at 2 × 10 4 per well as above. Cycling human mesangial cells were primed with IFN-γ (500 U/ml) for 24 hours, then prelabeled with CMFDA as described for rat mesangial cells. Once washed and trypsinized, they were added to wells in a 2:1 ratio (because of the larger size of human mesangial cells). After 4 hours, wells were washed and replaced with full DMEM/F12. Human co-cultures were activated with human IFN-γ (Peprotech) (500 U/ml) and LPS (1 μg/ml).
Assessment of Mesangial Cell Apoptosis in Co-Culture
For a detailed description, see our earlier work. 4 At the end of co-culture experiments, wells were either fixed with formaldehyde (4% final concentration) and stored for 48 hours at 4°C to allow firm adhesion of apoptotic cells to the plate, or they were assessed live by fluorescence microscopy after the addition of Hoechst 33342 (1 μg/ml) and PI (1 μg/ml). For assessment of live cells, green rounded-up cells were scored as apoptotic if they also were positive for Hoechst uptake, but excluded PI. For assessment of fixed (and permeabilized) cells by morphology, plates were first counterstained with PI (1 μg/ml) and Hoechst (1 μg/ml) in PBS for 5 minutes (which stains both Mφ and mesangial cells). After discarding the stain, wells were covered with a fluorescent mountant. Apoptotic mesangial cells were easily discernible by green fluorescence and characteristic morphology. For both live and fixed wells, apoptosis of mesangial cells was assessed blindly and five fields per well were randomly chosen without observer bias. Each experiment was performed in triplicate. Previous studies have shown this method to give similar results to flow cytometric assays. 4 However, the microscopical assay is reproducibly more sensitive because apoptotic mesangial cells tend to disintegrate during centrifugation once they have been fixed. Note that when exogenous apoptotic cells were added these were not labeled with fluorescent dyes, enabling confident identification of apoptosis in the previously healthy target mesangial cells.
Assays of TNF-α and Nitric Oxide
Culture supernatants (free from phenol red) were harvested, clarified by centrifugation (4000 × g, 5 minutes) then stored at −20°C. After complete thawing, 50 μl of each sample was assayed by Quantikine mouse TNF-α enzyme-linked immunosorbent assay (R&D Systems) according to the manufacturer’s instructions. A standard curve with absorbencies from 0.1 to 1.2 was achieved on each occasion. Samples (50 μl) were assayed for nitrite by mixing with an equal volume of the Griess reagent as previously described 6 and measuring absorbency at 540 nm and comparing with a sodium nitrite standard curve.
Chimeric Soluble Death Receptors
Fusion proteins were constructed using cDNAs for the extracellular domains of human receptors fused with the Fc portion of human IgG1. Proteins were expressed in insect cells infected with recombinant baculoviruses. Protein secreted into the culture supernatant was then purified by protein A-Sepharose column affinity. The protein was stored at −20°C in Hanks’ salt solution. 12
Knockout Mice
TNF-α/TNF-β double-knockout mice were generated by inserting a targeting vector between exon 1 and 2 of the murine TNF-β gene and the middle of exon 4 of the adjacent murine TNF-α gene of chromosome 17 of GS1 mouse embryonic stem cells. Mutant embryonic stem cells were selected and injected into C57/BL6 blastocysts. 13
Statistics
All experiments were performed on at least four separate occasions using at least four animals. The data were expressed as mean values with the SE of mean. Paired data were compared using the t-test and multiple comparisons using analysis of variance.
Results
Ingestion of Apoptotic Cells by Activated Rodent Mφ Selectively Suppresses Nitric Oxide-Independent Induction of Mesangial Cell Apoptosis
In our previous studies of IFN-γ/LPS-activated rodent bone marrow-derived Mφ killing of rodent mesangial cells primed with IFN-γ (Figure 1) ▶ we demonstrated that at least half of the mesangial cell killing was independent of Mφ-derived nitric oxide, being unaffected by inhibitors of inducible nitric oxide synthase (iNOS) such as L-NMMA at 500 μmol/L. 4 We also observed that the vast majority of mesangial cells induced into apoptosis by activated Mφ were not ingested, 4 presumably because of kinetic and spatial restraints in a two-dimensional culture system in which IFN-γ-primed mesangial cells were added above a sparse monolayer of Mφ before the co-culture was activated by addition of IFN-γ and LPS.
Figure 1.
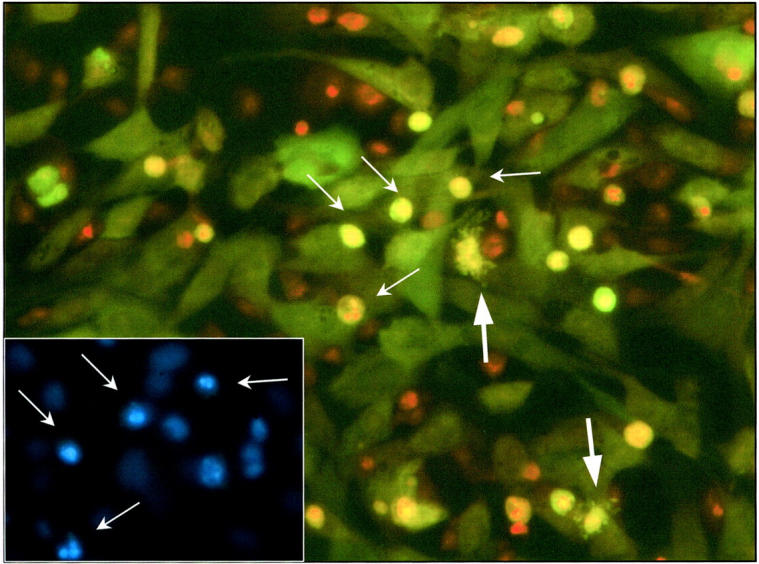
Fluorescent micrograph (original magnification, ×320) showing activated co-culture of CMFDA green-labeled mesangial cells with Mφ. The co-culture was established according to Materials and Methods and activated with IFN-γ (100 U/ml) plus LPS (1 μg/ml) after 4 hours of exposure to preformed unlabeled apoptotic mesangial cells. At 24 hours, the co-culture was fixed and later counterstained with PI (1 μg/ml). Apoptotic green mesangial cells are clearly seen with red/orange pyknotic nuclear material and are readily distinguished from Mφ (arrows). Before fixation, co-culture was exposed to Hoechst 33342 (1 μg/ml) for confirmation of apoptosis of the green mesangial cells (see inset). Note inset field and arrow-marked apoptotic cells correspond to central field of main figure. Note also two apoptotic mesangial cells showing cytoplasmic blebbing (large arrows).
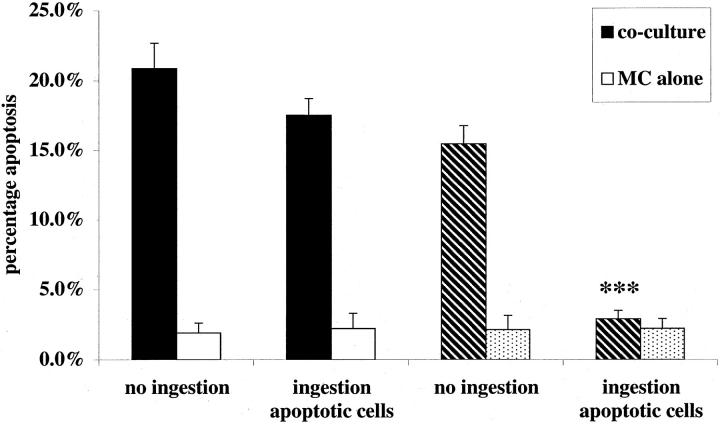
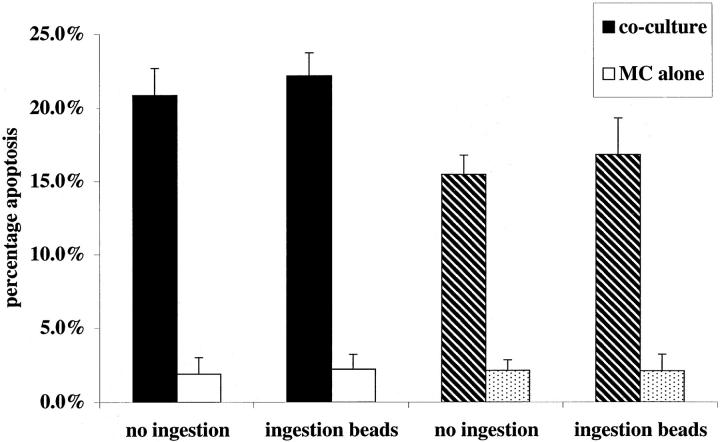
Therefore, to test whether ingestion of apoptotic cells diminished Mφ capacity to trigger apoptosis in mesangial cells, an established co-culture of IFN-γ-primed rat mesangial cells with rat Mφ was exposed to a fivefold excess of mesangial cells induced into apoptosis by UV irradiation; the apoptotic cells were administered at the same time as activation of the co-culture with IFN-γ and LPS. Under standard conditions, there was no significant effect of apoptotic cells on Mφ induction of mesangial cell apoptosis. However, in the presence of 200 μmol/L L-NMMA, apoptotic mesangial cells exerted a dramatic inhibitory effect on Mφ induction of mesangial cell apoptosis (Figure 2) ▶ . Addition of the same number (1 × 10 5 per well) of 10-μm latex beads, as a control phagocytic particle, had no inhibitory effect whether L-NMMA was present or not (Figure 3) ▶ . Furthermore, suppression of nitric oxide-independent triggering of apoptosis was observed with rodent thymocytes induced into apoptosis by UV or dexamethasone, but not when freshly isolated nonapoptotic thymocytes were used as controls (Table 1) ▶ . Finally, similar results were observed whether apoptotic cells were added simultaneously with activation of co-culture, as above, or if apoptotic cells were administered to co-culture before activation (Table 1) ▶ . This indicates that the suppressive effect was mediated by effects of apoptotic cells on the Mφ most likely consequent on ingestion of apoptotic cells, but not excluding an effect of binding alone.
Figure 2.
Induction of rodent mesangial cell apoptosis by activated Mφ is blocked by simultaneous phagocytosis of apoptotic mesangial cells when iNOS inhibitors are present. Established co-cultures of rodent Mφ and IFN-γ primed, CMFDA-labeled, mesangial cells, or mesangial cells growing alone (as control), were activated with IFN-γ (100 U/ml) and LPS (1 μg/ml). Simultaneously to activation, a fivefold excess of unlabeled apoptotic rodent mesangial cells was added (1 × 10 5 cells per well). In addition, to some wells (hatched bars), L-NMMA (200 μmol/L) was added at the same time as activation. After 24 hours of incubation, induction of CMFDA-labeled mesangial cells was scored in all wells. ***, P < 0.001 versus no ingestion (n = 5).
Figure 3.
Induction of rodent mesangial cell apoptosis by activated Mφ is not blocked by simultaneous phagocytosis of latex beads when iNOS inhibitors are present. Both established co-cultures of rodent Mφ- and IFN-γ-primed, CMFDA-labeled, mesangial cells, and wells of mesangial cells alone were activated with IFN-γ (100 U/ml) and LPS (1 μg/ml). Simultaneously a fivefold excess of unlabeled sterile 10-μm latex beads was added (1 × 10 5 cells per well). In addition, to some wells (hatched bars), L-NMMA (200 μmol/L) was added. After 24 hours of incubation, induction of CMFDA-labeled mesangial cells was scored (n = 5).
Table 1.
The Effect of Co-Culture with Apoptotic Cells upon Murine Mφ Induction of Apoptosis in IFN-γ Primed Mesangial Cells at 24 Hours in the Presence of Nitric Oxide Synthesis Inhibitor
| Cell type | % Mesangial cell apoptosis | |
|---|---|---|
| No added cells | Cells added | |
| Apoptotic mesangial cells (UV) | 10.7 ± 2.1 | 3.3 ± 1.0* |
| Apoptotic thymocytes (UV) | 16.1 ± 1.4 | 8.2 ± 0.1† |
| Apoptotic thymocytes (Dex) | 16.1 ± 1.4 | 8.3 ± 0.5† |
| Nonapoptotic thymocytes | 16.1 ± 1.8 | 14.2 ± 1.3 |
UV, Ultraviolet; Dex, dexamethasone.
Rodent co-culture was established as described in Materials and Methods. Apoptotic cells were incubated with the co-culture for 6 hours. Mφ were activated with IFN-γ plus LPS in the presence of L-NMMA 200 μmol/L after washing away noningested cells. Note that control cultures of mesangial cells alone exposed to apoptotic cells followed by activating cytokines underwent no greater than 3.42 ± 0.2% apoptosis.
*P < 0.01 compared with no added cells.
†P < 0.001 compared with no added cells.
Blockade of TNFR1 Also Inhibits Nitric Oxide-Independent Macrophage Induction of Mesangial Cell Apoptosis
Exposure of activated monocytes/macrophages to apoptotic cells exerts a well-established inhibitory effect on secretion of TNF. 14,15 It therefore seemed especially likely that nitric oxide-independent induction of IFN-γ-primed mesangial cell apoptosis by activated Mφ, which we had found to be inhibited by apoptotic cells (Figure 2) ▶ , was also mediated by Mφ-derived TNF.
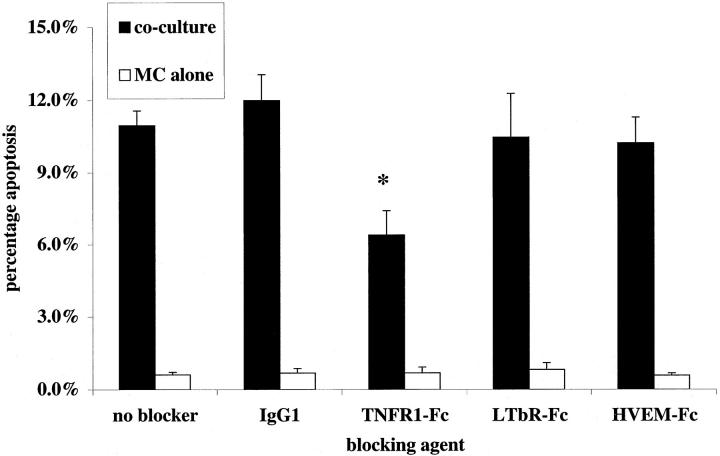
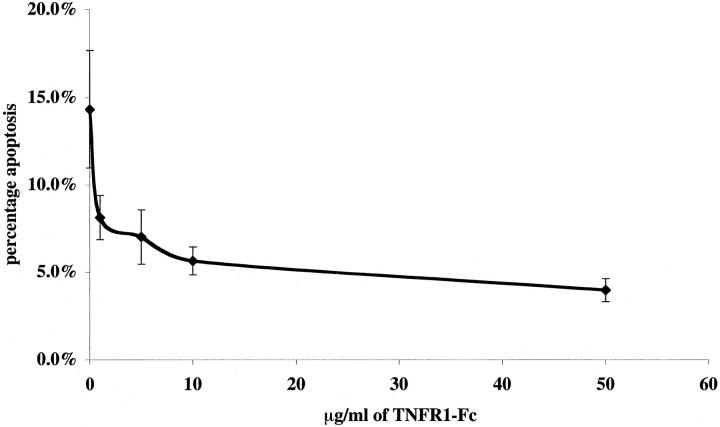
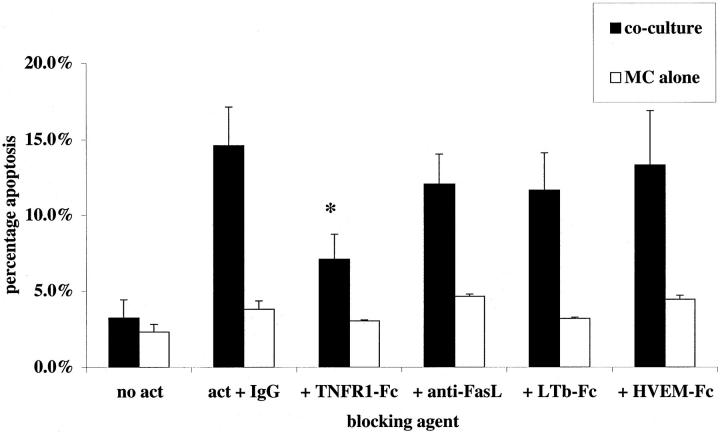
To seek a role for TNF in Mφ directed killing of mesangial cells, we examined the effect of soluble chimeric death receptors (fused with the Fc portion of human IgG1) on activated rat macrophage killing of primed rat mesangial cells in the presence of inhibitors of nitric oxide synthesis (in these experiments the selective iNOS inhibitor L-NIL at 30 μmol/L, as validated in our earlier work 4 ). A role for Mφ-derived FasL had already been ruled out in rodent co-culture 4 by using bone marrow Mφ from gld/gld mice (that lack active FasL) and therefore soluble inhibitors of Fas-FasL interaction were not used. Induction of rat mesangial cell apoptosis in activated co-culture was assessed at 24 hours. TNFR1-Fc (10 μg/ml) was able to reduce significantly the killing capacity of Mφ in co-culture (Figure 4) ▶ whereas lymphotoxin β-receptor fusion protein (LTβR-Fc 16 ) and herpesvirus entry mediator fusion protein (HVEM-Fc 17,18 ) had no effect. At higher concentrations, TNFR1-Fc (50 μg/ml) was able to increase further the reduction in killing capacity (14.3 ± 3.3% rat mesangial cell apoptosis in activated co-culture with IgG1 versus 3.9 ± 0.7% mesangial cell apoptosis with 50 μg/ml TNFR1-Fc), but the reduction was not complete. Control rat mesangial cells growing alone with 50 μg/ml TNFR1-Fc showed 1.3 ± 0.7% apoptosis at 24 hours. The IC50 for this fusion protein was 5.0 ± 1.2 μg/ml (Figure 5) ▶ .
Figure 4.
Soluble TNFR1 inhibits nitric oxide-independent rodent Mφ killing of primed rodent mesangial cells. Murine bone marrow-derived Mφ were co-cultured with mesangial cells as described in Materials and Methods. Experiments were activated with IFN-γ and LPS as described, with L-NIL (30 μmol/L) to block NOS 2-mediated killing, and soluble Fc-fusion proteins TNFR1-Fc, LTβR-Fc, HVEM-Fc, or isotype control IgG1 were added in culture medium at 10 μg/ml. Control wells containing mesangial cells but no Mφ received all reagents. Mesangial cell apoptosis was assessed at 24 hours. Note a reduction in the capacity of Mφ to induce apoptosis in the presence of TNFR1-Fc. *, P < 0.05.
Figure 5.
Concentration-dependent inhibition by sTNFR1 of nitric oxide independent rodent Mφ induction of rodent mesangial cell apoptosis. Using the same co-culture assay of mesangial cell apoptosis, a range of concentrations of TNFR1-Fc was added to wells and apoptosis quantified, compared with 50 μg/ml of IgG1 control shown as 0 μg/ml of TNFR1-Fc on the curve.
We were also interested in determining whether TNFR1 blockade might similarly inhibit activated human Mφ induction of mesangial cell apoptosis, because human Mφ production of nitric oxide is notoriously difficult to elicit. 9 Indeed, we confirmed that the IFN-γ/LPS activation regimen had no significant stimulatory effect on human monocyte-derived Mφ production of nitric oxide (data not shown) and that L-NMMA had no effect on activated human Mφ killing of IFN-γ-primed human mesangial cells (14.7 ± 2.1% apoptosis at 24 hours with 200 μmol/L L-NMMA versus 15.7 ± 1.9% under control conditions). In this physiologically nitric oxide-independent human cell system, soluble TNFR1 again demonstrated selective inhibition of activated Mφ direction of mesangial cell apoptosis (Figure 6) ▶ .
Figure 6.
Induction of apoptosis of human mesangial cells by human monocyte-derived Mφ is mediated by TNFR1 ligation. Co-culture was established using primed human mesangial cells and Mφ. On activation with IFN-γ plus LPS, soluble Fc-fusion proteins TNFR1-Fc, LTβR-Fc, HVEM-Fc, or isotype control IgG1 (10 μg/ml) were added in culture medium at 10 μg/ml. Also, the blocking anti-Fas ligand antibody (1 μg/ml) was applied to adjacent wells. Control wells containing human mesangial cells but no Mφ received all reagents. Note a reduction in the capacity of Mφ to induce apoptosis in the presence of TNFR1-Fc, but not in the presence of any of the other reagents. *, P < 0.05.
Nitric Oxide-Independent Induction of Mesangial Cell Apoptosis Is Restricted by Macrophage TNF
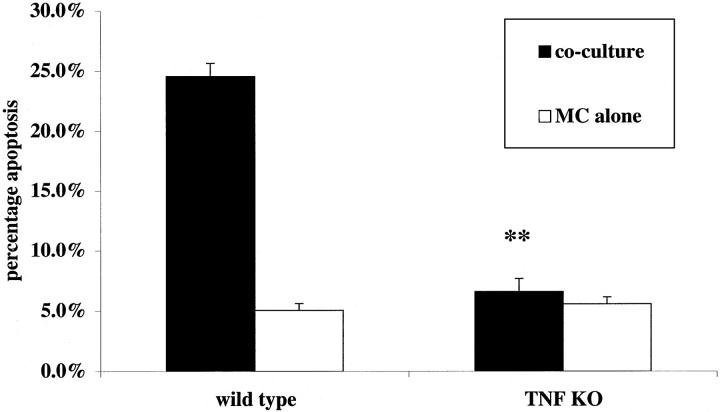
Although the foregoing studies provided strong evidence that ligation of TNFR1 played a major role in nitric oxide-independent Mφ killing of mesangial cells, the data did not provide direct evidence that Mφ-derived ligands for TNFR1 (TNF-α and TNF-β) were involved. Furthermore, the failure of sTNFR1 to exert complete inhibition of killing might have reflected its physical exclusion from regions of close contact between macrophages and mesangial cells. To examine this question definitively, we prepared bone marrow-derived (BMD) Mφ from double-knockout Tnfα−/−, Tnfβ−/− mice, and wild-type littermate controls; all animals used were C57/BL6 × 129sv to ensure that strain differences did not complicate interpretation. 13 Preliminary studies showed both knockout and wild-type Mφ produced similar amounts of nitric oxide in response to IFN-γ with LPS (50 ± 4.6 [wild type] versus 47.0 ± 3.3 [Tnfα/β−/−] nmol nitrite per 10 6 cells per 24 hours) and that this was almost completely inhibited by the iNOS inhibitor L-NIL at 30 μmol/L (to 3.7 ± 2.1 and 2.9 ± 1.9 nmol nitrite per 10 6 cells per 24 hours, respectively). Supernatants from Mφ activated with IFN-γ and LPS confirmed that the Tnfα/β−/− Mφ were completely unable to produce TNF-α, as assessed by enzyme-linked immunosorbent assay of culture supernatants at 24 hours (wild-type Mφ 3982 pg/ml Tnfα/β−/− Mφ< 0.00 pg/ml). Rat mesangial cells were primed for 24 hours with IFN-γ then co-cultured with either wild-type Mφ or Tnfα/β−/− Mφ. The co-cultures were activated with IFN-γ (100 U/ml) and LPS (1 μg/ml) in the presence of L-NIL (30 μmol/L). In these experiments knockout Mφ were completely unable to induce mesangial cell apoptosis compared with controls of mesangial cells growing alone in the presence of cytokines (Figure 7) ▶ . Wild-type Mφ induced mesangial cell apoptosis as expected. These data demonstrate that when nitric oxide synthesis was inhibited, and therefore used as a model of human Mφ-directed apoptosis, Mφ production of TNF-α/β accounted for all of the killing effect of rodent Mφ on mesangial cells.
Figure 7.
The comparative effect of activated wild-type and Tnfα/Tnfβ−/− knockout murine Mφ on apoptosis of mesangial cells primed with IFN-γ. Mesangial cells were primed as described for 24 hours in the presence of IFN-γ (300 U/ml). Co-culture was established using bone marrow-derived Mφ from either Tnfα/Tnfβ−/− knockout mice or wild-type littermate controls and activated with IFN-γ (100 U/ml) plus LPS (1 μg/ml) in the presence of L-NIL (30 μmol/L) to block NOS 2-mediated killing. Experiments were assayed after 24 hours. Although the wild-type Mφ were able to induce mesangial cell apoptosis, the knockout animals incapable of inducing apoptosis greater than that seen in control wells. **, P < 0.01 compared with wild-type co-culture (n = 5).
Discussion
Although it has been well documented that Mφ can kill tumor cells, 6-8 it has only very recently become apparent that macrophages can also direct tissue remodeling by inducing physiological cell death, in nontransformed resident cells. 4,19,20 Thus we have shown previously that activated Mφ, whether isolated directly from experimentally inflamed glomeruli or generated by IFN-γ/LPS activation in vitro can induce apoptosis in cultured primary mesangial cells. 4 In this report, we demonstrate for the first time that activated Mφ induction of apoptosis in nontransformed cells can be profoundly inhibited by interaction of Mφ with apoptotic cells. However, in a rodent system this effect could only be demonstrated when nitric oxide production was inhibited. Nevertheless, such nitric oxide-independent direction of mesangial cell apoptosis was inhibitable by soluble TNF receptor in both a rodent cell culture system and a physiologically nitric oxide-independent human cell culture system. Indeed, a major role for TNF in the rodent system was confirmed by the failure of knockout Mφ to induce nitric oxide-independent mesangial cell apoptosis. Therefore, our key conclusion is that macrophage-derived TNF may play a major role in directing apoptosis of primary, nontransformed glomerular mesangial cells.
We also regard it as interesting and important that TNF-restricted Mφ direction of mesangial cell apoptosis was selectively suppressed by interaction of Mφ with apoptotic cells but not when healthy cells or latex beads were used as control particles. These data are in keeping with the marked suppressive effects of apoptotic cells on activated rodent bone marrow-derived Mφ killing of tumor cells, in which the reported data favor a major role for a nitric oxide-independent cell killing mechanism because nitric oxide release was only modestly suppressed. 10 Both the latter study and the current work may have methodological differences from experiments in which nitric oxide-directed parasite killing was suppressed by Mφ binding of apoptotic cells. 21 Nevertheless, a second key conclusion of our work is that interaction with apoptotic cells can suppress TNF-restricted Mφ direction of mesangial cell apoptosis.
We propose that further work should examine the likelihood that activated Mφ deletion of resident cells at inflamed sites may be subject to negative feedback control, in which ingestion of apoptotic cells down-regulates Mφ capacity to induce apoptosis in resident cells. This new concept suggests additional deleterious consequences should Mφ clearance of apoptotic cells be defective. Previously it has been proposed 22,23 that reduced Mφ capacity for clearance of leukocytes and other cells undergoing apoptosis, as now demonstrated in C1q−/− knockout mice, 24 threatens tissue injury because of the likelihood that cellular contents escaping from noningested apoptotic cells undergoing secondary necrosis will injure tissues directly and indirectly 23 by stimulating Mφ release of injurious mediators. Our new data indicate that failure to ingest apoptotic cells might deprive activated Mφ of a crucial off-signal resulting in undesirably prolonged capacity to direct death of neighboring cells by TNF-restricted mechanisms. Nevertheless, it is likely to be some time before this hypothesis can be tested in vivo, because a growing body of evidence argues that redundancy in clearance mechanisms may require that a number are disabled before sustained defects in clearance of apoptotic cells can be demonstrated. 25,26
Furthermore, future work will also need to address whether Mφ ingestion of apoptotic cells down-regulates Mφ membrane TNF-α cell-surface expression, or whether integrin co-factors, necessary for successful transduction of the apoptotic signal, are down-regulated. 5 Preliminary data suggest that membrane-bound rather than soluble TNF-α is responsible for Mφ-directed apoptosis of mesangial cells (J. Duffield and J. Savill, unpublished data), as reported for Mφ-directed leukocyte apoptosis. 5
To conclude, when macrophage release of nitric oxide is minimal, as may be expected in human inflammation, these data demonstrate a major role for TNF in activated Mφ killing of cytokine-primed mesangial cells by apoptosis. Furthermore, inhibition of the capacity to direct apoptosis consequent on Mφ binding/ingestion of apoptotic cells reveals a new and potentially important negative feedback control mechanism in remodeling of the inflamed site.
Acknowledgments
We thank Mrs. Carolyn Gilchrist for expert secretarial assistance, Ms. Shonna MacCall for technical assistance, and Drs. Adam Lacy-Hulbert and Ian Dransfield for providing invaluable advice.
Footnotes
Address reprint requests to Dr. J. S. Duffield, M. R. C. Center for Inflammation Research, Medical School, University of Edinburgh, Teviot Place, Edinburgh, EH8 9AG, UK. E-mail: j.duffield@ed.ac.uk.
Supported by a Medical Research Council Clinical Training Fellowship (no. G84/4757, to J. S. D.) and a Wellcome Trust Program Grant (no. 047273, to J. S.).
References
- 1.Schocklmann HO, Lang S, Sterzel RB: Regulation of mesangial cell proliferation. Kidney Int 1999, 56:1199-1207 [DOI] [PubMed] [Google Scholar]
- 2.Baker AJ, Mooney A, Hughes J, Lombardi D, Johnson RJ, Savill J: Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest 1994, 94:2105-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N: Apoptosis in progressive crescentic glomerulonephritis. Lab Invest 1996, 74:941-951 [PubMed] [Google Scholar]
- 4.Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS: Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol 2000, 164:2110-2119 [DOI] [PubMed] [Google Scholar]
- 5.Meszaros AJ, Reichner JS, Albina JE: Macrophage-induced neutrophil apoptosis. J Immunol 2000, 165:435-441 [DOI] [PubMed] [Google Scholar]
- 6.Decker T, Lohmann-Matthes M-L, Gifford GE: Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol 1987, 138:957-962 [PubMed] [Google Scholar]
- 7.Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M: A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 1990, 63:251-258 [DOI] [PubMed] [Google Scholar]
- 8.Duerksen-Hughes PJ, Day DB, Laster SM, Zachariades NA, Aquino L, Gooding LR: Both tumor necrosis factor and nitric oxide participate in lysis of Simian Virus 40-transformed cells by activated macrophages. J Immunol 1992, 149:2114-2122 [PubMed] [Google Scholar]
- 9.Albina JE: On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukoc Biol 1995, 58:643-649 [DOI] [PubMed] [Google Scholar]
- 10.Reiter I, Krammer B, Schwamberger G: Cutting edge: differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol 1999, 163:1730-1732 [PubMed] [Google Scholar]
- 11.Savill JS, Henson PM, Haslett C: Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge sensitive” recognition mechanism. J Clin Invest 1989, 84:1518-1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe PD, VanArsdale TL, Walter BN, Dahms KM, Ware CF: Production of lymphotoxin (LT alpha) and a soluble dimeric form of its receptor using the baculovirus expression system. J Immunol Methods 1994, 168:79-89 [DOI] [PubMed] [Google Scholar]
- 13.Eugster HP, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, Zinkernagel R, Bluethmann H, Ryffel B: Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-alpha double-deficient mice. Int Immunol 1996, 8:23-36 [DOI] [PubMed] [Google Scholar]
- 14.Voll RE, Herrmann M, Roth EA, Strach C, Kalden JR: Immunosuppressive effects of apoptotic cells. Nature 1997, 390:350-351 [DOI] [PubMed] [Google Scholar]
- 15.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM: Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998, 101:890-898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC, Cohen GH, Eisenberg RJ, Ware CF: The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem 2000, 275:14307-14315 [DOI] [PubMed] [Google Scholar]
- 17.Harrop JA, Donnell PC, Brigham-Burke M, Lyn SD, Minton J, Tan KB, Dede K, Spampanato J, Silverman C, Hensley P, DiPrinzio R, Emery JG, Deen K, Eichman C, Chabot-Fletcher M, Truneh A, Young PR: Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem 1998, 273:27548-27556 [DOI] [PubMed] [Google Scholar]
- 18.Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon BS, Jiang GW, Lu J, Tan J, Ugustus M, Carter K, Rojas L, Zhu F, Lincoln C, Endress G, Xing L, Wang S, Oh KO, Gentz R, Ruben S, Lippman ME, Hsieh SL, Yang D: LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 1998, 102:1142-1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RA, Bishop JM: Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 1993, 74:453-462 [DOI] [PubMed] [Google Scholar]
- 20.Lang RA: Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ 1997, 4:12-20 [DOI] [PubMed] [Google Scholar]
- 21.Friere-de-Lima CG, Nasciemento DO, Soares MBP, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopez MF: Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 2000, 403:199-203 [DOI] [PubMed] [Google Scholar]
- 22.Savill J: Apoptosis in resolution of inflammation. J Leukoc Biol 1997, 61:375-380 [DOI] [PubMed] [Google Scholar]
- 23.Stern M, Savill J, Haslett C: Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol 1996, 149:911-921 [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ: A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 2000, 192:359-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savill J: Apoptosis: phagocytic docking without shocking. Nature 1998, 392:442-443 [DOI] [PubMed] [Google Scholar]
- 26.Savill J, Fadok V: Corpse clearance defines the meaning of cell death. Nature 2000, 407:784-788 [DOI] [PubMed] [Google Scholar]