Abstract
Type IV collagen is a major component of basement membranes and it provides structural and functional support to various cell types. Type IV collagen exists in a highly complex suprastructure form and recent studies implicate that protomer (the trimeric building unit of type IV collagen) assembly is mediated by the NC1 domain present in the C-terminus of each collagen α-chain polypeptide. Here we show that type IV collagen contributes to the maintenance of the epithelial phenotype of proximal tubular epithelial cells, whereas type I collagen promotes epithelial-to-mesenchymal transdifferentiation (EMT). In addition, the recombinant human α1NC1 domain inhibits assembly of type IV collagen NC1 hexamers and potentially disrupts the deposition of type IV collagen, facilitating EMT in vitro. Inhibition of type IV collagen assembly by the α1NC1 domain up-regulates the production of transforming growth factor-β1 in proximal tubular epithelial cells, an inducer of EMT. These results strongly suggest that basement membrane architecture is pivotal for the maintenance of epithelial phenotype and that changes in basement membrane architecture potentially lead to up-regulation of transforming growth factor-β1, which contributes to EMT during renal fibrosis.
Basement membranes are present throughout the human body. In contrast to interstitial extracellular matrix, it is a highly organized structure that consists primarily of laminin, nidogen, and collagen type IV, which as the most abundant matrix molecule serves as a scaffold for the basement membrane proteins. 1 Type IV collagen includes six genetically distinct isoforms named α1(IV) through α6(IV). 2 These isoforms organize themselves into a unique network that provides basement membrane specificity and inequality. 2 Assembly of type IV collagen is initiated by the formation of protomers (trimers). 1,3 Three α chains come together through associations among their noncollagenous (NC) domains followed by folding of the collagenous domains into triple helices. 4 Each protomer is associated with another protomer by its NC1 domain to form interlocking hexamers. 5 Along with lateral association of the collagenous triple helices and covalent binding of 7S domains, association of the α-chain of NC1 domain is essential to allow the formation of the protomeric network that serves as a network scaffold for other basement membrane proteins. 6 With six different α-chains known at present, 56 different combinations of triple-helical protomers are possible. In Engelberth Holm Swarm-sarcoma-derived type IV collagen, the most abundant protomers are preferentially those that contain only α1 and α2 chains in a 2:1 ratio, stressing that the NC1 domain of the α1 chain (α1NC1) has a central role in assembly of type IV collagen. 7 In human kidney, as in mouse, differences in type IV collagen composition may hint to specific roles of specialized basement membranes. 7 In adults, human renal tubular basement membrane (TBM) that surrounds proximal tubules, consists exclusively of α1/α2 protomers, whereas distal tubules also contain some α3 chain. 8,9 In the glomerular basement membrane the type IV collagen network involves the α3, α4, and α5 chains. 8 Type IV collagen binds various cells via surface receptors such as integrins, which is suggestive of its capacity to modulate specific cell behavior. 10
Renal interstitial fibrogenesis, as the common pathway in progressive chronic renal disease, is traditionally characterized by an increasing number of interstitial fibroblasts that mediate excessive deposition of interstitial matrix components leading to tubular atrophy. 11-16 Recent observations stress a pivotal role of tubular epithelial cells as mediators of renal scarring. 17,18 Tubular epithelial cells function as a source of fibrogenic growth factors and chemokines in the initiation of fibrogenesis, contribute to tubular atrophy by undergoing apoptosis, and potentially contribute to increased numbers of interstitial fibroblasts by epithelial-mesenchymal transdifferentiation (EMT). 19 EMT is defined as the acquisition of phenotypic as well as functional properties of mesenchymal fibroblasts by epithelial cells. 20 It occurs in development, carcinogenesis, and chronic diseases in different organs. 21-23 EMT is increasingly being considered as a possible mechanism leading to renal fibrogenesis. 24-26 The current concept of EMT postulates a mechanism in which tubular epithelial cells become activated by exogenous stimuli, followed by a loss of contact with neighboring cells and basement membrane. 20,27 After initiation of EMT, cells move through their basement membrane into the interstitial matrix where they become detectable as fibroblasts/myofibroblasts. 20 Thus, in this hypothetical model of EMT, the epithelial phenotype is clearly associated with TBM microenvironment whereas mesenchymal phenotype is associated with interstitial microenvironment.
Therefore, in the present study, we investigated the role of type IV collagen composition, assembly, and integrity on the phenotype of proximal tubular epithelial cells in vitro. Our studies suggest that disruption of TBM leads to increased expression of transforming growth factor (TGF)-β1 by mouse proximal tubular epithelial cells (MCT). Alterations of cell-matrix interactions potentially facilitate EMT and contribute to fibroblast population in the renal interstitium.
Materials and Methods
Materials
Recombinant human TGF-β1, human epithelial growth factor (EGF), and the neutralizing polyclonal goat antibodies to TGF-β and EGF were purchased from R&D Systems (Minneapolis, MN). Mouse monoclonal antibody to vimentin was obtained from Boehringer Mannheim (Mannheim, Germany). Rabbit polyclonal antibody to cytokeratin, fluorescein isothiocyanate-labeled F(ab′) goat anti-rabbit IgG, fluorescein isothiocyanate-conjugated anti-mouse IgG, alkaline-phosphatase-conjugated anti-rabbit IgG, and alkaline phosphatase-labeled anti-mouse IgG were purchased from Sigma (St. Louis, MO). Polyclonal rabbit antibody to type IV collagen was purchased from ICN (Aurora, OH). Polyclonal rabbit antibody to FSP-1 was generated as described elsewhere. 28 Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium were obtained from Gibco BRL Ltd. (Paisley, UK), fetal calf serum was purchased from BioWhittaker (Walkersville, MD), rat tail collagen type I and type IV collagen were obtained from Becton Dickinson (Franklin Lakes, NJ).
Cell Culture
Murine renal cell lines were established previously and had been cloned several times. 24,28-30 They were grown in recommended conditions: 24 MCTs and tubulointerstitial fibroblasts were cultured in DMEM supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. In EMT experiments the medium was replaced with serum-free K1 medium (1:1 Ham’s F12/DMEM with 5 μg/ml transferrin, 5 μg/ml insulin, and 5 × 10−8 mol/L hydrocortisone) containing cytokines or collagen domains. 31 Homogeneity, cell surface markers, and phenotype characteristics have been documented extensively for both MCT cells and tubulointerstitial fibroblast cells. 24,28
Expression of Soluble Type IV Collagen FLAG-α1NC1 Domains in 293 Embryonic Kidney Cells
A pDS plasmid containing α1NC1 domain cDNA was used to add a leader signal sequence in frame into the pcDNA3.1 (Invitrogen, Carlsbad, CA) eukaryotic expression vector by polymerase chain reaction amplification. The leader sequence from the 5′ end of the full-length α1 type IV collagen chain was cloned 5′ to the cDNA of α1NC1 domain to enable protein secretion into the culture medium. The α1NC1 domain was confirmed as described previously. 32 The α1NC1 domain containing plasmid and control plasmid were used to transfect 293 human embryonic kidney cells using the calcium chloride method. Transfected clones were selected by Geneticin (Life Technologies, Inc., Gaithersburg, MD) antibiotic treatment. The cells were passed for 3 weeks in the presence of the antibiotic until no cell death was evident. Clones were expanded into T-225 flasks and grown until confluent. The supernatant was collected and concentrated using an Amicon (Beverly, MA) concentrator. The concentrated supernatant was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), immunoblotting, and enzyme-linked immunosorbent assay (ELISA) with anti-α1NC1 domain and anti-FLAG antibodies. The α1NC1 domain-containing supernatant was subjected to affinity chromatography using α1NC1 domain-specific antibodies. 32
Western Blot Analysis
Renal cortex NC1 hexamer was prepared as described elsewhere. 33 After purification, native hexamers from the kidney cortex were subjected to treatment with 50 mmol/L formic acid followed by incubation for 10 minutes at room temperature to disassemble protein to monomers. Tris, 1 mol/L (pH 7.5), was added and incubation was extended for an additional 12-hour period at room temperature for reassembly of hexamers. For Western blot analysis proteins were transferred to trans-blot nitrocellulose membranes (Bio-Rad, Hercules, CA) and then probed with antibodies for 1 hour at room temperature. After extensive washes, bands were visualized using reagents for enhanced chemiluminescence (ECL Western blotting kit, Amersham, Arlington Heights, IL). For detection of incorporated FLAG-α1NC1 domain in hexamers, native hexamers were allowed to reassemble in presence of FLAG-α1NC1 domain and were run on a nondenaturing gel. The band corresponding to hexamers was cut out and protein was extracted. For detection protein was electrophoresed in denaturing polyacrylamide gel. FLAG-α1NC1 domain was detected by anti-FLAG antibody. For analysis of tissue culture supernatant, medium was removed and concentrated 10-fold using Centricon YM10 centrifugal filter devices (Millipore, Bedford, MA).
Direct ELISA
ELISAs were performed as described previously. 24 Cells (5 × 10 4 cells/well) were plated in 12-well plates and grown in DMEM with 10% fetal calf serum for 6 hours. Then the medium was replaced with serum-free K1 media containing cytokines, antibodies, or type IV collagen domains. After 24, 48, and 72 hours cells were harvested by trypsin/ethylenediaminetetraacetic acid, spun down, and resuspended in phosphate-buffered saline (PBS). The number of cells was counted using a hemocytometer. Cells (5 × 104) were pelleted, then lysed in 500 μl of 6 mol/L guanidine hydrochloride, pH 7.5. Microtiter ELISA plates (96-well) were coated in quadruplicate with 150 μl of cell lysate and were then incubated overnight at room temperature. After coating, the plates were washed with 0.15 mol/L NaCl 0.05% Tween-20 washing solution and blocked with 2% bovine serum albumin and 0.1% Tween-20 in PBS for 30 minutes at room temperature. The wells were incubated with 1:500 dilution of anti-FSP-1 or 1:200 dilution of anti-cytokeratin antibodies in the incubation buffer. After incubation with the primary antibody for 1 hour at room temperature, the plates were washed three times with washing solution and then incubated with alkaline phosphatase (ALP)-conjugated secondary antibody diluted 1:1000 in incubation buffer. Finally the plates were washed thoroughly and disodium p-nitrophenyl phosphate (5 μg/ml) was added. After color development the absorbance was measured with an ELISA plate reader at 450 nm.
Immunocytochemistry
The effect of α1NC1 domain on EMT was visualized by immunofluorescent staining of FSP-1 and vimentin after incubation for 48 hours as described above. At the end of incubation cells were washed twice with PBS and fixed with ethanol/acetic acid (50:50, v/v) at 4°C. The cells were subsequently washed again and incubated for 2 hours with a 1:40 dilution of antibody to FSP-1 or 1:8 dilution of antibody to vimentin. Cells were then washed again and incubated for 1 hour at room temperature with fluorescein isothiocyanate-conjugated secondary antibody. Staining was analyzed by fluorescence microscopy.
Cell Attachment Assay
This assay was performed as described previously with minor modifications. 34 Ninety-six-well plates were coated with 10 μg/ml of type IV collagen, type I collagen, or fibronectin in PBS or with 10% w/v bovine serum albumin/PBS as negative control at 37°C overnight. Before seeding cells, wells were blocked with 10% bovine serum albumin/PBS for 2 hours at 37°C. MCT cells were maintained in K1 medium or K1 medium that contained 3 ng/ml TGF-β1 and 10 ng/ml EGF for 48 hours in T75 flasks as described. Cells were harvested by trypsin, and 4000 cells per well were seeded in 100 μl of K1 medium. After 30 minutes, medium was removed, wells were washed once, and cells were incubated again for 1 hour at 37°C. The number of attached cells was determined with methylene blue staining.
Preparation of Probes and Northern Blot Analysis
MCT cells were grown for 6 hours in DMEM containing 10% fetal calf serum before the medium was replaced with serum-free medium or with medium containing growth factors or α1NC1 domains. Total cellular RNA was extracted using Trizol reagent (Gibco BRL Ltd.) according to instructions of the manufacturer. RNA concentrations were determined by absorbance at 260 nm and samples were stored at −80°C before use. Northern blot analysis was performed as described previously with minor modifications. 35 Forty μg of total RNA were electrophoresed on a 1.2% agarose gel containing 2.2 mol/L formaldehyde using 1×× MOPS, pH 7.0, as the running buffer. RNA was transferred to a nylon membrane by capillary transfer for 12 hours using 10× standard saline citrate as the transfer buffer. Blots were UV-crosslinked. TGF-β1 cDNA probes were linearized and labeled with [γ32p]-ATP by random labeling according to instructions of the manufacturer (Boehringer Mannheim,). Hybridizations were performed overnight at 68°C after 1 hour of prehybridization with hybridization buffer (0.5 mol/L NaPo4, 7% w/v SDS, 1% w/v bovine serum albumin, 1 mmol/L ethylenediaminetetraacetic acid). Washing steps were performed three times using a solution containing 0.25 mol/L Na2HPO4 at 68°C. After washing, autoradiograms were obtained. All blots were stripped and reprobed with isotope-labeled GAPDH cDNA as a control for equal loading and transfer. Quantitative analysis was performed relative to the GAPDH band using a densitometer and quantitation software (Bio-Rad).
Statistical Analysis
All values are expressed as mean ± SEM unless specified. Analysis of variance was used to determine statistical differences between groups using Sigma-Stat software (Jandel Scientific, San Rafael, CA). Further analysis was performed using t-test with Bonferroni correction to identify significant differences. A level of P < 0.05 was considered statistically significant.
Results
Tubular Epithelial Cell Interactions with Collagen Types I and IV
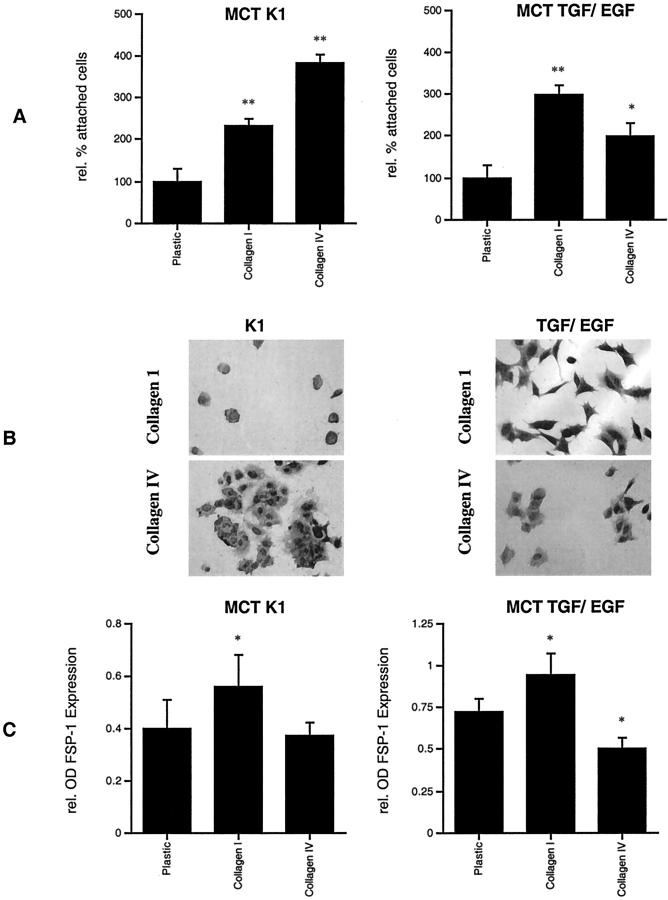
Tubular epithelial cells typically adhere tightly to the TBM, whereas interstitial fibroblasts are surrounded by type I collagen in vivo. 36 To test whether the microenvironment has specific effects on tubular cells, MCTs were exposed to culture dishes that were coated with either type I collagen or with type IV collagen composed exclusively of the α1 and α2 chains. In cell attachment assays, untreated MCT cells adhered better to type IV collagen (Figure 1A ▶ , left), whereas after induction of EMT with TGF-β1 and EGF, cells adhered preferably to type I collagen (Figure 1A ▶ , right) and appeared to display a more spindle-shaped morphology (Figure 1B) ▶ . Cultivation on type I collagen resulted in an increase in the fibroblast-specific marker FSP-1, whereas cultivation on type IV collagen did not alter the expression of FSP-1 compared to uncoated plates (Figure 1C ▶ , left). When EMT was induced with TGF-β1 and EGF, cultivation on type IV collagen reduced levels of FSP-1 expression and thus stabilized the epithelial phenotype, whereas cultivation on type I collagen further increased FSP-1 expression (Figure 1C ▶ , right).
Figure 1.
Tubular epithelial cell interactions with collagen types I and IV. MCT cells adhere preferably to type IV collagen (383 ±16.6% compared to uncoated plastic control) than to type I collagen (232.2 ± 20.5%) in cell adhesion assay (A, left). After induction of EMT with TGF-β1 and EGF, MCT cells with a fibroblast-like morphology attached increasingly to collagen type I (396.7 ± 24.3% compared to uncoated plastic control), whereas adhesion to type IV collagen was less abundant (299.5 ± 20.6%) (A, right). MCT cells grown in K1 medium adhere strongly to type IV collagen and seem to display a round-shaped, epithelial cell-like, morphology (B, left). MCT cells that were pretreated with TGF-β1 and EGF gain in capacity to attach to type I collagen and appear to have a more spindle-shaped morphology (B, right). Cultivation on type I collagen increased FSP-1 expression in MCT cells that were grown in K1 medium (140 ± 9.3% compared to uncoated plastic control), whereas cultivation on type IV collagen had no effect on FSP-1 expression in untreated cells (C, left) as was measured by ELISA of cell lysates. When EMT was induced in MCT cells with TGF-β1 and EGF, cultivation on type I collagen further increased levels of FSP-1 expression (130.6 ± 7.3% compared to uncoated plastic control), whereas coating with type IV collagen decreased levels of FSP-1 expression (58.1 ± 10.4%) and thus stabilized the epithelial phenotype (C, right). *, P < 0.05; **, P < 0.001. Original magnifications, ×400.
Recombinant Type IV Collagen α1NC1 Domain Incorporates within Native Type IV Collagen NC1-Hexamers
Type IV collagen exhibits self-assembly in vitro and potentially in vivo. 37-40 The initial process of type IV collagen assembly involves six α-chains and their NC1 domains to form NC1 hexamers. 5,41 Type IV collagen network is formed by lateral assembly of collagenous domains and by covalent association of 7S domains. 4,37,38
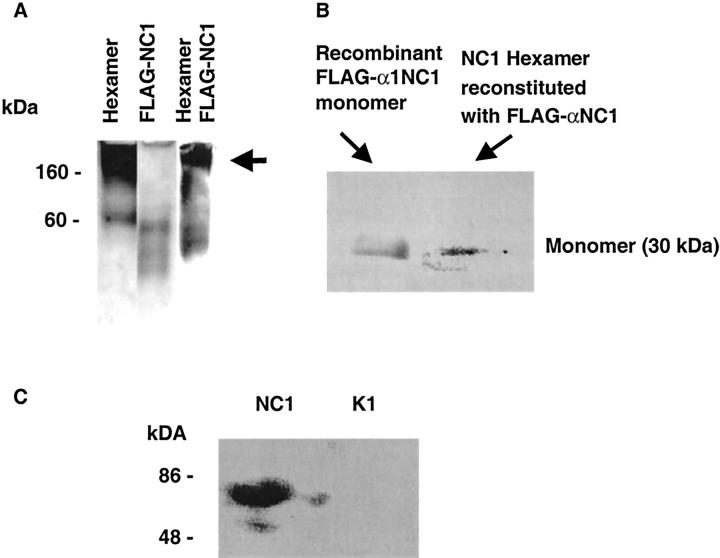
We hypothesized that type IV collagen α1NC1 domain, which lacks the collagenous- and 7S domain, will incorporate into hexamers and thus act in a dominant-negative manner on type IV collagen self-assembly involving the collagenous chain to form triple helices. 3,42 Type IV collagen hexamers, isolated from bovine kidney cortex, were used in hexamer association and dissociation experiments. In the presence of FLAG-tagged α1NC1 domain the reassembled hexamers incorporated the recombinant FLAG-tagged human α1NC1 domain, as determined by nondenaturing gel electrophoresis of the reconstituted hexamers (Figure 2A) ▶ . To further demonstrate the incorporation of FLAG-α1NC1 domain into the hexameric structure, the hexamer band (arrow in Figure 2A ▶ ) was excised and eluted from the nondenaturing gel and resolved by SDS-PAGE and immunoblotted with anti-FLAG antibodies. These results show that FLAG-α1NC1 is incorporated in the hexameric structure and detectable as a monomer band in dissociated hexamer in SDS-PAGE (Figure 2B) ▶ , similar to FLAG-tagged human α1NC1 domain (Figure 2B) ▶ . Thus FLAG-α1NC1 domain can potentially play a role in the disintegration of type IV collagen assembly and structure. When MCT cells were incubated with α1NC1 domain, an increase in type IV collagen degradation products was detectable in cell culture supernatant (Figure 2C) ▶ . 43 The degradation product of 80 kd has been reported in other experimental systems. 33 These results suggest that NC1 domain is capable of disrupting the organized self-assembly of type IV collagen and thus basement membranes. Figure 3 ▶ summarizes the model of type IV collagen assembly that evolves from these findings. NC1 hexamers can be dissociated by low pH treatment (Figure 3A) ▶ . When hexamers are reassembled by increasing pH in presence of FLAG-α1NC1 domain, it incorporates into the hexameric structure (Figure 3B) ▶ . Because of its lack of collagenous and 7S domains, FLAG-α1NC1 domain can potentially inhibit assembly of the type IV collagen network and lead to degradation of basement membranes (Figure 3C) ▶ .
Figure 2.
Recombinant α1NC1 domain is incorporated into hexamers of native NC1 protein. To demonstrate incorporation of recombinant FLAG-α1NC1 domain into hexamers, native hexamers of type IV collagen from kidney cortex were subjected to changing pH from 7.5 to 3.0 and subsequently allowed to reassemble in the presence of FLAG-α1NC1 monomers. Native hexamers and FLAG-α1NC1 display typical bands (A). When hexamers were subjected to changing of pH and allowed to reassemble in the presence of the FLAG-α1NC1 domain and were run on a denaturing gel FLAG-α1NC1 incorporated into hexamers. Bands corresponding to the full-size hexamer were cut out (A, arrow). Protein was extracted and subjected to dissociation by SDS-PAGE. Western Blot analysis with anti-FLAG antibody revealed positive staining of eluted protein (B) and thus proves incorporation of FLAG-α1NC1 domain into hexamers. When FLAG-α1NC1 domain was added to MCT cells, Western blot analysis of cell culture supernatant with antibodies to type IV collagen revealed an increased pattern that is typical of type IV collagen degradation (C, left lane) in contrast to untreated cells (C, right lane). Thus it demonstrates an increased disassembly of type IV collagen in cell culture.
Figure 3.
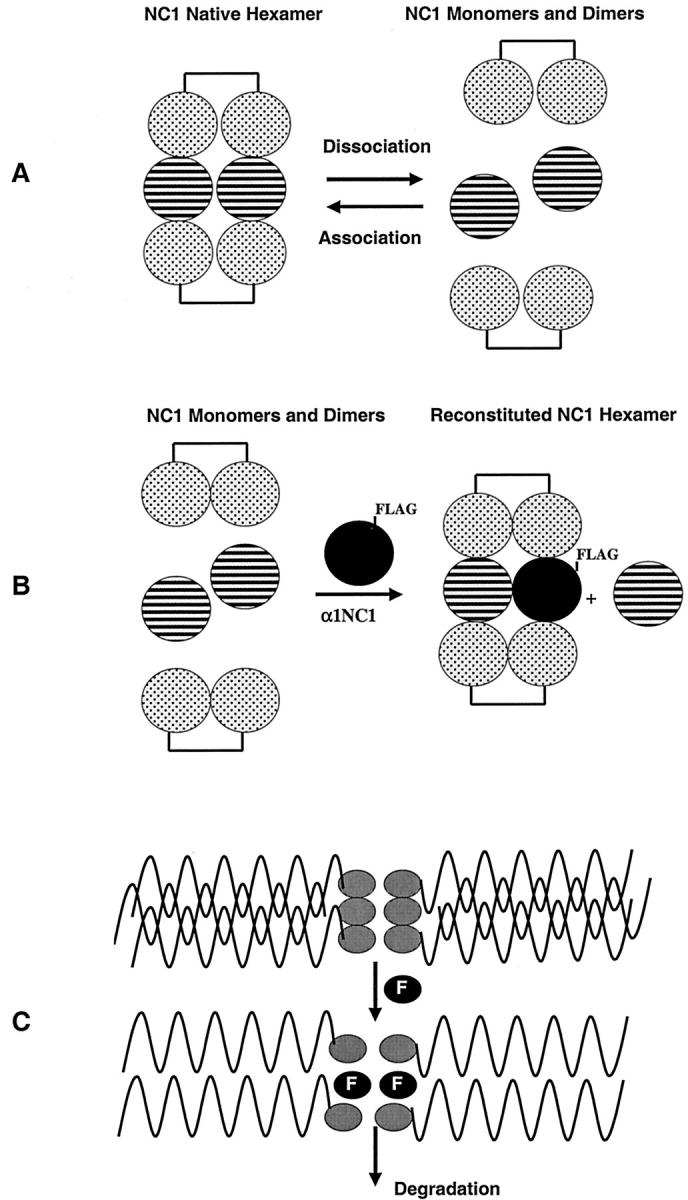
Schematic illustration of incorporation of recombinant FLAG-α1NC1 domain into type IV collagen hexamers and its potential capacity to degrade type IV collagen. When type IV collagen hexamers are subjected to low pH treatment they dissociate to monomers and dimers (A). When NC1 monomers and dimers are reassembled into hexamers in the presence of FLAG-tagged recombinant α1NC1 domain, it gets incorporated into the hexameric structure (B). Because of the lack of collagenous domain and 7S domain, incorporation of FLAG-α1NC1 domain potentially leads to increased degradation of type IV collagen (C).
Inhibition of Type IV Collagen Assembly Induces EMT in Vitro
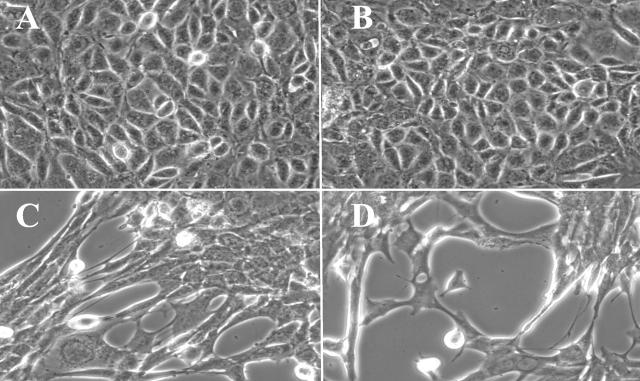
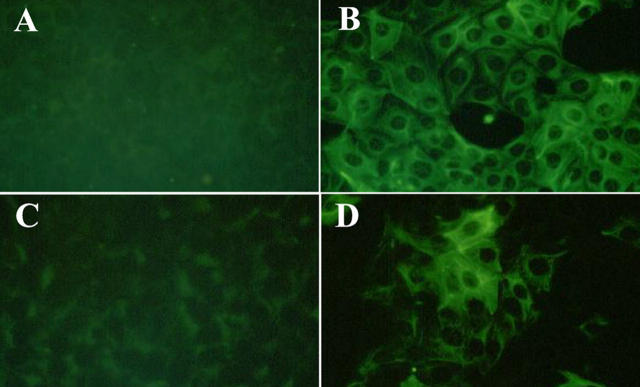
MCTs in culture produce type IV collagen and assemble it basolaterally. 44 This event is pivotal for the proliferation and survival of these cells. 45,46 We hypothesized that self-assembled type IV collagen in this experimental system plays a role in the maintenance of the epithelial phenotype. Thus, α1NC1 domain was used to potentially inhibit the basolateral assembly of type IV collagen by MCT cells and to evaluate its impact on the phenotype of these cells. After incubation with 20 μg/ml of α1NC1 domain for 48 hours, MCT cells acquired a spindle-shaped, fibroblast-like morphology similar to MCT cells incubated with 3 ng/ml TGF-β1 and 10 ng/ml EGF, a well-established stimulus for induction of EMT in vitro (Figure 4, C and D) ▶ . 24,47 Incubation with 7S domain of type IV collagen had no effect on cellular phenotype. MCT cells maintained their typical cobblestone morphology as compared to control cells (Figure 4, A and B) ▶ . When more than 20 μg/ml of α1NC1 domain was used in these experiments, the cells completely detached from the culture plate (data not shown). The EMT conversion remained stable, even after removal of α1NC1-containing K1 culture media and replacement with just K1 culture medium. These results demonstrate the potent effects of basement membrane collagen loss on the conversion of the epithelial phenotype of MCT cells. To confirm the induction of EMT, the cells were stained for vimentin and FSP-1 after 48 hours of α1NC1 treatment. Cells attained expression of vimentin (Figure 5, A and B) ▶ as well as of FSP-1 (Figure 5, C and D) ▶ , strongly suggestive of a mesenchymal phenotype. 48
Figure 4.
MCT cells acquire a fibroblast-like morphology after incubation with type IV collagen α1NC1 domain, similar to changes that were observed after incubation with TGF-β1 and EGF. Tubular epithelial MCT cells display their typical cobblestone-like morphology when cultured in K1 medium (A). When medium was supplemented with either soluble type IV collagen α1NC1 (C) domain or with 3 ng/ml TGF-β1 and 10 ng/ml EGF (D) cells acquired a typical spindle-shaped morphology, which is typically observed in the induction of EMT. Incubation with type IV collagen 7S domain did not alter the cellular phenotype (B). Original magnifications, ×400.
Figure 5.
Incubation with type IV collagen α1NC1 domain results in increased expression of mesenchymal markers FSP-1 and vimentin by MCT cells. MCT cells that were cultured in K1 medium displayed little staining for the mesenchymal markers vimentin (A) and FSP-1 (C) using indirect immunofluorescent staining. Treatment with type IV collagen α1NC1 domain resulted in a robust up-regulation of vimentin (B) and FSP-1 (D) expression. Original magnifications, ×400.
To further confirm induction of EMT by soluble α1NC1 domain, direct solid-phase ELISA for cytokeratin, as an epithelial marker, and FSP-1 as a mesenchymal marker, was performed. After 48 and 72 hours of treatment with α1NC1 domains a strong increase in FSP-1 expression was detected when compared to cells grown in control K1 culture medium (Figure 6, A and C) ▶ . This observation parallels the results of 48- and 72-hour incubations with TGF-β1 and EGF (Figure 6, A and C) ▶ . Also, after incubation for 48 and 72 hours with α1NC1 domain, a decrease in cytokeratin was observed (Figure 6, B and D) ▶ , similar to treatment with TGF-β1 and EGF (Figure 6, B and D) ▶ . In contrast to NC1 domain, incubation with 7S domain did not induce FSP-1 expression (Figure 6E) ▶ . The EMT-stimulating effect could be inhibited by addition of α1NC1-neutralizing antibodies, whereas addition of antibodies to 7S domain had no effect (Figure 6E) ▶ .
Figure 6.
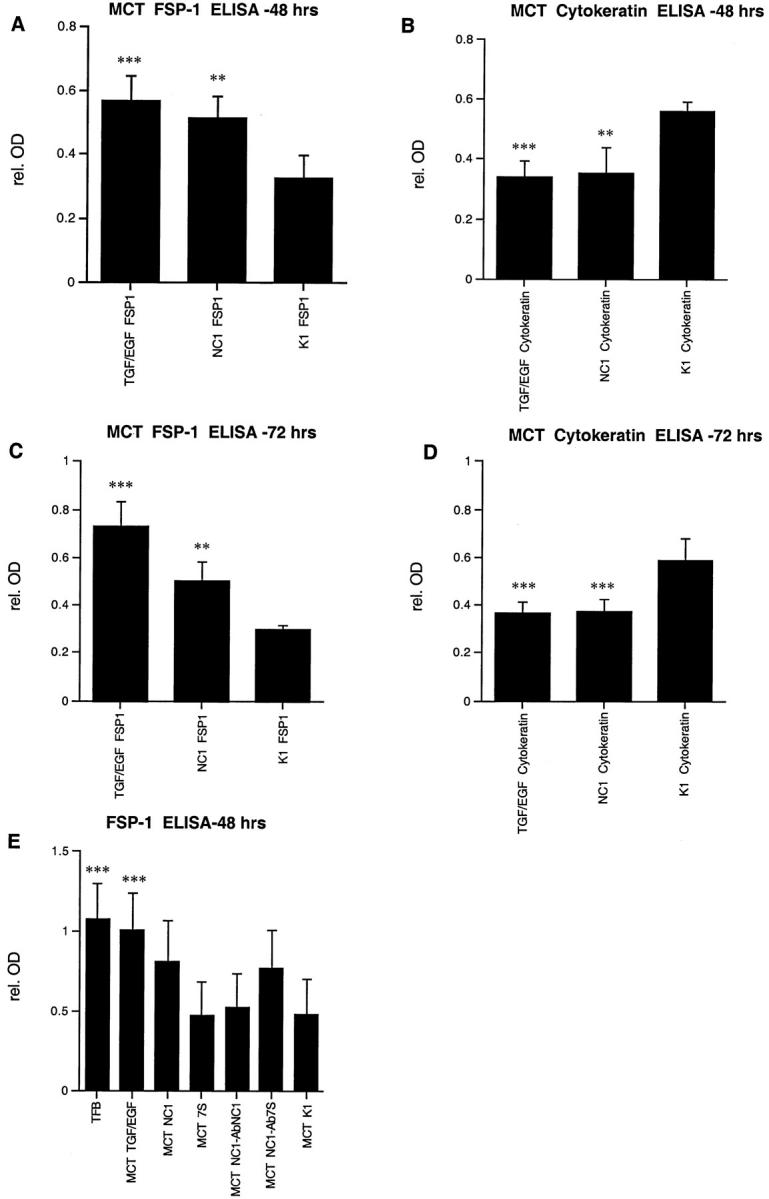
Solid phase direct ELISA for FSP-1 and cytokeratin. Treatment of MCT cells with type IV collagen α1NC1 domain resulted in an increase in FSP-1 expression (A and C) and a decrease in cytokeratin expression as compared to untreated K1 control (B and D). Effects were similar to those that were obtained by stimulation with TGF-β1 and EGF, which is currently the most established inductor of EMT in vitro. After 48 hours the α1NC1 domain induced an increase in FSP-1 expression (162.7 ± 14.1% compared to K1 control) (A) and decreased cytokeratin expression (76.1 ± 5.3% compared to K1 control) (B), whereas effects of TGF-β1 and EGF were 156.7 ± 23.6% and 65.0 ± 4.9%, respectively. Similar results were obtained after 72 hours (C and D). Type IV collagen α1NC1 domain induced FSP-1 expression (181.6 ± 23.6% compared to control) (C) and a decrease in cytokeratin expression by (61.4 ± 9.6% of K1 control) (D). Treatment with TGF-β1 and EGF for 72 hours resulted in an induction of FSP-1 expression (184.7 ± 28.1%) (C) and depression of cytokeratin expression (60.7 ± 10.1%) (D). In another series of experiments FSP-1 expression was determined by ELISA to test the specificity of α1NC1 domain-induced effects (E). ELISAs for FSP-1 display a strong expression in tubulointerstitial fibroblasts and low expression levels in untreated MCT cells (MCT-K1) that were used as a control. Increased expression of FSP-1 compared to untreated MCT control was detectable after treatment with TGF-β1 and EGF (259.9 ± 40.2% compared to MCT K1 control) as well as with α1NC1 domain (199.2 ± 33.1%). Incubation with type IV collagen 7S domain had no significant effect on FSP-1 expression levels (105.9 ± 1.4%). Co-incubation of α1NC1 domain with α1NC1 neutralizing antibodies (MCT NC1-abNC1) diminished the increase in FSP-1 expression (112.7 ± 7.2%), whereas 7S neutralizing antibodies (MCT NC1-ab7S) had no effect (184.1 ± 23.7%). *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Cumulatively, these results strongly suggest that α1NC1 domain serves to alter the epithelial phenotype and propagates conversion to fibroblast-like/myofibroblast-like cells via its ability to inhibit basement membrane formation. This conversion (EMT) by α1NC1 domain is demonstrated by the expression of vimentin and FSP-1, and decrease in the epithelial cell marker, cytokeratin. These changes mirror the effects seen by treatment of MCT cells by TGF-β1 and EGF as previously described. 24
TGF-β1 Mediates α1NC1-Induced EMT in Proximal Tubular Epithelial Cells
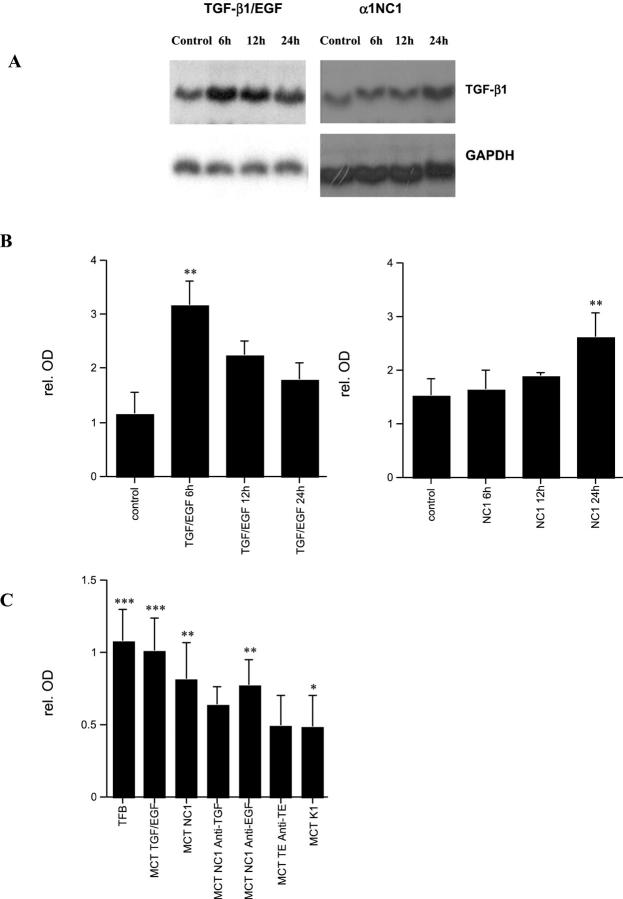
TGF-β1 is an important mediator of EMT. 49 In renal fibrogenesis it is highly abundant in tubules. 50 Thus, we speculated that autocrine expression of TGF-β1 may play a role in EMT. By Northern blotting we show that TGF-β1 mRNA is up-regulated significantly, when EMT was induced by stimulation with TGF-β1 and EGF (Figure 7A ▶ , left). Treatment of MCT cells with soluble α1NC1 domain also resulted in a significant increase in TGF-β1 mRNA expression (Figure 7A ▶ , right). Figure 7B ▶ summarizes densitometric analysis of experiments. Addition of neutralizing antibodies against TGF-β1 was sufficient to inhibit α1NC1-induced EMT and attenuated α1NC1 domain-mediated increase in FSP-1 (Figure 7C) ▶ . EGF-neutralizing antibodies had no effect on EMT or FSP-1 expression (Figure 7C) ▶ .
Figure 7.
TGF-β1 mRNA expression is up-regulated in EMT. Induction of EMT by TGF-β1/EGF or with type IV collagen α1NC1 domain results in up-regulation of TGF-β1 mRNA expression (A). Induction with TGF-β1/EGF resulted in a peak after 6 hours, whereas after incubation with type IV collagen α1NC1 domains resulted in an up-regulation after 24 hours (B) summarizes the densitometric analysis of experiments with TGF-β1/EGF (left) and with α1NC1 domain (right). Treatment of MCT cells with TGF-β1 and EGF results in an increase in TGF-β1 mRNA after 6 hours by (291 ± 48% compared to K1 control), and remains elevated after 12 hours (204 ± 34%) and 24 hours (162 ± 18%). Treatment with α1NC1 domain leads to a significant increase in TGF-β1 mRNA after 24 hours (175.2 ± 24%), however results after 6 hours (110.7 ± 8%) and 12 hours (125.7 ± 9%) were not significant. In solid-phase direct ELISA for FSP-1 (C) co-incubation of α1NC1 domain with neutralizing antibodies to TGF-β1 (MCT NC1 anti-TGF) reduced the increase in FSP-1 expression significantly (158.1 ± 23% compared to K1 control), whereas the addition of neutralizing EGF antibodies (MCT NC1 anti-EGF) had no significant effect (193.5 ± 29%). Addition of neutralizing antibodies to TGF and EGF (MCT TE anti-TE) abolished growth factor-induced EMT (111.8 ± 14%). These results suggest a role for TGF-β1 autocrine stimulation for mediation of EMT. *, P < 0.05); **, P < 0.001; ***, P < 0.0001.
Discussion
TBMs provide a tightly regulated microenvironment for normal function of tubular epithelial cells. 8 TBM facilitates numerous cell-matrix interactions that are pivotal for the maintenance of epithelial phenotype. 51,52 Type IV collagen is the most abundant protein in basement membranes and acts as a scaffold to provide structural and functional integrity. 53 We show that tubular epithelial cells adhere preferably to collagen type IV, whereas MCT cells treated with TGF-β1 and EGF, that display a fibroblast-like morphology, adhere better to type I collagen. Also type IV collagen stabilizes the epithelial phenotype, whereas cultivation on type I collagen promoted transdifferentiation to a mesenchymal phenotype. Thus, integrity of type IV collagen seems to affect tubular epithelial cells. These findings confirm observations with lens mesenchymal cells and Madin-Darby canine kidney cells on different matrices. 54,55
Basement membrane organization is provided by assembly of trimers that consist of three collagen α-chains. 56 Noncollagenous (NC) domains play a pivotal role in basement membrane self-assembly. 5,57,58 In the present study we show that recombinant human α1NC1 domain can interfere with type IV collagen hexamer formation, a pivotal step in the assembly of type IV collagen. Conceivably, the use of soluble α1NC1 domain without the collagenous domain leads to incorporation of this domain in the hexamer structure, leading to a dominant-negative effect of decreasing viable protomers and protomeric dimers. A dominant-negative effect of mutated NC1 domains has been reported for type X collagen, another nonfibrillar collagen, where mutations in the NC1 domain interfere with trimer formation and impaired structure in vivo and in vitro. 59-61 Presumably such a dominant-negative effect is achieved because of the lack of the collagenous domain to complete triple helix formation. 40,42 Impaired assembly of triple helices potentially leads to degradation of such incompletely assembled molecules. Our results with supernatants of α1NC1 domain-treated cells show increased degradation products, supporting this notion.
In the present study, disruption of basement membrane assembly induced acquisition of mesenchymal phenotype by MCT cells. Tubular epithelial cells transformed to spindle-shaped morphology with increased expression of mesenchymal markers FSP-1 and vimentin, whereas expression of the epithelial marker cytokeratin decreased. The transdifferentiation that was observed by incubation with α1NC1 domains was similar to those that were observed after stimulation with TGF-β1 and EGF, which is currently the best characterized stimulus for the induction of EMT. 24,47,49 The transdifferentiation of cells was concordant with findings by other groups that define induction of EMT in vitro. 26,62 This finding is also supported by the observation that tubular epithelial cells that undergo EMT loose contact with their disintegrated TBM in vivo. 63
Our findings suggest that induction of EMT by growth factors, as well as by disruption of TBM, is mediated via an autocrine loop that is driven by TGF-β1. Transdifferentiation through a TGF-β1-dependent mechanism has been previously reported in rat tubular epithelial cells. 64 Thus, our findings suggest a reciprocal dependency of tubular-epithelial cell phenotype, basement membrane integrity, and TGF-β1. Tubular epithelial cells that are plated on plastic have initially a much higher rate of type IV collagen expression than cells that are cultivated on Matrigel that consists of type IV collagen α1 and α2 chains. 44 Further impairment of basement membrane assembly leads to EMT or anoikis of epithelial cells, 65,66 TGF-β1 is known to induce EMT in tubular epithelial cells. 24 TGF-β1 also impairs formation of a continuous basement membrane architecture. 46 TGF-β1 increases synthesis of type IV collagen chains α1 and α2 and type I collagen, the predominant form of interstitial matrix. 67,68 In MCT cells that produce type IV collagen α1 and α2, 29 de novo ectopic expression of type IV collagen α3 expression has been observed after stimulation with angiotensin 2, an effect that was inhibited by TGF-β antisense oligonucleotides. 69 TGF-β1 and collagen type I up-regulate MMP-9 expression, 70,71 which mediates degradation of basement membranes. 43,72 Further, our findings suggest that impairment of basement membrane integrity leads to up-regulation of TGF-β1.
Collectively our results offer for the first time a central role for the structural integrity of TBM in maintenance of normal phenotype of proximal tubular cells. We speculate that very early events in progression of renal disease induce subtle disruptions of TBM, which leads to increased production of TGF-β1 by proximal tubular cells, which further enhances EMT. Such initial events are possibe potent drivers of EMT, leading eventually to renal interstitial fibrosis. Therefore, disruption of TBM is potentially an important facilitator of EMT. Mechanisms associated with such alterations and their impact on cell-matrix interaction need further clarification.
Acknowledgments
We thank Biogen, Inc. for their generous research gift and help; Dr. Helmut Hopfer for his help in constructing the FLAG-tagged recombinant human α1NC1 plasmid; and Dr. Eric G. Neilson for his gift of FSP-1 antibody.
Footnotes
Address reprint requests to Dr. Raghu Kalluri, Assistant Professor of Medicine, Nephrology Division, Department of Medicine, RW 563a, Beth Israel Deaconess Medical Center, 330 Brookline Ave., Boston, MA 02215. E-mail: rkalluri@caregroup.harvard.edu.
Supported in part by grants DK51711 and DK55001 from the National Institutes of Health; a research grant from Creative Biomolecules Inc./Curis, Inc.; a research grant from the Beth Israel Deaconess Liver Center; a research fund from the Beth Israel Deaconess Medical Center; and a grant from the German Research Foundation (DFG STR 388/3–1 to F. S. during his sabbatical at Beth Israel Deaconess Medical Center).
References
- 1.Yurchenco PD, O’Rear JJ: Basal lamina assembly. Curr Opin Cell Biol 1994, 6:674-681 [DOI] [PubMed] [Google Scholar]
- 2.Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993, 268:26033-26036 [PubMed] [Google Scholar]
- 3.Timpl R, Oberbaumer I, von der Mark H, Bode W, Wick G, Weber S, Engel J: Structure and biology of the globular domain of basement membrane type IV collagen. Ann NY Acad Sci 1985, 460:58-72 [DOI] [PubMed] [Google Scholar]
- 4.Yurchenco PD: Assembly of basement membranes. Ann NY Acad Sci 1990, 580:195-213 [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R, Cosgrove D: Assembly of type IV collagen. Insights from alpha3(IV) collagen-deficient mice. J Biol Chem 2000, 275:12719-12724 [DOI] [PubMed] [Google Scholar]
- 6.Siebold B, Deutzmann R, Kuhn K: The arrangement of intra- and intermolecular disulfide bonds in the carboxyterminal, non-collagenous aggregation and cross-linking domain of basement-membrane type IV collagen. Eur J Biochem 1988, 176:617-624 [DOI] [PubMed] [Google Scholar]
- 7.Timpl R: Structure and biological activity of basement membrane proteins. Eur J Biochem 1989, 180:487-502 [DOI] [PubMed] [Google Scholar]
- 8.Miner JH: Renal basement membrane components. Kidney Int 1999, 56:2016-2024 [DOI] [PubMed] [Google Scholar]
- 9.Van Vliet AI, Van Alderwegen IE, Baelde HJ, de Heer E, Killen PD, Kalluri RK, Bruijn JA, Bergijk EC: Differential expression of collagen type IV alpha-chains in the tubulointerstitial compartment in experimental chronic serum sickness nephritis. J Pathol 1999, 189:279-287 [DOI] [PubMed] [Google Scholar]
- 10.Hynes RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992, 69:11-25 [DOI] [PubMed] [Google Scholar]
- 11.Eddy AA: Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 1996, 7:2495-2508 [DOI] [PubMed] [Google Scholar]
- 12.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992, 20:1-17 [DOI] [PubMed] [Google Scholar]
- 13.Strutz F, Muller GA: On the progression of chronic renal disease. Nephron 1995, 69:371-379 [DOI] [PubMed] [Google Scholar]
- 14.Fogo AB: Pathology of progressive nephropathies. Curr Opin Nephrol Hypertens 2000, 9:241-246 [DOI] [PubMed] [Google Scholar]
- 15.Alpers CE, Pichler R, Johnson RJ: Phenotypic features of cortical interstitial cells potentially important in fibrosis. Kidney Int 1996, 54(Suppl):S28-S31 [PubMed] [Google Scholar]
- 16.Alpers CE: The evolving contribution of renal pathology to understanding interstitial nephritis. Ren Fail 1998, 20:763-771 [DOI] [PubMed] [Google Scholar]
- 17.Becker GJ, Hewitson TD: The role of tubulointerstitial injury in chronic renal failure. Curr Opin Nephrol Hypertens 2000, 9:133-138 [DOI] [PubMed] [Google Scholar]
- 18.D’Agati V, Appel GB: Renal pathology of human immunodeficiency virus infection. Semin Nephrol 1998, 18:406-421 [PubMed] [Google Scholar]
- 19.Muller GA, Zeisberg M, Strutz F: The importance of tubulointerstitial damage in progressive renal disease. Nephrol Dial Transplant 2000, 15:76-77 [DOI] [PubMed] [Google Scholar]
- 20.Hay ED, Zuk A: Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis 1995, 26:678-690 [DOI] [PubMed] [Google Scholar]
- 21.Birchmeier W, Birchmeier C: Epithelial-mesenchymal transitions in development and tumor progression. EXS 1995, 74:1-15 [DOI] [PubMed] [Google Scholar]
- 22.Wallner EI, Yang Q, Peterson DR, Wada J, Kanwar YS: Relevance of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol 1998, 275:F467-F477 [DOI] [PubMed] [Google Scholar]
- 23.Kanwar YS, Carone FA, Kumar A, Wada J, Ota K, Wallner EI: Role of extracellular matrix, growth factors and proto-oncogenes in metanephric development. Kidney Int 1997, 52:589-606 [DOI] [PubMed] [Google Scholar]
- 24.Okada H, Danoff TM, Kalluri R, Neilson EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol 1997, 273:F563-F574 [DOI] [PubMed] [Google Scholar]
- 25.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 1998, 339:1448-1456 [DOI] [PubMed] [Google Scholar]
- 26.Strutz F, Muller GA: Transdifferentiation comes of age. Nephrol Dial Transplant 2000, 15:1729-1731 [DOI] [PubMed] [Google Scholar]
- 27.Hay ED: An overview of epithelio-mesenchymal transformation. Acta Anat 1995, 154:8-20 [DOI] [PubMed] [Google Scholar]
- 28.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG: Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995, 130:393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, Kefalides NA, Neilson EG: Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 1988, 107:1359-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez RJ, Sun MJ, Haverty TP, Iozzo RV, Myers JC, Neilson EG: Biosynthetic and proliferative characteristics of tubulointerstitial fibroblasts probed with paracrine cytokines. Kidney Int 1992, 41:14-23 [DOI] [PubMed] [Google Scholar]
- 31.Taub N, Livingston D: The development of serum-free hormone-supplemented media for primary kidney cultures and their use in examining renal functions. Ann NY Acad Sci 1981, 372:406-421 [DOI] [PubMed] [Google Scholar]
- 32.Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R: Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 2000, 60:2520-2526 [PubMed] [Google Scholar]
- 33.Gunwar S, Ballester F, Kalluri R, Timoneda J, Chonko AM, Edwards SJ, Noelken ME, Hudson BG: Glomerular basement membrane. Identification of dimeric subunits of the noncollagenous domain (hexamer) of collagen IV and the Goodpasture antigen. J Biol Chem 1991, 266:15318-15324 [PubMed] [Google Scholar]
- 34.Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR, Kharbanda S, Kalluri R: Identification of the anti-angiogenic site within vascular basement membrane derived tumstatin. J Biol Chem 2001, 276:15240-15248 [DOI] [PubMed] [Google Scholar]
- 35.Rocco MV, Chen Y, Goldfarb S, Ziyadeh FN: Elevated glucose stimulates TGF-beta gene expression and bioactivity in proximal tubule. Kidney Int 1992, 41:107-114 [DOI] [PubMed] [Google Scholar]
- 36.Zuk A, Hay ED: Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn 1994, 201:378-393 [DOI] [PubMed] [Google Scholar]
- 37.Oberbaumer I, Wiedemann H, Timpl R, Kuhn K: Shape and assembly of type IV procollagen obtained from cell culture. EMBO J 1982, 1:805-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yurchenco PD, Ruben GC: Basement membrane structure in situ: evidence for lateral associations in the type IV collagen network. J Cell Biol 1987, 105:2559-2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurchenco PD, Ruben GC: Type IV collagen lateral associations in the EHS tumor matrix. Comparison with amniotic and in vitro networks. Am J Pathol 1988, 132:278-291 [PMC free article] [PubMed] [Google Scholar]
- 40.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC: New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo. J Biol Chem 2000, 275:8051-8061 [DOI] [PubMed] [Google Scholar]
- 41.Tsilibary EC, Reger LA, Vogel AM, Koliakos GG, Anderson SS, Charonis AS, Alegre JN, Furcht LT: Identification of a multifunctional, cell-binding peptide sequence from the a1(NC1) of type IV collagen. J Cell Biol 1990, 111:1583-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutaud A, Borza DB, Bondar O, Gunwar S, Netzer KO, Singh N, Ninomiya Y, Sado Y, Noelken ME, Hudson BG: Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J Biol Chem 2000, 275:30716-30724 [DOI] [PubMed] [Google Scholar]
- 43.Craig FM, Archer CW, Murphy G: Isolation and characterisation of a chicken gelatinase (type IV collagenase). Biochim Biophys Acta 1991, 1074:243-250 [DOI] [PubMed] [Google Scholar]
- 44.Gibbs SR, Goins RA, Belvin EL, Dimari SJ, Merriam AP, Bowling-Brown S, Harris RC, Haralson MA: Characterization of the collagen phenotype of rabbit proximal tubule cells in culture. Connect Tissue Res 1999, 40:173-188 [DOI] [PubMed] [Google Scholar]
- 45.Furuyama A, Mochitate K: Assembly of the exogenous extracellular matrix during basement membrane formation by alveolar epithelial cells in vitro. J Cell Sci 2000, 113:859-868 [DOI] [PubMed] [Google Scholar]
- 46.Furuyama A, Iwata M, Hayashi T, Mochitate K: Transforming growth factor-beta1 regulates basement membrane formation by alveolar epithelial cells in vitro. Eur J Cell Biol 1999, 78:867-875 [DOI] [PubMed] [Google Scholar]
- 47.Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999, 56:1455-1467 [DOI] [PubMed] [Google Scholar]
- 48.Strutz F, Muller GA, Neilson EG: Transdifferentiation: a new angle on renal fibrosis. Exp Nephrol 1996, 4:267-270 [PubMed] [Google Scholar]
- 49.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994, 331:1286-1292 [DOI] [PubMed] [Google Scholar]
- 50.Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN: Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol 2000, 278:F628-F634 [DOI] [PubMed] [Google Scholar]
- 51.Paulsson M: Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 1992, 27:93-127 [DOI] [PubMed] [Google Scholar]
- 52.Kanwar YS, Kumar A, Yang Q, Tian Y, Wada J, Kashihara N, Wallner EI: Tubulointerstitial nephritis antigen: an extracellular matrix protein that selectively regulates tubulogenesis vs. glomerulogenesis during mammalian renal development. Proc Natl Acad Sci USA 1999, 96:11323-11328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K: A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem 1981, 120:203-211 [DOI] [PubMed] [Google Scholar]
- 54.Zuk A, Matlin KS, Hay ED: Type I collagen gel induces Madin-Darby canine kidney cells to become fusiform in shape and lose apical-basal polarity. J Cell Biol 1989, 108:903-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuk A, Kleinman HK, Hay ED: Culture on basement membrane does not reverse the phenotype of lens derived mesenchyme-like cells. Int J Dev Biol 1989, 33:487-490 [PubMed] [Google Scholar]
- 56.Timpl R: Macromolecular organization of basement membranes. Curr Opin Cell Biol 1996, 8:618-624 [DOI] [PubMed] [Google Scholar]
- 57.Reddy GK, Gunwar S, Kalluri R, Hudson BG, Noelken ME: Structure and composition of type IV collagen of bovine aorta. Biochim Biophys Acta 1993, 1157:241-251 [DOI] [PubMed] [Google Scholar]
- 58.Netzer KO, Suzuki K, Itoh Y, Hudson BG, Khalifah RG: Comparative analysis of the noncollagenous NC1 domain of type IV collagen: identification of structural features important for assembly, function, and pathogenesis. Protein Sci 1998, 7:1340-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwan KM, Pang MK, Zhou S, Cowan SK, Kong RY, Pfordte T, Olsen BR, Sillence DO, Tam PP, Cheah KS: Abnormal compartmentalization of cartilage matrix components in mice lacking collagen X: implications for function. J Cell Biol 1997, 136:459-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan D, Freddi S, Weng YM, Bateman JF: Interaction of collagen alpha1(X) containing engineered NC1 mutations with normal alpha1(X) in vitro. Implications for the molecular basis of schmid metaphyseal chondrodysplasia. J Biol Chem 1999, 274:13091-13097 [DOI] [PubMed] [Google Scholar]
- 61.Chan D, Ho MS, Cheah KS: Aberrrant signal peptide cleavage of collage x in Schmid metaphyseal chondrodysplasia: implications for the molecular basis of the disease. J Biol Chem 2001, 276:7992-7997 [DOI] [PubMed] [Google Scholar]
- 62.Okada H, Inoue T, Suzuki H, Strutz F, Neilson EG: Epithelial-mesenchymal transformation of renal tubular epithelial cells in vitro and in vivo. Nephrol Dial Transplant 2000, 15:44-46 [DOI] [PubMed] [Google Scholar]
- 63.Ng YY, Huang TP, Yang WC, Chen ZP, Yang AH, Mu W, Nikolic-Paterson DJ, Atkins RC, Lan HY: Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats. Kidney Int 1998, 54:864-876 [DOI] [PubMed] [Google Scholar]
- 64.Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY: Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Kidney Dis 2001, 37:820-831 [DOI] [PubMed] [Google Scholar]
- 65.Frisch SM: Anoikis Methods Enzymol 2000, 322:472-479 [DOI] [PubMed] [Google Scholar]
- 66.Aoudjit F, Vuori K: Matrix attachment regulates fas-induced apoptosis in endothelial cells. A role for c-flip and implications for anoikis. J Cell Biol 2001, 152:633-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuncio GS, Alvarez R, Li S, Killen PD, Neilson EG: Transforming growth factor-beta modulation of the alpha 1(IV) collagen gene in murine proximal tubular cells. Am J Physiol 1996, 271:F120-F125 [DOI] [PubMed] [Google Scholar]
- 68.Orphanides C, Fine LG, Norman JT: Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int 1997, 52:637-647 [DOI] [PubMed] [Google Scholar]
- 69.Wolf G, Kalluri R, Ziyadeh FN, Neilson EG, Stahl RA: Angiotensin II induces alpha3(IV) collagen expression in cultured murine proximal tubular cells. Proc Assoc Am Physicians 1999, 111:357-364 [DOI] [PubMed] [Google Scholar]
- 70.Wahl SM, Allen JB, Weeks BS, Wong HL, Klotman PE: Transforming growth factor beta enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci USA 1993, 90:4577-4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarret Y, Woodley DT, Goldberg GS, Kronberger A, Wynn KC: Constitutive synthesis of a 92-kDa keratinocyte-derived type IV collagenase is enhanced by type I collagen and decreased by type IV collagen matrices. J Invest Dermatol 1992, 99:836-841 [DOI] [PubMed] [Google Scholar]
- 72.Xia Y, Garcia G, Chen S, Wilson CB, Feng L: Cloning of rat 92-kDa type IV collagenase and expression of an active recombinant catalytic domain. FEBS Lett 1996, 382:285-288 [DOI] [PubMed] [Google Scholar]