Abstract
The myosin superfamily of molecular motor proteins includes conventional myosins and several classes of unconventional myosins. Recent studies have characterized the human and mouse unconventional myosin XVA, which has a role in the formation and/or maintenance of the unique actin-rich structures of inner ear sensory hair cells. Myosin XVA is also highly expressed in human anterior pituitary cells. In this study we examined the distribution of myosin XVA protein and mRNA in normal and neoplastic human pituitaries and other neuroendocrine cells and tumors. Myosin XVA was expressed in all types of normal anterior pituitary cells and pituitary tumors and in other neuroendocrine cells and tumors including those of the adrenal medulla, parathyroid, and pancreatic islets. Most nonneuroendocrine tissues examined including liver cells were negative for myosin XVA protein and mRNA, although the distal and proximal tubules of normal kidneys showed moderate immunoreactivity for myosin XVA. Ultrastructural immunohistochemistry localized myosin XVA in association with secretory granules of human anterior pituitary cells and human pituitary tumors. These data suggest that in neuroendocrine cells myosin XVA may have a role in secretory granule movement and/or secretion.
Myosins are molecular motors that hydrolyze ATP to produce force and movement along actin filaments. The myosin superfamily includes conventional class II myosins and at least 16 classes of unconventional myosins, based on the degree of sequence divergence of the motor domain. 1,2 Unconventional myosins have functions that include transportation of intracellular organelles, phagocytosis, endocytosis, secretion, muscular contraction, and cellular movement. 1-8 The myosin family members share similar structural organization consisting of a motor domain, a neck region with myosin light chain-binding sites (IQ motifs), and a tail domain that varies in length and sequence among myosin family classes. 1,2,4
Myosin XVA (accession no. NM_016239) is a large protein (∼395 kd) consisting of a unique ∼1200 amino acid N-terminal domain preceding the motor and 1631 amino acids in the tail domain. 9 We reported that mutations linked to the human DFNB3 locus (MIM 600316) chromosome 17p11.2 is associated with profound congenital deafness. Mutations of myosin XVA are responsible for DFNB3 and the shaker 2 phenotype. 10-14 Recent light microscopic studies on the characterization of the human and mouse unconventional myosin XVA genes in the auditory system suggest a role for myosin XVA protein in the formation or maintenance of stereocilia, unique actin-rich structures of inner ear sensory hair cells. 9 Surprisingly, myosin XVA was found to be expressed at high levels in the anterior pituitary glands of humans and mice. 9 However, no obvious pituitary-related phenotype has been yet found in deaf individuals homozygous for mutant alleles of myosin XVA.
Previous studies examined myosins in the pituitary gland by cell fractionation methods. 15,16 Analysis of myosins in secretory tissues including the pituitary showed that the myosin that was present could not be accounted for by the myosin protein in the vascular structures of smooth muscle in these tissues. 15 Isolation of myosin from the GH3 pituitary cell line reinforced this observation. 16 Myosin-like substances have also been found in the neurosecretory synaptic vesicles in the brain. 17
To begin an exploration of the function of myosin XVA in the pituitary, we studied the distribution of myosin XVA in normal and neoplastic human pituitary tissues and other tissues by immunohistochemistry (IHC) and in situ hybridization. Ultrastructural immunolocalization was used for the subcellular localization of myosin XVA in human pituitary cells. These results suggest an important role for myosin XVA in neuroendocrine granule intracytoplasmic movement and/or secretion.
Materials and Methods
Tissues
Normal pituitary tissues (four cases) were obtained within 6 hours postmortem from patients who did not have any endocrine diseases. Pituitary adenomas (22 cases) were obtained from surgically resected tumors. All tissues were fixed in phosphate-buffered formalin, pH 7.4, and embedded in paraffin. Five-μm sections were cut on positively charged slides and used for IHC and in situ hybridization. In addition, normal (n = 18) and neoplastic endocrine tissues (n = 25) and other nonendocrine tissues (n = 10) were also analyzed.
Antibodies
PB78 and PB48 antisera were produced in rabbits against synthetic peptides synthesized by Princeton Biomolecules (Langhorne, PA) designed from conserved regions of mouse myosin XVA corresponding to amino acid residues 547 to 575 (GFGPEFGHPTPRPATSLARFLKKTLSEKK) from the N-terminal extension and residue 2379 to 2402 (CGDADLEKPTAIAYRMKGGGQPGG) 9 from the tail region of myosin XVA, respectively (accession no. Q9QZZ4). Each of the antisera was affinity-purified on a Pierce AminoLink column (Rockford, IL) to which the peptides were attached via an amino terminal cysteine.
Polyclonal antibodies to human pituitary hormones (Rockford, IL) were obtained from the National Pituitary Distribution, Agency, Bethesda, MD, and used as previously described. 18 Normal human anterior pituitary was used as a positive control tissue. Monoclonal antibodies against human growth hormone (GH) and human prolactin (PRL) were obtained from Biogenex, San Ramon, CA. Monoclonal antibodies against human luteinizing hormone (LH), thyroid-stimulating hormone, and adrenocorticotropic hormone (ACTH) were obtained from DAKO, Carpinteria, CA. The dilutions of the antibodies for visualization with the diaminobenzidine peroxidase or the alkaline-phosphatase systems were ACTH, 1/800 or 1/1600; GH, 1/400 or 1/800; PRL, 1/400 or 1/800; thyroid-stimulating hormone, 1/2000 or 1/4000; and LH, 1/800 or 1/1600.
Probes
The myosin XVA cDNA, containing 1316 bp of the myosin XVA tail region, (6906 to 8222 nucleotides, amino acids 2190 to 2,628; accession no. NM_016239) was cloned into pGEM-T Easy vector with reverse orientation. The in vitro transcription and digoxigenin 11-UTP (Boehringer Mannheim, Indianapolis, IN) labeling were performed with T7 RNA polymerase, provided in the riboprobe-labeling kit following the manufacturer’s instruction (Promega, Madison, WI). The transcription reaction produced either antisense or sense RNA probe depending on the orientation of the cloned insert. The labeled probes were digested with deoxyribonuclease, extracted with phenol/chloroform, and precipitated with ethanol.
Immunohistochemistry
Single IHC staining and double IHC localization were performed. The PB48 antibody to myosin XVA was used at a 1/2000 dilution and the PB78 antibody at 1/1000 dilution. IHC was done as previously described. 18,19 The avidin-biotin-peroxidase and avidin-biotin alkaline-phosphatase reagents for IHC were from Vector Laboratories (Burlingame, CA).
The specificity of the PB48 and PB78 antibodies was examined by absorption with 10 μg/ml of purified antigen. Staining with the absorbed antibody resulted in no staining of tissues. Another control for IHC was substituting normal serum for co-localization of myosin XVA and pituitary hormones. The polyclonal PB48 antibody and monoclonal pituitary hormone antibodies were used for combined IHC by either peroxidase/diaminobenzidine or alkaline-phosphatase/NBT and BCIP detection systems.
In Situ Hybridization
In situ hybridization was done as previously described. 18,19 Briefly after deparaffinizing, sections were placed in an 800-mW microwave oven in 10 mmol/L citrate buffer, pH 6.0, 18,19 and proteinase K, followed by prehybridization (as previously reported). Hybridization was done at 50°C for 16 hours followed by washing in sodium citrate, sodium chloride buffer. Sections were then reacted with anti-digoxigenin linked to alkaline phosphatase followed by nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3 indolyl phosphate (BCIP).
Combined Immunohistochemistry and In Situ Hybridization
For combined in situ hybridization and IHC slides were hybridized for in situ hybridization with the myosin XVA riboprobe followed by IHC with antibodies to pituitary hormones after the in situ hybridization procedure was completed. The digoxigenin-alkaline phosphatase reagents with NBT/BCIP was used for in situ hybridization while avidin-biotin-peroxidase reagents with diaminobenzidine were used for immunostaining.
Ultrastructural Immunohistochemistry
Human pituitary specimens from 19 cases were examined. Three were normal (nontumorous) anterior pituitary removed in association with an adenoma. Based on immunohistochemical and ultrastructural studies, the samples included adenomas, as previously classified by Horvath and Kovacs, 20 four GH (two sparsely and two densely granulated), three PRL, three ACTH, three thyroid-stimulating hormone, three follicle-stimulating hormone/LH, and three oncocytic null cell adenomas.
All specimens were fixed in 2.5% glutaraldehyde in Sorensen’s phosphate buffer (pH 7.4). Portions of the tissues were postfixed in 1% osmium tetroxide, whereas other portions remained unosmified. After thorough washing in Sorensen’s buffer, the samples were dehydrated in a graded ethanol series, embedded in an Epon-Araldite mixture, and investigated by transmission electron microscopy.
The postembedding double-immunogold-labeling technique was used for the simultaneous detection of myosin XVA and adenohypophysial hormones 20-22 . One side of the grids was incubated at 37°C for 24 hours with specific antisera directed toward human myosin XVA with antibody PB48 at a dilution 1:10. Subsequently, the grids were treated at 37° for 1 hour with biotinylated goat anti-rabbit IgG (Sigma-Aldrich Ltd., St. Louis, MO) diluted 1:100; the grids were subsequently treated for 60 minutes in 10-nm streptavidin gold complex (Nanoprobes Inc., Stony Brook, NY) diluted 1:20. Between each step, grids were washed in 0.2 mol/L phosphate-buffered saline (PBS) (pH 7.5) admixed with 0.2% cold water fish gelatin (Sigma-Aldrich Ltd.). For double immunostaining, the other side of the grid was labeled by the postembedding immunogold-labeling technique of Roth 21,22 using antisera directed against adenohypophysial hormones. Subsequently, the grids were treated at 37° for 1 hour with gold-labeled, goat anti-rabbit IgG (Sigma-Aldrich Ltd.); the gold particle used measured 20 nm in diameter. After immunolabeling, sections were stained with uranyl acetate and examined on a Philips 410 LS electron microscope.
To test for specificity, three control procedures were used successively with each immunolabeling procedure: 1) the specific primary antibody was replaced with the antibody diluent (0.2 mol/L PBS admixed with 0.2% cold water fish gelatin); 2) the specific primary antibody was substituted by normal rabbit serum; and 3) preabsorption of the specific polyclonal antiserum with homologous and heterologous antigens. Absorption tests were performed as described previously. 23,24
To detect myosin XVA in tumorous pituitary a simple immunolabeling procedure using the previous described streptavidin-biotin-gold complex method was used. 23
Analysis of Staining
The IHC and in situ hybridization staining was graded on a scale of 0 to 3 as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, strong staining.
Results
Immunohistochemistry
IHC staining with antisera directed to the tail (PB48) localized myosin XVA in most cells in the anterior pituitary (Figure 1, A to D) ▶ . There was variable staining in the different cell types. Combined IHC staining for pituitary hormones and myosin XVA showed the most intense staining in ACTH, PRL, and glycoprotein hormone-producing cells with slightly less intense staining in GH cells (Table 1) ▶ . The posterior pituitary showed weak staining (1+) for myosin XVA (not shown). IHC analysis of pituitary adenomas showed a similar distribution of staining in different anterior pituitary cell types with ACTH, PRL, and glycoprotein hormone-producing cells showing the strongest staining (Table 1 ▶ and Figure 1E ▶ ). A similar distribution of immunostaining with the PB78 antibody to an epitope in the N-terminal extension was seen in the normal and neoplastic pituitaries (Figure 1) ▶ . Liver tissue was negative for myosin XVA, but the renal tubules stained positively with moderate immunoreactivity (2+), while the renal glomeruli were negative for myosin XVA (not shown). The specificity of both antibodies was confirmed by absorption studies with purified peptides (Figure 1B) ▶ .
Figure 1.
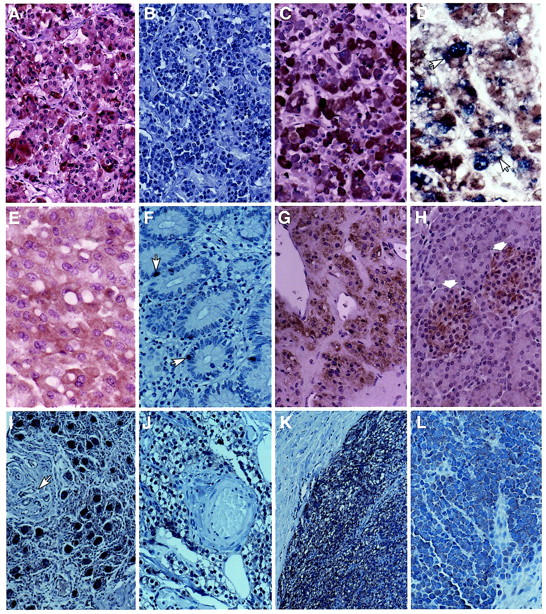
Immunohistochemical localization of myosin XVA in neuroendocrine tissues. A: Normal anterior pituitary staining strongly (3+) with antibody PB48 in most cells. B: Preabsorption of the antibody with 10 μg/ml purified antigen abolished staining. C: Antibody PB78 also produced strong (3+) staining in normal anterior pituitary cells. D: Combined staining with anti-GH antibody (blue) and myosin XV showed localization of myosin XVA in some GH-producing cell (arrows). E: ACTH adenoma showing diffuse positive staining with PB78. F: Normal endocrine cells (arrows) in the ileum are positive for myosin XV with the PB48 antibody. G: The adrenal medulla shows moderate positive staining (2+) for myosin XVA. H: The islet cells (arrows) are positive (1+) for myosin XVA and the exocrine pancreas is negative. I: The ganglion cells from the retroperitoneum are strongly positive (3+) for myosin XVA. The blood vessel and connective tissues (arrows) are negative. J: Normal parathyroid gland tissue shows positive staining (1+) for myosin XVA. K: Parathyroid adenoma showing strong positive staining (3+) with PB48 while the adjacent fibrous connective tissue is negative. L: Merkel cell carcinoma showing positive cytoplasmic staining (1+) for myosin XVA. Original magnifications: ×250 (A–D, F–L), ×300 (E).
Table 1.
Immunohistochemical and in Situ Hybridization Analysis of Normal and Neoplastic Human Pituitaries for Myosin XVA
| Tissue | Immunohisto- chemistry* | In situ hybridization* |
|---|---|---|
| Normal pituitary cells | ||
| PRL | 2+ | 2+ |
| GH | 1+ | 1+ |
| ACTH | 3+ | 3+ |
| LH/FSH | 2+ | 2+ |
| TSH | 2+ | 2+ |
| Pituitary adenomas | ||
| PRL | 2+ | 3+ |
| GH | 1+ | 2+ |
| ACTH | 3+ | 3+ |
| LH/FSH | 2+ | 2+ |
| TSH | 2+ | 2+ |
| Null cell | 2+ | 2+ |
*Staining intensity: 1+, weakly positive; 2+, moderately positive; 3+, strongly positive.
PRL, prolactin; GH, growth hormone; ACTH, adrenocorticotropic hormone; LH/FSH, luteinizing hormone/follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
Four normal anterior pituitaries and 22 adenomas were examined. Immunohistochemical staining results for PB48 are shown. Similar results were obtained with antibody PB78.
Immunostaining of normal neuroendocrine cells including small bowel, gastric, adrenal medulla, paraganglionic tissue, and pancreatic islets showed positive immunoreactivity with both PB48 and PB78 antibodies (Table 2 ▶ and Figure 1 ▶ ; F to J). The staining intensity was variable, but was generally less than in the normal anterior pituitary (1 to 2+). The adrenal cortex, exocrine pancreas, blood vessels, nerves, stromal cells, and liver were negative for myosin XVA. Neuroendocrine tumors showed weak to moderate immunoreactivity for myosin XVA (Table 2 ▶ and Figure 1, K and L ▶ ).
Table 2.
Immunohistochemical Staining of Normal and Neoplastic Human Endocrine Tissues for Myosin XVA
| Normal cell type | n* | Neuroendocrine tumors | n* |
|---|---|---|---|
| Pituitary† | 4 | Pituitary adenoma† | 22 |
| Adrenal medulla | 3 | Parathyroid adenoma | 3 |
| Pancreatic islet | 5 | Paraganglioma | 5 |
| Parathyroid | 3 | Carcinoid tumors–small intestine | 3 |
| Endocrine cells, small intestine | 3 | Lung small cell carcinoma | 2 |
| Endocrine cells, stomach | 2 | Islet cell tumor | 4 |
| Paraganglionic tissue | 2 | Medullary thyroid carcinoma | 4 |
| Lung carcinoid | 2 | ||
| Merkel cell carcinoma | 2 |
*n, Number of cases examined. These cases were all positive for myosin XVA by immunohistochemistry.
†Data for normal pituitary and pituitary adenomas are summarized in greater detail in Table 1.
In Situ Hybridization
Detection of myosin XVA mRNA by in situ hybridization showed strong staining in most normal anterior pituitary cells and in pituitary adenomas. (Figure 2 ▶ ; A to C). There was no staining with the sense probe (Figure 2B) ▶ . In situ hybridization with liver tissue was also negative with the anti-sense probe. Localization of myosin XVA mRNA and pituitary hormone proteins in the same section showed the strongest staining in ACTH, PRL, and glycoprotein hormone-producing cells (not shown).
Figure 2.

In situ hybridization localization of myosin XVA mRNA in neuroendocrine tissues. A: The anterior pituitary cells are diffusely positive (3+) for myosin XVA mRNA with the antisense probe. B: The sense probe is negative in the pituitary cell. C: A PRL-producing adenoma shows strong positive staining (3+) for myosin XVA mRNA. D: The adrenal medulla (arrows) is moderately positive (2+) for myosin XVA mRNA whereas the adjacent cortex shows some background staining. E: The pancreatic islet cells (arrows) are weakly positive (1+) for myosin XVA mRNA. F: The normal liver tissue is negative for myosin XVA mRNA. Original magnifications: ×250 (A–C, E), ×200 (D).
Myosin XVA mRNA was also detected in a small number of cases (n = 8) of normal and neoplastic neuroendocrine cells and tumors analyzed including adrenal medulla (Figure 2D) ▶ , and pancreatic islet cells (Figure 2E) ▶ . In situ hybridization also showed myosin XVA in renal tubular cells, but not in hepatocytes (Figure 2F) ▶ , adrenal cortex, blood vessel wall, or nerves.
Ultrastructure
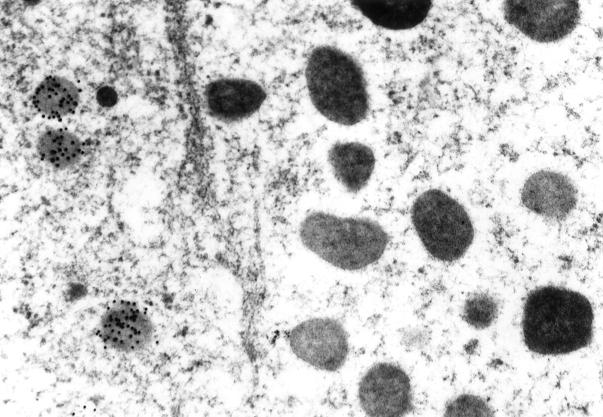
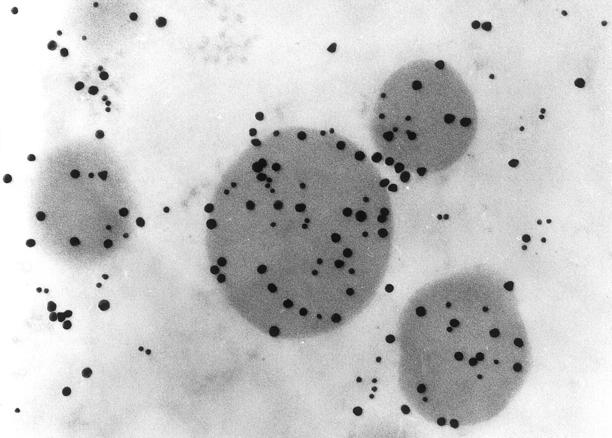
Ultrastructural immunolabeling with colloidal gold particles of 10 nm and 20 nm in normal and neoplastic human pituitaries showed labeling of the secretory granules of anterior pituitary cells for myosin XVA (Figures 3 to 5) ▶ ▶ ▶ . The ACTH and GH cells were the most frequent cell types labeled (Figures 4 and 5) ▶ ▶ . In these cell types, most of the secretory granules were labeled for myosin XVA. There were less secretory granules with myosin XVA in pituitary adenoma cells compared to the normal anterior pituitary cells with an estimate of 70% of the total GH secretory granules and 40% of the total ACTH secretory granules labeled.
Figure 3.
Myosin XVA ultrastructural localization was done to identify the subcellular localization of this protein with 10-nm colloidal gold particles. Localization of myosin XVA in the secretory granule of a pituitary cell on the left is shown while the secretory granules in an adjacent cell on the right are negative (original magnification, ×53,000).
Figure 4.
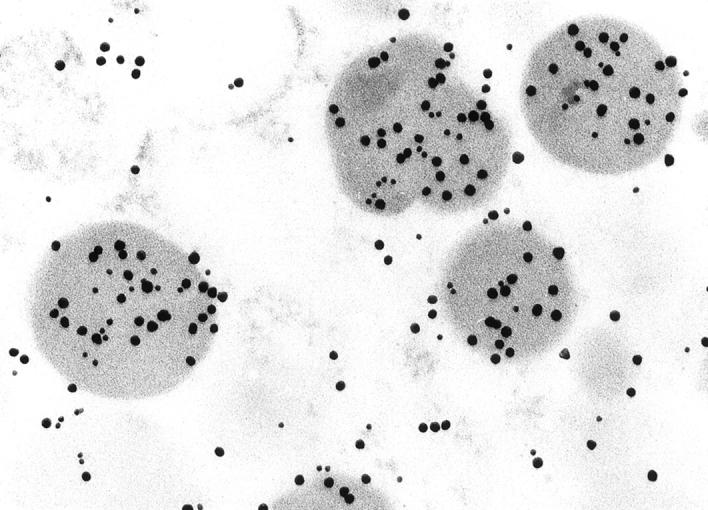
To determine the subcellular location of myosin XVA ultrastructural localization to characterize the specific pituitary cell type was done with colloidal gold particles of two sizes. 20 Double localization of ACTH (20-nm gold particles) and myosin XVA (10 nm) in a normal human pituitary showing both colloidal gold particles in the same secretory granules (original magnification, ×127,500).
Figure 5.
Double localization of GH (20-nm gold particles) and myosin XVA (10-nm gold particles) in the same secretory granule in a pituitary adenoma (original magnification, ×132,000).
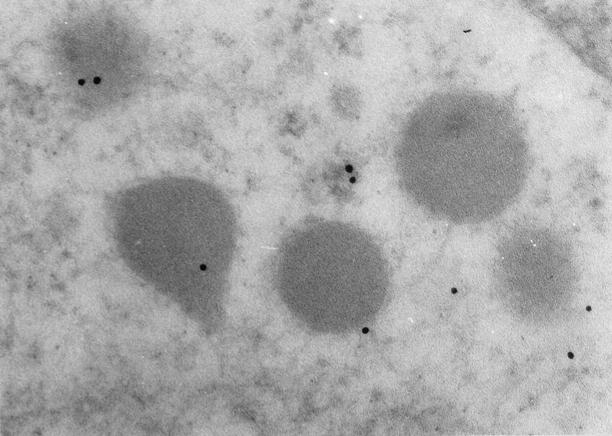
Negative controls including preabsorption of myosin XVA with purified antigen, substitution of primary antibody with PBS, and with normal rabbit serum resulted in a significant decrease in the labeling of secretory granule for myosin XVA confirming the specificity of the immunolabeling (Figure 6) ▶ .
Figure 6.
Negative control in which the PB48 primary antiserum was absorbed with purified antigen resulted in significantly reduced labeling (original magnification, ×120,000).
Discussion
Recent studies on the characterization of the human and mouse unconventional myosin XVA genes responsible for hereditary congenital, profound deafness at the human DFNB3 locus on chromosome 17p11.2 and in shaker 2 indicated that myosin XVA mRNA and protein were localized in the inner and outer hair cells of the inner ear as well as in anterior pituitary cells. 9 The present study extended these earlier findings by showing for the first time that myosin XVA was associated with the secretory granules of normal and neoplastic pituitaries. Myosin XVA mRNA and protein were widely distributed in normal anterior pituitary gland cells and in pituitary adenomas as well as in other neuroendocrine cells and tumors. The detection of myosin XVA protein with two different antibodies as well as myosin XVA mRNA by in situ hybridization in pituitary and other neuroendocrine tissues supports the specificity of myosin XVA localizations.
Myosin XVA seems to be associated with secretory granules in the pituitary (Figures 3 to 5) ▶ ▶ ▶ . Although all anterior pituitary cell types expressed myosin XVA by IHC and in situ hybridization at the light microscope level, by ultrastructural labeling only the secretory granules of ACTH and GH cells were labeled. This probably reflects the lower degree of sensitivity of the ultrastructural labeling. Earlier studies of myosins in secretory tissues by cell fractionation showed that myosins were present in the cytoplasm, although the class of myosin was unknown. 16 Specific myosin ATPase activity measured in 0.6 mol/L/KCl was present in these secretory tissues. Although the function of myosin in secretory tissue is not known, recent studies have shown that myosin light chain kinase (MLCK) stimulated exocytosis and that secretion in these cells was associated with dephosphorylation of myosin light chain by MLCK and phosphorylation by protein kinase C. 25,26 In a study by Rao and colleagues 25 cultured rat pituitary cells were shown to increase cytosolic calcium concentration and LH secretion by activation of gonadotropin hormone-releasing hormone (GnRH) receptors. Treatment of the pituitary cells with wortmannin, which inhibits MLCK, led to an attenuation of GnRH-induced LH release. These observations and other experiments suggest that pituitary hormone exocytosis was dependent on phosphorylation of nonmuscle myosin II B light chains by MLCK. 25 Similarly in bovine anterior pituitary there was an enrichment of MLCK and other calmodulin-binding proteins in the pituitary secretory granule membrane fractions suggesting a role of calmodulin and calmodulin-binding proteins in granule membrane function and possibly in exocytosis. 27
Earlier studies showed that actin filaments that are intimately associated with myosins were localized in the cytoplasm of pituitary cells beneath the plasma membrane, whereas some actin filaments were associated with the intracellular transport of the secretory granules. 28 The actin filaments localized in the peripheral cytoplasmic matrix were thought to control the approach of secretory granules to the plasma membrane and their release. 28 Immunofluorescent studies combined with confocal microscopy of actin, α-actinin, and myosin-like immunoreactivities in pituitary cells localized these filaments along stress fiber-like structures in cultured pituitary cells. 27 Differences in the localization of cytoskeletal proteins between cells in vivo and in vitro were observed suggesting that the substrate on which the cells are growing may influence cytoskeletal protein expression. 29
Myosin-like substances have been detected in platelet membranes 30 and in fibroblast plasma membranes 31 as well as in the neurosecretory synaptic vesicles in the brain. 17 Myosin V has also been found associated with melanosomes in mouse melanocytes implicating this myosin as an organelle motor for the outward movement of melanosomes within dendritic extensions. 5 Although these structures may not be completely analogous to the pituitary secretory granules, they may have similar functions such as cellular movement and/or secretion.
The presence of myosin XVA in many normal and neoplastic endocrine tissues raises the possibility that myosin XVA may be a useful marker for the characterization of neuroendocrine cells and tumors similarly to chromogranin A. 32,33 The use of myosin XVA as a diagnostic marker would be more restricted, because myosin XVA, unlike chromogranin, is also present in some nonneuroendocrine tissues, such as inner ear and renal tubular cells. Myosin VIIa and myosin VI are also expressed in renal tubules 34,35 suggesting that these myosins may have important functions in renal tubular cells.
At present, we cannot definitely rule out the possibility that our two different antisera to myosin XVA described in this report may be cross-reacting with a related novel unconventional myosin in neuroendocrine granules and in renal tubules. However, several different antisera to three different unique regions of myosin XVA consistently give similar results. 9 A related concern is the possibility that there is a second member of the class XV myosins encoding a protein sufficiently similar to myosin XVA at 17p11.2 that antisera would cross-react. With the sequences of the human genome primarily completed, indeed we identified genomic sequence and cDNA clones for a second class XV myosin gene located on chromosome 17q25. However, a detailed analysis of the sequence of myosin XVB cDNAs and genomic sequence (E. Boger, unpublished data) indicates that there are many in-frame translation stop codons and thus it is a transcribed pseudogene now designated myosin XVB (putative pseudogene) by the HUGO Nomenclature committee.
In summary, these studies have localized myosin XVA to secretory granules of normal and neoplastic human anterior pituitary cells. The presence of the unconventional myosin XVA protein associated with secretory granules of anterior pituitary cells as well as localization of myosin XVA immunoreactivity and mRNA in other neuroendocrine cells and tumors strongly suggests that this unconventional myosin may have an important role in secretory granule movement and/or secretion within the cytoplasm of neuroendocrine cells.
Acknowledgments
We thank the National Pituitary Distribution Agency for the antibodies to pituitary hormones used in this study.
Footnotes
Address reprint requests to Dr. Ricardo V. Lloyd, Laboratory Medicine and Pathology, Mayo Foundation, Rochester, MN 55905. E-mail: lloyd.ricardo@mayo.edu.
Supported in part by the National Institute on Deafness and Other Communication Disorders Intramural Research Projects 201 DC00035-04 and by National Institutes of Health grants CA 90249 and a grant from the Jarislowky Foundation.
Current address of R. A. F.: Virology Department, Bristol-Myers Squibb, 5 Research Parkway, Wallingford, CT 06492.
References
- 1.Yamashita RA, Sellers JR, Anderson JB: Identification and analysis of the myosin superfamily in Drosophila: a database approach. J Muscle Res Cell Motil 2000, 21:491-505 [DOI] [PubMed] [Google Scholar]
- 2.Sellers JR: Myosins. A diverse superfamily 2. Biochim Biophys Acta 2000, 1496:3-22 [DOI] [PubMed] [Google Scholar]
- 3.Cheney RE, Riley MA, Mooseker MS: Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton 1993, 24:215-223 [DOI] [PubMed] [Google Scholar]
- 4.Mooseker MS, Cheney RE: Unconventional myosins. Annu Rev Cell Dev Biol 1995, 11:633-675 [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Bowers B, Wei Q, Kocher B, Hammer JA, III: Myosin V associates with melanosomes in mouse melanocytes: evidence that myosin V is an organelle motor. J Cell Science 1997, 110:847-859 [DOI] [PubMed] [Google Scholar]
- 6.Catlett NL, Weisman LS: The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc Natl Acad Sci USA 1998, 95:14799-14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cope MJT, Witsstock J, Rayment I, Kendrick Jones J: Conservation within the myosin motor domain: implications for structure and function. Structure 1996, 4:969-987 [DOI] [PubMed] [Google Scholar]
- 8.Mermall V, Post PL, Mooseker MS: Unconventional myosins in cell movement, membrane traffic and signal transduction. Science 1998, 279:527-533 [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Wang A, Belyantseva IA, Anderson DW, Probst FJ, Barber TD, Miller W, Touchman JW, Jin L, Sullivan SL, Sellers JR, Camper SA, Lloyd RV, Kachar B, Friedman TB, Fridell RA: Characterization of the human and mouse unconventional myosin XV genes responsible for hereditary deafness DFNB3 and shaker 2. Genomics 1999, 61:243-258 [DOI] [PubMed] [Google Scholar]
- 10.Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, Friedman TB, Camper SA: Correction of deafness in shaker 2 mice by an unconventional myosin in a BAC transgene. Science 1998, 280:1444-1447 [DOI] [PubMed] [Google Scholar]
- 11.Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB: Association of unconventional myosin MYO 15 mutations with human nonsyndromic deafness DFNB3. Science 1998, 280:1447-1451 [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi Y, Kikkawa Y, Matsumoto Y, Shinbo T, Kosugi S, Chou D, Furuya M, Jishage K, Noda T, Yonekawa H, Kominami R: Genetic and physical delineation of the region of the mouse deafness mutation shaker-2. Biochem Biophys Res Commun 1997, 234:107-110 [DOI] [PubMed] [Google Scholar]
- 13.Willems PJ: Genetic causes of hearing loss. N Engl J Med 2000, 342:1101-1109 [DOI] [PubMed] [Google Scholar]
- 14.Friedman TB, Liang Y, Weber JL, Hinnant JT, Barber TD, Winata S, Arhya N, Asher AH, Jr: A gene for congenital, recessive deafness DFNB3 maps to the pericentromeric region of chromosome 17. Nat Genet 1995, 9:86-91 [DOI] [PubMed] [Google Scholar]
- 15.Ostlund RE, Pastan I: The purification and quantitation of myosin from cultured cells. Biochem Biophys Acta 1976, 453:37-47 [DOI] [PubMed] [Google Scholar]
- 16.Ostlund RE, Jr, Leung JT, Kipnis DM: Myosins of secretory tissues. J Cell Biol 1978, 77:827-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berl S, Puszkin S, Niklas WJ: Actomyosin-like protein in brain. Science 1973, 179:441-446 [DOI] [PubMed] [Google Scholar]
- 18.Lloyd RV, Jin L, Qian X, Scheithauer BW, Young WF, Jr, Davis DH: Analysis of the chromogranin A post-translation and cleavage product pancreastatin and the prohormone convertases PC2 and PC3 in normal and neoplastic human pituitaries. Am J Pathol 1995, 146:1188-1198 [PMC free article] [PubMed] [Google Scholar]
- 19.Qian X, Jin L, Grande JP, Lloyd RV: Transforming growth factor-β and p27 expression in pituitary cells. Endocrinology 1996, 137:3051-3060 [DOI] [PubMed] [Google Scholar]
- 20.Horvath E, Kovacs K: Ultrastructural diagnosis of pituitary adenomas and hyperplasia. Surgical pathology of the pituitary gland. Lloyd RV eds. Major Problems in Pathology, 1993, vol 27.:pp 52-84 W. B. Saunders, Co., Philadelphia [Google Scholar]
- 21.Roth J: Application of lectin-gold complexes for electron microscopic localization of glycoconjugates on thin sections. J Histochem Cytochem 1983, 31:987-999 [DOI] [PubMed] [Google Scholar]
- 22.Roth J: Light and electron microscopic localization of antigenic sites in tissue sections by the protein A-gold technique. Acta Histochem 1984, (Suppl)29:S9-S22 [PubMed] [Google Scholar]
- 23.Vidal S, Cohen SM, Horvath E, Kovacs K, Scheithauer BW, Burguera B, Lloyd RV: Subcellular localization of leptin in non-tumorous and adenomatous human pituitaries. An immuno-ultrastructural study. J Histochem Cytochem 2000, 48:1147-1152 [DOI] [PubMed] [Google Scholar]
- 24.Vidal S, Oliveira MC, Kovacs K, Scheithauer BW, Lloyd RV: Immunolocalization of vascular endothelial growth factor in the GH3 cell line. Cell Tissue Res 2000, 300:83-88 [DOI] [PubMed] [Google Scholar]
- 25.Rao K, Park W-Y, Zheng L, Jobin RM, Tomic M, Jiang H, Nakanishi S, Stojilkovic SS: Wortmannin-sensitive and-insensitive steps in calcium-controlled exocytosis in pituitary gonadotrophs evidence that myosin light chain kinase mediates calcium-dependent and wortmannin-sensitive gonadotropin secretion. Endocrinology 1997, 138:1440-1449 [DOI] [PubMed] [Google Scholar]
- 26.Ohara-Imaizumi M, Sakurai T, Nakamura S, Nakanishi S, Matsuda Y, Muramatsu S, Nonomura Y, Kumakura K: Inhibition of Ca2+-dependent catecholamine release by myosin light chain kinase inhibitor, wortmannin, in adrenal chromaffin cells. Biochem Biophys Res Commun 1992, 185:1016-1021 [DOI] [PubMed] [Google Scholar]
- 27.Nelson TY, Lorenson MY, Jacobs LS, Boyd AE, III: Distribution of calmodulin and calmodulin-binding proteins in bovine pituitary: association of myosin light chain kinase with pituitary secretory granule membranes. Mol Cell Biochem 1987, 74:83-94 [DOI] [PubMed] [Google Scholar]
- 28.Senda T, Fujita H, Ban T, Zhong C, Ishimura K, Kanda K, Sobue K: Ultrastructural and immunocytochemical studies on the cytoskeleton in the anterior pituitary of rats, with special regard to the relationship between actin filaments and secretory granules. Cell Tissue Res 1989, 258:25-30 [DOI] [PubMed] [Google Scholar]
- 29.Shimada O, Ishikawa H: Morphological methods for analyzing hormone secretory mechanism in the pituitary cells. Nippon Rinsho 1993, 51:2536-2543 [PubMed] [Google Scholar]
- 30.Booyse FM, Sternberger LA, Zschocke D, Rafelson ME: Ultrastructural localization of contractile protein (thrombosthenin) in human platelets using an unlabeled antibody-peroxidase staining technique. J Histochem Cytochem 1971, 19:540-550 [DOI] [PubMed] [Google Scholar]
- 31.Willingham MC, Ostlund RE, Pastan I: Myosin is a component of the cell surface of cultured cells. Proc Natl Acad Sci USA 1974, 71:4144-4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd RV, Wilson BS: Specific endocrine tissue marker defined by a monoclonal antibody. Science 1983, 222:628-630 [DOI] [PubMed] [Google Scholar]
- 33.Winkler H, Fischer-Colbri R: The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 1992, 49:497-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redowizs MJ: Myosins and deafness. J Muscle Res Cell Motil 1999, 20:241-248 [DOI] [PubMed] [Google Scholar]
- 35.Breckler J, Au K, Cheng J, Hasson T, Burnside B: Novel myosin VI isoform is abundantly expressed in retina. Exp Eye Res 2000, 70:121-134 [DOI] [PubMed] [Google Scholar]