Abstract
Urokinase-type plasminogen activator (uPA) is increased in human abdominal aortic aneurysm (AAA). Chronic infusion of angiotensin II (Ang II) results in AAA in apolipoprotein E-deficient mice. We tested the hypothesis that Ang II infusion results in an elevation of uPA expression contributing to aneurysm formation. Ang II or vehicle was infused by osmotic pumps into apoE-KO mice. All mice treated with Ang II developed a localized expansion of the suprarenal aorta (75% increase in outer diameter), accompanied by an elevation of blood pressure (22 mmHg), compared to the vehicle-treated group. Histological examination of the dilated aortic segment revealed similarities to human AAA including focal elastin fragmentation, macrophage infiltration, and intravascular hemorrhage. Ang II treatment resulted in a 13-fold increase in the expression of uPA mRNA in the AAA segment in contrast to a twofold increase in the atherosclerotic aortic arch. Increased uPA protein was detected in the abdominal aorta as early as 10 days after Ang II infusion before significant aorta expansion. Thus, Ang II infusion results in macrophage infiltration, increased uPA activity, and aneurysm formation in the abdominal aorta of apoE-KO mice. These data are consistent with a causal role for uPA in the pathogenesis of AAA.
Abdominal aortic aneurysm (AAA) is a chronic degenerative disease characterized by segmental weakening and dilation of the vascular wall. Recent estimates indicate that the prevalence of AAA is 4 to 9% in adults older than 65 years of age and is known to be associated with atherosclerosis, aging, hypertension, and cigarette smoking. Continued tissue remodeling results in silent expansion of the AAA with an increased risk of spontaneous rupture. Currently the only available treatments for AAAs are surgical resection and replacement or, more recently, insertion of an endovascular stent. The etiology of AAA is unclear. The extracellular matrix plays an essential role in maintaining the integrity of the vascular wall. Elastin and collagen fibers are the major components of this extracellular matrix. Both plasmin and matrix metalloproteinases (MMPs) are capable of degrading extracellular matrix, including collagen, elastin, and fibrin. Urokinase-type plasminogen activator (uPA) hydrolyzes plasminogen to form plasmin, which in turn activates MMPs. The in vivo activity of uPA is also regulated by local concentrations of its major inhibitor, PAI-1. Biochemical studies have demonstrated increased proteolytic activity in the aortic wall of AAA. Schneiderman and colleagues 1 showed that uPA mRNA as well as the tissue-type plasminogen activator (tPA), co-localized with infiltrating macrophages, is significantly increased in human AAA. Increased activities of MMP-2, -3, -9, and -12 in AAA have also been reported. 2-5 Atherosclerotic aortic lesions from high-cholesterol diet-fed apoE-KO mice show fragmentation of the elastic lamellae and rupture of the media resulting in pseudomicroaneurysm formation. These pathological changes are not observed in mice deficient in both apoE and uPA, 6 suggesting that uPA may play a key role in matrix destruction and aneurysm formation.
Recently, Daugherty and colleagues 7 reported that chronic infusion of angiotensin II (Ang II) induces AAA in 33% of female apoE-KO mice. The aneurysms were characterized by complex tissue remodeling in the adventitia similar to human disease. In addition, the aneurysmal tissue also showed exacerbated vascular inflammation, such as monocyte/macrophage infiltration in the vascular wall. Ang II has been shown to induce the expression of interleukin (IL)-6. 8-10 IL-6 is a marker of vascular inflammation and it has been shown to increase in atherosclerotic lesions in apoE-KO mice in association with the infiltrating macrophages. 11 In human AAAs, the circulating level of IL-6 has been reported to be elevated 12 and positively correlated with aortic diameter expansion. 13 Therefore, the aim of this study was to test the hypothesis that Ang II promotes vascular inflammation that in turn activates the uPA-plasmin-MMP system, thus causing aneurysm in apoE-KO mice.
Materials and Methods
Animal Preparation
Osmotic minipumps (Alzet, model 2004, Palo Alto, CA) containing either phosphate-buffered saline (PBS) or Ang II (1.44 mg/kg/day) were implanted subcutaneously in 6-month-old male apoE-KO mice. After 30 days, blood pressure was measured noninvasively by the tail-cuff method. The diameter of the abdominal aorta was measured noninvasively by miniaturized ultrasound, and validated postmortem by direct measurement of cross sections of the suprarenal aorta. Serum cholesterol and triglycerides levels were measured by Consolidated Veterinary Diagnostics (West Sacramento, CA).
Noninvasive Measurement of Systolic Blood Pressure
Systolic blood pressure and heart rate were measured in conscious mice using a tail-cuff system (Kent Scientific, Litchfield, CT). Mice were trained to lie quietly in a restrainer placed on a warm pad for a period of at least 30 minutes for 1 to 4 days before the study. On the day of the study, the mice were placed in the temperature-controlled restrainer for 15 minutes. Blood pressure was then measured repeatedly and recorded on a data acquisition system (PowerLab, 16/s; ADInstruments, Australia). Systolic blood pressure and heart rate were averaged from consecutive five measurements.
Measurement of Aortic Diameter
Noninvasive Measurement by Ultrasound
A cardiovascular ultrasound system (GE Vingmed System FiVE, Fairfield, CT) and a linear transducer (10 MHz) were used to image the suprarenal aorta noninvasively in mice anesthetized with 2.5% isoflurane via an anesthesia machine (IMPAC6; VetEquip, Pleasanton, CA). A longitudinal view of the suprarenal aorta was obtained by sagittally scanning the abdomen with the transducer. Confirmation of the correct location of the aorta was achieved by pulsatile flow-velocity waveforms from the middle of the aortic chamber on the image. The segment with the maximum diameter and the adjacent segment of the suprarenal aorta were located on the longitudinal image. The two cross-sections were scanned transversely to obtain images for measurement of the luminal diameters of the aneurysmal and adjacent portions of the suprarenal aorta. The images were stored digitally and analyzed with an on board computer with a resolution of 0.01 mm.
Postmortem Direct Measurement
Direct measurements from the suprarenal aorta were made histologically (as described in the next section). The lumen and adventitial circumferences at the maximal expended portion of the suprarenal aorta were quantified by CSimple Imaging Systems (Compix, Mars, PA) that was then used to calculate the luminal and outer diameters of the vessel. The wall thickness was calculated from the difference between the luminal and outer diameters.
Histopathological Examination
Tissue Preparation
At the end of each experiment, the aortae were perfused at a constant pressure of 100 mmHg through the heart with PBS followed by warm (37°C) agarose (SeaPlaque GTG Agarose, low-melt; FMC BioProducts, Rockland, ME) diluted in saline (3% w/v) and colored with a green tissue dye. After the agarose had solidified, the abdominal aorta was dissected free from the surrounding connective tissue and pinned onto a wax block before fixation in 10% formalin. Cross-sections of aorta (2.5 mm in thickness) were made between the superior mesenteric and right renal arteries. A small portion of the right renal artery was left attached to the samples to facilitate orientation of the specimen. These tissues were dehydrated through a graded ethanol series, cleared with xylene, infiltrated with warm paraffin, embedded in paraffin blocks, cut at 5-μm thickness, and stained with hematoxylin and eosin. Elastin and collagen were stained by the elastin-van Gieson method.
Quantification of Atherosclerotic Plaques in the Carotid Arteries
The left and right carotid arteries were dissected, cut open longitudinally, and pinned down individually on silicon-coated Petri dishes. Atherosclerotic plaques are visible without staining. The images of the open luminal surface of the vessels were captured with a digital camera (Sony, Japan) mounted on a dissecting microscope. The plaque area was quantified using C-Simple system (Compix, Mars, PA) and expressed as a percentage of the total luminal surface area as described in detail previously. 14
Recombinant Mouse uPA
Mouse uPA from amino acids 146 to 434 (with deletion of most of the A chain and cysteine 296 to serine mutation) was expressed in a BaculoGold Baculovirus system (Pharmingen, San Diego, CA). The expressed recombinant protein was purified by benzamidine-Sepharose affinity chromatography.
Determination of uPA, PAI-1, tPA, and MMP Expression
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Tissue and Total RNA Preparation: Mice were anesthetized in CO2/O2 and the entire aorta rapidly dissected from fat and connective tissues. The arch and abdominal aorta were separated, transferred to vials, and snap-frozen in liquid nitrogen. Tissues were homogenized with an Omni TH homogenizer (Omni International, Warrenton, VA) at 4°C in denaturing solution (Ambion, Austin, TX). Total RNA was isolated using a RNAaqueous-4 PCR kit from Ambion.
Primers and Probe Design: Primers and probes for the uPA, tPA, and PAI-1 genes were designed to recognize the mouse uPA, tPA, and PAI-1 sequences by using the computer program Primer Express (Perkin-Elmer Applied Biosystems, Foster City, CA). The oligonucleotide primers and TaqMan probes were purchased from Synthetic Genetics (San Diego, CA). The primers and a probe for the housekeeping gene, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were purchased from Perkin-Elmer as rodent GAPDH control reagent kits (vic-labeled probe). Specific uPA sequences for TaqMan primers and probe were forward primer: CGA TTC TGG AGG ACC GCT TA, reverse primer: CCA GCT CAC AAT CCC ACT CA, and probe CTG TAA CAT CGA AGG CCG CCC AAC T. Those for PAI-1 were forward primer: TGC ATC GCC TGC CAT T, reverse primer: CTT GAG ATA GGA CAG TGC TT, and probe: TGG CCC ATG GCA CCC TCC A. Those for tPA were forward primer: CAA CAG CGG CCT GGT ACA A, reverse primer: CCC CAT TGA AGC ATC TTG GTT, and probe: CTC AGT GCC TGT CCG AAG TTG CAG C.
Procedures: Reverse transcriptase (RT) generation of cDNA and PCR was performed by one-step RT-PCR using the TaqMan Gold RT-PCR kit (Perkin-Elmer Applied Biosystems, Foster City, CA). The thermal cycling parameters were: 30 minutes at 48°C for RT, AmpliTag Gold activation for 10 minutes at 95°C, and 40 cycles of PCR (denature for 15 seconds at 95°C and annealing/extension for 1 minute at 60°C). A standard curve was constructed with a 4-point dilution curve (0.3, 3, 30, 300 ng) of total RNA from mouse testis. A control without template was included with each PCR. Samples and standard curves were run in triplicate for each PCR experiment. The quality of the PCR data were determined from the correlation coefficient of the standard curve, and the data were then normalized to the mRNA level of the housekeeping gene, GAPDH.
Immunoblotting
Mouse AAA segments were pooled together (average of 10 mice) from each experimental group. Tissues were homogenized in PBS containing 0.1% Tween 20 (PBST) to extract proteins. Protein concentrations were determined by the method of Bradford using the Coomassie protein assay reagent from Pierce (Rockford, IL). Six hundred μg of protein sample was mixed with sample buffer (in a final concentration of 63 mmol/L Tris-HCl, 10% glycerol, and 2% sodium dodecyl sulfate) and separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred to a nitrocellulose membrane (BioRad, Hercules, CA) that was blocked with a 10% solution of nonfat dried milk in PBST for 1 hour at room temperature. The membrane was incubated with rabbit anti-rodent uPA antibody (1190, 5 μg/ml in PBST; American Diagnostica Inc., Greenwich, CT) overnight at 4°C, and washed three times with PBST. The membrane was subsequently incubated for 1 hour at room temperature with horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After washing four times with PBST, the immunoreactive protein bands were detected by using the enhanced chemiluminescence kit (Amersham, Piscataway, NJ).
Zymography
Protein extracts were prepared from aortic tissues as described above. Samples were resolved under nonreducing, denaturing conditions, on a 12% acrylamide gel (cast with 1% nonfat dry milk and 5 μg/ml human plasminogen) for uPA activity and on a 10% gel containing 0.1% gelatin as the substrate for MMP activity. After electrophoresis proteins were renatured by washing in Triton X-100. The gels were then incubated overnight at 37°C in 50 mmol/L Tris-HCl, 200 mmol/L NaCl, 5 mmol/L CaCl2. Zones of lysis were visualized by staining with 0.5% Coomassie blue R-250.
Ex Vivo Aorta Organ Culture and IL-6 Secretion Assay
The aortas from apoE-KO mice treated with vehicle (n = 6) or Ang II (n = 5) were dissected aseptically and cut into three segments: aortic arch, suprarenal aorta, and the rest of the abdominal aorta. The fresh aortic segments were immediately placed in 1 ml of Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Gaithersburg, MD) containing 1× ITS (insulin, transferrin, and selenium; Life Technologies, Inc.) and 0.1% bovine serum albumin (Sigma Chemical Co., St. Louis, MO). The secretion of IL-6 was measured at different time points by using a commercially available murine IL-6 enzyme-linked immunosorbent assay (Biotrak IL-6 ELISA kit; Amersham Life Sciences, Arlington Heights, IL) as described. 11 The IL-6 values were normalized to the weight of the aortic tissue segment and expressed as pg/ml per mg tissue.
Statistics
Results are presented as means ± SE for the number of animals (n) indicated. Comparison between two groups with different treatments was performed by Student’s t-test. The differences were considered statistical significant when the P value was less than 0.05.
Results
As shown in Table 1 ▶ , chronic infusion of Ang II in apoE-KO mice for 1 month did not significantly affect body weight, or cholesterol and triglyceride levels. However, systolic blood pressure was higher and the atherosclerotic plaque area in the carotid arteries was significantly greater in apoE-KO mice treated with Ang II compared to vehicle treatment.
Table 1.
Effects of Angiotensin II in ApoE-KO Mice
| Vehicle (n = 8) | Ang II (n = 13) | |
|---|---|---|
| Body weight (g) | 31 ± 1 | 32 ± 1 |
| Serum cholesterol (mg/dl) | 655 ± 63 | 615 ± 34 |
| Triglycerides (mg/dl) | 161 ± 20 | 140 ± 18 |
| Systolic blood pressure (mmHg) | 120 ± 3 | 142 ± 5* |
| Lesion area (%) | 5 ± 2 | 31 ± 4* |
*, P < 0.001, versus vehicle-treated apoE-KO mice.
Chronic Ang II Infusion Induced Abdominal Aneurysm in apoE-KO Mice
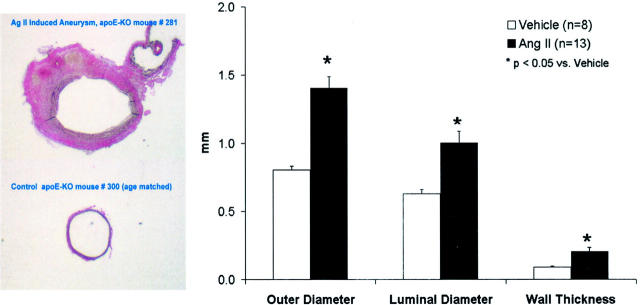
Chronic infusion of Ang II for 1 month resulted in development of AAA in all animals. Fifty-eight percent of the mice had large aneurysms (>100% expansion) that were consistently localized to the left lateral aspect of the suprarenal region of the aorta (Figure 1) ▶ . None of the vehicle-treated apoE-KO mice developed an aneurysm. Figure 2 ▶ shows an ultrasound image from a mouse treated with Ang II measured noninvasively. The longitudinal view shows the characteristic dilatation of the abdominal aorta in the suprarenal region (Figure 2 ▶ , top). The inner diameter of the aneurysm (Figure 2 ▶ , bottom left) was ∼2.5-fold larger than the adjacent nonaneurysmal portion measured in a cross-section (Figure 2 ▶ , bottom right). Direct measurements of histological sections of the suprarenal aorta of the apoE-KO mice treated with either Ang II or vehicle demonstrate that Ang II increased the outer diameter, inner diameter, and vascular wall thickness by 75%, 60%, and 129%, respectively (Figure 3) ▶ .
Figure 1.
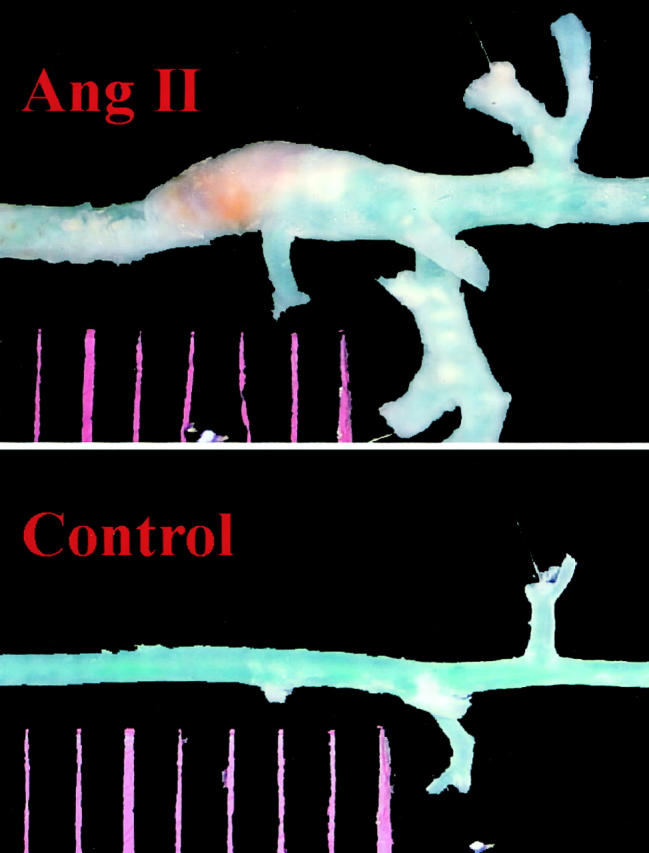
Representative pictures show that localized expansion of the abdominal aorta between the diaphragm and the renal artery was found only in mouse infused with Ang II for 1 month (top) but not in control animal infused with PBS (bottom).
Figure 2.
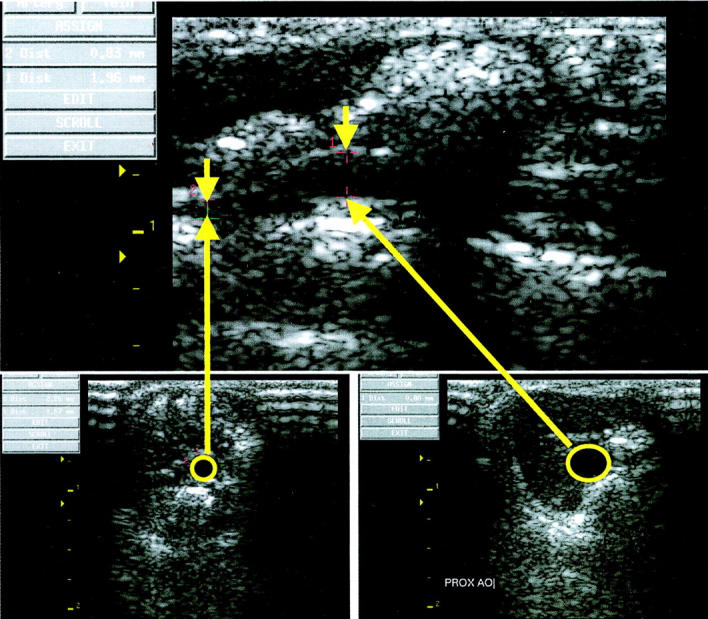
Ultrasound images obtained noninvasively from a live apoE-KO mouse treated with Ang II for 1 month. Top: Longitudinal view of the suprarenal aorta including both the aneurysmal and adjacent nonaneurysmal segment. Bottom left: Cross-sectional view of the adjacent nonaneurysmal segment of the suprarenal aorta with an inner diameter of 0.88 mm. Bottom right: Cross-sectional view of the aneurysmal portion of the suprarenal aorta with an inner diameter of 2.26 mm.
Figure 3.
Direct measurement of the cross-section of the suprarenal aorta in apoE-KO mice treated with Ang II or vehicle for 1 month. Left: Representative sections of the suprarenal aorta from a mouse treated with Ang II (top) and one with vehicle (bottom). Right: Quantification of the outer and luminal diameters, and wall thickness of suprarenal aortas in apoE-KO mice treated with Ang II or vehicle. *, P < 0.05 between two groups.
Pathological Examination of the Aneurysms
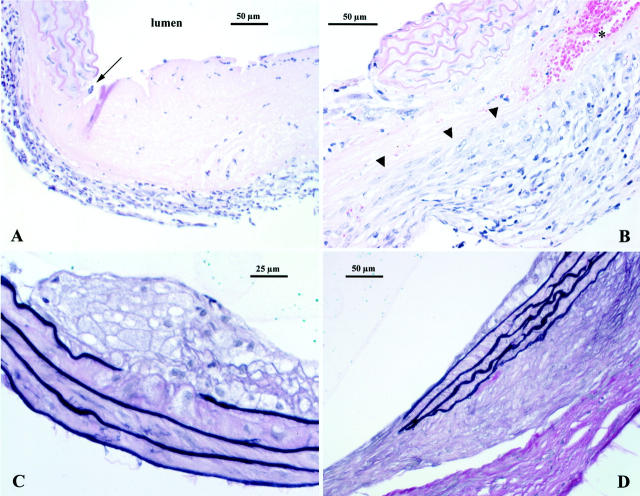
Ang II treatment produced a distinct pathology in the supra renal aorta. Pathological changes could be seen as early as 10 days of treatment with four out of six mice showing inflammation in the adventitia. In one case, there was focal, full-thickness necrosis of the aortic wall characterized by an acellular, pale eosinophilic area (Figure 4A) ▶ . One of six aortae appeared normal whereas another was characterized by mild atheroma formation with adjacent focal elastin fragmentation. At day 20, inflammatory cell infiltrates, both lymphocytic and monocytic cells, were seen entering the lesion from the adventitial area. There were also foci of acute hemorrhage present (Figure 4B) ▶ .
Figure 4.
A: Day 10 lesion. Inflammatory cell infiltration of the adventitia is prominent. There is also an abrupt, focal, full-thickness necrosis of the arterial wall (arrow). This lesion appears as an acellular, pale, eosinophilic area. B: Day 20 lesion, again there is an abrupt lesion involving destruction of the vascular media. Note that activated monocytes in tissue are infiltrating into the lesion from the adventitial side (arrowheads). An area of acute hemorrhage is seen at asterisk. H&E stain (both A and B). C: Small atheroma containing foamy macrophages. Note that there is destruction of the top layer of the elastic laminae directly below the lesion. D: Extensive fibrosis in the adventitia. These changes are seen around areas where there has been destruction of the vascular media as well as beneath areas with intact elastic laminae. Note that in both C and D smooth muscle cells in the media are hypertrophied (elastin van Gieson stain, tissues from day 30).
After 30 days of treatment, all abdominal aorta segments showed some pathology, the most common finding being adventitial reactive changes. These changes were characterized by fibrosis, mononuclear cell inflammatory infiltrates. Atheromata, when present, were typically small, focal, and composed almost entirely of foam cells (Figure 4C) ▶ . There was evidence of both acute and chronic hemorrhage in the adventitia. In one mouse, a large neo-lumen formed within the adventitia directly adjacent to a relatively undamaged segment of the aorta. The wall of this neo-lumen was composed of a mature granulation tissue containing histiocytes and a few lymphocytes embedded in a thin collagen matrix. The neo-lumen appeared to be connected to the aorta as shown by the presence of green-colored agarose in the space.
Elastin fragmentation and degeneration of the media sometimes occurred in the outer lamellae close to areas of adventitial inflammation. In other vessels, focal destruction of the inner elastic lamina was found in association with focal atheroma formation (Figure 4C) ▶ . In over half the cases there was focal, full thickness destruction of the elastic lamellae (Figure 4D) ▶ . In addition, even in areas of intact media, the smooth muscle cells appeared hypertrophied as compared to controls. The aortae of the age-matched apoE-KO mice treated with vehicle appeared completely normal.
Secretion of IL-6 Protein from the Isolated Aorta
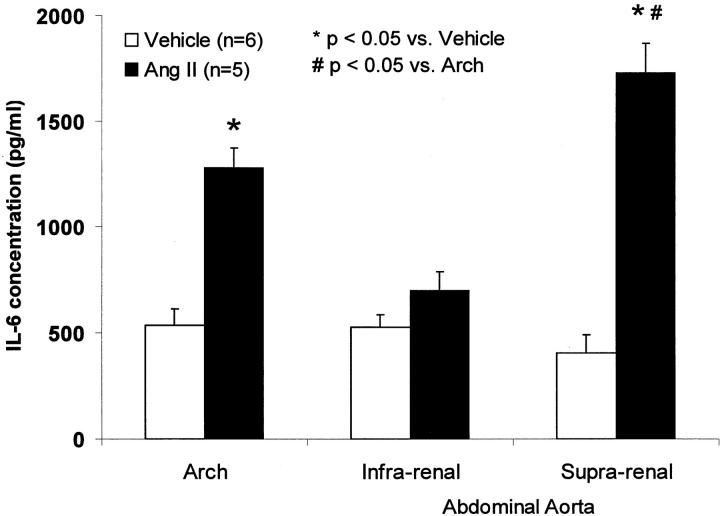
To determine the IL-6 level in Ang II-induced aneurysm, we measured the ex vivo secretion of IL-6 protein from the freshly isolated aortae separated into the atherosclerotic aortic arch, suprarenal, and infrarenal abdominal aorta. Ang II increased secretion of IL-6 protein from the aneurysmal segment and aortic arch by 4.2-fold and 2.4-fold, respectively, compared to the comparable aortic segments from vehicle-treated mice (Figure 5) ▶ . Ang II had no effect on IL-6 production in the infrarenal nonaneurysmal segments of the abdominal aorta.
Figure 5.
Ex vivo IL-6 secretion in the atherosclerotic aortic arch, and suprarenal and infrarenal aortas in apoE-KO mice infused with Ang II or vehicle for 1 month.
uPA Expression and Activity Was Increased in the Aneurysmal Tissue
The expression of uPA, tPA, and their major inhibitor, PAI-1, was studied with real-time quantitative RT-PCR. As shown in Figure 6A ▶ , the expression of uPA mRNA was increased significantly (13-fold) in the aneurysmal portion of the abdominal aortae in apoE-KO mice treated with Ang II compared to vehicle-treated controls. This compares with a twofold increase in uPA mRNA in the atherosclerotic aortic arch from Ang II-treated animals. In addition, the expression of PAI-1 mRNA was increased sixfold in the aneurysmal tissue compared to that of vehicle-treated controls (Figure 6B) ▶ . On the other hand, the expression of tPA was decreased ∼50% in the aneurysmal tissue (Figure 6C) ▶ .
Figure 6.
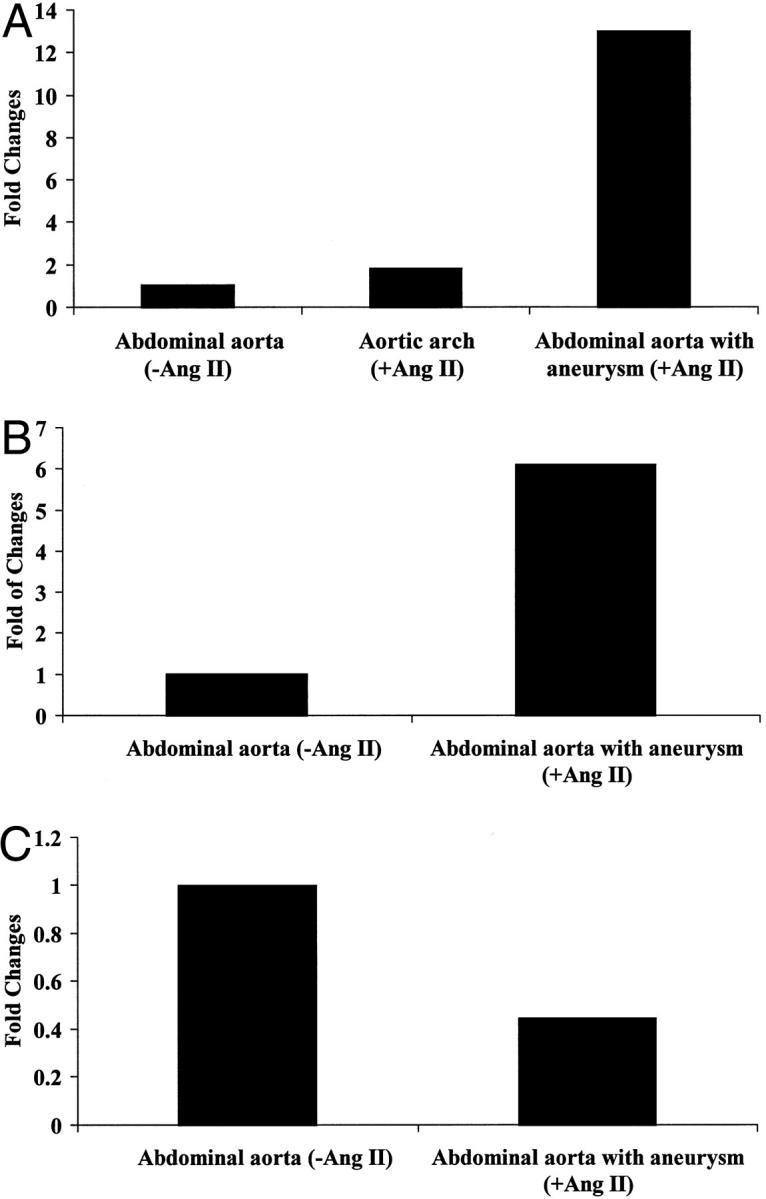
Expression of uPA, PAI-1, and tPA in aneurysm. Total RNA extracted from pooled aortic tissues from 10 mice was subjected to quantitative RT-PCR using primers and probes specific for uPA (A), PAI-1 (B), and tPA (C).
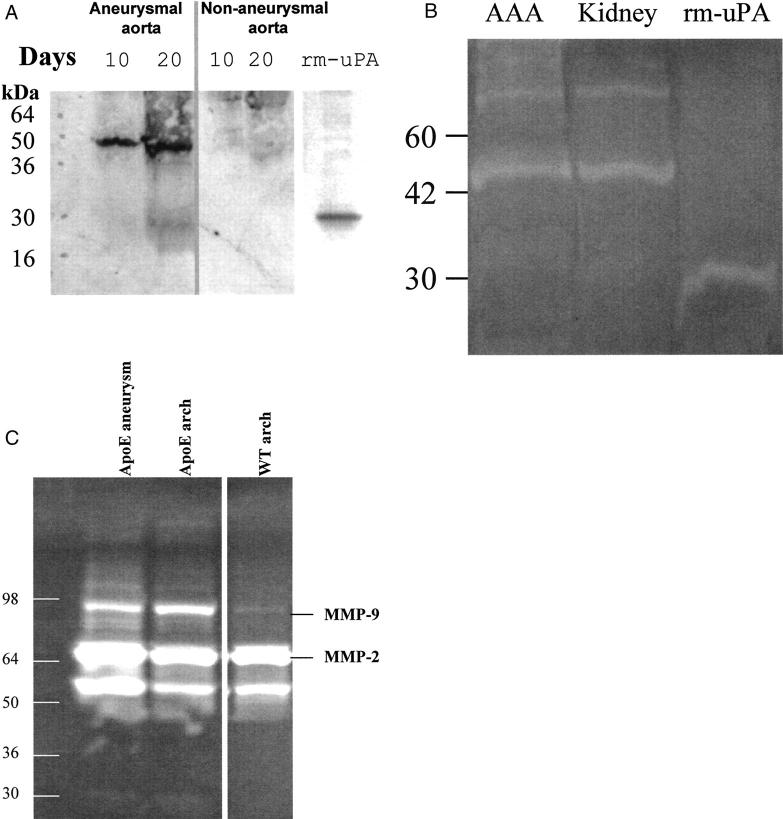
The time-course of uPA protein induction was studied in the aneurysmal segments of the aortae from apoE-KO mice infused with Ang II for 10 and 20 days. The uPA protein was detected as early as 10 days after AngII infusion and was further increased at 20 days, although little uPA protein was detected in adjacent nonaneurysmal segments (Figure 7A) ▶ . The recombinant mouse uPA (rm-uPA) was blotted with the same antibody showing that the antibody is recognizing mouse uPA. Activated uPA was detected from the aneurysm tissues on the zymographic gel comparable to a mouse kidney extract containing high uPA activity (Figure 7B) ▶ . MMP-2 and -9 activity was also examined by zymography. Although the MMP-9 activity was increased in the aorta of apoE-KO mice compared with that of wild type, there was no significant difference in MMP-9 activity between the aneurysmal section of the abdominal aorta and the nonaneurysmal aortic arch in apoE-KO mice (Figure 7B) ▶ .
Figure 7.
Expression of uPA and MMP protein in aneurysm. A: Immunoblotting to detect uPA protein in the aneurysmal (left) and nonaneurysmal (right) portion of the abdominal aorta in the mice treated with Ang II for 10 or 20 days. Two-chain uPA is detected as a 50-kd band. The recombinant mouse uPA (rm-uPA) B chain (5 μg) appears as a 30-kd band. B: Zymographic analysis of uPA activity. Extract from aneurysm tissues (AAA) (containing 10 μg of protein) was compared with a kidney extract from a wild-type mouse containing abundant uPA and with rm-uPA (0.1 μg). C: Gelatin zymography of tissue extracts of aneurysm and aortic arch from Ang II-infused mice. The pro- and activated forms of MMP-9 and MMP-2 were detected.
Discussion
The major findings in this study are that uPA expression is markedly increased in the aneurysmal segment but not in the adjacent, nonaneurysmal segment of the abdominal aorta in apoE-KO mice after Ang II infusion. Aneurysms were induced in 100% of the male apoE-KO mice. The lesions were consistently localized in the suprarenal region of the abdominal aorta and associated with mononuclear inflammatory cell infiltration, neovascularization, intra-adventitial hemorrhage, and elastic laminal destruction. The features are commonly seen in human AAAs. 1,5,15 In addition, Ang II accelerated atherosclerosis and resulted in a modest increase in blood pressure.
Daugherty and colleagues 7 reported that approximately one-third of the female apoE-KO mice developed bulbous aneurysm after chronic infusion of Ang II for 1 month. By using the same method (1.44 mg/kg/day Ang II for 1 month) in male 6-month-old apoE-KO mice, we observed that 100% of the mice developed aneurysms; 58% with large aneurysms (>100% expansion of the aorta) and the rest with small aneurysms (<100% expansion). The discrepancy between the two laboratories may be the results of the sex difference and from the subjectively different criteria in determination of the bulbous and large aneurysm. To quantify the diameter of the vessel, we measured the cross-sectional diameter directly in the aorta perfused with agarose under normal pressure to prevent collapse of the vessel after animal death. This measurement showed not only the expansion of the outer diameter by 75%, but also an expansion by 60% of the lumen in apoE-KO mice treated with Ang II compared to those with vehicle. Most importantly, it revealed that the aortic wall thickness increased 125%. The thickening was because of a reactive fibrosis with a predominantly chronic (mononuclear) inflammatory cell infiltrate. In addition, we also developed an ultrasound method to measure the diameters of the aorta with a resolution of 0.01 mm. This is the first reported application of this technique in mice. The luminal expansion was observed noninvasively under normal pressure. Despite increased aortic wall thickness and localized intramural hemorrhage, the lumen was found uniformly dilated in aneurysm (Figure 2) ▶ . The measurement was validated by postmortem direct measurement. Therefore, this technique can be used for longitudinal monitoring of AAAs in small animals, such as mice and rats, as well as following the progressive effects of potential therapies.
Ang II is a potent vasocontractor and can cause hypertension. In the present study in conscious male apoE-KO mice Ang II increased blood pressure by 22 mmHg at 1 month. In a recent report, active uPA was also found to promote vascular smooth muscle contraction. 16 It could be that the increased active uPA in aneurysm contributes to the elevation of blood pressure. It is also possible that the hemodynamic stress resulting from hypertension may contribute to aneurysm formation in our model.
Macrophages and foam cells are the major source of proteases including uPA and pro-MMPs in the atherosclerotic plaque. 1,2,4,17 Many pro-MMPs are activated by plasmin which, in turn, is activated by uPA. uPA expression was increased 13-fold in the aneurysmal segment of Ang II-treated apoE-KO mice compared to vehicle-treated controls. In contrast, the expression of uPA was increased only twofold in the macrophage-rich but not dilated atherosclerotic aortic arch of the Ang II-treated mice. We also demonstrated the increased protein and activity of uPA in aneurysm tissues, suggesting that the increased PAI-1 (with a lesser increase in mRNA compared to that of uPA) was not enough to inhibit uPA activity (Figure 7) ▶ . Consistent with these findings, increased expression of uPA, 18,19 MMPs, 20,21 as well as PAI-1, 1 have been reported in human aortic aneurysms.
We also found that the expression of tPA mRNA was reduced in the aneurysm tissues. This is most likely caused by the depletion of smooth muscle cells and endothelial dysfunction in the aneurysm as histology showing the replacement of smooth muscle cell by noncellular structures in the media (Figure 4) ▶ . In the vehicle-treated wild-type mouse, we found that the majority of plasminogen activities are from tPA but not uPA on a zymographic gel (data not shown). These data are consistent with previous reports showing that tPA is mainly produced by smooth muscle cells and that uPA mainly by inflammatory cells.
Both MMP-2 and MMP-9 are expressed in human AAA tissue and are the major elastolytic enzymes secreted by human AAA tissues in organ culture. 4,22 Recently, MMP-12 was reported to be prominently expressed by aneurysm-infiltrating macrophages within the media of human AAA. 5 The roles of MMP-9 and MMP-12 in the pathogenesis of AAA have been investigated in a mouse model of elastase-induced aneurysm. In this model, Pyo and colleagues 23 found that MMP-9- but not MMP-12-deficient mice were protected from aneurysm formation, suggesting that MMP-9 plays a more critical role than MMP-12 in this experimental model. In the present study, whereas MMP-9 was increased in both the aneurysmal segment of the aorta and in the atherosclerotic aortic arch compared to that of wild-type mice, MMP-9 activity was not specifically increased in the aneurysm (Figure 7B) ▶ .
Although the exact mechanism(s) by which Ang II induces aneurysm in apoE-KO mice is not clearly understood, its proinflammatory actions may be important. Chronic vascular wall inflammation has been proposed to play an important role in the pathogenesis of AAA formation. 24-26 Ang II promotes recruitment of inflammatory cells to the vessel wall by inducing the expression of monocyte chemoattractant protein-1. 27 Ang II increases low-density lipoprotein oxidation 28 and by interacting with the Ang II subtype-1 (AT1) receptor on macrophage increases 12/15-lipoxygenase activity, 29 suggesting that it may exacerbate atherosclerosis. Indeed, the present study also demonstrated that Ang II exacerbates atherosclerosis in apoE-KO mice. Histologically, the chronic inflammation and reactive collagen formation found in the adventitia suggests a chronic, resolving process whose initial event is unknown. The presence of hemosiderin in some adventitiae suggests that hemorrhage may have played a role in eliciting the reactive adventitial changes. Moreover, Ang II has also been shown to induce the expression of IL-6. 8-10 IL-6 is increased in atherosclerotic lesions in apoE-KO mice in association with the infiltrating macrophages. 11 In human AAAs, the circulating level of IL-6 has been reported to be elevated 12 and positively correlated with aortic diameter expansion. 13 Consistent with these observations, we showed that ex vivo secretion of IL-6 from the aneurysmal segment of the aorta was increased compared to the aortic arch in Ang II-treated mice and to the aortic segments from the vehicle-treated mice (Figure 5) ▶ . These results suggest that the severity of vascular inflammation measured as IL-6 production may be important in aneurysm formation.
In summary, we demonstrated that chronic infusion of Ang II in male apoE-KO mouse led to induction of AAAs in 100% of the animals, accompanied by a significant increase in uPA in the aneurysmal segment of the aorta. Macrophage infiltration and focal elastin fragmentation were also observed in the aneurysm. These findings are in agreement with the observations in human AAA, and support a key role for uPA in the pathogenesis of AAA.
Acknowledgments
We thank Dr. Ronald Cobb and Wei Xia for expressing the recombinant mouse uPA; Kieu Chu for assistance on purification of the recombinant uPA; and Minh Le for assistance on uPA zymography.
Footnotes
Address reprint requests to Gary G. Deng, M.D., Ph.D., 15049 San Pablo Ave., Richmond, CA 94804. E-mail: gary_deng@berlex.com.
References
- 1.Schneiderman J, Bordin GM, Engelberg I, Adar R, Seiffert D, Thinnes T, Bernstein EF, Dilley RB, Loskutoff DJ: Expression of fibrinolytic genes in atherosclerotic abdominal aortic aneurysm wall. A possible mechanism for aneurysm expansion. J Clin Invest 1995, 96:639-645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT: Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1995, 15:1145-1151 [DOI] [PubMed] [Google Scholar]
- 3.Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, Ghorpade A, Baxter BT: Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 1998, 18:1625-1633 [DOI] [PubMed] [Google Scholar]
- 4.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC: Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 1995, 96:318-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW: Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest 1998, 102:1900-1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D: Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 1997, 17:439-444 [DOI] [PubMed] [Google Scholar]
- 7.Daugherty A, Manning MW, Cassis LA: Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 2000, 105:1605-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W: Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1999, 19:1623-1629 [DOI] [PubMed] [Google Scholar]
- 9.Funakoshi Y, Ichiki T, Ito K, Takeshita A: Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 1999, 34:118-125 [DOI] [PubMed] [Google Scholar]
- 10.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R: Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1999, 19:2364-2367 [DOI] [PubMed] [Google Scholar]
- 11.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM: Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol 1998, 18:1498-1505 [DOI] [PubMed] [Google Scholar]
- 12.Juvonen J, Surcel HM, Satta J, Teppo A-M, Bloigu A, Syrkälä H, Airaksinen J, Leinonen M, Saikku P, Juvonen T: Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1997, 17:2843-2847 [DOI] [PubMed] [Google Scholar]
- 13.Rohde LE, Arroyo LH, Rifai N, Creager MA, Libby P, Ridker PM, Lee RT: Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arterioscler Thromb Vasc Biol 1999, 19:1695-1699 [DOI] [PubMed] [Google Scholar]
- 14.Wang YX, Halks-Miller M, Vergona R, Sullivan ME, Fitch R, Mallari C, Martin-McNulty B, da Cunha V, Freay A, Rubanyi GM, Kauser K: Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am J Physiol 2000, 278:H428-H434 [DOI] [PubMed] [Google Scholar]
- 15.Shah PK: Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation 1997, 96:2115-2117 [DOI] [PubMed] [Google Scholar]
- 16.Haj-Yehia A, Nassar T, Sachais BS, Kuo A, Bdeir K, Al-Mehdi AB, Mazar A, Cines DB, Higazi AA: Urokinase-derived peptides regulate vascular smooth muscle contraction in vitro and in vivo. FASEB J 2000, 14:1411-1422 [DOI] [PubMed] [Google Scholar]
- 17.Schneiderman J, Bordin GM, Adar R, Smolinsky A, Seiffert D, Engelberg I, Dilley RB, Thinnes T, Loskutoff DJ: Patterns of expression of fibrinolytic genes and matrix metalloproteinase-9 in dissecting aortic aneurysms. Am J Pathol 1998, 152:703-710 [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly JM: Plasminogen activators in abdominal aortic aneurysmal disease. Ann NY Acad Sci 1996, 800:151-156 [DOI] [PubMed] [Google Scholar]
- 19.Shireman PK, McCarthy WJ, Pearce WH, Shively VP, Cipollone M, Kwaan HC: Elevations of tissue-type plasminogen activator and differential expression of urokinase-type plasminogen activator in diseased aorta. J Vasc Surg 1997, 25:157-164 [DOI] [PubMed] [Google Scholar]
- 20.Elmore JR, Keister BF, Franklin DP, Youkey JR, Carey DJ: Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg 1998, 12:221-228 [DOI] [PubMed] [Google Scholar]
- 21.Knox JB, Sukhova GK, Whittemore AD, Libby P: Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 1997, 95:205-212 [DOI] [PubMed] [Google Scholar]
- 22.Herron GS, Unemori E, Wong M, Rapp JH, Hibbs MH, Stoney RJ: Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb 1991, 11:1667-1677 [DOI] [PubMed] [Google Scholar]
- 23.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW: Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest 2000, 105:1641-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szekanecz Z, Shah MR, Pearce WH, Koch AE: Intercellular adhesion molecule-1 (ICAM-1) expression and soluble ICAM-1 (sICAM-1) production by cytokine-activated human aortic endothelial cells: a possible role for ICAM-1 and sICAM-1 in atherosclerotic aortic aneurysms. Clin Exp Immunol 1994, 98:337-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szekanecz Z, Shah MR, Pearce WH, Koch AE: Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions 1994, 42:159-162 [DOI] [PubMed] [Google Scholar]
- 26.Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE: Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology 1994, 62:134-139 [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM: Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 1998, 83:952-959 [DOI] [PubMed] [Google Scholar]
- 28.Keidar S: Angiotensin, LDL peroxidation and atherosclerosis. Life Sci 1998, 63:1-11 [DOI] [PubMed] [Google Scholar]
- 29.Scheidegger KJ, Butler S, Witztum JL: Angiotensin II increases macrophage-mediated modification of low density lipoprotein via a lipoxygenase-dependent pathway. J Biol Chem 1997, 272:21609-21615 [DOI] [PubMed] [Google Scholar]