Abstract
Cardiac valves arise from endocardial cushions, specialized regions of the developing heart that are formed by an endothelial-to-mesenchymal cell transdifferentiation. Whether and to what extent this transdifferentiation is retained in mature heart valves is unknown. Herein we show that endothelial cells from mature valves can transdifferentiate to a mesenchymal phenotype. Using induction of α-smooth muscle actin (α-SMA), an established marker for this process, two distinct pathways of transdifferentiation were identified in clonally derived endothelial cell populations isolated from ovine aortic valve leaflets. α-SMA expression was induced by culturing clonal endothelial cells in medium containing either transforming growth factor-β or low levels of serum and no basic fibroblast growth factor. Cells induced to express α-SMA exhibited markedly increased migration in response to platelet-derived growth factor-BB, consistent with a mesenchymal phenotype. A population of the differentiated cells co-expressed CD31, an endothelial marker, along with α-SMA, as seen by double-label immunofluorescence. Similarly, this co-expression of endothelial markers and α-SMA was detected in a subpopulation of cells in frozen sections of aortic valves, suggesting the transdifferentiation may occur in vivo. Hence, the clonal populations of valvular endothelial cells described here provide a powerful in vitro model for dissecting molecular events that regulate valvular endothelium.
The aortic and pulmonic valves of the adult heart are highly specialized trileaflet structures that develop from the endocardial cushions of the fetal heart. 1 These semilunar valves consist of three principal layers, the ventricularis at the inflow surface, spongiosa in the center, and fibrosa at the outflow surface. 2 The extracellular matrix components have been well characterized; the ventricularis contains radially aligned collagen and is rich in elastin, the spongiosa is primarily composed of glycosaminoglycans with some collagen, and the fibrosa contains densely packed collagen fibers aligned in parallel with the free edge of the valve cusp. 2 The cellular mechanisms that produce and maintain the highly ordered structure of the leaflets are clearly of great importance for understanding normal valve function and pathophysiology, and for developing new strategies to produce improved heart valve substitutes.
During development, the heart begins as a tube consisting of two layers, the endocardium and the myocardium, separated by an extracellular matrix known as cardiac jelly. 3 Studies of avian heart development indicate that endothelial cells (ECs) from the endocardium are initially activated by an unknown factor secreted by the myocardium. 4 Sequential signaling by transforming growth factor (TGF)-β2 and -β3 then promotes transdifferentiation of a subset of ECs to mesenchymal cells. 5 These cells migrate into the cardiac jelly 4,6 and further develop into mature valves. A biochemical marker for this transdifferentiation is the expression of α-smooth muscle actin (α-SMA), which is not normally expressed by ECs. α-SMA has been shown to be important for mesenchymal formation from ECs in embryonic explants of endocardial cushion tissue, 7 suggesting that α-SMA may play a functional role in this process. Alterations in cell phenotype, such as that occurring in the developing valve, are referred to by various terms including phenotypic modulation, transformation, or transdifferentiation. 8 We use transdifferentiation here to denote an irreversible alteration from one cell lineage to that characteristic of a different cell lineage.
In contrast to embryological studies, little is known about the cellular properties of mature valves. Valve leaflets are composed of an outer layer of ECs that cover interstitial mesenchymal cells located throughout the leaflet. Both the ECs 9 and interstitial cells 10 of valves have been isolated and cultured in vitro. ECs from human cardiac valves appear spindle-shaped in culture, but like all ECs, they express inducible intercellular adhesion molecule-1 and E-selectin. 9 The interstitial cells, although not well characterized, have been reported to share some characteristics with both fibroblasts and smooth muscle cells. 11
Because valvular disease leads to 60,000 valve replacement surgeries every year in the United States and current replacement valves are not optimal, 2 understanding the cells that make up valves is essential if better options for correcting valvular defects are to be developed. To pursue this, we isolated ECs from the aortic valve leaflets of healthy mature sheep. Although the cells in primary culture formed typical cobblestone endothelial monolayers, other cellular morphologies, reminiscent of mitral valve interstitial cells, 10 appeared within 2 to 3 weeks of subculture. We therefore isolated clonal EC populations from adult aortic valve leaflets that express the endothelial markers CD31/PECAM-1 and E-selectin. Herein we demonstrate that clonal ECs can transdifferentiate to a mesenchymal cell phenotype in a manner that resembles one of the early events during valve development.
Materials and Methods
Materials
Material used were endothelial basal medium (EBM) (CC-3121; Clonetics, San Diego, CA); fetal bovine serum (FBS) (Hyclone, Logan, UT); 100× GPS (29.2 mg/ml l-glutamine, 10,000 U/ml penicillin G, 10,000 μg/ml streptomycin sulfate); gentamicin sulfate and 100× PSF (10,000 U/ml penicillin G, 10,000 μg/ml streptomycin sulfate, 25 μg/ml amphotericin B) (Life Technologies, Inc., Grand Island, NY); collagenase A (Boehringer Mannheim, Indianapolis, IN); Immobilon-P membrane (Millipore, Bedford, MA); Hyperfilm ECL, fluorescein-streptavidin, and Texas Red-streptavidin (Amersham Life Sciences, Arlington Heights, IL); Lumiglo (KPL); human TGF-β1, recombinant human TGF-β2 and -β3, recombinant human platelet-derived growth factor (PDGF)-BB, and anti-PDFG-BB (R&D Systems, Minneapolis, MN); Vectastain Elite ABC kit, avidin/biotin blocking kit, fluorescein anti-mouse IgG, Texas red anti-rabbit IgG, peroxidase-conjugated anti-goat IgG, biotinylated horse anti-mouse IgG, avidin-peroxidase, peroxidase-conjugated anti-mouse IgG, and 3,3′5,5′-tetramethylbenzidine (Vector Laboratories, Burlingame, CA); 3-amino-9-ethyl carbazol and mouse anti-human α-SMA (clone 1A4 12 ) (Sigma Chemical Co., St. Louis, MO); goat anti-human CD31/PECAM-1 IgG (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-human von Willebrand factor (vWF) and mouse anti-human CD31/PECAM-1 (DAKO, Carpinteria, CA); polycarbonate PVP-F membranes (Neuro Probe, Inc, Gaithersburg, MD). Recombinant human bFGF was kindly provided by Scios Nova Inc., Mountain View, CA; soluble recombinant TGF-β type II receptor, prepared as described, 13 was kindly provided by Philip Gotwals, Biogen, Cambridge, MA; SM1 antibody was kindly provided by Masanori Aikawa, Brigham and Women’s Hospital, Boston; rabbit anti-bovine CD31/PECAM-1 was kindly provided by Steven Albelda, University of Pennsylvania. Figures were prepared from scanned images using Adobe Photoshop version 5.5.
Tissue Procurement
Ovine tissues from animals weighing 20 to 25 kg and 8 to 10 months of age were obtained under approved guidelines for animal experimentation at Children’s Hospital, Boston. Human valvular cells were isolated from pulmonary valve leaflets obtained from children undergoing open-heart surgery at Children’s Hospital, Boston. Valve tissue was obtained in accordance with the Committee on Clinical Investigation, Children’s Hospital, Boston. Adult human aortic valve tissue was obtained in accordance with the Human Investigation Review Committee, Brigham and Women’s Hospital, Boston, MA.
Cell Culture
Ovine and human valve cells were grown on 1% gelatin-coated dishes in EBM, 10% heat inactivated FBS, 1× GPS, and 2 ng/ml bFGF (growth medium). Cells were passaged 1:3 or 1:4 every 6 to 14 days and used between passages 8 to 14. Human dermal microvascular ECs (HDMECs) and human umbilical vein ECs (HUVECs) were isolated and cultured as described. 14,15
Isolation of Valve ECs
Valve leaflets were incubated in media with 5% FBS, PSF, 2 mmol/L l-glutamine, and 100 μg/ml gentamicin sulfate for 1 to 4 hours, then minced into 2 mm 2 pieces, incubated with 0.2% collagenase A in EBM for 5 minutes at 37°C, and diluted with Hanks’ balanced salt solution containing 5% FBS, 1.26 mmol/L CaCl2, 0.8 mmol/L MgSO4, and 1× PSF (wash buffer). The supernatant containing released cells was sedimented at 200 × g, resuspended in growth medium, and plated. The following day, primary cultures were washed to remove unattached cells and refed. ECs from primary cultures of human pulmonic valve were isolated using Ulex europaeus I-coated Dynabeads. 16
Clonal Cell Populations
Primary cultures were trypsinized, resuspended in growth medium at 3.3 cells/ml, and 100 μl plated in each well of a 96-well plate, yielding a distribution, on average, of one cell in every third well. Each well was then checked to confirm that only one cell colony grew up in each well. When the colonies covered two-thirds of the well, cells were split into 24-well dishes.
Indirect Immunofluorescence
Cells on gelatin-coated glass coverslips were fixed with −20°C methanol, incubated with primary antibodies followed by species-specific fluorescein-conjugated and Texas Red-conjugated secondary antibodies, and then analyzed using a Zeiss Axiophot II fluorescence microscope. The rabbit polyclonal antibody against bovine E-selectin described previously 17 was shown to cross-react with ovine E-selectin (data not shown). A rabbit polyclonal against bovine CD31 or a goat anti-human CD31/PECAM-1 was used to detect ovine CD31 by immunofluorescence.
Western Blots
Cells were lysed with 4 mol/L urea, 0.5% sodium dodecyl sulfate, 0.5% Nonidet P-40, 100 mmol/L Tris, and 5 mmol/L ethylenediaminetetraacetic acid, pH 7.4, containing 100 μmol/L leupeptin, 10 mmol/L benzamidine, 1 mmol/L phenylmethyl sulfonyl fluoride, and 12.5 μg/ml aprotinin. Lysates were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10 μg of protein per lane) and transferred to Immobilon-P membrane. Membranes were incubated with primary antibody diluted in 1× phosphate-buffered saline (PBS), 2% dry milk, 0.1% Tween-20, and then with secondary antibody (peroxidase-conjugated anti-mouse or anti-goat). Antigen-antibody complexes were visualized using Lumiglo and chemiluminescent sensitive film.
Migration Assay
Cellular migration was measured using the Boyden chamber assay. 18 Briefly, cells in EBM and 0.1% bovine serum albumin were pipetted into the upper wells of the chamber at 10,000 cells/well. Lower wells contained EBM and 0.1% bovine serum albumin (control) or 5% FBS, or growth factors diluted in EBM and 0.1% bovine serum albumin. Each condition was performed in quadruplicate. To quantitate, the number of cells in four high-power fields, each of which corresponded to 1.25 mm2, was counted. Therefore, the migration data represents the mean ± SE of 16 high-power fields from four different wells. Total area of the well equals 12.5 mm2.
Flow Cytometry
Cells were fixed in 0.1% paraformaldehyde in PBS at 4°C for 16 hours, permeabilized with 0.1% Triton X-100, incubated with either 2.6 μg/ml mouse anti-α-SMA or 2.6 μg/ml isotype matched IgG2a for 1 hour at 4°C, and then with fluorescein-conjugated goat anti-mouse IgG for 30 minutes at 4°C. Cells were resuspended in 0.1% sodium citrate, 20 μg/ml RNase A, 0.3% Nonidet P-40, and 50 μg/ml propidium iodide at 200,000 cells/ml and analyzed on a Becton Dickinson FACScan flow cytometer (Becton Dickinson, Mountain View, CA) for fluorescein (α-SMA) and propidium iodide (DNA).
Immunohistochemistry of Human and Sheep Valves
Hearts were obtained from healthy sheep (n = 7) and aortic valves were dissected. A portion of each valve including the aortic wall was embedded in OCT compound. Human aortic valves were obtained from autopsy cases (n = 3). Serial frozen sections (6 μm) were prepared, fixed in 4% paraformaldehyde for 5 minutes, and stained by the avidin-biotin-peroxidase method. The peroxidase reaction was visualized with 3-amino-9-ethyl carbazole. Sections were counterstained with Gill’s hematoxylin solution. Frozen sections were incubated with anti-α-SMA, followed by biotinylated secondary horse anti-mouse, and Texas Red-conjugated streptavidin. Subsequently, specimens were treated with an avidin-biotin blocking kit and stained with vWF or CD31 antibodies overnight at 4°C, then appropriate secondary antibodies, and finally fluorescein-conjugated streptavidin was applied for 30 minutes. Specimens of human aortic valves were fixed in 10% formalin and embedded in paraffin. Sections (6 μm) were stained by the avidin-peroxidase method as described above. Immune complexes were visualized using 3,3′5,5′-tetramethylbenzidine and counterstained with methyl green.
Results
Clonal Valve-Derived Endothelial Cells
A limiting dilution technique was used to isolate clonal EC populations from primary cultures of ovine aortic valve leaflets. We used immunofluorescence to examine whether the clonal cell populations expressed the endothelial markers CD31 and E-selectin (Figure 1) ▶ . Clone av 10 cells express CD31 at cell-cell borders as expected for ECs (Figure 1a) ▶ , but only diffuse background staining was observed with control serum (Figure 1b) ▶ . E-selectin was detected in av 10 cells treated with lipopolysaccharide (Figure 1d) ▶ but only limited expression was detected in untreated cells (Figure 1c) ▶ , consistent with the known regulation of E-selectin in ECs. α-SMA was not detected in av 10 under normal culture conditions (Figure 1e) ▶ . A classic endothelial cobblestone morphology was consistently observed in confluent cells (Figure 1f) ▶ . Based on: 1) CD31 expression at cell-cell borders, 2) lipopolysaccharide-induced up-regulation of E-selectin, 3) lack of α-SMA, and 4) cobblestone morphology, the clonal cells were designated ECs. Similar results were obtained with six different clonal populations derived from aortic valve and additional clones from ovine pulmonary valve leaflets (data not shown).
Figure 1.
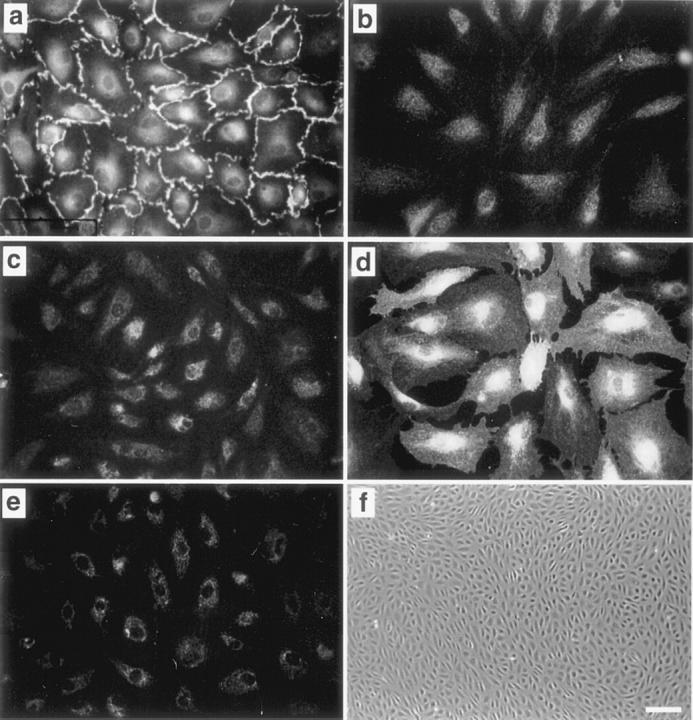
Clonal cells from ovine aortic valve express endothelial-specific markers. Av 10 cells were incubated with anti-human CD31 (a), control serum (b), anti-bovine E-selectin (c and d), or mouse anti-α-SMA (e). d: Av 10 cells were treated with 1 μg/ml lipopolysaccharide for 5 hours to induce E-selectin expression. f: Phase contrast micrograph of clonal aortic valve ECs. Original magnifications: ×400 (a–e), ×40 (f). Scale bar in f, 100 μm.
Endothelial Transdifferentiation Induced by TGF-β and Non-TGF-β-Dependent Pathways
To determine whether clonal aortic valve-derived ECs could be induced to transdifferentiate to a mesenchymal phenotype, we tested whether TGF-β1–3 isoforms could induce α-SMA 19 , a marker of valvular transdifferentiation in heart development. A second approach was to culture cells in low-serum medium, which is known to promote differentiation in many cell types. Thus, we cultured clonal cell populations in either growth medium with 1 ng/ml TGF-β1 or in medium containing no bFGF and 0.5 to 1% serum (reduced medium) for 6 days. The cells were then assayed for expression of CD31 and α-SMA by double-label immunofluorescence and by Western blot (Figure 2) ▶ . Individual cells co-expressing CD31 and α-SMA, appearing as yellow/orange, were seen in clone av 10 cells treated with TGF-β1 and in clone av 17 cells cultured in reduced medium (Figure 2A) ▶ , providing direct evidence for transdifferentiation. Because TGF-β1 and -β3 at 1 or 10 ng/ml had similar effects on av 10 cells, TGF-β1 at 1 ng/ml was used for subsequent experiments.
Figure 2.
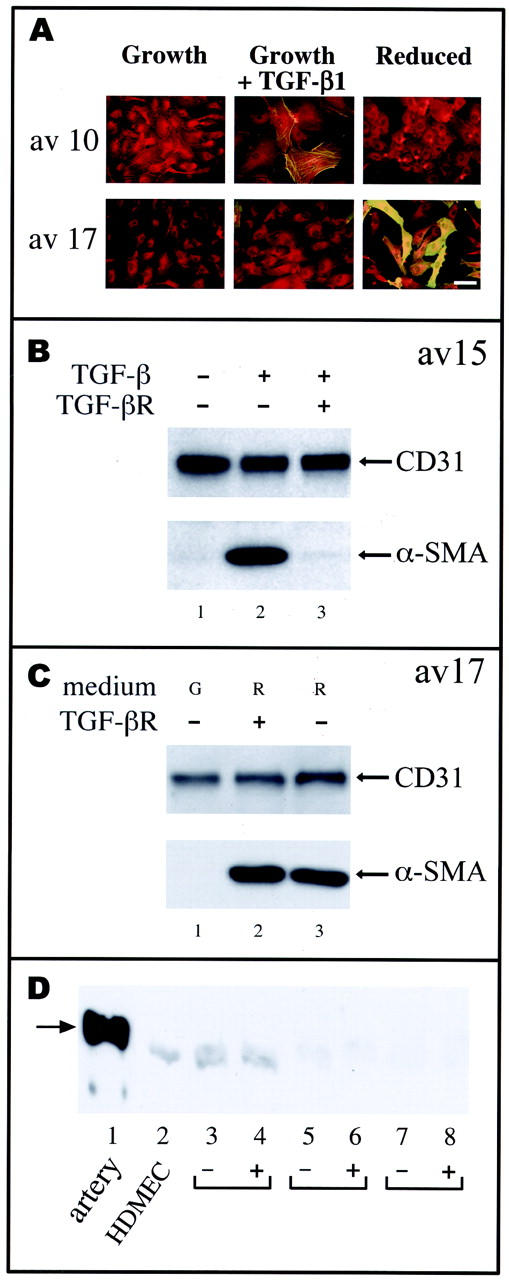
TGF-β-dependent and TGF-β-independent pathways for induction of α-SMA. A: Clones av 10 and av 17 were grown for 6 days in growth medium (growth, G), in growth medium with 1 ng/ml TGF-β1 (growth plus TGF-β1, T), or in medium with 0.5% serum and without bFGF (reduced, R). Cells were double-labeled with anti-CD31 and a Texas Red-conjugated secondary antibody and with anti-α-SMA and fluorescein-conjugated anti-mouse IgG for simultaneous detection of these two antigens. Original magnification, ×400. Scale bar, 10 μm. B: Clone av 15 cells cultured in growth medium (lane 1), in growth medium with 1 ng/ml TGF-β1 (lane 2), or in growth medium with TGF-β1 plus 36 μg/ml recombinant soluble TGF-β type II receptor antagonist (lane 3) for 6 days. C: Clone av 17 cells were cultured in growth medium (G) (lane 1), in reduced medium (R) with 36 μg/ml soluble TGF-β receptor (lane 2), and in reduced medium (R) (lane 3). Cell lysates were analyzed by Western blot for expression of CD31 and α-SMA. D: Lysates from ovine carotid artery (lane 1), cultured HDMECs (lane 2), aortic valve clones av 14 (lanes 3 and 4), av 17 (lanes 5 and 6), and av 10 (lanes 7 and 8) were probed by Western blot using anti-smooth muscle myosin heavy chain SM-1 monoclonal antibody. 22 Av clones were in growth medium (−) or in reduced medium (+) for 7 days. The arrow denotes the 204-kd smooth muscle myosin heavy chain detected in ovine carotid artery. The faint band detected in HDMECs and aortic valve clones was designated nonspecific because of its presence in cultured ECs.
Two types of response to TGF-β and low serum were found among the different clonal populations. Clone av 10 and av 15 are representative of one type of response in which the cells were induced to express α-SMA by the addition of TGF-β1 but not when cultured in reduced medium (Figure 2A) ▶ . Clone av 17 is representative of a second type of response in which α-SMA was not induced in response to TGF-β1 (Figure 2A) ▶ . All three TGF-β isoforms were tested at 1 and 10 ng/ml and found to have no effect on inducing α-SMA in av 17 (data not shown). However, av 17 expressed high levels of α-SMA after culture in reduced medium (Figure 2, A and C) ▶ . Time course experiments showed that TGF-β-mediated and reduced serum-mediated induction of α-SMA in av 15 and av 17, respectively, peaked at 5 to 7 days (data not shown).
The TGF-β-induced up-regulation of α-SMA in av 15 could be blocked by adding a soluble TGF-β type II receptor antagonist to the medium concurrently with TGF-β1 (Figure 2B) ▶ . Similar results were obtained with av 10 (data not shown). In contrast, the soluble TGF-β receptor antagonist had no effect on the induction of α-SMA when either clone av 17 (Figure 2C) ▶ or av 14 (data not shown) was cultured in reduced medium. This indicates that the induction of α-SMA in these clones does not occur by the generation of extracellular TGF-β, but instead occurs by a TGF-β-independent pathway. It is possible, however, that signaling pathways induced by culturing the cells in TGF-β or in reduced medium converge intracellularly to stimulate expression of α-SMA.
Besides its induction during valve development, α-SMA is expressed in smooth muscle cells, myofibroblasts, and dermal fibroblasts. 20,21 To determine whether induction of α-SMA in valvular ECs reflects a conversion to the smooth muscle lineage, we examined expression of smooth muscle myosin heavy chain, a definitive marker of the smooth muscle cell lineage, 21 in valve-derived cells (Figure 2D) ▶ using a monoclonal antibody against the SM-1 isoform of smooth muscle myosin heavy chain. 22 Lysates of freshly resected ovine artery were used to demonstrate that the antibody cross-reacts with ovine SM-1 (lane 1). Lysates from cultured human dermal microvascular ECs were used as a negative control (lane 2). Smooth muscle-specific myosin heavy chain was not detected in clones av 14 (lanes 3 and 4), av 17 (lanes 5 and 6), or av 10 (lanes 7 and 8) before (−) or after (+) induction of α-SMA in reduced medium for 7 days or up to 21 days (data not shown), indicating that the valvular α-SMA-positive cells obtained using our methods are distinct from classic arterial or venous smooth muscle cell. This is not surprising given that smooth muscle myosin heavy chain in valve interstitial cells has not been reported.
Increased Cellular Migration Coincides with Transdifferentiation
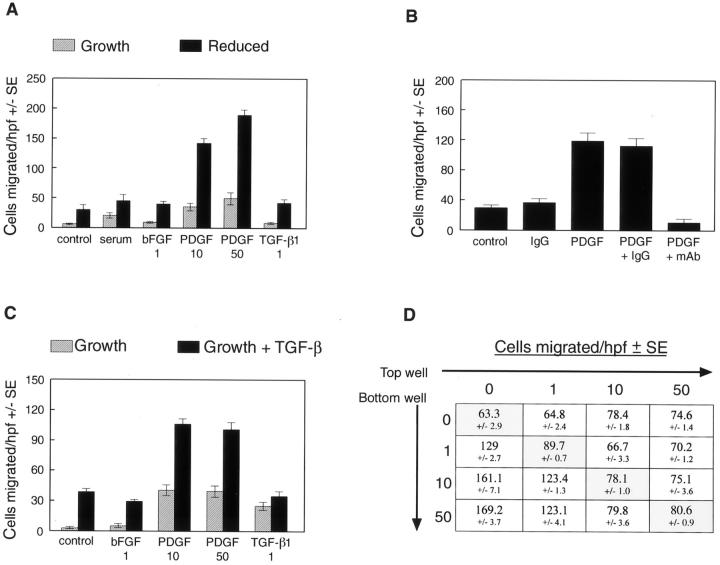
The induction of α-SMA and loss of cobblestone morphology suggested transdifferentiation to a mesenchymal cell phenotype. To determine whether these observations coincided with alterations in cell function, or with activities consistent with a mesenchymal phenotype, we measured cell migration of clonal populations of transdifferentiated cells. Basic FGF was tested because it is known to stimulate EC migration, PDGF-BB was tested because of its role in recruitment and assembly of mesenchymal cells during blood vessel development, 23 and TGF-β was tested to determine whether it also plays a role in cellular migration. Figure 3A ▶ shows the results of a migration assay using av 17 in response to 5% serum, 1 ng/ml bFGF, 10 or 50 ng/ml PDGF-BB, or 1 ng/ml TGF-β1. Clone av 17 cultured in reduced medium exhibited greatly increased migration in response to PDGF-BB, but not to TGF-β or bFGF when compared to the control. The PDGF-BB-induced migration in transdifferentiated av 17 cells was effectively blocked by a neutralizing antibody against PDGF-BB, whereas control IgG at the same concentration had no effect (Figure 3B) ▶ . Clone av 15 cells cultured in growth medium plus TGF-β1 also exhibited increased migration in response to PDGF-BB (Figure 3C) ▶ . Results similar to those shown in Figure 3, A and C ▶ , were obtained with clones av 14 and av 10, respectively (data not shown). Checkerboard analysis demonstrated that the PDGF-induced migration of av 17 cells grown in reduced medium for 6 days was because of chemotaxis rather than random cell migration because cells migrated directionally toward PDGF-BB in the bottom well (Figure 3D) ▶ . These results demonstrate that increased chemotaxis toward PDGF-BB coincides with the induction of α-SMA and the conversion to a mesenchymal phenotype. The precise role that PDGF plays in valve development and in normal ongoing function of postnatal valve leaflets warrants further investigation.
Figure 3.
Transdifferentiated cells exhibit increased migration in response to PDGF-BB. A: Av 17 cells cultured in growth medium (hatched bars) or in reduced medium (solid bars) for 6 days were tested for the ability to migrate toward EBM with 0.1% bovine serum albumin (control), EBM with 5% FBS (serum), 1 ng/ml bFGF, 10 ng/ml PDGF-BB, 50 ng/ml PDGF-BB, or 1 ng/ml TGF-β1. B: A neutralizing anti-PDGF-BB monoclonal antibody (mAb) and an isotype-matched IgG were tested for the ability to block the migration of av 17 cells, which were grown in reduced medium for 6 days, toward 10 ng/ml PDGF-BB. C: Av 15 cells cultured in growth medium (hatched bars) or in growth medium with 1 ng/ml TGF-β1 (solid bars) for 6 days were tested as in A. D: Checkerboard analysis was performed on av 17 cells grown in reduced medium for 6 days. PDGF-BB at 0, 1, 10, or 50 ng/ml was added to top and bottom wells as indicated, and cells allowed to migrate for 4 hours. The shaded boxes on the diagonal highlight the finding that PDGF-BB does not elicit random cell migration toward increasing concentrations of PDGF-BB in the upper and lower chambers.
Characterization of Endothelial Transdifferentiation
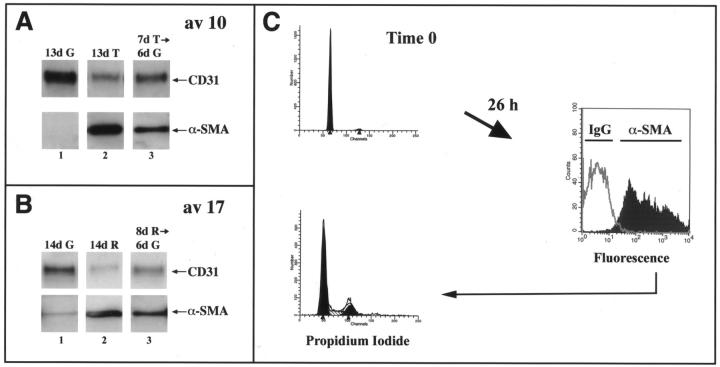
To determine whether or not the induction of α-SMA was reversible, cells that had up-regulated α-SMA in response to TGF-β1 or reduced medium were trypsinized and replated on fresh gelatin-coated plates in normal growth medium for 6 to 8 days. In Figure 4A ▶ , clone av 10 cells were plated in growth medium and 24 hours later either transferred to medium containing TGF-β1 (lanes 2 and 3) or maintained in growth medium (lane 1) for 7 days. At day 7, cells were trypsinized and replated in normal growth medium (lanes 1 and 3) or in growth medium containing TGF-β (lane 2) for another 6 days. A parallel experiment was performed using clone av 17 cells cultured in reduced medium (Figure 4B) ▶ . The induction of α-SMA by TGF-β1 or reduced medium was not reversible throughout 6 days (Figure 4, A and B ▶ ; lane 3) or 8 days (data not shown). Thus, the induction of α-SMA seems to reflect a permanent alteration in cellular phenotype rather than a transient modulation of phenotype.
Figure 4.
Characterization of endothelial transdifferentiation. A: Av 10 cells were cultured in growth medium (G) (lane 1) or in growth medium with 1 ng/ml of TGF-β1 (T) for 7 days (lanes 2 and 3). After 7 days, cells were trypsinized and replated on new gelatin-coated dishes in growth medium (lanes 1 and 3) or in growth medium with TGF-β1 (lane 2) for 6 additional days. B: A similar time series was used with av 17 cells and reduced medium (R) instead of TGF-β. Cell lysates were analyzed by Western blot using anti-CD31 and anti-α-SMA. C: Av 17 cells were grown in reduced medium for 6 days. A portion of the cells was stained with propidium iodide to determine the cell-cycle distribution (top left). The rest of the cells were replated in growth medium containing 10% FBS and 2 ng/ml of bFGF at 30,000 cells/cm 2 for 26 hours. The cells were then harvested, permeabilized with 0.1% Triton-X-100 for immunostaining of intracellular α-SMA by flow cytometry (right), and simultaneously stained with propidium iodide (bottom left). The gray line at the right represents cells stained with an isotype-matched IgG2a control.
We also examined the ability of cells that had transdifferentiated to progress through the cell cycle to determine whether transdifferentiation was a terminal differentiation process. Av 17 cells were cultured in reduced medium for 6 days and either harvested for flow cytometry to determine the distribution of cells in G0/G1, S, and G2/M or replated in growth medium for 26 hours (Figure 4C) ▶ . After 6 days in reduced medium, the av 17 cells were in G0/G1 (top left panel). The portion of quiescent cells that was replated in normal growth medium with 10% serum and 2 ng/ml bFGF for 26 hours was harvested and analyzed for α-SMA expression. More than 90% of the cells expressed α-SMA (right panel). When these α-SMA-positive cells were analyzed for cell cycle distribution, approximately one third of the cells had progressed into S and G2/M in 26 hours (bottom left panel). These results demonstrate that av 17 cells induced to transdifferentiate can proliferate in response to serum and bFGF. In additional experiments, we found that mitomycin C did not block the induction of α-SMA (data not shown), indicating that cell proliferation is not required for transdifferentiation.
To determine whether ECs from other vascular sites could also be induced to transdifferentiate, we tested ECs derived from human pulmonic valve, HDMECs, HUVECs, and ovine peripheral vein cultured in either growth medium (G) or in reduced medium (R) (Figure 5A) ▶ . Increased α-SMA was seen in human pulmonic valve ECs and in the positive control, av 17 cells, but not in HDMECs, HUVECs, or ECs isolated from ovine peripheral vein (Figure 5A) ▶ . The modest level of induction of α-SMA in human pulmonic valve ECs in response to reduced medium, compared to av 17, was most likely because of the fact that the pulmonary valve ECs were not clonal, and therefore represented a heterogeneous population of ECs in which only some cells were capable of transdifferentiation. As expected, TGF-β1 caused no induction of α-SMA in HDMECs or HUVECs (Figure 5B) ▶ . In summary, of the vascular ECs tested, the induction of α-SMA in response to reduced medium or TGF-β1 was specific to valve-derived ECs.
Figure 5.
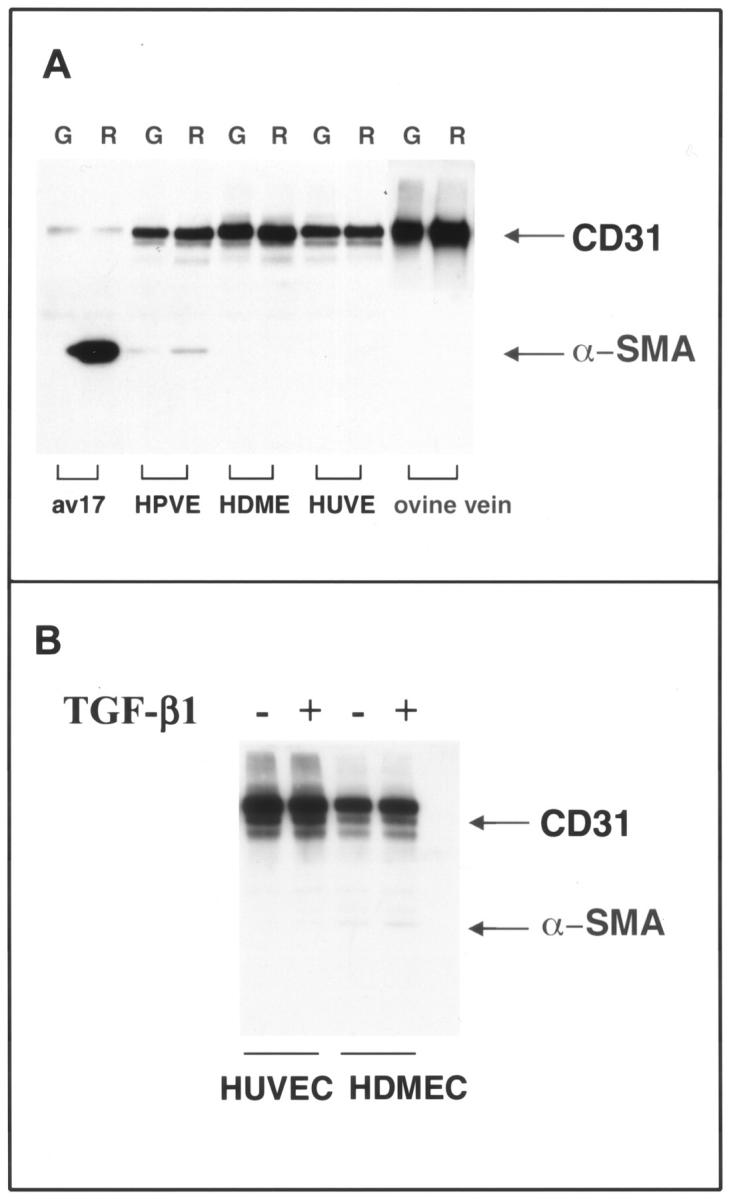
Induction of α-SMA is restricted to valvular ECs. A: Human pulmonary valve ECs (HPVEs), HDMECs, HUVECs, and ovine peripheral vein ECs (ovine vein) were grown for 6 days in growth medium (G) or in reduced medium (R). Av 17 cells served as a positive control. B: HDMECs and HUVECs were cultured in the absence or presence of 1 ng/ml TGF-β1 for 6 days. For both A and B, cell lysates were analyzed by Western blot using anti-CD31 and anti-α-SMA.
Transdifferentiation in Vivo
To determine whether evidence of the transdifferentiation process could be detected in vivo in mature valve leaflets, we stained frozen sections of aortic valves from mature sheep with anti-CD31 and anti-α-SMA (Figure 6) ▶ . Figure 6, A and B ▶ , are adjacent sections stained with anti-CD31 and anti-α-SMA, respectively, that demonstrate co-localization of these two antigens in focal regions along the leaflet endothelium, suggesting these ECs may be transdifferentiating. Figure 6, C and D ▶ , show low-power photomicrographs of the same sections to demonstrate co-localization of CD31 and α-SMA occurs in subregions of the endothelial layer, and that CD31 was expressed predominantly along one side of the leaflet, whereas α-SMA was most abundant in the subendothelial region adjacent to the base of the leaflet.
Figure 6.
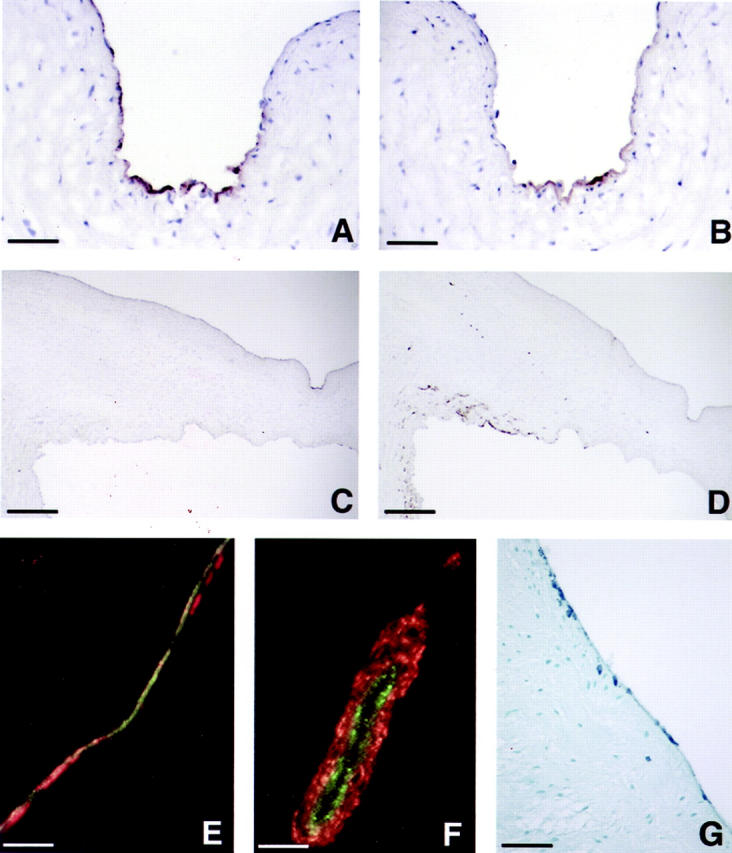
Transdifferentiation in mature valve leaflets. Immunoperoxidase staining of adjacent frozen sections of ovine aortic valve with anti-CD31 (A and C) and anti-α-SMA (B and D) is shown at ×400 original magnification (A and B) to focus on regions of co-localization of the two antigens and ×40 original magnification (C and D). The indented region seen on the upper surface of the leaflet on the right of C and D corresponds to the region shown in A and B. Scale bars: 12.5 μm (A and B) and 125 μm (C and D). E and F: Double-label immunofluorescence of ovine aortic valve leaflets with anti-vWF (green) and anti-α-SMA (red). F: A double-labeled vessel located toward the base of the leaflet as an internal control. G: A paraffin section of human aortic valve leaflet stained with anti-α-SMA at ×100 original magnification. Scale bar, 50 μm.
To identify directly cells undergoing transdifferentiation, we performed double-label immunofluorescence for vWF, an endothelial-specific protein, and α-SMA on frozen sections of ovine aortic valve (Figure 6, E and F) ▶ . In Figure 6E ▶ , vWF-positive ECs (green), double-labeled vWF-positive/α-SMA-positive cells (yellow/orange) and α-SMA-positive cells (red) were observed. The double-labeled cells and cells expressing only α-SMA seem to represent intermediates in the transdifferentiation process. Similar results were observed in human aortic valve sections (data not shown). Classic blood vessel architecture was observed in the ovine aortic root and served as a positive control in this analysis (Figure 6F) ▶ . In paraffin sections from human aortic valve leaflets, α-SMA-positive cells were seen along the endothelium and also seemed to be migrating into the subendothelial regions (Figure 6G) ▶ , similar to what was observed in ovine aortic valve (Figure 6E) ▶ .
Discussion
Our studies demonstrate that ECs are present in mature cardiac semilunar valves that transdifferentiate in vitro to a mesenchymal phenotype. A soluble TGF-β antagonist revealed two distinct pathways, one TGF-β-mediated and the other reduced serum-mediated, in clonal EC populations from aortic valve leaflets. Transdifferentiation by either pathway coincided with markedly increased migration toward PDGF-BB, consistent with the increased migratory phenotype associated with mesenchymal cells. Further analyses showed that the endothelial-to-mesenchymal transdifferentiation: 1) peaked at 5 to 7 days, 2) seemed to be irreversible, and 3) did not prevent cells from progressing through the cell cycle. In all experiments, CD31 protein expression was retained in cells that had transdifferentiated, suggesting that the process was incomplete and that the experimental conditions we used were not sufficient to allow down-regulation of this endothelial marker.
To our knowledge, the only nonvalvular ECs reported to express α-SMA are bovine aortic ECs treated with TGF-β1. 19 In this study, the α-SMA expression induced by TGF-β1 was reversible. Endothelial-to-mesenchymal transdifferentiation has also been suggested to occur in the embryonic quail dorsal aorta, raising the possibility that transdifferentiation may give rise to subendothelial α-SMA-positive cells in other vascular beds. 24 We cultured ECs from several sources to determine whether any of these cells would transdifferentiate in response to TGF-β1 or reduced medium. We saw transdifferentiation only in valvular ECs and not in ECs from microvascular or venular sources. Hence, the valve-derived clonal cell populations described here provide a unique and powerful in vitro model for elucidating the molecular events that regulate valvular endothelium.
TGF-β and the type II and type III TGF-β receptors have been implicated in the endothelial-to-mesenchymal transdifferentiation that underlies the formation of the endocardial cushions during heart development. 3-6,25,26 Furthermore, attenuation of TGF-β signaling seems to be critical for proper development of the aortic and pulmonary valve leaflets, because mice lacking the inhibitor of TGF-β signaling, SMAD6, have an overabundance of mesenchymal cells that results in hyperplastic thickening of the valves. 27 In addition, a number of genes, such as neurofibromin−1, 28 NF-ATc, 29,30 and endoglin 31 have been found to be essential for normal valve development based on phenotypes observed in mice genetically deficient in these gene products. For the most part, the valvular defects in these mice were not anticipated and underscore how little is known about the molecular basis of valve development.
Based on our observations, we hypothesize that adult valvular endothelium contains a subset of cells capable of undergoing transdifferentiation to a mesenchymal cell phenotype. These cells may serve to replenish the interstitial cells, which in turn synthesize the extracellular matrix needed for maintenance of the leaflet architecture. Such cells would need to be capable of prolonged self-renewal and might, therefore, be thought of as progenitor-like cells. This speculation is supported by the fact that clonal EC populations were readily obtained from ovine pulmonary and aortic valve leaflets and were expanded easily for at least 20 passages. Further studies will be required to determine the significance of these cells for valve function and durability in vivo.
Acknowledgments
We thank Dmitri Zvagelsky for technical assistance with preparing tissue sections; and Dr. John E. Mayer, Jr., Cardiovascular Surgery, Children’s Hospital, Boston, for ovine and human valve leaflet tissue and support for this project.
Footnotes
Address reprint requests to Joyce Bischoff, Ph.D., Department of Surgery, Children’s Hospital, 300 Longwood Ave., Boston, MA 02115. E-mail: joyce.bischoff@tch.harvard.edu.
Supported by the National Heart Lung and Blood Institute (grant R01 HL60490), the Vascular Pathology Core at the Brigham and Women’s Hospital (HL48743 PPG), and a fellowship from the Sarnoff Foundation (to S. K.).
References
- 1.Eisenberg LM, Markwald RR: Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res 1995, 77:1-6 [DOI] [PubMed] [Google Scholar]
- 2.Schoen FJ, Levy RJ: Tissue heart valves: current challenges and future research perspectives. J Biomed Mat Res 1999, 47:439-465 [DOI] [PubMed] [Google Scholar]
- 3.Mjaatvedt CH, Yamamura H, Wessels A, Ramsdell A, Turner D, Markwald RR: Mechanisms of segmentation, septation, and remodeling of the tubular heart: endocardial cushion fate and cardiac looping. Harvery RP Rosenthal N eds. Heart Development. 1999, :pp 159-177 Academic Press, San Diego [Google Scholar]
- 4.Ramsdell AF, Markwald RR: Induction of endocardial cushion tissue in the avian heart is regulated in part by TGF-beta3-mediated autocrine signalling. Dev Biol 1997, 188:64-74 [DOI] [PubMed] [Google Scholar]
- 5.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB: TGF beta 2 and TGF beta 3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol 1999, 208:530-545 [DOI] [PubMed] [Google Scholar]
- 6.Nakajima Y, Yamagishi T, Nakamura H, Markwald RR, Krug EL: An autocrine function for transforming growth factor (TGF)-beta3 in the transformation of atrioventricular canal endocardium into mesenchyme during chick heart development. Dev Biol 1998, 194:99-113 [DOI] [PubMed] [Google Scholar]
- 7.Nakajima Y, Yamagishi T, Yoshimura K, Nomura M, Nakamura H: Antisense oligonucleotide complementary to smooth muscle alpha-actin inhibits endothelial-mesenchymal transformation during chick cardiogenesis. Dev Dyn 1999, 216:489-498 [DOI] [PubMed] [Google Scholar]
- 8.Schor AM, Schor SL, Arciniegas E: Phenotypic diversity and lineage relationships in vascular endothelial cells. Potten GS eds. Stem Cells. 1997, :pp 119-146 Academic Press Limited, London [Google Scholar]
- 9.Simon A, Zavazava N, Sievers HH, Muller-Ruchholtz W: In vitro cultivation and immunogenicity of human cardiac valve endothelium. J Card Surg 1993, 8:656-665 [DOI] [PubMed] [Google Scholar]
- 10.Mulholland DL, Gotlieb AI: Cell biology of valvular interstitial cells. Can J Cardiol 1996, 12:231-236 [PubMed] [Google Scholar]
- 11.Filip DA, Radu A, Simionescu M: Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res 1986, 59:310-320 [DOI] [PubMed] [Google Scholar]
- 12.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G: A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986, 103:2787-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JD, Bryant SR, Couper LL, Vary CPH, Gotwals PJ, Koteliansky VE, Lindner V: Soluble transforming growth factor-beta type II receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res 1999, 84:1212-1222 [DOI] [PubMed] [Google Scholar]
- 14.Kraling BM, Bischoff J: A simplified method for growth of human microvascular endothelial cells results in decreased senescence and continued responsiveness to cytokines and growth factors. In Vitro Cell Dev Biol 1998, 33:308-315 [DOI] [PubMed] [Google Scholar]
- 15.Gimbrone MA, Cotran RS, Folkman J: Human vascular endothelial cells in culture. J Cell Biol 1974, 60:673-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson CJ, Garbett PK, Nissen B, Schrieber L: Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci 1990, 96:257-262 [DOI] [PubMed] [Google Scholar]
- 17.Bischoff J, Brasel C, Kraling B, Vranovska K: E-selectin is upregulated in proliferating endothelial cells in vitro. Microcirculation 1997, 4:279-287 [DOI] [PubMed] [Google Scholar]
- 18.Banyard J, Anand-Apte B, Symons M, Zetter BR: Motility and invasion are differentially modulated by Rho family GTPases. Oncogene 2000, 19:580-591 [DOI] [PubMed] [Google Scholar]
- 19.Arciniegas E, Sutton AB, Allen TD, Schor AM: Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci 1992, 103:521-529 [DOI] [PubMed] [Google Scholar]
- 20.Moulin V, Garrel D, Auger FA, O’Connor-Mccourt M, Castilloux G, Germain L: What’s new in human wound healing myofibroblasts. Curr Top Pathol 1999, 93:123-133 [DOI] [PubMed] [Google Scholar]
- 21.Owens GK: Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995, 75:487-516 [DOI] [PubMed] [Google Scholar]
- 22.Aikawa M, Sivam PN, Kuro OM, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, Nagai R: Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res 1993, 73:1000-1012 [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom M, Kal MN, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 24.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC: Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 1997, 80:444-451 [DOI] [PubMed] [Google Scholar]
- 25.Brown CB, Boyer AS, Runyan RB, Barnett JV: Antibodies to the type II TGFbeta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Biol 1996, 174:248-257 [DOI] [PubMed] [Google Scholar]
- 26.Brown CB, Boyer AS, Runyan RB, Barnett JV: Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science 1999, 283:2080-2082 [DOI] [PubMed] [Google Scholar]
- 27.Galvin KM, Donavan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA, Falb D, Huszar D: A role for Smad6 in development and homeostasis of the cardiovascular system. Nat Genet 2000, 24:171-174 [DOI] [PubMed] [Google Scholar]
- 28.Lakkis MM, Epstein JA: Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development 1998, 125:4359-4367 [DOI] [PubMed] [Google Scholar]
- 29.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW: Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 1998, 392:182-185 [DOI] [PubMed] [Google Scholar]
- 30.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH: The transcription factor NF-ATc is essential for cardiac valve formation. Nature 1998, 392:186-190 [DOI] [PubMed] [Google Scholar]
- 31.Bourdeau A, Dumont DJ, Letarte M: A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999, 104:1343-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]